Abstract
Bartonella is a Gram-negative pathogen that is unique among bacteria in being able to induce angioproliferative lesions. Cultured human endothelial cells have provided an in vitro system in which to study the basis of angioproliferation. Previous studies have attributed the organism's ability to induce angioproliferative lesions to direct mitotic stimulation of endothelial cells by these bacteria. Here we show that Bartonella inhibits apoptosis of endothelial cells in vitro, and that its ability to stimulate proliferation of endothelial cells depends to a large extent on its antiapoptotic activity. Bartonella suppresses both early and late events in apoptosis, namely caspase activation and DNA fragmentation, respectively. Its ability to inhibit death of endothelial cells after serum starvation can be recapitulated by media conditioned by bacteria, indicating that direct cell contact is not necessary. Among tested strains, the activity is produced only by Bartonella species that are significant human pathogens and are associated with angioproliferative lesions. We suggest that endothelial cells normally respond to infection by undergoing apoptosis and that Bartonella evolved the antiapoptotic activity to enhance survival of the host cells and therefore itself. We propose that Bartonella's antiapoptotic mechanism accounts at least in part for its ability to induce vascular proliferation in vivo.
Bartonella are unique among bacterial pathogens in their ability to cause angioproliferative lesions (1–3). These lesions are comprised of proliferating endothelial cells admixed with bacteria and can be found in many organs of the body. The disease process has been named bacillary angiomatosis when Bartonella henselae and Bartonella quintana are present. In the liver and spleen, the lesions can assume a cystic form referred to as bacillary peliosis. Bacillary angiomatosis and peliosis occur exclusively in immunocompromised patients, predominantly affecting patients with AIDS, and can be life-threatening.
In immunocompetent individuals, B. henselae and B. quintana cause “cat-scratch disease” and “trench fever” (1–3). These prolonged febrile illnesses are associated with infection in blood and tissues that may last for months and can result in infection and destruction of heart valves. Approximately 20,000 cases of cat-scratch disease occur a year, with an estimated 2,000 requiring hospitalization. Trench fever predominantly affects homeless alcoholics but in the past was responsible for an epidemic of over 1 million cases during World War I. An additional species, Bartonella bacilliformis, causes disease in immunocompetent individuals within a geographically confined region in South America. This infection is associated with red cell destruction, immune suppression, and multiple angioproliferative skin lesions. Cat fleas, body lice, and sand flies serve as vectors for human infection by B. henselae, B. quintana, and B. bacilliformis, respectively.
In these disease states, Bartonella has been found associated with endothelial cells. For example, in cat-scratch disease, B. henselae has been found in vessel walls (4), and in angioproliferative lesions, Bartonella is found both within and in clusters surrounding endothelial cells (5, 6). Presumably, endothelial association is necessary for the organism's survival in vivo. It allows replication of an extremely slow growing organism in a compartment protected from the host immune response. This association also must help account for the sustained blood infections that allow bloodsucking insect vectors to transmit infection from one host to another. In vitro studies have confirmed an ability to invade and multiply within endothelial cells (7–9). After actin-dependent invasion, intracellular replication occurs within a vacuole termed an invasome (10).
Very little is known currently about how Bartonella causes disease at the molecular level. However, it is thought that direct endothelial association underlies Bartonella's ability to cause angioproliferative lesions. These lesions only occur in association with bacteria (5, 6), suggesting the action of bacterial factors that act locally or are bacterial cell-associated. The disappearance of proliferative lesions after antibiotic treatment confirms a primary role for Bartonella in their formation. A number of studies have examined whether Bartonella could similarly stimulate endothelial proliferation in vitro (11–14). In each of these studies, human umbilical cord endothelial cells (HUVECs) were infected with Bartonella in a medium lacking endothelial mitogens. In all the studies, a relative increase in the number of endothelial cells was found in infected cultures. This effect was proposed to result from a bacterial mitogenic activity that also would account for angioproliferative lesions observed in vivo. However, the mechanism underlying this phenomenon remains unclear. Here we examined the underlying basis for this increased endothelial proliferation in the presence of Bartonella. We reasoned that the increase in cell numbers could be caused by either increased cell division or reduced cell death. To our surprise, we found that increased numbers were caused predominantly by decreased cell death. Furthermore, the decrease in cell death was specifically the result of a bacterial antiapoptotic activity. Below we describe details of this phenomenon and hypothesize that endothelial proliferation in vivo also relies on antiapoptotic activity.
Materials and Methods
Strains and Cell Lines.
Unless otherwise indicated, infections were with B. henselae (ATCC 49882). B. henselae strains 88-712, 87-66 (lower passage than corresponding ATCC 49793), and Bartonella vinsonii VR-152 were a kind gift of Denise Pickett-Robison (Univ. of Oklahoma Medical Center, Oklahoma City, OK). Other strains were purchased from the ATCC. Bacteria were cultured at 37°C with 5% CO2 on tryptic soy agar with 5% sheep blood (BD Biosciences, Sparks, MD) or heart infusion agar with 5% rabbit blood (BBL) for more fastidious strains. HUVECs and neonatal human dermal microvascular endothelial cells (Clonetics, San Diego) were grown in EGM-2 and used at passage number 5–7. MRC-5, Hep-2, A549, HEL, WI-38, RD, and Madin–Darby canine kidney cell lines were from BioWhittaker.
Infections.
To achieve an estimated ratio of 500 bacteria per mammalian cell based on optical density (OD600 = 1 estimated at 1E9 bacteria per ml), 6–7-day patches of bacteria were suspended in Media 199 with Earle's salts (M199) + 10% FBS (Life Technologies, Rockville, MD). Unless otherwise indicated, cell lines plated at a density of 12,000 per cm2 in the same medium were infected for 24 h followed by serum starvation or exposure to actinomycin D (Sigma–Aldrich).
Proliferation Assays.
Endothelial cells plated at 3,000 per cm2 (1,000 cells per assay) were infected or treated with human vascular endothelial growth factor-165 and bFGF (R&D Systems) in the presence of BrdUrd. BrdUrd incorporation was determined by using the cell-proliferation BrdUrd ELISA (Roche Molecular Biochemicals). For microscopic determination of proliferation rate, HUVECs infected in M199 with 10% FBS were fed daily with the same medium. Z-VAD-FMK (Biomol) was added daily at 50 μM. At 48 and 96 h, cells were fixed in 4% paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) for 10 min. Microscopy was performed with a Nikon Eclipse fluorescent microscope, and ×40 images were captured with a Nikon DXM 1200 digital camera. The cell number was quantified by using IPLAB for Macintosh 3.5 (Scanalytics, Billerica, MA). Anaphase and telephase nuclei were identified easily from low-power images and counted manually to determine a mitotic rate (anaphase + telephase nuclei/total cell number). This procedure allowed us to scan large numbers of cells. We determined the relationship between mitotic rate and doubling time in highly supplemented medium (EGM-2 MV) in which almost no apoptosis occurred and doubling took place in ≈24 h (data not shown).
Cell-Death Assays.
The viability of mammalian cells was determined by assaying for total cytoplasmic lactate dehydrogenase (LDH) activity by using the Cytotox 96 nonradioactive cytotoxicity assay (Promega; ref. 15). To ensure that only cytoplasmic LDH was measured, mammalian cells were washed three times with PBS and then lysed in 0.9% Triton X-100 in PBS. The washing step served to remove unbound cells and wash away cytoplasmic LDH from cells that no longer maintain an intact cell membrane, including cells that have undergone necrosis and those in the later stages of apoptosis when cell-membrane integrity is lost (15, 16). The majority of apoptotic cells would be accounted for by a reduction in the total LDH measured, because they would have already lost cell-membrane integrity or their attachment to the substratum and therefore not contribute to LDH activity. Absorbance values correlated directly with cell number over the range of our assays (data not shown). The CellTiter 96 Aqueous One Solution cell-proliferation assay (MTS reduction, Promega) was performed according to manufacturer instructions.
For terminal dUTP nick end-labeling (TUNEL) assays, HUVECs were plated on CC2 chamber slides (Nunc), infected overnight, and serum-starved or treated with actinomycin D for 6 h. Slides were fixed in 4% paraformaldehyde in PBS. TUNEL assays were performed by using the fluorescein-dUTP in situ cell-death detection kit (Roche), and slides were counterstained for 5 min with DAPI. Images (×100) were captured by using FITC (TUNEL-positive cells) and DAPI (total cells) filter sets, and the percentage of TUNEL positivity was determined. Low molecular weight, histone-complexed DNA fragments were quantified by using the Cell-Death Detection ElisaPLUS (Roche). For specific caspase assays, infected cultures were serum-starved for 5 h and assayed as described previously (17) with minor modifications. Briefly, 250,000 cells per assay were lysed in 150 mM Tris⋅HCl, pH 7.4/1% Triton X-100 and incubated in a reaction buffer containing 50 mM Hepes, pH 7.4, 75 mM NaCl, 0.1% CHAPS, 5 mM DTT, and 25 μM of the indicated caspase substrates (Alexis Biochemicals, San Diego) ± 10 μM of specific caspase inhibitors; 7-amino-4-methylcoumarin (Molecular Probes) standards were used to calculate enzymatic units (μmol/h of cleaved substrate). Alternatively, a broad-spectrum caspase assay (DEVD-R110 substrate) was performed directly on infected endothelial cells in a 96-well plate format by using the fluorometric homogenous caspase assay (Roche).
Results
Bartonella Has a Minimal Effect on Endothelial Mitosis.
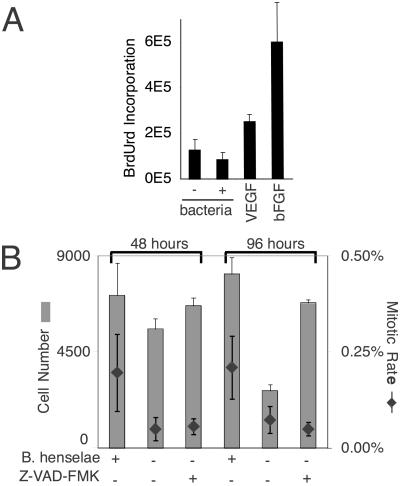
We first asked whether Bartonella-induced cell proliferation (11–13) is a result of increased mitosis. Mitotic stimulation of HUVECs was examined by incorporation of the thymidine analog, BrdUrd, under conditions previously reported to result in cell proliferation. During a 48-h labeling period, B. henselae-infected cultures did not exhibit BrdUrd incorporation beyond that observed for uninfected cells (Fig. 1A). Similar results were obtained with other Bartonella species, including B. quintana, B. vinsonii, and Bartonella elizabethae (data not shown). To demonstrate that the endothelial cells were competent for mitosis, we showed that BrdUrd incorporation was induced by two known endothelial mitogens, vascular endothelial growth factor and basic fibroblastic growth factor. Thus, Bartonella did not appear to stimulate mitosis at a level detectable by this assay.
Figure 1.
Analysis of mitosis. (A) HUVECs were infected or treated with 10 ng/ml vascular endothelial growth factor (VEGF) or 25 ng/ml basic fibroblastic growth factor (bFGF) for 48 h in medium containing BrdUrd (in sextuplicate). (B) Number of HUVECs and mitotic rate at 48 and 96 h after infection with B. henselae 49882. Mean and SD of cells from three different assays were used, and six ×40 fields were counted per assay.
We then used a more sensitive microscopic assay to look further for possible mitotic stimulation. At 48 and 96 h, we observed a relative increase in mitotic rate of 0.15% for infected cultures (Fig. 1B). However, by 96 h there were three times as many infected as uninfected cells. This 3-fold difference could not be explained completely by the small 0.15% increase in relative mitotic rate. In this system, a mitotic rate of 1.4% corresponded to a doubling time of 24 h (data not shown). At this rate, the infected cultures would double relative to uninfected cultures only after 9 days in culture. Thus, the relative difference in cell numbers could be explained only partially by mitotic stimulation.
An Antiapoptotic Activity in Bartonella.
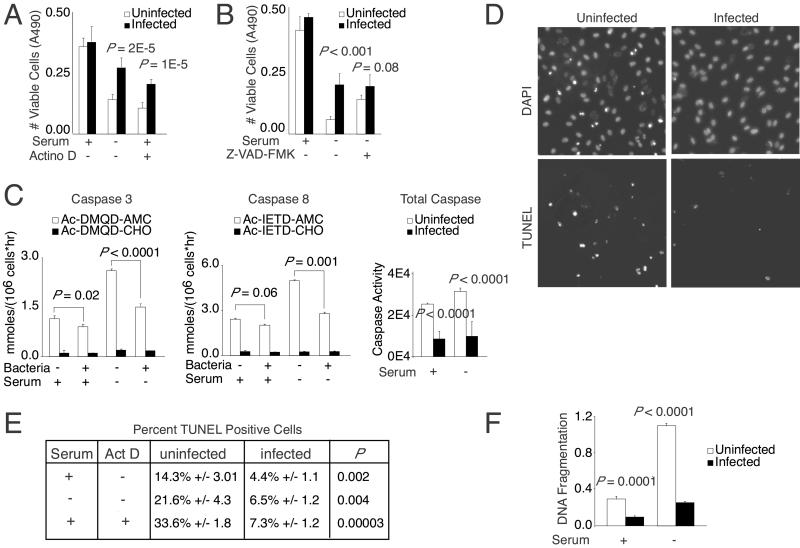
We next asked whether this difference could be accounted for by an inhibition of cell death. To test whether Bartonella inhibited cell death, we stressed infected endothelial cells by either serum starvation or actinomycin D treatment in the presence of serum, two conditions that are known to induce apoptosis in endothelial cells (18, 19). Using a cytoplasmic LDH assay to assess cell viability, we found that B. henselae infection significantly reduced cell death induced by these treatments (Fig. 2A). We confirmed these results by using an alternative method to measure viable cell number. Specifically, the MTS reduction assay also showed an ≈2-fold increase in viability of Bartonella-infected cells as compared with uninfected controls (data not shown). This difference was observed under both serum starvation and actinomycin D treatment conditions.
Figure 2.
Bartonella inhibits apoptosis. (A) Endothelial viability after serum starvation or treatment with 400 nM actinomycin D in the presence of serum for 18 h (in sextuplicate). The P values were determined by using an unpaired Student's t test. (B) Endothelial viability after serum starvation for 18 h in the presence of 50 μM Z-VAD-FMK (in sextuplicate). (C) Caspase activity after 6 h of serum starvation (in triplicate). The values shown are proteolytic activity for caspase 3 and 8 substrates [Ac-DMQD-7-amino-4-methylcoumarin (AMC) and Ac-IETD-7-amino-4-methylcoumarin, respectively] in the presence or absence of their respective inhibitors (Ac-DMQD-CHO and Ac-IETD-CHO). Proteolysis of caspase 3 family substrate DEVD-R110 is expressed in relative fluorescent units (in octuplicate). (D) After serum starvation or treatment with 50 nM actinomycin D for 6 h, endothelial cells were labeled by using a TUNEL assay and counterstained with DAPI. Representative images of actinomycin D-treated cells are shown. (E) Percentage of TUNEL positivity [mean and SD of three separate assays with three ×100 fields (>1,000 cells) counted per assay]. (F) DNA fragmentation measured by immunological ELISA after 18 h of serum starvation (in triplicate).
We then asked whether this effect on HUVEC death was caused by a block specifically in apoptosis. To this end, we compared Bartonella infection to the effect of adding Z-VAD-FMK, an antiapoptotic agent with broad-spectrum anti-caspase activity (20). In all the experiments described below, apoptosis was induced by serum starvation or actinomycin D as indicated in the figure legends. We found that B. henselae suppressed endothelial cell death as effectively as the caspase inhibitor Z-VAD-FMK (Fig. 2B). In addition, when added in the presence of bacteria, Z-VAD-FMK did not lead to increased viability of endothelial cells beyond that afforded by bacteria alone. Because the effects were nonadditive, Bartonella most likely acts in the same pathway as Z-VAD-FMK. Therefore, Bartonella seems to preserve HUVEC viability by inhibiting apoptosis. Interestingly, neither Z-VAD-FMK nor bacteria could prevent cell loss completely, suggesting that either Z-VAD-FMK and Bartonella could not inhibit apoptosis completely or a subpopulation of refractory cells might be dying in an apoptosis-independent pathway.
We sought further proof of an antiapoptotic effect by assessing effects on caspase activation (Fig. 2C), one of the first steps of the apoptosis pathway in which initial signals are amplified by a cascade of sequentially activated proteases. Specific caspase assays showed that caspases 3 and 8 were inhibited significantly by bacterial infection. Although caspase 8 generally is considered to act upstream of caspase 3, it is unclear whether Bartonella inhibited either caspase directly, because both early and late caspases participate in a positive feedback cycle that amplifies total caspase activity (21). Caspase 1 activity was not induced significantly under our assay conditions (data not shown). Using a general caspase assay, we found that B. henselae also inhibited members of the caspase 3 family. Therefore, B. henselae inhibited the activation of the caspase proteolytic cascade, indicating that early events of apoptosis are blocked by Bartonella.
To test whether later events were disrupted also, we examined Bartonella's effect on endonuclease-mediated chromatin fragmentation, a common endpoint of the apoptotic pathway. Using the TUNEL assay (22), we found that infection significantly reduced the number of HUVECs with fragmented chromatin occurring after serum starvation or actinomycin D treatment (Fig. 2 D and E). Corresponding DAPI images were consistent with these results, as indicated by the characteristic condensed and fragmented nuclei (Fig. 2D; refs. 16 and 23). Similar results were observed using an immunological assay that detects low molecular weight, histone-associated DNA fragments (Fig. 2F). Thus, Bartonella infection also blocked late events of apoptosis.
Taken together, these assays demonstrated that Bartonella inhibits endothelial cell death by disrupting the apoptosis pathway. The evidence also argues that the relative difference in endothelial cell numbers observed during prolonged infection (Fig. 1B) can be accounted for largely by this effect on apoptosis. More specifically, although a 0.15% mitotic increase could not explain the relative increase in cell number at 96 h postinfection, blocking apoptosis with Z-VAD-FMK produced a quantitatively similar result to Bartonella infection (Fig. 1B). These results therefore provided in vitro evidence that Bartonella-induced endothelial proliferation relies on a bacterially associated antiapoptotic factor. It is worthy to note also that an antiapoptotic effect of Bartonella could be seen in serum-plus conditions (Fig. 2 C–F). This result was completely expected, because culture conditions generally induce some degree of apoptosis in all cell types. In the case of endothelial cells, ≈14.3% in serum were TUNEL-positive (Fig. 2E). Serum starvation increased the number of apoptotic cells to ≈21.6%. The fact that Bartonella infection resulted in a 3-fold decrease under both culture conditions indicates that the antiapoptotic factor is not induced by the lack of serum per se.
Antiapoptotic Activity Required Bacterial RNA and Protein Synthesis.
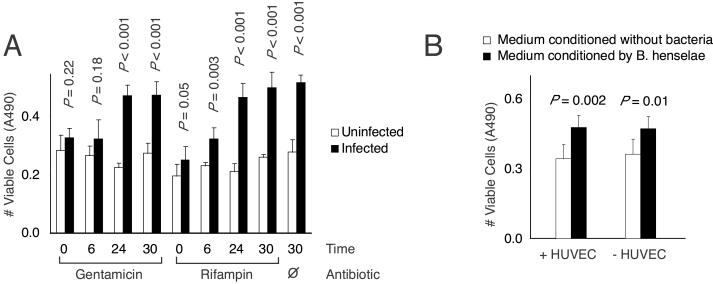
To assess whether new synthesis was necessary for antiapoptotic activity, infections were carried out in the presence of bacterial RNA and protein synthesis inhibitors. When added immediately after infection, both rifampin (a bacterial RNA polymerase inhibitor) and gentamicin (a bacterial protein synthesis inhibitor) completely abolished Bartonella's antiapoptotic activity (Fig. 3A). When added 6 h after infection, rifampin now only partially interfered with antiapoptotic activity, whereas gentamicin was still fully inhibitory. When added 24 or 30 h after infection, neither antibiotic was inhibitory. Although we cannot exclude an indirect effect of these inhibitors, these results suggested that either the antiapoptotic factor is not preformed and is synthesized during the first 24 h after infection or a preformed antiapoptotic factor requires additional synthetic activity for its eventual release.
Figure 3.
(A) Effect of antibiotics. Gentamicin (100 μg/ml) or rifampin (5 μg/ml) was added at indicated times after infection. At 30 h, HUVEC cultures were serum-starved overnight in the presence of antibiotics, and cell viability was determined (in quadruplicate). (B) Endothelial viability after exposure to conditioned medium. Tester endothelial cells were treated with bacterially conditioned media made in the presence (+) or absence (−) of HUVECs and then serum-starved for 18 h (in sextuplicate).
Conditioned Medium Contained Antiapoptotic Activity.
The antiapoptotic factor could be either released into the medium or closely associated with the bacteria. In the latter case, direct bacterial contact might be required to prevent endothelial cell death in response to serum starvation. To distinguish between these possibilities, we generated culture medium that was preconditioned by Bartonella during a 24-h infection of endothelial cells (Fig. 3B, +HUVECs histograms). The medium was passed through a 0.2-μm filter to remove all bacteria and then fed for 24 h to test cells (endothelial cells that were not exposed previously to bacteria). When test cells subsequently were serum-starved for 18 h, we observed a modest protection from cell death. This result suggested that the antiapoptotic factor is released into the culture medium, and bacteria contact with endothelial cells was not necessary. However, the protective effect was not as robust as that seen during direct bacterial infection (Fig. 2A). This difference could mean that either the secreted antiapoptotic activity is labile or other bacterial factors, perhaps themselves labile or requiring direct bacterial contact, are needed to maximize the antiapoptotic effect.
To determine whether the antiapoptotic factor is synthesized in response to endothelial cells, we generated culture medium that was preconditioned by Bartonella in the absence of endothelial cells (Fig. 3B, −HUVECs histograms). Surprisingly, a modest antiapoptotic effect could also be observed on test endothelial cells. Therefore, the factor appeared to be made constitutively or was at least induced in response to something present in serum-containing tissue culture medium. This effect could be elicited by both standard M199 and DMEM. These data combined argued that the antiapoptotic factor is made by bacteria and released into the extracellular environment, but the factor does not seem to be induced specifically by endothelial cells.
Host-Pathogen Specificity of the Antiapoptotic Activity.
Next, we tested whether antiapoptotic activity was limited to cells of human umbilical origin. We found that Bartonella could also reduce cell death of human dermal microvascular endothelial cells significantly (Table 1), an effect also dependent on inhibition of apoptosis as shown by suppression of caspase activation (P < 0.001) and DNA fragmentation (P = 0.02) (data not shown). Therefore, we found that endothelial cells of dermal microvascular origin (human dermal microvascular endothelial cells) also responded to Bartonella. On the other hand, there was no significant inhibition of cell death (P > 0.1) for fibroblast lines (MRC-5, WI-38, and HEL), carcinoma cell lines (HEp-2, A549, and Madin–Darby canine kidney), and an RD cell line (Table 1), suggesting that among the cell types tested, antiapoptotic activity was restricted to cells of endothelial origin.
Table 1.
Cell survival (LDH absorbance) of different cell lines after infection with B. henselae
| Cell line | Uninfected (SD) | Infected (SD) | P, t test | |
|---|---|---|---|---|
| HUVEC | Serum + | 0.36 (0.04) | 0.42 (0.06) | 0.57 |
| Serum − | 0.14 (0.02) | 0.27 (0.04) | 2E-5 | |
| Actino D | 0.11 (0.02) | 0.21 (0.02) | 1E-5 | |
| Human dermal microvascular endothelial | Serum + | 0.24 (0.01) | 0.27 (0.03) | 0.04 |
| Serum − | 0.19 (0.01) | 0.23 (0.02) | 0.001 | |
| Actino D | 0.19 (0.02) | 0.24 (0.02) | 0.002 | |
| MRC-5 | Serum + | 0.66 (0.04) | 0.73 (0.06) | 0.68 |
| Serum − | 0.50 (0.07) | 0.54 (0.04) | 0.78 | |
| Actino D | 0.61 (0.05) | 0.63 (0.03) | 0.93 | |
| WI-38 | Serum + | 0.51 (0.03) | 0.47 (0.03) | 0.78 |
| Serum − | 0.36 (0.02) | 0.38 (0.03) | 0.77 | |
| Actino D | 0.45 (0.06) | 0.50 (0.03) | 0.67 | |
| HEL | Serum + | 1.09 (0.05) | 1.14 (0.04) | 0.14 |
| Serum − | 0.67 (0.07) | 0.63 (0.03) | 0.38 | |
| Actino D | 0.86 (0.05) | 0.91 (0.04) | 0.13 | |
| A549 | Serum + | 1.03 (0.12) | 1.14 (0.08) | 0.17 |
| Serum − | 0.97 (0.08) | 0.93 (0.09) | 0.61 | |
| Actino D | 0.92 (0.07) | 0.92 (0.02) | 0.92 | |
| Modin–Darby canine kidney | Serum + | 0.82 (0.04) | 0.80 (0.07) | 0.6 |
| Serum − | 0.80 (0.03) | 0.76 (0.07) | 0.35 | |
| Actino D | 0.21 (0.02) | 0.24 (0.05) | 0.34 | |
| Hep-2 | Serum + | 1.65 (0.07) | 1.67 (0.12) | 0.8 |
| Serum − | 1.54 (0.06) | 1.51 (0.09) | 0.65 | |
| Actino D | 1.51 (0.03) | 1.55 (0.07) | 0.29 | |
| RD | Serum + | 1.24 (0.07) | 1.03 (0.21) | 0.11 |
| Serum − | 0.89 (0.04) | 0.86 (0.05) | 0.38 | |
| Actino D | 0.39 (0.07) | 0.46 (0.04) | 0.13 |
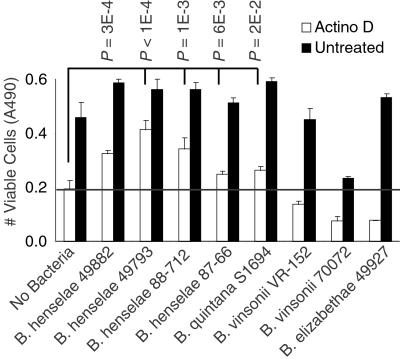
Because the ability to cause angioproliferative lesions is associated with a small subset of Bartonella, whether antiapoptotic effects were restricted to these species was also of interest. Among the strains tested, we found that multiple B. henselae isolates and B. quintana S1694 were able to inhibit significantly cell death induced by actinomycin (Fig. 4) and serum starvation (data not shown). These species have been associated with serious human disease and angioproliferative lesions. Antiapoptotic activity from B. quintana S1694 was confirmed by demonstrating significant suppression of DNA fragmentation (P < 1E-5; data not shown). In contrast, nonangiogenic species, B. vinsonii VR-152, B. vinsonii 700727, B. elizabethae 49927, and Bartonella clarridgeiae 51734, did not inhibit cell death (Fig. 4; data not shown for B. clarridgeiae). Interestingly, these species have rarely, if ever, been associated with human infection (24). Therefore, angioproliferative ability and human pathogenicity correlated with antiapoptotic potential in the genus Bartonella.
Figure 4.
Presence of antiapoptotic activity in different Bartonella strains. Endothelial viability was determined after treatment with actinomycin D (in quadruplicate). The P values shown for actinomycin D-treated samples are pairwise comparisons between uninfected HUVECs and HUVECs infected with indicated Bartonella strains. The horizontal bar indicates baseline survival in uninfected cells.
Discussion
We have studied an in vitro model of Bartonella pathogenesis and found that the bacterium enhances growth of human endothelial cells through an as yet unidentified antiapoptotic factor. We provide evidence that this antiapoptotic factor is found in species that are highly pathogenic in humans. Although future studies will need to examine a greater range of cell lines, our present study suggests that the antiapoptotic factor may act preferentially on cells of endothelial origin. We propose that this antiapoptotic property of Bartonella accounts at least in part for its previously described proliferative effects on endothelial cells in vitro.
In vivo, Bartonella causes angioproliferative diseases involving the formation of numerous immature vessels (6, 25–27). The results of our study raise the obvious question of whether inhibition of apoptosis also might lead to endothelial proliferation and, hence, angioproliferation in vivo. Indeed, emerging evidence shows that cells of the neovasculature are critically dependent on antiapoptotic signals for their survival (28–30). Experimentally suppressing apoptosis by increasing Bcl-2 levels promotes angiogenesis in tumors (31), whereas withdrawal of antiapoptotic survival signals leads to regression of vessels (28). In contrast, mature vessels become relatively independent of antiapoptotic survival signals and are largely quiescent (32). Prevalent models for angiogenesis therefore envision a dynamic interplay between opposing forces that promote or inhibit vessel formation (33). On the basis of our present findings, we predict that Bartonella-associated angioproliferation depends on the antiapoptotic factor uncovered by our experiments.
However, as suggested by previous in vivo observations, antiapoptotic activity by itself cannot explain angioproliferative lesions completely. Endothelial cells within these lesions show increased levels of mitotic activity (6, 25–27), suggesting that mitotic stimulation of endothelial cells plays a significant role, which is in contrast to our in vitro studies in which only a small degree of mitotic activity was observed. This difference could be explained in one of three ways. First, it is possible that our in vitro assay was insensitive for detecting bacterial mitogenic activity. Second, Bartonella may stimulate mitosis indirectly via cell types that were not present in our culture system. In vivo, bacillary angiomatosis is associated with the accumulation of inflammatory cells (25–27) that can secrete potent endothelial mitogens and are believed to be partly responsible for angiogenesis at inflammatory sites (30, 34). In this case, Bartonella's antiapoptotic activity would amplify the angiogenesis that normally occurs during inflammation. Finally, our in vitro assay did detect a small but measurable increase in mitotic activity between 48–96 h. Although relatively weak in vitro, this mitogenic activity may be significant when acting in vivo over the course of weeks and months, especially if combined with the type of antiapoptotic activity reported here. The development of an in vivo model for bacillary angiomatosis and assessment of the relative contributions of mitogenic and antiapoptotic activities will help differentiate among these possibilities.
In conclusion, nature has demonstrated repeatedly that hosts and pathogens evolve side by side. Host organisms seek to minimize damage, and pathogens attempt to evade host defenses, propagate, and spread. Because Bartonella has a long generation time (weeks to grow on bacterial medium), it is particularly vulnerable to rapid elimination by host defenses. Despite this vulnerability, Bartonella can cause sustained infections in the blood stream for many months, perhaps partly because of its association with endothelial cells. Here, Bartonella likely finds a hospitable environment in which it is protected from clearance by the immune system. One common defense strategy against this type of intracellular parasitism is the elimination of infected cells via apoptosis (35, 36). Our work shows that Bartonella has evolved an antiapoptotic activity, presumably to combat this host defense. An antiapoptotic strategy apparently has been adopted by many other human pathogens including Mycobacteria (37), Salmonella (38), and Rickettsia (39) and numerous viruses (35). In the case of Bartonella, antiapoptotic activity may contribute to the development of angioproliferative lesions and lead to potentially fatal complications in immunocompromised hosts, perhaps as an unintended consequence of Bartonella's evolution to coexist with endothelial cells. Further elucidation of the interaction between this pathogen and endothelial cells will give us additional insights into apoptosis, angiogenesis, and survival strategies of highly evolved pathogens.
Acknowledgments
We thank Jeannie T. Lee and Shirley Stiver for critical reading of the manuscript. J.E.K. was supported by the Beth Israel Hospital Pathology Foundation and National Institutes of Health Grant AI-01402.
Abbreviations
- HUVEC
human umbilical vein endothelial cell
- DAPI
4′,6-diamidino-2-phenylindole
- LDH
lactate dehydrogenase
- TUNEL
terminal dUTP nick end-labeling
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Spach D H, Koehler J E. Infect Dis Clin North Am. 1998;12:137–155. doi: 10.1016/s0891-5520(05)70414-1. [DOI] [PubMed] [Google Scholar]
- 2.Maurin M, Birtles R, Raoult D. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 3.Maguina C G E. Infect Dis Clin North Am. 2000;14:1–22. doi: 10.1016/s0891-5520(05)70215-4. [DOI] [PubMed] [Google Scholar]
- 4.Guccion J G, Gibert C L, Ortega L G, Hadfield T L. Ultrastruct Pathol. 1996;20:195–202. doi: 10.3109/01913129609016315. [DOI] [PubMed] [Google Scholar]
- 5.Reed J A, Brigati D J, Flynnn S D, McNutt N S, Min K, Welch D F, Slater L N. Am J Surg Pathol. 1992;16:650–657. doi: 10.1097/00000478-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Arias-Stella J L, P H, Erlandson R A, Arias-Stella J., Jr Am J Surg Pathol. 1986;10:595–610. doi: 10.1097/00000478-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Brouqui P, Raoult D. Res Microbiol. 1996;147:719–731. doi: 10.1016/s0923-2508(97)85119-4. [DOI] [PubMed] [Google Scholar]
- 8.Kempf V A, Schaller M, Behrendt S, Volkmann B, Aepfelbacher M, Cakman I, Autenrieth I B. Cell Microbiol. 2000;2:431–441. doi: 10.1046/j.1462-5822.2000.00072.x. [DOI] [PubMed] [Google Scholar]
- 9.Verma A, Davis G E, Ihler G M. Infect Immun. 2000;68:5960–5969. doi: 10.1128/iai.68.10.5960-5969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. J Cell Sci. 1997;110:2141–2154. doi: 10.1242/jcs.110.18.2141. [DOI] [PubMed] [Google Scholar]
- 11.Conley T, Slater L, Hamilton K. J Lab Clin Med. 1994;124:521–528. [PubMed] [Google Scholar]
- 12.Garcia F U, Wojta J, Broadley K N, Davidson J M, Hoover R L. Am J Pathol. 1990;136:1125–1136. [PMC free article] [PubMed] [Google Scholar]
- 13.Maeno N, Oda H, Yoshiie K, Wahid M R, Fujimura T, Matayoshi S. Microb Pathog. 1999;27:419–427. doi: 10.1006/mpat.1999.0315. [DOI] [PubMed] [Google Scholar]
- 14.Palmari J, Teysseire N, Dussert C, Raoult D. Anal Cell Pathol. 1996;11:13–30. [PubMed] [Google Scholar]
- 15.Hohenwarter O, Waltenberg A, Katinger H. Anal Biochem. 1996;235:56–69. doi: 10.1006/abio.1996.0049. [DOI] [PubMed] [Google Scholar]
- 16.McGahon A J, Martin S J, Bissonnette R P, Mahboubi A, Shi Y, Mogil R J, Nishioka W K, Green D R. Methods Cell Biol. 1995;46:153–185. doi: 10.1016/s0091-679x(08)61929-9. [DOI] [PubMed] [Google Scholar]
- 17.Gurtu V, Kain S R, Zhang G. Anal Biochem. 1997;251:98–102. doi: 10.1006/abio.1997.2220. [DOI] [PubMed] [Google Scholar]
- 18.Grafe M, Steinheider G, Desaga U, Warnecke C, Lehmkuhl H B, Regitz-Zagrosek V, Hildebrandt A G, Fleck E. Clin Chem Lab Med. 1999;37:505–510. doi: 10.1515/CCLM.1999.081. [DOI] [PubMed] [Google Scholar]
- 19.Madge L A, Pober J S. J Biol Chem. 2000;275:15458–15465. doi: 10.1074/jbc.M001237200. [DOI] [PubMed] [Google Scholar]
- 20.Shao R G, Cao C X, Pommier Y. J Biol Chem. 1997;272:31321–31325. doi: 10.1074/jbc.272.50.31321. [DOI] [PubMed] [Google Scholar]
- 21.Bratton S B, MacFarlane M, Cain K, Cohen G M. Exp Cell Res. 2000;256:27–33. doi: 10.1006/excr.2000.4835. [DOI] [PubMed] [Google Scholar]
- 22.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr J F R, Gobe G C, Winterford C M, Harmon B V. Methods Cell Biol. 1995;46:1–27. doi: 10.1016/s0091-679x(08)61921-4. [DOI] [PubMed] [Google Scholar]
- 24.Schwartzman W. Annu Rev Med. 1996;47:355–364. doi: 10.1146/annurev.med.47.1.355. [DOI] [PubMed] [Google Scholar]
- 25.Cockerell C J, Tierno P M, Friedman-Kien A E, Kim K S. J Invest Dermatol. 1991;97:812–817. doi: 10.1111/1523-1747.ep12487507. [DOI] [PubMed] [Google Scholar]
- 26.LeBoit P E, Berger T G, Egbert B M, Beckstead J H, Yen T S, Stoler M H. Am J Surg Pathol. 1989;13:909–920. [PubMed] [Google Scholar]
- 27.Perkocha L A, Geaghan S M, Yen T S, Nishimura S L, Chan S P, Garcia-Kennedy R, Honda G, Stoloff A C, Klein H Z, Goldman R L, et al. N Engl J Med. 1990;323:1581–1586. doi: 10.1056/NEJM199012063232302. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin L E. Cancer Metastasis Rev. 2000;19:75–81. doi: 10.1023/a:1026552415576. [DOI] [PubMed] [Google Scholar]
- 29.Darland D C, D'Amore P A. J Clin Invest. 1999;103:157–158. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimmeler S, Zeiher A M. Circ Res. 2000;87:434–439. doi: 10.1161/01.res.87.6.434. [DOI] [PubMed] [Google Scholar]
- 31.Nor J E, Christensen J, Liu J, Peters M, Mooney D J, Strieter R M, Polverini P J. Cancer Res. 2001;61:2183–2188. [PubMed] [Google Scholar]
- 32.Benjamin L E, Golijanin D, Itin A, Pode D, Keshet E. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 34.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. J Leukocyte Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 35.Roulston A, Marcellus R C, Branton P E. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 36.Weinrauch Y, Zychlinsky A. Annu Rev Microbiol. 1999;53:155–187. doi: 10.1146/annurev.micro.53.1.155. [DOI] [PubMed] [Google Scholar]
- 37.Maiti D, Bhattacharyya A, Basu J. J Biol Chem. 2001;276:329–333. doi: 10.1074/jbc.M002650200. [DOI] [PubMed] [Google Scholar]
- 38.Steele-Mortimer O, Knodler L A, Marcus S L, Scheid M P, Goh B, Pfeifer C G, Duronio V, Finlay B B. J Biol Chem. 2000;275:37718–37724. doi: 10.1074/jbc.M008187200. [DOI] [PubMed] [Google Scholar]
- 39.Clifton D R, Goss R A, Sahni S K, van Antwerp D, Baggs R B, Marder V J, Silverman D J, Sporn L A. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]