Abstract
Many G protein-coupled receptor-mediated responses desensitize within minutes. Sustained stimulation of μ-opioid receptors (MORs), which primarily signal through Gi/o proteins, leads to activation and subsequent desensitization of G protein-coupled inwardly rectifying potassium (GIRK) currents. We observed that in neurons of the locus coeruleus, which express among the highest levels of MORs in the brain, the degree of desensitization depended on the intensity of receptor stimulation, indicating that the process is initiated at the receptor. Interestingly, while GIRK-mediated postsynaptic inhibition substantially desensitized within 15 min, presynaptic inhibition of afferent transmission, which involves other effector systems, remained constant, suggesting that the postsynaptic desensitization we observed is expressed at the effector. We show that desensitized GIRK currents can gradually be reactivated by additional G protein signals of increasing intensity and present evidence that desensitization is a G protein-mediated process. Finally, desensitization of MOR-induced GIRK currents had heterologous effects on responses mediated by other G protein-coupled receptors converging onto the same population of GIRK channels. Taken together, our results provide evidence for a form of desensitization mediated by a slowly developing G protein-dependent pathway, initiated at the MORs and leading to competitive inhibition of GIRK channel activation. This implies that MORs exert a bidirectional action on GIRK channels.
Induction of tolerance and dependence to opiates needs the activation of MORs (1). As for other G protein-coupled receptors (GPCRs) coupled to Gi/o proteins, stimulation of MORs leads to inhibition of adenylyl cyclase by the α subunit of the heterotrimeric G proteins, as well as inhibition of voltage-dependent calcium channels and activation of G protein-coupled inwardly rectifying potassium (GIRK) channels directly by the βγ dimers (2, 3) through a membrane-delimited process (4, 5).
Like many other GPCR-mediated responses, the MOR-induced GIRK currents display acute desensitization (6), the molecular mechanisms of which still remain elusive. In analogy with the β2 adrenergic receptor (7, 8), MOR–G protein uncoupling resulting from receptor phosphorylation by G protein-coupled receptor kinase (GRK), followed by β-arrestin recruitment and subsequent endocytosis via clathrin-coated vesicles has been proposed to underlie desensitization. This model is based on studies in reconstituted systems and correlation between desensitization and internalization (for reviews, see refs. 9 and 10), but to date has not been scrutinized in neurons of acute brain slices.
Understanding the mechanisms leading to desensitization and internalization is of great importance because their absence has recently been suggested to explain the induction of tolerance and dependence by morphine, the prototypical opioid of abuse (11, 12). However, the reason why morphine in contrast to most endogenous opioids fails to induce both desensitization and internalization of wild-type MORs still remains unresolved.
In the present study, we focused on the acute desensitization of MOR-mediated response by monitoring membrane currents of locus coeruleus (LC) neurons in acute slices of rat brain and observed that pre- and postsynaptic inhibition show a remarkable dissociation in regard to desensitization. This casts doubt on the hypothesis that MOR desensitization in LC neurons is mediated by G protein uncoupling. Our data in turn suggest a G protein-dependent pathway, which leads to desensitization through competitive inhibition of GIRK channel activation. This pathway, which is differentially activated by various agonists, may indeed explain why some induce desensitization (and eventually internalization) of MORs, while others do not.
Methods
Electrophysiology in Acute Slices.
Horizontal pontine slices (300 μm) were prepared from P10-P21 Sprague–Dawley rat brains in cooled external solution containing (in mM) NaCl 119, KCl 2.5, MgCl2 1.3, CaCl2 2.5, NaH2PO4 1.0, NaHCO3 26.2, and glucose 11, and bubbled with 95% O2 and 5% CO2. Whole-cell voltage-clamp recording techniques were used (32–34°C, 2 ml/min, submerged slices) to measure holding currents and synaptic responses of LC adrenergic neurons. Synaptic currents were evoked by stimuli (0.1 ms) at 0.05 Hz through bipolar stainless steel electrodes positioned caudal to the LC. The internal solution contained (in mM): K-Gluconate 140, NaCl 4, MgCl2 2, EGTA 1.1, Hepes 5, Na2ATP 2, Na2-Creatine-phosphate 5, and Na3GTP 0.6, supplemented in some experiments with caged-GTPγS (0.5 mM, Molecular Probes). Currents were amplified (Visual patch 500, Biologic, Grenoble, France), filtered at 1 kHz, and digitized at 5 kHz (National Instruments Board PCI-MIO-16E4, Igor, WaveMetrics, Lake Oswego, OR). A liquid junction potential of −13 mV was corrected.
Caged-GTPγS was photolyzed by a brief UV flash (330–385 nm), using the epifluorescence attachment of the Olympus BX50WI microscope controlled by a Uniblitz shutter (Vincent Associates, Rochester, NY). Typically, a 100-ms flash (attenuated by a ND25 filter) elicited maximal responses. In cells not loaded with GTPγS, a UV flash of similar intensity and duration did not induce any detectable current.
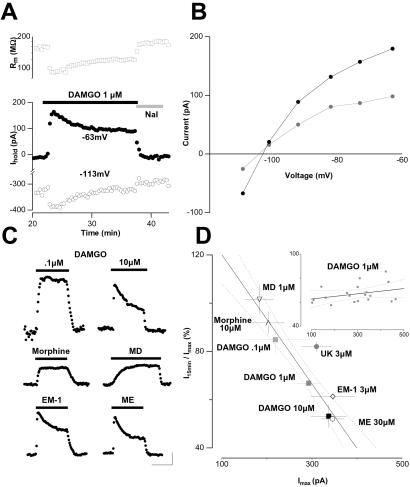
In many LC neurons voltage stepped to −113 mV (20 of 30), [D-Ala-2, NMe-Phe-4, Gly-5-ol]-enkephalin (DAMGO)-elicited currents did not reverse polarity. This is indicative of a limited space-clamp, which has been explained by electrotonic coupling of LC neurons with other cells of the LC via gap junctions (13, 14). Other cells however were found to have nice space-clamp as indicated by a reversal potential (Erev) of the DAMGO-elicited response close to the calculated potential equilibrium for potassium ions (EK = −106 mV; see Fig. 1). The degree of desensitization in response to DAMGO in coupled and uncoupled cells was similar (<5% difference, no significance) and data therefore compiled.
Figure 1.
Desensitization of the MOR-induced GIRK currents is associated with decreased GIRK conductance, and depends on the intensity of receptor stimulation. (A) Example of a MOR-induced response in an electrotonically uncoupled LC neuron (see Methods). Application of the selective MOR-agonist DAMGO (1 μM) for 15 min evoked an outward current at −63 mV and an inward current at −113 mV (Lower). This current desensitizes substantially within 15 min at both holding potentials. Activation and desensitization are associated with a decrease and subsequent partial recovery of the membrane resistance (Rm; Upper) (B) I–V relationships of maximal (Imax) and desensitized (I15min) DAMGO-elicited currents of the LC neuron shown in A. Both I–V curves reverse at the same potential, which is close to the calculated EK (−106 mV) and show inward rectification. (C) Examples of responses elicited by the application of different MOR-agonists at various concentrations for 15 min [25 min for methadone (MD)]. (Scale bars, 100 pA and 10 min; Vh = −73 mV.) (D) Mean (±SE) normalized residual response (I15min/Imax) after application for 15 min of various MOR agonists and UK14,304 as a function of respective mean (±SE) maximal responses (Imax, n = 5–19). Inset shows I15min/Imax as a function of respective Imax for each cell stimulated with 1 μM DAMGO. Regression line (r2 = 0.95 for D, UK values excluded, r2 = 0.1 for Inset) and 95% confidence intervals (dotted lines) were calculated.
Compiled data are expressed as means ± SE. For statistical comparisons the nonparametric Mann–Whitney or Wilcoxon matched tests were used and the level of significance was taken at P = 0.05.
Drugs.
Baclofen was bought from Tocris Neuroamin (Bristol, U.K.), somatostatin (SST) from Bachem (Bubendorf, Switzerland), DAMGO, [Met-5]-enkephalin (ME), endomorphin1 (EM-1), naloxone (Nal), ICI174,864, UK14,304 (UK), Yohimbine (Yoh), kynurenic acid, U73,122, bestatin, and thiorphan from Sigma, and morphine and MD from Amino AG (Neuenhof, Switzerland). Because desensitization of 30-μM ME-elicited responses were unaffected by enkephalinase inhibitors (20 μM bestatin plus 2 μM thiorphan), alone or in combination with the δ-opioid receptor (DOR) antagonist ICI174,864 (1 μM), data were compiled.
Results
Desensitization of MOR-Elicited GIRK Currents Is due to Channel Closure.
In line with previous reports (6, 15–17), application of the selective MOR-agonist DAMGO at 1 μM evoked an outward current that showed substantial desensitization associated with an increase of the membrane resistance within 15 min in the continuous presence of the agonist (Fig. 1A). The current–voltage (I–V) relationships of the response at the peak and after desensitization showed both inward rectification and a reversal potential (Erev) close to the calculated equilibrium potential for potassium ions (EK = −106 mV; Fig. 1B). In addition, the response was completely abolished by 1 mM extracellular barium (Fig. 5B), a GIRK channel blocker. These observations indicate that the MOR-elicited response and its desensitization are mediated by the opening and subsequent closure of GIRK channels.
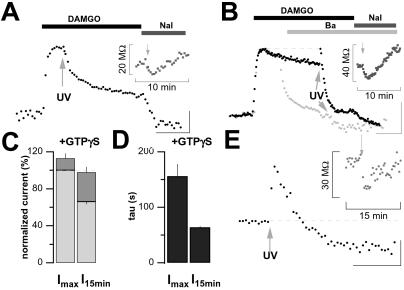
Figure 5.
Photo release of GTPγS completely reactivates desensitized GIRK currents, and subsequently enhances desensitization. (A) Flash photolysis of caged-GTPγS (100 ms, arrow) at the peak amplitude of the DAMGO-mediated response did not activate any additional GIRK current, indicating that 1 μM DAMGO was sufficient to trigger the activation of all cellular GIRK channels. Note that the desensitization is apparently enhanced in the presence of GTPγS. (Inset) Decrease and subsequent slowly increase of membrane resistance (Rm) after GTPγS photolysis (arrow), indicating channel opening and subsequent closure. (B) Uncaging GTPγS (arrow, UV: 100 ms) after desensitization of the DAMGO-mediated response transiently restored the initial maximal GIRK current amplitude, indicating that GIRK channels can be fully reactivated from the desensitized state by a strong G protein-mediated signal. Again, the subsequent desensitization was increased. (Inset) Rm as in A. Superimposed is the scaled response of another cell (light gray) to the same protocol, except that 1 mM Barium (Ba) was added 5 min after the DAMGO application. Note that uncaged GTPγS triggered a small inward current in these conditions, equal in size to the small shift in baseline observed in A and B. (Scale bars, 100 pA and 5 min; Vh = −73 mV.) (C) Bar graph representation of mean (±SE) normalized outward current amplitudes evoked GTPγS photo release (dark gray bars) stacked on bars representing MOR-elicited responses (light gray bars) at the peak (Imax) or in the desensitized state (I15min). Responses are expressed as percent of the peak DAMGO-elicited response in the same cell. (D) Bar graph representation of mean (±SE) time constant of the exponential desensitization induced by GTPγS photo release at the peak (Imax) or in the desensitized state (I15min). (C and D) n = 4 for each condition. (E) Flash photolysis of caged-GTPγS (100 ms, arrow) in the absence of any agonist application triggered an outward current that desensitized within minutes. It also triggered a small inward current as observed above. (Scale bars, 50 pA and 5 min; Vh = −73 mV.) (Inset) Concomitant decrease and subsequent recovery of the cell membrane resistance.
Desensitization Depends on the Intensity of MOR Activation.
To investigate the relationship between receptor activation and response desensitization, we first examined the effect of various DAMGO concentrations (Fig. 1 A and C). After 15 min of 0.1 μM DAMGO application, the residual outward current was 85 ± 6% (n = 4) of Imax, while it dropped to 53 ± 5% (n = 5) with 10 μM. Both values were significantly different from the desensitization observed with an intermediate concentration of DAMGO (1 μM, 67 ± 2%, n = 19, P < 0.05).
We next tested MOR-agonists of various efficacies at saturating concentration (Fig. 1C). The partial agonists morphine (10 μM) and MD (1 μM) elicited submaximal GIRK currents, which showed almost no desensitization (92 ± 5%, n = 5 and 102 ± 6%, n = 4, respectively; P > 0.05 for a hypothesized mean of 100%). At the other end of the spectrum, the full agonists met-enkephalin (ME, 30 μM) and endomorphin-1 (EM-1, 3 μM) triggered responses that strongly desensitized (52 ± 2%, n = 15 and 61 ± 2%, n = 5, respectively).
Plotting mean normalized residual MOR-elicited currents at the end of agonist application (I15min/Imax) as a function of the corresponding averaged maximal current (Imax) reveals an inverse correlation (r2 = 0.95; Fig. 1D). Maximal activation of another Gi/o-coupled receptor expressed on LC neurons, the α2 adrenergic receptor (α2AR) elicited hardly desensitizing GIRK currents (Fig. 1D), arguing against induction at the level of the GIRK channels. Furthermore, plotting normalized residual MOR-elicited currents after 15 min of 1 μM DAMGO as a function of Imax for each cell does not reveal any correlation (r2 = 0.1; Fig. 1D Inset). This indicates that the degree of desensitization depends neither on the number of MORs and GIRK channels activated nor the identity of the agonist, but mainly on the intensity of receptor stimulation (6).
MOR-Mediated Presynaptic Inhibition Does Not Desensitize.
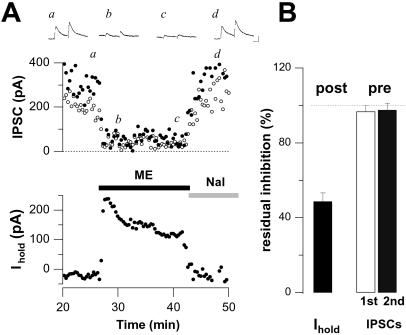
It is well known that DAMGO causes a depression of the fast GABAA-mediated receptor inhibitory postsynaptic currents (IPSCs) due to an inhibition of transmitter release by presynaptic MORs (18). We examined the time course of this presynaptic inhibition with ME, the MOR-agonist found to induce the highest degree of GIRK current desensitization. In addition, these experiments were carried out in the presence of the δ-opioid receptor (DOR) antagonist ICI174,864 (1 μM) to discard possible interference from DOR-mediated effects, and transmitter release was boosted (extracellular calcium 5 mM and paired-pulse stimulation) to ensure that IPSCs were not completely abolished. As shown in Fig. 2, monitoring presynaptic inhibition in such a dynamic range revealed a uniform level of depression throughout the agonist application (97 ± 3% and 98 ± 4% of initial inhibition after 15 min for the first and second IPSCs, respectively), despite a substantial concomitant desensitization of the GIRK currents (Imax/I15min = 49 ± 5%, n = 6).
Figure 2.
MOR-mediated presynaptic inhibition does not desensitize. (A) ME-induced presynaptic inhibition of GABAA-mediated IPSCs remained constant while the concomitant postsynaptic GIRK response substantially desensitized. GABAA-mediated IPSCs were boosted with 5 mM extracellular calcium and paired pulse facilitation to avoid complete inhibition. AMPA receptor, NMDA receptor, and DORs were blocked by 2 mM kynurenic acid and 1 μM ICI174,864 throughout the experiment (Vh = −53 mV). (Insets) Averaged traces of paired evoked IPSCs at referred time (a, b, c, and d). (Scale bars, 100 pA and 20 ms.) (B) Bar graph representation of mean (±SE) normalized residual post- (Left) and presynaptic (Right) effects of 30 μM ME after 15 min application (n = 6). Residual GIRK response was normalized as I15min/Imax, whereas presynaptic inhibition was normalized as inhibition after 15 min per initial inhibition. Responses from three consecutive measures were binned.
It has previously been shown that postsynaptic inhibition is mediated by the activation of GIRK channels (19), whereas presynaptic inhibition is caused by an inhibition of Ca channels, direct effects on the release machinery downstream of Ca entry (20), or activation of voltage-gated potassium channels (21). Given these different effector systems, our observation that presynaptic inhibition did not change while postsynaptic inhibition strongly desensitized argues against uncoupling of G proteins from MORs as the main underlying mechanism. Moreover, it suggests a locus of desensitization downstream of the MORs, possibly at the level of the GIRK channels themselves.
Reactivation of Desensitized GIRK Currents.
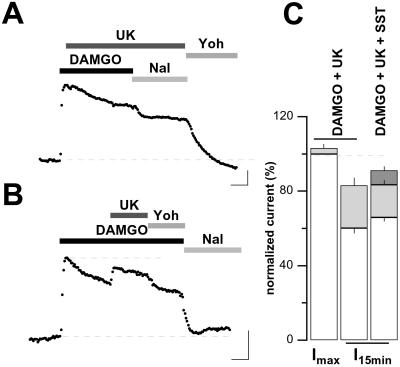
To test whether desensitization might be due to GIRK channel inactivation, we first tested whether phosphatidylinositol 4,5-bisphosphate (PIP2), essential for GIRK channel gating (22), may deplete during sustained MOR activation and could account for desensitization (23). However, the application of the selective phospholipase C (PLC) inhibitor U73,122 (30 μM, preapplied for 10 min) did not affect desensitization (70 ± 4%, n = 3, vs. 67 ± 2%; data not shown). We then attempted to reactivate desensitized GIRK currents by providing an additional G protein signal. Neurons of the LC are known to express α2 adrenergic, GABAB, and SST2 receptors [α2ARs, GABABRs, and SST2Rs (15)], which also signal through G proteins of the Gi/o family. In accordance with the literature (24), responses mediated by these three receptors and MORs proved to be mutually occlusive. For example, the α2AR-agonist UK14,304 (UK) at a saturating concentration of 3 μM, when applied at the peak of a maximal DAMGO-elicited current, did not elicit any substantial additional current (3 ± 2%, n = 4; Fig. 3 A and C), confirming that α2ARs and MORs signals converge onto the same GIRK channel population. We next applied UK (in addition to DAMGO) in the desensitized state of the MOR-elicited response and were able to partially restore the initial DAMGO-elicited GIRK currents (from 60 ± 3% to 83 ± 4% of ImaxDAMGO; Fig. 3 B and C). Further reactivation could be obtained when SST (3 μM) was added onto DAMGO plus UK (91 ± 2% of ImaxDAMGO, n = 4; Fig. 3C).
Figure 3.
Desensitized DAMGO-elicited GIRK currents may be reactivated. (A) Stimulating α2ARs at the peak amplitude of the DAMGO-mediated response did not activate any additional GIRK current, indicating that both receptor systems share the same population of GIRK channels. (B) Stimulating α2ARs after desensitization of the DAMGO-mediated response partially restored the initial GIRK current amplitude. (Scale bars in A and B, 100 pA and 5 min; Vh = −73 mV.) (C) Bar graph representation of mean (±SE) normalized current amplitudes evoked by α2ARs stimulation (light gray bars) or SST (dark gray bar) stacked on bars representing MOR-elicited responses (empty bars) at the peak or in the desensitized state. Note that addition of SST onto DAMGO plus UK further increases the reactivation of the desensitized GIRK currents. Responses are expressed as percent of the peak DAMGO-elicited response in the same cell (n = 4 for each condition). UK, 3 μM; Yoh, 10 μM; DAMGO, 1 μM; Nal, 1 μM.
These results indicate that desensitization is not due to GIRK channel inactivation, but suggest competitive inhibition of GIRK channel activation because it can be overcome by additional G protein signals.
Heterologous Desensitization.
The partial reactivation of desensitized GIRK response by addition of UK onto DAMGO suggested heterologous effects because in naive cells, full stimulation of α2ARs with 3 μM UK elicited currents of similar amplitude as 1 μM DAMGO (94 ± 2%, n = 13; Fig. 4 A and E). Heterologous desensitization was confirmed by the observation that the maximal current elicited by a UK application was of similar amplitude as the preceding desensitized DAMGO response (ImaxUK/I15minDAMGO = 97 ± 7%, n = 13; Fig. 4 C and E). In other words, on naive slices α2AR-elicited responses were significantly larger than in slices exposed to 15 min of DAMGO (P < 0.01). This heterologous desensitization was not induced by Nal because if the MOR-induced response was reversed by Nal before substantial desensitization occurred, subsequent α2AR-elicited response was of similar amplitude as the MOR response (Fig. 4B, n = 3). As for homologous desensitization, heterologous desensitization could be overcome by an additional G protein signal through SST2Rs stimulation (Fig. 4 D and E).
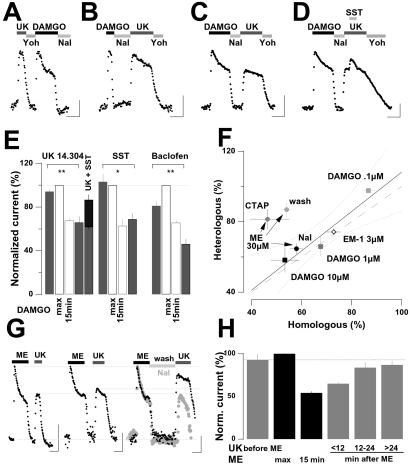
Figure 4.
Heterologous effects of MOR-induced desensitization. (A) Example of responses elicited successively by UK and DAMGO. Stimulation of α2ARs triggers a large outward current reversed by Yoh. Subsequent stimulation of MORs triggers an outward current of similar amplitude that desensitizes substantially within 15 min. (B) The application of the above agonists in the reversed order also elicited outward currents of similar amplitude, provided that DAMGO was applied only briefly. (C) However, if DAMGO is applied for 15 min and Nal used to reverse the response after desensitization occurred, subsequent application of UK elicited an outward current with a maximal amplitude similar to the preceding MOR-induced desensitized response. (D) Same as in C, except that addition of 3 μM SST onto the UK-induced response partially reactivated this heterologously desensitized response. (A–D) (Concentrations: DAMGO 1 μM, UK 3 μM, Nal 1 μM, Yoh 10 μM; scale bars, 100 pA and 10 min; Vh = −73 mV.) (E) Bar graph representation of mean (±SE) normalized responses evoked by 3 μM UK (n = 13), 3 μM somatostatin (SST; n = 3), or 100 μM Baclofen (GABAB agonist; n = 5) before 1 μM DAMGO for 15 min or conversely. The fifth bar from the left represents the responses to UK plus SST (n = 4), reflecting partial reactivation of heterologous desensitization. Responses are expressed as percent of the peak DAMGO-elicited response in the same cell. *, P < 0.05; **, P < 0.01. (F) Mean (±SE) normalized heterologously desensitized 3 μM UK-elicited response (ImaxUK/ImaxMORagonist) as a function of respective mean (±SE) normalized homologously desensitized responses (I15min/Imax) after 15 min applications of various MOR agonists followed by Nal (n = 3–13). Regression line (r2 = 0.91), 95% confidence intervals (dotted lines), and line of identity are superimposed (dashed line). The ME data, where the agonist was washed from the slice (ME 30 μM wash, also see below) or antagonized with CTAP, were not included for the calculation of the regression. (G) Representative records of currents evoked by ME (30 μM, 15 min) and subsequent UK (3 μM) applications separated by increasing duration of ME washout (from left to right: 5, 12, and 30 min). The right trace is scaled and superimposed onto a recording (light gray) where the MOR-induced currents were reversed by 30 min of Nal (1 μM) application. (Scale bars, 100 pA and 10 min; Vh = −73 mV.) (H) Bar graph representation of mean (±SE) normalized responses evoked by 3 μM UK (n = 3) before 30 μM ME and after increasing ME washout duration (n = 3–4). Responses are expressed as percent of the peak ME-elicited response in the same cell.
Moreover, heterologous desensitization is not specific for α2ARs because it was also observed with other receptors coupled to Gi/o proteins, such as SST2Rs and GABABRs (Fig. 4E). Finally, examining the degree of heterologous desensitization of α2AR-mediated response as a function of the degree of homologous desensitization induced by various MOR agonists (see Fig. 1) revealed a close correlation (r2 = 0.91; Fig. 4F). These observations suggest that homologous and heterologous desensitization are mediated mainly, if not exclusively, by the same molecular mechanisms.
These results differ quantitatively from previous reports, which found homologous to exceed heterologous desensitization (6, 15, 17). The main methodological difference is that in the present study Nal was used to terminate MOR-induced responses. This seemed to delay recovery from heterologous desensitization. Indeed, in experiments with ME, which is easily washed from the slices, subsequent UK-mediated responses elicited at increasing time intervals showed that heterologous desensitization almost completely recovered within 30 min (Fig. 4 G and H), while it persisted for at least for 30 min if ME was reversed with Nal (Fig. 4 F and G). This effect may be linked to the role of Nal as an inverse agonist. Indeed, reversal of desensitized GIRK currents with the selective antagonist CTAP did not prevent resensitization over the same time period (Fig. 4F).
GTPγS Enhanced Desensitization.
To test whether desensitized GIRK currents could be reactivated by amplifying the G protein signal initiated by MORs, instead of other GPCRs as in Fig. 3, we loaded the cells with caged-GTPγS, a photo-releasable nonhydrolyzable GTP analogue. This compound becomes biologically active on brief exposure to UV light and strongly and irreversibly amplifies G protein signals. When photo-released at the peak of the DAMGO-elicited current, only a small additional current could be recruited (13 ± 5%, n = 5; Fig. 5 A and C), indicating that 1 μM DAMGO activates virtually all GIRK channels in LC neurons and that GTPγS does not activate any other outward current. However, when performed after desensitization, a UV flash was sufficient to fully restore maximal DAMGO-elicited GIRK current amplitude (from 65 ± 2% to 99 ± 5% of ImaxDAMGO, n = 5; Fig. 5 B and C). This GTPγS-reactivated current is mediated almost exclusively by GIRK channels, because in the presence of 1 mM barium, photolysis of caged GTPγS did not evoke any outward current, but a small inward current (20–80 pA, n = 3; Fig. 5B).
Surprisingly, the reactivation induced by GTPγS (Fig. 5B) was only transient and, over 10 min, the current “re-desensitized,” as reflected by an increase of the membrane resistance (Fig. 5B Inset). Analysis of the experiments, where GTPγS was photo-released at the peak, also showed an apparent increased desensitization and concomitant increase of membrane resistance (Fig. 5A Inset). Interestingly, the desensitization that followed photolysis of caged GTPγS was developing more quickly when the UV flash was performed in the desensitized state (Fig. 5B) rather than at the peak (Fig. 5A) of the DAMGO-elicited response (τ = 64 ± 2 s vs. 156 ± 22 s, n = 4, P < 0.01; Fig. 5D). This suggests that the desensitization is itself a G protein dependent process because photolysis of GTPγS led to stronger amplification when the process was fully activated after 15 min. Finally, examination of the effect of GTPγS photo-release in absence of any agonist application revealed that it activated both an outward current and its desensitization, as well as a small inward current (Fig. 5E), indicating that in LC neurons, G proteins involved in the desensitization of GIRK currents do show a basal activity independent of receptor stimulation.
Discussion
Desensitization Is Initiated at the MOR.
In LC neurons, partial agonists like morphine or MD hardly induced desensitization, while full agonists such as DAMGO, ME, and endomorphin-1 (EM-1) yielded strong desensitization when applied at saturating concentrations and weaker desensitization when applied at lower concentrations (Fig. 1; ref. 6). Desensitization therefore appears to be a function of the intensity of receptor stimulation, which implies that desensitization is actually initiated by the receptor.
Desensitization Is Expressed at the Effector.
In addition to activating GIRK channels, MORs also inhibit presynaptic release of GABA through other effectors (25, 26). Indeed, in the hippocampal slice, it has been shown that GPCR-mediated postsynaptic hyperpolarization is mediated by GIRK channel activation, whereas presynaptic inhibition of transmitter release works via other effectors (27). Our observation that, over 15 min, MOR-elicited presynaptic inhibition remained unchanged while postsynaptic GIRK currents strongly desensitized, suggests that desensitization is expressed downstream of the MORs, possibly at the level of the GIRK channels.
The observation that GIRK currents elicited by α2ARs, GABABRs, and SST2Rs are decreased after MOR-induced desensitization supports this hypothesis.
We also showed that the heterologous desensitization of α2AR-mediated GIRK currents correlates very closely with the degree of desensitization of the MOR-induced response (Fig. 4F). Taken together, these data argue for a common mechanism of both homologous and heterologous desensitization leading to inhibition of GIRK channel activation. This interpretation differs from the previous one that the two forms of inhibition were independent (6, 15). This conclusion was based on the observation that homologous desensitization exceeded heterologous desensitization. However, as shown here, direct comparison within the same cell under conditions that induced full desensitization (longer applications of saturating agonist concentrations) and rapid reversal with Nal reveal that there is a close correlation between the two forms of desensitization (Fig. 4F). It seems that under these conditions very closely overlapping pools of GIRK channels were activated, which may not be the case at the lower concentrations (28).
Desensitization Occurs Through Competitive Inhibition of GIRK Channel Activation.
Providing an additional G protein signal by means of α2AR stimulation onto the desensitized MOR-mediated signal led to partial reactivation of desensitized GIRK currents (Fig. 3). Furthermore, providing a further additional G protein signal through stimulation of both α2ARs and SST2Rs or a much stronger one through photo-release of GTPγS led to increased or full reactivation of MOR-induced desensitized currents, respectively (Figs. 3 and 5). A first sight, these observations seem to contradict the heterologous desensitization data (Fig. 4). In the reactivation experiments, however, the G protein signals generated by MORs and α2ARs add to activate GIRK channels, whereas in the heterologous desensitization experiments the α2AR signal followed inhibition of the MOR. Reactivation therefore depended on the intensity of the additional G protein signal, suggesting that desensitization is due to competitive inhibition of GIRK channel activation.
Desensitization May Be a G Protein-Dependent Process.
Interestingly, MOR-induced desensitization of GIRK currents was enhanced after photo release of GTPγS. This is in contrast to results obtained in oocytes coexpressing MOR with GIRK1/2 where nonhydrolyzable analogues of GTP eliminated a much faster form of desensitization occurring within 30 s (29), suggesting that desensitization in our system is determined by additional mechanisms that may not be present in oocytes. Furthermore, given the bidirectional effect of GTPγS on GIRK currents when photo released, it seems possible that both effects were active simultaneously when GTPγS is slowly applied. Moreover, we find that in slices where some GIRK channels are activated under basal conditions (19), photo release of GTPγS, in the absence of any agonist, rapidly activates GIRK currents but also triggered their desensitization (Fig. 5E). In contrast, in cultured cells of the LC, dialysis with GTPγS activated GIRK currents only in combination with agonist applications, and had only very little effect on basal currents (30).
We show that flash photolysis of caged GTPγS also triggered a small inward current, which is not related to MOR activation. On its own, the inward current is insufficient to explain the increased desensitization observed when GTPγS was uncaged during MOR stimulation. Moreover, desensitization was associated with an increase of membrane resistance.
We cannot exclude the possibility that photo-released GTPγS activated G proteins other than Gi/o, such as Gq proteins, which have been suggested to inhibit GIRK channels (31). This may indeed explain the intriguing observation that desensitization of MOR-induced GIRK currents is actually enhanced in the presence of type 2 muscarinic receptor activation (ref. 15 and data not shown). Such a scenario would however not explain the observation that uncaging GTPγS in the desensitized state led to faster and more intense desensitization compared with photo release at the peak of the DAMGO-elicited response, unless these inhibitory G proteins participate in the desensitization process. Indeed, it is important to recall that GTPγS does not activate G protein per se but only amplifies underlying G protein-dependent signals. The increased rate of desensitization induced by GTPγS photo released after desensitization of the MOR-elicited response implies that an underlying G protein signal at this time is stronger than at the peak of the response. Our results therefore suggest that desensitization of the MOR-elicited GIRK currents may be a G protein-dependent process.
A Model for Desensitization of MOR Signaling.
Based on our results, we propose a model in which sustained intense activation of MORs would activate a G protein-dependent pathway leading to competitive inhibition of GIRK channel activation. The nature of the inhibitory factor is presently unknown, but there are a few candidate mechanisms known to inhibit GIRK channel activation that could potentially be involved in MOR-induced desensitization.
First, phosphatidylinositol 4,5-bisphosphate (PIP2) has been shown to be essential for gating of GIRK channels (22) and MOR activation may stimulate phospholipase C (PLC) (32). However, inhibiting PLC did not affect desensitization. Moreover, PIP2 depletion would be expected to induce noncompetitive inhibition and would therefore be difficult to reconcile with our observation that desensitized GIRK currents can be gradually reactivated by additional G protein signal of increasing intensities. For these reasons, we do not think that PIP2 depletion would explain desensitization.
Competitive inhibition of GIRK channel activation can be achieved either by decreasing free βγ or by displacing βγ from its binding site at the GIRK channels. The former possibility may result from the activation of a βγ scavenger, such as GRK2 or GRK3, which through their carboxyl terminus, in a phosphorylation-independent process, act as βγ sinks (33).
Alternatively, it is possible that sustained receptor activation would induce a switch in the type of G proteins recruited, a mechanism that has been reported for β2ARs (34). This may lead to the activation of βγ dimers composed of different subunits that may have different functional consequences on GIRK channels. For instance, it has been reported that, in heterologous expression systems, β5γ2 dimers inhibit GIRK currents (35).
Functional Implications and Conclusions.
It has recently been suggested that the induction of tolerance and dependence may result from the absence of desensitization and internalization of MORs (11, 12). The MOR-signaling pathway we describe may explain why some agonists (e.g., DAMGO) induce both desensitization and internalization of wild-type MORs, while others (e.g., morphine) do not. Indeed, only the formers would activate this pathway that induce desensitization and may trigger MOR phosphorylation and internalization. Moreover, heterologous desensitization and GIRK current reactivation from desensitization indicates that opioid and other GPCRs systems can undergo cross-talk in a complex fashion that may participate in the induction of dependence. Dissociation of the desensitization of MOR-mediated presynaptic and postsynaptic inhibitions may also have important consequences in the induction or the maintenance of dependence. Finally, the observation that Nal delays resensitization suggests that it has effects beyond the termination of the MOR signal, possibly through affinity to a particular state of the receptor, and hence raises the possibility that the withdrawal syndrome precipitated by Nal may have specific characteristics.
Taken together, our data suggest that desensitization of MOR-induced GIRK currents is mediated by a G protein dependent pathway, which inhibits GIRK channel activation. This implies that MOR is capable of bi-directional signaling. This pathway may reveal targets for drugs that induce analgesia without dependence.
Acknowledgments
We thank the members of the Muller and Lüscher labs for helpful discussions, M. Frerking, M. Scanziani, P. Slesinger, and R. Nicoll for comments on an earlier version of the manuscript, and G. Gilliéron for technical support. C.L. is supported by a career development award from the Swiss National Science Foundation and a Young Investigator's Grant from the Human Frontier Science Program Organization.
Abbreviation
- GPCR
G protein-coupled receptor
- MOR
μ-opioid receptor
- DAMGO
[D-Ala-2, NMe-Phe-4, Gly-5-ol]-enkephalin
- GIRK
G protein-coupled inwardly rectifying potassium
- SST
somatostatin
- α2AR
α2 adrenergic receptor
- MD
methadone
- ME
[Met-5]-enkephalin
- UK
UK14,304
- Nal
naloxone
- IPSC
inhibitory postsynaptic currents
- LC
locus coeruleus
References
- 1.Matthes H W, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, et al. Nature (London) 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 2.Reuveny E, Slesinger P A, Inglese J, Morales J M, Iniguez-Lluhi J A, Lefkowitz R J, Bourne H R, Jan Y N, Jan L Y. Nature (London) 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- 3.Huang C L, Slesinger P A, Casey P J, Jan Y N, Jan L Y. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 4.Williams J T, North R A, Tokimasa T. J Neurosci. 1988;8:4299–4306. doi: 10.1523/JNEUROSCI.08-11-04299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyake M, Christie M J, North R A. Proc Natl Acad Sci USA. 1989;86:3419–3422. doi: 10.1073/pnas.86.9.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris G C, Williams J T. J Neurosci. 1991;11:2574–2581. doi: 10.1523/JNEUROSCI.11-08-02574.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson S S. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 8.Pitcher J A, Freedman N J, Lefkowitz R J. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 9.Law P Y, Wong Y H, Loh H H. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 10.Tsao P, Zastrow M. Curr Opin Neurobiol. 2000;10:365–369. doi: 10.1016/s0959-4388(00)00096-9. [DOI] [PubMed] [Google Scholar]
- 11.Whistler J L, Chuang H H, Chu P, Jan L Y, von Zastrow M. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- 12.Finn A K, Whistler J L. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Maubecin V, Garcia-Hernandez F, Williams J T, Van Bockstaele E J. J Neurosci. 2000;20:4091–4098. doi: 10.1523/JNEUROSCI.20-11-04091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie M J, Williams J T, North R A. J Neurosci. 1989;9:3584–3589. doi: 10.1523/JNEUROSCI.09-10-03584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorillo C D, Williams J T. J Neurosci. 1996;16:1479–1485. doi: 10.1523/JNEUROSCI.16-04-01479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie M J, Williams J T, North R A. Mol Pharmacol. 1987;32:633–638. [PubMed] [Google Scholar]
- 17.Osborne P B, Williams J T. Br J Pharmacol. 1995;115:925–932. doi: 10.1111/j.1476-5381.1995.tb15899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen G A, Doze V A, Madison D V. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- 19.Lüscher C, Jan L Y, Stoffel M, Malenka R C, Nicoll R A. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 20.Scanziani M, Capogna M, Gähwiler B H, Thompson S M. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- 21.Vaughan C W, Christie M J. J Physiol (London) 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C L, Feng S, Hilgemann D W. Nature (London) 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 23.Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis D E. Nat Cell Biol. 2000;2:507–514. doi: 10.1038/35019544. [DOI] [PubMed] [Google Scholar]
- 24.Aghajanian G K, Wang Y Y. Neuropharmacology. 1987;26:793–799. doi: 10.1016/0028-3908(87)90054-2. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan C W, Ingram S L, Connor M A, Christie M J. Nature (London) 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- 26.Capogna M, Gähwiler B H, Thompson S M. J Physiol. 1993;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller R J. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- 28.Sodickson D L, Bean B P. J Neurosci. 1998;18:8153–8162. doi: 10.1523/JNEUROSCI.18-20-08153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuang H H, Yu M, Jan Y N, Jan L Y. Proc Natl Acad Sci USA. 1998;95:11727–11732. doi: 10.1073/pnas.95.20.11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velimirovic B M, Koyano K, Nakajima S, Nakajima Y. Proc Natl Acad Sci USA. 1995;92:1590–1594. doi: 10.1073/pnas.92.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill J J, Peralta E G. J Biol Chem. 2001;276:5505–5510. doi: 10.1074/jbc.M008213200. [DOI] [PubMed] [Google Scholar]
- 32.Jin W, Lee N M, Loh H H, Thayer S A. J Neurosci. 1994;14:1920–1929. doi: 10.1523/JNEUROSCI.14-04-01920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch W J, Hawes B E, Inglese J, Luttrell L M, Lefkowitz R J. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 34.Daaka Y, Luttrell L M, Lefkowitz R J. Nature (London) 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 35.Lei Q, Jones M B, Talley E M, Schrier A D, McIntire W E, Garrison J C, Bayliss D A. Proc Natl Acad Sci USA. 2000;97:9771–9776. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]