Abstract
Background
Intake of glucose and sucrose accelerates the development of dental caries in children. Scardovia wiggsiae (S. wiggsiae) is a potential pathogen of early childhood caries (ECC). Previous research conducted by our group has demonstrated that rubusoside, a non-cariogenic sweetener, inhibits the caries pathogen Streptococcus mutans (S. mutans). To investigate the effects of rubusoside as a sucrose substitute and sweet additive on the growth and metabolism of S.wiggsiae and to predict the underlying mechanisms.
Results
The minimum inhibitory concentration (MIC) of rubusoside was determined to be 1% using the microdilution method. Both as a standalone agent and in combination with sucrose, rubusoside significantly inhibited the growth, acid-producing capability, and adhesion of S.wiggsiae. Utilizing techniques such as crystal violet staining, anthrone-sulfuric acid reaction, BCA protein quantification, scanning electron microscopy (SEM), and confocal laser scanning microscopy (CLSM), we demonstrated that rubusoside effectively suppressed the formation of S. wiggsiae biofilms and reduced the synthesis of extracellular polysaccharides (EPS) and soluble proteins. The whole genome sequencing of S. wiggsiae was conducted for the first time and combined with RNA-seq analysis, the potential mechanism of rubusoside inhibiting bacterial biofilm formation was predicted: It might exert bacteriostatic effects by influencing bacterial carbohydrate metabolic pathways (such as nucleotide sugar synthesis, amino sugar and nucleotide sugar metabolism, galactose metabolism, and phosphotransferase system), and downregulating the expression of virulence genes (clpP and groEL) related to adhesion, invasion, and biofilm formation.
Conclusions
Rubusoside exhibits a significant bacteriostatic effect against S. wiggsiae, a potential pathogen associated with early childhood dental caries. Notably, its inhibitory activity remains unaffected even in the presence of sucrose. Consequently, as a sucrose substitute or sweetener additive, rubusoside holds broad application prospects in both the food industry and oral hygiene products, offering a promising approach to the prevention and treatment of dental caries.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12915-025-02321-9.
Keywords: Rubusoside, Scardovia wigggsiae, Early childhood caries, RNA sequencing, Metabolism
Background
ECC, previously referred to as baby bottle tooth decay, is a severe chronic disease affecting children. ECC is a prevalent dental condition observed in children and is defined as the presence of one or more decayed, missing, or filled tooth surfaces (dmfs) in any primary tooth of children aged 71 months or younger due to caries. According to the guidelines provided by the American Academy of Pediatric Dentistry, if the dmfs score for children under 3 years of age reaches or exceeds specific age thresholds (4 at age 3, 5 at age 4, and 6 at age 5), it indicates severe early childhood caries [1]. A study has revealed that the incidence rate of deciduous teeth caries ranked fifth among 328 diseases, including low back pain [2]. According to a summary paper presented at the 2018 International Association of Pediatric Dentistry conference, ECC has shown an annual increasing trend among children aged 1–5 years, with rates of 36, 43, 55, and 63%, respectively, underscoring the urgent need for attention and intervention in early childhood oral health [3]; Additionally, data from the 2018 Fourth National Oral Health Epidemiological Survey in China revealed that the prevalence of ECC among 3–5-year-old children in China is as high as 62.5% [4]. These studies indicate that ECC is a chronic infectious disease with a relatively high incidence and prevalence among children [5].
These findings highlight the importance of children’s oral health and call for enhanced implementation of oral health education and early preventive measures to reduce the occurrence and progression of ECC, thereby safeguarding children’s overall health. Therefore, the prevention of caries is the most effective strategy to reduce the adverse consequences of ECC. Research has shown that ECC is primarily caused by long-term consumption of sugary substances and an imbalance in oral microbiota. For the former, if the daily sugar intake of children aged 1 to 3 is more than 32 g and that of children aged 4 to 6 is more than 44 g, it will increase the risk of dental caries in children [6]. The latter is rich in acid-producing bacteria [7], which primarily metabolize sugar to generate lactic acid and synthesize extracellular polysaccharide polymers, further lowering the pH of the local oral environment. This acidic environment promotes the formation of biofilms, providing protection for bacteria and allowing them to thrive and reproduce stably in the oral cavity. This process ultimately leads to the demineralization of dental hard tissues, where mineral substances are lost from the tooth structure, thereby causing dental caries [8, 9]. Extensive research indicates that acid-tolerant S. mutans is the main pathogen of ECC [10–13]. However, in clinical practice, S. mutans is not always detected in cases of childhood dental caries [14, 15]. This finding challenges the view that S. mutans is the sole indicative pathogen of childhood dental caries and suggests that the development of caries may involve multiple pathogens. Several studies indicate a correlation between ECC and an unnamed species of Bifidobacteria [16–18], which was named S.wiggsiae in 2011 [19]. Tanner et al. [20] found that S. wiggsiae was present in the carious oral cavities of children without S. mutans infection, suggesting its unique role in the destructive process of tooth decay. S. wiggsiae has also been found in the necrotic pulp of primary teeth [21]. A recent study revealed higher levels of S. wiggsiae among children who underwent orthodontic treatment during the early stages of caries development [22]. The aforementioned studies indicate a significant correlation between S. wiggsiae and ECC, thereby identifying it as a potential pathogen.
Studies on the epidemiology, etiology, and clinical observations of ECC have demonstrated that dairy and starchy foods consumed by children contain high levels of sucrose [3, 23]. Frequent intake can enable the plaque biofilm to continuously metabolize and produce acid, maintaining a low pH environment. When the stimulation exceeds the host’s defense ability (such as saliva buffering), it can erode tooth enamel, representing the most direct cause of caries [24]. Consuming a diet rich in exogenous sugars plays a crucial role in the development and progression of ECC. [25–27]. Strict and long-term restriction of sucrose consumption would undoubtedly reduce the incidence of caries. However, due to children’s dietary structure characteristics, it is not practical to limit sucrose usage without providing alternatives. Thus, finding a sugar substitute that is both highly sweet and non-cariogenic has been a focal point in both domestic and international research on dental caries prevention.
The exploration and application of natural sweeteners are becoming increasingly widespread. These sweet components, primarily extracted from plants, grains, fruits, and vegetables [28], include sugar alcohols and glycosides. In daily life, they are predominantly utilized as food additives and flavor enhancers. Additionally, certain natural sweeteners, such as stevia, can serve as flavor correctors in traditional Chinese medicine preparations to mitigate the bitterness of medicinal products [29]. Rubusoside is extracted from the leaves of Rubus suavissimus S. Lee, a plant belonging to the Rosaceae family. Its sweetness is approximately 300 times greater than that of sucrose while containing only 1% of the caloric content of sucrose [30]. Both animal and cell experiments have confirmed its minimal toxic side effects [31]. Our research group has previously confirmed that rubusoside downregulates the adhesion genes spaP and gbpB of the S. mutans biofilm, as well as the polysaccharide synthesis genes gtfB, gtfC, gtfD, and ftf, the acid production and acid resistance genes ldh and atpF, and the stress regulatory genes vicR and comD, thereby inhibiting the growth and metabolism of the S. mutans biofilm [32]. However, to date, all previous studies have focused on the effect of rubusoside on the cariogenic properties of S. mutans without investigating those of S.wiggsiae. To investigate the cariogenic potential of S. wiggsiae, we reviewed existing studies that examined the bacterium’s specific mechanisms of sugar metabolism: the absence of arginine deiminase limits the increase in ammonia production, which maintains a low pH environment; the acid produced during metabolism lowers the pH of the oral environment, inducing demineralization of hydroxyapatite; even when the environmental pH value decreased to 3.5, it did not compromise the acid-producing ability, indicating a robust tolerance to acidic conditions [33]. In summary, given that S. wiggsiae is an acid-producing bacterium similar to S. mutans, we hypothesized that rubusoside may influence its growth pattern along with other biological characteristics.
In conclusion, it is imperative to investigate the impact of non-cariogenic sweeteners on oral microbiota. Consequently, our research group will continue to explore the effects of the natural sweetener rubusoside on the growth and metabolism of S. wiggsiae. For this purpose, we have established in vitro models of planktonic bacterial cultures and biofilms. This study marks the first investigation to demonstrate the efficacy of rubusoside, a natural sweetener, in effectively inhibiting the cariogenic effects caused by S. wiggsiae, a recently identified bacterium associated with dental caries. Consequently, rubusoside exhibits promising potential as an innovative and advanced alternative to xylitol because of its cariostatic properties.
Results
MIC measurement
The MIC determined by the microdilution method revealed that rubusoside inhibits the growth of planktonic S. wiggsiae DSMZ22547 bacteria. The minimal inhibitory concentration is the absence of visible bacterial growth to the naked eye at a concentration of 1% rubusoside (Additional file 1: Fig. S1).
Rubusoside inhibits the planktonic growth of S. wiggsiae
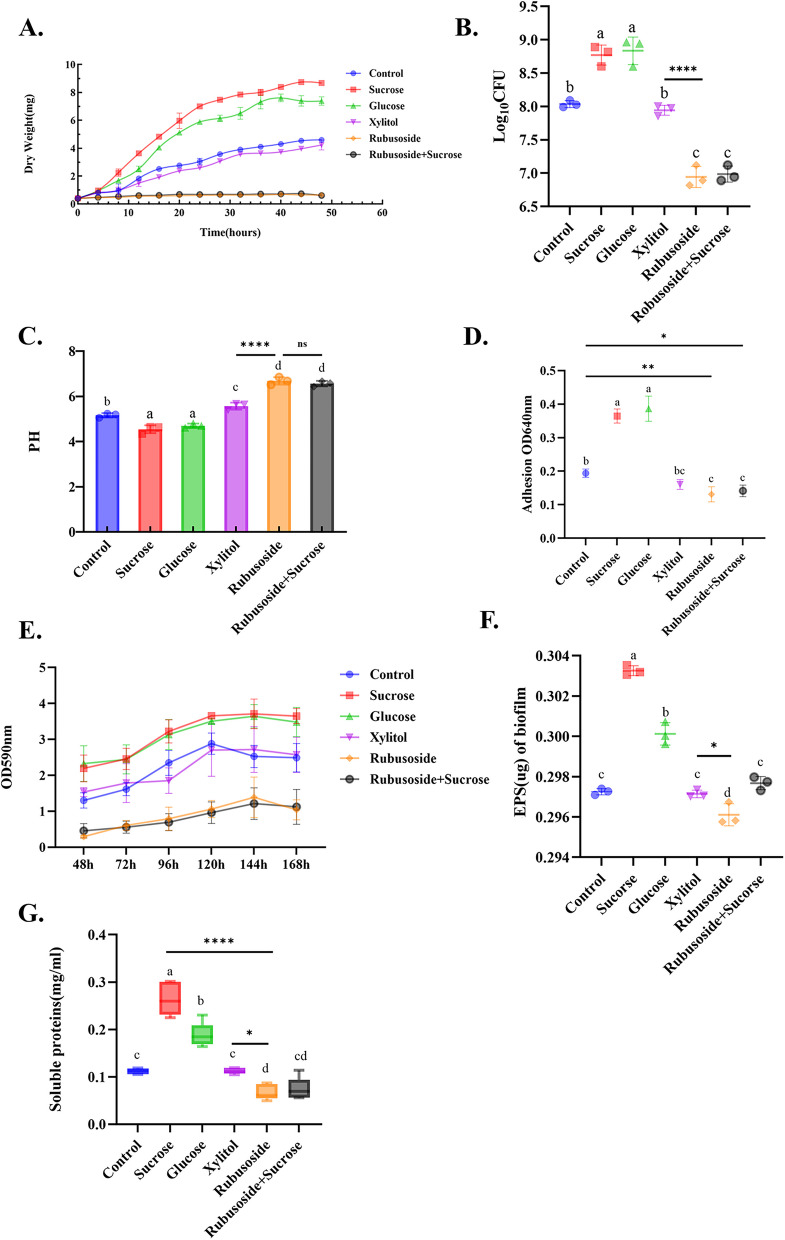
The cell dry weight of each group was compared (Fig. 1A). It was found that the concentration of 1% saccharides, sucrose > glucose > xylitol > rubusoside, rubusoside + sucrose. Furthermore, the cumulative biological production of the first three groups showed a steady increase over time. The xylitol group demonstrated a similar yield to the blank control group (P > 0.05), rubusoside exhibited the lowest yield and a significant inhibitory effect (P < 0.001). Interestingly, when sucrose was used in combination with rubusoside at an equivalent concentration, rubusoside inhibited the promoting effect of sucrose (P < 0.001).
Fig. 1.
Effects of rubusoside,sucrose + rubusoside, xylitol, glucose, and sucrose on the cell growth of S. wiggsiae in an anaerobic environment at 35 °C. Dynamic dry weight of cells at 48 h (A), quantitative statistics of plate colony counts (B), PH value at 48 h (C), and determination of bacterial adhesion (D). The growth curves (E) of S. wiggsiae biofilm formation in each group at 48 h, 72 h, 96 h, 120 h, 144 h, and 168 h were determined by crystal violet staining. The contents of EPS (F) and soluble protein (G) in the 96 h S. wiggsiae biofilm. All experiments were conducted with three replicates , n=3, and the values are presented as mean ± SD. Specific values are provided in Additional file 2. Significance was determined by using one-way analysis of variance and Tukey's multiple comparison test. Different letters represent significant differences among treatments. Statistical significance is marked with asterisks: *, P<0.05; **, P<0.01; ***, P<0.001
Figure 1B shows the viable bacteria counts of colonies cultured in the planktonic state for each group. Our findings revealed that the two groups added with rubusoside significantly reduced the viable bacterial counts compared to xylitol, glucose, sucrose, and blank control (P < 0.05). The group treated with xylitol showed slightly lower results compared to the blank control group.
Comparisons were made regarding the pH of the culture medium after 48 h of S. wiggsiae growth in different treatment groups (Fig. 1C). The results demonstrated that the pH values in both the sucrose and glucose groups were significantly lower than those in the other groups (P < 0.001), with both values falling below 5.5. Previous research has established that the critical pH threshold for apatite dissolution in saliva is 5.5 [34]. The pH level in the xylitol group was significantly higher compared to both the rubusoside and rubusoside + sucrose groups (P < 0.001), which indicated that the ability of S. wiggsiae to produce acid by using rubusoside was weak.
Influence of rubusoside on adherence
The impact of various saccharides on the adhesion ability of S. wiggsiae is shown in Fig. 1D. The glucose and sucrose groups exhibited the highest adhesion ability, with no significant difference between them (P > 0.05). Rubusoside resulted in the lowest bacterial adhesion, even when co-cultured with sucrose (P < 0.001). Additionally, there was no significant difference in adhesion between the xylitol and blank control groups. Planktonic bacteria adhere to the tooth surface and aggregate with epithelial cells, proteins, and other matrix components to form a pathogenic biofilm [35]. These results suggest that rubusoside decreased biofilm formation to some extent (P < 0.05).
Rubusoside inhibits S. wiggsiae biofilm accumulation
As shown in Fig. 1E, crystal violet staining analysis of biofilm growth revealed that the blank control group reached its peak biofilm formation at 120 h, whereas the other groups reached their peak at 144 h. The biofilm formation in the sucrose treatment group was not significantly different from that in the glucose group, yet it was markedly higher compared to the blank control group, the xylitol group, the rubusoside group, and the rubusoside + sucrose group (P < 0.05). Notably, rubusoside treatment resulted in the lowest amount of biofilm formation, indicating that it inhibited the formation and accumulation of bacterial biofilm (P < 0.05).
Table 1 presents the biofilm pH values of six groups for each time point. The acid production ability of the sucrose group was significantly higher than that of the xylitol and rubusoside groups at each time point (P < 0.05), while that of the xylitol group was not significantly different compared to the blank control group (P > 0.05). The pH of the sucrose and xylitol groups decreased gradually over time (P < 0.05). The pH values of the rubusoside and rubusoside + sucrose groups also slightly decreased but remained closer to 7 (neutral environment) compared to the other groups (P < 0.05), which did not change with time (P > 0.05).
Table 1.
Acid production of S. wiggsiae biofilm
| Time (h) | pH of spent medium at the end of each time points | |||||
|---|---|---|---|---|---|---|
| Control | Sucrose* | Glucose | Xylitol* | Rubusoside | Rubusoside + Sucrose | |
| 48h | 5.08 ± 0.03b | 4.52 ± 0.05a | 4.48 ± 0.03a | 4.82 ± 0.29c | 6.55 ± 0.01d | 6.48 ± 0.02d |
| 72h | 4.58 ± 0.02b | 4.08 ± 0.04a | 4.09 ± 0.15a | 4.55 ± 0.09b | 6.45 ± 0.07c | 6.48 ± 0.04c |
| 96h | 4.39 ± 0.02b | 4.06 ± 0.03a | 4.08 ± 0.02a | 4.39 ± 0.02b | 6.44 ± 0.03c | 6.34 ± 0.05c |
| 120h | 4.37 ± 0.02b | 4.05 ± 0.06a | 4.03 ± 0.03a | 4.38 ± 0.02b | 6.39 ± 0.07c | 6.32 ± 0.07d |
| 144h | 4.34 ± 0.02b | 3.96 ± 0.02a | 3.92 ± 0.02a | 4.36 ± 0.03b | 6.31± 0.06c | 6.22 ± 0.05c |
*, P < 0.05, indicates that the pH of this group changes statistically over time
The pH value was analyzed by linear regression analysis, repeated measures analysis of variance, and Tukey’s test. All experiments were conducted with three replicates, n = 3, and the values are presented as mean ± standard deviation. Different letters represent that there are statistically significant differences in pH between groups at the same time point. *, P < 0.05, indicates that the pH of this group changes statistically over time.
The amount of water-insoluble exopolysaccharides produced by the bacterial biofilms is shown in Fig. 1F. Among the groups, the sucrose and glucose groups showed significant increases in the number of water-insoluble exopolysaccharides compared to the blank control group (P < 0.0001). The yield of rubusoside group was significantly lower than that of the xylitol and blank control groups (P < 0.05) but not significantly different from that of the xylitol and blank control groups (P > 0.05). In addition, the yield of the combination of rubusoside and sucrose group was higher than that of rubusoside alone (P < 0.05).
Figure 1G shows the protein content in the biofilm. The content of biofilm protein was the highest in the sucrose medium, which was significantly higher than that in the glucose and blank control groups (P < 0.0001). No significant difference in protein content was found between the xylitol and blank control groups (P > 0.05). Compared to the xylitol group, the protein yield decreased in the rubusoside group; however, when rubusoside was combined with sucrose, the inhibitory effect was not obvious (P > 0.05).
All experiments were conducted with three replicates, n = 3, and the values are presented as mean ± SD. Specific values are provided in Additional file 2. Significance was determined by using one-way analysis of variance and Tukey’s multiple comparison test. Different letters represent significant differences among treatments. Statistical significance is marked with asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Observation of biofilms by SEM
Biofilm formation and bacterial morphology were observed using SEM. As shown in Fig. 2, compared with the blank control group, the biofilm formed by cell stacks in the sucrose and glucose groups exhibited a dense and thicker structure, with the extracellular matrix showing small spherical structures. Under microscopic observation, most cells were bifurcated in a “Y” shape, a small portion were rod or stick shaped, and the cell length was longer than that of the blank control group. No significant difference was found between the xylitol and blank control groups. In the xylitol group, most cells showed a short rod shape, whereas a few showed a “V” shape. Furthermore, the number of bacteria was significantly reduced in the two experimental groups with rubusoside, and the biofilm was scattered and relatively sparse. Under a × 10,000 microscope, it was found that the bacteria were scattered in short rod shapes, and the cell length was shorter than that in the sucrose and glucose groups.
Fig. 2.
Characteristics of the effects of rubusoside on S.wiggsiae biofilm morphology and bacterial distribution by scanning electron microscopy. The microimages were taken at × 1000 (scale bar: 10 μm), × 5000 (scale bar: 2 μm), and × 10,000 (scale bar: 1 μm) magnifications. The red arrows indicate the formation of extracellular matrix. For each treatment, the images were acquired from three different areas
Confocal laser scanning microscopy analysis
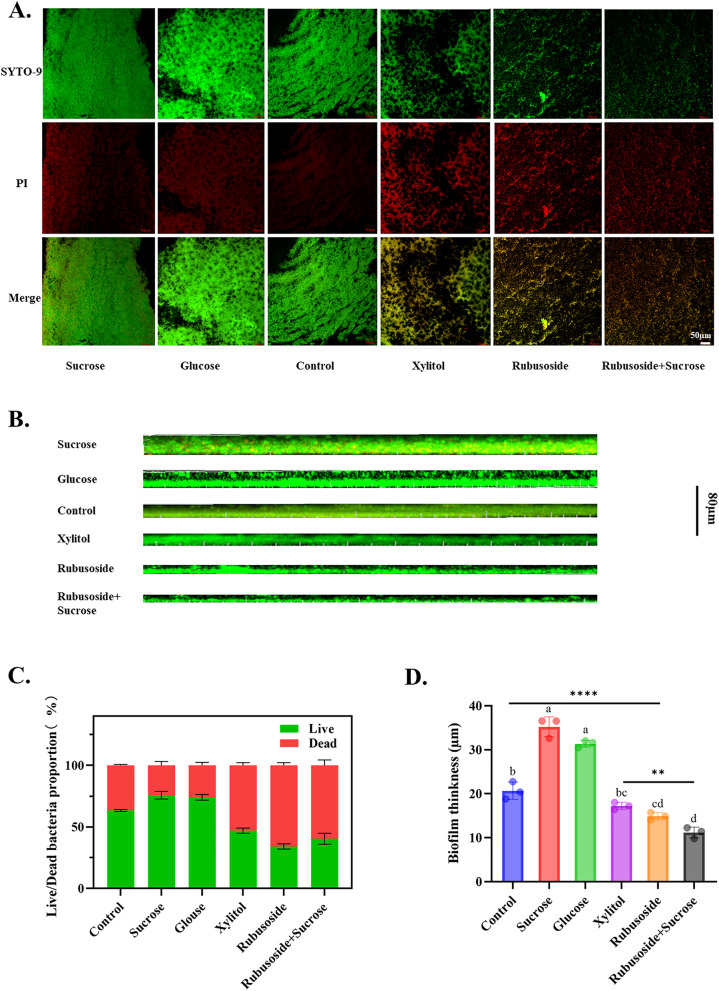
As depicted in Fig. 3A and C, the biofilms in the sucrose, glucose, and blank control groups exhibited consistent green fluorescence overall, indicating intact cell membranes that were predominantly occupied by viable bacteria. Conversely, treatment with xylitol resulted in a yellow-green fluorescence with an increased intensity of red fluorescence, suggesting an elevated proportion of dead bacteria. Both rubusoside alone and rubusoside combined with sucrose mainly showed heterogeneous yellow–red fluorescence, and the fluorescence area was reduced. This suggests that the biofilms in these groups had reduced integrity and a sparse distribution, coinciding with an increased proportion of dead bacteria. Figure 3B and D show that both sucrose and glucose significantly increased the biofilm thickness compared to the blank control group. However, both xylitol and rubusoside effectively inhibited biofilm formation, decreased bacterial aggregation, and led to a significant reduction in biofilm thickness. Notably, even when rubusoside was used in conjunction with sucrose, it still resulted in a markedly thinner biofilm.
Fig. 3.
Live/dead staining images of S. wiggsiae biofilms cultured with rubusoside. A Images of live/dead stained biofilms. Green (SYTO-9) and red (PI) represent live and dead bacteria, respectively. Scale bar: 50 μm. B Effect of rubusoside on S. wiggsiae biofilm thickness. Scale bar: 80 μm. C The proportion of live and dead bacteria in the S. wiggsiae biofilm. D To evaluate the biofilm thickness of S. wiggsiae. Live, dead, and total bio-area were calculated based on image analysis and data from the ImageJ software. For each treatment, the images were acquired from three different areas. Significance was determined by using one-way analysis of variance and Tukey’s multiple comparison test. Specific values are provided in Additional file 2. Different letters represent significant differences among treatments. Statistical significance is marked with asterisks: **, P < 0.01; ****, P < 0.0001
Characteristics of the whole genome
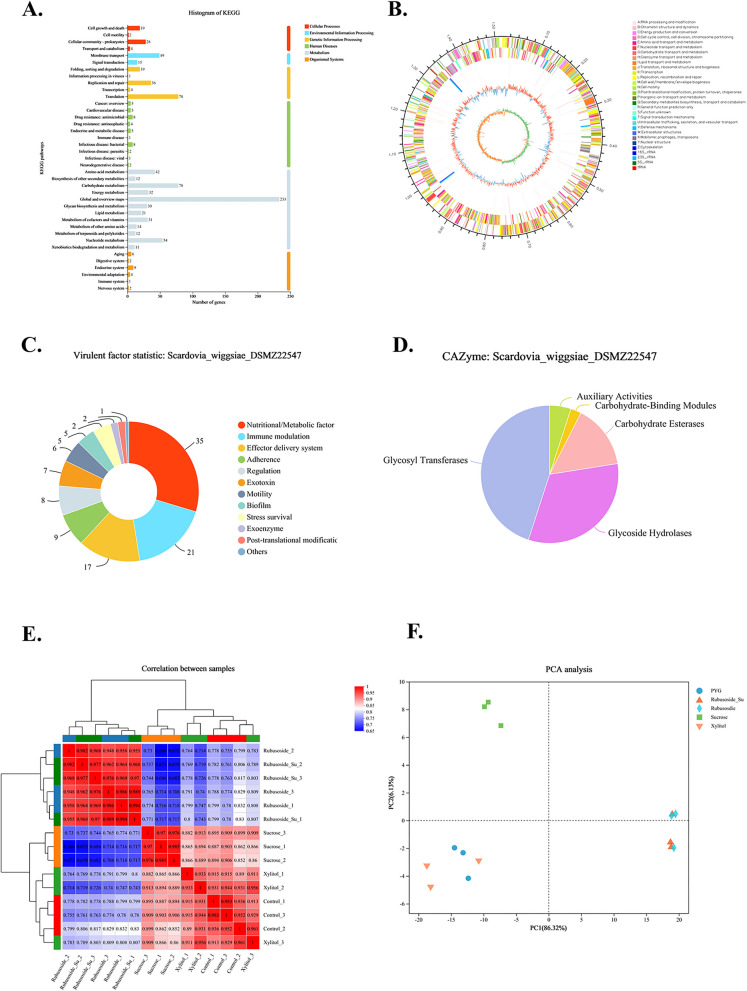
The complete genome of S. wiggsiae DSMZ22547 was assembled using Illumina and PacBio sequencing data, resulting in the successful acquisition of a single chromosome devoid of any plasmids (Fig. 4B). According to Additional file 1: Table S1, the genome size was 1,518,528 bp, with a GC content of 52.99%. The Q30 base percentage exceeded 96.94%, and 957 and 743 genes were annotated in the GO and KEGG databases, respectively. KEGG pathway analysis revealed 12 metabolic pathways, with membrane transport accounting for the largest proportion, while the extracellular polysaccharide-related functional genes were labeled with membrane transport function (Fig. 4A). A total of 118 virulence genes were annotated in the Virulence Factor Database (VFDB), with metabolism, immunity, effector delivery system, and adhesion being the major contributing factors (Fig. 4C). We annotated five gene classes in the Carbohydrate Active Enzyme database (CAZy, http://www.cazy.org/) (Fig. 4D), among which glycoside hydrolase and glycosyltransferase-related genes accounted for the largest proportion. These results indicate that S. wiggsiae has strong carbohydrate metabolism, amino acid metabolism, and membrane transport ability.
Fig. 4.
The whole genome of S.wiggsiae DSMZ22547. A KEGG annotation of the S. wiggsiae DSMZ22547. The ordinate represents the level2-level classification of the KEGG pathway, and the abscissa represents the number of genes annotated under this classification. Different column colors represent the level1-level classification of the KEGG pathway. The rightmost column indicates the number of genes under different level1 classifications. B Ring chromosome of S. wiggsiae DSMZ22547. Six circles make up the genomic map. Information about the genome of each circle is displayed from the outside to the inner: (1) genome size, (2) GOC functional classification of forward CDS, (3) GOC functional classification of reverse CDS, (4) rRNA and tRNA, (5) G + C content, and (6) GC skew. Summary statistics of virulence factor gene annotation (C) and carbohydrate active enzyme annotations (D). Gene expression patterns of samples from each group, n = 3. E Gene expression correlation plots. F Principal component analysis (PCA) plot of gene expression
Transcriptome sequencing analysis
In this experiment, a total of 15 samples of sucrose, xylitol, rubusoside、rubusoside + sucrose, and blank control groups were sequenced to complete the transcriptome analysis. A total of 58.04 GB of clean data were obtained. Base quality value is a critical metric for assessing sequencing quality; Q30 is used to evaluate sequencing quality based on the probability of incorrect base recognition. A high Q30 (above 96.94%) indicates high-quality data (Additional file 1: Table S2) [36].
As shown in Fig. 4E, the Pearson correlation coefficient (R2) between samples within the same group was consistently above 0.91. The Pearson correlation coefficient (R2) among samples within the same group was consistently above 0.91, indicating a good correlation between biological replicates. In Fig. 4F, PCA revealed that samples treated with rubusoside were negatively distributed along PC2, while the xylitol-treated group shifted along PC1 and the sucrose-treated group was predominantly positively distributed along PC2. These findings suggest that different treatments led to distinct patterns of gene expression at the transcriptome level.
Effect of rubusoside on the transcription profile of S. wiggsiae
The names of the differentially expressed gene sets compared to sucrose and xylitol are “rubusoside vs. sucrose” and “rubusoside vs. xylitol,” respectively.
GO enrichment analysis of differentially expressed genes (DEGs)
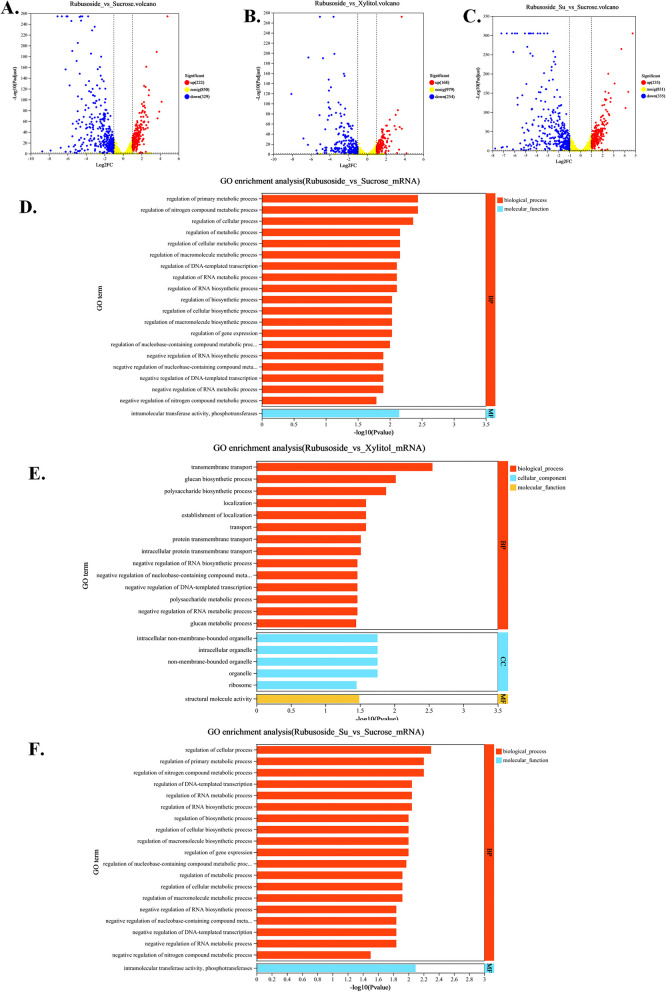
As shown in Fig. 5A and B, there were 551 and 422 differential genes in “rubusoside vs. sucrose” and “rubusoside vs. xylitol,” respectively. Compared to sucrose, the DEGs following rubusoside treatment were significantly enriched in the top 20 GO terms (Fig. 5D), notably in the regulation of nitrogen compound metabolic processes, regulation of cellular processes, and regulation of metabolic processes. This indicates that rubusoside significantly influences cellular growth and metabolism. The DEGs from “rubusoside vs. xylitol” were enriched in the top 20 GO terms, including transmembrane transport, cellulose and polysaccharide biosynthetic processes, and establishment of localization, intracellular organelles, and non-membrane-bounded organelles (Fig. 5E). Rubusoside affects energy transport, cell growth, and aggregation.
Fig. 5.
Volcano plot (log2(|fold change|) ≥ 1, P value < 0.05) of DEGs, GO, and KEGG enrichment analysis were performed for different comparison groups. Volcano plot of DEGs between the A rubusoside and sucrose treatments, B rubusoside and xylitol treatments, and C Rubusoside + Sucrose and Sucrose treatments. Upregulated and downregulated genes are highlighted by red and blue dots, respectively. GO enrichment analyses of D the gene set rubusoside vs sucrose, E the gene set rubusoside vs xylitol, and F the gene set rubusoside + sucrose vs sucrose. Additional file 1: Fig. S2: KEGG enrichment analysis of up and down-regulated genes in the gene set rubusoside vs sucrose. Additional file 1: Fig. S3: KEGG enrichment analysis of up and down-regulated genes in the gene set rubusoside vs xylitol. Additional file 1: Fig. S4: KEGG enrichment analysis of up and down-regulated genes in the gene set rubusoside+sucrose vs sucrose
-
(2)
KEGG pathway analysis
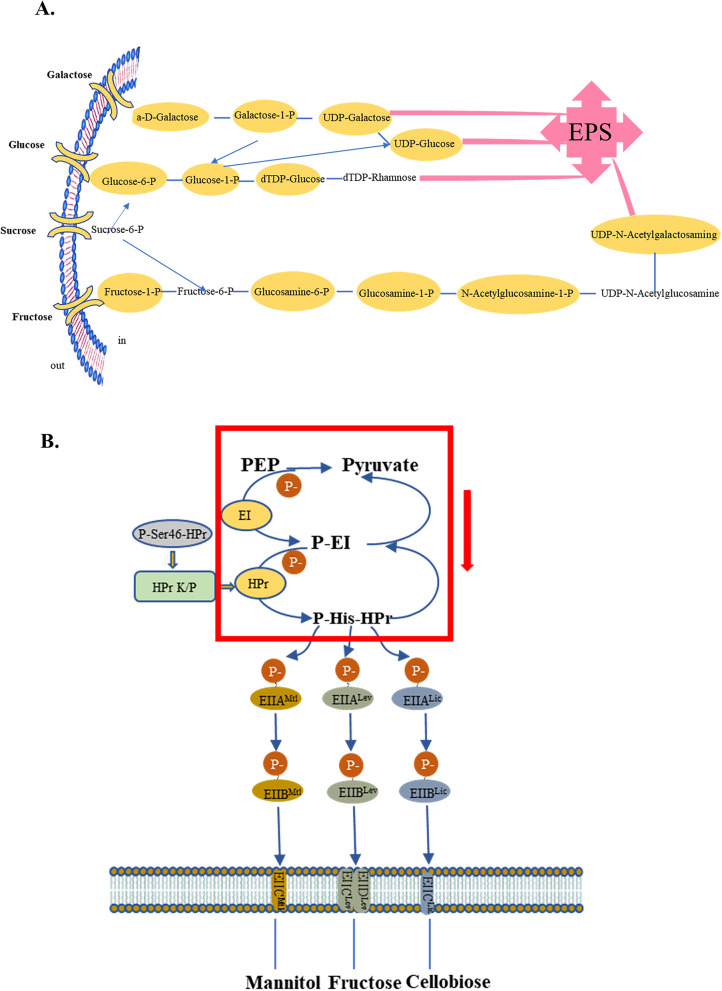
The KEGG database was used to determine the impact of various treatment factors on metabolic pathways in this study. As shown in Additional file 1: Fig. S2B, “rubusoside vs. sucrose” was among the top 20 KEGG pathways enriched by downregulated genes. In the longevity-regulating pathway, clpP and groEL were downregulated, both of which function as virulence genes with adhesion properties. Table 2 presents the key enzymes involved in nucleotide sugar synthesis according to the KEGG database annotation. Among them, scrk (encoding fructokinase), glmM (encoding phosphoglucosamine mutase), glucokinase (glk), galK (encoding galactosidase), and galT (encoding lactose 1-phosphate uridylyl transferase) play a crucial role in metabolizing sucrose, glucose, and galactose to nucleotide sugars. The downregulation of these genes affects the compound highlighted in the yellow box in Fig. 7A, thereby influencing the production of EPS. In addition, galactose metabolism, fructose, and mannose metabolism, phosphotransferase system (PTS), starch and sucrose metabolism, and quorum sensing were not significantly enriched in KEGG, but they might play an important role under the influence of rubusoside.
Table 2.
Key enzymes of the nucleotide sugar biosynthesis process of S. wiggsiae DSMZ22547 annotated in the KEGG database
| Gene Name | KO ID | KO Description |
|---|---|---|
| pgm | K01835 | Phosphoglucomutase [EC:5.4.2.2] |
| scrK | K00847 | Fructokinase [EC:2.7.1.4] |
| galE | K01784 | UDP-glucose 4-epimerase [EC:5.1.3.2] |
| galU | K00963 | UTP–glucose-1-phosphate uridylyltransferase [EC:2.7.7.9] |
| galM | K01785 | Aldose 1-epimerase [EC:5.1.3.3] |
| galT | K00965 | UDP-glucose–hexose-1-phosphate uridylyltransferase [EC:2.7.7.12] |
| galK | K00849 | Galactokinase [EC:2.7.1.6] |
| glgC | K00975 | Glucose-1-phosphate adenylyltransferase [EC:2.7.7.27] |
| glk | K25026 | Glucokinase [EC:2.7.1.2] |
| nagB | K02564 | Glucosamine-6-phosphate deaminase [EC:3.5.99.6] |
| glmM | K03431 | Phosphoglucosamine mutase [EC:5.4.2.10] |
| glmU | K04042 | Glucosamine-1-phosphate N-acetyltransferase [EC:2.7.7.23 2.3.1.157] |
Fig. 7.
Differentially expressed genes affect pathways. A Nucleotide sugar biosynthesis pathways. The downregulation of the expression of the key enzymes in Table 2 will affect the formation of the intermediate products shown in the yellow box in the figure, which may thereby influence the synthesis of the final product EPS. B Phosphorylation cascades. The red box in Figure E and the green box in Fig. 6C represent the same inhibition process. This may affect the subsequent pentose phosphate pathway and glycolysis processes
Compared to xylitol (Additional file 1: Fig. S3B), rubusoside exerted a significant impact on starch and sucrose metabolism. As is shown in Fig. 6A, the operon containing glgB (1,4-α-glucan branching enzyme), glgC (encoding glucose-1-phosphate adenosine transferase), glgE (encoding DUF3416 domain protein), and glgM (encoding glycogen synthase) exhibited downregulation, which has been demonstrated to be associated with the biosynthesis and degradation of intracellular polysaccharides. Furthermore, scrk expression was also decreased, thereby affecting the conversion of fructose to fructose-6-phosphate (Fig. 6A). Moreover, in the galactose metabolism pathway (Fig. 6B), the genes galK, galT,and galA (encoding α-galactosidase) were also downregulated, thereby affecting the Leloir pathway responsible for D-galactose degradation. In addition, ptsI (encoding cytosolic Phosphotransferase) and ptsH (encoding histidine phospho carrier protein) were downregulated in PTS, further influencing the cascade reaction process within this pathway (Fig. 6C). Rubusoside also influenced KEGG pathways, including nucleotide sugar biosynthesis, ABC transport protein, and amino sugar.
Fig. 6.
Differentially expressed genes enriched KEGG pathway. A “Starch and sucrose metabolism.” B “Galactose metabolism.” C “Phosphotransferase system.” The yellow background color represents downregulation. glg operons (glgB, glgC, glgE, glgM), scrk, galK, galT, galA, PtsI, and PtsH genes are highlighted with a green border
Compared to sucrose, upregulated genes enriched in KEGG pathways (Additional file 1: Fig. S2A) involve quorum sensing, protein secretion systems, and ribosomes. Among these, 11 genes are enriched in the quorum sensing pathway, which is likely to affect the biofilm formation of S. wiggsiae. In contrast to xylitol, upregulated genes significantly enrich bacterial secretion systems, ribosomes, homologous recombination, RNA degradation and quorum sensing (Additional file 1: Fig. S3A).
KEGG enrichment analysis revealed that rubusoside may exert antibacterial effects through the regulation of amino acid metabolism, carbohydrate metabolism, cell growth, and other signaling pathways.
Global response of rubusoside as a sweet flavor supplement
Next, the DEGs in the sucrose and rubusoside + sucrose groups were subjected to GO and KEGG enrichment analyses. As shown in Fig. 5C, 570 DEGs were identified between the group treated with rubusoside + sucrose and the group treated with the same concentration of sucrose. The gene set was named “rubusoside + sucrose vs. sucrose.”
After conducting GO analysis annotation (Fig. 5F), we observed significant enrichment in the metabolic pathways of pyruvate, glycolysis, and regulation of cellular processes. The KEGG enrichment analysis results are presented in Additional file 1: Fig. S4A and B and resemble the enrichment outcomes obtained for "rubusoside vs. sucrose".
Discussion
Dental caries, commonly referred to as tooth decay, is a multifactorial disease characterized by plaque biofilm-mediated, sugar-driven progressive, and destructive changes in the hard tissues of teeth [37]. The formation of dental plaque biofilm refers to the continuous dynamic process of adsorption, growth, movement, and re-adsorption of free bacteria on the tooth surface within the oral cavity [38]. If not promptly removed from the tooth surface, long-term accumulation of plaque can gradually lead to macroscopic pigmentation formation and subsequent enamel demineralization and damage [39]. In addition, some plaque will combine with the minerals in saliva, calcify, and harden to form odontoliths and cause periodontal inflammation.
In recent years, it has been discovered that S. wigggsiae plays a significant role in ECC and may even be a pathogenic bacterium [16, 17, 19, 20, 22]. Compared to adults, children lack awareness and the ability to clean their mouths, and parents lack the awareness and knowledge of oral hygiene for children, so the diet is particularly important. Consequently, if we can reduce the intake of refined carbohydrates and sugars in our daily diet, and reduce the energy required for bacterial growth and the production of acid metabolites, we can largely control the occurrence and progression of caries. Rubusoside is predominantly cultivated in provinces such as Guangdong, Guangxi, Hunan, and Jiangxi in China [40], and it possesses the triple attributes of tea, sugar substitute, and medicinal herb. However, limited research hinders the application. Our group has previously demonstrated that rubusoside effectively inhibits the growth of S. mutans, a cariogenic pathogen, demonstrating promising anti-caries properties. In this study, we aimed to assess the inhibitory effects of xylitol, glucose, sucrose, and rubusoside individually and their combination on both planktonic bacteria and biofilms. Furthermore, we aimed to elucidate the antibacterial properties of rubusoside and explore its specific mechanism through transcriptomics.
In this study, we demonstrate for the first time that rubusoside exerts inhibitory effects on S. wiggsiae. To determine the experimental concentration, we conducted studies on samples in both planktonic and biofilm states based on the results of the microdilution experiment. By comparing the growth and metabolism of S. wiggsiae when cultivated with sucrose, glucose, and xylitol, we observed that rubusoside had a significant inhibitory effect on this bacterium. Specifically, rubusoside significantly reduced the number of viable bacteria; decreased bacterial acid production, lowered yields of water-insoluble extracellular polysaccharides and proteins, and diminished bacterial adhesion ability, thereby inhibiting biofilm formation. Notably, even when combined with sucrose, its inhibitory effect remains significant. These findings provide robust theoretical support for the potential of rubusoside as a sucrose substitute and sweetener supplement. It is anticipated that rubusoside can be applied in various dental products, such as toothpaste (as an alternative to sugar in sugar-containing toothpaste) [41], bitter medicines (to enhance palatability) [42], and other oral care products like xylitol.
Our research results indicate that the MIC of rubusoside is 1%. The cell dry weight and viable bacteria counts, when exposed to 1% sugar in each group, reveal that both sucrose and glucose promote bacterial growth. Conversely, rubusoside and rubusoside + sucrose exhibit inhibitory effects on bacterial growth, which are more pronounced than those observed with xylitol. Additionally, a similar trend was observed in terms of acid production by cellular metabolism. Notably, our experimental findings show that xylitol also allows bacteria to maintain significant acid-producing ability. This phenomenon may be attributed to the absence of an “ineffective metabolic cycle” during xylitol metabolism, thereby providing energy for cellular functions and creating favorable living conditions; alternatively, it could be that the MIC of xylitol has not been reached.
Bacterial adhesion is a prerequisite for the formation of bacterial biofilms. However, preventing bacterial adhesion and reducing biofilm formation effectively mitigate disease risk [35]. The results of the bacterial adhesion experiment provide us with a conjecture that “rubusoside significantly inhibits the formation of S. wiggsiae biofilms compared with sucrose, glucose, and xylitol”, and this conjecture has finally been confirmed. The formation of bacterial biofilms is a dynamic process in which EPS plays a crucial role in developmental stages such as adhesion, structural support, mechanical stability, and protection [43]. Thus, the increase in EPS is generally associated with the enhancement of S. wiggsiae biofilms. As shown in Fig. 1F, the EPS produced by bacteria under the influence of rubusoside is significantly lower compared to those in the sucrose and glucose groups. Furthermore, SEM revealed that bacterial biofilms formed under rubusoside treatment are dispersed and sparse, whereas biofilms generated from sucrose and glucose treatments exhibit a stacked and dense structure. CLSM also demonstrated a significant increase in dead cells within biofilms treated with rubusoside, along with a notably reduced biofilm thickness compared to the sucrose group. In summary, rubusoside effectively inhibited the growth and metabolic activity of S. wiggsiae, decreased biofilm accumulation, and prevented the onset of dental caries. This is similar to the result of our previous finding that rubusoside has a good antibacterial effect on S. mutans, providing a reference for the subsequent exploration of related antibacterial mechanisms, including the mining of virulence genes such as adhesion and the changes of key enzymes in metabolic pathways. Importantly, rubusoside maintains its inhibitory effect even in the presence of sucrose supplementation. Therefore, rubusoside can be used not only as a non-cariogenic sweetener but also as a supplement to reduce sucrose consumption by bacteria.
The transcriptome analysis initially compared the effects of rubusoside with those of sucrose and xylitol on bacteria. Based on the GO enrichment results, it was observed that rubusoside potentially inhibits S. wiggsiae by modulating biological processes such as RNA synthesis and metabolism, intracellular nitrogen metabolism, and cell metabolism. Compared to xylitol (Additional file 1: Fig. S3B), rubusoside may exert its impact on bacteria through the regulation of proteins and polysaccharides involved in transmembrane transport (ABC transport system).
KEGG enrichment analysis shows that rubusoside decreased the expression of clpP encoding genes of longevity pathway compared with sucrose, control bacterial invasion, biofilm formation, and stress tolerance. Consequently, it actively participates in the regulation of cell growth processes and homeostasis [44, 45]. Furthermore, clpP has also been annotated as a virulence factor of S. wiggsiae. The clpP gene has been identified as a potential target for antibacterial activity in studies on Staphylococcus aureus [46]. The groEL gene in this pathway is also associated with virulence, and GroEL is its cognate gene. At present, several broad-spectrum antibacterial drugs targeting this gene as an antibacterial candidate have been developed and tested [47, 48]. The GroEL gene of Actinomycetes, Helicobacter pylori, and Fusobacterium difficile is involved in the adhesion or invasion of various target cells or tissues [49–51]. YAO Ling [52] found that the molecular chaperone GroEL affects the folding and assembly of the nascent peptide chain and the renormalization process of the denatured protein. If protein folding occurs slowly or incorrectly, it not only affects the structure and function of the cell but can also lead to cell death. Lastra and Sanchez [53] found that in the biofilm of Porphyromonas gingivalis, its expression was upregulated compared with planktonic bacteria, which has strong autoimmunity [54]. This may be a pathogen of chronic inflammation such as that present in periodontitis. In conclusion, we hypothesize that the action of rubusoside on S. wiggsiae modulates longevity pathways, internal homeostasis, protein stability, and adhesion processes, reducing the production of exopolysaccharides thus reducing the number of microbial communities [55], thereby influencing its own immunogenicity and potentially mitigating the occurrence of chronic inflammation such as that present in dental caries.
Furthermore, rubusoside affects nucleotide sugar synthesis as well as amino sugar and nucleotide sugar metabolic pathways. The former pathway is associated with bacterial EPS formation [56], while the latter pathway is linked to acid production [57]. whereas the latter pathway is linked to acid production [57]. Bacteria can utilize different carbon sources such as glucose and sucrose to synthesize different types of nucleotide sugars. Nucleotide sugars are precursors for EPS biosynthesis. However, under the action of rubusoside, it fails to provide sufficient carbon sources for bacteria, thereby inhibiting the generation of EPS. Interestingly, the glycosyltransferases that are closely related to the regulation of EPS biosynthesis were not enriched. However, they may still play an important role in the action of rubusoside. In the future, we will conduct more in-depth research in this direction to explore. EPS enhances colony adhesion [58] and constitutes approximately 80% of the biofilm dry weight. Through the abovementioned pathways, rubusoside leads to decreased S. wiggsiae EPS and protein production along with diminished bacterial adhesion and biofilm formation, ultimately resulting in reduced acid production.
The carbohydrate metabolic pathways associated with dental caries exhibited significant enrichment, thereby diminishing the carbohydrate metabolism capacity of S. wiggsiae in the rubusoside group. These pathways resulted in reduced energy production and acid products, such as lactic and acetic acid. Bacteria require nutrients and energy from their environment for growth and metabolism. When exposed to rubusoside, bacteria cannot obtain sufficient carbon sources comparable to those provided by sucrose, which inhibits bacterial growth from the outset. As this condition persists throughout the culture period, it maintains the bacteria in a state of nutrient deprivation, leading to diminished overall vitality and increased mortality, ultimately resulting in reduced biofilm formation compared to other groups. Enzymes encoded by DEGs in the starch and sucrose metabolic pathways catalyze the production of carbohydrate intermediates. Inhibition of fructose transferase to impede sucrose metabolism; glgC affects the synthesis of ADP-glucose, which is the exclusive donor of glucose synthesis from starch [59], and may also inhibit and reduce IPS production. Galactose plays a crucial role in glycosylation processes, where it serves as an energy source [60]. Inhibition of intermediates through the downregulation of galK, galT, and galA within the Leloir pathway hampers its metabolism. PTS serves as a pivotal signal transduction pathway that regulates central carbon metabolism. This enzymatic reaction converts phosphoenolpyruvate (PEP) into pyruvate and triggers a phosphorylation cascade [61]. The phosphorylated carbohydrates are readily released into the cytoplasm for sugar metabolism and transport [62]. Figure 7B shows that the pathways within the highlighted red box are downregulated after treatment with rubusoside. This may affect the subsequent pentose phosphate pathway and glycolysis processes. Moreover, it has been reported that this pathway can also regulate bacterial sodium–potassium balance, virulence factor expression, oral bacterial biofilm formation, stress response, and other cellular functions [63–66]. Previous studies have highlighted PTS as an important target for antimicrobial agents [66, 67]. ABC transporters, one of the largest and most ancient protein superfamilies with ATP dependence, facilitate the transmembrane transport of diverse substrates such as metal ions, carbohydrates, amino acids, and inorganic salts by harnessing energy from ATP hydrolysis [67]. After exposure to rubusoside, insufficient energy supply in the environment leads to a decrease in the ability of ABC transport proteins in S. wiggsiae, which is necessary for amino acid transport and thus affects bacterial growth.
Both groups exhibited enrichment of ribosomes in the KEGG pathways associated with upregulated genes. The bacterial 70S ribosome comprises a small 30S subunit responsible for genetic information transmission and a large 50S subunit responsible for peptide bond formation [67]. Previous studies have demonstrated that to ensure the survival of bacterial cells under adverse conditions, macromolecular complexes such as ribosomes need to be preserved and their functions restored once the environment improves [68]. Therefore, it is reasonable to hypothesize that when the surrounding environment is unfavorable for growth, cells allocate a portion of their energy resources to overcome these challenges. Other upregulated genes involved in processes such as glycolysis/gluconeogenesis and the pentose phosphate pathway may also be attributed to similar reasons.
Notably, the addition or omission of sucrose did not affect the functionality of rubusoside, and the differential gene enrichment results of the two groups were similar. We believe that the promotional effect of sucrose is inhibited when sucrose and rubusoside are added in equal proportions. All of the mechanistic pathway analyses mentioned above corroborated our in vitro findings.
In summary, this study provides a comprehensive genome annotation of S. wiggsiae DSMZ22547 for the first time and evaluates the impact of rubusoside on its planktonic state and biofilm. We also explored the bacteriostatic mechanism of rubusoside for the first time. Our findings suggest that rubusoside not only serves as an excellent substitute for sucrose but also functions as a sweet supplement. In children’s daily diet, even if it is impossible to completely avoid sucrose intake (such as in pasta), the use of a natural sweetener can effectively inhibit caries bacteria, which has reference value for the research of caries-prevention foods. Furthermore, the above affected gene as a therapeutic target may be the future of preventing caries and developing new drugs.
Conclusions
In this study, we discovered that rubusoside, a natural non-cariogenic sweetener, exhibited significant bacteriostatic effects against S. wiggsiae, a potential pathogen of ECC. Transcriptomics methods were employed to comprehensively assess changes in DEGs expression and predict the bacteriostatic mechanism. Specifically, rubusoside affects carbohydrate metabolic pathways including nucleotide sugar synthesis, amino acid sugar and nucleotide sugar metabolism, galactose metabolism, and PTS. Rubusoside also influences virulence genes associated with adhesion and invasion, biofilm formation, and extracellular polysaccharide production (clpP, groEL), thereby impeding bacterial growth. Notably, sucrose supplementation did not affect the bacteriostatic effect of rubusoside but regulated pathways associated with carbohydrate and amino acid metabolism. These findings provide new insights into the antibacterial mechanism of rubusoside along with sucrose. As a promising natural non-cariogenic substance, rubusoside, as a sucrose substitute and sweet additive, is better than xylitol and has wide application value in the food industry and oral preventive cleaning products industry. However, the environment of ECC consists of complex microbial communities rather than a single bacterial model, These microorganisms form symbiotic relationships that facilitate their collective survival and proliferation [69]. Among these, polymicrobial biofilms formed by bacterial and fungal pathogens, such as the symbiotic biofilm of Candida albicans and S. mutans, are frequently detected in cariogenic environments. This coexistence enhances the expression of cariogenic virulence factors, resulting in a more potent cariogenic potential. These interactions render polymicrobial biofilms a significantly greater threat compared to single-species biofilms [70]. Therefore, exploring the mechanisms by which rubusoside inhibits both single-species and multi-species microbial biofilm formation will aid in preventing the onset and progression of dental caries. Future research should focus on conducting more in-depth studies to explore and validate these mechanisms.
Methods
Bacterial strains and growth conditions
In this study, we used lyophilized cultures of S.wiggsiae DSMZ22547, provided by the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, https://www.dsmz.de/de/dsmz). Bacteria were cultured on Colombian blood group agar plates for 72 h at 35 °C in an anaerobic chamber (N2, 80%; H2, 10%; CO2, 10%; Bcatron). Following a 72-h incubation period, several colonies were injected into the modified Peptone Yeast Glucose (PYG) Broth liquid medium (DSMZ NO.104), supplemented with 5% fetal bovine serum (FBS), 5 mg/L hemin chloride, and 0.5 mg/L vitamin K, and cultured for 48 h in an anaerobic environment.
Following the method proposed by Hans Christian Gram [71] and the instructions provided in the staining kit, we performed Gram staining before each experiment to microscopically examine the color and morphology of the samples, thereby assessing potential microbial contamination. First, apply 1–2 drops of the bacterial suspension to be tested onto a clean glass slide and perform heat fixation. Then, successively perform primary staining with crystal violet for 1 min, wash with water, and perform mordant staining with iodine solution for 1 min. After washing with water, decolorize with 95% alcohol by shaking the slide for 20–60 s. Wash with water again and perform counterstaining with acid fuchsin for 1 min. After washing and drying, observe the stained bacterial morphology under an oil immersion microscope (OLYMPUS CX23, China). Phosphate buffer solution (PBS, Servicebio, China) was used to wash the bacteria after they were extracted by centrifugation (8000 × g, 10 min, 4 °C). Subsequently, the bacterial cells were resuspended in PBS to achieve a cell density of 1 × 108 CFU/mL, which served as the standard bacterial suspension. All procedures were conducted in an anaerobic chamber.
Test compound preparation
The microbial growth medium utilized in this investigation was DSMZ NO.104 modified PYG liquid medium (Mingzhou Biotechnology Co., Ltd., Ningbo, China), supplemented with 5% FBS, 5 mg/L hemin chloride, and 0.5 mg/L vitamin K. The sweeteners employed in the study, namely sucrose, glucose, xylitol, and rubusoside (Desite Biotechnology Co., Ltd., Chengdu, China) were all confirmed to be of high purity (98%) through high-performance liquid chromatography analysis (Additional file 1: Table S3). Bacteria were cultured in six distinct media containing additional sugars, such as sucrose, glucose, xylitol, rubusoside, or a combination of rubusoside and sucrose, with one group remaining untreated.
Determination of the minimal inhibitory concentration (MIC)
We employed the microbroth dilution method recommended by the Clinical and Laboratory Standards Institute guideline M07-A9 to determine the MIC.
Culture media with different concentrations of rubusoside (8%, 4%, 2%, 1%, 0.5%, 0.25%, and 0.125%) were prepared, along with a bacterial suspension of 1 × 106 CFU/mL. One hundred microliters of bacterial suspension was inoculated into 96-well polystyrene culture plates (Nest), followed by adding 100 μL of rubusoside at various concentrations to each well. The modified PYG medium (100 μL) served as the negative control. The plates were then incubated in an anaerobic chamber at 35 °C for 48 h [72]. The MIC was determined by visually identifying the lowest concentration of rubusoside that completely inhibited visible microbial growth. At least three biological replicates were determined for each group (n = 6), and all experiments were conducted with three replicates.
Growth activity of planktonic bacteria
The inhibitory effect of rubusoside on S. wiggsiae cell growth was assessed by dynamically monitoring biomass growth, specifically the dry weight of the cells. The liquid medium was supplemented with 1% (MIC) sucrose, glucose, xylitol, rubusoside, or rubusoside and sucrose. A mixture of 5 mL liquid medium and 250 μL standard bacterial suspension (1 × 108 CFU/mL) was incubated in a 10-mL test tube for 48 h at 35 °C in an anaerobic chamber. Samples were collected every 4 h and mixed ethanol at − 20℃ for 15 min, followed by centrifugation at 4 °C and 12,000g for 10 min to remove to remove the supernatant. After performing two washes with 75% ethanol, the centrifugation step was repeated, and the weight was determined after freeze-drying [32, 73]. Three biological replicates were set up in each group at each time point (n = 3).
The growth inhibition of the planktonic cells was quantified by enumerating viable bacteria. In brief, standard bacterial suspension(1 × 108 CFU/mL) and different groups of culture media were incubated anaerobically at 1:20 for 48 h, before centrifuging and determining the pH using a pH meter (Mettler-Toledo, Columbus, OH, USA) [74]. Subsequently, the precipitate was washed twice with PBS. A 100 μL aliquot of the solution was diluted stepwise, and 50 μL each of the 10−5, 10−6, and 10−7 dilutions were evenly applied to the Columbia blood plate with a Beekman coating rod. Plate counts were performed after 1 week of incubation in an anaerobic chamber at 35℃ (the optimal range for the number of colonies was 20–200) [75]. Three biological replicates were determined for each group (n = 3).
Bacterial adhesion analysis
The effect of rubusoside on bacterial adhesion was observed in 96-well cell culture plates (NEST). The S. wiggsiae with a concentration of 1 × 108 CFU/mL was placed in wells at a 1:10 ratio with the culture medium of each group. The cells were incubated in an anaerobic chamber (80% N2, 10% CO2, and 10% H2) at 35 °C for 48 h. Subsequently, the wells were washed with PBS to retain adherent cells. To ensure complete resuspension of the adherent bacteria, 100 μl of PBS was added to each well. Each well was then gently aspirated and mixed for 1 min per cycle (to prevent bubble formation), repeating this process 10 times to ensure thorough resuspension. The samples were measured at Optical Density (OD) 640 [76]. Three biological replicates were determined for each group (n = 3).
Determination of the biofilm growth progress
Biofilm production was quantitatively assessed in vitro using crystal violet staining. The standard bacterial suspension (1 × 108 CFU/mL) and medium of each group were anaerobically incubated in 96-well plates for 168 h, and the fresh medium was replaced once at 48, 72, 96, 120, 144, and 168 h. The wells were washed with sterile PBS to remove unadhered bacteria. Then, the biofilms at the bottom of the wells were fixed with methanol for 15 min, washed again, and dried. They were stained with 0.1% (wt/vol) crystal violet–glacial acetic acid solution for 15 min, and then rinsed with sterile PBS until the liquid became clear; after drying, they were dissolved with 33% glacial acetic acid solution (200 µl) [32]. After completion, the solution in each well was transferred to a new 96-well plate, and the absorbance at 590 nm was measured using a microplate spectrophotometer to quantitatively analyze the total biomass of the biofilms in each well. Three biological replicates were determined for each group (n = 3).
Two milliliters of the medium, containing sucrose, glucose, xylitol, rubusoside, and rubusoside + sucrose, was inoculated with 100 μl of a standardized bacterial suspension (1 × 108 CFU/mL) in each well of a 24-well plate on the sterile frosted glass to evaluate the acid production of biofilm. The fresh medium was replaced every 24 h from 48 h, and the pH of the old medium was measured using a pH meter. The pH was not measured during the first 48 h because of the existence of planktonic bacteria and the bacterial adhesion process of this stage [77]. Three biological replicates were determined for each group (n = 3).
The water-insoluble EPS content was determined using the anthrone-sulfuric acid method, starting with the establishment of a standard curve to correlate the sugar concentration with OD625nm absorbance. Biofilms were obtained for 96 h as described above, the supernatant was removed and the slides were taken out. The slides were washed three times with sterile PBS to remove the unattached cells on the slides. The biofilm was scraped off the slides with a sterile cell scraper and suspended in 1 mL PBS. The bacterial suspension was collected in a 1.5 mL centrifuge tube and centrifuged at 4 °C and 6000 × g for 10 min, and the process was repeated once. The precipitate was resuspended in 4 mL 0.4 M NaOH and stirred constantly at 37 °C for 2 h. Then, 3 volumes of anthrone-sulfuric acid reagent were added and further mixed with the supernatant. The mixture was heated in a 95 °C water bath for 6 min until the reaction was completed. Finally, equal amounts of each sample were placed in a 96-well cell culture plate, measured at OD625nm using a microplate spectrophotometer [78]. Three biological replicates were determined for each group (n = 3).
The bicinchoninic acid protein assay kit (BCA protein assay; Vazyme, Nanjing, China) was used to determine the protein yield in the presence of rubusoside. Biofilms were grown and cell suspensions were added to fine grinding beads as previously described and then ground with 2 M NaOH containing 1 mM EDTA for 1 h at 0 °C, followed by a reaction at 100 °C for 15 min. The supernatant and BCA protein working solution were mixed by centrifugation and incubated at room temperature for 30 min to measure at OD595nm [79]. Five biological replicates were determined for each group.
SEM observations of biofilms by scanning electron microscopy
The structure and distribution density of the S. wiggsiae biofilm were observed using SEM. In brief, sterile frosted glass slides were placed in 24-well plates, and 200 µl of standard bacterial suspension (1 × 108 CFU/mL) and 2 mL of different media were added for 72 h at an anaerobic temperature of 35°C. After washing three times with sterile PBS, the samples were fixed in the dark at 4 °C for 12 h using 2.5% glutaraldehyde solution. Subsequently, the samples were fixed again with a 1% osmic acid solution for approximately 1–2 h. The samples were then subjected to gradient dehydration using eight concentrations of ethanol (ranging from 30 to 100%). Next, the samples were treated with a mixture of ethanol and isobutyl acetate (V/V = 1/1) for a duration of 30 min, followed by treatment with pure isobutyl acetate for 1 h. Finally, the samples were critically dried, gold-sputtered, and examined by SEM (Zeiss Sigma 300, Germany). Three random regions on each sample were selected and photographed at three different magnifications (× 1000, × 2000, and × 5000) [80].
Confocal laser scanning microscope (CLSM) biofilm characterization using confocal laser scanning microscopy
The cell viability was assessed by observing the cell membrane integrity using a staining method with SYTO-9 and propidium iodide (Invitrogen, USA). Two dyes were each diluted 50-fold with 0.3% (v/v) dimethyl sulfoxide and thoroughly mixed for subsequent use. Briefly, sterile frosted glass plates were placed in 24-well plates and inoculated with a standard bacterial suspension (1 × 108 CFU/mL) in a 1% liquid medium with various growth media for co-cultivation for 72 h, the slides were washed three times with sterile PBS to remove the unattached cells. After drying, staining it with the mixed dye under light-proof conditions. The stained cells were then examined under a CLSM (Olympus FV3000, Japan) to differentiate live bacteria (green fluorescence) from dead bacteria (red fluorescence). Dual-channel scanning was performed using the green channel (excited at 488 nm) and the red channel (excited at 561 nm) [81]. Three random fields from each group were selected and captured through photography. ImageJ COMSTAT software was used for quantitative analysis to determine the live-to-dead cell ratio and biofilm thickness [82, 83].
Genomic DNA isolation and whole-genome sequencing
To further understand S. wiggsiae DSMZ22547, pick single colonies and incubate them in modified PYG broth for 48 h. Subsequently, inoculate 1 mL of the resulting culture into 100 mL of fresh medium at a ratio of 1:100. According to the manufacturer’s protocol, the genomic DNA was extracted using Wizard® Genomic DNA Purification Kit (Promega, USA) [84, 85]. Whole genome sequencing was performed on Illumina HiSeq and PacBio RSII platforms (Shanghai Meji Bio-Pharmaceutical Technology Co., Ltd) [86]. After performing quality control on the raw data, the sequencing fragments were assembled into a complete, circular genome using the HGAP4 Analysis Application/SMRT Link (V6.0.0.47841) [87]. The potential virulence genes were annotated by conducting a Basic Local Alignment Search Tool (BLAST) BlastP protein search against the VFDB (http://www.mgc.ac.cn/VFs/main.htm), with E-value ≤ 1e–5 [88, 89]. Further annotation of coding sequences (CDS) was performed in databases such as NR (http://ftp.ncbi.nlm.nih.gov/blast/db), Swiss-Prot (https://web.expasy.org/docs/swiss-prot_guideline.html), Pfam (http://pfam.xfam.org/), COG (http://eggnogdb.embl.de/#/app/home), GO (http://current.geneontology.org/ontology/index.html), and KEGG (http://www.genome.jp/kegg/) [85, 90]. The complete nucleotide sequence has been submitted to GenBank with the accession number CP157350.
RNA extraction, Illumina sequencing, and data analysis
In brief, 96-h biofilms of sucrose, xylitol, rubusoside, rubusoside + sucrose, and blank control were grown as described above and used for RNA extraction. Each group has three biological replicates, a total of 15 samples. Total bacterial RNA was extracted using TRIzol® Reagent following the manufacturer’s instructions (Invitrogen, USA), and genomic DNA was removed by DNase I (TaKaRa, Japan). Total RNA was extracted according to the instructions of TRIzol® Reagent (Invitrogen, USA), and genomic DNA was removed by DNase I (TaKaRa, Japan) [85]. The purity of RNA (with OD260/280 ≥ 1.8 and OD260/230 ≥ 1.0) was assessed using a NanoDrop2000 (Thermo Fisher Scientific, USA).
To construct transcriptome sequencing libraries, raw data were trimmed and quality controlled using Sickle (https://github.com/najoshi/sickle), SeqPrep (https://github.com/jstjohn/SeqPrep), and fastp (https://github.com/OpenGene/fastp) to obtain high-quality and clean reads [91]. The original sequencing data obtained often contain reads with relatively low quality. Additionally, a small fraction of the raw data may include artificial sequences such as sequencing primers and adapters. To ensure more accurate subsequent assembly, the raw data undergo quality trimming. Specifically, adapter sequences are removed from the reads, and any non-A, G, C, or T bases at the 5'end are trimmed. Ends of reads with quality values below Q20 are also trimmed, and reads where the proportion of N bases exceeds 10% are discarded. Furthermore, fragments shorter than 25 bp after adapter removal and quality trimming are eliminated. The high-quality reads obtained through this series of quality control measures are referred to as Clean data. Functional annotations were obtained by searching protein databases such as KEGG, COG, GO, and CAZymes (http://www.cazy.org/) [92].
DESeq2 (https://bioconductor.org/packages/release/bioc/html/DESeq2.html) [93] was used for gene expression normalization and differential gene expression analysis with the significance threshold set at P < 0.05, | log2(FoldChange) |≥ 1. GO enrichment analysis and KEGG pathway enrichment analyses were employed to elucidate key metabolic pathways influencing S. wiggsiae growth at the molecular level, which provided insights into its inhibitory mechanism [94]. The sequence reads were submitted in the NCBI Sequence Read Archive under the accession PRJNA1129267.
Statistical analysis
GraphPad Prism 9.0 software was used for one-way analysis of variance (ANOVA) and Tukey’s multiple comparison test. All experiments were repeated three times, and data are presented as the mean ± SD. A statistically significant difference was considered when P < 0.05.
Supplementary Information
Additional file 1: Fig. S1: MIC of rubusoside against S.wiggsiae. Row 3–11:8%, 4%, 2%, 1%, 0.5%, 0.25%, 0.125% of rubusoside, negative control, blank group. No visible bacterial growth was observed at 1% concentration, which was the minimum inhibitory concentration. Fig. S2: KEGG enrichment analysis of up and down-regulated genes in the gene set rubusoside vs sucrose. Fig. S3: KEGG enrichment analysis of up and down-regulated genes in the gene set rubusoside vs xylitol. Fig. S4: KEGG enrichment analysis of up and down-regulated genes in the gene set rubusoside+sucrose vs sucrose. Table S1: Genome features of S.wiggsiae DSMZ22547. Table S2: The data statistics of transcriptome sequencing. Table S3: HPLC-quantified purity of compound(s).
Additional file 2: Table S1: Single data value of cell dry weight. Table S2: Single data value of 48 h colony counts. Table S3: Single data value of 48 h pH. Table S4: Single data value of adhesion. Table S5: Crystal violet staining of biofilms. Table S6: The content of EPS in biofilms. Table S7: The content of soluble proteins in biofilms. Table S8: Live Dead bacteria proportion. Table S9: Biofilm thinkness.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. We sincerely thank the editors and all reviewers for their valuable feedback, which was valuable in refining our paper.
Abbreviations
- S. wiggsiae
Scardovia wiggsiae
- ECC
Early Childhood Caries
- S. mutans
Streptococcus mutans
- MIC
Minimum inhibitory concentration
- SEM
Scanning electron microscopy
- CLSM
Confocal laser scanning microscope
- EPS
Extracellular polysaccharide
- dmfs
Decayed, missing, or filled tooth surfaces
- DSMZ
Deutsche SammLung von Mikroorganismen und Zellkulturen
- VFDB
Virulence Factor Database
- CAZy
Carbohydrate Active Enzyme database
- PCA
Principal component analysis
- DEGs
Differentially expressed genes
- PYG
Peptone Yeast Glucose Broth
- OD
Optical Density
- FBS
Fetal bovine serum
- PBS
Phosphate buffer solution
- CFU
Colony forming units
- PTS
Phosphotransferase system
- PEP
Phosphoenolpyruvat
Authors’ contributions
P.W. and J.C. designed the project. P.W. and R.M. conducted the experiments; Y.L., D.M. and Y.Z. carried out the bioinformatics analysis; PW wrote the manuscript; P.W., R.M. and Y.W. revised the manuscript. All authors read and approved the final manuscript.
Funding
Our work was supported by was supported by the Henan Province’s Health Science and Technology Innovation Leading Talent Cultivation Program for Middle-aged and Young Professionals (grant no. LJRC2023006) and the Natural Science Foundation of Henan Province (grant no. 202300410448).
Data availability
All data generated or analyzed in this study are included in this published article, its supplementary information files, and publicly available databases. The complete nucleotide sequences have been submitted to GenBank with the accession number CP157350; the sequence read data generated in this study have been submitted to the NCBI Sequence Read Archive with the accession number PRJNA1129267. All individual data values can be found in an Excel table named “Additional file 2”.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Academy on Pediatric Dentistry; American Academy of Pediatrics. Policy on early childhood caries (ECC): classifications, consequences, and preventive strategies. Pediatr Dent. 2017;39:59–61. [PubMed]
- 2.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators.Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. [DOI] [PMC free article] [PubMed]
- 3.Tinanoff N, Baez RJ, Diaz Guillory C, Donly KJ, Feldens CA, McGrath C, et al. Early childhood caries epidemiology, aetiology, risk assessment, societal burden, management, education, and policy: Global perspective. Int J Paediatr Dent. 2019;29:238–48. [DOI] [PubMed] [Google Scholar]
- 4.Zou J, Du Q, Ge L, Wang J, Wang X, Li Y, et al. Expert consensus on early childhood caries management. Int J Oral Sci. 2022;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oubenyahya H, Bouhabba N. General anesthesia in the management of early childhood caries: an overview. J Dent Anesth Pain Med. 2019;19:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moynihan PJ, Kelly SAM. Effect on Caries of Restricting Sugars Intake. J Dent Res. 2014;93:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quivey RG, O’Connor TG, Gill SR, Kopycka-Kedzierawski DT. Prediction of Early Childhood Caries Onset and Oral Microbiota. Mol Oral Microbiol. 2021;36:255–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preza D, Olsen I, Aas JA, Willumsen T, Grinde B, Paster BJ. Bacterial profiles of root caries in elderly patients. J Clin Microbiol. 2008;46:2015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sbordone L, Bortolaia C. Oral microbial biofilms and plaque-related diseases: microbial communities and their role in the shift from oral health to disease. Clin Oral Investig. 2003;7:181–8. [DOI] [PubMed] [Google Scholar]
- 10.Beighton D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Comm Dent Oral Epid. 2005;33:248–55. [DOI] [PubMed] [Google Scholar]
- 11.Caufield PW, Li Y, Dasanayake A. Dental caries: an infectious and transmissible disease. Compend Contin Educ Dent. 2005;26(5 Suppl 1):10–6. [PubMed] [Google Scholar]
- 12.Marchant S, Brailsford SR, Twomey AC, Roberts GJ, Beighton D. The predominant microflora of nursing caries lesions. Caries Res. 2001;35:397–406. [DOI] [PubMed] [Google Scholar]
- 13.Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65:1028–37. [PubMed] [Google Scholar]
- 14.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, et al. Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults. J Clin Microbiol. 2008;46:1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loesche WJ, Rowan J, Straffon LH, Loos PJ. Association of Streptococcus mutants with human dental decay. Infect Immun. 1975;11:1252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, et al. Molecular Analysis of Bacterial Species Associated with Childhood Caries. J Clin Microbiol. 2002;40:1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng F, Yang F, Huang S, Bo C, Xu ZZ, Amir A, et al. Prediction of Early Childhood Caries via Spatial-Temporal Variations of Oral Microbiota. Cell Host Microbe. 2015;18:296–306. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wang S, Wu C, Chen X, Duan Z, Xu Q, et al. Oral Microbiome Alterations Associated with Early Childhood Caries Highlight the Importance of Carbohydrate Metabolic Activities. mSystems. 2019;4:e00450-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downes J, Mantzourani M, Beighton D, Hooper S, Wilson MJ, Nicholson A, et al. Scardovia wiggsiae sp. nov., isolated from the human oral cavity and clinical material, and emended descriptions of the genus Scardovia and Scardovia inopinata. Int J Syst Evol Microbiol. 2011;61:25–9. [DOI] [PubMed] [Google Scholar]
- 20.Tanner ACR, Mathney JMJ, Kent RL, Chalmers NI, Hughes CV, Loo CY, et al. Cultivable Anaerobic Microbiota of Severe Early Childhood Caries. J Clin Microbiol. 2011;49:1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledezma-Rasillo G, Flores-Reyes H, Gonzalez-Amaro AMA, Garrocho-Rangel A, Ruiz-Rodriguez Ma del S, Pozos-Guillen A. Identification of Cultivable Microorganisms from Primary Teeth with Necrotic Pulps. J Clin Pediatr Dent. 2010;34:329–33. [DOI] [PubMed] [Google Scholar]
- 22.Tanner ACR, Kent RL, Holgerson PL, Hughes CV, Loo CY, Kanasi E, et al. Microbiota of Severe Early Childhood Caries before and after Therapy. J Dent Res. 2011;90:1298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalmers NI, Oh K, Hughes CV, Pradhan N, Kanasi E, Ehrlich Y, et al. Pulp and plaque microbiotas of children with severe early childhood caries. J Oral Microbiol. 2015;7:25951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janakiram C, Deepan Kumar CV, Joseph J. Xylitol in preventing dental caries: A systematic review and meta-analyses. J Nat Sci Biol Med. 2017;8:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharbanda OP, Moynihan P, Priya H, Ivaturi A, Gupta A, Haldane D. Report from a symposium on accelerating policy-driven action against excessive sugar consumption for the prevention of early childhood caries and noncommunicable diseases. Indian J Public Health. 2018;62:305–7. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro CCC, da Silva MCB, Nunes AMM, Thomaz EB de AF, Carmo CDS, Ribeiro MRC, et al. Overweight, obese, underweight, and frequency of sugar consumption as risk indicators for early childhood caries in Brazilian preschool children. Int J Paediatr Dent. 2017;27:532–9. [DOI] [PubMed] [Google Scholar]
- 27.Sun HB, Zhang W, Zhou XB. Risk Factors associated with Early Childhood Caries. Chin J Dent Res. 2017;20:97–104. [DOI] [PubMed] [Google Scholar]
- 28.Castro-Muñoz R, Correa-Delgado M, Córdova-Almeida R, Lara-Nava D, Chávez-Muñoz M, Velásquez-Chávez VF, et al. Natural sweeteners: Sources, extraction and current uses in foods and food industries. Food Chem. 2022;370:130991. [DOI] [PubMed] [Google Scholar]
- 29.Cai Q, Xiang N, Qiu Z. Research Progress of Sugar-Free Traditional Chinese Medicine Preparations. Pharmacy Today. 2024;34(11):877–80. [Google Scholar]
- 30.Local Food Safety Standard - Determination of Rubusoside Content in Sweet Tea. Guangxi Zhuang Autonomous Region: DBS45/ 067-2020[S] Health Commission of Guangxi Zhuang Autonomous Region 2021–05–30.
- 31.Zhou Z, Sui X, Xia Y. Enzymatic Preparation of Rubusoside and Its Inhibitory Effect on Liver, Gastric and Intestinal Cells. Chinese Journal of Applied Chemistry. 2020;37:785–92. [Google Scholar]
- 32.Guan C, Che F, Zhou H, Li Y, Li Y, Chu J. Effect of Rubusoside, a Natural Sucrose Substitute, on Streptococcus mutans Biofilm Cariogenic Potential and Virulence Gene Expression In Vitro. Appl Environ Microbiol. 2020;86:e01012-e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kameda M, Abiko Y, Washio J, Tanner ACR, Kressirer CA, Mizoguchi I, et al. Sugar Metabolism of Scardovia wiggsiae, a Novel Caries-Associated Bacterium. Front Microbiol. 2020;11:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen MJ, Pearce IF. A computer program for correlating dental plaque ph values, cH+, plaque titration, critical ph, resting ph and the solubility of enamel apatite. Arch Oral Biol. 1997;42:475–80. [DOI] [PubMed] [Google Scholar]
- 35.Jakubovics NS, Goodman SD, Mashburn-Warren L, Stafford GP, Cieplik F. The dental plaque biofilm matrix. Periodontol. 2000;2021(86):32–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan X, Qi J, Zhou B, Mao B. Metabolomic and transcriptomic analyses reveal the regulation of pigmentation in the purple variety of Dendrobium officinale. Sci Rep. 2020;10:17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries Nat Rev Dis Primers. 2017;3:17030. [DOI] [PubMed] [Google Scholar]
- 38.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2:1599–607. [DOI] [PubMed] [Google Scholar]
- 39.Valm AM. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J Mol Biol. 2019;431:2957–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang G, Wen S. Research progress of sweet plants. Anhui Agricultural Science. 2006;18:4712–3.
- 41.Riley P, Moore D, Ahmed F, Sharif MO, Worthington HV. Xylitol-containing products for preventing dental caries in children and adults. Cochrane Database Syst Rev. 2015;2015:CD010743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hesari Z, Shafiee A, Hooshfar S, Mobarra N, Mortazavi SA. Formulation and Taste Masking of Ranitidine Orally Disintegrating Tablet. Iran J Pharm Res. 2016;15:677–86. [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Fang J, Zhu J, Liu J. A brief review of biofilm matrix in structured microbial communities. Chinese Journal of Microbiology. 2022;62:47–56. [Google Scholar]
- 44.Huang J, Wang X, Cao Q, Feng F, Xu X, Cai X. ClpP participates in stress tolerance and negatively regulates biofilm formation in Haemophilus parasuis. Vet Microbiol. 2016;182:141–9. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Wang T, Chen W, Yilmaz O, Park Y, Jung I-Y, et al. Differential protein expression by Porphyromonas gingivalis in response to secreted epithelial cell components. Proteomics. 2005;5:198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Wei L, Wang L, Chen X, Kong X, Luan Y, et al. Scutellarin potentiates vancomycin against lethal pneumonia caused by methicillin-resistant Staphylococcus aureus through dual inhibition of sortase A and caseinolytic peptidase P. Biochem Pharmacol. 2022;199:114982. [DOI] [PubMed] [Google Scholar]
- 47.Kirk JA, Banerji O, Fagan RP. Characteristics of the Clostridium difficile cell envelope and its importance in therapeutics. Microb Biotechnol. 2017;10:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romero-Lastra P, Sánchez MC, Llama-Palacios A, Figuero E, Herrera D, Sanz M. Gene expression of Porphyromonas gingivalis ATCC 33277 when growing in an in vitro multispecies biofilm. PLoS ONE. 2019;14:e0221234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hennequin C, Porcheray F, Waligora-Dupriet A, Collignon A, Barc M, Bourlioux P, et al. GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology (Reading). 2001;147(Pt 1):87–96. [DOI] [PubMed] [Google Scholar]
- 50.Johnson SM, Sharif O, Mak PA, Wang H-T, Engels IH, Brinker A, et al. A biochemical screen for GroEL/GroES inhibitors. Bioorg Med Chem Lett. 2014;24:786–9. [DOI] [PubMed] [Google Scholar]
- 51.Yoshioka M, Grenier D, Hinode D, Fukui M, Mayrand D. Antigenic cross-reactivity and sequence homology between Actinobacillus actinomycetemcomitans GroEL protein and human fibronectin. Oral Microbiol Immunol. 2004;19:124–8. [DOI] [PubMed] [Google Scholar]
- 52.Yao L, Lin Z, Fu Z. GroEL-Assisted Protein Folding by Utilizing The Energy From ATP. Prog Biochem Biophys. 2015;42(2):154–60.
- 53.Skår CK, Krüger PG, Bakken V. Characterisation and subcellular localisation of the GroEL-like and DnaK-like proteins isolated from Fusobacterium nucleatum ATCC 10953. Anaerobe. 2003;9:305–12. [DOI] [PubMed] [Google Scholar]
- 54.Ueki K, Tabeta K, Yoshie H, Yamazaki K. Self-heat shock protein 60 induces tumour necrosis factor-alpha in monocyte-derived macrophage: possible role in chronic inflammatory periodontal disease. Clin Exp Immunol. 2002;127:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong G-J, Khan F, Tabassum N, Kim Y-M. Alteration of oral microbial biofilms by sweeteners. Biofilm. 2024;7:100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao X, Liang Q, Song X, Zhang Y. Whole genome sequence of Lactiplantibacillus plantarum MC5 and comparative analysis of eps gene clusters. Front Microbiol. 2023;14:1146566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jia Y-J, Liao Y, He Y-Q, Zheng M-Q, Tong X-T, Xue W-Q, et al. Association Between Oral Microbiota and Cigarette Smoking in the Chinese Population. Front Cell Infect Microbiol. 2021;11:658203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao W, Walker SL, Huang Q, Cai P. Contrasting effects of extracellular polymeric substances on the surface characteristics of bacterial pathogens and cell attachment to soil particles. Chem Geol. 2015;410:79–88. [Google Scholar]
- 59.Liu Q, Zhang H, Chang F, Qiu J, Duan L, Hu G, et al. The effect of graphene photocatalysis on microbial communities in Lake Xingyun, southwestern China. Environ Sci Pollut Res Int. 2022;29:48851–68. [DOI] [PubMed] [Google Scholar]
- 60.Zielińska D, Turemko M. Electroactive Phenolic Contributors and Antioxidant Capacity of Flesh and Peel of 11 Apple Cultivars Measured by Cyclic Voltammetry and HPLC-DAD-MS/MS. Antioxidants (Basel). 2020;9:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen TT, Ghirlando R, Roche J, Venditti V. Structure elucidation of the elusive Enzyme I monomer reveals the molecular mechanisms linking oligomerization and enzymatic activity. Proc Natl Acad Sci U S A. 2021;118:e2100298118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeckelmann J-M, Erni B. Carbohydrate Transport by Group Translocation: The Bacterial Phosphoenolpyruvate: Sugar Phosphotransferase System. Subcell Biochem. 2019;92:223–74. [DOI] [PubMed] [Google Scholar]
- 63.Abranches J, Candella MM, Wen ZT, Baker HV, Burne RA. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J Bacteriol. 2006;188:3748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi J, Shin D, Yoon H, Kim J, Lee C-R, Kim M, et al. Salmonella pathogenicity island 2 expression negatively controlled by EIIANtr-SsrB interaction is required for Salmonella virulence. Proc Natl Acad Sci U S A. 2010;107:20506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi J, Ryu S. Regulation of Iron Uptake by Fine-Tuning the Iron Responsiveness of the Iron Sensor Fur. Appl Environ Microbiol. 2019;85:e03026-e3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu S, Yu P-L, Wheeler D, Flint S. Transcriptomic study on persistence and survival of Listeria monocytogenes following lethal treatment with nisin. J Glob Antimicrob Resist. 2018;15:25–31. [DOI] [PubMed] [Google Scholar]
- 67.Huang K, Zeng J, Liu X, Jiang T, Wang J. Structure of the mannose phosphotransferase system (man-PTS) complexed with microcin E492, a pore-forming bacteriocin. Cell Discov. 2021;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zegarra V, Bedrunka P, Bange G, Czech L. How to save a bacterial ribosome in times of stress. Semin Cell Dev Biol. 2023;136:3–12. [DOI] [PubMed] [Google Scholar]
- 69.Jeong G-J, Rather MA, Khan F, Tabassum N, Mandal M, Kim Y-M. pH-responsive polymeric nanomaterials for the treatment of oral biofilm infections. Colloids Surf, B. 2024;234:113727. [DOI] [PubMed] [Google Scholar]
- 70.Jeong G-J, Khan F, Tabassum N, Cho K-J, Kim Y-M. Strategies for controlling polymicrobial biofilms: A focus on antibiofilm agents. Int J Antimicrob Agents. 2024;64:107243. [DOI] [PubMed] [Google Scholar]
- 71.Gressner AM, Gressner OA. Gram-Färbung. In: Gressner AM, Arndt T, editors. Lexikon der Medizinischen Laboratoriumsdiagnostik. Berlin, Heidelberg: Springer; 2019. p. 1017–1017. [Google Scholar]
- 72.Cannon ML, Merchant M, Kabat W, Catherine L, White K, Unruh B, et al. In Vitro Studies of Xylitol and Erythritol Inhibition of Streptococcus Mutans and Streptococcus Sobrinus Growth and Biofilm Production. J Clin Pediatr Dent. 2020;44:307–14. [DOI] [PubMed] [Google Scholar]
- 73.Yun EJ, Lee AR, Kim JH, Cho KM, Kim KH. 3,6-Anhydro-l-galactose, a rare sugar from agar, a new anticariogenic sugar to replace xylitol. Food Chem. 2017;221:976–83. [DOI] [PubMed] [Google Scholar]
- 74.Chu J, Zhang T, He K. Cariogenicity features of Streptococcus mutans in presence of rubusoside. BMC Oral Health. 2016;16:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCutcheon CR, Pell ME, Gaddy JA, Aronoff DM, Petroff MG, Manning SD. Production and Composition of Group B Streptococcal Membrane Vesicles Vary Across Diverse Lineages. Front Microbiol. 2021;12:770499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Han L, Yang H, Liu S, Huang C. Effect of apigenin on surface-associated characteristics and adherence of Streptococcus mutans. Dent Mater J. 2020;39:933–40. [DOI] [PubMed] [Google Scholar]
- 77.Xie X, Wang L, Xing D, Zhang K, Weir MD, Liu H, et al. Novel dental adhesive with triple benefits of calcium phosphate recharge, protein-repellent and antibacterial functions. Dent Mater. 2017;33:553–63. [DOI] [PubMed] [Google Scholar]
- 78.Yin W, Zhang Z, Shuai X, Zhou X, Yin D. Caffeic Acid Phenethyl Ester (CAPE) Inhibits Cross-Kingdom Biofilm Formation of Streptococcus mutans and Candida albicans. Microbiol Spectr. 2022;10(5):e0157822. [DOI] [PMC free article] [PubMed]
- 79.Chen W, Liang J, He Z, Jiang W. Preliminary study on total protein extraction methods from Enterococcus faecalis biofilm. Genet Mol Res. 2016;15(3):10.4238. [DOI] [PubMed]
- 80.Niu Y, Wang K, Zheng S, Wang Y, Ren Q, Li H, et al. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob Agents Chemother. 2020;64:e00251-e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tawakoli PN, Al-Ahmad A, Hoth-Hannig W, Hannig M, Hannig C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin Oral Invest. 2013;17:841–50. [DOI] [PubMed] [Google Scholar]
- 82.Morris AJ, Li A, Jackson L, Yau YCW, Waters V. Quantifying the Effects of Antimicrobials on In vitro Biofilm Architecture using COMSTAT Software. J Vis Exp. 2020;166:61759. [DOI] [PubMed]
- 83.Veloz JJ, Alvear M, Salazar LA. Antimicrobial and Antibiofilm Activity against Streptococcus mutans of Individual and Mixtures of the Main Polyphenolic Compounds Found in Chilean Propolis. Biomed Res Int. 2019;2019:7602343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Li R, Qi C, Gao H, Wei Q, Tan L, et al. Mechanisms of polymyxin resistance induced by Salmonella typhimurium in vitro. Vet Microbiol. 2021;257:109063. [DOI] [PubMed] [Google Scholar]
- 85.Yang Z, Liu Z, Zhao F, Yu L, Yang W, Si M, et al. Organic acid, phosphate, sulfate and ammonium co-metabolism releasing insoluble phosphate by Klebsiella aerogenes to simultaneously stabilize lead and cadmium. J Hazard Mater. 2023;443:130378. [DOI] [PubMed] [Google Scholar]
- 86.Ren Y, Yu G, Shi C, Liu L, Guo Q, Han C, et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta. 2022;1:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng Z, Lei Y, Wang Y, Lin C, Lin J. Occurrence of mcr Positive Strains and Molecular Characteristics of Two mcr-1 Positive Salmonella typhimurium and Escherichia coli from a Chinese Women’s and Children’s Hospital. Infect Drug Resist. 2021;14:2925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L, Yang J, Yu J, Yao Z, Sun L, Shen Y, et al. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res. 2005;33(Database Issue):D325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon T, Kim J-B, Bak Y-S, Yu Y-B, Kwon KS, Kim W, et al. Draft genome sequence of non-shiga toxin-producing Escherichia coli O157 NCCP15738. Gut Pathog. 2016;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niu Y, Hu X, Song Y, Wang C, Luo P, Ni S, et al. Blautia Coccoides is a Newly Identified Bacterium Increased by Leucine Deprivation and has a Novel Function in Improving Metabolic Disorders. Adv Sci (Weinh). 2024;11:e2309255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan J, Tang Z, Li Y, Wang H, Hsu JC, Shi M, et al. Molybdenum Nanodots for Acute Lung Injury Therapy. ACS Nano. 2023;17:23872–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan X, Qin N, Wu C, Sheng J, Yang R, Zheng B, et al. Transcriptome analysis of the biofilm formed by methicillin-susceptible Staphylococcus aureus. Sci Rep. 2015;5:11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y, Xiang L, Wang H, Ma L, Qiu X, Liu D, et al. Transcriptome analysis of an arsenite-/antimonite-oxidizer, Bosea sp. AS-1 reveals the importance of the type 4 secretion system in antimony resistance. Sci Total Environ. 2022;826:154168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1: MIC of rubusoside against S.wiggsiae. Row 3–11:8%, 4%, 2%, 1%, 0.5%, 0.25%, 0.125% of rubusoside, negative control, blank group. No visible bacterial growth was observed at 1% concentration, which was the minimum inhibitory concentration. Fig. S2: KEGG enrichment analysis of up and down-regulated genes in the gene set rubusoside vs sucrose. Fig. S3: KEGG enrichment analysis of up and down-regulated genes in the gene set rubusoside vs xylitol. Fig. S4: KEGG enrichment analysis of up and down-regulated genes in the gene set rubusoside+sucrose vs sucrose. Table S1: Genome features of S.wiggsiae DSMZ22547. Table S2: The data statistics of transcriptome sequencing. Table S3: HPLC-quantified purity of compound(s).
Additional file 2: Table S1: Single data value of cell dry weight. Table S2: Single data value of 48 h colony counts. Table S3: Single data value of 48 h pH. Table S4: Single data value of adhesion. Table S5: Crystal violet staining of biofilms. Table S6: The content of EPS in biofilms. Table S7: The content of soluble proteins in biofilms. Table S8: Live Dead bacteria proportion. Table S9: Biofilm thinkness.
Data Availability Statement
All data generated or analyzed in this study are included in this published article, its supplementary information files, and publicly available databases. The complete nucleotide sequences have been submitted to GenBank with the accession number CP157350; the sequence read data generated in this study have been submitted to the NCBI Sequence Read Archive with the accession number PRJNA1129267. All individual data values can be found in an Excel table named “Additional file 2”.