ABSTRACT
The human microbiome is a unique organ and maintains host immunomodulation and nutrient metabolism. Structural and functional microbiome alterations are commonly known as dysbiosis, which is strongly associated with disease progression. Ferroptosis is a novel iron‐dependent cell death mode characterized by intracellular iron accumulation, increased reactive oxygen species (ROS), and lipid peroxidation (LPO). Importantly, the complex crosstalk between the microbiome and ferroptosis in disease has attracted considerable research attention. The microbiome influences ferroptosis by regulating host iron homeostasis, mitochondrial metabolism, and LPO, among many other pathways. Thus, the in‐depth analysis of microbiome–ferroptosis crosstalk and associated mechanisms could provide new strategies to treat human diseases. Therefore, understanding this crosstalk is critical. Here, we systematically explore the associations between gut microbiome and ferroptosis across multiple diseases. We show that the oral microbiome also influences disease progression by regulating ferroptosis. Furthermore, we provide a potential for certain disease therapies by targeting the crosstalk between the microbiome and ferroptosis.
Keywords: fecal microbiota transplantation, ferroptosis, gut‐organ‐axis, microbiome, probiotics
The human microbiome, a key homeostatic hub, interacts with ferroptosis in diseases. This review dissects the crosstalk between the microbiome and ferroptosis, providing strategies for disease therapy. FMT, fecal microbiota transplantation; ROS, reactive oxygen species; TCM, traditional Chinese medicine.

Abbreviations
- AA‐PE
arachidonic acid‐phosphatidylethanolamine
- ACSL4
acyl‐CoA synthetase long‐chain family member 4
- AD
Alzheimer's disease
- AHR
aryl hydrocarbon receptor
- AIEC
adherent‐invasive E. coli
- ALD
alcoholic liver disease
- ALDH1A3
aldehyde dehydrogenase 1 family member A3
- ALI
acute liver injury
- APAP
acetaminophen
- ATF3
transcription factor 3
- Aβ
β‐amyloid
- BAs
bile acids
- CagA
cytotoxin‐associated gene A
- CAT
capsiate
- CD
Crohn's disease
- CDCA
chenodeoxycholic acid
- CNS
central nervous system
- CoQ
ubiquinone
- CoQH2
ubiquinol
- CRC
colorectal cancer
- DA
dopaminergic
- DAMPs
danger‐associated molecular patterns
- DCA
deoxycholic acid
- DMT1
divalent metal transporter 1
- DSS
dextran sodium sulfate
- Ferritinophagy
ferritin autophagy
- FMT
fecal microbiota transplant
- FPN
ferroportin
- FSP1
ferroptosis suppressor protein 1
- FXR
farnesoid X receptor
- Glu
glutamate
- GLUT1
glucose transporter‐1
- GPX4
glutathione peroxidase 4
- GSH
glutathione
- H2O2
hydrogen peroxide
- HCC
hepatocellular carcinoma
- HDAC
histone deacetylase
- HIF‐1α
hypoxia‐inducible factor‐1α
- HIF‐2α
hypoxia‐inducible factor‐2α
- IBD
inflammatory bowel disease
- ICC
intrahepatic cholangiocarcinoma
- IDA
trans‐3‐indoleacrylic acid
- IEC
intestinal epithelial cell
- IRI
ischemia reperfusion injury
- IS
ischemic stroke
- KC
Kupffer cells
- KEAP1
Kelch‐associated protein 1
- ALOX
lipoxygenase
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- LPO
lipid peroxidation
- LPS
lipopolysaccharide
- MAFLD
metabolic dysfunction‐associated fatty liver disease
- NAFLD
nonalcoholic fatty liver disease
- NCOA4
nuclear receptor co‐activator 4
- NOX2
NADPH oxidases 2
- NOXs
NADPH oxidases
- NRF2
nuclear factor erythroid 2‐related factor 2
- OCA
obeticholic acid
- OFG
oral fecal gavage
- OPG
pasteurized fecal gavage
- PD
Parkinson's disease
- PDLSCs
periodontal ligament stem cells
- POR
oxidoreductase cytochrome P450 reductase
- PPAR
peroxisome proliferator‐activated receptor
- PUFA
polyunsaturated fatty acid
- PUFA‐ePLs
polyunsaturated ether phospholipids
- PUFA‐PL
polyunsaturated fatty acid‐phospholipid
- PUFA‐PL‐OOH
PUFA‐PL hydroperoxides
- RA
rheumatoid arthritis
- SCFAs
short‐chain fatty acids
- SFXN1
siderofexin
- SLC2A1
solute carrier family 2 member 1
- SLC3A2
solute carrier family 3 member 2
- SLC7A11
solute carrier family 7a member 11
- SN
substantia nigra
- SOD2
superoxide dismutase 2
- TBI
total body irradiation
- TCM
traditional Chinese medicine
- TFR1
transferrin receptor 1
- TLR4
toll‐like receptor‐4
- TNF‐α
tumor necrosis factor α
- UC
ulcerative colitis
- α‐syn
α‐synuclein
- ROS
reactive oxygen species
1. Introduction
Ferroptosis is a form of nonapoptotic cell death that depends on iron accumulation in cells (Dixon et al. 2012) and leads to increased toxic lipid peroxide reactive oxygen species (ROS) (Alves et al. 2025). The biochemical mechanisms underpinning ferroptosis involve lethal lipid peroxidation (LPO), ROS overload, altered cell metabolism, and imbalanced redox homeostasis (Dixon and Pratt 2023). Ferroptosis is induced by decreased glutathione peroxidase 4 (GPX4) activity, which leads to lipid peroxide accumulation. Ferroptosis sensitivity is closely associated with multiple biological processes, including iron (Anandhan et al. 2023), amino acid (Swanda et al. 2023), and polyunsaturated fatty acid (PUFA) metabolisms (Doll et al. 2017), mitochondrial function (Yamashita et al. 2024), glutathione (GSH) synthesis (Jiang et al. 2024), and phospholipid processing (Qiu, Zandkarimi, et al. 2024). Furthermore, ferroptosis is associated with multiple benign and malignant gut (Huang, Yang, et al. 2024), liver (Yu et al. 2020), brain (Wang, Liu, Zhang, et al. 2024), oral (Torres et al. 2024), and bone diseases (Feng et al. 2024).
As a unique organ, the human microbiome has been gradually revealed (Baquero and Nombela 2012). The ecological stability of a healthy human microbiome depends on many factors, such as the environment (Liu et al. 2025), the diet (Lin et al. 2025), medication (Daruka et al. 2025), genetics (Liu et al. 2025), gender (Dunham et al. 2024), age (Pasolli et al. 2019), and delivery mode (Zhou et al. 2023). Human microbiome composition and diversity may be altered by habitat environmental changes and may manifest as decreased beneficial symbionts and increased opportunistic pathogens, or dysbiosis (Qing et al. 2025). Microbiome dysbiosis also induces multiple human diseases (Ambat et al. 2024; Thibaut et al. 2025; Bosch et al. 2024; Kambara et al. 2024; Essex et al. 2024).
Iron is a key trace element that maintains normal human physiological activities and microbiome homeostasis (Pereira et al. 2024), whereas iron overload causes ferroptosis and dysbiosis (Gu et al. 2024). It was previously reported that dysbiosis induces ferroptosis in different diseases (He et al. 2023; Liu, Liu, et al. 2024; Fu et al. 2024; Zha et al. 2025; Wang, Wang, et al. 2023; Wang, Pan, et al. 2025). Furthermore, therapies targeting crosstalk between the microbiome and ferroptosis have been efficacious in several human diseases and attracted considerable research attention (Davenport et al. 2017; Sun et al. 2023). However, the specific interaction mechanisms between the microbiome and ferroptosis, as well as their impact on diseases, remain unclear. This review first systematically introduces the mechanisms of ferroptosis and the composition of the microbiome. Subsequently, we summarize the crosstalk between the microbiome and ferroptosis and its influence on diseases across various systems. Finally, we review the methods and advancements in targeting both the microbiome and ferroptosis for disease treatment.
2. Ferroptosis Mechanisms
2.1. Iron Metabolism Mechanisms
The balance of intracellular iron (Fe2+/Fe3+) concentration is a key link in maintaining cell function, involving the uptake, storage, utilization, and excretion of iron. Iron is involved in many biochemical processes, including oxygen transport, energy production, immune regulation, DNA synthesis, and antioxidant defense (Sun et al. 2023; Bogdan et al. 2016). Iron mainly comes from aging red blood cells, which are phagocytosed by macrophages, and also from dietary supplements absorbed by intestinal cells (Ahlqvist et al. 2015; von Siebenthal et al. 2023). Through the complex modulation of key molecules such as transferrin, divalent metal transporter 1 (DMT1), ferritin, and ferroportin (FPN), iron uptake, storage, and use are dynamically balanced (Galy et al. 2024). Dietary gut iron enters cells via DMT1 (Noordine et al. 2024), whereas absorbed iron is exported to the peripheral blood by FPN (Lim et al. 2018). In plasma, Fe3+ is tightly bound to transferrin for transport, which reduces free iron production and protects the iron from oxidation (Li, Li, et al. 2025). After reaching target cells, transferrin enters cells via the transferrin receptor 1 (TFR1) (Feng et al. 2020); after which, Fe3+ dissociates from transferrin and is converted to Fe2+ by the six‐transmembrane epithelial antigen of prostate 3 in DMT1 (Meng et al. 2022). Inside cells, iron is mainly stored by ferritin; however, when a cell is iron‐deficient, ferritin converts Fe3+ into Fe2+ via ferritin autophagy (ferritinophagy) (Zhou et al. 2022). When iron dissolves, FPN transports it outside the cell, where it is converted to Fe3+ by ceruloplasmin for further recycling (Fuqua et al. 2018). Critically, these iron metabolism and circulation processes are strictly modulated to ensure a balance exists between physiological requirements and toxicity.
Balanced iron metabolism is regulated by multiple factors, such as intracellular iron concentrations and partial oxygen pressure (Maniscalchi et al. 2024; Mastrogiannaki et al. 2009). When iron is deficient, anemia and other diseases may occur; however, iron overload aggravates oxidative stress in cells and eventually causes ferroptosis (Ru et al. 2024). In 2012, ferroptosis was first described as a form of cell death induced by the small molecule erastin, which was characterized by GSH depletion and phosphoperoxidase GPX4 inactivation (Dixon et al. 2012). Distinct from apoptosis, pyroptosis, and autophagy, ferroptosis is characterized by iron overload and unlimited LPO accumulation (Stockwell et al. 2017). Morphologically, ferroptotic cells exhibit necrosis‐like changes, including lost plasma membrane integrity, cytoplasm, and organelle swelling, but a normal nucleus (Dixon and Olzmann 2024). Structurally, ferroptosis alters mitochondrial structures, including mitochondrial condensation, reducing or missing cristae, increasing membrane density, and rupturing mitochondrial outer membranes (Zhang, Zhou, Gu, et al. 2024). Ferroptosis is highly complex and depends on an imbalance between oxidation and antioxidant systems, which involve several signal pathways and molecules (Tang et al. 2021; Conrad and Pratt 2019).
2.2. Ferroptosis Driver Pathways
2.2.1. Iron Overload
Imbalanced iron homeostasis causes intracellular iron overload and ferroptosis. Specifically, Fe3+ enters cells and is converted to Fe2+ via TFR1 and DMT1 actions, while ferritinophagy increases Fe2+ levels (Chen, Zhang, et al. 2024). These reactive Fe2+ enter the labile iron pool (LIP) to maintain intracellular iron homeostasis (Camarena et al. 2021). Excessive Fe2+ catalyzes hydroxyl radical and lipid peroxide formation in a non‐enzymatic manner via Fenton reactions or lipid autoxidation (Li, Han, et al. 2025). These molecules (hydroxyl radicals and lipid peroxides) further promote LPO, ultimately leading to ferroptosis (Jinson et al. 2024). Also, free radicals may damage intracellular proteins, nucleic acids, and lipids and further promote ferroptosis. Notably, the FerroOrange fluorescent probe and ferrozine (detect intracellular Fe2+ levels) or Prussian blue staining (verifies tissue iron overload) were used to monitor host iron homeostasis, which helps the experimenters determine the relationship between iron metabolism and ferroptosis (Yang, Gao, et al. 2024; Huang et al. 2025). Although the precise mechanisms underpinning iron involvement in ferroptosis remain elusive, its role as a key medium for ROS production emphasizes its importance (Zhang, Li, Wang, et al. 2024; Co et al. 2024).
2.2.2. Mitochondrial Metabolism
Most unstable intracellular iron is directly sent to the mitochondria to meet its normal functions (Das et al. 2016). However, excessive Fe2+ intake may increase ROS production via Fenton reactions, making the mitochondria the main ROS source in cells (Zheng et al. 2018; Willems et al. 2015). Among them, electrons are catalyzed via transfer by NADPH oxidases (NOXs) (Liu, Shi, et al. 2024) or leakage from electron transfer chain complexes I and III to produce superoxide, which is converted to hydrogen peroxide (H2O2) via superoxide dismutase 2 (SOD2) (Qiu, Sun, et al. 2024). H2O2 is further reduced by GPX4 (Schwarz et al. 2023) or it reacts with unstable iron via Fenton reactions to generate hydroxyl radicals (Illés et al. 2019). This promotes increased ROS levels and drives PUFA‐phospholipid (PUFA‐PL) peroxidation to induce ferroptosis (Zheng and Conrad 2020). Furthermore, free iron overload causes mitochondrial damage, which causes uncontrolled mitophagy, resulting in large quantities of free iron, ROS, and lipid peroxides entering the cytoplasm and exacerbating ferroptosis (Yu et al. 2022). When ferroptosis occurs, mitochondrial condensation and membrane rupture (distinct from apoptosis/necrosis) can be directly observed using transmission electron microscopy (Dixon et al. 2012). Therefore, limiting excess Fe2+ availability could strategically facilitate cellular antioxidant defenses.
2.2.3. LPO
Lipid metabolism disorders are closely associated with ferroptosis (Feng and Stockwell 2018). LPO refers to the oxidation, fragmentation, and shortening of PUFAs or allyl‐rich phosphatidylethanolamine on lipid membranes by oxygen free radicals (Li, Hu, et al. 2024). This process generates cytotoxic substances such as lipid free radicals and active aldehydes, which cause cell disintegration and death by destroying cell lipid bilayers, proteins, and DNA structures (Yan et al. 2021). PUFA peroxidation via enzymatic reactions is the main cause of ferroptosis. Free PUFA reacts with CoA via acyl‐CoA synthetase long‐chain family member 4 (ACSL4) (Doll et al. 2017) to produce PUFA‐CoA, which generates PUFA‐PL via lysophosphatidylcholine acyltransferase 3 (LPCAT3) (Kagan et al. 2017). PUFA‐PL then produces high ROS levels via different enzymes such as oxidoreductase cytochrome P450 reductase (POR) (Xie et al. 2024) and lipoxygenases (ALOXs) (Zou, Li, et al. 2020), which cause LPO and promote ferroptosis. In this enzymatic reaction pathway, ACSL4 is the most thoroughly studied regulator and a vital initiator of ferroptosis (Mishima et al. 2025). The incorporation of PUFAs into phospholipids is a vital step in ferroptosis, and this process requires the joint participation of ACSL4 and LPCAT3 to determine ferroptosis sensitivity (Cui et al. 2023). Increased expression of ACSL4 and LPCAT3 facilitates the conversion of PUFAs to phospholipids. The iron‐containing enzyme ALOXs is a key target for intracellular Fe2+ to induce ferroptosis in a non‐enzymatic manner. Intracellular iron overload will increase the catalytic activity of ALOXs and trigger LPO (Shintoku et al. 2017). Furthermore, ALOXs are also associated with the p53‐mediated ferroptosis pathway that is independent of ACSL4 (Chu et al. 2019). Notably, NOXs also initiate membrane‐associated ROS production, which is another source of ROS, to mediate LPO (Poursaitidis et al. 2017; Reis et al. 2023). By inhibiting the expression of these enzymes, the levels of ROS and LPO can be effectively reduced, endowing cells with more resistance to ferroptosis (Doll et al. 2017). These findings suggest that pharmacological inhibition or gene knockout of these key enzymes may be a potential strategy to reduce the occurrence of ferroptosis. In addition, by using methods such as the DCFH‐DA fluorescent probe and C11‐BODIPY, researchers can assess intracellular ROS levels to evaluate the efficacy of measures targeting these enzymes (Dixon et al. 2012; Yang, Gao, et al. 2024).
2.3. Ferroptosis Suppressor Pathways
2.3.1. The Xc−‐GSH‐GPX4 Axis
This axis was the first ferroptosis prevention system to be identified (Lei et al. 2022). Xc− is a membrane sodium‐dependent amino acid antiporter, which exchanges glutamate (Glu) and cystine at a 1:1 ratio via solute carrier family 3 member 2 (SLC3A2) and solute carrier family 7A member 11 (SLC7A11), while reducing cystine to cysteine and promoting cysteine (raw material for GSH synthesis) transport to cells (Koppula et al. 2018; Liu et al. 2020). GPX4 is a lipid repair enzyme that converts and reduces reactive PUFA‐PL hydroperoxides (PUFA‐PL‐OOH) to non‐reactive and non‐lethal PUFA‐PL alcohols (Rodencal et al. 2024), while oxidizing reduced GSH to oxidized GSH to maintain a balanced intracellular free radical content and regulating ferroptosis (Zhang, Dai, et al. 2023). The use of imidazole ketone erastin, erastin, and RSL3 can pharmacologically remove cystine, deplete GSH, and inhibit GPX4, respectively, which can reduce the antioxidant capacity in cells and increase lipid ROS levels, ultimately leading to ferroptosis (Badgley et al. 2020; Yang et al. 2014).
2.3.2. The FSP1‐CoQH2 System
The ferroptosis suppressor protein 1 (FSP1)‐ubiquinone (CoQ) axis was identified as a second endogenous mechanism that inhibited LPO and ferroptosis (Bersuker et al. 2019). FSP1 is localized to the plasma membrane and converts CoQ to its reduced form, ubiquinol (CoQH2), due to its NADH: ubiquinone oxidoreductase activity (Doll et al. 2019). CoQH2 acts as a lipid‐soluble antioxidant that prevents LPO and inhibits ferroptosis in cell membranes by reducing lipid radicals (Alves et al. 2025; Nakamura et al. 2023).
2.3.3. Other Pathways
Thanks to in‐depth ferroptosis analyses, ferroptosis prevention mechanisms have been increasingly unraveled. Apart from the Xc−‐GSH–GPX4 axis and the FSP1‐CoQH2 system, DHODH‐CoQH2 (Mao et al. 2021), GCH1‐BH4 (Kraft et al. 2020; Soula et al. 2020), MBOAT1/2‐MUFA (Liang et al. 2023), and the SC5D‐7‐DHC axis systems (Freitas et al. 2024; Li, Ran, et al. 2024) have been identified and characterized as novel ferroptosis prevention systems (Figure 1).
FIGURE 1.
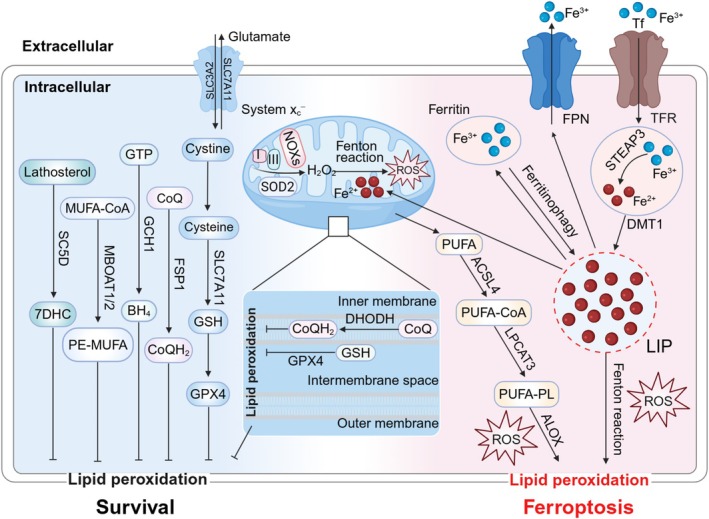
Ferroptosis driver or suppressor pathways. Ferroptosis driver pathways: TFR and DMT1 transport Fe3+ into cells and convert it to Fe2+. Intracellular iron is exported to extracellular sites by FPN. Fe2+ released by intracellular ferritinophagy promotes ROS production and lipid peroxidation via Fenton reactions. Most intracellular Fe2+ is used by the mitochondria. Electrons produce superoxide from electron transfer chain complexes I and III leak or NOXs transfer, which can be reduced to H2O2 by SOD2. H2O2 also reacts with unstable iron to produce abundant ROS (Fenton reactions). Additionally, free iron overload causes uncontrolled mitophagy, resulting in high free iron, ROS, and lipid peroxide levels entering the cytoplasm and exacerbating ferroptosis. PUFA is catalyzed by ACSL4 to produce PUFA‐CoA, which produces PUFA‐PL under LPCAT3. PUFA‐PL produces many ROS molecules through POR and ALOX actions, causing LPO and promoting ferroptosis. Ferroptosis suppressor pathways: Ferroptosis inhibition pathways include: Xc−‐GSH‐GPX4, FSP1‐CoQH2, DHODH‐CoQH2, GCH1‐BH4, MBOAT1/2‐MUFA, and SC5D‐7‐DH axis systems. SLC3A2 and SLC7A11 exchange glutamate and cystine, while reducing cystine to cysteine to synthesize GSH and promote GPX4 production. ACSL4, acyl‐CoA synthetase long‐chain family member 4; ALOX, lipoxygenase; CoQH2, ubiquinol; DMT1, divalent metal transporter 1; FPN, ferroportin; FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; GSH, glutathione; LPCAT3, lysophosphatidylcholine acyltransferase 3; LPO, lipid peroxidation; NOXs, NADPH oxidases; POR, oxidoreductase cytochrome P450 reductase; PUFA, polyunsaturated fatty acid; PUFA‐PL, polyunsaturated fatty acid‐phospholipid; ROS, reactive oxygen species; SLC3A2, solute carrier family 3 member 2; SLC7A11, solute carrier family 7a member 11; SOD2, superoxide dismutase 2; STEAP3, six‐transmembrane epithelial antigen of prostate 3.
3. The Microbiome
3.1. General Properties
Broadly, the human microbiome consists of various microbiota systems, including not only microbiotas themselves (such as bacteria, archaea, fungi, viruses, phages, and small protists) but also their structural elements and various microbiota‐derived metabolites (such as toxins, amino acids, nucleic acids, lipopolysaccharide [LPS], short‐chain fatty acids [SCFAs], and bile acids [BAs]) (Berg et al. 2020; Glorieux et al. 2023; McCann and Rawls 2023; Carasso et al. 2024; Brown and Heneka 2024; Mann et al. 2024; Li, Ding, et al. 2024). The microbiome is distributed across different parts of the body, such as the digestive tract (oral, esophagus, and the gut), the respiratory tract (oral or nose, or the lung), the urogenital tract (kidney, vagina, uterus, and testes), the skin, and also various tumor tissues (Hou et al. 2022; Aggarwal et al. 2023), which maintain overall homeostasis by communicating within or between species and with the host (Manos 2022) (Figure 2A). Microbiome homeostasis is essential for human health, helping hosts to digest food and absorb nutrients, maintain intestinal barrier function, regulate the immune system, promote metabolism, and perform other assorted functions (Bolte et al. 2025; Zhao et al. 2023). Therefore, when dysbiosis occurs, diseases can arise (Aggarwal et al. 2023).
FIGURE 2.
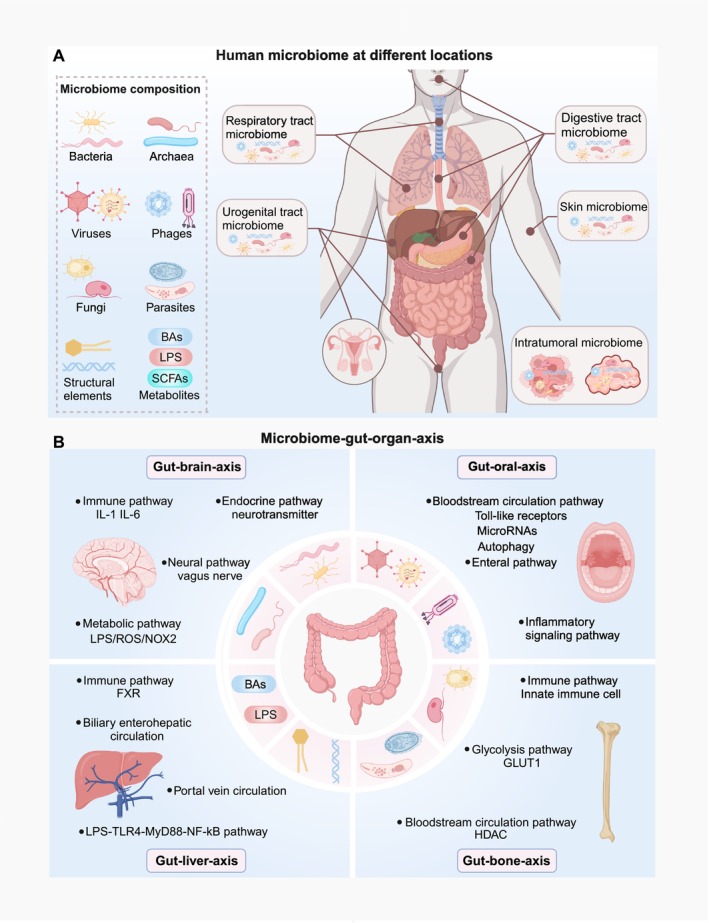
General microbiome properties and the microbiome‐gut‐organ axis. (A) The microbiome is distributed across different parts of the body, such as the digestive, respiratory, urogenital tract, and skin systems, and also tumor tissues. Microbiome composition includes bacteria, archaea, viruses, phages, fungi, and parasites, their structural elements, and various metabolites (e.g., LPS, SCFAs, and BAs). (B) The gut microbiome communicates with the liver, brain, oral system, and bone through immune, inflammatory, bloodstream, and other pathway systems to establish microbiome‐gut‐organ axes. BAs, bile acids; FXR, farnesoid X receptor; GLUT1, glucose transporter‐1; HDAC, histone deacetylase; LPS, lipopolysaccharide; NOX2, NADPH oxidases 2; SCFAs, short‐chain fatty acids; TLR4, toll‐like receptor‐4.
3.2. The Microbiome–Gut–Organ Axis
Gut microbiome homeostasis influences multiple human diseases (Lee et al. 2022). Such may be related to the complex interaction between the gut and other remote organs, called the gut‐organ axis (Zhang, Wang, Sang, et al. 2024; Kim and Sung 2024). Thanks to the communication ability of the microbiome, these axes are also known as microbiome‐gut‐organ axes, which are critical for human microbiome and host homeostasis (Hsu and Schnabl 2023; He et al. 2025; Baker et al. 2024; Zaiss et al. 2019). Here, we describe microbiome‐gut‐liver/brain/oral/bone axes, which have been relatively well‐studied in ferroptosis (Figure 2B).
3.2.1. The Microbiome–Gut–Liver Axis
This axis depends on biliary enterohepatic circulation, portal vein circulation, and systemic mediators (Hsu and Schnabl 2023). Enterohepatic BA circulation is a major communication route between the liver and the gut. Specifically, BAs and other metabolites synthesized by the liver are released through the biliary tract into the gut and influence gut microbiome composition and intestinal barrier function (Tripathi et al. 2018). Gut BAs effectively inhibit microbial overgrowth by disrupting bacterial cell membranes (Kurdi et al. 2006) or binding to farnesoid X receptor (FXR) (Inagaki et al. 2006). Moreover, oral BAs reduce bacterial translocation and endotoxemia (Lorenzo‐Zúñiga et al. 2003), which may be related to the protective effects of BA‐mediated FXR activation on intestinal barrier integrity (Li, Chaudhari, et al. 2022). Gadaleta et al. reported that FXR activation reduced goblet cell loss, decreased intestinal permeability, and alleviated intestinal inflammation to improve inflammatory bowel disease (IBD) prognosis (Gadaleta et al. 2011).
The gut microbiome can be translocated to the liver via reabsorbed BAs or increased intestinal permeability, thereby inducing liver inflammation and damage (Tilg et al. 2022). Studies have confirmed that patients with liver diseases experience intestinal inflammation and gut microbiome dysbiosis (Boursier et al. 2016), which are inducible factors for increased intestinal permeability (Kang et al. 2022). Increased intestinal permeability causes microbes and microbial metabolites (e.g., LPS) to translocate to the liver through portal vein circulation (Pabst et al. 2023). Microbial metabolites also influence liver disease progression by regulating liver immune responses via FXR (Wen et al. 2024). LPS and acetaldehyde (ethanol metabolites) are recognized by immune receptors on Kupffer cells (KCs) and induce these cells to release pro‐inflammatory cytokines and chemokines, leading to host immune responses (Yan et al. 2011; Remetic et al. 2022). Also, commensal‐derived D‐lactate enhances clearance in KCs and helps these cells to establish an intravascular immune firewall to prevent pathogen transmission from the bloodstream (McDonald et al. 2020). Moreover, LPS accumulation in the liver induces myeloid differentiation factor‐88‐mediated nuclear factor‐kappa B activation via toll‐like receptor‐4 (TLR4) activation. These actions release inflammatory cytokines (Nighot et al. 2017), trigger liver inflammation and damage, and further deteriorate liver disease (Luther et al. 2015). Notably, probiotic supplementation can restore BAs and SCFAs, which reduces intestinal epithelial damage (Li, Wang, et al. 2022), regulates tight junctions (Zheng et al. 2017), and improves the intestinal barrier to reduce blood endotoxin levels and inhibit liver disease progression (Yu et al. 2021).
3.2.2. The Microbiome–Gut–Brain Axis
Bidirectional communications between the gut and the brain are known as the microbiome‐gut‐brain axis, with the microbiome having key roles in this process (He et al. 2025). The gut microbiome affects physiological and pathological brain mechanisms mainly through metabolic, endocrine, neural, and immune pathways (Shandilya et al. 2022) Physiologically, the gut microbiome regulates the vagus nerve and several cytokines (e.g., interleukin (IL)‐1 and IL‐6) (Powell et al. 2017) by producing neurotransmitters and various metabolites (e.g., LPS and SCFAs) to regulate host immunity and neurodevelopment (Wang, Yang, and Liu 2023). The pathological regulation of the gut microbiome on the central nervous system (CNS) is mainly mediated by microglia (Loh et al. 2024), which, with either pro‐inflammatory M1 or anti‐inflammatory M2 phenotypes, are resident macrophages in the CNS (Yu, Chang, et al. 2023). It was previously reported that LPS crossed the intestinal barrier into the systemic circulation and across the blood–brain barrier, promoting microglia M1 polarization, which then released abundant pro‐inflammatory cytokines (e.g., tumor necrosis factor (TNF)‐α, IL‐1, and IL‐6), exacerbating neuroinflammation and neurodegenerative processes (Guo et al. 2022). Also, LPS‐induced macrophage ROS production and NADPH oxidase 2 (NOX2) activation were shown to further increase brain oxidative stress and damage (Wu et al. 2020). Yang, Hao, et al. (2023) observed that inhibited LPS‐induced NOX2 microglia activation effectively reduced oxidative stress and neuroinflammation caused by ischemia–reperfusion brain damage. In contrast, SCFAs promoted microglia polarization to M2 phenotypes, which facilitated anti‐inflammatory cytokine release (e.g., IL‐4 and IL‐13) (Ahuja and Lazar 2021) to inhibit neuroinflammation (Qian et al. 2022). Notably, reduced SCFA and increased LPS levels may cause α‐synuclein (α‐syn) aggregation, which increases ROS and LPO in dopaminergic (DA) neurons and also microglia activation and M1 polarization. This process was shown to generate more ROS and inflammatory cytokine levels and exacerbate neuroinflammation and neuronal injury (Kalyanaraman et al. 2024; Fang et al. 2024).
3.2.3. The Microbiome–Gut–Oral Axis
The intimate relationship between the oral and gut microbiome supports the notion of a microbiome‐oral‐gut axis (Derrien et al. 2010; Chen, Peng, et al. 2024). Liao et al. (2024) reported that gut microbiome depletion increased relative oral microbiome abundance in the gut. Furthermore, oral inflammation and mucosal damage caused by periodontal disease allowed oral pathogens and their metabolites to enter the bloodstream (Sedghi et al. 2021), which potentially allowed the oral microbiome to participate in gut or systemic diseases (Baker et al. 2024). The oral pathogen F. nucleatum is a key pathogenic factor and colorectal cancer (CRC) biomarker (Wang and Fang 2023). The evidence now suggests that F. nucleatum is mainly colonized in the gut via an enteral route and promotes CRC and chemotherapy resistance by regulating TLRs, microRNAs, and inflammatory signaling pathways (Wang and Fang 2023; Yu et al. 2017; Zhang, Zhang, et al. 2022). Interestingly, F. nucleatum colonization can also be detected in CRC tissues by intravenously injected F. nucleatum , indicating that it can also be translocated to the gut through the bloodstream circulation (Abed et al. 2020). In contrast, gut microbes rarely colonize oral spaces and only appear in special cases, such as poor hygiene or hypoimmunity (Liu, Su, et al. 2022).
Notably, the links between the oral microbiome and liver diseases may be mediated by a novel oral‐gut‐liver axis (Albuquerque‐Souza and Sahingur 2022). Porphyromonas gingivalis ( P. gingivalis ) infection was reported as a risk factor for alcoholic liver disease (ALD) (Gao et al. 2023). Specifically, P. gingivalis , which enters the liver by inducing intestinal microbiome dysbiosis, was shown to aggravate liver inflammation in ALD mice via TLR4/IL‐6/TNF‐α/TGF‐β1 signaling (Gao et al. 2023). Moreover, P. gingivalis directly affects nonalcoholic fatty liver disease (NAFLD) progression via the circulation. P. gingivalis ‐associated LPS also promoted TLR2 and pro‐inflammatory cytokine expression in steatotic hepatocytes, leading to intracellular lipid accumulation and insulin resistance, which worsened NAFLD (Kim et al. 2024).
3.2.4. The Microbiome–Gut–Bone Axis
The establishment of the microbiome‐gut‐bone axis depends on the regulation of the gut microbiome to remote organs through the bloodstream circulation (Zaiss et al. 2019). Previous studies reported that the gut microbiome could maintain bone homeostasis by regulating host immunity and metabolism (Guo et al. 2023; Lin et al. 2023), whereas, during dysbiosis, the gut microbiome caused bone disease by changing intestinal permeability (Zhang et al. 2021). Gut metabolites that reach the bone via the systemic circulation can impact bone metabolism through multiple mechanisms. For example, glucose transporter 1 (GLUT1) is mainly responsible for glucose transport in osteoblasts and provides materials for osteoblast glycolysis (Lee et al. 2017). A recent study reported that gut metabolites may regulate GLUT1 expression to regulate bone energy metabolism, thus influencing bone health (Guan et al. 2023). Furthermore, butyrate has important roles in bone diseases, as it inhibits histone deacetylase (HDAC) to stimulate osteoblast or anti‐osteoclast differentiation (Perego et al. 2018).
4. The Microbiome and Ferroptosis
The impact of the gut microbiome on ferroptosis in disease has gained considerable research traction (Yao and Li 2023; Liu, Wang, et al. 2024). Pathogenic bacteria usually exhibit promoting ferroptosis, of which mechanisms include promoting pro‐inflammatory cytokine secretion, labile iron accumulation, mitochondrial dysfunction, ROS production, and LPO. Escherichia coli‐derived LPS promotes IL‐6 secretion to increase the level of intracellular oxidative stress and Fe2+, thereby triggering ROS‐mediated ferroptosis in goat mammary epithelial cells (Zhu et al. 2022). While probiotics can enhance the levels of GSH and antioxidant enzymes by modulating iron absorption, generating SCFAs, and improving intestinal barrier function, they reduce oxidative stress and LPO and inhibit ferroptosis. Lactobacillus‐produced SCFAs enhance GSH levels, thereby suppressing ferroptosis in ischemia reperfusion injury (IRI) mice (Wang, Wang, et al. 2025). Notably, the oral microbiome can also influence multiple diseases by directly regulating ferroptosis.
4.1. Iron Homeostasis
Iron homeostasis is maintained by complex iron metabolic activities (uptake, storage, and utilization) (Galy et al. 2024). An imbalanced iron metabolism causes several health issues, such as iron deficiency‐related anemia (Pasricha et al. 2021) due to iron deficiency or ferroptosis (Yang et al. 2020) due to iron overload. Host iron metabolism affects microbiome diversity, distribution, and function. Because intestinal iron absorption (dietary iron or iron agents) is the sole external exogenous iron source in a physiological situation, controlling intestinal iron absorption is key to maintaining iron homeostasis (Bao et al. 2024). Lactobacillus species are reportedly implicated in intestinal iron levels, while their relative abundance decreases with iron overload (Shah et al. 2019) but increases with iron deficiency (Das et al. 2020). Several clinical trial studies have demonstrated that probiotics (such as Lactobacillus) and prebiotics can enhance intestinal iron absorption and alleviate intestinal inflammation (Hoppe et al. 2015; Mikulic et al. 2024). Furthermore, the microbiome regulates ferroptosis by regulating iron homeostasis in several ways (Xiao et al. 2023; Teschke 2024).
Siderophores are a type of metabolite with a high affinity for iron. Studies have shown that microbes indirectly regulate iron metabolism and ferroptosis by secreting siderophores to compete with the host for iron absorption (Schalk 2025). For instance, the siderophore pyoverdine secreted by Pseudomonas aeruginosa significantly reduces the intracellular iron concentration by chelating iron (Fe2+/Fe3+), thereby inhibiting ferroptosis in tumor cells (Yeung et al. 2024).
Hepcidin is a primary iron homeostasis regulator and limits iron release from cells by inducing FPN degradation, thus facilitating cellular iron overload (Taylor et al. 2011). While the microbiome induces hepcidin expression (hepatocyte‐derived or conventional dendritic cell‐derived) (Shanmugam et al. 2015; Bessman et al. 2020), there is no direct evidence that it regulates ferroptosis via hepcidin. What is known is that FPN expression is significantly reduced in LPS‐induced endotoxemia in rat atrial tissues, resulting in intracellular iron overload and triggering ferroptosis by increasing atrial oxidative stress (Fang et al. 2021). Notably, intestinal iron absorption by the hepcidin/FPN axis is mediated by hypoxia‐inducible factor‐2α (HIF‐2α) (Schwartz et al. 2019), which may facilitate intestinal iron absorption by modulating DMT1 and FPN (Taylor et al. 2011). Das et al. (2020) reported that in intestinal iron deficiency, elevated microbial metabolites regulated host iron homeostasis by inhibiting HIF‐2α activity, which limited intestinal iron absorption and upregulated ferritin expression to increase cellular iron storage. Another gut bacterial metabolite, deoxycholic acid (DCA), was reported to up‐regulate HIF‐2α and DMT1 expression, resulting in intracellular Fe2+ overload, which produced excessive ROS (Fenton reactions) and triggered oxidative stress, thus causing intestinal epithelial cell (IEC) ferroptosis (Wang, Chu, Dong, et al. 2024). In contrast, hypoxia‐inducible factor‐1α (HIF‐1α) is generally considered a ferroptosis inhibitor (Shen et al. 2024); however, HIF‐1α mediates ferritinophagy in promoting ferroptosis (Zhao et al. 2020). Nuclear receptor co‐activator 4 (NCOA4)‐dependent ferritinophagy is another regulatory process for maintaining intracellular and systemic iron homeostasis (Galy et al. 2024; Santana‐Codina et al. 2021). Butyrate secreted by oral pathogens, such as P. gingivalis , Tannerella forsythia , and Prevotella intermedia , promoted NCOA4‐mediated ferritinophagy, which was dependent on HIF‐1α, resulting in high Fe2+ quantities entering the transient LIP and producing ROS via Fenton reactions, thereby inducing ferroptosis (Zhao et al. 2020). Similarly, F. nucleatum ‐induced ferroptosis in periodontal ligament stem cells (PDLSCs) by increasing unstable iron levels (Wang, Wang, et al. 2023) (Figure 3A).
FIGURE 3.
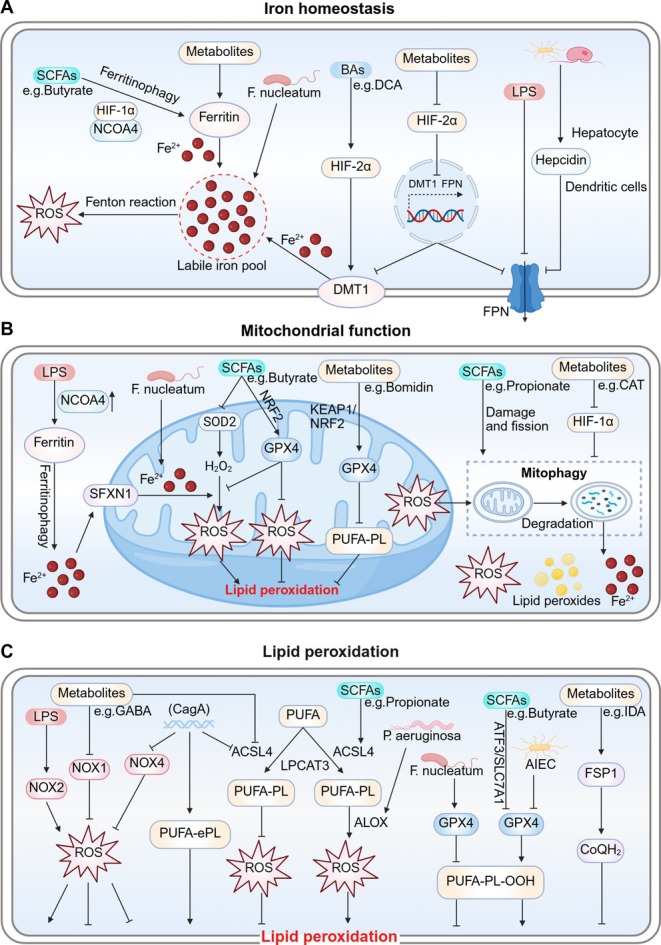
Microbial regulation of ferroptosis. (A) The microbiome inhibits FPN expression by inducing hepcidin or inhibiting HIF‐2α expression, and inducing intracellular iron overload. DCA promotes DMT1 expression via HIF‐2α, leading to increased labile iron levels. Microbial metabolites promote ferritin storage. Butyrate promotes HIF‐1α‐NCOA4‐mediated ferritinophagy, resulting in high Fe2+ levels entering the LIP and producing ROS, thereby inducing ferroptosis. F. nucleatum induces ferroptosis by increasing unstable iron levels in cells. (B) LPS induces NCOA4‐mediated ferritinophagy to activate SFXN1, and transfers excess Fe2+ to mitochondria, resulting in ferroptosis. F. nucleatum ‐induced iron overload may cause mitochondrial dysfunction. Excess iron accumulation in the mitochondria leads to the ROS, LPO, and mitophagy‐mediated aggravation of intracellular labile iron, which further induces ferroptosis. Additionally, propionate promotes mitochondrial damage, fission, and mitophagy. CAT also inhibits mitophagy and oxidative stress by inhibiting HIF‐1α expression to ultimately inhibit ferroptosis. Bomidin and butyrate increase GPX4 expression by upregulating the KEAP1/NRF2 pathway, which reduces mitochondrial damage and subsequent LPO. Tumoral butyrate increases intracellular ROS levels and mitochondrial metabolic damage by decreasing SOD2 expression. (C) Propionate elevates PUFA‐PL levels by increasing ACSL4 expression, while CagA reduces ACSL4 levels. CagA promotes PUFA‐ePLs synthesis, causing ferroptosis sensitivity in cells. P. aeruginosa promotes lipid oxidation by enhancing ALOX expression to induce ferroptosis. LPS‐induced NOX2 activation increases oxidative stress. Nevertheless, CagA or glutamine reduces NOX4 or NOX1 expression, respectively, both of which decrease ROS production and inhibit ferroptosis. AIEC colonization may decrease GPX4 expression, thus aggravating LPO and ferroptosis. F. nucleatum inhibits ferroptosis by elevating GPX4. Butyrate depletes intracellular GPX4 by inhibiting the ATF3/SLC7A11 axis to promote lipid peroxidation and ferroptosis. IDA increases FSP1‐CoQH2 levels and contributes to ferroptosis resistance. ACSL4, acyl‐CoA synthetase long‐chain family member 4; AIEC, adherent‐invasive E. coli ; ALOX, lipoxygenase; ATF3, transcription factor 3; CagA, cytotoxin‐associated gene A; CAT, Capsiate; CoQH2, ubiquinol; DCA, deoxycholic acid; DMT1, divalent metal transporter 1; FPN, ferroportin; FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; HIF‐1α, hypoxia‐inducible factor‐1α; HIF‐2α, hypoxia‐inducible factor‐2α; IDA, trans‐3‐indoleacrylic acid; KEAP1, Kelch‐associated protein 1; LIP, labile iron pool; LPO, lipid peroxidation; LPS, lipopolysaccharide; NCOA4, nuclear receptor co‐activator 4; NOX1/2/4, NADPH oxidases 1/2/4; NRF2, nuclear factor erythroid 2‐related factor 2; P. aeruginosa , Pseudomonas aeruginosa ; PUFA‐ePLs, polyunsaturated ether phospholipids; PUFA‐PL, polyunsaturated fatty acid‐phospholipid; SFXN1, siderofexin; SOD2, superoxide dismutase 2.
4.2. Mitochondrial Functions
As an intermediate link between iron overload and LPO, mitochondrial dysfunction is implicated in ferroptosis (Lei et al. 2022). Mitochondria are the main ROS source in the cell, and the microbiome regulates ferroptosis by regulating mitochondrial function. LPS‐induced NCOA4 overexpression induces the ferritinophagy‐mediated release of high Fe2+ quantities into the cytoplasm, resulting in siderofexin (SFXN1) activation on outer mitochondrial membranes (Li et al. 2020). Subsequently, the transfer of excess Fe2+ to mitochondria through SFXN1 is a self‐protection mechanism of cells in the early stage of iron overload (Zhang, Xin, et al. 2022). However, excess iron accumulation in the mitochondria leads to excess ROS and LPO, as well as extensive mitophagy, at the cost of aggravating intracellular unstable iron and further inducing ferroptosis (Chen et al. 2020; Li et al. 2023). For instance, F. nucleatum ‐induced iron overload leads to mitochondrial dysfunction, which induces ferroptosis in PDLSCs (Wang, Wang, et al. 2023). Propionate derived from the gut microbiome also promotes acute myeloid leukemia (AML) cell ferroptosis by inducing mitochondrial damage, fission, and mitophagy (Wei, Liu, et al. 2024). Under hypoxic conditions, HIF‐1 promoted mitophagy, resulting in increased oxidative stress and accelerated LPO to amplify ferroptosis (Li et al. 2023). Additionally, the gut metabolite capsiate (CAT) inhibits mitophagy and oxidative stress by inhibiting HIF‐1α expression, ultimately inhibiting ferroptosis in knee osteoarthritis mice (Guan et al. 2023).
Furthermore, the microbiome regulates ferroptosis by influencing mitochondrial antioxidant proteins. The antioxidant nuclear factor erythroid 2‐related factor 2 (NRF2), which is inhibited by kelch‐associated protein 1 (KEAP1) transcription (Bonkhoff et al. 2022), induces antioxidant proteins such as SOD2 and GPX4, which are cell‐protection defenses against ferroptosis (Gan 2021). During oxidative stress, NRF2 dissociates from KEAP1 to trigger downstream antioxidant protein transcription, which is a self‐protection mechanism in cells (Dodson et al. 2019). The Escherichia coli metabolite bomidin increases Xc−/GPX4 axis expression by upregulating KEAP1/NRF2 signaling, thus exerting anti‐ferroptosis and anti‐inflammatory effects in TNF‐α‐induced PDLSCs (Wu et al. 2024). H2O2 reduction by SOD2 and GPX4 cuts off mitochondrial PUFA‐PL peroxidation to reduce oxidative stress and maintain mitochondrial homeostasis (Kenny et al. 2019; Brigelius‐Flohé and Maiorino 2013). Gut butyrate was also shown to inhibit ferroptosis by inducing NRF2/GPX4 to reduce mitochondrial damage and subsequent LPO in colitis mice (Chen, Qian, et al. 2024). Additionally, the tumoral butyrate‐producing Clostridium butyricum enhances oxidative stress and mitochondrial metabolic damage in pancreatic ductal adenocarcinoma cells by decreasing SOD2 expression and significantly increasing intracellular ROS levels (Yang, Zhang, et al. 2023). These observations suggest that butyrate may have opposite roles at different sites via different mechanisms (Figure 3B). This may be related to the intricate tumor microenvironment and the cooperation between the host and microbiome.
4.3. LPO
Uncontrollable LPO is a critical hallmark of ferroptosis. The evidence now suggests that the microbiome influences ferroptosis by regulating LPO. PUFA‐PL susceptibility to LPO is due to its bis‐allylic hydrogen atom structure and ACSL4 catalysis (Conrad and Pratt 2019). Therefore, regulating ACSL4 expression can be an effective strategy to influence ferroptosis. Wei, Liu, et al. (2024) investigated the effects of different propionate concentrations on the progression of AML in vivo or in vitro, including oral administration (200 mM), intravenous injection (5 mg/100 μL/per mouse), and cell culture (0, 2.5, 5.0, 7.5, 10 mM). They found that different dosages of propionate induced AML cell ferroptosis by upregulating ACSL4 expression. Additionally, Helicobacter pylori cytotoxin‐associated gene A (CagA) (Lu et al. 2024) reduces ACSL4 levels, with potential anti‐ferroptosis roles. However, CagA was also shown to promote polyunsaturated ether phospholipid (PUFA‐ePL) synthesis, endowing gastric cancer cells with ferroptosis sensitivity (Peng, Lei, et al. 2024). This susceptibility was related to PUFA‐ePLs serving as additional substrates for LPO (Zou, Henry, et al. 2020). Unfortunately, there is little evidence on the impact of CagA on ferroptosis. Excess ROS produced by PUFA‐PL peroxidation is mediated by ALOX. The evidence now suggests that P. aeruginosa promotes LPO by enhancing ALOX expression to induce ferroptosis in human bronchial epithelial cells and mice IECs and shows how the microbiome affects ferroptosis‐associated iron‐containing enzymes (Dar et al. 2018, 2022). Additionally, NOX enzymes, which drive membrane‐associated ROS, are reportedly regulated by the microbiome. LPS‐induced NOX2 activation increased oxidative stress (Wu et al. 2020). Nevertheless, CagA overexpression or supplementation with glutamine reduced NOX4 or NOX1 expression, respectively, both of which significantly decreased ROS production and exerted ferroptosis inhibitory effects (Lu et al. 2024; Zhang, Zhou, Zhai, et al. 2024).
The microbiome‐mediated regulation of ferroptosis suppressor pathways is another important factor affecting lipid peroxide accumulation and ferroptosis. GPX4 is a well‐known ferroptosis defender with key roles in defending against LPO by hydrolyzing PUFA‐PL‐OOH (Seibt et al. 2019). Adherent‐invasive Escherichia coli (AIEC) cell colonization potentially decreased GPX4 expression; thus aggravating LPO and ferroptosis (Wen et al. 2023). In contrast, the gut microbial metabolite vinyl‐ether phospholipid (plasmalogen) inhibited ferroptosis by increasing GPX4 expression (Liu et al. 2023); unlike the previous PUFA‐ePLs, the absence of PUFA results in its insensitivity to H2O2 (Vítová et al. 2021). Interestingly, F. nucleatum also inhibited ferroptosis in CRC cells and a xenograft mouse model by elevating GPX4 levels, which were associated with GSH accumulation (Li, Wei, et al. 2024). GSH is an indispensable cofactor for correct GPX4 function, and its depletion inactivated GPX4 (Yang et al. 2014). Moreover, GSH synthesis was limited by cysteine availability, which depended on the Xc− (SLC7A11) system. Cystine removal or interceding with SLC7A11‐mediated cystine transport (e.g., using the ferroptosis inducer erastin) also promoted ferroptosis (Koppula et al. 2021). Bi et al. (2024) reported that butyrate depleted intracellular GSH levels by inhibiting SLC7A11 expression via transcription factor 3 (ATF3) (Wang et al. 2020), which was possibly related to the HDAC inhibition of butyrate (Duncan et al. 2020), thereby promoting LPO and ferroptosis in lung cancer cells.
Notably, when GPX4 was inactivated, anti‐ferroptosis actions were mainly mediated by FSP1 (Doll et al. 2019), which in turn inhibited lipid peroxides by producing CoQH2, trapping lipid radicals, and thus inhibiting ferroptosis in various cell membrane structures (Bersuker et al. 2019). Previous studies have confirmed that several tryptophan metabolites, such as indole‐3‐pyruvate, serotonin, and 3‐hydroxyo‐aminobenzoic acid, can counteract ferroptosis by scavenging oxygen free radicals or activating NRF2/GSH synthesis (Zheng and Conrad 2025). In addition, microbial tryptophan metabolites have exhibited great potential in protecting intestinal barrier function and controlling intestinal inflammation by activating aryl hydrocarbon receptor (AHR) (Scott et al. 2020; Pernomian et al. 2020). Recently, a tryptophan metabolite derived from P. anaerobius , trans‐3‐indoleacrylic acid (IDA), increased FSP1‐CoQH2 levels via the AHR‐aldehyde dehydrogenase 1 family member A3 (ALDH1A3) axis, thus contributing to ferroptosis resistance in CRC mouse models (Zhang, Kang, and Tang 2024; Cui et al. 2024) (Figure 3C).
5. Microbiome Influences on Ferroptosis in Disease
With increasing research, the regulatory role of the microbiome in ferroptosis is becoming increasingly clear. Not only does it play a role in gut diseases, but the microbiome can also participate in the progression of various non‐gut diseases (such as liver, brain, oral, and bone diseases) through the gut‐organ axis. In addition to the gut microbiome, crosstalk between the oral microbiome and ferroptosis has received considerable research attention (Figure 4). Notably, the interaction between the microbiome and ferroptosis in disease contexts has a dual role; that is, it both potentially exacerbates pathology and offers therapeutic avenues. For instance, in benign diseases, ferroptosis usually leads to disease progression, while in tumors, ferroptosis reverses chemotherapy resistance and promotes tumor cell death, providing new opportunities for cancer therapy. Therefore, it is highly necessary to identify specific microbial elements associated with different disease contexts.
FIGURE 4.
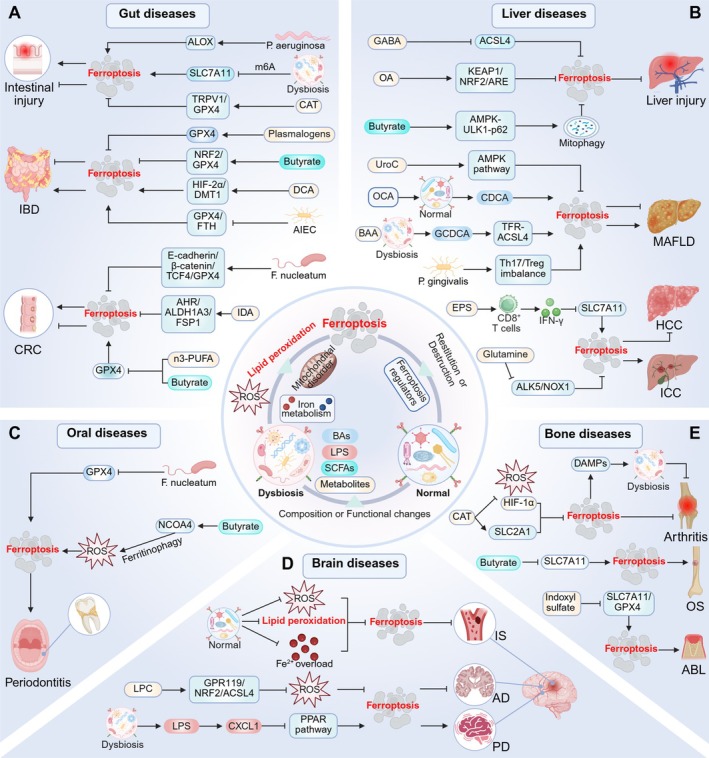
Crosstalk between the microbiome and ferroptosis in disease. The microbiome regulates ferroptosis in several ways to influence gut, liver, brain, oral, bone, and other diseases. ABL, alveolar bone loss; ACSL4, acyl‐CoA synthetase long‐chain family member 4; AD, Alzheimer's disease; AHR, aryl hydrocarbon receptor; AIEC, adherent‐invasive E. coli ; ALDH1A3, aldehyde dehydrogenase 1 family member A3; ALK5, activin receptor‐like kinase 5; ALOX, lipoxygenase; BAA, bromoacetic acid; BAs, bile acids; CAT, capsiate; CDCA, chenodeoxycholic acid; CRC, colorectal cancer; CXCL1, C‐X‐C motif chemokine ligand‐1; DAMPs, danger‐associated molecular patterns; DCA, deoxycholic acid; DMT1, divalent metal transporter 1; EPS, exopolysaccharides; FSP1, ferroptosis suppressor protein 1; GCDCA, glycochenodeoxycholate; GPX4, glutathione peroxidase 4; HCC, hepatocellular carcinoma; HIF‐1α, hypoxia‐inducible factor‐1α; HIF‐2α, hypoxia‐inducible factor‐2α; IBD, inflammatory bowel disease; ICC, intrahepatic cholangiocarcinoma; IDA, trans‐3‐indoleacrylic acid; IS, ischemic stroke; KEAP1, Kelch‐associated protein 1; LPC, lysophosphatidylcholine; LPS, lipopolysaccharide; MAFLD, metabolic dysfunction‐associated fatty liver disease; n‐3 PUFA, omega‐3 polyunsaturated fatty acids; NCOA4, nuclear receptor co‐activator 4; NOX1, NADPH oxidases 1; NRF2, nuclear factor erythroid 2‐related factor 2; NRF2, nuclear factor erythroid 2‐related factor 2; OA, oleanolic acid; OCA, obeticholic acid; OS, osteosarcoma; P. gingivalis , Porphyromonas gingivalis ; PD, Parkinson's disease; PPAR, peroxisome proliferator‐activated receptor; SCFAs, short‐chain fatty acids; SLC2A1, solute carrier family 2 member 1; TRPV1, transient receptor potential cation channel subfamily V member 1; UroC, urolithin C.
5.1. Gut Diseases
5.1.1. Microbiota Regulates Ferroptosis
Intestinal injury is closely associated with ferroptosis and caused by several factors, including environmental toxicants (Tang et al. 2023), ionizing radiation (Zhou et al. 2022), and intestinal IRI (Li et al. 2019). Fluoride is a common environmental toxicant (Stockbridge and Wackett 2024), with fluoride‐induced gut microbiome dysbiosis decreasing SLC7A11 expression via m6A modification, triggering ferroptosis in IECs, and causing IEC damage (Huang, Lin, et al. 2024). Total body irradiation (TBI) is another factor in gut microbiome dysbiosis and intestinal epithelial injury (Zhao et al. 2021). In a TBI environment, P. aeruginosa increases its intestinal colonization and catalyzes the peroxidation of arachidonic acid‐phosphatidylethanolamine (AA‐PE) to 15‐hydroperoxy‐AA‐PE via ALOX, resulting in IEC‐based ferroptosis (Dar et al. 2022). IBD, which includes Crohn's disease (CD) and ulcerative colitis (UC), is closely related to microbial metabolism (Shan et al. 2022). Notably, the gut microbiome not only influences IBD progression through metabolic pathways but also participates in the onset and development of the disease by regulating ferroptosis. Studies have shown that ferroptosis destroys intestinal barriers and is associated with dextran sodium sulfate (DSS)‐induced colitis (Wu et al. 2023). In CD, gut microbiome dysbiosis is characterized by reduced microbial diversity and the increased abundance of specific mucosal‐associated bacteria, particularly AIEC (de Souza et al. 2012). Notably, such colonization of AIEC contributes to the pathogenesis of CD through regulating ferroptosis; it exacerbates LPO and ferroptosis in IECs by reducing the levels of GPX4 and FTH, thereby inducing intestinal inflammation (Wen et al. 2023). The microbiome and ferroptosis are reportedly strongly involved in CRC pathogenesis (Permain et al. 2024). The oral microbiome has been shown to regulate ferroptosis in CRC via the gut‐oral axis. Ferroptosis is well known for its anti‐drug resistance to cancer therapy (Hassannia et al. 2019). F. nucleatum , a carcinogenic oral microbe, is believed to be involved in CRC initiation, progression, and chemotherapy resistance (Kong et al. 2023). Li, Wei, et al. (2024) reported that F. nucleatum inhibits ferroptosis through the E‐cadherin/β‐catenin/TCF4/GPX4 axis and promotes oxaliplatin resistance in CRC.
5.1.2. Microbial Metabolites Regulate Ferroptosis
Different gut microbial metabolites exert different effects on ferroptosis and the above gut diseases. IRI, caused by oxidative stress in the ischemic intestines after blood supply restoration, is often accompanied by intestinal barrier destruction and microbiome changes (Chen, Han, et al. 2024). A previous study reported that CAT inhibited ferroptosis and reduced IRI‐mediated small intestinal mucosal damage by promoting TRPV1 and GPX4 expression (Deng et al. 2021). Obligate anaerobes, such as Bifidobacterium longum and Clostridium butyricum and their products, are potentially beneficial to the IBD colonic environment (Sharma et al. 2023; Wu et al. 2022). Plasmalogen, the most common ether phospholipid form in Bifidobacterium longum (Vítová et al. 2021), inhibits ferroptosis and reduces intestinal inflammation by increasing GPX4 levels but inhibiting COX‐2 expression (Liu et al. 2023). Clostridium butyricum ‐derived butyrate inhibited ferroptosis via NRF2/GPX4 signaling, improved intestinal barrier integrity, and effectively alleviated DSS‐induced colitis in mice (Chen, Qian, et al. 2024). In contrast, DCA potentially impairs the mucus barrier and aggravates UC progression (Liu et al. 2018). Long‐term high‐fat diets can increase DCA levels, which cause Fe2+ accumulation and ferroptosis in IECs by upregulating HIF‐2α and DMT1 expression and aggravating colitis (Wang, Chu, Dong, et al. 2024). A significant increase in P. anaerobius abundance was observed in the feces from CRC patients (Long et al. 2019). Its metabolite IDA inhibits ferroptosis through the AHR‐ALDH1A3‐FSP1 axis and promotes CRC progression (Cui et al. 2024). Additionally, the n‐3 PUFA and butyrate combination inhibits CRC development by weakening mitochondrial antioxidant defenses and promoting mitochondrial GPX4‐dependent ferroptosis (Chapkin et al. 2020).
5.2. Liver Diseases
Gut microbiome effects on liver diseases are mainly realized via the gut‐liver axis (Tilg et al. 2022; Aron‐Wisnewsky et al. 2020). As the role of ferroptosis in liver diseases becomes clearer, the interactions between the gut microbiome and ferroptosis are being uncovered. Notably, the novel oral‐gut‐liver axis links oral to liver diseases and provides strong support for oral pathogens in regulating hepatocyte ferroptosis (Albuquerque‐Souza and Sahingur 2022).
5.2.1. Microbiota Regulates Ferroptosis
The gut microbiome can influence liver tumor progression by modulating ferroptosis. A recent study indicates that the gut microbiota inhibits ferroptosis in intrahepatic cholangiocarcinoma (ICC) cells by altering glutamine metabolism to inhibit the ALK5/NOX1 axis, which promotes ICC (Zhang, Zhou, Zhai, et al. 2024). Also, Enterobacter faecium induces CD8+ T cells to secrete IFN‐γ, which promotes ferroptosis in hepatocellular carcinoma (HCC) cells by down‐regulating SLC7A11, thus hindering disease progression (Yu, Lin, et al. 2024). In recent years, guidelines have recommended that NAFLD should be replaced with metabolic dysfunction‐associated fatty liver disease (MAFLD) (Rinella et al. 2023; Yang, Zhu, et al. 2024); therefore, MAFLD is used to describe NAFLD in this review. Accumulated studies now show that microbiome dysbiosis, one of the main MAFLD features (Saeed et al. 2024), influences MAFLD progression by modulating ferroptosis (Bu et al. 2024). Notably, oral microbes modulate hepatocellular ferroptosis, possibly via the oral‐gut‐liver axis (Albuquerque‐Souza and Sahingur 2022; Chen et al. 2023). In a study, oral P. gingivalis administration alters the gut microbiome and metabolites to induce a Th17/Treg imbalance and hepatocyte ferroptosis, thus causing MAFLD in mice (Yao et al. 2023). Interestingly, another study reported that the gut microbiome in mice orally administered P. gingivalis had colonized hepatocytes and induced ferroptosis, thereby exacerbating alcoholic liver disease (Yao et al. 2024).
5.2.2. Microbial Metabolites Regulate Ferroptosis
In addition, microbial metabolites also affect MAFLD progression by regulating ferroptosis. The gut metabolite urolithin C inhibits ferroptosis via AMPK pathway activation and counteracts gut microbiome dysbiosis, showing an ameliorative potential for MAFLD (Xu et al. 2023). Obeticholic acid (OCA) increases chenodeoxycholic acid (CDCA) production by remodeling the gut microbiome; subsequently, CDCA promotes MAFLD fibrosis by inducing hepatocyte ferroptosis. This action was possibly related to OCA inducing LPO and impairing its antifibrotic effects (Zhuge et al. 2023). Additionally, environmental toxins induce gut microbiome dysbiosis and hepatocyte ferroptosis (Mu et al. 2022). Under bromoacetic acid exposure, the gut microbial metabolite glycochenodeoxycholate activates TFR‐ACSL4‐mediated ferroptosis to accelerate MAFLD (Liu, Gao, et al. 2022). IRI‐related ferroptosis is one major cause of liver injury (Liu et al. 2019; Ye et al. 2020; Jiao et al. 2020). A recent study reported that the microbial metabolite oleanolic acid inhibits hepatocyte ferroptosis via KEAP1/NRF2/ARE signaling and rescues severe IRI after liver transplantation (Pi et al. 2024). Acetaminophen (APAP)‐induced liver injury is the leading cause of acute liver failure (Reuben et al. 2016). Butyrate, a Lachnospiraceae metabolite, reportedly inhibits ferroptosis by activating AMPK‐ULK1‐p62 signaling and mitophagy, protecting mice from APAP‐induced acute liver injury (ALI) (Yang, Chang, et al. 2024).
5.3. Brain Diseases
The gut microbiome is involved in regulating oxidative stress in the CNS via the gut‐brain axis and affects brain neuron injury (Shandilya et al. 2022).
5.3.1. Microbiota Regulates Ferroptosis
Ischemic stroke (IS) is a cerebrovascular disease with a significant global health burden, and its pathogenesis is associated with ferroptosis (Tuo et al. 2022). Currently, drug reperfusion or surgery is the major therapeutic strategy (Widimsky et al. 2023), but ischemia–reperfusion may induce ferroptosis by increasing oxidative stress, thus aggravating brain tissue injury (Cui et al. 2021). A recent animal study reported that changes in specific gut microbe abundance in mice significantly reduce oxidative stress and inhibit ferroptosis and alleviate neuronal damage (Wang, Zhang, et al. 2023). Moreover, restoring normal gut microbiota composition with fecal microbiota transplantation (FMT) effectively reduces LPO and iron overload levels, inhibiting ferroptosis and improving IS prognosis outcomes (Wei, Wang, et al. 2024). Parkinson's disease (PD) is mainly caused by α‐syn accumulation and DA neuron damage in the substantia nigra (SN) (Tansey et al. 2022). The α‐syn originates from the gut and enters the brain via the vagus nerve of the gut‐brain axis, where it is aggregated by activated microglia (Fang et al. 2024; Ebedes and Borlongan 2024). Notably, α‐syn is also a key factor inducing ferroptosis in neuronal cells (Dong‐Chen et al. 2023). Ma et al. (2024) found that the gut microbiota in rotenone‐treated mice were changed, manifested as enrichment of Proteobacteria, which raised the circulatory levels of pro‐inflammatory cytokines LPS and C‐X‐C motif chemokine ligand‐1 (CXCL1). Increased CXCL1 effectively induces microglial activation, α‐syn aggregation, iron accumulation, and ferroptosis in SN, thus causing neuroinflammation and dopaminergic neurodegeneration. Furthermore, they also demonstrated that supplementing linoleic acid to activate PPAR alleviated PD symptoms in CXCL1 mice. This indicates that modulation of gut microbiota reduces brain inflammation, oxidative stress, and neuronal ferroptosis in PD models (Ma et al. 2024).
5.3.2. Microbial Metabolites Regulate Ferroptosis
The β‐amyloid (Aβ) deposition is a widely recognized pathological mechanism in Alzheimer's disease (AD) (Blennow et al. 2006). Evidence now suggests that GSH and GPX4 depletion, represented by ferroptosis, is closely related to the Aβ burden (Wang et al. 2022; Fan et al. 2024). Also, microglia M1 polarization caused by increased iron levels intensified brain neuroinflammation in AD patients (Alrouji et al. 2024). A recent study reported that the probiotic Bacteroides ovatus metabolite lysophosphatidylcholine inhibits ferroptosis by reducing intracellular ROS and LPO through the GPR119‐NRF2‐ACSL4 axis, thus reducing brain Aβ deposition and alleviating AD symptoms in mice (Zha et al. 2025).
5.4. Oral Diseases
The oral microbiome, the second largest microbiome in the body, directly regulates ferroptosis in oral diseases (Sedghi et al. 2021). Periodontitis is characterized by oral microbiome dysbiosis and immune inflammatory responses, which are caused by excessive P. gingivalis and F. nucleatum colonization (Teles et al. 2022). P. gingivalis releases inflammatory cytokines and triggers host immune inflammatory responses, thus promoting periodontitis and its pathological characteristics (Baker et al. 2024). Ferroptosis may be another contributing factor to periodontitis (Fu et al. 2023). An enhanced ferroptosis pathway was observed in patients with periodontitis and was possibly related to increased butyrate and decreased GSH and GPX4 levels (Ding et al. 2024). For example, F. nucleatum has been reported to induce ferroptosis in PDLSCs by regulating cellular iron metabolism, mitochondrial function, and GPX4 expression, thereby activating early‐stage inflammatory responses in hosts (Wang, Wang, et al. 2023). Unlike the anti‐inflammatory effects of butyrate at the gut level (Kang et al. 2023), butyrate secreted by periodontal pathogens disrupts iron homeostasis by promoting NCOA4‐mediated ferritinophagy, resulting in excessive ROS production and depleted GSH levels, which trigger ferroptosis in periodontal ligament fibroblasts and accelerate periodontitis (Zhao et al. 2020).
5.5. Bone and Joint Diseases
5.5.1. Microbiota Regulates Ferroptosis
The gut‐bone axis allows the gut microbiome to influence bone diseases. Furthermore, the microbiome‐immunity axis has important roles in rheumatoid arthritis (RA) (Wang and Xu 2019). RA is an autoimmune disease that is activated by danger‐associated molecular patterns (DAMPs) in the innate immune system (Yasuda et al. 2024). P2X7 receptor (P2X7R) is a receptor on immune cells, inducing cellular inflammation and immunity activation (Di Virgilio et al. 2017). Notably, ferroptosis may alter the distribution and abundance of the gut microbiome and increase cell immunogenicity by activating DAMPs to activate P2X7R in a mouse model of RA (Ma et al. 2023). Equally, gut microbiome dysbiosis may cause immune dysfunction, which worsens joint destruction and inflammation and promotes RA progression (Ma et al. 2023).
5.5.2. Microbial Metabolites Regulate Ferroptosis
Indoxyl sulfate is a gut toxin produced by tryptophan metabolism in the gut bacteria and induces IEC damage by promoting ROS production and interfering with mitophagy (Huang et al. 2020). The latest study reported that indoxyl sulfate activates AHR to inhibit osteoblast differentiation and reduce SLC7A11/GPX4 expression, which induces ferroptosis and aggravates alveolar bone loss (Chen et al. 2025). Solute carrier family 2 member 1 (SLC2A1), also known as GLUT1, is explicitly involved in ferroptosis, and its responses to hypoxia‐induced oxidative stress are mediated by HIF‐1α. Guan et al. (2023) observed that CAT shows good alleviating effects toward osteoarthritis; such remission effects are achieved by restraining ferroptosis, which is related to increased SLC2A1 expression, and inhibiting HIF‐1α signaling and oxidative stress levels. Additionally, butyrate (HDAC inhibitor) inhibits SLC7A11 transcription by upregulating ATF3 expression and promoting ferroptosis in osteosarcoma cells, thus alleviating osteosarcoma (Nie et al. 2023).
5.6. Other Diseases
In addition to the aforementioned microbiome sites, microbiome and ferroptosis crosstalk has been reported elsewhere in the body. For example, the microbial metabolite Urolithin A inhibited LPS‐induced acute lung injury and ferroptosis by upregulating the Keap1/NRF2/HO‐1 pathway (Lou et al. 2023). LPS induces ferroptosis‐mediated esophageal mucosal injury by increasing the expression of the special protein 1/ACSL4 and the accumulation of iron (Liu, Tang, et al. 2022). Probiotic Prevotella histicola reduces ferroptosis by inhibiting ACSL4 and activating the Xc−/GPX4 axis, thereby attenuating ethanol‐induced gastric mucosal lesions in mice (Peng, Lei, et al. 2024). Gut bacteria metabolite 3‐hydroxyphenylacetic acid inhibits ferroptosis and restores spermatogenesis in aged mice testicles (Teles et al. 2022). High‐fat diet‐induced gut microbiome dysbiosis increased circulating LPS level in mice, which promotes ferroptosis by decreasing GPX4 expression, thereby leading to testicular inflammation (Fu et al. 2023) and atrial inflammation (Ding et al. 2024). Bacteroidaceae mediates iron overload and lipid peroxidation to induce ferroptosis, thus promoting systemic inflammatory responses and benzene‐induced hematopoietic toxicity. Oral probiotics can reverse elevated Bacteroidaceae and ferroptosis, ultimately alleviating hematopoietic damage (Kang et al. 2023). These mechanisms are summarized (Table 1). However, crosstalk at these sites remains understudied. More evidence is required to explain these interactions.
TABLE 1.
Microbiome influences on ferroptosis in disease.
| Organs | Diseases | Microbiotas and metabolites | Relevant mechanisms | Result of ferroptosis | Progression of diseases | References |
|---|---|---|---|---|---|---|
| Gut | Intestinal injury | Lactobacillus and Ileibacterium | The m6A level of SLC7A11 mRNA is upregulated, which promotes YTHDF2 to bind to it and drive SLC7A11 mRNA degradation | Promote | Promote intestinal epithelial cell injury | (Huang, Lin, et al. 2024) |
| Intestinal IRI | Capsiate | Activation of TRPV1 promotes GPX4 expression | Suppress | Suppress intestinal IRI | (Deng et al. 2021) | |
| IBD | Plasmalogens (anaerobes) | Regulates the expression of ferroptosis‐related proteins such as GPX4 and COX2 | Suppress | Suppress colitis | (Liu et al. 2023) | |
| Butyrate | Upregulate the NRF2/GPX4 pathway | Suppress | Suppress colitis | (Chen, Qian, et al. 2024) | ||
| Deoxycholic acid | Upregulate the HIF‐2α/DMT1 pathway | Promote | Strengthening of colitis | (Wang, Chu, Dong, et al. 2024) | ||
| AIEC | Exacerbate lipid peroxidation by reducing GPX4 and FTH levels | Promote | Promote intestinal epithelial cell death | (Wen et al. 2023) | ||
| CRC | IDA ( P. anaerobius ) | Up‐regulate the AHR‐ALDH1A3‐FSP1 axis | Suppress | Promote tumorigenesis | (Cui et al. 2024) | |
| Butyrate | Downregulated GPX4 expression | Promote | Suppress tumor formation | (Chapkin et al. 2020) | ||
| F. nucleatum | Up‐regulate the E‐cadherin/β‐catenin/TCF4/GPX4 axis | Suppress | Promote drug resistance | (Li, Wei, et al. 2024) | ||
| Liver | Liver IRI | Gamma‐aminobutyric acid | Reduces ACSL4, TFR1 and increases anti‐ferroptotic FTH1 levels | Suppress | Suppress liver IRI | (Wang, Liu, Huang, et al. 2024) |
| Oleanolic acid | Upregulate the KEAP1/NRF2 pathway | Suppress | Alleviate liver IRI | (Pi et al. 2024) | ||
| Acute liver injury | Butyrate (Lachnospiracease) | Activates the AMPK‐ULK1‐p62 pathway and mitophagy | Suppress | Alleviate acute liver injury | (Yang, Chang, et al. 2024) | |
| MAFLD | Urolithin C | Activates the AMPK pathway | Suppress | Suppress liver injury | (Xu et al. 2023) | |
| Chenodeoxycholic acid | Induces lipid peroxidation | Promote | Promote liver fibrosis | (Zhuge et al. 2023) | ||
| Glycochenodeoxycholate | Activates TFR‐ACSL4 | Promote | Promote hepatic steatosis and inflammation | (Liu, Gao, et al. 2022) | ||
| Porphyromonas gingivalis | Th17/Treg imbalance | Promote | Induce the occurrence of NAFLD | (Yao et al. 2023) | ||
| ALD | Porphyromonas gingivalis | Increased the expression of ACSL4 and decreased the expression of GPX4 and SLC7A11 | Promote | Aggravate ALD | (Yao et al. 2024) | |
| ICC | Glutamine | Inhibits the ALK5/NOX1 axis | Suppress | Promote tumor growth | (Zhang, Zhou, Zhai, et al. 2024) | |
| HCC | EPS (Enterobacter faecium) | EPS promotes the secretion of IFN‐γ by CD8T cells, thereby down‐regulating SLC7A11 | Promote | Enhance the efficacy of treatment | (Yu, Lin, et al. 2024) | |
| Brain | IS | Bacteroidaceae Enterobacteriaceae | Reduce oxidative stress | Suppress | Suppress cerebral IRI | (Wang, Zhang, et al. 2023) |
| Restoring normal gut microbiota composition | Reduce the levels of lipid peroxidation and iron overload | Suppress | Ameliorate IS | (Wei, Wang, et al. 2024) | ||
| PD | Enterobacterium (Proteobacteria) | Elevated CXCL1 causes PPAR pathway inhibition, promoting microglia activation, α‐syn aggregation, and iron accumulation | Promote | Promote dopaminergic neurodegeneration in PD | (Ma et al. 2024) | |
| AD | Lysophosphatidylcholine | Reduces ROS and lipid peroxidation through the GPR119‐NRF2‐ACSL4 axis | Suppress | Ameliorate cognitive impairment | (Zha et al. 2025) | |
| Oral | Periodontitis | Butyrate (Periodontal pathogens) | Promotes NCOA4‐mediated ferritinophagy, causing excessive ROS and depletion of GSH | Promote | Promote periodontitis development | (Zhao et al. 2020) |
| Bone | Alveolar bone loss | Indoxyl sulfate | Activates AHR to inhibit osteoblast differentiation and reduce SLC7A11/GPX4 expression | Promote | Promote alveolar bone deterioration | (Chen et al. 2025) |
| Osteoarthritis | Capsiate | Inhibition of HIF‐1α and activation of SLC2A1 | Suppress | Improve osteoarthritis | (Guan et al. 2023) | |
| Osteosarcoma | Butyrate | Inhibits SLC7A11 expression through ATF3 | Promote | Suppress tumor growth | (Nie et al. 2023) | |
| Lung | Lung cancer | Butyrate (Gut microbiota) | Inhibits SLC7A11 expression through ATF3 | Promote | Suppress cancer cell survival | (Bi et al. 2024) |
| Acute lung injury | Urolithin A | Activates the Keap1‐NRF2/HO‐1 signaling pathway | Suppress | Suppress acute lung injury | (Lou et al. 2023) | |
| Esophagus | RE | LPS (Gram‐negative bacteria) | Promotes ACSL4 by elevating the expression of Sp1 through the Caspase11/GSDMD pathway | Promote | Promote esophageal mucosal damage | (Liu, Tang, et al. 2022) |
| Stomach | GC | Helicobacter pylori with CagA | Activates the MEK/ERK/SRF pathway | Promote | Increase drug sensitivity | (Peng, Lei, et al. 2024) |
| Reduced NOX4 and ACSL4 expression, upregulated GPX4 expression | Suppress | Promote tumor growth | (Lu et al. 2024) | |||
| Testicle | Oligospermia | 3‐HPAA | Reduce oxidative stress and elevate the expression of GPX4 | Suppress | Alleviated spermatogenic dysfunction | (Jin et al. 2023) |
| Testicular injury | LPS | The expression of NRF2 was downregulated | Promote | Induce testicular inflammation | (Zhang, Chen, and Li 2024) | |
| Heart | AF | LPS (Desulfovibrionaceae) | Activates ferroptosis and the TLR4/NF‐κB/NLRP3 pathway | Promote | Increase susceptibility to AF | (Kong et al. 2022) |
| Blood system | AML | Propionate | Caused excessive ROS to induce mitochondrial damage and fission and mitophagy; increased ACSL4 | Promote | Increase the sensitivity to immunotherapy | (Wei, Liu, et al. 2024) |
| Hematopoietic toxicity | Bacteroidaceae | Mediated systemic inflammatory responses, iron overload, and lipid peroxidation | Promote | Increase hematopoietic toxicity | (Zhang, Kang, et al. 2023) |
Abbreviations: 3‐HPAA, 3‐hydroxyphenylacetic acid; ACSL4, acyl‐CoA synthetase long‐chain family member 4; AF, atrial fibrillation; AHR, aryl hydrocarbon receptor; AIEC, adherent‐invasive E. coli ; ALD, alcoholic liver disease; ALDH1A3, aldehyde dehydrogenase 1 family member A3; ALK5, activin receptor‐like kinase 5; AML, acute myeloid leukemia; ARA‐PEs, phosphatidylethanolamine‐anchored arachidonic acids; ATF3, transcription factor 3; COX2, cyclooxygenase 2; CRC, colorectal cancer; CXCL1, C‐X‐C motif chemokine ligand‐1; CYP2E1, cytochrome P450‐2E1; DMT1, divalent metal transporter 1; EPS, exopolysaccharides; FSP1, ferroptosis suppressor protein 1; FTH, ferritin heavy chain; GC, gastric cancer; GPX4, glutathione peroxidase 4; GSH, glutathione; HCC, hepatocellular carcinoma; HIF‐2α, hypoxia‐inducible factor‐2α; IBD, inflammatory bowel disease; ICC, intrahepatic cholangiocarcinoma; IDA, trans‐3‐indoleacrylic acid; IRI, ischemia reperfusion injury; IS, ischemic stroke; KEAP1, Kelch‐associated protein 1; LPS, lipopolysaccharide; LPS, lipopolysaccharide; m6A, N6‐methyladenosine; MAFLD, metabolic dysfunction‐associated fatty liver disease; NASH, nonalcoholic steatohepatitis; NCOA4, nuclear receptor co‐activator 4; NOX1, NADPH oxidases 1; NRF2, nuclear factor erythroid 2‐related factor 2; PD, Parkinson's disease; PPAR, peroxisome proliferator‐activated receptor; PUFA, polyunsaturated fatty acid; RE, reflux esophagitis; ROS, reactive oxygen; SLC7A11, solute carrier family 7 member 11; Sp1, special protein 1; SREBP1, sterol‐regulatory element binding protein 1; TCF4, transcription factor; TFR, transferrin receptor; TLR4, toll‐like receptor‐4; TRPV1, transient receptor potential cation channel subfamily V member 1; YTHDF2, YTH domain‐containing family protein 2.
6. Disease Treatments
In recent years, considering the critical roles of the microbiome and ferroptosis in disease progression, therapeutic strategies targeting their crosstalk have become increasingly common in the disease treatment field. Therefore, we conclude this review with current and major prevention and treatment interventions targeting this crosstalk (Table 2).
TABLE 2.
Therapeutic interventions targeting crosstalk between the microbiome and ferroptosis in disease.
| Type | Names | Model | Related targets | Mechanism action | Diseases | References |
|---|---|---|---|---|---|---|
| FMT | Fecal bacterial suspension | Rats with MCAO | Restores normal gut microbiota composition | Reduces cerebral infarction volume and ferroptosis after stroke | IS | (Wei, Wang, et al. 2024) |
| OFG or OPG | APAP‐induced liver injury in mice | ↑ Lachnospiraceae and butyrate | Changes the gut microbiome composition | ALI | (Yang, Chang, et al. 2024) | |
| Fecal microbiota gavage | Mice and GC‐2 spd cells | ↑ 3‐hydroxyphenylacetic acid | Upregulates GPX4 to decrease oxidative stress | Oligospermia | (Jin et al. 2023) | |
| Probiotics | L. lactis MG1363‐pMG36e‐GLP‐1 | MTPT‐induced PD mice | ↑ Akkermansia, Oscillospira, and Sutterella | Remodels gut microbiome homeostasis and decreases oxidative stress | PD | (Yue et al. 2022) |
| LGG | DSS‐ or WAI‐induced colitis mice |
↑ Lactobacillus, Blautia, and Akkermansia ↓ pathogenic E. coli and Bacteroides |
Produces SCFAs and increases GPX4 to clear ROS | Colitis | (Sun et al. 2024) | |
| Bifico | Benzene‐induced hematopoietic toxicity in mice | ↓ Bacteroidaceae | Inhibits gut ferroptosis and Th2‐type systemic inflammation | Hematopoietic toxicity | (Zhang, Kang, et al. 2023) | |
| Bifidobacterium bifidum BGN4 | PA‐induced‐MAFLD LO2 cells | ↓ CYP2E1 | Decreases oxidative stress | MAFLD | (Bu et al. 2024) | |
| Clostridium butyricum | KPC1199 cells mice | ↑ Butyrate | Maintains gut homeostasis | PDAC | (Yang, Zhang, et al. 2023) | |
| P. histicola | Ethanol‐induced EGML mice | Consolidate the gastric microbiota | Inhibits ACSL4 and activates the Xc−/GPX4 axis | EGML | (Wang, Wu, et al. 2023) | |
| Prebiotics | TA 2–1 | DSS‐induced UC mice | ↑ Akkermansia, Adlercreutzia, and Lactobacillus | Increased amino acid levels to promote Xc−‐GSH‐GPX4 defense | UC | (Peng, Wang, et al. 2024) |
| L‐Fucose | OTA‐induced ovarian damage in mice | ↓ Sutterella | Counteracts microbial dysbiosis and inflammatory/ROS response | Ovarian damage | (Wang, Song, Cai, et al. 2024) | |
| EPS | Hepa1‐6 cell mice | ↑ Enterobacter faecium | Promotes the secretion of IFN‐γ+ by CD8+ T cells to enhance sorafenib activity, increasing ROS | HCC | (Yu, Lin, et al. 2024) | |
| TCM | JFG | CFA‐induced RA rat | ↑ α diversity and SCFAs | Activates AMPK to inhibit lipid oxidative stress | RA | (Wang, Pan, et al. 2025) |
| Berberine | Cerebral ischemia–reperfusion injury mice |
↑ Bacteroidaceae and Enterobacteriaceae ↓ Muribaculaceae, Erysipelotrichaceae, Helicobacteraceae, Streptococcaceae, and Tannerellaceae |
Produces SCFAs and increases GPX1 to decrease lipid peroxidation and oxidative stress | IS | (Wang, Zhang, et al. 2023) | |
| SHTB | MCAO/R rats |
↑ Lactobacillus ↓ Escherichia‐Shigella |
Increase SCFAs to induce PPARγ and reduce lipid oxidative stress | IS | (Wei et al. 2025) | |
| SDG | TMAO‐induced NRK‐52E cells and adenine‐induced CKD rats | ↓ Muribaculaceae, Bacteroides, and Ruminococcaceae_UCG‐010 | Decreases TMAO to reduce ROS | CKD | (Ge et al. 2024) | |
| Nobiletin | CLP‐induced SALI mice |
↑ Ligilactobacillus, Akkermansia, and Lactobacillus ↓ Dubosiella and Bacteroides |
Increase NRF2‐GPX4 axis | SALI | (Huang et al. 2023) |
Abbreviations: APAP, acetaminophen; Bifico, Bifidobacterium longum , Lactobacillus acidophilus , and Enterococcus faecalis ; CFA, complete Freud's adjuvant; CKD, chronic kidney disease; CLP, cecal ligation and puncture; DSS, dextran sodium sulfate; EGML, ethanol‐induced gastric mucosal lesions; FMT, fecal microbiota transplant; JFG, Jingfang Granule; L. lactis , Lactococcus lactis ; LGG, Lactobacillus rhamnosus GG; MCAO, middle cerebral artery occlusion; MCAO/R, middle cerebral artery occlusion/reperfusion; MPTP, 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine; OFG, oral fecal gavage; OPG, pasteurized fecal gavage; P. histicola , Prevotella histicola ; PA, palmitic acid; PDAC, pancreatic ductal adenocarcinoma; SALI, sepsis‐associated acute liver injury; SDG, suyin detoxification granule; SHTB, Shouhui Tongbian capsules; TCM, traditional Chinese medicine; TMAO, trimethylamine N‐oxide; WAI, whole‐abdominal irradiation.
6.1. Fecal Microbiota Transplantation
FMT is a promising treatment for disorders associated with gut microbiome dysbiosis, as it restores healthy gut microbiome diversity by transferring feces from healthy donors to recipients (Porcari et al. 2023). The evidence now shows that FMT generates satisfactory results in treating several diseases, including recurrent Clostridioides difficile infections (Kao et al. 2017), IBD (Lopetuso et al. 2023), and cancers (Yu, Feng, et al. 2024; Wang, Fang, et al. 2025). FMT improves intestinal inflammatory status, reduces oxidative stress, and restores intestinal barrier function by increasing beneficial microbes and reducing potentially pathogenic bacteria (Zu et al. 2024), which in turn reduces ferroptosis risks. For example, FMT reversed gut microbiota dysbiosis, reduced ferroptosis after IS, and relieved cerebral infarction symptoms (Wei, Wang, et al. 2024). However, limitations can occur in terms of patient acceptability and adherence to FMT, thus restraining its application to clinical practice. However, oral fecal gavage (OFG) or pasteurized fecal gavage (OPG) are more acceptable approaches for patients, while the efficacy is comparable to FMT administration by colonoscopy (Kao et al. 2017; Vaughn et al. 2023). The study authors reported that OFG or OPG improved ALI prognosis outcomes by altering gut microbiome structures and inhibiting ferroptosis (Yang, Chang, et al. 2024). Additionally, fecal microbiota component (mainly bacteria or the virome) transplantation, accompanied by higher patient acceptability when compared with crude FMT, may represent improved trends for the next generation of FMT (Yu, Wang, and Zhang 2023).
6.2. Probiotics and Prebiotics
Probiotics are living microbes that, when administered in sufficient quantities, provide health benefits to hosts (Fentie et al. 2024). Increasing evidence now shows that short‐term probiotic gut colonization alleviates gut microbiome dysbiosis and inhibits intracellular iron overload and LPO by improving intestinal barrier function, SCFA production, and reducing intestinal ROS levels to slow disease progression (Cao et al. 2023; Verstraeten et al. 2024). Lactobacillus and Bifidobacterium are the most common probiotics. Lactobacillus produces GSH and many different antioxidant enzymes (e.g., SOD) to reduce oxidative stress (Liu, Yue, et al. 2024). For instance, L. lactis MG1363‐PMGP36E‐GLP‐1 exerted neurotrophic effects by activating the Keap1/NRF2/GPX4 pathway to inhibit ferroptosis (Yue et al. 2022). Additionally, Lactobacillus rhamnosus GG improves colitis in mice by synthesizing SOD and SCFAs to inhibit ferroptosis (Sun et al. 2024). Moreover, Bifico (which contains Bifidobacterium longum , Lactobacillus acidophilus , and Enterococcus faecalis ) alleviates benzene‐induced hematopoietic toxicity by inhibiting Bacteroidaceae‐mediated ferroptosis (Zhang, Kang, et al. 2023).
Prebiotics are organic food substances that are not digested, are absorbed by hosts, and selectively support beneficial microbe growth and metabolism (e.g., Bifidobacterium, Lactobacillus, and Fecalibacterium) to improve host health (Sanders et al. 2019). Since the main role of prebiotics is to play a permissibility role on beneficial microbiomes in the gut, their efficacy against disease is inextricably linked to probiotic function (Quigley 2019). Studies have shown that TA 2–1 (Peng, Wang, et al. 2024) and L‐fucose (Wang, Song, Cai, et al. 2024) enhance intestinal barrier integrity by increasing intestinal probiotic proliferation, supporting IEC development, inhibiting ferroptosis in cells, and relieving UC and ovarian damage. In contrast, E. faecium exopolysaccharides synergistically induce ferroptosis in HCC cells with sorafenib by promoting IFN‐γ+ secretion from CD8+ T cells and reducing SLC7A11 levels (Yu, Lin, et al. 2024). Such is related to the anti‐resistance properties of ferroptosis in tumor treatment.
6.3. Traditional Chinese Medicine (TCM)
There is growing evidence that TCM effectively influences disease progression via the microbiome to regulate ferroptosis. Accordingly, Jingfang granules improve fatty acid metabolism disorders by regulating the gut microbiome and SCFA production to activate AMPK, inhibiting ferroptosis caused by lipid oxidative stress, and attenuating RA (Wang, Pan, et al. 2025). Berberine (Wang, Zhang, et al. 2023) and Shouhui Tongbian capsules (Wei et al. 2025) inhibit LPO and oxidative stress by increasing SCFA levels and preventing ferroptosis, thus improving IS symptoms. Suyin detoxification granules reduce ROS levels via the gut microbiome to reduce trimethylamine N‐oxide levels, thus suppressing renal tubular ferroptosis and preventing chronic kidney disease progression (Ge et al. 2024). Furthermore, nobiletin decayed ferroptosis via the gut microbiome to activate the NRF2‐GPX4 pathway and then mitigate sepsis‐associated ALI (Huang et al. 2023). This evidence underscores TCM as a promising treatment option for different diseases.
7. Conclusions and Prospects
Ferroptosis represents an imbalance in intracellular redox reactions and is a form of programmed cell death resulting from iron‐dependent LPO due to a ruptured cytoplasmic membrane. Although ferroptosis usually heralds tissue damage and necroinflammation, it has received considerable research attention in precision tumor therapy and as a novel strategy for combating therapeutic resistance. It is worth noting that there are physiological thresholds for the iron ratio, lipid oxidation, and total ROS levels in healthy cells. Once these thresholds are exceeded, the cells become more sensitive to ferroptosis. Using ferroptosis detection methods, such as the FerroOrange fluorescent probe, ferrozine, Prussian blue staining, the DCFH‐DA fluorescent probe, and C11‐BODIPY, it can be observed that when iron death occurs, the iron ratio in cells increases, and the fluorescence intensity of lipid oxidation and total ROS levels significantly enhances. Additionally, since ferroptosis shares similarities with other forms of cell death, combined detection of multiple markers (e.g., Fe2++lipid peroxidation+mitochondrial morphological changes) can effectively help researchers confidently determine that ferroptosis is the primary contributor to cell death rather than other forms of cell death (Zeng et al. 2023).
It is now accepted that microbiome dysbiosis facilitates disease onset and progression, which is related to the regulation of the host's physiological processes, such as metabolism, redox reactions, and the immune system. Iron is an essential micronutrient for normal host physiological and microbiome metabolism. The cooperation and competition between the host and the microbiome for iron uptake have provided a theoretical basis for the microbiome‐mediated regulation of iron‐related diseases (Noordine et al. 2024; Das et al. 2020). Indeed, increasing evidence now suggests that toxic pathogen metabolites can induce ferroptosis and influence disease progression, whereas probiotics secrete beneficial metabolites that inhibit ferroptosis and alleviate disease symptoms. However, this evidence is not enough to fully elucidate the complex relationships between the microbiome and ferroptosis in disease. Most studies have posited that ferroptosis is regulated by the microbiome, but notably, the microbiome and ferroptosis may be in a mutual regulatory relationship. Some evidence now suggests that ferroptosis may, in turn, influence disease onset and progression by modulating the ecological balance of the gut microbiome. For example, triggering ferroptosis with a ferroptosis inducer (erastin) exacerbates gut microbiome dysbiosis in collagen‐induced arthritis mice (Ma et al. 2023; Zhu et al. 2025). Nevertheless, this is insufficient for a comprehensive and detailed discussion of the specific regulatory relationships between the microbiome and ferroptosis. More research is required to elucidate the sequential and causal relationships between microbiome dysbiosis and ferroptosis in disease.
Therapies targeting the crosstalk between microbiome and ferroptosis hold significant clinical value. Specifically, FMT, probiotics, prebiotics, and TCM have shown great potential in disease management by influencing ferroptosis primarily by modulating microbiome composition. Compared with probiotics and prebiotics, which only colonize the gut temporarily, FMT offers considerable therapeutic advantages due to its sustained effects. Furthermore, unlike antibiotics that disrupt gut microbiome balance and potentially increase drug resistance, FMT gently restores microbial homeostasis. However, poor accessibility and patient acceptability remain major limitations of FMT. Additionally, the crosstalk between the gut microbiome and ferroptosis may also provide a potential target for glucose‐lowering drugs to fulfill their therapeutic potential. For instance, metformin alleviates hepatic IRI symptoms by inhibiting ferroptosis through gut microbiome remodeling (Wang, Liu, Huang, et al. 2024). Furthermore, drugs such as ferrostatin‐1 (Wang, Li, et al. 2023), deferasirox (Wu et al. 2023), and L‐citrulline (Zhao et al. 2024) can reshape gut microbiome homeostasis while inhibiting ferroptosis, thus mitigating disease progression. However, the exact relationship between ferroptosis inhibition and reshaping microbiome homeostasis has not yet been elucidated. This may be related to the fact that ferroptosis has not been shown to definitively regulate the microbiome.
In conclusion, crosstalk between the microbiome and ferroptosis across different diseases is highly complex. When compared with its regulation of gut diseases, gut microbiome influences on liver, brain, oral, bone, and other parenteral diseases depend on gut‐organ axes. The oral microbiome influences disease progression by regulating ferroptosis. This novel finding will undoubtedly deepen our understanding of the crosstalk between the microbiome and ferroptosis. Future studies should explore this crosstalk at other body sites. Notably, human cells are more vulnerable to iron overload compared to rodent cells, which is related to their lower antioxidant defense efficiency, higher irreversible damage, and weaker response to repair pathways (Zheng et al. 2018; Ma et al. 2021; Du et al. 2025). Therefore, a cautious attitude still needs to be maintained regarding the extrapolation of translational research on mouse data. To fully exploit the therapeutic potential of the microbiome and ferroptosis, more comprehensive basic, preclinical, and clinical studies are necessary. Continued foundational and clinical research in this field may facilitate the translation from mechanistic understanding to clinical therapies in the future, though a cautiously optimistic approach remains essential at present.
Author Contributions
D.‐Q.D. conceived the review. C.‐D.Z. provided guidance on the writing of the manuscript. Y.‐S.L. provided guidance on drawing figures. J.‐K.Z. provided guidance on the revision and correction of the manuscript. S.‐Q.D. was a major contributor to writing the manuscript. Y.‐L. organized the tables and was responsible for some of the writing. Z.‐M.Z. was responsible for the creation and modification of the figures. X.‐Y.L. collected the literature. J.‐X.L. collated the literature. All authors contributed to the discussion and revision and the final version.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We sincerely appreciate the potential editors and reviewers for their succinct comments on improving this manuscript. We also thank BioRender software (https://biorender.com) for creating figures. Declaration of generative AI and AI‐assisted technologies in the writing process: This work was prepared without the help of AI.
Ding, S.‐Q. , Lei Y., Zhao Z.‐M., et al. 2025. “Crosstalk Between Microbiome and Ferroptosis in Diseases: From Mechanism to Therapy.” Comprehensive Physiology 15, no. 4: e70042. 10.1002/cph4.70042.
Funding: This work was supported by grants from the National Natural Science Foundation of China (81972322); the Scientific Study Project for Institutes of Higher Learning, Ministry of Education, Liaoning Province (JYTMS20230108); the Young Backbone Talents of China Medical University (RXXM202302); and the Liaoning Provincial Natural Science Foundation (2023‐MS‐163).
Si‐Qi Ding, Yun Lei, and Zhe‐Ming Zhao contributed equally to this work and shared the first authorship.
Jia‐Kui Zhang, Yong‐Shuang Li, Chun‐Dong Zhang, and Dong‐Qiu Dai contributed equally to this work and shared the last authorship.
Contributor Information
Jia‐Kui Zhang, Email: 20071283@cmu.edu.cn.
Yong‐Shuang Li, Email: liyongshuang@cmu.edu.cn.
Chun‐Dong Zhang, Email: cdzhang@cmu.edu.cn.
Dong‐Qiu Dai, Email: dqdai@cmu.edu.cn.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- Abed, J. , Maalouf N., Manson A. L., et al. 2020. “Colon Cancer‐Associated Fusobacterium nucleatum May Originate From the Oral Cavity and Reach Colon Tumors via the Circulatory System.” Frontiers in Cellular and Infection Microbiology 10: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal, N. , Kitano S., Puah G. R. Y., Kittelmann S., Hwang I. Y., and Chang M. W.. 2023. “Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies.” Chemical Reviews 123, no. 1: 31–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlqvist, K. J. , Leoncini S., Pecorelli A., et al. 2015. “MtDNA Mutagenesis Impairs Elimination of Mitochondria During Erythroid Maturation Leading to Enhanced Erythrocyte Destruction.” Nature Communications 6: 6494. [DOI] [PubMed] [Google Scholar]
- Ahuja, S. , and Lazar I. M.. 2021. “Systems‐Level Proteomics Evaluation of Microglia Response to Tumor‐Supportive Anti‐Inflammatory Cytokines.” Frontiers in Immunology 12: 646043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque‐Souza, E. , and Sahingur S. E.. 2022. “Periodontitis, Chronic Liver Diseases, and the Emerging Oral‐Gut‐Liver Axis.” Periodontology 2000 89, no. 1: 125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrouji, M. , Anwar S., Venkatesan K., et al. 2024. “Iron Homeostasis and Neurodegeneration in the Ageing Brain: Insight Into Ferroptosis Pathways.” Ageing Research Reviews 102: 102575. [DOI] [PubMed] [Google Scholar]
- Alves, F. , Lane D., Nguyen T. P. M., Bush A. I., and Ayton S.. 2025. “In Defence of Ferroptosis.” Signal Transduction and Targeted Therapy 10, no. 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambat, A. , Antony L., Maji A., et al. 2024. “Enhancing Recovery From Gut Microbiome Dysbiosis and Alleviating DSS‐Induced Colitis in Mice With a Consortium of Rare Short‐Chain Fatty Acid‐Producing Bacteria.” Gut Microbes 16, no. 1: 2382324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandhan, A. , Dodson M., Shakya A., et al. 2023. “NRF2 Controls Iron Homeostasis and Ferroptosis Through HERC2 and VAMP8.” Science Advances 9, no. 5: eade9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron‐Wisnewsky, J. , Warmbrunn M. V., Nieuwdorp M., and Clément K.. 2020. “Nonalcoholic Fatty Liver Disease: Modulating Gut Microbiota to Improve Severity?” Gastroenterology 158, no. 7: 1881–1898. [DOI] [PubMed] [Google Scholar]
- Badgley, M. A. , Kremer D. M., Maurer H. C., et al. 2020. “Cysteine Depletion Induces Pancreatic Tumor Ferroptosis in Mice.” Science 368, no. 6486: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, J. L. , Mark Welch J. L., Kauffman K. M., McLean J. S., and He X.. 2024. “The Oral Microbiome: Diversity, Biogeography and Human Health.” Nature Reviews. Microbiology 22, no. 2: 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, H. , Wang Y., Xiong H., Xia Y., Cui Z., and Liu L.. 2024. “Mechanism of Iron Ion Homeostasis in Intestinal Immunity and Gut Microbiota Remodeling.” International Journal of Molecular Sciences 25, no. 2: 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero, F. , and Nombela C.. 2012. “The Microbiome as a Human Organ.” Clinical Microbiology and Infection 18, no. Suppl 4: 2–4. [DOI] [PubMed] [Google Scholar]
- Berg, G. , Rybakova D., Fischer D., et al. 2020. “Microbiome Definition Re‐Visited: Old Concepts and New Challenges.” Microbiome 8, no. 1: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker, K. , Hendricks J. M., Li Z., et al. 2019. “The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis.” Nature 575, no. 7784: 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman, N. J. , Mathieu J. R. R., Renassia C., et al. 2020. “Dendritic Cell‐Derived Hepcidin Sequesters Iron From the Microbiota to Promote Mucosal Healing.” Science 368, no. 6487: 186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, R. , Hu R., Jiang L., et al. 2024. “Butyrate Enhances Erastin‐Induced Ferroptosis of Lung Cancer Cells via Modulating the ATF3/SLC7A11 Pathway.” Environmental Toxicology 39, no. 2: 529–538. [DOI] [PubMed] [Google Scholar]
- Blennow, K. , de Leon M. J., and Zetterberg H.. 2006. “Alzheimer's Disease.” Lancet 368, no. 9533: 387–403. [DOI] [PubMed] [Google Scholar]
- Bogdan, A. R. , Miyazawa M., Hashimoto K., and Tsuji Y.. 2016. “Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease.” Trends in Biochemical Sciences 41, no. 3: 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte, L. A. , Björk J. R., Gacesa R., and Weersma R. K.. 2025. “Pharmacomicrobiomics: The Role of the Gut Microbiome in Immunomodulation and Cancer Therapy.” Gastroenterology. 10.1053/j.gastro.2025.04.025. [DOI] [PubMed] [Google Scholar]
- Bonkhoff, A. K. , Ullberg T., Bretzner M., et al. 2022. “Deep Profiling of Multiple Ischemic Lesions in a Large, Multi‐Center Cohort: Frequency, Spatial Distribution, and Associations to Clinical Characteristics.” Frontiers in Neuroscience 16: 994458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch, M. E. , Dodiya H. B., Michalkiewicz J., et al. 2024. “Sodium Oligomannate Alters Gut Microbiota, Reduces Cerebral Amyloidosis and Reactive Microglia in a Sex‐Specific Manner.” Molecular Neurodegeneration 19, no. 1: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursier, J. , Mueller O., Barret M., et al. 2016. “The Severity of Nonalcoholic Fatty Liver Disease Is Associated With Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota.” Hepatology 63, no. 3: 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius‐Flohé, R. , and Maiorino M.. 2013. “Glutathione Peroxidases.” Biochimica et Biophysica Acta 1830, no. 5: 3289–3303. [DOI] [PubMed] [Google Scholar]
- Brown, G. C. , and Heneka M. T.. 2024. “The Endotoxin Hypothesis of Alzheimer's Disease.” Molecular Neurodegeneration 19, no. 1: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu, G. , Chen G., Li J., Wu D., and Liao J.. 2024. “ Bifidobacterium bifidum BGN4 Fractions Ameliorate Palmitic Acid‐Induced Hepatocyte Ferroptosis by Inhibiting SREBP1‐CYP2E1 Pathway.” Journal of Investigative Medicine 72, no. 1: 67–79. [DOI] [PubMed] [Google Scholar]
- Camarena, V. , Huff T. C., and Wang G.. 2021. “Epigenomic Regulation by Labile Iron.” Free Radical Biology & Medicine 170: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, F. , Jin L., Gao Y., et al. 2023. “Artificial‐Enzymes‐Armed Bifidobacterium longum Probiotics for Alleviating Intestinal Inflammation and Microbiota Dysbiosis.” Nature Nanotechnology 18, no. 6: 617–627. [DOI] [PubMed] [Google Scholar]
- Carasso, S. , Zaatry R., Hajjo H., et al. 2024. “Inflammation and Bacteriophages Affect DNA Inversion States and Functionality of the Gut Microbiota.” Cell Host & Microbe 32, no. 3: 322–334.e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapkin, R. S. , Navarro S. L., Hullar M. A. J., and Lampe J. W.. 2020. “Diet and Gut Microbes Act Coordinately to Enhance Programmed Cell Death and Reduce Colorectal Cancer Risk.” Digestive Diseases and Sciences 65, no. 3: 840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Peng L., Wang Z., He Y., and Zhang X.. 2024. “Exploring the Causal Relationship Between Periodontitis and Gut Microbiome: Unveiling the Oral‐Gut and Gut‐Oral Axes Through Bidirectional Mendelian Randomization.” Journal of Clinical Periodontology 51, no. 4: 417–430. [DOI] [PubMed] [Google Scholar]
- Chen, H. , Qian Y., Jiang C., et al. 2024. “Butyrate Ameliorated Ferroptosis in Ulcerative Colitis Through Modulating Nrf2/GPX4 Signal Pathway and Improving Intestinal Barrier.” Biochimica et Biophysica Acta—Molecular Basis of Disease 1870, no. 2: 166984. [DOI] [PubMed] [Google Scholar]
- Chen, H. , Zhou Y., Liu Y., et al. 2025. “Indoxyl Sulfate Exacerbates Alveolar Bone Loss in Chronic Kidney Disease Through Ferroptosis.” Oral Diseases 31, no. 1: 264–277. [DOI] [PubMed] [Google Scholar]
- Chen, T. P. , Yu H. C., Lin W. Y., and Chang Y. C.. 2023. “The Role of Microbiome in the Pathogenesis of Oral‐Gut‐Liver Axis Between Periodontitis and Nonalcoholic Fatty Liver Disease.” Journal of Dental Sciences 18, no. 3: 972–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Yu C., Kang R., and Tang D.. 2020. “Iron Metabolism in Ferroptosis.” Frontiers in Cell and Development Biology 8: 590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Han B., Guan X., et al. 2024. “Enteric Fungi Protect Against Intestinal Ischemia‐Reperfusion Injury via Inhibiting the SAA1‐GSDMD Pathway.” Journal of Advanced Research 61: 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Zhang J., Tian Y., et al. 2024. “Iron Accumulation in Ovarian Microenvironment Damages the Local Redox Balance and Oocyte Quality in Aging Mice.” Redox Biology 73: 103195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, B. , Kon N., Chen D., et al. 2019. “ALOX12 Is Required for p53‐Mediated Tumour Suppression Through a Distinct Ferroptosis Pathway.” Nature Cell Biology 21, no. 5: 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co, H. K. C. , Wu C. C., Lee Y. C., and Chen S. H.. 2024. “Emergence of Large‐Scale Cell Death Through Ferroptotic Trigger Waves.” Nature 631, no. 8021: 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, M. , and Pratt D. A.. 2019. “The Chemical Basis of Ferroptosis.” Nature Chemical Biology 15, no. 12: 1137–1147. [DOI] [PubMed] [Google Scholar]
- Cui, J. , Wang Y., Tian X., et al. 2023. “LPCAT3 Is Transcriptionally Regulated by YAP/ZEB/EP300 and Collaborates With ACSL4 and YAP to Determine Ferroptosis Sensitivity.” Antioxidants & Redox Signaling 39, no. 7–9: 491–511. [DOI] [PubMed] [Google Scholar]
- Cui, W. , Guo M., Liu D., et al. 2024. “Gut Microbial Metabolite Facilitates Colorectal Cancer Development via Ferroptosis Inhibition.” Nature Cell Biology 26, no. 1: 124–137. [DOI] [PubMed] [Google Scholar]
- Cui, Y. , Zhang Y., Zhao X., et al. 2021. “ACSL4 Exacerbates Ischemic Stroke by Promoting Ferroptosis‐Induced Brain Injury and Neuroinflammation.” Brain, Behavior, and Immunity 93: 312–321. [DOI] [PubMed] [Google Scholar]
- Dar, H. H. , Epperly M. W., Tyurin V. A., et al. 2022. “ P. aeruginosa Augments Irradiation Injury via 15‐Lipoxygenase‐Catalyzed Generation of 15‐HpETE‐PE and Induction of Theft‐Ferroptosis.” JCI Insight 7, no. 4: e156013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar, H. H. , Tyurina Y. Y., Mikulska‐Ruminska K., et al. 2018. “ Pseudomonas aeruginosa Utilizes Host Polyunsaturated Phosphatidylethanolamines to Trigger Theft‐Ferroptosis in Bronchial Epithelium.” Journal of Clinical Investigation 128, no. 10: 4639–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daruka, L. , Czikkely M. S., Szili P., et al. 2025. “ESKAPE Pathogens Rapidly Develop Resistance Against Antibiotics in Development in Vitro.” Nature Microbiology 10, no. 2: 313–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, A. , Nag S., Mason A. B., and Barroso M. M.. 2016. “Endosome‐Mitochondria Interactions Are Modulated by Iron Release From Transferrin.” Journal of Cell Biology 214, no. 7: 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, N. K. , Schwartz A. J., Barthel G., et al. 2020. “Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis.” Cell Metabolism 31, no. 1: 115–130.e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport, E. R. , Sanders J. G., Song S. J., Amato K. R., Clark A. G., and Knight R.. 2017. “The Human Microbiome in Evolution.” BMC Biology 15, no. 1: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, H. L. , de Carvalho V. R., Romeiro F. G., Sassaki L. Y., Keller R., and Rodrigues J.. 2012. “Mucosa‐Associated but Not Luminal Escherichia coli Is Augmented in Crohn's Disease and Ulcerative Colitis.” Gut Pathogens 4, no. 1: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, F. , Zhao B. C., Yang X., et al. 2021. “The Gut Microbiota Metabolite Capsiate Promotes Gpx4 Expression by Activating TRPV1 to Inhibit Intestinal Ischemia Reperfusion‐Induced Ferroptosis.” Gut Microbes 13, no. 1: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien, M. , van Passel M. W. J., van de Bovenkamp J. H. B., Schipper R., de Vos W., and Dekker J.. 2010. “Mucin‐Bacterial Interactions in the Human Oral Cavity and Digestive Tract.” Gut Microbes 1, no. 4: 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio, F. , Dal Ben D., Sarti A. C., Giuliani A. L., and Falzoni S.. 2017. “The P2X7 Receptor in Infection and Inflammation.” Immunity 47, no. 1: 15–31. [DOI] [PubMed] [Google Scholar]
- Ding, J. , Li J., Zhang C., Tan L., Zhao C., and Gao L.. 2024. “High‐Throughput Combined Analysis of Saliva Microbiota and Metabolomic Profile in Chinese Periodontitis Patients: A Pilot Study.” Inflammation 47, no. 3: 874–890. [DOI] [PubMed] [Google Scholar]
- Dixon, S. J. , Lemberg K. M., Lamprecht M. R., et al. 2012. “Ferroptosis: An Iron‐Dependent Form of Nonapoptotic Cell Death.” Cell 149, no. 5: 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, S. J. , and Olzmann J. A.. 2024. “The Cell Biology of Ferroptosis.” Nature Reviews. Molecular Cell Biology 25, no. 6: 424–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, S. J. , and Pratt D. A.. 2023. “Ferroptosis: A Flexible Constellation of Related Biochemical Mechanisms.” Molecular Cell 83, no. 7: 1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson, M. , Castro‐Portuguez R., and Zhang D. D.. 2019. “NRF2 Plays a Critical Role in Mitigating Lipid Peroxidation and Ferroptosis.” Redox Biology 23: 101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll, S. , Freitas F. P., Shah R., et al. 2019. “FSP1 Is a Glutathione‐Independent Ferroptosis Suppressor.” Nature 575, no. 7784: 693–698. [DOI] [PubMed] [Google Scholar]
- Doll, S. , Proneth B., Tyurina Y. Y., et al. 2017. “ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition.” Nature Chemical Biology 13, no. 1: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong‐Chen, X. , Yong C., Yang X., Chen‐Yu S., and Li‐Hua P.. 2023. “Signaling Pathways in Parkinson's Disease: Molecular Mechanisms and Therapeutic Interventions.” Signal Transduction and Targeted Therapy 8, no. 1: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Z. Q. , Xie J. B., Ji S. Y., Zhou W., and Tao Z. S.. 2025. “Spermidine Prevents Iron Overload‐Induced Impaired Bone Mass by Activating SIRT1/SOD2 Signaling in Senile Rat Model.” Redox Report 30, no. 1: 2485666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, R. M. , Reyes L., Moats K., et al. 2020. “ATF3 Coordinates Antitumor Synergy Between Epigenetic Drugs and Protein Disulfide Isomerase Inhibitors.” Cancer Research 80, no. 16: 3279–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham, S. J. B. , Avelar‐Barragan J., Rothman J. A., et al. 2024. “Sex‐Specific Associations Between AD Genotype and the Microbiome of Human Amyloid Beta Knock‐In (hAβ‐KI) Mice.” Alzheimers Dementia 20, no. 7: 4935–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebedes, D. , and Borlongan C. V.. 2024. “Going Straight for the Gut: Gut‐Brain Axis Pathology and Treatment of Parkinson's Disease.” Neural Regeneration Research 19, no. 10: 2111–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex, M. , Rios Rodriguez V., Rademacher J., et al. 2024. “Shared and Distinct Gut Microbiota in Spondyloarthritis, Acute Anterior Uveitis, and Crohn's Disease.” Arthritis and Rheumatology 76, no. 1: 48–58. [DOI] [PubMed] [Google Scholar]
- Fan, Y. G. , Ge R. L., Ren H., et al. 2024. “Astrocyte‐Derived Lactoferrin Inhibits Neuronal Ferroptosis by Reducing Iron Content and GPX4 Degradation in APP/PS1 Transgenic Mice.” Pharmacological Research 209: 107404. [DOI] [PubMed] [Google Scholar]
- Fang, J. , Kong B., Shuai W., et al. 2021. “Ferroportin‐Mediated Ferroptosis Involved in New‐Onset Atrial Fibrillation With LPS‐Induced Endotoxemia.” European Journal of Pharmacology 913: 174622. [DOI] [PubMed] [Google Scholar]
- Fang, X. , Liu S., Muhammad B., et al. 2024. “Gut Microbiota Dysbiosis Contributes to α‐Synuclein‐Related Pathology Associated With C/EBPβ/AEP Signaling Activation in a Mouse Model of Parkinson's Disease.” Neural Regeneration Research 19, no. 9: 2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, H. , Schorpp K., Jin J., et al. 2020. “Transferrin Receptor Is a Specific Ferroptosis Marker.” Cell Reports 30, no. 10: 3411–3423.e3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, H. , and Stockwell B. R.. 2018. “Unsolved Mysteries: How Does Lipid Peroxidation Cause Ferroptosis?” PLoS Biology 16, no. 5: e2006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Z. , Meng F., Huo F., et al. 2024. “Inhibition of Ferroptosis Rescues M2 Macrophages and Alleviates Arthritis by Suppressing the HMGB1/TLR4/STAT3 Axis in M1 Macrophages.” Redox Biology 75: 103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentie, E. G. , Lim K., Jeong M., and Shin J. H.. 2024. “A Comprehensive Review of the Characterization, Host Interactions, and Stabilization Advancements on Probiotics: Addressing the Challenges in Functional Food Diversification.” Comprehensive Reviews in Food Science and Food Safety 23, no. 5: e13424. [DOI] [PubMed] [Google Scholar]
- Freitas, F. P. , Alborzinia H., dos Santos A. F., et al. 2024. “7‐Dehydrocholesterol Is an Endogenous Suppressor of Ferroptosis.” Nature 626, no. 7998: 401–410. [DOI] [PubMed] [Google Scholar]
- Fu, E. , Kuo C. Y., Hsia Y. J., et al. 2023. “Role of Ferroptosis in Periodontitis: An Animal Study in Rats.” Journal of Periodontal Research 58, no. 5: 1031–1040. [DOI] [PubMed] [Google Scholar]
- Fu, J. , Zhang P., Sun Z., et al. 2024. “A Combined Nanotherapeutic Approach Targeting Farnesoid X Receptor, Ferroptosis, and Fibrosis for Nonalcoholic Steatohepatitis Treatment.” Acta Pharmaceutica Sinica B 14, no. 5: 2228–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua, B. K. , Lu Y., Frazer D. M., et al. 2018. “Severe Iron Metabolism Defects in Mice With Double Knockout of the Multicopper Ferroxidases Hephaestin and Ceruloplasmin.” Cellular and Molecular Gastroenterology and Hepatology 6, no. 4: 405–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta, R. M. , van Erpecum K. J., Oldenburg B., et al. 2011. “Farnesoid X Receptor Activation Inhibits Inflammation and Preserves the Intestinal Barrier in Inflammatory Bowel Disease.” Gut 60, no. 4: 463–472. [DOI] [PubMed] [Google Scholar]
- Galy, B. , Conrad M., and Muckenthaler M.. 2024. “Mechanisms Controlling Cellular and Systemic Iron Homeostasis.” Nature Reviews. Molecular Cell Biology 25, no. 2: 133–155. [DOI] [PubMed] [Google Scholar]
- Gan, B. 2021. “Mitochondrial Regulation of Ferroptosis.” Journal of Cell Biology 220, no. 9: e202105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. , Zhang P., Wei Y., et al. 2023. “ Porphyromonas gingivalis Exacerbates Alcoholic Liver Disease by Altering Gut Microbiota Composition and Host Immune Response in Mice.” Journal of Clinical Periodontology 50, no. 9: 1253–1263. [DOI] [PubMed] [Google Scholar]
- Ge, H. , Wei Y., Zhang W., Yong C., Chen Y., and Zhou E.. 2024. “Suyin Detoxification Granule Alleviates Trimethylamine N‐Oxide‐Induced Tubular Ferroptosis and Renal Fibrosis to Prevent Chronic Kidney Disease Progression.” Phytomedicine 135: 156195. [DOI] [PubMed] [Google Scholar]
- Glorieux, G. , Nigam S. K., Vanholder R., and Verbeke F.. 2023. “Role of the Microbiome in Gut‐Heart‐Kidney Cross Talk.” Circulation Research 132, no. 8: 1064–1083. [DOI] [PubMed] [Google Scholar]
- Gu, K. , Wu A., Liu C., et al. 2024. “Absence of Gut Microbiota Alleviates Iron Overload‐Induced Colitis by Modulating Ferroptosis in Mice.” Journal of Advanced Research. 10.1016/j.jare.2024.12.030. [DOI] [PubMed] [Google Scholar]
- Guan, Z. , Jin X., Guan Z., Liu S., Tao K., and Luo L.. 2023. “The Gut Microbiota Metabolite Capsiate Regulate SLC2A1 Expression by Targeting HIF‐1α to Inhibit Knee Osteoarthritis‐Induced Ferroptosis.” Aging Cell 22, no. 6: e13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, M. , Liu H., Yu Y., et al. 2023. “ Lactobacillus rhamnosus GG Ameliorates Osteoporosis in Ovariectomized Rats by Regulating the Th17/Treg Balance and Gut Microbiota Structure.” Gut Microbes 15, no. 1: 2190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, S. , Wang H., and Yin Y.. 2022. “Microglia Polarization From M1 to M2 in Neurodegenerative Diseases.” Frontiers in Aging Neuroscience 14: 815347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassannia, B. , Vandenabeele P., and Vanden Berghe T.. 2019. “Targeting Ferroptosis to Iron Out Cancer.” Cancer Cell 35, no. 6: 830–849. [DOI] [PubMed] [Google Scholar]
- He, X. , Hu M., Xu Y., et al. 2025. “The Gut‐Brain Axis Underlying Hepatic Encephalopathy in Liver Cirrhosis.” Nature Medicine 31: 627–638. [DOI] [PubMed] [Google Scholar]
- He, Y. , Ling Y., Zhang Z., et al. 2023. “Butyrate Reverses Ferroptosis Resistance in Colorectal Cancer by Inducing c‐Fos‐Dependent xCT Suppression.” Redox Biology 65: 102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe, M. , Önning G., Berggren A., and Hulthén L.. 2015. “Probiotic Strain Lactobacillus plantarum 299v Increases Iron Absorption From an Iron‐Supplemented Fruit Drink: A Double‐Isotope Cross‐Over Single‐Blind Study in Women of Reproductive Age.” British Journal of Nutrition 114, no. 8: 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, K. , Wu Z. X., Chen X. Y., et al. 2022. “Microbiota in Health and Diseases.” Signal Transduction and Targeted Therapy 7, no. 1: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, C. L. , and Schnabl B.. 2023. “The Gut‐Liver Axis and Gut Microbiota in Health and Liver Disease.” Nature Reviews. Microbiology 21, no. 11: 719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, G. , Cai Y., Ren M., et al. 2025. “Salidroside Sensitizes Triple‐Negative Breast Cancer to Ferroptosis by SCD1‐Mediated Lipogenesis and NCOA4‐Mediated Ferritinophagy.” Journal of Advanced Research 74: 589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Lin Y., Xin J., et al. 2024. “Fluoride Exposure‐Induced Gut Microbiota Alteration Mediates Colonic Ferroptosis Through N(6)‐Methyladenosine (m(6)A) Mediated Silencing of SLC7A11.” Ecotoxicology and Environmental Safety 283: 116816. [DOI] [PubMed] [Google Scholar]
- Huang, W. , Chen H., He Q., et al. 2023. “Nobiletin Protects Against Ferroptosis to Alleviate Sepsis‐Associated Acute Liver Injury by Modulating the Gut Microbiota.” Food & Function 14, no. 16: 7692–7704. [DOI] [PubMed] [Google Scholar]
- Huang, X. , Yang X., Zhang M., et al. 2024. “SELENOI Functions as a Key Modulator of Ferroptosis Pathway in Colitis and Colorectal Cancer.” Advanced Science (Weinh) 11, no. 28: e2404073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Zhou J., Wang S., et al. 2020. “Indoxyl Sulfate Induces Intestinal Barrier Injury Through IRF1‐DRP1 Axis‐Mediated Mitophagy Impairment.” Theranostics 10, no. 16: 7384–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illés, E. , Mizrahi A., Marks V., and Meyerstein D.. 2019. “Carbonate‐Radical‐Anions, and Not Hydroxyl Radicals, Are the Products of the Fenton Reaction in Neutral Solutions Containing Bicarbonate.” Free Radical Biology & Medicine 131: 1–6. [DOI] [PubMed] [Google Scholar]
- Inagaki, T. , Moschetta A., Lee Y. K., et al. 2006. “Regulation of Antibacterial Defense in the Small Intestine by the Nuclear Bile Acid Receptor.” Proceedings of the National Academy of Sciences of the United States of America 103, no. 10: 3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, D. , Guo Y., Wang T., et al. 2024. “IRE1α Determines Ferroptosis Sensitivity Through Regulation of Glutathione Synthesis.” Nature Communications 15, no. 1: 4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, Z. , Liu X., Ma Y., et al. 2020. “Adipose‐Derived Stem Cells Protect Ischemia‐Reperfusion and Partial Hepatectomy by Attenuating Endoplasmic Reticulum Stress.” Frontiers in Cell and Development Biology 8: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Z. , Yang Y., Cao Y., et al. 2023. “The Gut Metabolite 3‐Hydroxyphenylacetic Acid Rejuvenates Spermatogenic Dysfunction in Aged Mice Through GPX4‐Mediated Ferroptosis.” Microbiome 11, no. 1: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinson, S. , Zhang Z., Lancaster G. I., Murphy A. J., and Morgan P. K.. 2024. “Iron, Lipid Peroxidation and Ferroptosis Play Pathogenic Roles in Atherosclerosis.” Cardiovascular Research 121: 44–61. [DOI] [PubMed] [Google Scholar]
- Kagan, V. E. , Mao G., Qu F., et al. 2017. “Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis.” Nature Chemical Biology 13, no. 1: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman, B. , Cheng G., and Hardy M.. 2024. “Gut Microbiome, Short‐Chain Fatty Acids, Alpha‐Synuclein, Neuroinflammation, and ROS/RNS: Relevance to Parkinson's Disease and Therapeutic Implications.” Redox Biology 71: 103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara, Y. , Fujiwara H., Yamamoto A., et al. 2024. “Oral Inflammation and Microbiome Dysbiosis Exacerbate Chronic Graft‐Versus‐Host Disease.” Blood 145, no. 8: 881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, X. , Liu C., Ding Y., et al. 2023. “ Roseburia intestinalis Generated Butyrate Boosts Anti‐PD‐1 Efficacy in Colorectal Cancer by Activating Cytotoxic CD8(+) T Cells.” Gut 72, no. 11: 2112–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Y. , Kang X., Yang H., et al. 2022. “ Lactobacillus acidophilus Ameliorates Obesity in Mice Through Modulation of Gut Microbiota Dysbiosis and Intestinal Permeability.” Pharmacological Research 175: 106020. [DOI] [PubMed] [Google Scholar]
- Kao, D. , Roach B., Silva M., et al. 2017. “Effect of Oral Capsule‐ vs Colonoscopy‐Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial.” JAMA 318, no. 20: 1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, T. C. , Gomez M. L., and Germain D.. 2019. “Mitohormesis, UPR(Mt), and the Complexity of Mitochondrial DNA Landscapes in Cancer.” Cancer Research 79, no. 24: 6057–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. Y. , Lim Y., Jang J. S., et al. 2024. “Extracellular Vesicles From Periodontal Pathogens Regulate Hepatic Steatosis via Toll‐Like Receptor 2 and Plasminogen Activator Inhibitor‐1.” Journal of Extracellular Vesicles 13, no. 1: e12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, R. , and Sung J. H.. 2024. “Recent Advances in Gut‐ and Gut‐Organ‐Axis‐On‐a‐Chip Models.” Advanced Healthcare Materials 13, no. 21: e2302777. [DOI] [PubMed] [Google Scholar]
- Kong, B. , Fu H., Xiao Z., Zhou Y., Shuai W., and Huang H.. 2022. “Gut Microbiota Dysbiosis Induced by a High‐Fat Diet Increases Susceptibility to Atrial Fibrillation.” Canadian Journal of Cardiology 38, no. 12: 1962–1975. [DOI] [PubMed] [Google Scholar]
- Kong, C. , Liang L., Liu G., et al. 2023. “Integrated Metagenomic and Metabolomic Analysis Reveals Distinct Gut‐Microbiome‐Derived Phenotypes in Early‐Onset Colorectal Cancer.” Gut 72, no. 6: 1129–1142. [DOI] [PubMed] [Google Scholar]
- Koppula, P. , Zhang Y., Zhuang L., and Gan B.. 2018. “Amino Acid Transporter SLC7A11/xCT at the Crossroads of Regulating Redox Homeostasis and Nutrient Dependency of Cancer.” Cancer Communications (London) 38, no. 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula, P. , Zhuang L., and Gan B.. 2021. “Cystine Transporter SLC7A11/xCT in Cancer: Ferroptosis, Nutrient Dependency, and Cancer Therapy.” Protein & Cell 12, no. 8: 599–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft, V. A. N. , Bezjian C. T., Pfeiffer S., et al. 2020. “GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis Through Lipid Remodeling.” ACS Central Science 6, no. 1: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdi, P. , Kawanishi K., Mizutani K., and Yokota A.. 2006. “Mechanism of Growth Inhibition by Free Bile Acids in Lactobacilli and Bifidobacteria.” Journal of Bacteriology 188, no. 5: 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. Y. , Tsolis R. M., and Bäumler A. J.. 2022. “The Microbiome and Gut Homeostasis.” Science 377, no. 6601: eabp9960. [DOI] [PubMed] [Google Scholar]
- Lee, W. C. , Guntur A. R., Long F., and Rosen C. J.. 2017. “Energy Metabolism of the Osteoblast: Implications for Osteoporosis.” Endocrine Reviews 38, no. 3: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, G. , Zhuang L., and Gan B.. 2022. “Targeting Ferroptosis as a Vulnerability in Cancer.” Nature Reviews. Cancer 22, no. 7: 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B. , Wei Z., Wang Z., et al. 2024. “ Fusobacterium nucleatum Induces Oxaliplatin Resistance by Inhibiting Ferroptosis Through E‐Cadherin/β‐Catenin/GPX4 Axis in Colorectal Cancer.” Free Radical Biology & Medicine 220: 125–138. [DOI] [PubMed] [Google Scholar]
- Li, D. K. , Chaudhari S. N., Lee Y., et al. 2022. “Inhibition of Microbial Deconjugation of Micellar Bile Acids Protects Against Intestinal Permeability and Liver Injury.” Science Advances 8, no. 34: eabo2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Jia Y. C., Ding Y. X., Bai J., Cao F., and Li F.. 2023. “The Crosstalk Between Ferroptosis and Mitochondrial Dynamic Regulatory Networks.” International Journal of Biological Sciences 19, no. 9: 2756–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Wang W., Zhou H., et al. 2020. “Ferritinophagy‐Mediated Ferroptosis Is Involved in Sepsis‐Induced Cardiac Injury.” Free Radical Biology & Medicine 160: 303–318. [DOI] [PubMed] [Google Scholar]
- Li, T. , Ding N., Guo H., et al. 2024. “A Gut Microbiota‐Bile Acid Axis Promotes Intestinal Homeostasis Upon Aspirin‐Mediated Damage.” Cell Host & Microbe 32, no. 2: 191–208.e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Li Y., Xu J., et al. 2025. “Terahertz Wave Desensitizes Ferroptosis by Inhibiting the Binding of Ferric Ions to the Transferrin.” ACS Nano 19: 6876–6889. [DOI] [PubMed] [Google Scholar]
- Li, X. , Wang C., Zhu J., et al. 2022. “Sodium Butyrate Ameliorates Oxidative Stress‐Induced Intestinal Epithelium Barrier Injury and Mitochondrial Damage Through AMPK‐Mitophagy Pathway.” Oxidative Medicine and Cellular Longevity 2022: 3745135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Feng D., Wang Z., et al. 2019. “Ischemia‐Induced ACSL4 Activation Contributes to Ferroptosis‐Mediated Tissue Injury in Intestinal Ischemia/Reperfusion.” Cell Death and Differentiation 26, no. 11: 2284–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Han S., Zhao Y., et al. 2025. “A Redox‐Triggered Polymeric Nanoparticle for Disrupting Redox Homeostasis and Enhanced Ferroptosis.” Small 21, no. 5: e2404299. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Ran Q., Duan Q., et al. 2024. “7‐Dehydrocholesterol Dictates Ferroptosis Sensitivity.” Nature 626, no. 7998: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Hu Y., Zheng H., et al. 2024. “LPCAT1‐Mediated Membrane Phospholipid Remodelling Promotes Ferroptosis Evasion and Tumour Growth.” Nature Cell Biology 26, no. 5: 811–824. [DOI] [PubMed] [Google Scholar]
- Liang, D. , Feng Y., Zandkarimi F., et al. 2023. “Ferroptosis Surveillance Independent of GPX4 and Differentially Regulated by Sex Hormones.” Cell 186, no. 13: 2748–2764.e2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, C. , Rolling T., Djukovic A., et al. 2024. “Oral Bacteria Relative Abundance in Faeces Increases due to Gut Microbiota Depletion and Is Linked With Patient Outcomes.” Nature Microbiology 9, no. 6: 1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, D. , Kim K. S., Jeong J. H., et al. 2018. “The Hepcidin‐Ferroportin Axis Controls the Iron Content of Salmonella‐Containing Vacuoles in Macrophages.” Nature Communications 9, no. 1: 2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X. , Xiao H. M., Liu H. M., et al. 2023. “Gut Microbiota Impacts Bone via Bacteroides vulgatus ‐Valeric Acid‐Related Pathways.” Nature Communications 14, no. 1: 6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Z. , Li T., Huang F., et al. 2025. “Comparison of Diet and Exercise on Cardiometabolic Factors in Young Adults With Overweight/Obesity: Multiomics Analysis and Gut Microbiota Prediction, a Randomized Controlled Trial.” MedComm (2020) 6, no. 1: e70044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. , Liu L., Tian Z., et al. 2024. “ Bacteroides uniformis Ameliorates Pro‐Inflammatory Diet‐Exacerbated Colitis by Targeting Endoplasmic Reticulum Stress‐Mediated Ferroptosis.” Journal of Advanced Research. 10.1016/j.jare.2024.11.025. [DOI] [PubMed] [Google Scholar]
- Liu, D. , Yue Y., Ping L., et al. 2024. “ Lactobacillus delbrueckii Subsp. Bulgaricus 1.0207 Exopolysaccharides Attenuate Hydrogen Peroxide‐Induced Oxidative Stress Damage in IPEC‐J2 Cells Through the Keap1/Nrf2 Pathway.” Antioxidants (Basel) 13, no. 9: 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F. , Su D., Zhang H., et al. 2022. “Clinical Implications of the Oral‐Gut Microbiome Axis and Its Association With Colorectal Cancer (Review).” Oncology Reports 48, no. 5: 192. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Dong W., Wang S., et al. 2018. “Deoxycholic Acid Disrupts the Intestinal Mucosal Barrier and Promotes Intestinal Tumorigenesis.” Food & Function 9, no. 11: 5588–5597. [DOI] [PubMed] [Google Scholar]
- Liu, R. , Wang J., Liu Y., Gao Y., and Yang R.. 2024. “Regulation of Gut Microbiota on Immune Cell Ferroptosis: A Novel Insight for Immunotherapy Against Tumor.” Cancer Letters 598: 217115. [DOI] [PubMed] [Google Scholar]
- Liu, S. , Gao Z., He W., et al. 2022. “The Gut Microbiota Metabolite Glycochenodeoxycholate Activates TFR‐ACSL4‐Mediated Ferroptosis to Promote the Development of Environmental Toxin‐Linked MAFLD.” Free Radical Biology & Medicine 193, no. Pt 1: 213–226. [DOI] [PubMed] [Google Scholar]
- Liu, S. , Tang Y., Liu L., et al. 2022. “Proteomic Analysis Reveals That ACSL4 Activation During Reflux Esophagitis Contributes to Ferroptosis‐Mediated Esophageal Mucosal Damage.” European Journal of Pharmacology 931: 175175. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Olszewski K., Zhang Y., et al. 2020. “Cystine Transporter Regulation of Pentose Phosphate Pathway Dependency and Disulfide Stress Exposes a Targetable Metabolic Vulnerability in Cancer.” Nature Cell Biology 22, no. 4: 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Shi Y., Liu R., Song K., and Chen L.. 2024. “Structure of Human Phagocyte NADPH Oxidase in the Activated State.” Nature 627, no. 8002: 189–195. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Jiao C., Zhang T., et al. 2023. “Early‐Life Gut Microbiota Governs Susceptibility to Colitis via Microbial‐Derived Ether Lipids.” Research (Washington, D.C.) 6: 0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Lu T., Zhang C., et al. 2019. “Activation of YAP Attenuates Hepatic Damage and Fibrosis in Liver Ischemia‐Reperfusion Injury.” Journal of Hepatology 71, no. 4: 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Yao X., Chen C., et al. 2025. “Growth of Microbes in Competitive Lifestyles Promotes Increased ARGs in Soil Microbiota: Insights Based on Genetic Traits.” Microbiome 13, no. 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh, J. S. , Mak W. Q., Tan L. K. S., et al. 2024. “Microbiota‐Gut‐Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases.” Signal Transduction and Targeted Therapy 9, no. 1: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, X. , Wong C. C., Tong L., et al. 2019. “ Peptostreptococcus anaerobius Promotes Colorectal Carcinogenesis and Modulates Tumour Immunity.” Nature Microbiology 4, no. 12: 2319–2330. [DOI] [PubMed] [Google Scholar]
- Lopetuso, L. R. , Deleu S., Godny L., et al. 2023. “The First International Rome Consensus Conference on Gut Microbiota and Faecal Microbiota Transplantation in Inflammatory Bowel Disease.” Gut 72, no. 9: 1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo‐Zúñiga, V. , Bartolí R., Planas R., et al. 2003. “Oral Bile Acids Reduce Bacterial Overgrowth, Bacterial Translocation, and Endotoxemia in Cirrhotic Rats.” Hepatology 37, no. 3: 551–557. [DOI] [PubMed] [Google Scholar]
- Lou, L. , Wang M., He J., et al. 2023. “Urolithin A (UA) Attenuates Ferroptosis in LPS‐Induced Acute Lung Injury in Mice by Upregulating Keap1‐Nrf2/HO‐1 Signaling Pathway.” Frontiers in Pharmacology 14: 1067402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Sun J., Yang M., et al. 2024. “Myricetin Induces Ferroptosis and Inhibits Gastric Cancer Progression by Targeting NOX4.” Journal of Agricultural and Food Chemistry 72, no. 12: 6178–6188. [DOI] [PubMed] [Google Scholar]
- Luther, J. , Garber J. J., Khalili H., et al. 2015. “Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability.” Cellular and Molecular Gastroenterology and Hepatology 1, no. 2: 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, S. , Dubin A. E., Zhang Y., et al. 2021. “A Role of PIEZO1 in Iron Metabolism in Mice and Humans.” Cell 184, no. 4: 969–982.e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. Z. , Chen L. L., Qu L., et al. 2024. “Gut Microbiota‐Induced CXCL1 Elevation Triggers Early Neuroinflammation in the Substantia Nigra of Parkinsonian Mice.” Acta Pharmacologica Sinica 45, no. 1: 52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Li W., Niu S., et al. 2023. “BzATP Reverses Ferroptosis‐Induced Gut Microbiota Disorders in Collagen‐Induced Arthritis Mice.” International Immunopharmacology 124, no. Pt A: 110885. [DOI] [PubMed] [Google Scholar]
- Maniscalchi, A. , Benzi Juncos O. N., Conde M. A., et al. 2024. “New Insights on Neurodegeneration Triggered by Iron Accumulation: Intersections With Neutral Lipid Metabolism, Ferroptosis, and Motor Impairment.” Redox Biology 71: 103074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, E. R. , Lam Y. K., and Uhlig H. H.. 2024. “Short‐Chain Fatty Acids: Linking Diet, the Microbiome and Immunity.” Nature Reviews. Immunology 24, no. 8: 577–595. [DOI] [PubMed] [Google Scholar]
- Manos, J. 2022. “The Human Microbiome in Disease and Pathology.” APMIS 130, no. 12: 690–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, C. , Liu X., Zhang Y., et al. 2021. “DHODH‐Mediated Ferroptosis Defence Is a Targetable Vulnerability in Cancer.” Nature 593, no. 7860: 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiannaki, M. , Matak P., Keith B., Simon M. C., Vaulont S., and Peyssonnaux C.. 2009. “HIF‐2alpha, but Not HIF‐1alpha, Promotes Iron Absorption in Mice.” Journal of Clinical Investigation 119, no. 5: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann, J. R. , and Rawls J. F.. 2023. “Essential Amino Acid Metabolites as Chemical Mediators of Host‐Microbe Interaction in the Gut.” Annual Review of Microbiology 77: 479–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, B. , Zucoloto A. Z., Yu I. L., et al. 2020. “Programing of an Intravascular Immune Firewall by the Gut Microbiota Protects Against Pathogen Dissemination During Infection.” Cell Host & Microbe 28, no. 5: 660–668.e664. [DOI] [PubMed] [Google Scholar]
- Meng, F. , Fleming B. A., Jia X., Rousek A. A., Mulvey M. A., and Ward D. M.. 2022. “Lysosomal Iron Recycling in Mouse Macrophages Is Dependent Upon Both LcytB and Steap3 Reductases.” Blood Advances 6, no. 6: 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulic, N. , Uyoga M. A., Stoffel N. U., et al. 2024. “Prebiotics Increase Iron Absorption and Reduce the Adverse Effects of Iron on the Gut Microbiome and Inflammation: A Randomized Controlled Trial Using Iron Stable Isotopes in Kenyan Infants.” American Journal of Clinical Nutrition 119, no. 2: 456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima, E. , Nakamura T., Doll S., et al. 2025. “Recommendations for Robust and Reproducible Research on Ferroptosis.” Nature Reviews. Molecular Cell Biology 26: 615–630. [DOI] [PubMed] [Google Scholar]
- Mu, Y. , Sun J., Li Z., et al. 2022. “Activation of Pyroptosis and Ferroptosis Is Involved in the Hepatotoxicity Induced by Polystyrene Microplastics in Mice.” Chemosphere 291, no. Pt 2: 132944. [DOI] [PubMed] [Google Scholar]
- Nakamura, T. , Hipp C., Santos Dias Mourão A., et al. 2023. “Phase Separation of FSP1 Promotes Ferroptosis.” Nature 619, no. 7969: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie, J. , Ling Y., Jin M., et al. 2023. “Butyrate Enhances Erastin‐Induced Ferroptosis of Osteosarcoma Cells via Regulating ATF3/SLC7A11 Pathway.” European Journal of Pharmacology 957: 176009. [DOI] [PubMed] [Google Scholar]
- Nighot, M. , al‐Sadi R., Guo S., et al. 2017. “Lipopolysaccharide‐Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll‐Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression.” American Journal of Pathology 187, no. 12: 2698–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordine, M. L. , Seyoum Y., Bruneau A., et al. 2024. “The Microbiota and the Host Organism Switch Between Cooperation and Competition Based on Dietary Iron Levels.” Gut Microbes 16, no. 1: 2361660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst, O. , Hornef M. W., Schaap F. G., Cerovic V., Clavel T., and Bruns T.. 2023. “Gut‐Liver Axis: Barriers and Functional Circuits.” Nature Reviews. Gastroenterology & Hepatology 20, no. 7: 447–461. [DOI] [PubMed] [Google Scholar]
- Pasolli, E. , Asnicar F., Manara S., et al. 2019. “Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes From Metagenomes Spanning Age.” Geography, and Lifestyle, Cell 176, no. 3: 649–662.e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasricha, S. R. , Tye‐Din J., Muckenthaler M. U., and Swinkels D. W.. 2021. “Iron Deficiency.” Lancet 397, no. 10270: 233–248. [DOI] [PubMed] [Google Scholar]
- Peng, G. , Wang S., Zhang H., et al. 2024. “Tremella Aurantialba Polysaccharides Alleviate Ulcerative Colitis in Mice by Improving Intestinal Barrier via Modulating Gut Microbiota and Inhibiting Ferroptosis.” International Journal of Biological Macromolecules 281, no. Pt 4: 135835. [DOI] [PubMed] [Google Scholar]
- Peng, Y. , Lei X., Yang Q., et al. 2024. “ Helicobacter pylori CagA‐Mediated Ether Lipid Biosynthesis Promotes Ferroptosis Susceptibility in Gastric Cancer.” Experimental & Molecular Medicine 56, no. 2: 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego, S. , Sansoni V., Banfi G., and Lombardi G.. 2018. “Sodium Butyrate Has Anti‐Proliferative, Pro‐Differentiating, and Immunomodulatory Effects in Osteosarcoma Cells and Counteracts the TNFα‐Induced Low‐Grade Inflammation.” International Journal of Immunopathology and Pharmacology 32: 394632017752240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, F. C. , Ge X., Kristensen J. M., et al. 2024. “The Parkinson's Disease Drug Entacapone Disrupts Gut Microbiome Homoeostasis via Iron Sequestration.” Nature Microbiology 9, no. 12: 3165–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permain, J. , Hock B., Eglinton T., and Purcell R.. 2024. “Functional Links Between the Microbiome and the Molecular Pathways of Colorectal Carcinogenesis.” Cancer Metastasis Reviews 43, no. 4: 1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernomian, L. , Duarte‐Silva M., and de Barros Cardoso C. R.. 2020. “The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Control of Intestinal Inflammation: Insights From an Immune and Bacteria Sensor Receptor.” Clinical Reviews in Allergy and Immunology 59, no. 3: 382–390. [DOI] [PubMed] [Google Scholar]
- Pi, Y. , Zuo H., Wang Y., et al. 2024. “Oleanolic Acid Alleviating Ischemia‐Reperfusion Injury in Rat Severe Steatotic Liver via KEAP1/NRF2/ARE.” International Immunopharmacology 138: 112617. [DOI] [PubMed] [Google Scholar]
- Porcari, S. , Benech N., Valles‐Colomer M., et al. 2023. “Key Determinants of Success in Fecal Microbiota Transplantation: From Microbiome to Clinic.” Cell Host & Microbe 31, no. 5: 712–733. [DOI] [PubMed] [Google Scholar]
- Poursaitidis, I. , Wang X., Crighton T., et al. 2017. “Oncogene‐Selective Sensitivity to Synchronous Cell Death Following Modulation of the Amino Acid Nutrient Cystine.” Cell Reports 18, no. 11: 2547–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, N. , Walker M. M., and Talley N. J.. 2017. “The Mucosal Immune System: Master Regulator of Bidirectional Gut‐Brain Communications.” Nature Reviews. Gastroenterology & Hepatology 14, no. 3: 143–159. [DOI] [PubMed] [Google Scholar]
- Qian, X. H. , Xie R. Y., Liu X. L., Chen S. D., and Tang H. D.. 2022. “Mechanisms of Short‐Chain Fatty Acids Derived From Gut Microbiota in Alzheimer's Disease.” Aging and Disease 13, no. 4: 1252–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing, W. , Chen H., Ma X., et al. 2025. “Gut Dysbiosis‐Induced Vitamin B6 Metabolic Disorder Contributes to Chronic Stress‐Related Abnormal Behaviors in a Cortisol‐Independent Manner.” Gut Microbes 17, no. 1: 2447824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, B. , Zandkarimi F., Bezjian C. T., et al. 2024. “Phospholipids With Two Polyunsaturated Fatty Acyl Tails Promote Ferroptosis.” Cell 187, no. 5: 1177–1190.e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, W. , Sun Q., Li N., et al. 2024. “Superoxide Dismutase 2 Scavenges ROS to Promote Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Regulating Smad3 in Alveolar Bone‐Defective Rats.” Journal of Periodontology 95, no. 5: 469–482. [DOI] [PubMed] [Google Scholar]
- Quigley, E. M. M. 2019. “Prebiotics and Probiotics in Digestive Health.” Clinical Gastroenterology and Hepatology 17, no. 2: 333–344. [DOI] [PubMed] [Google Scholar]
- Reis, J. , Gorgulla C., Massari M., et al. 2023. “Targeting ROS Production Through Inhibition of NADPH Oxidases.” Nature Chemical Biology 19, no. 12: 1540–1550. [DOI] [PubMed] [Google Scholar]
- Remetic, J. , Ghallab A., Hobloss Z., et al. 2022. “Loss of Bile Salt Export Pump Aggravates Lipopolysaccharide‐Induced Liver Injury in Mice due to Impaired Hepatic Endotoxin Clearance.” Hepatology 75, no. 5: 1095–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben, A. , Tillman H., Fontana R. J., et al. 2016. “Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study.” Annals of Internal Medicine 164, no. 11: 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinella, M. E. , Lazarus J. V., Ratziu V., et al. 2023. “A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature.” Journal of Hepatology 79, no. 6: 1542–1556. [DOI] [PubMed] [Google Scholar]
- Rodencal, J. , Kim N., He A., et al. 2024. “Sensitization of Cancer Cells to Ferroptosis Coincident With Cell Cycle Arrest.” Cell Chemical Biology 31, no. 2: 234–248.e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru, Q. , Li Y., Chen L., Wu Y., Min J., and Wang F.. 2024. “Iron Homeostasis and Ferroptosis in Human Diseases: Mechanisms and Therapeutic Prospects.” Signal Transduction and Targeted Therapy 9, no. 1: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed, H. , Díaz L. A., Gil‐Gómez A., et al. 2024. “Microbiome‐Centered Therapies for the Management of Metabolic Dysfunction‐Associated Steatotic Liver Disease.” Clinical and Molecular Hepatology 31(Suppl): S94–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, M. E. , Merenstein D. J., Reid G., Gibson G. R., and Rastall R. A.. 2019. “Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic.” Nature Reviews. Gastroenterology & Hepatology 16, no. 10: 605–616. [DOI] [PubMed] [Google Scholar]
- Santana‐Codina, N. , Gikandi A., and Mancias J. D.. 2021. “The Role of NCOA4‐Mediated Ferritinophagy in Ferroptosis.” Advances in Experimental Medicine and Biology 1301: 41–57. [DOI] [PubMed] [Google Scholar]
- Schalk, I. J. 2025. “Bacterial Siderophores: Diversity, Uptake Pathways and Applications.” Nature Reviews. Microbiology 23, no. 1: 24–40. [DOI] [PubMed] [Google Scholar]
- Schwartz, A. J. , das N., Ramakrishnan S. K., et al. 2019. “Hepatic Hepcidin/Intestinal HIF‐2α Axis Maintains Iron Absorption During Iron Deficiency and Overload.” Journal of Clinical Investigation 129, no. 1: 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, M. , Löser A., Cheng Q., et al. 2023. “Side‐By‐Side Comparison of Recombinant Human Glutathione Peroxidases Identifies Overlapping Substrate Specificities for Soluble Hydroperoxides.” Redox Biology 59: 102593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, S. A. , Fu J., and Chang P. V.. 2020. “Microbial Tryptophan Metabolites Regulate Gut Barrier Function via the Aryl Hydrocarbon Receptor.” Proceedings of the National Academy of Sciences of the United States of America 117, no. 32: 19376–19387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedghi, L. , DiMassa V., Harrington A., Lynch S. V., and Kapila Y. L.. 2021. “The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease.” Periodontology 2000 87, no. 1: 107–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibt, T. M. , Proneth B., and Conrad M.. 2019. “Role of GPX4 in Ferroptosis and Its Pharmacological Implication.” Free Radical Biology & Medicine 133: 144–152. [DOI] [PubMed] [Google Scholar]
- Shah, T. M. , Patel J. G., Gohil T. P., Blake D. P., and Joshi C. G.. 2019. “Host Transcriptome and Microbiome Interaction Modulates Physiology of Full‐Sibs Broilers With Divergent Feed Conversion Ratio.” npj Biofilms and Microbiomes 5, no. 1: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan, Y. , Lee M., and Chang E. B.. 2022. “The Gut Microbiome and Inflammatory Bowel Diseases.” Annual Review of Medicine 73: 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandilya, S. , Kumar S., Kumar Jha N., Kumar Kesari K., and Ruokolainen J.. 2022. “Interplay of Gut Microbiota and Oxidative Stress: Perspective on Neurodegeneration and Neuroprotection.” Journal of Advanced Research 38: 223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam, N. K. , Chen K., and Cherayil B. J.. 2015. “Commensal Bacteria‐Induced Interleukin 1β (IL‐1β) Secreted by Macrophages Up‐Regulates Hepcidin Expression in Hepatocytes by Activating the Bone Morphogenetic Protein Signaling Pathway.” Journal of Biological Chemistry 290, no. 51: 30637–30647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , Bhatia R., Devi K., et al. 2023. “A Synbiotic Combination of Bifidobacterium longum Bif10 and Bifidobacterium breve Bif11, Isomaltooligosaccharides and Finger Millet Arabinoxylan Prevents Dextran Sodium Sulphate Induced Ulcerative Colitis in Mice.” International Journal of Biological Macromolecules 231: 123326. [DOI] [PubMed] [Google Scholar]
- Shen, Z. , Yu N., Zhang Y., et al. 2024. “The Potential Roles of HIF‐1α in Epithelial‐Mesenchymal Transition and Ferroptosis in Tumor Cells.” Cellular Signalling 122: 111345. [DOI] [PubMed] [Google Scholar]
- Shintoku, R. , Takigawa Y., Yamada K., et al. 2017. “Lipoxygenase‐Mediated Generation of Lipid Peroxides Enhances Ferroptosis Induced by Erastin and RSL3.” Cancer Science 108, no. 11: 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soula, M. , Weber R. A., Zilka O., et al. 2020. “Metabolic Determinants of Cancer Cell Sensitivity to Canonical Ferroptosis Inducers.” Nature Chemical Biology 16, no. 12: 1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockbridge, R. B. , and Wackett L. P.. 2024. “The Link Between Ancient Microbial Fluoride Resistance Mechanisms and Bioengineering Organofluorine Degradation or Synthesis.” Nature Communications 15, no. 1: 4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell, B. R. , Friedmann Angeli J. P., Bayir H., et al. 2017. “Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease.” Cell 171, no. 2: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, R. , du S., Wang M., et al. 2024. “Colonic Long‐Term Retention and Colonization of Probiotics by Double‐Layer Chitosan/Tannic Acid Coating and Microsphere Embedding for Treatment of Ulcerative Colitis and Radiation Enteritis.” International Journal of Biological Macromolecules 280, no. Pt 1: 135757. [DOI] [PubMed] [Google Scholar]
- Sun, S. , Shen J., Jiang J., Wang F., and Min J.. 2023. “Targeting Ferroptosis Opens New Avenues for the Development of Novel Therapeutics.” Signal Transduction and Targeted Therapy 8, no. 1: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanda, R. V. , Ji Q., Wu X., et al. 2023. “Lysosomal Cystine Governs Ferroptosis Sensitivity in Cancer via Cysteine Stress Response.” Molecular Cell 83, no. 18: 3347–3359.e3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, D. , Chen X., Kang R., and Kroemer G.. 2021. “Ferroptosis: Molecular Mechanisms and Health Implications.” Cell Research 31, no. 2: 107–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J. , Bu W., Hu W., et al. 2023. “Ferroptosis Is Involved in Sex‐Specific Small Intestinal Toxicity in the Offspring of Adult Mice Exposed to Polystyrene Nanoplastics During Pregnancy.” ACS Nano 17, no. 3: 2440–2449. [DOI] [PubMed] [Google Scholar]
- Tansey, M. G. , Wallings R. L., Houser M. C., Herrick M. K., Keating C. E., and Joers V.. 2022. “Inflammation and Immune Dysfunction in Parkinson Disease.” Nature Reviews. Immunology 22, no. 11: 657–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, M. , Qu A., Anderson E. R., et al. 2011. “Hypoxia‐Inducible Factor‐2α Mediates the Adaptive Increase of Intestinal Ferroportin During Iron Deficiency in Mice.” Gastroenterology 140, no. 7: 2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles, F. , Collman R. G., Mominkhan D., and Wang Y.. 2022. “Viruses, Periodontitis, and Comorbidities.” Periodontology 2000 89, no. 1: 190–206. [DOI] [PubMed] [Google Scholar]
- Teschke, R. 2024. “Hemochromatosis: Ferroptosis, ROS, Gut Microbiome, and Clinical Challenges With Alcohol as Confounding Variable.” International Journal of Molecular Sciences 25, no. 5: 2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut, M. M. , Roumain M., Piron E., et al. 2025. “The Microbiota‐Derived Bile Acid Taurodeoxycholic Acid Improves Hepatic Cholesterol Levels in Mice With Cancer Cachexia.” Gut Microbes 17, no. 1: 2449586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg, H. , Adolph T. E., and Trauner M.. 2022. “Gut‐Liver Axis: Pathophysiological Concepts and Clinical Implications.” Cell Metabolism 34, no. 11: 1700–1718. [DOI] [PubMed] [Google Scholar]
- Torres, A. , Michea M. A., Végvári Á., et al. 2024. “A Multi‐Platform Analysis of Human Gingival Crevicular Fluid Reveals Ferroptosis as a Relevant Regulated Cell Death Mechanism During the Clinical Progression of Periodontitis.” International Journal of Oral Science 16, no. 1: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi, A. , Debelius J., Brenner D. A., et al. 2018. “The Gut‐Liver Axis and the Intersection With the Microbiome.” Nature Reviews. Gastroenterology & Hepatology 15, no. 7: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo, Q. Z. , Zhang S. T., and Lei P.. 2022. “Mechanisms of Neuronal Cell Death in Ischemic Stroke and Their Therapeutic Implications.” Medicinal Research Reviews 42, no. 1: 259–305. [DOI] [PubMed] [Google Scholar]
- Vaughn, B. P. , Fischer M., Kelly C. R., et al. 2023. “Effectiveness and Safety of Colonic and Capsule Fecal Microbiota Transplantation for Recurrent Clostridioides Difficile Infection.” Clinical Gastroenterology and Hepatology 21, no. 5: 1330–1337. [DOI] [PubMed] [Google Scholar]
- Verstraeten, S. , Layec S., Auger S., et al. 2024. “Faecalibacterium Duncaniae A2‐165 Regulates the Expression of Butyrate Synthesis, Ferrous Iron Uptake, and Stress‐Response Genes Based on Acetate Consumption.” Scientific Reports 14, no. 1: 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vítová, M. , Palyzová A., and Řezanka T.. 2021. “Plasmalogens—Ubiquitous Molecules Occurring Widely, From Anaerobic Bacteria to Humans.” Progress in Lipid Research 83: 101111. [DOI] [PubMed] [Google Scholar]
- von Siebenthal, H. K. , Moretti D., Zimmermann M. B., and Stoffel N. U.. 2023. “Effect of Dietary Factors and Time of Day on Iron Absorption From Oral Iron Supplements in Iron Deficient Women.” American Journal of Hematology 98, no. 9: 1356–1363. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Chu Q., Dong W., et al. 2024. “Microbial Metabolite Deoxycholic Acid‐Mediated Ferroptosis Exacerbates High‐Fat Diet‐Induced Colonic Inflammation.” Molecular Metabolism 84: 101944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. , Liu X., Huang F., et al. 2024. “Gut Microbiota‐Derived Gamma‐Aminobutyric Acid From Metformin Treatment Reduces Hepatic Ischemia/Reperfusion Injury Through Inhibiting Ferroptosis.” eLife 12: RP89045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. , Wang J., Shen Y., Li H., Rausch W. D., and Huang X.. 2022. “Iron Dyshomeostasis and Ferroptosis: A New Alzheimer's Disease Hypothesis?” Frontiers in Aging Neuroscience 14: 830569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. , Wang X., Wang C., et al. 2025. “Gut Microbiota‐Derived Glutathione From Metformin Treatment Alleviates Intestinal Ferroptosis Induced by Ischemia/Reperfusion.” BMC Medicine 23, no. 1: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Liu Y., du T., et al. 2020. “ATF3 Promotes Erastin‐Induced Ferroptosis by Suppressing System Xc().” Cell Death and Differentiation 27, no. 2: 662–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, N. , and Fang J. Y.. 2023. “ Fusobacterium nucleatum , A Key Pathogenic Factor and Microbial Biomarker for Colorectal Cancer.” Trends in Microbiology 31, no. 2: 159–172. [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Liu J., Zhang Y., et al. 2024. “Microglial CR3 Promotes Neuron Ferroptosis via NOX2‐Mediated Iron Deposition in Rotenone‐Induced Experimental Models of Parkinson's Disease.” Redox Biology 77: 103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , and Xu R.. 2019. “Data‐Driven Multiple‐Level Analysis of Gut‐Microbiome‐Immune‐Joint Interactions in Rheumatoid Arthritis.” BMC Genomics 20, no. 1: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Yang Q., and Liu X.. 2023. “The Microbiota‐Gut‐Brain Axis and Neurodevelopmental Disorders.” Protein & Cell 14, no. 10: 762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Song J., Cai M., et al. 2024. “Gut Microbiota Modulation by L‐Fucose as a Strategy to Alleviate Ochratoxin A Toxicity on Primordial Follicle Formation.” Journal of Hazardous Materials 480: 136469. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Wu D., Wu F., et al. 2023. “ Prevotella histicola Suppresses Ferroptosis to Mitigate Ethanol‐Induced Gastric Mucosal Lesions in Mice.” BMC Complementary Medicine and Therapies 23, no. 1: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Fang Y., Liang W., et al. 2025. “Gut‐Liver Translocation of Pathogen Klebsiella pneumoniae Promotes Hepatocellular Carcinoma in Mice.” Nature Microbiology 10, no. 1: 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Li W., Dong Y., et al. 2023. “Ferrostatin‐1 Mitigates Ionizing Radiation‐Induced Intestinal Injuries by Inhibiting Apoptosis and Ferroptosis: An In Vitro and In Vivo Study.” International Journal of Radiation Biology 99, no. 10: 1607–1618. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Pan L., Niu D., et al. 2025. “Jingfang Granules Alleviates the Lipid Peroxidation Induced Ferroptosis in Rheumatoid Arthritis Rats by Regulating Gut Microbiota and Metabolism of Short Chain Fatty Acids.” Journal of Ethnopharmacology 339: 119160. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Zhang J., Wang S., et al. 2023. “Berberine Modulates Gut Microbiota to Attenuate Cerebral Ferroptosis Induced by Ischemia‐Reperfusion in Mice.” European Journal of Pharmacology 953: 175782. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Wang L., Sun T., et al. 2023. “Study of the Inflammatory Activating Process in the Early Stage of Fusobacterium nucleatum Infected PDLSCs.” International Journal of Oral Science 15, no. 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, F. , Zhou J., Pan L., et al. 2025. “Integrative Microbiomics, Proteomics and Lipidomics Studies Unraveled the Preventive Mechanism of Shouhui Tongbian Capsules on Cerebral Ischemic Stroke Injury.” Journal of Ethnopharmacology 337, no. Pt 2: 118874. [DOI] [PubMed] [Google Scholar]
- Wei, J. , Wang G., Lai M., et al. 2024. “Faecal Microbiota Transplantation Alleviates Ferroptosis After Ischaemic Stroke.” Neuroscience 541: 91–100. [DOI] [PubMed] [Google Scholar]
- Wei, Y. , Liu W., Wang R., et al. 2024. “Propionate Promotes Ferroptosis and Apoptosis Through Mitophagy and ACSL4‐Mediated Ferroptosis Elicits Anti‐Leukemia Immunity.” Free Radical Biology & Medicine 213: 36–51. [DOI] [PubMed] [Google Scholar]
- Wen, W. , Xu Y., Qian W., et al. 2023. “PUFAs Add Fuel to Crohn's Disease‐Associated AIEC‐Induced Enteritis by Exacerbating Intestinal Epithelial Lipid Peroxidation.” Gut Microbes 15, no. 2: 2265578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, Y. Q. , Zou Z. Y., Zhao G. G., et al. 2024. “FXR Activation Remodels Hepatic and Intestinal Transcriptional Landscapes in Metabolic Dysfunction‐Associated Steatohepatitis.” Acta Pharmacologica Sinica 45, no. 11: 2313–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widimsky, P. , Snyder K., Sulzenko J., Hopkins L. N., and Stetkarova I.. 2023. “Acute Ischaemic Stroke: Recent Advances in Reperfusion Treatment.” European Heart Journal 44, no. 14: 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems, P. H. , Rossignol R., Dieteren C. E., Murphy M. P., and Koopman W. J.. 2015. “Redox Homeostasis and Mitochondrial Dynamics.” Cell Metabolism 22, no. 2: 207–218. [DOI] [PubMed] [Google Scholar]
- Wu, H. , Wang Y., Zhang Y., et al. 2020. “Breaking the Vicious Loop Between Inflammation, Oxidative Stress and Coagulation, a Novel Anti‐Thrombus Insight of Nattokinase by Inhibiting LPS‐Induced Inflammation and Oxidative Stress.” Redox Biology 32: 101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Zhou B., Pang X., et al. 2022. “ Clostridium butyricum , A Butyrate‐Producing Potential Probiotic, Alleviates Experimental Colitis Through Epidermal Growth Factor Receptor Activation.” Food & Function 13, no. 13: 7046–7061. [DOI] [PubMed] [Google Scholar]
- Wu, W. , Li G., Dong S., et al. 2024. “Bomidin Attenuates Inflammation of Periodontal Ligament Stem Cells and Periodontitis in Mice via Inhibiting Ferroptosis.” International Immunopharmacology 127: 111423. [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Ran L., Yang Y., et al. 2023. “Deferasirox Alleviates DSS‐Induced Ulcerative Colitis in Mice by Inhibiting Ferroptosis and Improving Intestinal Microbiota.” Life Sciences 314: 121312. [DOI] [PubMed] [Google Scholar]
- Xiao, L. , Tang R., Wang J., Wan D., Yin Y., and Xie L.. 2023. “Gut Microbiota Bridges the Iron Homeostasis and Host Health.” Science China. Life Sciences 66, no. 9: 1952–1975. [DOI] [PubMed] [Google Scholar]
- Xie, H. , Tang J., Song L., et al. 2024. “Mitochondria‐Endoplasmic Reticulum Crosstalk in Apoptosis: The Interactions of Cytochrome c With Monooxygenase and Its Reductase.” International Journal of Biological Macromolecules 279, no. Pt 1: 135160. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Tian H., Ji Y., et al. 2023. “Urolithin C Reveals Anti‐NAFLD Potential via AMPK‐Ferroptosis Axis and Modulating Gut Microbiota.” Naunyn‐Schmiedeberg's Archives of Pharmacology 396, no. 10: 2687–2699. [DOI] [PubMed] [Google Scholar]
- Yamashita, S. I. , Sugiura Y., Matsuoka Y., et al. 2024. “Mitophagy Mediated by BNIP3 and NIX Protects Against Ferroptosis by Downregulating Mitochondrial Reactive Oxygen Species.” Cell Death and Differentiation 31, no. 5: 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, A. W. , Fouts D. E., Brandl J., et al. 2011. “Enteric Dysbiosis Associated With a Mouse Model of Alcoholic Liver Disease.” Hepatology 53, no. 1: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, B. , Ai Y., Sun Q., et al. 2021. “Membrane Damage During Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1.” Molecular Cell 81, no. 2: 355–369.e310. [DOI] [PubMed] [Google Scholar]
- Yang, A. , Zhu X., Zhang L., and Ding Y.. 2024. “Transitioning From NAFLD to MAFLD and MASLD: Consistent Prevalence and Risk Factors in a Chinese Cohort.” Journal of Hepatology 80, no. 4: e154–e155. [DOI] [PubMed] [Google Scholar]
- Yang, C. J. , Chang H. C., Sung P. C., et al. 2024. “Oral Fecal Transplantation Enriches Lachnospiraceae and Butyrate to Mitigate Acute Liver Injury.” Cell Reports 43, no. 1: 113591. [DOI] [PubMed] [Google Scholar]
- Yang, L. , Wang H., Yang X., et al. 2020. “Auranofin Mitigates Systemic Iron Overload and Induces Ferroptosis via Distinct Mechanisms.” Signal Transduction and Targeted Therapy 5, no. 1: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R. , Gao W., Wang Z., et al. 2024. “Polyphyllin I Induced Ferroptosis to Suppress the Progression of Hepatocellular Carcinoma Through Activation of the Mitochondrial Dysfunction via Nrf2/HO‐1/GPX4 Axis.” Phytomedicine 122: 155135. [DOI] [PubMed] [Google Scholar]
- Yang, W. S. , SriRamaratnam R., Welsch M. E., et al. 2014. “Regulation of Ferroptotic Cancer Cell Death by GPX4.” Cell 156, no. 1–2: 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Zhang Z., Shen X., et al. 2023. “Clostridium Butyricum and Its Metabolite Butyrate Promote Ferroptosis Susceptibility in Pancreatic Ductal Adenocarcinoma.” Cellular Oncology (Dordrecht) 46, no. 6: 1645–1658. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Hao T., Yao X., et al. 2023. “Crebanine Ameliorates Ischemia‐Reperfusion Brain Damage by Inhibiting Oxidative Stress and Neuroinflammation Mediated by NADPH Oxidase 2 in Microglia.” Phytomedicine 120: 155044. [DOI] [PubMed] [Google Scholar]
- Yao, C. , Lan D., Li X., Wang Y., Qi S., and Liu Y.. 2023. “ Porphyromonas gingivalis Is a Risk Factor for the Development of Nonalcoholic Fatty Liver Disease via Ferroptosis.” Microbes and Infection 25, no. 1–2: 105040. [DOI] [PubMed] [Google Scholar]
- Yao, C. , Lu L., Lan D., et al. 2024. “ Porphyromonas gingivalis as a Promotor in the Development of the Alcoholic Liver Disease via Ferroptosis.” Microbes and Infection 26, no. 3: 105250. [DOI] [PubMed] [Google Scholar]
- Yao, T. , and Li L.. 2023. “The Influence of Microbiota on Ferroptosis in Intestinal Diseases.” Gut Microbes 15, no. 2: 2263210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda, K. , Shimodan S., Maehara N., et al. 2024. “AIM/CD5L Ameliorates Autoimmune Arthritis by Promoting Removal of Inflammatory DAMPs at the Lesions.” Journal of Autoimmunity 142: 103149. [DOI] [PubMed] [Google Scholar]
- Ye, L. , He S., Mao X., Zhang Y., Cai Y., and Li S.. 2020. “Effect of Hepatic Macrophage Polarization and Apoptosis on Liver Ischemia and Reperfusion Injury During Liver Transplantation.” Frontiers in Immunology 11: 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, Y. W. S. , Ma Y., Deng Y., Khoo B. L., and Chua S. L.. 2024. “Bacterial Iron Siderophore Drives Tumor Survival and Ferroptosis Resistance in a Biofilm‐Tumor Spheroid Coculture Model.” Advanced Science (Weinh) 11, no. 39: e2404467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, F. , Zhang Q., Liu H., et al. 2022. “Dynamic O‐GlcNAcylation Coordinates Ferritinophagy and Mitophagy to Activate Ferroptosis.” Cell Discovery 8, no. 1: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H. , Chang Q., Sun T., et al. 2023. “Metabolic Reprogramming and Polarization of Microglia in Parkinson's Disease: Role of Inflammasome and Iron.” Ageing Research Reviews 90: 102032. [DOI] [PubMed] [Google Scholar]
- Yu, H. , Lin G., Jiang J., et al. 2024. “Synergistic Activity of Enterococcus Faecium ‐Induced Ferroptosis via Expansion of IFN‐γ(+)CD8(+) T Cell Population in Advanced Hepatocellular Carcinoma Treated With Sorafenib.” Gut Microbes 16, no. 1: 2410474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Feng L., Luo Z., et al. 2024. “Interleukin‐10 Deficiency Suppresses Colorectal Cancer Metastasis by Enriching Gut Parabacteroides distasonis .” Journal of Advanced Research. 10.1016/j.jare.2024.11.024. [DOI] [PubMed] [Google Scholar]
- Yu, J. S. , Youn G. S., Choi J., et al. 2021. “Lactobacillus Lactis and Pediococcus pentosaceus ‐Driven Reprogramming of Gut Microbiome and Metabolome Ameliorates the Progression of Non‐Alcoholic Fatty Liver Disease.” Clinical and Translational Medicine 11, no. 12: e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, T. , Guo F., Yu Y., et al. 2017. “ Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy.” Cell 170, no. 3: 548–563.e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Jiang L., Wang H., et al. 2020. “Hepatic Transferrin Plays a Role in Systemic Iron Homeostasis and Liver Ferroptosis.” Blood 136, no. 6: 726–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Wang W., and Zhang F.. 2023. “The Next Generation Fecal Microbiota Transplantation: To Transplant Bacteria or Virome.” Advanced Science (Weinh) 10, no. 35: e2301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, M. , Wei J., Chen W., Hong D., Chen T., and Fang X.. 2022. “Neurotrophic Role of the Next‐Generation Probiotic Strain L. lactis MG1363‐pMG36e‐GLP‐1 on Parkinson's Disease via Inhibiting Ferroptosis.” Nutrients 14, no. 22: 4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss, M. M. , Jones R. M., Schett G., and Pacifici R.. 2019. “The Gut‐Bone Axis: How Bacterial Metabolites Bridge the Distance.” Journal of Clinical Investigation 129, no. 8: 3018–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, F. , Nijiati S., Tang L., Ye J., Zhou Z., and Chen X.. 2023. “Ferroptosis Detection: From Approaches to Applications.” Angewandte Chemie (International Ed. in English) 62, no. 35: e202300379. [DOI] [PubMed] [Google Scholar]
- Zha, X. , Liu X., Wei M., et al. 2025. “Microbiota‐Derived Lysophosphatidylcholine Alleviates Alzheimer's Disease Pathology via Suppressing Ferroptosis.” Cell Metabolism 37, no. 1: 169–186.e169. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Kang H., Zhang W., et al. 2023. “Probiotics Ameliorate Benzene‐Induced Systemic Inflammation and Hematopoietic Toxicity by Inhibiting Bacteroidaceae‐Mediated Ferroptosis.” Science of the Total Environment 899: 165678. [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Zhou J., Zhai D., Jiang Q., Yang M., and Zhou M.. 2024. “Gut Microbiota Regulates the ALK5/NOX1 Axis by Altering Glutamine Metabolism to Inhibit Ferroptosis of Intrahepatic Cholangiocarcinoma Cells.” Biochimica et Biophysica Acta—Molecular Basis of Disease 1870, no. 5: 167152. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Kang R., and Tang D.. 2024. “Gut Microbiome Mediates Ferroptosis Resistance for Colorectal Cancer Development.” Cancer Research 84, no. 6: 796–797. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Xin W., Anderson G. J., et al. 2022. “Double‐Edge Sword Roles of Iron in Driving Energy Production Versus Instigating Ferroptosis.” Cell Death & Disease 13, no. 1: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Dai J., Hou G., et al. 2023. “SMURF2 Predisposes Cancer Cell Toward Ferroptosis in GPX4‐Independent Manners by Promoting GSTP1 Degradation.” Molecular Cell 83, no. 23: 4352–4369.e4358. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Li H., Wang H., et al. 2024. “Iron/ROS/Itga3 Mediated Accelerated Depletion of Hippocampal Neural Stem Cell Pool Contributes to Cognitive Impairment After Hemorrhagic Stroke.” Redox Biology 71: 103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Wang H., Sang Y., et al. 2024. “Gut Microbiota in Health and Disease: Advances and Future Prospects.” MedComm (2020) 5, no. 12: e70012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Zhang L., Zheng S., et al. 2022. “ Fusobacterium nucleatum Promotes Colorectal Cancer Cells Adhesion to Endothelial Cells and Facilitates Extravasation and Metastasis by Inducing ALPK1/NF‐κB/ICAM1 Axis.” Gut Microbes 14, no. 1: 2038852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Chen H., and Li Q.. 2024. “High‐Fat Diet Led to Testicular Inflammation and Ferroptosis via Dysbiosis of Gut Microbes.” International Immunopharmacology 142, no. Pt B: 113235. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Lin T., Meng Y., et al. 2021. “FOS/GOS Attenuates High‐Fat Diet Induced Bone Loss via Reversing Microbiota Dysbiosis, High Intestinal Permeability and Systemic Inflammation in Mice.” Metabolism 119: 154767. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Zhou H., Gu W., et al. 2024. “CGI1746 Targets σ(1)R to Modulate Ferroptosis Through Mitochondria‐Associated Membranes.” Nature Chemical Biology 20, no. 6: 699–709. [DOI] [PubMed] [Google Scholar]
- Zhao, D. , Gao Y., Chen Y., et al. 2024. “L‐Citrulline Ameliorates Iron Metabolism and Mitochondrial Quality Control via Activating AMPK Pathway in Intestine and Improves Microbiota in Mice With Iron Overload.” Molecular Nutrition & Food Research 68, no. 6: e2300723. [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Chen Y., Huang W., Zhou H., and Zhang W.. 2023. “Drug‐Microbiota Interactions: An Emerging Priority for Precision Medicine.” Signal Transduction and Targeted Therapy 8, no. 1: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, T. S. , Xie L. W., Cai S., et al. 2021. “Dysbiosis of Gut Microbiota Is Associated With the Progression of Radiation‐Induced Intestinal Injury and Is Alleviated by Oral Compound Probiotics in Mouse Model.” Frontiers in Cellular and Infection Microbiology 11: 717636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Li J., Guo W., Li H., and Lei L.. 2020. “Periodontitis‐Level Butyrate‐Induced Ferroptosis in Periodontal Ligament Fibroblasts by Activation of Ferritinophagy.” Cell Death Discovery 6, no. 1: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , and Conrad M.. 2020. “The Metabolic Underpinnings of Ferroptosis.” Cell Metabolism 32, no. 6: 920–937. [DOI] [PubMed] [Google Scholar]
- Zheng, J. , and Conrad M.. 2025. “Ferroptosis: When Metabolism Meets Cell Death.” Physiological Reviews 105, no. 2: 651–706. [DOI] [PubMed] [Google Scholar]
- Zheng, L. , Kelly C. J., Battista K. D., et al. 2017. “Microbial‐Derived Butyrate Promotes Epithelial Barrier Function Through IL‐10 Receptor‐Dependent Repression of Claudin‐2.” Journal of Immunology 199, no. 8: 2976–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Q. , Zhao Y., Guo J., et al. 2018. “Iron Overload Promotes Mitochondrial Fragmentation in Mesenchymal Stromal Cells From Myelodysplastic Syndrome Patients Through Activation of the AMPK/MFF/Drp1 Pathway.” Cell Death & Disease 9, no. 5: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Zhou Y. L., Mao J. A., et al. 2022. “NCOA4‐Mediated Ferritinophagy Is Involved in Ionizing Radiation‐Induced Ferroptosis of Intestinal Epithelial Cells.” Redox Biology 55: 102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L. , Qiu W., Wang J., et al. 2023. “Effects of Vaginal Microbiota Transfer on the Neurodevelopment and Microbiome of Cesarean‐Born Infants: A Blinded Randomized Controlled Trial.” Cell Host & Microbe 31, no. 7: 1232–1247.e1235. [DOI] [PubMed] [Google Scholar]
- Zhu, G. , Sui S., Shi F., and Wang Q.. 2022. “Inhibition of USP14 Suppresses Ferroptosis and Inflammation in LPS‐Induced Goat Mammary Epithelial Cells Through Ubiquitylating the IL‐6 Protein.” Hereditas 159, no. 1: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Lu H., Li W., et al. 2025. “Ferroptosis Induces Gut Microbiota and Metabolic Dysbiosis in Collagen‐Induced Arthritis Mice via PAD4 Enzyme.” Gene 936: 149106. [DOI] [PubMed] [Google Scholar]
- Zhuge, A. , Li S., Yuan Y., et al. 2023. “Microbiota‐Induced Lipid Peroxidation Impairs Obeticholic Acid‐Mediated Antifibrotic Effect Towards Nonalcoholic Steatohepatitis in Mice.” Redox Biology 59: 102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, Y. , Henry W. S., Ricq E. L., et al. 2020. “Plasticity of Ether Lipids Promotes Ferroptosis Susceptibility and Evasion.” Nature 585, no. 7826: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, Y. , Li H., Graham E. T., et al. 2020. “Cytochrome P450 Oxidoreductase Contributes to Phospholipid Peroxidation in Ferroptosis.” Nature Chemical Biology 16, no. 3: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu, M. , Liu G., Xu H., et al. 2024. “Extracellular Vesicles From Nanomedicine‐Trained Intestinal Microbiota Substitute for Fecal Microbiota Transplant in Treating Ulcerative Colitis.” Advanced Materials 36, no. 41: e2409138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


