Abstract
Lactobacillus helveticus is a probiotic bacterium widely used in the food industry. In this study, we evaluate the safety and probiotic properties of L. helveticus strain KM7, isolated from the gut of Apis cerana, through whole-genome sequencing and in vitro experiments. The complete genome consists of 2,164,024 bp, encoding 2192 genes with an average GC content of 36.82%. The strain lacks identifiable virulence factor genes and antibiotic resistance genes, shows no hemolytic activity, and cannot produce biogenic amines such as spermine, cadaverine, or putrescine. L. helveticus KM7 exhibited strong acid and bile salt tolerance, superior adhesion capacity, and remarkable antioxidant activity, with its genome harboring genes associated with these traits. It contains gene clusters potentially involved in the biosynthesis of bacteriocins Helveticin J and Enterolysin A, as well as encoding multiple genes of carbohydrate-active enzymes. Moreover, KM7 demonstrated excellent exopolysaccharide-producing capability, and glucose supplementation in the medium enhanced yield. These findings indicate that KM7 is a safe strain with promising probiotic properties, highlighting its potential for food applications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-025-04286-9.
Keywords: Lactobacillus helveticus, Whole-genome sequencing, Probiotic properties, Safety, Exopolysaccharide
Introduction
Probiotics are defined as living microorganisms that are beneficial to the health of the host when administered in adequate amounts, and lactic acid bacteria (LAB) are one of the most common groups [1]. Lactobacillus helveticus is a homofermentative, Gram-positive, rod-shaped, thermophilic LAB predominantly isolated from cheese, fermented milk, and other dairy products [2, 3]. Its remarkable adaptability to diverse environmental stresses, including high temperature, low pH, low osmotic pressure, and low oxygen levels, has enabled L. helveticus to be widely used as a probiotic [4, 5]. As a dietary supplement, L. helveticus has been demonstrated to improve mood, alleviate alcohol-induced liver injury, and ameliorate intestinal inflammation [6–9]. Furthermore, owing to its unique proteolytic system and capacity for exopolysaccharide (EPS) production, L. helveticus is widely employed in food fermentation [10, 11]. For example, during cheese production, it not only suppresses the growth of gas-producing microorganisms by metabolizing residual carbohydrates, but it also effectively reduces bitterness and accelerates the generation of flavor compounds [12, 13]. These multifaceted probiotic and technological properties of L. helveticus underscore its broad application potential, which has recently attracted increased research attention.
With the expanding applications of probiotics, safety assessment has become increasingly critical [14]. Systematic evaluation of strain safety and probiotic properties is a fundamental prerequisite for their use in human and animal health [15]. Beyond the ability to survive gastrointestinal transit, ideal probiotics should have effective intestinal adhesion and colonization capabilities, along with functional characteristics such as antimicrobial activity [16]. Furthermore, growing concerns regarding food safety and antibiotic resistance have underscored the importance of assessing potential virulence factors and antimicrobial resistance profiles in probiotic strains. Whole-genome sequencing (WGS) has recently emerged as an indispensable tool for strain characterization, offering unprecedented resolution for phylogenetic analysis, functional gene identification, and metabolic pathway elucidation [15]. Consequently, it has become a highly reliable approach for comprehensive safety and probiotic trait assessment.
The honeybee gut microbiota is highly specific and functionally diverse, playing crucial roles in carbohydrate metabolism, antioxidation, and immune modulation [17, 18]. In a previous study, we isolated L. helveticus KM7 from the Apis cerana gut and demonstrated its notable antimicrobial activity and antibiotic susceptibility [19]. However, most currently characterized L. helveticus strains originate from fermented dairy products, while insect-derived isolates (particularly those from honeybees) remain understudied and their potential probiotic traits and genomic features poorly characterized. Therefore, we here employ WGS combined with phenotypic assays to systematically evaluate the safety, probiotic properties and EPS-producing capacity of L. helveticus KM7. Our findings provide a theoretical foundation for developing this strain as both a probiotic candidate and a microorganism for fermented food production.
Materials and methods
Bacterial strains and growth conditions
We previously isolated L. helveticus KM7 from the intestinal tract of A. cerana, collected in Kunming, China (102°45’30.5’’E, 25°8’5.8’’N). KM7 was revived and anaerobically cultured in MRS medium (Guangdong Huankai Microbial Sci. & Tech. Co., Ltd., Guangzhou, China; cat. no. 027312) at 37℃ for 24 h. The pathogenic strains (Escherichia coli K88, Staphylococcus aureus ATCC 49521, and Salmonella typhi) were revived and aerobically cultured in LB medium (Guangdong Huankai Microbial Sci. & Tech. Co., Ltd., Guangzhou, China; cat. no. 028320) at 37℃ for 24 h.
Genome sequencing and analysis
The activated KM7 culture was inoculated into MRS broth at a 1% (v/v) concentration and incubated at 37 ℃ until reaching the mid-logarithmic growth phase (8 h). The bacteria were then harvested by centrifugation at 2,800 × g for 10 min, followed by three washes with sterile PBS (phosphate-buffered saline) buffer for subsequent DNA extraction, which was performed with a Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA; cat. no. A1620). Purified genomic DNA was quantified with a TBS-380 fluorometer (Turner BioSystems Inc., CA, USA). High quality DNA (OD260/280 = 1.8 ~ 2.0, >20 µg) was used for further research.
We sequenced the KM7 genome using a combination of PacBio RS II Single Molecule Real-Time (SMRT) and Illumina platforms. For Illumina sequencing, at least 1 µg of genomic DNA per strain was sheared into 400–500 bp fragments using a Covaris M220 Focused Acoustic Shearer (Covaris, Inc., MA, USA). Sequencing libraries were prepared using the NEXTflex™ Rapid DNA-Seq Kit (Bioo Scientific, TX, USA), including end-repair, phosphorylation, A-tailing, adapter ligation, and PCR enrichment. Paired-end sequencing (2 × 150 bp) was performed on an Illumina HiSeq X Ten. For PacBio sequencing, 15 µg of DNA was fragmented using a Covaris g-TUBE (Covaris, Inc., MA, USA) at 6,000 g for 60 s. The fragments were then purified, end-repaired, and ligated with SMRTbell adapters (Pacific Biosciences, CA, USA). The library was purified three times using 0.45 × volumes of Agencourt AMPure XP beads (Beckman Coulter, Inc., CA, USA). An ~ 10 kb insert library was prepared and sequenced on one SMRT cell using standard protocols and the complete genome sequence assembled using both PacBio and Illumina reads. Raw sequence data, generated via base calling, were saved as FASTQ files containing sequence read and quality information. Quality trimming was performed to remove low-quality reads. We assembled the genome using the hierarchical genome assembly process (HGAP) and Canu, followed by manual verification to ensure circularization, resulting in a complete genome with seamless chromosomes and plasmids. Final error correction of the PacBio assembly was performed using Illumina reads with Pilon.
Coding sequences (CDSs) were predicted using Glimmer. The predicted CDSs were functionally annotated by aligning against the NR, GO, KEGG and CAZy databases using BLAST, Diamond, Hmmscan and HMMER. Annotations were assigned based on the best-matched hits with an E-value threshold of 10−5. Putative genomic islands, prophages, and CRISPR arrays were identified bioinformatically using IslandPath-DIMOB, PHASTER, and CRISPRCasFinder, respectively, applied to the complete genome sequence. Genes associated with probiotic functions were identified following the approaches and utilizing the databases described by Sabino et al. and Rocha et al. [20, 21]. Putative bacteriocin biosynthesis gene clusters were identified using the BAGEL4 web server (http://bagel4.molgenrug.nl).
Comparative genome analysis and reconstruction of the pan-core gene set
We performed comparative genome analysis and pan-core gene set reconstruction of L. helveticus KM7 and six other L. helveticus strains from different sources: DS3_8 from commercial dietary supplements (GenBank accession: GCA_003053085.1), CAUH18 from koumiss (GCA_001308285.1), CGMCC from Emmental cheese (GCA_001434945.1), LBGT 162 from sorghum beer fermentation (GCA_019455825.1), LZ-R-5 from yoghurt (GCA_009498395.1), and TMW 12,315 from rice vinegar fermentation (GCA_019455865.1). We calculated the pairwise average nucleotide identity (ANI) between KM7 and the other strains using the integrated prokaryotes genome and pan-genome analysis service (IPGA) v1.09. We reconstructed the pan-genome also using the IPGA, which incorporates the Panaroo tool (v1.3.2) for gene clustering and annotation. Orthologous gene clusters were identified using a 70% nucleotide identity threshold, and core genes were defined as those present in ≥ 95% of the analyzed strains. A stringent support value of 1.0 was applied to ensure high-confidence clustering. Finally, we reconstructed a core gene-based phylogenetic tree using VBCG v1.3 with FastTree to assess sequence divergence among strains.
Safety assessment
Identification of safety-relevant genes
We predicted potential virulence factor-related genes in the genome of KM7 using the Virulence Factor Database (VFDB) v20240301 (https://www.mgc.ac.cn/VFs/) and annotated antibiotic resistance genes via the Comprehensive Antibiotic Resistance Database (CARD) v3.2.9 (https://card.mcmaster.ca). In accordance with the Guidelines for Identification and Safety Evaluation of Strains Used in Direct Feeding of Microorganisms and Fermented Products [22], we set the screening criteria for virulence factors and antibiotic resistance genes as follows: E value < 10−5, coverage > 70%, similarity > 80%.
Hemolytic activity assessment
The activated cultures of L. helveticus KM7 and S. aureus ATCC 49521 (negative control) were streaked onto Columbia blood agar plates (Guangdong Huankai Microbial Sci. & Tech. Co., Ltd., Guangzhou, China) and incubated at 37℃ for 48 h. Hemolytic activity was assessed by observing hemolysis zones around the colonies: A clear zone surrounding the colony signifies β-hemolysis, whereas a greenish discoloration indicates α-hemolysis; in contrast, the absence of any hemolytic activity is classified as γ-hemolysis.
Assessment of the ability to produce biogenic amines
The capacity to produce putrescine, cadaverine, and spermidine was assessed using biochemical test tubes (Hangzhou Binhe Microbial Reagent Co., Ltd., Hangzhou, China, cat. nos. A139, A140, A141). KM7 was streaked onto MRS agar plates and incubated at 37℃ for 24 h. Single colonies were then inoculated into test tubes for arginine decarboxylase, lysine decarboxylase, and ornithine decarboxylase assays, along with control tubes, followed by sealing with paraffin oil. After 24 h of incubation at 37℃, color changes were observed. A negative result was recorded if both the test and control tubes turned yellow, whereas a positive result was indicated by a color change in the test tube with the control tube remaining yellow.
Probiotic characterization
Acid and bile salt tolerance
We adjusted the pH of MRS medium to different levels (2.0, 3.0, and 4.0) using 1 mol/L diluted hydrochloric acid (HCl) (Tianjin Fengchuan Chemical Reagent Technology Co., Ltd., Tianjin, China) and inoculated activated KM7 into the adjusted medium at 5% (v/v) concentration, followed by culturing at 37℃. Viable cell counts were determined by plating on MRS agar at 0, 1, 2, and 3 h, followed by survival rate calculation. For bile salt tolerance assessment, we inoculated KM7 at 5% (v/v) concentration into MRS medium containing 0.3% (w/v) bile salt (bovine) (Solarbio Biotechnology Co., Ltd., Beijing, China, cat. no. B8210) and cultured it at 37 ℃. Survival rates were determined at 0, 1, 2, and 3 h as follows:
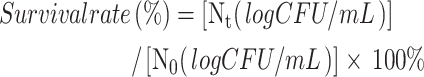 |
where Nt refers to the number of viable bacteria corresponding to 1, 2 and 3 h and N0 refers to the number of viable bacteria corresponding to 0 h.
Bile salt hydrolase activity
KM7 was cultured in MRS broth at 37℃ for 2, 4, 6, 8, 10, and 12 h. Subsequently, the cultures were centrifuged at 8,000 × g for 10 min at 4℃. The resulting pellets were washed twice with PBS (pH 7.4) and resuspended in the same buffer. The suspensions were subjected to ultrasonication using an ultrasonic cell disruptor (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China, cat. no. 950E) operated at 200 W with cycles of 5 s pulse and 5 s pause for 15 min. Cell debris was removed by centrifugation. The Bile salt hydrolase (BSH) activity in the supernatant was quantified using a commercial BSH Activity Assay Kit (Gerisi Bio-Technologies Co., Ltd., Suzhou, China, cat. no. G0933F). One unit of enzyme activity (U) was defined as the amount of enzyme required to catalyze the production of 1 µg of taurine per minute per milliliter of supernatant under the assay conditions.
Adhesion and aggregation
Cell-surface hydrophobicity
KM7 suspension cultured for 24 h was centrifuged at 4,000 × g for 10 min at 4℃. The collected cells were washed twice with PBS and resuspended in 2 mL PBS to a concentration of 108 CFU/mL, followed by measurement of the optical density at 600 nm (A0). Then, 2 mL cell suspension was mixed with 2 mL xylene (C8H10) (Tianjin Zhiyuan Chemical Reagent Co., Ltd., Tianjin, China), vortexed for 5 min, and allowed to stand for 30 min for phase separation. The optical density of the aqueous phase was measured at 600 nm (A1). Cell surface hydrophobicity was calculated as follows:
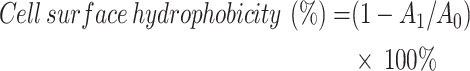 |
Auto-aggregation and co-aggregation
We cultured KM7 anaerobically in MRS broth at 37℃ until the late exponential phase (OD600 ≈ 1.0). Cells were harvested by centrifugation (5,000 × g, 10 min, 4℃), washed twice with PBS, and resuspended in the same buffer to a final concentration of 108 CFU/mL. The suspension was then vortexed for 10 s and allowed to incubate at 37℃. Aliquots (200 µl) from the upper layer were collected at 0 h (A0) and 3 h (A2), and OD600 measured using a spectrophotometer (METASH Co., Ltd., Shanghai, China, cat. no. UV-5100). Self-aggregation ability was calculated as follows:
 |
The selected pathogenic strains (E. coli K88, S. aureus ATCC 49521, and S. typhi) were cultured aerobically in LB medium at 37℃ for 8 h and adjusted to a concentration of 108 CFU/mL. Equal volumes (1:1) of KM7 suspension (108 CFU/mL) and each pathogen suspension (108 CFU/mL) were thoroughly mixed and co-cultured at 37℃. OD600 measurements of the upper suspension were performed at identical time (5 h) intervals. Co-aggregation efficiency was determined using the formula:
 |
where A2 is the OD600 (108 CFU/mL) of the KM7 suspension, A3 is the OD600 (108 CFU/mL) of each pathogen suspension, and A4 is the OD600 of the mixed suspension after standing.
Antioxidant capacity
The total antioxidant capacity (T-AOC), hydroxyl radical scavenging ability, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging ability, reduced glutathione (GSH) content, and superoxide dismutase (SOD) activity of both fermentation supernatant and cell-free extract of L. helveticus KM7 were quantified using commercial assay kits (Solarbio Biotechnology Co., Ltd., Beijing, China, cat. nos. BC1310, BC1320, BC4750, BC1170, BC0170). For T-AOC determination, FeSO4 (40 µmol/mL) was used as the standard compound; DPPH radical scavenging ability was quantified using vitamin C (10 mg/mL) as the standard reference; Reduced glutathione (10 mg/mL) served as the standard compound for GSH content measurement. Fermentation supernatant was collected by centrifugation of the bacterial culture supernatant (8,000 × g, 15 min, 4℃). To obtain cell-free extract, cell pellets were washed twice with PBS, disrupted via ultrasonication (200 W, 5 s pulse/5 s pause, 15 min) in ice-cold lysis buffer, and clarified by centrifugation (8,000 × g, 20 min, 4℃).
Extraction and quantification of crude exopolysaccharides
Crude EPS were extracted following the method of Senanayake [23]. with modifications. Bacterial cultures were centrifuged (8,000 × g, 20 min, 4℃), and the supernatant was treated with an equal volume of 10% (w/v) trichloroacetic acid (C2HCl3O2) (Chengdu Kelong Chemical Co., Ltd., Chengdu, China) to precipitate proteins (homogenized at 90 rpm for 30 min). After centrifugation (8,000 × g, 20 min, 4℃), EPS was precipitated from the clarified supernatant using ice-cold ethanol (1:2 v/v, 4 ℃, 24 h), recovered by centrifugation (8,000 × g, 20 min, 4℃), and lyophilized. The crude EPS were dissolved in deionized water to prepare an aqueous solution for further analysis.
Quantification of crude EPS was performed using the sulfuric acid-phenol method. A glucose standard curve was prepared by mixing 0–2.0 mL (in 0.2 mL increments) of 100 µg/mL glucose solution with distilled water (final volume: 2 mL), followed by addition of 2 mL 6% phenol (C6H5OH) (Guangdong Guanghua Sci-Tech Co., Ltd., Shantou, China) and 10 mL concentrated sulfuric acid (H2SO4) (Tianjin Fengchuan Chemical Reagent Technology Co., Ltd., Tianjin, China). After vortexing and 30 min incubation at 25℃, absorbance was measured at 490 nm. For crude EPS quantification, 2 mL of aqueous solution of crude exopolysaccharides was similarly processed, and concentration was calculated using the standard curve. The strain was cultured statically in MRS broth (1% inoculum, 37℃), and the content of extracellular polysaccharides was measured at 4, 8, 12, 16, 20 and 24 h. Following the method of Tenea [24] with minor modifications, the strain was statically cultured on MRS broth supplemented with 20% (w/v) each of glucose (BioFroxx GmbH, Heidelberg, Germany, cat. no. 1179GR500), sucrose (Macklin Biochemical Co., Ltd., Shanghai, China, cat. no. S818046), fructose (Macklin Biochemical Co., Ltd., Shanghai, China, cat. no. D809612), and lactose (Macklin Biochemical Co., Ltd., Shanghai, China, cat. no. L812298) (1% inoculum, 37℃, 24 h). The crude EPS content was subsequently quantified.
Statistical analysis
Data were analyzed using SPSS. Group differences were evaluated by Student’s t-test or one-way ANOVA followed by Tukey’s post hoc test. Results are reported as mean ± SD. Statistical significance was defined as a two-tailed p-value < 0.05.
Results and discussion
Genome characteristics of L. helveticus KM7
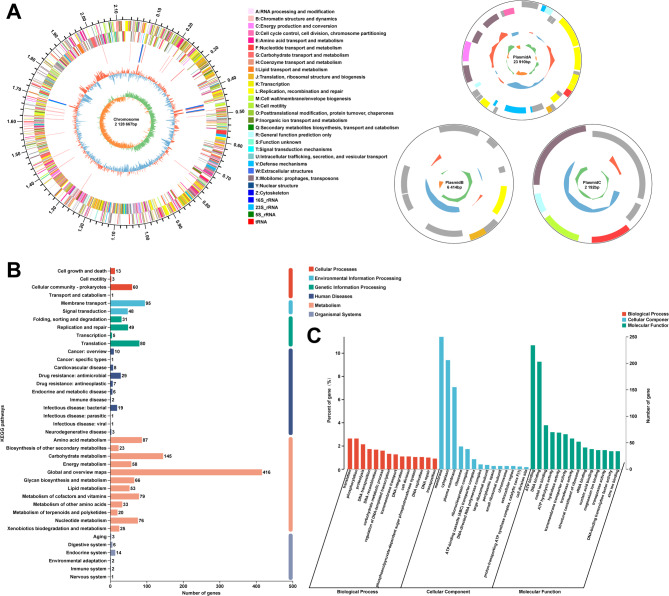
The genome of L. helveticus typically consists of a single circular chromosome ~ 2 Mbp in size, with some variation among strains [3]. The KM7 genome has a total length of 2,164,024 bp with a GC content of 36.82%, distributed on one chromosome and three plasmids (Fig. 1A). It encodes 2,192 genes with an average coding sequence length of 856.32 bp (Table 1). Of these, 2,145 genes are located on the chromosome, while the remaining 30, 11, and 6 genes are found on plasmid A, B, and C, respectively. The total length of coding sequences is 1,877,052 bp, representing 86.74% of the genome. Genomic analysis identified fifteen genomic islands (GIs) in the KM7 genome, fourteen located on the chromosome and one on plasmidA (Table S1). Three CRISPR arrays were also detected, two on the chromosome and one on plasmidA (Table S2). No genes encoding Cas proteins associated with the CRISPR arrays were detected. The absence of these essential components results in a standalone, defective CRISPR structure, compromising formation of a functional CRISPR-Cas defense system. Consequently, this abolishes the capacity of the system for targeted cleavage of exogenous genetic elements, such as plasmids harboring antibiotic resistance or virulence genes [25]. Furthermore, no prophages were identified.
Fig. 1.
Genome overview of L. helveticus KM7 and functional annotation in different databases. A Chromosomal and plasmid genomic circular map. B GO function classification. C KEGG function classification
Table 1.
Characteristics of the L. helveticus KM7 genome
| Attributes | Values |
|---|---|
| Genome Size (bp) | 2,164,024 |
| Chromosome | 2,128,667 |
| PlasmidA | 23,910 |
| PlasmidB | 6,414 |
| PlasmidC | 2,192 |
| G + C Content (%) | 36.82 |
| Chromosome | 36.81 |
| PlasmidA | 38.53 |
| PlasmidB | 34.49 |
| PlasmidC | 36.7 |
| Coding Gene Number | 2,192 |
| Coding Gene Average Length (bp) | 856.32 |
| Coding Gene Total Length (bp) | 1,877,052 |
The Gene Ontology (GO) database offers standardized terms and hierarchical classifications to describe gene and protein functions across diverse organisms, and is continuously updated to enhance biological research [26]. GO annotation of the KM7 genome revealed that 1,498 genes (68.34%) were successfully annotated (Fig. 1B). Within the Biological Process category, 1,194 genes were annotated, with translation-related and phosphorylation-related genes being the most abundant (58 genes each). For Cellular Component, 819 genes were annotated, with membrane-associated functions representing the predominant category (250 genes). Furthermore, 2,423 genes were assigned to Molecular Function, among which ATP-binding proteins constituted the largest group (234 genes). These genes may contribute to the adhesive colonization capacity and environmental stress resistance of L. helveticus KM7.
KEGG functional annotation enables the mapping of genes, proteins, and other biomolecules to pathways and functional modules within the KEGG (Kyoto Encyclopedia of Genes and Genomes) database, elucidating their roles and interactions in biological processes such as metabolism and signal transduction [27]. Our KEGG analysis of the L. helveticus KM7 genome successfully annotated 1581 genes (72.13%), with 77, 143, 165, 87, 1,081, and 28 assigned to Cellular Processes, Environmental Information Processing, Genetic Information Processing, Human Diseases, Metabolism, and Organismal Systems, respectively (Fig. 1C). Notably, metabolism-related genes constituted the predominant category, including 145 genes involved in carbohydrate metabolism. This metabolic profile aligns remarkably well with the characteristic fermentative properties of LAB. The efficient carbohydrate metabolic capacity of strain likely drives substantial lactate production, suggesting that KM7 holds promising potential for dairy fermentation and functional food applications [28].
Comparative genomic analysis of L. helveticus KM7 and other L. helveticus strains
Average Nucleotide Identity (ANI) analysis compares homologous sequences between pairwise genomes to evaluate phylogenetic relationships at the whole-genome level, with an ANI threshold > 95% typically indicating conspecificity [29]. Our comparative analysis of KM7 and six other L. helveticus strains from diverse origins revealed that all pairwise comparisons exceeded the 95% threshold, confirming the accurate taxonomic classification of KM7 as L. helveticus at the genome level (Fig. 2). Notably, KM7 had the highest ANI value with DS3_8 (99.81%), followed by CAUH18 (98.01%) and LBGT 162 (98.26%). This suggests that these strains have a closer evolutionary relationship with KM7 and thus may have similar metabolic capabilities due to the conservation of specific functional genes.
Fig. 2.
Comparative genomic analysis of L. helveticus KM7 and other L. helveticus strains. A Heatmap of ANI analysis. B Collinearity analysis
Genomic synteny analysis enables the elucidation of genetic locus alterations caused by various mechanisms, including inversions and translocations between sequenced and reference strains, as well as sequence insertions and deletions [30]. Comparative synteny analysis of L. helveticus KM7 with the six other strains mentioned above revealed extensive collinear conservation of most genes between KM7 and both CAUH18 and LZ-R-5, with only localized genomic rearrangements (e.g., inversions, translocations, and positional variations). This conservation pattern suggests that these strains have undergone relatively constrained genomic evolution. In contrast, KM7 had more extensive genomic rearrangements when compared with DS_8, TMW 12,315, LBGT 162, and CGMCC. These differences in genomic architecture may potentially correlate with the strains’ distinct isolation sources, environmental adaptation histories, or genetic backgrounds.
Construction of the pan-core gene set
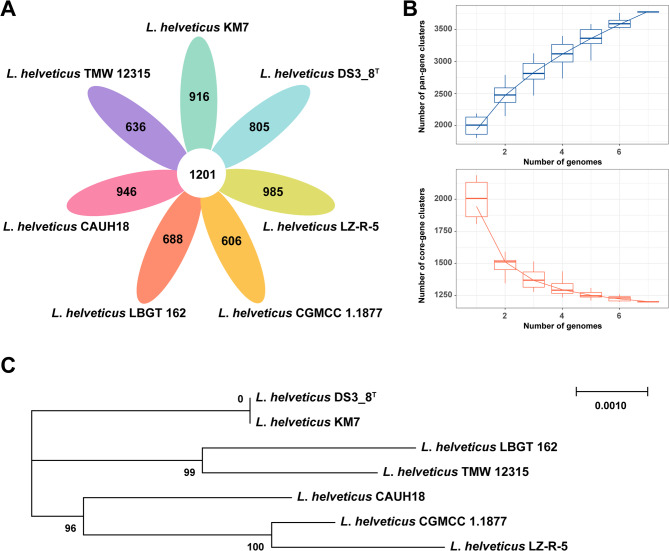
Core and pan-genome analyses have been widely employed to study bacterial evolution [31]. The pan-genome of L. helveticus KM7 and the six additional strains comprises 6,783 genes, including 1,201 core and 5,582 strain-specific genes (Fig. 3A). The rarefaction curve revealed decreasing core-gene clusters and increasing pan-gene clusters with the addition of more strains (Fig. 3B), indicating an open genome architecture and high genetic diversity [32]. Open pan-genomes are typically associated with broad environmental adaptability and frequent horizontal gene transfer (HGT), often facilitated by the acquisition of adaptive genes (e.g., related to carbohydrate metabolism and stress response) from mobile genetic elements such as phages, plasmids, or transposons in heterogeneous niches like dairy products or the host gut [33]. This genomic flexibility underlines the functional versatility of L. helveticus in industrial fermentation and probiotic applications. Our phylogenetic analysis of core gene sequences demonstrates a close evolutionary relationship between KM7 and DS_8 (Fig. 3C), suggesting that they share biological and physiological traits. As a model strain isolated from commercial dietary supplements, DS_8 is likely to have enhanced probiotic properties and metabolic capabilities for host health benefits [34]. The genetic similarity between KM7 and DS_8 implies that KM7 may have comparable beneficial traits, warranting further investigation.
Fig. 3.
Construction of the pan-core genome of L. helveticus KM7 and six other L. helveticus strains. A Core and unique genes of the seven strains. B Dilution curves of the core-genes and pan-genes clusters. C Phylogenetic tree reconstructed from core genes
Safety assessment of L. helveticus KM7
Antibiotic resistance
To ensure the safety and efficacy of probiotic strains, they must be devoid of antibiotic resistance genes (ARGs) [35]. No ARGs meeting the screening criteria of the “Guidelines for Identification and Safety Evaluation of Strains Used in Direct Feeding of Microorganisms and Fermented Products” (Ministry of Agriculture and Rural Affairs of China) were detected in our analysis of the L. helveticus KM7 genome. Although bacteria may acquire ARGs through HGT, mobile genetic elements (MGEs), or chromosomal mutations, KM7 carries only one transposon located on a plasmid, suggesting a low risk of plasmid-mediated ARG dissemination [36]. The transporter proteins detected in GIs (e.g., the MFS transporter in GI11 and the ABC transporter in GI15) are membrane-associated but show no apparent linkage to mobile genetic elements (MGEs), suggesting a low risk of ARG dissemination [37]. Moreover, consistent with the absence of annotated resistance genes in its genome, our prior phenotypic assays confirmed that KM7 is sensitive to cefotaxime, amoxicillin, cephalothin, penicillin G, ampicillin, kanamycin, and vancomycin, as well as moderately sensitive to novobiocin [19]. These results demonstrate that L. helveticus KM7 meets probiotic safety standards at both genomic and phenotypic levels, aligning with regulatory requirements for fermented products.
Virulence factors
Virulence factors play crucial roles in bacterial pathogenesis, including host invasion, colonization, and disease progression [38]. Our genomic analysis of L. helveticus KM7 against the Virulence Factor Database identified only one putative virulence factor (capsule). Importantly, many genes annotated in virulence databases may participate in routine metabolic processes or environmental adaptation rather than directly mediating pathogenicity [39]. For instance, certain bacterial adhesion-related genes in KM7 might solely facilitate intestinal colonization, which is a desirable trait for potential probiotic applications [40]. Notably, the capsule virulence factor is commonly present across Lactobacillus species. As surface polysaccharide structures, bacterial capsules in non-pathogenic strains typically contribute to environmental stress resistance and biofilm formation rather than virulence [41]. The GIs within the KM7 strain lack detectable canonical virulence genes (e.g., hemolysins, invasins) and harbor only toxin-antitoxin (TA) systems (GI02/GI03). These systems play a significant role in cellular stress responses, enhancing genomic stability and cell viability without exerting detrimental effects on the host or environment [42]. Furthermore, transposases (e.g., those in GI04/GI12) are primarily associated with metabolic genes (such as the GI06 fatty acid biosynthesis cluster) and do not carry exogenous pathogenic islands. These findings demonstrate that KM7 lacks definite virulence factors and complies with safety standards for microbial strains used in fermented food production.
Hemolytic activity
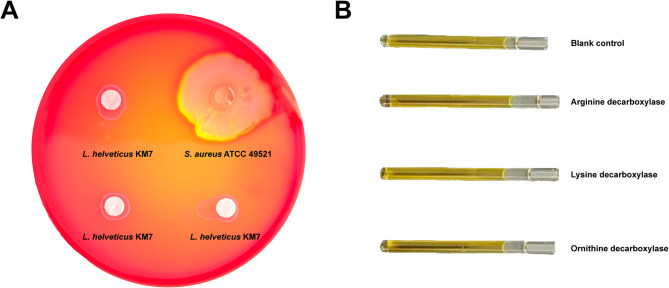
Assessment of hemolytic activity is a critical safety evaluation for probiotic strains [43]. Our hemolysis assays revealed distinct zones of β-hemolysis surrounding S. aureus colonies (positive control), indicating hemolysin production (e.g., α-toxin or staphylolysin) capable of erythrocyte lysis and systemic infection [44] (Fig. 4A). In contrast, no hemolytic zones were observed around L. helveticus KM7 colonies, demonstrating γ-hemolysis (i.e., a non-hemolytic phenotype). These results confirm that L. helveticus KM7 lacks the capacity to damage host erythrocytes, consistent with the safety profile expected of LAB strains.
Fig. 4.
Safety assessment of L. helveticus KM7. A Hemolytic activity assay. B Production capacity assays for spermine, cadaverine, and putrescine
Biogenic amine production
Biogenic amines (BAs) in food are primarily formed through decarboxylation of free amino acids by microbial amino acid decarboxylases, and their excessive accumulation may lead to food poisoning. For instance, in fermented foods such as cheese and sausages, certain LAB and Enterococcus species can pose safety risks by producing BAs [45]. Therefore, we evaluated the BA-producing capacity of L. helveticus KM7. According to our results, the arginine decarboxylase, lysine decarboxylase, and ornithine decarboxylase test tubes showed no color change, indicating that KM7 lacks the ability to decarboxylate arginine, lysine, or ornithine, and thus cannot produce spermine, cadaverine, or putrescine (Fig. 4B). This further shows the suitability of L. helveticus KM7 for applications in the food industry.
Probiotic characterization of L. helveticus KM7
Acid and bile salt tolerance
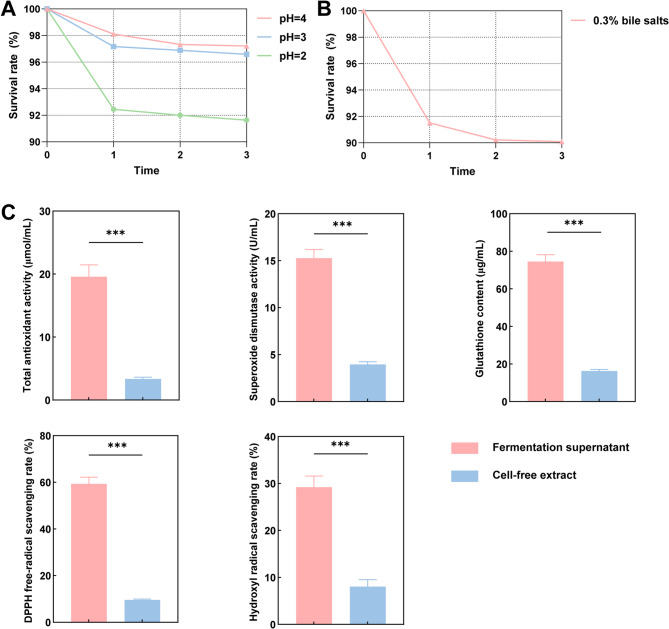
LAB encounter acidic environments during fermentation, and their acid tolerance is crucial for survival in the host gut or fermented foods [46]. Our results demonstrate that L. helveticus KM7 is highly viable under acidic conditions (pH 3 and 4) (Fig. 5A). To investigate the mechanisms underlying this acid resistance, we further analyzed the strain’s probiotic-related genes (Table S3). The genome of KM7 harbors a complete F-type H+/Na+-transporting ATPase system (atpA-atpH genes). This proton pump expels excess intracellular H+ by ATP hydrolysis, thus maintaining cytoplasmic pH homeostasis [47]. The integrity of this system is a key determinant of bacterial acid tolerance, as studies have shown that downregulation of the F-type ATPase β subunit significantly reduces the growth and survival of Streptococcus mutans under acidic conditions [48]. The genomic integrity of the F-type ATPase system in KM7 ensures its high survival rate under acidic conditions. The arginine deiminase (ADI) pathway, regulated by arginine deiminase (arcA), ornithine carbamoyltransferase (arcB), and carbamate kinase (arcC), is involved in modulating intracellular pH homeostasis in LAB under acid stress [49]. When exposed to adverse environmental conditions, LAB can activate the ADI pathway to enhance acid resistance [50]. The presence of partial ADI pathway genes (arcA, arcC) in the KM7 genome suggests its potential reliance on ADI pathway activation for acid stress tolerance. The synergistic action of the F-type ATPase system and the ADI pathway likely underpins the robust acid stress response in L. helveticus KM7. The KM7 genome also encodes additional genes supporting acid adaptation. Key glycolytic enzymes (gapA, pgi, tpiA, pyk) ensure sustained ATP generation, facilitating proton extrusion via the F-type ATPase [51]. Furthermore, the DNA repair function mediated by uvrA and cell wall synthesis regulated by ddl contribute to maintaining structural integrity [52, 53]. Notably, lactate dehydrogenase (ldh) activity contributes to environmental acidification, which could potentially enhance competitive fitness [51]. These molecular adaptations not only enhance its survival in harsh environments but also support its potential as a functional probiotic for industrial applications.
Fig. 5.
Probiotic characterization of L. helveticus KM7. A Acid tolerance. B Bile salt tolerance. C Antioxidant activity of fermentation supernatant and cell-free extract. Data are presented as mean ± SD (n = 3) and *** indicates P < 0.001
Bile salt tolerance represents a fundamental prerequisite for probiotic survival within the host gastrointestinal tract. According to our results, L. helveticus KM7 had good survival rates when exposed to bile salts (Fig. 5B). We identified the bile salt hydrolase gene cbh in the genome of KM7, suggesting that the strain may directly degrade bile salts enzymatically (Table S3). Bile salt hydrolase (BSH) activity is a central mechanism of bile salt tolerance in L. helveticus, enhancing its survival in the intestine by reducing bile salt damage to cell membranes and proteins [54]. The BSH activity assay showed that strain KM7 has the ability to produce bile salt hydrolase (BSH), and its BSH activity level gradually increased with the advancement of the growth phase under the experimental conditions (Fig. S1). This result suggests that strain KM7 is able to continuously express and accumulate BSH enzyme during growth. Our results are consistent with previous studies that BSH activity correlates with bile salt resistance in L. helveticus, and that its expression level and substrate specificity may lead to differences in tolerance among strains [55]. The cbh is both present in the KM7 genome, so this strain may expand bile salt hydrolysis capacity through synergistic effects of the two genes to increase the adaptation to the complex bile salt environment. It is worth noting that its adaptability to this environment not only stems from direct detoxification mechanisms, but is also closely related to its ability to maintain the structural integrity of the cell membrane and wall [56]. Concerning the regulation of cell membrane stability, genomic analysis of KM7 revealed that the cyclopropane fatty acid synthase encoded by the cfa gene may play a key role [57]. This enzyme can maintain the stability of the cell membrane through fatty acid cyclopropanation, thus limiting the permeability damage of bile salt molecules. GlcN-6-P deaminase encoded by nagB may affect peptidoglycan synthesis by regulating the N-acetylglucosamine (NAG) metabolic network [58]. We hypothesize that KM7 may form a physical barrier against bile salts by dynamically regulating the rate of cell wall synthesis and repair. Moreover, peptidases (pepF, pepO, pepP) contribute to amino acid acquisition for the synthesis of repair proteins, while pyrophosphatase (ppaC) could potentially provide energy for these processes [59, 60]. Notably, the DNA-binding protein Dps counteracts bile-induced oxidative DNA damage [61]. Thus, the bile salt tolerance in L. helveticus KM7 is not dependent on a single gene function but is the result of synergistic effects of multiple pathways.
Adhesion and aggregation
The hydrophobicity and aggregation capacity of bacterial strains are closely associated with their adhesion properties, serving as crucial prerequisites for probiotic functionality. Our investigations revealed that L. helveticus KM7 has high surface hydrophobicity and auto-aggregation abilities (Table 2). These characteristics imply the presence of abundant hydrophobic surface molecules, such as lipoteichoic acids (LTAs) and proteins, which may be attributed to genes such as ltaS (encoding lipoteichoic acid synthase) and eno (encoding enolase) we identified in the KM7 genome [62]. As a key component of Gram-positive bacterial cell walls, LTAs enhance bacterial adhesion through interactions with host cell surfaces [63]. Enolase, a multifunctional surface protein, not only participates in glycolytic metabolism but also functions as an adhesin by directly binding to host extracellular matrix proteins, thereby mediating cellular adhesion [64]. These molecular features collectively account for the observed auto-aggregation phenotype and potential intestinal epithelial adhesion advantages of L. helveticus KM7. Furthermore, we identified scpA and scpB in the KM7 genome. While these genes encode fibronectin-binding proteins known to promote bacterial-host adhesion in certain streptococci (typically associated with pathogenic processes), their presence in this probiotic strain may enhance adhesion capacity without conferring pathogenicity [65]. Notably, the co-aggregation ability of probiotic strains further facilitates biofilm formation in the intestinal tract, thereby enhancing their inhibitory effects against pathogenic bacteria [66]. Studies have shown that various Lactobacillus species including L. crispatus, L. gasseri, L. reuteri, and L. coryniformis can co-aggregate with pathogenic bacteria [67, 68]. Here, we found that L. helveticus KM7 was able to co-aggregate (at a rate > 30%) with S. aureus, E. coli K88, and S. typhi, suggesting that it can inhibit pathogenic bacterial colonization through competitive binding [69].
Table 2.
Adhesion and aggregation ability of L. helveticus KM7
| Item | Value (%) |
|---|---|
| Hydrophobicity | 53.67 ± 1.28 |
| Self-coagulation | 78.27 ± 2.46 |
| Co-aggregation with S. aureus ATCC 49521 | 49.40 ± 1.42 |
| Co-aggregation with E. coli K88 | 32.05 ± 1.95 |
| Co-aggregation with S. typhi | 36.32 ± 1.15 |
Antioxidant capacity
Maintaining effective metabolic and oxidative homeostasis can counteract aging-related diseases [70]. Probiotics have significant potential as antioxidants to preserve intestinal redox balance [71]. Here, we have evaluated the antioxidant capacity of L. helveticus KM7. Our results reveal that both fermentation supernatant and cell-free extract of KM7 have antioxidant (including SOD and GSH) activity, as well as scavenging effects on DPPH and hydroxyl radicals (Fig. 5C). This enhanced antioxidant capability may be attributed to the synergistic effects of multiple pathways encoded by genes such as trxB, the suf operon, and msrA/B (Table S3). The trxB gene encodes thioredoxin reductase, a core component of the thioredoxin system. This enzyme catalyzes NADPH-dependent reduction reactions to continuously regenerate the reduced form of thioredoxin (Trx-(SH)₂), thereby facilitating electron transfer to peroxiredoxins and other antioxidant enzymes to maintain cellular redox homeostasis [72]. The suf operon genes encode proteins involved in the synthesis and repair of Fe-S clusters, which are essential for the function of key antioxidant enzymes such as thioredoxin and glutathione reductase [73]. The Suf system enhances cellular antioxidant capacity by modulating sulfur metabolism, thereby conferring protection against oxidative stress-induced damage [74]. The msrA/B genes encode the methionine sulfoxide reductase system, a critical mechanism for repairing oxidative protein damage. This enzyme system specifically reverses methionine sulfoxidation caused by oxidative stress, restoring the function of damaged proteins [75]. Notably, in our experiments, the fermentation supernatant had higher antioxidant capacity than the cell-free extract. LAB can secrete extracellular vesicles to deliver proteins into the surrounding environment [76]. L. helveticus KM7 may employ a similar mechanism, potentially contributing to the stronger free radical scavenging capacity of its extracellular products. The coordinated action of these genetic systems suggests that KM7 not only maintains its own oxidative homeostasis through enzymatic antioxidant pathways (SOD/GSH) but may also regulate extracellular redox balance via the secretion of bioactive compounds.
Antibacterial activity and potential bacteriocin genes
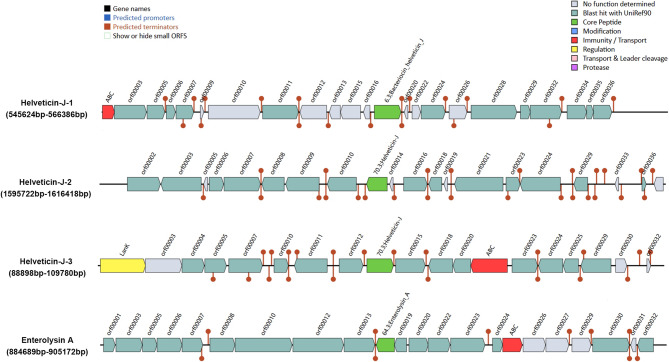
Antimicrobial activity is a key mechanism by which probiotics eliminate or inhibit harmful intestinal microorganisms, either through the production of antimicrobial peptides or by lowering environmental pH to kill pathogens [77]. In our previous studies, we found that L. helveticus KM7 exhibits antimicrobial activity against E. coli, Shigella flexneri, and Salmonella typhimurium, surpassing that of the reference strain LGG [19]. Bacteriocins are one of the natural defense mechanisms bacteria use to compete with other microbes in shared environments. To identify the bacteriocins responsible for the antimicrobial effects of KM7, we performed a genome-wide bacteriocin gene cluster analysis using BAGEL4 (Fig. 6). Our results revealed three putative gene clusters (Helveticin-J-1, -J-2, and -J-3) potentially involved in the biosynthesis of bacteriocin Helveticin J, with core peptide sequence similarities of 90.980%, 42.066%, and 39.481%, respectively. Helveticin J is a Class III bacteriocin and likely exerts its antimicrobial effects by disrupting bacterial cell walls and increasing membrane permeability [78]. NX371, a novel bacteriocin with high sequence similarity to Helveticin J, exhibits potent activity against both Gram-positive and Gram-negative bacteria [79]. In our study, Helveticin J-1 had the highest similarity to the known Helveticin J, suggesting it may be the primary contributor to the antimicrobial activity of KM7. Notably, sequence variations within bacteriocin families may influence target-binding specificity or membrane penetration efficiency, thereby expanding or modulating their antimicrobial spectrum [80]. The lower sequence similarity of Helveticin J-2 and J-3 suggests potential functional divergence, though further in vitro expression and mechanistic studies are required to validate this. Furthermore, we identified a gene cluster associated with the biosynthesis of the Class II bacteriocin Enterolysin A (EnlA) in the KM7 genome, with a core peptide similarity of 60.248%, indicating possible production of an EnlA-like bacteriocin. EnlA, a peptidase-type bacteriocin, induces bacterial lysis by hydrolyzing the peptidoglycan layer of Gram-positive cell walls [81]. The synergistic action of multiple bacteriocins may underlie the broad-spectrum activity of L. helveticus KM7 against pathogenic bacteria.
Fig. 6.
Potential antimicrobial gene clusters of L. helveticus KM7
Carbohydrate-active enzyme analysis
Through comprehensive analysis of carbohydrate-active enzymes (CAZymes) in the L. helveticus KM7 genome, we elucidated its functional characteristics in carbohydrate metabolism. Among the 71 identified CAZyme genes, glycoside hydrolases (GHs, 33 genes) and glycosyl transferases (GTs, 23 genes) predominated, indicating the robust capacity strain for decomposing complex polysaccharides and synthesizing functional oligosaccharides or exopolysaccharides (Fig. 7). The diversity of GH families (particularly GH13) likely reflects metabolic adaptation to plant-derived polysaccharides and host mucopolysaccharides in the intestinal environment. GH13 family α-amylases may provide carbon sources by degrading α-glucans while competitively limiting carbohydrate utilization by pathogens [82]. Furthermore, GT families (including GT2 and GT4) potentially contribute to cell wall biosynthesis or prebiotic-like molecule production, thereby enhancing intestinal colonization capacity [83]. Notably, the identification of carbohydrate esterases (CEs, 7 genes) and auxiliary activities (AAs, 8 genes) suggests that KM7 may facilitate degradation of recalcitrant polysaccharides (e.g., chitin or lignocellulose) through deacetylation or redox reactions that complement GH activity [84]. This metabolic versatility toward diverse substrates positions L. helveticus KM7 as a promising candidate for probiotic formulations or starter cultures, with potential applications in dairy texture modification and dietary fiber-enriched food development.
Fig. 7.
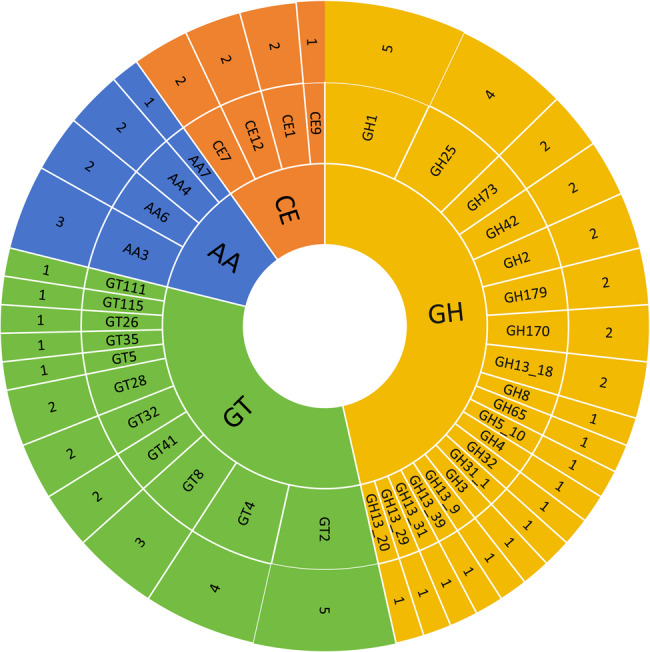
Classification of predicted carbohydrate-active enzymes (CAZy) from L. helveticus KM7. AA: Auxiliary Activities; CE: Carbohydrate Esterases; GH: Glycoside Hydrolases; GT: Glycosyl Transferases
EPS production capacity of L. helveticus KM7
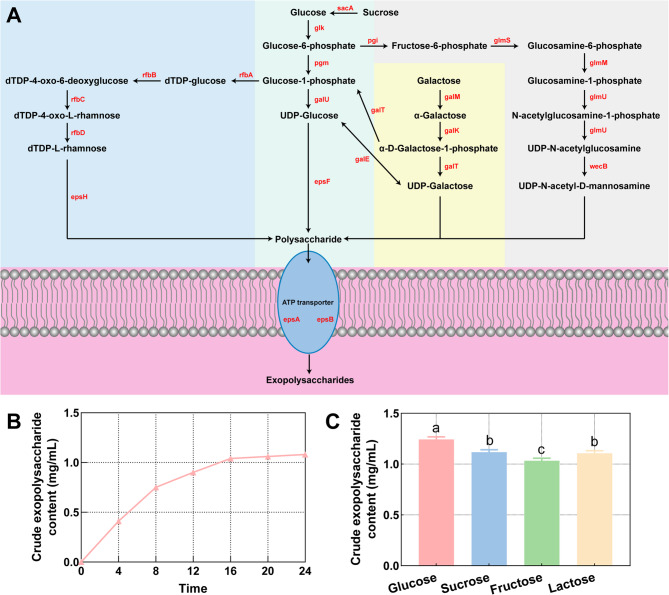
Genome annotation revealed that KM7 possesses complete gene clusters for sugar metabolism (rfb, gal, and glm clusters) and EPS biosynthesis (epsA/B/F/H/J), indicating genetic basis for complex EPS production (Table 3). Metabolic network predictions demonstrate ability of KM7 to convert monosaccharides (e.g., glucose, galactose) into diverse nucleotide sugars through four pathways, generating four key EPS precursors: UDP-glucose, UDP-galactose, dTDP-L-rhamnose, and UDP-N-acetyl-D-mannosamine (Fig. 8A). Unlike most lactic acid bacteria that utilize the wzx/wzy-dependent pathway, KM7 lacks wzx/wzy genes but retains complete ABC transporter-dependent genes (epsA/B), suggesting its EPS secretion primarily relies on the ABC transporter pathway [23]. Therefore, we hypothesized that the extracellular polysaccharides produced by KM7 are mainly capsular polysaccharides (CPS). CPS production likely contributes to multifaceted functional profile of KM7, including demonstrated antimicrobial effects through interference with pathogen adhesion and biofilm formation, as well as significant antioxidant capacity via both direct free radical scavenging and modulation of antioxidant enzyme activity [85, 86]. Furthermore, these CPS molecules also improve the functional properties of dairy products by increasing water retention and viscosity, which enhances texture and mouthfeel [87, 88]. These properties position KM7 as a potential candidate strain for functional food development.
Table 3.
Genes related to EPS production of L. helveticus KM7
| Gene locus | Gene name | Definition |
|---|---|---|
| gene0693 | glk | glucokinase |
| gene1548 | pgm | phosphoglucomutase |
| gene1616, gene1840 | galU | UTP–glucose-1-phosphate uridylyltransferase |
| gene1874 | rfbA | glucose-1-phosphate thymidylyltransferase |
| gene1875 | rfbB | dTDP-glucose 4,6-dehydratase |
| gene1873 | rfbC | dTDP-4-dehydrorhamnose 3,5-epimerase |
| gene1854, gene1872 | rfbD | dTDP-4-dehydrorhamnose reductase |
| gene0198, gene0734, gene1234 | galM | aldose 1-epimerase |
| gene0732 | galK | galactokinase |
| gene0733 | galT | UDPglucose–hexose-1-phosphate uridylyltransferase |
| gene0708 | galE | UDP-glucose 4-epimerase |
| gene0709, gene0710 | lacZ | beta-galactosidase |
| gene0408 | sacA | beta-fructofuranosidase |
| gene1485 | pgi | glucose-6-phosphate isomerase |
| gene1772 | glmS | glutamine—fructose-6-phosphate transaminase (isomerizing) |
| gene1516 | glmM | phosphoglucosamine mutase |
| gene0233 | glmU | bifunctional UDP-N-acetylglucosamine pyrophosphorylase/glucosamine-1-phosphate N-acetyltransferase |
| gene1621 | wecB | UDP-N-acetylglucosamine 2-epimerase (non-hydrolysing) |
| gene1900 | epsA | protein tyrosine kinase modulator |
| gene1899 | epsB | protein-tyrosine kinase |
| gene1893 | epsF | glycosyltransferase EpsF |
| gene0137 | epsH | glycosyltransferase EpsH |
| gene1892 | epsJ | glycosyltransferase EpsJ |
Fig. 8.
Predicted EPS metabolic network and production capacity of KM7. A Predicted EPS metabolic network. B Crude EPS yield by KM7 at different time points. C Crude EPS yield of KM7 supplemented with various carbon sources. Different letters indicate significant differences between age stages (P < 0.05)
KM7 exhibited a short lag phase, entering the exponential growth phase after 4 h of cultivation with concomitant EPS production (Fig. 8B). The EPS accumulation reached a plateau (1.08 mg/mL crude EPS) at 24 h when the culture transitioned into the stationary phase, a yield superior to several documented L. helveticus strains and other lactic acid bacteria [11, 89]. This high productivity may be attributed to strain-specific glycosyltransferases enhancing monosaccharide polymerization efficiency, and gene dosage effects (e.g., multi-copy galU) augmenting sugar nucleotide biosynthesis. The high-EPS-producing phenotype likely underpins enhanced adhesion of capacity and antioxidant activity. Carbon source utilization significantly influenced EPS yield, with glucose supporting the highest production, followed by sucrose, lactose, and fructose (Fig. 8C). This hierarchy aligns with genomic predictions of carbon transport and metabolic pathways. Notably, KM7 demonstrated reduced EPS biosynthesis efficiency from sucrose relative to glucose, along with compromised fructose metabolism. Genomic analysis revealed the absence of a fructose-6-phosphate isomerase (gpi) gene, potentially explaining the fructose assimilation bottleneck [90]. Given these findings, glucose supplementation represents the most effective strategy for maximizing EPS production in KM7. Furthermore, its efficient lactose metabolism supports its potential application in dairy fermentations.
Conclusions
In summary, we characterized the safety, probiotic properties and EPS-producing capacity of L. helveticus KM7 through genomic analysis and in vitro assays. The absence of identifiable virulence factor genes, antibiotic resistance genes, hemolytic activity, and biogenic amine production confirmed the safety of this strain. Its bile salt and acid tolerance, strong adhesion capacity, notable antioxidant activity, and the presence of genes potentially encoding bacteriocin and diverse carbohydrate-active enzymes endowed it with outstanding probiotic attributes. Furthermore, KM7 has an excellent EPS-producing capacity. Collectively, our findings demonstrate that L. helveticus KM7 is a safe and highly promising probiotic candidate with substantial potential for food applications.
Supplementary Information
Acknowledgements
Not applicable.
Authors' contributions
C.L.: Investigation, Data Curation, Formal analysis, Visualization, Writing - Original Draft. L.X.: Investigation, Data Curation, Formal analysis, Visualization. S.C.: Investigation, Data Curation, Formal analysis. S.W.: Data Curation, Formal analysis. M.L.: Data Curation, Formal analysis. K.D.: Conceptualization, Methodology, Supervision, Funding acquisition. Q.L.: Conceptualization, Methodology, Supervision, Writing - review & editing, Funding acquisition. Z.C.: Conceptualization, Methodology, Funding acquisition, Project administration, Supervision.
Funding
This project was supported by the Yunnan Provincial Middle-Young Academic and Technical Leader Candidate (202305AC160040), Young Talent of Yunnan Xingdian Support Project for High Level Talents (YNWR-QNBJ-2018-137), and the China Agriculture Research System of MOF and MARA (CARS-44-KXJ13).
Data availability
The KM7 genome data were deposited at the NCBI database, with the BioProject accession number: PRJNA1271779.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chen Liu and Le Xu contributed contributed equally to this study.
Contributor Information
Qiuye Lin, Email: linqiuye@ymu.edu.cn.
Zhenhui Cao, Email: caozhenhui@ynau.edu.cn.
References
- 1.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 2.Skrzypczak K, Gustaw W, Waśko A. Health-promoting properties exhibited by Lactobacillus helveticus strains. Acta Biochim Pol. 2015;62(4):713–20. [DOI] [PubMed] [Google Scholar]
- 3.Cremonesi. Genome sequence and analysis of Lactobacillus helveticus. Front Microbiol. 2012;3:435. [DOI] [PMC free article] [PubMed]
- 4.Valentina T, Simone G. Health-promoting properties of Lactobacillus helveticus. Front Microbiol. 2012;3:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zago M, Massimiliano L, Bonvini B, Penna G, Giraffa G, Rescigno M. Functional characterization and immunomodulatory properties of Lactobacillus helveticus strains isolated from Italian hard cheeses. PLoS One. 2021;16(1):e0245903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao K, Chen CL, Ke XQ, Yu YX, Chen S, Liu GC, Wang HF, Li YJ. Ingestion of Lactobacillus helveticus WHH1889 improves depressive and anxiety symptoms induced by chronic unpredictable mild stress in mice. Benef Microbes. 2022;13(6):473–88. [DOI] [PubMed] [Google Scholar]
- 7.Mutoh N, Kakiuchi I, Kato K, Xu C, Iwabuchi N, Ayukawa M, Kiyosawa K, Igarashi K, Tanaka M, Nakamura M. Heat-killed L. helveticus enhances positive mood states: a randomized, double-blind, placebo-controlled study. Brain Sci. 2023;13(6):973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv J, Lang G, Wang Q, Zhao W, Shi D, Zhou Z, Shen Y, Xia H, Han S, Li L. Lactobacillus helveticus attenuates alcoholic liver injury via regulation of gut microecology in mice. Microb Biotechnol. 2024;17(10): e70016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X, Lin T, Jiang W, Lin Y, Xiao L, Tian Y, Ma K, Zhang C, Ji F, Mahsa GC, et al. Lactobacillus helveticus LZ-R-5 ameliorates DSS-induced colitis in mice by modulating gut microbiota and enhancing intestinal barrier function. J Agric Food Chem. 2025;73(1):464–77. [DOI] [PubMed] [Google Scholar]
- 10.Sadat-Mekmene L, Genay M, Atlan D, Lortal S, Gagnaire V. Original features of cell-envelope proteinases of Lactobacillus helveticus. a review. Int J Food Microbiol. 2011;146(1):1–13. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Ji J, Rui X, Yu J, Tang W, Chen X, Jiang M, Dong M. Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. LWT. 2014;59(2):732–9. [Google Scholar]
- 12.Broadbent JR, Cai H, Larsen RL, Hughes JE, Welker DL, Carvalho VGD, Tompkins TA, Ard Y, Vogensen F, Lorentiis AD. Genetic diversity in proteolytic enzymes and amino acid metabolism among Lactobacillus helveticus strains. J Dairy Sci. 2011;94(9):4313–28. [DOI] [PubMed] [Google Scholar]
- 13.O’Sullivan DJ, Mcsweeney PLH, Cotter PD, Giblin L, Sheehan JJ. Compromised Lactobacillus helveticus starter activity in the presence of facultative heterofermentative Lactobacillus casei DPC6987 results in atypical eye formation in Swiss-type cheese. J Dairy Sci. 2016;99(4):2625–40. [DOI] [PubMed] [Google Scholar]
- 14.Merenstein D, Pot B, Leyer G, Ouwehand A, Preidis GA, Elkins CA, Hill C, Lewis ZT, Shane A, Zmora N. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes. 2023;15(1):2185034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JJ, Zhou Qy L, Dm, Xiong J, Liang MH, Tang J, Xu Y. Evaluation of the safety and probiotic properties of Lactobacillus gasseri LGZ1029 based on whole genome and phenotype analysis. LWT. 2023;184:114759. [Google Scholar]
- 16.Yang J, Sun Y, Lei X, Zhao L, Luo R, Liu W. Evaluation of novel isolates of Lacticaseibacillus rhamnosus Probio-M9 derived through space mutagenesis. Food Biosci. 2023;52:102456. [Google Scholar]
- 17.Engel P, Kwong WK, Mcfrederick Q, Anderson KE, Dainat B. The bee microbiome: impact on bee health and model for evolution and ecology of host-microbe interactions. mBio. 2016;7(2):e02164–02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonilla-Rosso G, Engel P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr Opin Microbiol. 2018;43:69–76. [DOI] [PubMed] [Google Scholar]
- 19.Lv M, Wang S, Yin H, Dong K, Liu Y, Pan H, Lin Q, Cao Z. Probiotic potential and effects on gut microbiota composition and immunity of Indigenous gut lactobacilli in Apis Cerana. Probiotics Antimicrob Proteins. 2022;14:252–62. [DOI] [PubMed] [Google Scholar]
- 20.Sabino YNV, Paiva AD, Fonseca BR, Medeiros JD, Machado ABF. Deciphering probiotic potential: a comprehensive guide to probiogenomic analyses. Future Microbiol. 2025;20(7–9):611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha BMO, Sabino YNV, de Almeida TC, Palacio FB, Rotta IS, Dias VC, da Silva VL, Diniz CG, Azevedo VAC, Brenig B, et al. Unlocking probiotic potential: genomic insights into Weissella paramesenteroides UFTM 2.6.1. Probiotics Antimicrob Proteins. 2024. [DOI] [PubMed]
- 22.MARA. Notice from the general office of the ministry of agriculture and rural affairs on the issuance of the guidelines for identification and safety evaluation of strains used in direct feeding of microorganisms and fermented products. Bull Ministry Agric Rural Affairs People’s Repub China. 2021;11:97–111. [Google Scholar]
- 23.Senanayake D, Ramarao-Milne P, Pandey G, Hlaing MM, Chandrapala J, Torley PJ, Terefe NS. Genomic insights into exopolysaccharide biosynthesis pathways in novel Lactiplantibacillus plantarum and Leuconostoc mesenteroides strains. LWT. 2025;225:117863. [Google Scholar]
- 24.Tenea GN, Hidalgo J, Pepinos J, Ortega C. Genome characterization of Leuconostoc pseudomesenteroides UTNElla29 isolated from Morus Nigra (L.) fruits: a promising exopolysaccharides producing strain. LWT. 2024;206:116594. [Google Scholar]
- 25.Zhang Q, Ye Y. Not all predicted CRISPR-Cas systems are equal: isolated Cas genes and classes of CRISPR like elements. BMC Bioinformatics. 2017;18(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vu TTD, Jung J. Protein function prediction with gene ontology: from traditional to deep learning models. PeerJ. 2021;9:e12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dan T, Chen H, Li T, Tian J, Ren W, Zhang H, Sun T. Influence of Lactobacillus plantarum P-8 on fermented milk flavor and storage stability. Front Microbiol. 2019;9:3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chirag J, Luis M, Rodriguez -R, Adam M, Phillippy, Konstantinos T, Konstantinidis. Srinivas, aluru. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun. 2018;9(1):5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, Liu S, Ren H, Afriyie OE, Zhang M, Xu D, Huang X. Genome-wide identification and comparative evolution of 14-3-3 gene family members in five brassicaceae species. BMC Genomics. 2025;26(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JY, Han GG, Kim EB, Choi YJ. Comparative genomics of Lactobacillus salivarius strains focusing on their host adaptation. Microbiol Res. 2017;205:48–58. [DOI] [PubMed] [Google Scholar]
- 32.Tettelin H, Masignani V, Cieslewicz M, Donati C, Medini D, Ward N, Angiuoli S, Crabtree J, Jones A, Durkin A. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial pan-genome. Proc Natl Acad Sci U S A. 2005;102(39):13950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oksana L, David W, Ussery, Trudy M. Wassenaar. Comparative genomics of Bifidobacterium, Lactobacillus and related probiotic genera. Microb Ecol. 2011;63(3):651–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latif A, Shehzad A, Niazi S, Zahid A, Ashraf W, Iqbal MW, Rehman A, Riaz T, Aadil RM, Khan IM, et al. Probiotics: mechanism of action, health benefits and their application in food industries. Front Microbiol. 2023;14:1216674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duche RT, Singh A, Wandhare AG, Sangwan V, Sihag MK, Nwagu TNT, Panwar H, Ezeogu LI. Antibiotic resistance in potential probiotic lactic acid bacteria of fermented foods and human origin from Nigeria. BMC Microbiol. 2023;23(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rana C, Vikas V, Awasthi S, Gautam D, Vats A, Rajput S, Behera M, Ludri A, Berwal A, Singh D. Antimicrobial resistance genes and associated mobile genetic elements in Escherichia coli from human, animal and environment. Chemosphere. 2024;369:143808. [DOI] [PubMed]
- 37.Drew D, North RA, Nagarathinam K, Tanabe M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem Rev. 2021;121(9):5289–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma AK, Dhasmana N, Dubey N, Kumar N, Gangwal A, Gupta M, Singh Y. Bacterial virulence factors: secreted for survival. Indian J Microbiol. 2017;57(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad M, Aduru SV, Smith RP, Zhao Z, Lopatkin AJ. The role of bacterial metabolism in antimicrobial resistance. Nat Rev Microbiol. 2025;23(7):439–54. [DOI] [PMC free article] [PubMed]
- 40.Colautti A, Arnoldi M, Comi G, Iacumin L. Antibiotic resistance and virulence factors in lactobacilli: something to carefully consider. Food Microbiol. 2022;103:103934. [DOI] [PubMed] [Google Scholar]
- 41.Yao P, Mohd Esah E, Zhao C. Regulatory mechanisms and applications of Lactobacillus biofilms in the food industry. Front Microbiol. 2025;15:1465373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, Wang W, Yao J, Wang X, Liu D, Wang P. The HipAB toxin-antitoxin system stabilizes a composite genomic island in Shewanella putrefaciens CN-32. Front Microbiol. 2022;13:858857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasmin I, Saeed M, Khan WA, Khaliq A, Tanweer S. In vitro probiotic potential and safety evaluation (hemolytic, cytotoxic activity) of Bifidobacterium strains isolated from raw camel milk. Microorganisms. 2020;8(3):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Z, Hu Z, Li S, Fang R, Ono HK, Hu DL. Molecular characteristics and pathogenicity of Staphylococcus aureus exotoxins. Int J Mol Sci. 2024;25(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linares DM, Martin MC, Ladero V, Alvarez MA, Fernández M. Biogenic amines in dairy products. Crit Rev Food Sci Nutr. 2011;51(7):691–703. [DOI] [PubMed] [Google Scholar]
- 46.Derunets AS, Selimzyanova AI, Rykov SV, Kuznetsov AE, Berezina OV. Strategies to enhance stress tolerance in lactic acid bacteria across diverse stress conditions. World J Microbiol Biotechnol. 2024;40(4):126. [DOI] [PubMed] [Google Scholar]
- 47.Zharova TV, Grivennikova VG, Borisov VB. F-1·F-0 ATP synthase/atpase: contemporary view on unidirectional catalysis. Int J Mol Sci. 2023;24(6):5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sekiya M, Ikeda K, Yonai A, Ishikawa T, Shimoyama Y, Kodama Y, Sasaki M, Nakanishi-Matsui M. F-type proton-pumping ATPase mediates acid tolerance in Streptococcus mutans. J Appl Microbiol. 2023;134(4): lxad073. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Wang D, Jin Y, Zhou R, Huang J, Wu C. Arginine deiminase pathway of Tetragenococcus halophilus contributes to improve the acid tolerance of lactic acid bacteria. Food Microbiol. 2023;113:104281. [DOI] [PubMed] [Google Scholar]
- 50.Lorena Díez, Ana S, Fernández-Pérez Rocío. Miriam, gonzález, carmen, tenorio. Transcriptome analysis shows activation of the arginine deiminase pathway in Lactococcus lactis as a response to ethanol stress. Int J Food Microbiol. 2017;257:41–8. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Chen X, Sun W, Nie T, Quanquin N, Sun Y. Escherichia coli increases its ATP concentration in weakly acidic environments principally through the glycolytic pathway. Genes. 2020;11(9):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pilhofer M, Rappl K, Eckl C, Bauer AP, Ludwig W, Schleifer KH, Petroni G. Characterization and evolution of cell division and cell wall synthesis genes in the bacterial phyla verrucomicrobia, lentisphaerae, chlamydiae, and planctomycetes and phylogenetic comparison with rRNA genes. J Bacteriol. 2008;190(9):3192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pakotiprapha D, Samuels M, Shen K, Hu JH, Jeruzalmi D. Structure and mechanism of the UvrA-UvrB DNA damage sensor. Nat Struct Mol Biol. 2012;19(3):291–8. [DOI] [PubMed] [Google Scholar]
- 54.Chen C, Yu L, Tian F, Zhao J, Zhai Q. Identification of novel bile salt-tolerant genes in Lactobacillus using comparative genomics and its application in the rapid screening of tolerant strains. Microorganisms. 2022;10(12):2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fontana A, Falasconi I, Molinari P, Treu L, Basile A, Vezzi A, Campanaro S, Morelli L. Genomic comparison of Lactobacillus helveticus strains highlights probiotic potential. Front Microbiol. 2019;10:1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu T, Jiang J, Zhang H, Liu J, Ruan H. Transcending membrane barriers: advances in membrane engineering to enhance the production capacity of microbial cell factories. Microb Cell Fact. 2024;23(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng J, Long J, Hu Q, Liu M, Ge A, Du Y, Zhang T, Jin Y, Yang H, Chen S. Current status of cyclopropane fatty acids on bacterial cell membranes characteristics and physiological functions. Microb Pathog. 2025;200:107295. [DOI] [PubMed] [Google Scholar]
- 58.Muhammad A, Sulman S, Irfan M, Birgitta HN, Kuipers OP. N-acetylglucosamine-mediated expression of NagA and NagB in Streptococcus pneumoniae. Front Cell Infect Microbiol. 2016;6:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Xu M, Hu S, Zhao H, Zhang B. The enzyme gene expression of protein utilization and metabolism by Lactobacillus helveticus CICC 22171. Microorganisms. 2022;10(9):1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.García-Contreras R, de la Mora J, Mora-Montes HM, Martínez-Álvarez JA, Vicente-Gómez M, Padilla-Vaca F, Vargas-Maya NI, Franco B. The inorganic pyrophosphatases of microorganisms: a structural and functional review. PeerJ. 2024;12:e17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamon E, Horvatovich P, Izquierdo E, Bringel F, Marchioni E, Aoudé-Werner D, Ennahar S. Comparative proteomic analysis of Lactobacillus plantarum for the identification of key proteins in bile tolerance. BMC Microbiol. 2011;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8(3):171–84. [DOI] [PubMed]
- 63.Payen S, Giroux MC, Gisch N, Schombel U, Fittipaldi N, Segura M, Gottschalk M. Lipoteichoic acids influence cell shape and bacterial division of Streptococcus suis serotype 2, but play a limited role in the pathogenesis of the infection. Vet Res. 2024;55(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schreiner SA, Sokoli A, Felder KM, Wittenbrink MM, Schwarzenbach S, Guhl B, Hoelzle K, Hoelzle LE. The surface-localised α-enolase of Mycoplasma suis is an adhesion protein. Vet Microbiol. 2012;156(1–2):88–95. [DOI] [PubMed] [Google Scholar]
- 65.Lindahl G, Stalhammar-Carlemalm M, Areschoug T. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev. 2005;18(1):102–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J, Jo J, Wan J, Seo H, Han SW, Shin YJ, Kim DH. In vitro evaluation of probiotic properties and anti-pathogenic effects of Lactobacillus and Bifidobacterium strains as potential probiotics. Foods. 2024;13(14):2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schachtsiek M, Hammes WP, Hertel C. Characterization of Lactobacillus coryniformis DSM 20001T surface protein Cpf mediating coaggregation with and aggregation among pathogens. Appl Environ Microbiol. 2004;70(12):7078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mizuno K, Mizuno M, Yamauchi M, Takemura AJ, Medrano Romero V, Morikawa K. Adjacent-possible ecological niche: growth of Lactobacillus species co-cultured with Escherichia coli in a synthetic minimal medium. Sci Rep. 2017;7(1):12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vinayamohan P, Joseph D, Viju LS, Baskaran SA, Venkitanarayanan K. Efficacy of probiotics in reducing pathogenic potential of infectious agents. Fermentation. 2024;10(12):599. [Google Scholar]
- 70.Rusu ME, Fizean I, Vlase L, Popa DS. Antioxidants in age-related diseases and anti-aging strategies. Antioxidants. 2022;11(10):1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mafe AN, Iruogheneedo G, Akpoghelie PO, Gaaz TS, Yousif E, Zainulabdeen K, Isoje EF, Igbuku UA, Opiti RA, Garba Y. Probiotics and food bioactives: unraveling their impact on gut microbiome, inflammation, and metabolic health. Probiotics Antimicrob Proteins. 2025:1–42. [DOI] [PubMed]
- 72.Tanaka R, Kosugi K, Mizukami M, Ishibashi M, Tokunaga H, Tokunaga M. Expression and purification of thioredoxin (TrxA) and thioredoxin reductase (TrxB) from Brevibacillus choshinensis. Protein Expr Purif. 2004;37(2):385–91. [DOI] [PubMed] [Google Scholar]
- 73.Lee JH, Yeo WS, Roe JH. Induction of the SufA operon encoding Fe-S assembly proteins by superoxide generators and hydrogen peroxide: involvement of oxyr, IHF and an unidentified oxidant‐responsive factor. Mol Microbiol. 2004;51(6):1745–55. [DOI] [PubMed] [Google Scholar]
- 74.Riboldi GP, Bierhals CG, Mattos EPD, Frazzon APG, D’Azevedo PA, Frazzon J. Oxidative stress enhances the expression of sulfur assimilation genes: preliminary insights on the Enterococcus faecalis iron-sulfur cluster machinery regulation. Mem Inst Oswaldo Cruz. 2018;109(4):408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh V, Singh K, Baum K. The role of methionine sulfoxide reductases in oxidative stress tolerance and virulence of Staphylococcus aureus and other bacteria. Antioxidants. 2018;7(10):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plavec TV, Žagar Soderžnik K, Della Pelle G, Zupančič Š, Vidmar R, Berlec A. Incorporation of recombinant proteins into extracellular vesicles by Lactococcus cremoris. Sci Rep. 2025;15(1):1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szajewska H, Scott KP, Meij TD, Forslund-Startceva SK, Knight R, Koren O, Little P, Johnston BC, Ukasik J, Suez J. Antibiotic-perturbed microbiota and the role of probiotics. Nat Rev Gastroenterol Hepatol. 2025;22(3):155–72. [DOI] [PubMed] [Google Scholar]
- 78.Angelescu IR, Grosu-Tudor S, Cojoc L, Maria G, Zamfir M. Isolation, characterization, and applicability of helveticin 34.9, a class III bacteriocin produced by Lactobacillus helveticus 34.9. World J Microbiol Biotechnol. 2021;38(12):220. [DOI] [PubMed] [Google Scholar]
- 79.Meng F, Zhu X, Zhao H, Nie T, Lu F, Lu Z, Lu Y. A class III bacteriocin with broad-spectrum antibacterial activity from Lactobacillus acidophilus NX2-6 and its preservation in milk and cheese. Food Control. 2021;121:107597. [Google Scholar]
- 80.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3(10):777–88. [DOI] [PubMed] [Google Scholar]
- 81.Nilsen T, Nes Ingolf F, Holo Helge. Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl Environ Microbiol. 2003;69(5):2975–84. [DOI] [PMC free article] [PubMed]
- 82.Cantarel BL, Coutinho PM, Corinne R, Thomas B, Vincent L, Bernard H. The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 2009;37:D233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vincent L, Hemalatha GR, Elodie D, Coutinho PM, Bernard H. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levasseur A, Drula E, Lombard V. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol Biofuels. 2013;6(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen HT, Pham TT, Nguyen PT, Le-Buanec H, Rabetafika HN, Razafindralambo HL. Advances in microbial exopolysaccharides: present and future applications. Biomolecules. 2024;14(9):1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen PT, Nguyen HT. Antioxidant property and structural characteristics of exopolysaccharides derived from Lactiplantibacillus plantarum VAL6. Discover Bacteria. 2024;1(1):1–10. [Google Scholar]
- 87.Yang Z, Li S, Zhang X, Zeng X, Li D, Zhao Y, Zhang J. Capsular and slime-polysaccharide production by Lactobacillus rhamnosus JAAS8 isolated from Chinese sauerkraut: potential application in fermented milk products. J Biosci Bioeng. 2010;110(1):53–7. [DOI] [PubMed] [Google Scholar]
- 88.Behera HT, Mojumdar A, Mohapatra C, Ray L. Microbial polysaccharides with potential industrial applications: diversity, synthesis, and their applications. Microb Polym. 2021;521:546. [Google Scholar]
- 89.Sørensen HM, Rochfort KD, Maye S, MacLeod G, Brabazon D, Loscher C, Freeland B. Exopolysaccharides of lactic acid bacteria: production, purification and health benefits towards functional food. Nutrients. 2022;14(14):2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang M, Zhao D, Yang H, Jiao X, Zhou R, Zheng J, Wu C. Lactic acid bacteria-derived exopolysaccharide: biosynthesis and antibacterial characterization. Trends Food Sci Technol. 2025;160:105033. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The KM7 genome data were deposited at the NCBI database, with the BioProject accession number: PRJNA1271779.