Abstract
Background
The treatment of Cutaneous Squamous Cell Carcinoma (CSCC) has undergone significant changes with the introduction of Immune Checkpoint Inhibitors (ICIs). While promising results have been observed, their efficacy remains limited to a subset of patients. Soluble immune checkpoint molecules, Interferon-gamma (IFN-γ), and cell-free DNA (cfDNA) could serve as potential biomarkers, particularly in ICI-based therapies.
Methods
In this study, we assessed the prognostic role of serum PD-L1, LAG-3, TIM-3, CTLA-4, IFN-γ, and plasma cfDNA in a cohort of 41 advanced (locally-advanced or metastatic) CSCC patients treated with the anti-PD-1 inhibitor cemiplimab.
Results
Our findings indicate that elevated baseline serum levels of IFN-γ are significantly associated with poorer treatment response and shorter Progression-Free Survival (PFS) in patients receiving cemiplimab. Moreover, patients with worse outcomes in terms of response and PFS exhibited a post-treatment decrease in IFN-γ levels. Higher baseline levels of plasma cfDNA and serum CTLA-4 demonstrated a trend toward association with unfavourable clinical outcomes.
Conclusions
These results highlight the potential of baseline and post-treatment IFN-γ levels as significant prognostic indicators for advanced CSCC patients undergoing cemiplimab therapy.
Keywords: cfDNA, PD-L1, LAG-3, TIM-3, CTLA-4, IFN-γ, CSCC, Cemiplimab, Soluble biomarkers
Background
Cutaneous Squamous Cell Carcinoma (CSCC) is a form of keratinocyte carcinoma and represents the second most common skin malignancy diagnosed worldwide, accounting for about 20–25% of all diagnoses of skin tumors, after basal cell carcinoma [1]. The incidence of CSCC is estimated to be around 2.4 million new cases per year worldwide, and it is growing exponentially due to the aging of the general population and the increase of immunosuppressed patients, with a rate of 50–200% during the last 3 decades in the United States [2]. In Italy, the incidence of this neoplasm is estimated to be about 19,000 new cases per year [3], with a high heterogeneity throughout the Italian territory. Although the majority of cases are identified when the lesions are still resectable, approximately 5% of CSCC cases are diagnosed at a locally advanced or metastatic stage. Once the disease is no longer amenable to curative surgery or radiotherapy, patients require systemic medical therapy. Until recently, no standard systemic therapy was able to offer a meaningful survival benefit. Patients with advanced CSCC treated with chemotherapy or targeted therapies, such as EGFR inhibitors, have demonstrated poor response rates and limited Overall Survival (OS) following disease progression, along with significant toxicity [4, 5]. This is especially concerning in frail patients with multiple comorbidities, including immunosuppression. However, the introduction of immunotherapy has markedly improved treatment outcomes for patients with advanced CSCC who lack targetable oncogenic drivers. The use of PD-1 inhibitors in CSCC is supported by a strong biological rationale, including the exceptionally high Tumor Mutational Burden (TMB) driven by chronic UV exposure, the resulting abundance of neoantigens that may enhance immune recognition, clinical evidence of efficacy independent of intra-tumoral PD-L1 expression, with no clear correlation between PD-L1 expression and objective response rates. Additionally, approximately half of CSCCs exhibit low or absent PD-L1 expression [6]. Together, these features define CSCC as an immunogenic tumor and provide a solid foundation for immune checkpoint blockade [7–9]. In 2018, the FDA approved cemiplimab, a PD-1 inhibitor, for the treatment of advanced/metastatic CSCC based on a combined analysis of data from an open-label, multicenter, non-randomized Phase 2 study (EMPOWER-CSCC-1, Study 1540; NCT02760498) and two expansion cohorts from a Phase I, multicenter, open-label, non-randomized study (Study 1423; NCT02383212) [10]. Cemiplimab has shown efficacy independent of intra-tumoral PD-L1 expression, with no clear correlation between PD-L1 expression and objective response rates [11]. Cemiplimab has also demonstrated rapid, deep, and durable tumor responses in a substantial proportion of patients with advanced CSCC, while maintaining a favourable safety profile. Despite these clinical benefits, approximately 50% of patients do not achieve an objective response [11–15]. Although the therapeutic indication for cemiplimab is clearly established in patients not eligible for curative surgery or radiotherapy, the ability to predict individual treatment response remains limited. To date, no validated biomarkers are available to guide treatment selection or to anticipate primary resistance. As a result, the identification of reliable prognostic and predictive factors, particularly in frail or comorbid populations, represents a pressing unmet clinical need [16–18]. This study aims to explore the prognostic significance of soluble serum biomarkers sPD-L1 (also known as B7-H1), sLAG-3, sTIM-3, sCTLA-4, and cell-free DNA (cfDNA) in CSCC. Indeed, beyond PD-L1, other immune checkpoints, including LAG-3, TIM-3, CTLA-4, B7-H3, and B7-H4, also play critical roles in regulating the immune response to cancer [19, 20]. Although their roles remain controversial, these molecules have been reported to have prognostic value in solid tumors. They can also be found in their soluble forms in the blood of patients with CSCC, and their functions are currently under investigation. To date, no data are available on these soluble markers or cfDNA levels in CSCC patients treated with cemiplimab. Additionally, Interferon-gamma (IFN-γ) plays a critical role in the immune system by promoting activation, maturation, proliferation, cytokine expression, and effector functions in immune cells, while also inducing antigen presentation, growth arrest, and apoptosis in tumor cells [21, 22]. In light of these unmet needs, we also explored the potential prognostic value of serum IFN-γ (sIFN-γ) levels in patients with advanced CSCC undergoing treatment with cemiplimab.
Methods
Patient selection, enrolment, treatment, and biospecimen collection
Cemiplimab was administered to patients considered unsuitable for curative surgery or radiotherapy, as determined by a multidisciplinary team discussion. Cemiplimab was administered intravenously (IV) at a dose of 350 mg flat dose every 3 weeks. The clinical response was assessed radiologically according to the RECIST 1.1 criteria [23] or clinically according to the WHO criteria [24]. Clinical lesions were considered measurable if their superficial extension on the skin (as nodules and/or palpable lymph nodes) was at least 10 mm in diameter. Patients were clinically examined at each cycle of cemiplimab. Patients underwent individualized reassessment according to the site of the tumor, its superficial extension on the skin, or deep extension in the surrounding tissues, according to the feasibility of undergoing an iodinated contrast scan or according to the compliance of each patient. Based on these characteristics, patients were monitored via Computed Tomography (CT) scan, Magnetic Resonance Imaging (MRI), or clinical photography. Importantly, radiological or clinical assessment of response was performed approximately every 12 ± 3 weeks (according to standard of care logistics) during treatment to maintain a consistent evaluation interval. Patients underwent follow-up clinical visits and radiological assessments every four to six months, in accordance with clinical practice and tailored to individual characteristics and compliance, even after discontinuation of cemiplimab. A total of 41 consecutive patients with advanced (locally-advanced or metastatic) CSCC who received cemiplimab between August 2019 and January 2025 were enrolled in this study. Written informed consent was obtained from each participant in accordance with the protocol approved by the Local Ethics Committee (CER No. 622/2022). For each patient, peripheral blood samples were collected at baseline (prior to cemiplimab initiation) and every three weeks thereafter.
Biomarkers evaluation
Baseline serum levels of sPD-L1, sLAG-3, sTIM-3, and sCTLA-4, were measured in 38 out of 41 patients using a custom multiplex cartridge on the Ella automated microfluidic platform (Bio-Techne, Minneapolis, MN, USA). sIFN- γ levels were also assessed at baseline in the same 38 patients using the Simple Plex Human IFN-gamma (3rd Gen) cartridge, also on the Ella platform, with 50 µL of the diluted serum samples (1:2 dilution with the provided diluent). sPD-L1, sLAG-3, sTIM-3, sCTLA-4, and sIFN-γ concentrations (pg/mL) were calculated using the manufacturer-calibrated standard curve and analyzed with Ella software. In addition to baseline values for each patient, a total of 119, 118, and 120 measurements after the first cycle of cemiplimab treatment were obtained for sPD-L1/sLAG-3/sCTLA-4, sTIM-3 and sIFN- γ, respectively. Specifically, on average, each patient underwent 3.2 measurements (range: 0–6) of soluble checkpoint molecules and sIFN-γ following treatment initiation. In 40 out of 41 patients, cfDNA was isolated at baseline from 5 mL of plasma using MagMAX™ Cell-Free DNA Isolation Kit (ThermoFisher Scientific, Waltham, MA) following the manufacturer’s instructions, and quantified with the Qubit® dsDNA HS Assay Kit on the Qubit 2.0 fluorometer (ThermoFisher Scientific). Additionally, cfDNA was also evaluated after the first treatment cycle (T1) in 33 out of 40 patients (Fig. 1).
Fig. 1.
Study design: Prognostic evaluation of soluble serum immune checkpoint molecules (sCTLA-4, sTIM-3, sLAG-3, sPD-L1/B7-H1) and IFN-γ in patients with locally advanced or advanced Cutaneous Squamous Cell Carcinoma (CSCC) treated with cemiplimab (anti-PD-1). A total of 41 consecutive patients were enrolled; serum levels of sPD-L1, sLAG-3, sTIM-3, sCTLA-4, and sIFN-γ were longitudinally assessed every 21 days in 38 patients. Plasma cell-free DNA (cfDNA) levels were analyzed in 40 patients at baseline, and in 33 patients also following the first cycle of therapy. Thirty-seven patients overlapped between the immune checkpoints/IFN-γ and cfDNA analyses (indicated as bordeaux-colored individuals)
Statistical analysis
Categorical baseline patient characteristics were summarized using absolute and relative frequencies. Age at the start of therapy and biomarker level distributions were described by mean, standard deviation, median, interquartile range (IQR: 25th–75th percentile), and minimum and maximum values. Non-Responders (NRs) were defined as patients whose best response was Progressive Disease (PD), or Stable Disease (SD) with progression or death occurring within 6 months from treatment initiation (C1D1; Cycle 1, Day 1). Responders (Rs) included patients with Complete Response (CR), Partial Response (PR), or SD lasting at least 6 months.
The prognostic role of the soluble biomarkers (sPD-L1, sCTLA-4, sLAG-3, sTIM-3, sIFN-γ) and cfDNA on Progression-Free Survival (PFS) and Best Overall Response (BOR) was respectively estimated through the Cox and the modified Poisson regressions using Hazard Ratio (HR) and incidence-rate ratio as measures of Relative Risk (RR) [25]. Each biomarker was evaluated for unit increases or specific concentration increments as follows: sPD-L1 and sCTLA-4 for a 10 pg/mL increase; sLAG-3 for a 200 pg/mL increase; sTIM-3 for a 500 pg/mL increase; IFN-γ for a 1 pg/mL increase; and cfDNA for a 10 ng/mL increase. These increments were chosen based on the range and distribution of values observed in the study cohort, in order to yield interpretable effect sizes in regression analyses and to better capture the variation in risk associated with changes in each biomarker level. In addition, the Geometric Mean Ratio (GMR), derived from a log-normal mixed-effects regression analysis, was used to evaluate the linear trend of each biomarker over the follow-up period.
In all cases, regression estimates were adjusted for potential imbalances in baseline demographic and clinical characteristics (age, gender, stage, ECOG PS, and LDH levels) and reported with corresponding 95% Confidence Intervals (95% CI). Statistical significance (p ≤ 0.05) was assessed using the likelihood ratio test. All analyses were performed using Stata software (StataCorp. Stata: Release 17. Statistical Software. College Station, TX, 2021).
Results
Clinicopathologic features of the patients’ cohort
Patients’ characteristics are summarized in Table 1.
Table 1.
Summary of the most relevant clinicopathological characteristics of the patient cohort
| Characteristics | N (%) |
|---|---|
| Gender | |
| Male | 26 (63.4) |
| Female | 15 (36.6) |
| Immunosuppression | |
| No | 31 (75.6) |
| Yes | 10 (24.4) |
| Previous treatments | |
| Surgery | 15 (36.6) |
| RT | 1 (2.4) |
| ECT | 1 (2.4) |
| Surgery + RT | 3 (7.3) |
| Surgery + CT | 1 (2.4) |
| CT and RT | 1 (2.4) |
| None | 19 (46.3) |
| ECOG PS | |
| 0 | 13 (31.7) |
| 1 | 23 (56.1) |
| 2–3 | 5 (12.2) |
| Primary tumor anatomical location | |
| Neck-Head | 34 (82.9) |
| Other | 7 (17.1) |
| Stage | |
| Locally advanced | 27 (65.9) |
| Metastatic | 14 (34.1) |
| LDH basal | |
| < UNL | 28 (68.3) |
| >UNL | 12 (29.3) |
| missing | 1 (2.4) |
| BOR | |
| Non-responders | 8 (19.5) |
| Responders | 30 (73.2) |
| Not evaluable | 3 (7.3) |
| Total sample | 41 (100.0) |
BOR best overall response; CT chemotherapy; ECOG eastern cooperative oncology group performance status (ECOG PS); ECT electrochemotherapy; LDH lactate dehydrogenase; NRs non-responders; Rs responders; RT radiotherapy; UNL upper normal limit. Non-Responders (NRs) were defined as patients whose best response was Progressive Disease (PD), or Stable Disease (SD) with progression or death occurring within 6 months from treatment initiation (C1D1). Responders (Rs) included patients with Complete Response (CR), Partial Response (PR), or SD lasting at least 6 months
The median age at the start of cemiplimab was 81.1 years. The median follow-up was 19.7 months (95% CI 14.07–25.80). Median PFS was not reached in the overall cohort; the estimated PFS at 24 months was 0.70 (95% CI 0.51–0.83). Similarly, in responders, median PFS was not reached, with a 24-month PFS of 0.87 (95% CI 0.66–0.96). In contrast, non-responders had a median PFS of 2.67 months (95% CI 1.00–3.80). Most patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1, while 5 patients (12.2%) had a performance status of ≥ 2. Based on geriatric assessment, the cohort was generally in good physical condition, with a median modified Katz Activities of Daily Living (ADL) score of 5 out of a maximum of 6 [26].
A history of immunosuppression was documented in 24.4% of patients (n = 10), due to hematological malignancies (n = 8), including chronic lymphocytic leukemia (n = 2), multiple myeloma (n = 3; one post-autologous stem cell transplant), mantle cell lymphoma (n = 1), follicular lymphoma (n = 1), myelofibrosis (n = 1), and myelodysplasia (n = 1); or to ongoing immunosuppressive therapy, namely methotrexate plus corticosteroids for psoriasis (n = 1) or capecitabine chemotherapy (n = 1).
At diagnosis, 65.9% of patients (n = 27) had locally advanced CSCC confined to the primary tumor site, while 34.1% (n = 14) presented with metastatic disease, 12 with nodal metastases only and 2 with visceral involvement. The most frequently involved anatomical region was the head and neck (83.2%, n = 34), followed by the trunk (n = 5) and lower limbs (n = 2).
Regarding prior treatments, 36.6% of patients (n = 15) had undergone surgery, 2.4% (n = 1) had received Radiotherapy (RT), 2.4% (n = 1) had undergone Electrochemotherapy (ECT), 7.3% (n = 3) had received combined surgery and RT, 2.4% (n = 1) had undergone surgery with Chemotherapy (CT), and 2.4% (n = 1) had received combined CT and RT. Notably, 46.3% of patients (n = 19) had not received any prior treatment before initiating cemiplimab.
Among evaluable patients, 73.2% (n = 30) had a CR or PR as BOR, while 19.5% (n = 8) were classified as non-responders. Three patients (7.3%) were not evaluable for response.
Soluble prognostic biomarkers at baseline
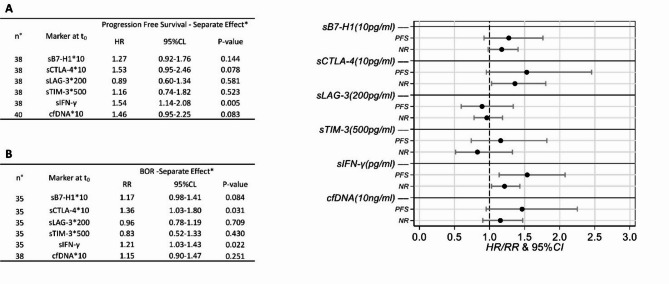
cfDNA was extracted from baseline plasma samples of 40 CSCC patients, yielding an average concentration of 7.5 ng/mL (range: 0.2–74.6 ng/mL). NRs showed a higher average cfDNA concentration at baseline compared to Rs (12.2 vs. 6.6 ng/mL). Baseline levels of cfDNA, sIFN-γ, and four soluble immune checkpoint molecules (sPD-L1, sCTLA-4, sTIM-3, and sLAG-3) in blood circulation were correlated with BOR and PFS achieved during cemiplimab treatment (Fig. 2).
Fig. 2.
Caterpillar plot of Hazard Ratios (HR)/Relative Risk (RR) for Progression-Free Survival (PFS) (A) and Best Overall Response (BOR) (B), adjusted for sex, age, stage, LDH, and ECOG performance status (*). Non-Responders were defined as patients whose best response was Progressive Disease (PD), or Stable Disease (SD) with progression or death occurring within 6 months from treatment initiation (C1D1). Responders included patients with Complete Response (CR), Partial Response (PR), or SD lasting at least 6 months. Baseline serum was available for 38 patients for the assessment of sPDL1 (also known as B7-H1), sLAG-3, sTIM-3, sCTLA-4, and sIFN-γ levels, whereas baseline plasma was available for 40 patients for cfDNA analysis. BOR data (B) were missing for 3 patients regarding sPD-L1, sLAG-3, sTIM-3, sCTLA-4, and sIFN-γ, and for 2 patients concerning cfDNA, as 1 patient lacking a response was the one without baseline plasma, and was therefore excluded from the analysis. NR non-response
Concordant positive associations between baseline biomarker levels and adverse prognostic events (PFS and no-response) were pointed out for sPD-L1 (HR = 1.27, 95% CI 0.92–1.76; RR = 1.17, 95% CI 0.98), sCTLA-4 (HR = 1.53, 95% CI 0.95–2.46; RR = 1.36, 95% CI 1.03–1.80), sIFN-γ (HR = 1.54, 95% CI 1.14–2.08; RR = 1.21, 95% CI 1.03–1.43) and cfDNA (RR = 1.15, 95% CI 0.90–1.47; HR = 1.46, 95% CI 0.95–2.25), whereas sLAG-3 showed negative associations (HR = 0.89, 95% CI 0.60–1.34; RR = 0.96, 95% CI 0.78–1.19) and sTIM-3 discordant behaviours (HR = 1.16, 95% CI 0.74–1.82; RR = 0.83, 95% CI 0.52–1.33) (Fig. 2).
Dynamic changes in soluble biomarkers during Anti-PD-1 therapy
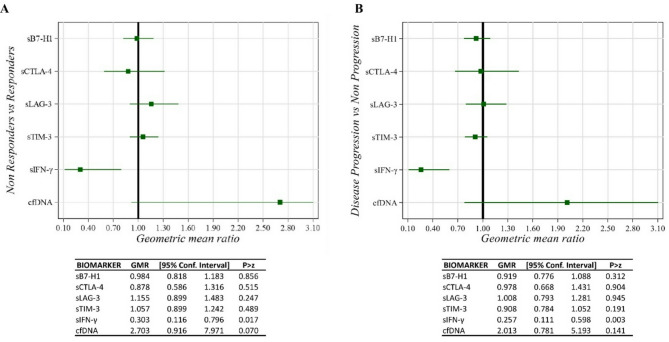
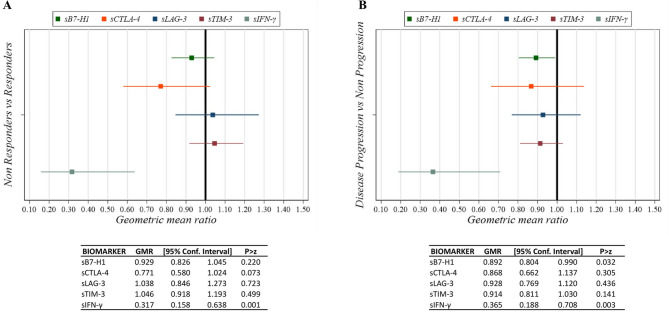
We evaluated whether changes in soluble biomarkers during treatment were associated with BOR and PFS. Serum samples were collected at baseline and before each treatment cycle for longitudinal analysis. In NRs and progressor patients, the sIFN-γ GMRs between the first cycle and baseline (T1-T0) and in trend of the first three cycles compared to baseline were significantly lower than in Rs and non-progressors (Figs. 3 and 4). Specifically, after the first treatment cycle, sIFN-γ levels decreased by 70% in NRs compared to Rs (GMR = 0.30, 95% CI 0.12–0.80, p = 0.017) and by 74% in progressors compared to non-progressors (GMR = 0.26, 95% CI 0.11–0.60, p = 0.003) (Fig. 3). Moreover, after the first treatment cycle, cfDNA levels increased in NR compared to R (GMR = 2.70, 95% CI 0.92–7.97) and in progressors compared to non-progressors (GMR = 2.01, 95% CI 0.78–5.19), although these differences were not statistically significant (Fig. 3). After the first three cycles, sIFN-γ levels decreased by 68% in NR (GMR = 0.32, 95% CI 0.16–0.64, p = 0.001) and by 64% in progressors (GMR = 0.36, 95% CI 0.19–0.71, p = 0.003) (Fig. 4).
Fig. 3.
Caterpillar plot of Geometric Mean Ratios (GMR) between the first cycle (T1) and baseline in Best Overall Response (BOR) (A, left) and Progression Free Survival (PFS) (B, right) for sPD-L1 (also known as B7-H1), sLAG-3, sTIM-3, sCTLA-4, and sIFN-γ levels, adjusted for sex, age, stage, LDH, and ECOG PS. Four patients did not have plasma/serum available for the T1 evaluation
Fig. 4.
Caterpillar plot of Geometric Mean Ratios (GMR) of trend for the first three cycles relative to baseline in Best Overall Response (BOR) (A, left) and Progression Free Survival (PFS) (B, right) for sPD-L1 (also known as B7-H1), sLAG-3, sTIM-3, sCTLA-4, and sIFN-γ levels, adjusted for sex, age, stage, LDH, and ECOG PS. Four patients did not have plasma/serum available for the trend evaluation
Discussion
To the best of our knowledge, this study represents the first comprehensive evaluation of the prognostic value of soluble immune checkpoint molecules (sPD-L1, sCTLA-4, sLAG-3, and sTIM-3), the pro-inflammatory cytokine IFN-γ, and cfDNA levels as blood-based immunological and tumor biomarkers. These biomarkers were investigated for their potential association with treatment response and clinical outcomes in patients with locally advanced or advanced CSCC undergoing therapy with the anti-PD-1 agent cemiplimab. Recent studies have linked increased serum IL6 levels after cemiplimab treatment in CSCC patients to poorer clinical responses. Non-responders showed elevated IL6 after just one treatment cycle, while responders’ levels remained stable [17]. Similarly, protumor cytokines IL9, IL27, and IL33 increased in non-responders but stayed unchanged in responders, suggesting their potential as biomarkers of poor prognosis. Additionally, responders exhibited slight decreases in IL13, IL11, and TNF-α levels, whereas these increased in non-responders, consistent with their roles in immune evasion and tumor progression [16]. IFN-γ is a pro-inflammatory cytokine that plays a pivotal role in antitumor immunity, being primarily secreted by activated T lymphocytes and natural killer cells. Its functions include enhancing antigen presentation, upregulating MHC expression, and activating macrophages [27].
Among all the soluble biomarkers analyzed in our study, we identified a positive association between the sIFN-γ marker and unfavourable outcomes. Specifically, CSCC patients have a 21% higher risk of non-response and a 54% higher risk of progression per unit increment of sIFN-γ at baseline. Furthermore, a significant decrease in sIFN-γ levels during therapy was also observed in NR and patients with progressive disease. This decrease likely reflects a complex interplay of immune activation, tumor-induced immune escape mechanisms, chronic inflammation, and immune dysregulation. While the immune system is attempting to fight the tumor, it may not be sufficient or may be thwarted by the immune evasion tactics of the tumor or a suppressive tumor microenvironment. These findings highlight the potential of sIFN-γ as a dynamic marker of treatment resistance. Although traditionally regarded as a marker of beneficial immune activation [22], recent evidence reveals a more nuanced role [21, 28]. Although our study focuses on sIFN-γ, previous studies have shown that chronic IFN-γ signaling within the tumor microenvironment may contribute to immune escape mechanisms. For instance, sustained IFN-γ exposure in tumor or immune cells has been reported to induce PD-L1 expression, T cell exhaustion, and the upregulation of immunosuppressive mediators such as IDO1, TGF-β, and galectin-9. Moreover, prolonged IFN-γ signaling may facilitate the selection of immune-resistant tumor clones, particularly those harboring mutations in the JAK/STAT pathway, contributing to a tumor microenvironment marked by chronic, non-productive inflammation that undermines the efficacy of immune checkpoint blockade. Such mechanisms, although not directly measured here, may underlie the associations observed with elevated sIFN-γ levels [29, 30].
In line with these observations, in melanoma patients with high baseline sIFN-γ levels have shown reduced responses to Immune Checkpoint Inhibitors (ICIs), possibly due to pre-existing dysfunctional T cell states [31]. Moreover, high sIFN-γ levels have also been associated with shorter OS in advanced NSCLC patients treated with anti-PD1, particularly among those whose tumors exhibit high PD-L1 expression [32]. In hepatocellular carcinoma, elevated sIFN-γ levels have been associated with accelerated disease progression and poor therapeutic outcomes, even with combination immunotherapies [33]. These observations suggest that persistently elevated sIFN-γ may reflect an overstimulated but ineffective immune response, correlating with poor survival despite ICI treatment.
In our patient cohort, sIFN-γ levels increased during therapy in responders, suggesting its potential as a reliable dynamic biomarker for predicting treatment response, particularly when assessed longitudinally. Effective PD-1 blockade may reinvigorate effector T cells, resulting in enhanced IFN-γ production within the tumor or peripheral immune system, which may be reflected by increased circulating sIFN-γ as part of an anti-tumor immune response. However, it could be integrated into multiparametric immune profiling, alongside other cytokines (e.g., IL-6, CXCL9/10), TMB, or PD-L1 expression. Overall, although IFN-γ has traditionally been considered a marker of anti-tumor immunity, our data suggests that it may represent a double-edged sword in oncology. Elevated levels may reflect a dysfunctional, exhausted immune landscape and are associated with poor prognosis and resistance to immunotherapy. Future studies should focus on integrating IFN-γ into comprehensive immune-monitoring panels to enable better patient selection and optimize treatment strategies in precision oncology.
In this study we did not observe significant baseline associations between sPDL1, sLAG-3, sTIM-3, or cfDNA and patient prognosis. However, our patient’s cohort showed a 36% higher risk of non-response and 53% higher risk of progression per 10 ng/mL increase in sCTLA-4 at baseline, although the latter was not statistically significant. High sCTLA-4 levels may neutralize CD80/CD86, thereby suppressing T-cell activation and contributing to immune evasion, even in the presence of PD-1 inhibition. This hypothesis should be further explored through immunophenotyping of tumor tissue or peripheral blood to assess Treg and CTLA-4 expression levels, which could support the rationale for using combination immunotherapy (anti-PD-1 + anti-CTLA-4).
Elevated cfDNA levels at baseline are often observed in tumors characterized by low T-cell infiltration and immune-suppressive microenvironments, both of which are associated with resistance to PD-1 inhibitors. In our cohort, patients with CSCC treated with cemiplimab exhibited a 46% increased risk of progression per 10 ng/mL increment in baseline cfDNA levels, although this association was not statistically significant. We acknowledge that cfDNA is not a specific marker of non-response, but of overall tumor burden, tumor shedding, and possibly more aggressive or immune-excluded tumor phenotypes. cfDNA analysis holds significant yet largely untapped potential in CSCC. Indeed, studies investigating cfDNA levels in CSCC patients treated with immunotherapy are currently lacking, whereas research on ctDNA mutational profiling is emerging [34].
Despite the potential advances provided by this study in identifying novel non-invasive prognostic markers and elucidating the role of sIFN-γ in the immune response, the results should be interpreted in light of several limitations. First, these findings should be interpreted in the context of the tumor immunophenotype, an aspect that requires further investigation. Additionally, although our cohort is relatively large given the rarity of this tumor type, the single-center design and absence of an independent validation cohort limit the assessment of the prognostic value of each marker, underscoring the need for validation in larger, multicenter studies.
One limitation of this study is the inclusion of immunosuppressed patients (comprising about one-fourth of the cohort). Their altered immune responses may affect biomarker performance, but the small number of cases and events has limited subgroup analyses. Nonetheless, as CSCC often affects elderly and immunocompromised individuals, our cohort reflects a real-world population, thereby underscoring the public health relevance of this population for future research and intervention strategies.
Another key consideration is that most patients with CSCC are diagnosed at an advanced age, which limits the ability to correlate these biomarkers with overall survival.
Conclusions
In conclusion, this exploratory study identifies IFN-γ as a promising prognostic and dynamic biomarker in patients with advanced CSCC treated with cemiplimab. Our findings demonstrate that elevated baseline levels of sIFN-γ are associated with an increased risk of non-response and disease progression. Additionally, a decrease in sIFN-γ levels during treatment was observed in NRs and progressors. These results underscore the potential value of integrating sIFN-γ into immune-monitoring strategies for guiding patient stratification and optimizing immunotherapy in CSCC. Other markers, such as sCTLA-4 and cfDNA, showed trends suggesting their potential associations with disease progression and treatment response, emphasizing the need for further investigation in larger, multicenter cohorts and in conjunction with immunogenomic analyses. In particular, while cfDNA is not a specific biomarker of non-response, elevated baseline levels might suggest a higher tumor burden, a more aggressive disease course, or an immunologically ‘cold’ tumor microenvironment—features that could be associated with reduced sensitivity to PD-1 blockade.
The lack of prognostic value for other soluble immune checkpoint molecules highlights the complexity of immune regulation in CSCC. Importantly, from a clinical perspective, the assessment of sIFN-γ and other soluble immune markers is feasible using currently available automated platforms, such as the Ella™ system. These technologies offer standardized workflows, require minimal sample volumes, and provide high reproducibility, making them suitable not only for translational research but also for potential clinical implementation. Overall, our study highlights the promise of non-invasive liquid biomarkers as valuable tools for advancing precision oncology in this clinically challenging patient population.
Acknowledgements
Not applicable.
Abbreviations
- ADL
Activities of daily living
- BOR
Best overall response
- cfDNA
cell-free DNA
- CL
Confidence level
- CR
Complete response
- CSCC
Cutaneous squamous cell carcinoma
- CT
Chemotherapy
- ECOG PS
Eastern cooperative oncology group performance status
- ECT
Electrochemotherapy
- GMR
Geometric mean ratio
- HR
Hazard ratio
- ICIs
Immune checkpoint inhibitors
- IDO1
Indoleamine 2,3-dioxygenase
- IQR
Interquartile range
- LDH
Lactate dehydrogenase
- MRI
Magnetic resonance imaging
- NRs
Non-responders
- ORR
Overall response rate
- OS
Overall survival
- PD
Progressive disease
- PFS
Progression-free survival
- PR
Partial response
- Rs
Responders
- RR
Relative risk
- RT
Radiotherapy
- sB7-H3
Serum B7 homolog 3
- sB7-H4
Serum B7 homolog 4
- SD
Stable Disease
- sIFN-γ
Serum interferon-gamma
- sLAG-3
Serum lymphocyte activation gene 3 protein
- sTIM-3
Serum T-cell immunoglobulin mucin domain 3
- TGF-β
Transforming growth factor beta
- TMB
Tumor mutational burden
Author contributions
I.V., E.T.T., F.F., and P.G. study design, data analysis and interpretation, draft and revision of the manuscript. I.V., M.C., S.C. and S.S. acquisition and data analysis, final revision of the manuscript. E.T.T., A.G.B., A.B., F.F. patient data collection and data interpretation and final revision of the manuscript. E.A. and F.B. serum/plasma collection and final revision of the manuscript. V.F. and A.R. statistical analyses and final revision of the manuscript.
Funding
This work was supported by the Italian Ministry of Health (Ricerca Corrente 2023 to E.T.T. and Ricerca Corrente 2025 to P.G.).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by Institutional Review Boards (IRB)-approved protocol 622/2022.
Consent for publication
Not applicable.
Competing interests
The authors declare no financial or non-financial competing interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stanganelli I, Spagnolo F, Argenziano G, Ascierto PA, Bassetto F, Bossi P, et al. The multidisciplinary management of cutaneous squamous cell carcinoma: a comprehensive review and clinical recommendations by a panel of experts. Cancers. 2022;14:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wysong A. Squamous-cell carcinoma of the skin. Longo DL, editor. N Engl J Med. 2023;388:2262–73. [DOI] [PubMed]
- 3.I numeri del cancro in Italia, AIOM. 2018. (2019) [Internet]. [cited 2019 June 8]. Available from: https://www.aiom.it/i-numeri-del-cancro-in-italia/
- 4.Gellrich FF, Hüning S, Beissert S, Eigentler T, Stockfleth E, Gutzmer R, et al. Medical treatment of advanced cutaneous squamous-cell carcinoma. J Eur Acad Dermatol Venereol. 2019;33:38–43. [DOI] [PubMed] [Google Scholar]
- 5.Pham JP, Rodrigues A, Goldinger SM, Sim H, Liu J. Epidermal growth factor receptor inhibitors in advanced cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Exp Dermatol. 2024;33:e14978. [DOI] [PubMed] [Google Scholar]
- 6.Lehmer L, Choi F, Kraus C, Shiu J, de Feraudy S, Elsensohn A. Histopathologic PD-L1 tumor expression and prognostic significance in nonmelanoma skin cancers: a systematic review. Am J Dermatopathol. 2021;43:321–30. [DOI] [PubMed] [Google Scholar]
- 7.Amoils M, Kim J, Lee C, Sunwoo JB, Colevas AD, Aasi SZ, et al. PD-L1 expression and Tumor‐Infiltrating lymphocytes in high‐risk and metastatic cutaneous squamous cell carcinoma. Otolaryngol Neck Surg. 2019;160:93–9. [DOI] [PubMed] [Google Scholar]
- 8.Slater NA, Googe PB. PD-L1 expression in cutaneous squamous cell carcinoma correlates with risk of metastasis. J Cutan Pathol. 2016;43:663–70. [DOI] [PubMed] [Google Scholar]
- 9.Yülek Ö, Batur Ş, Özcan K, Yol C, Aydın Ülgen Ö. Relationship between PD-L1 expression and prognostic factors in high-risk cutaneous squamous and basal cell carcinoma. Bosn J Basic Med Sci. 2022;22:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman DT. Cemiplimab and cutaneous squamous cell carcinoma: from bench to bedside. JPRAS Open. 2022;33:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 Blockade with Cemiplimab in advanced cutaneous Squamous-Cell carcinoma. N Engl J Med. 2018;379:341–51. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Townes T, Na’ara S. Current advances and challenges in the management of cutaneous squamous cell carcinoma in immunosuppressed patients. Cancers. 2024;16:3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM, et al. Phase 2 study of Cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer. 2020;8:e000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rischin D, Khushalani NI, Schmults CD, Guminski A, Chang ALS, Lewis KD, et al. Integrated analysis of a phase 2 study of Cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow-up of outcomes and quality of life analysis. J Immunother Cancer. 2021;9:e002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito D, Napolitano F, Maresca DC, Scala M, Amato A, Belli S, et al. Early assessment of IL8 and PD1 + Treg predicts response and guides treatment monitoring in cemiplimab-treated cutaneous squamous cell carcinoma. J Immunother Cancer. 2025;13:e010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallardo D, Simeone E, Festino L, Tuffanelli M, Vanella V, Trojaniello C, et al. IL-6 as new prognostic factor in patients with advanced cutaneous squamous cell carcinoma treated with Cemiplimab. J Transl Med. 2023;21:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Zhong A, Chen J. Immune checkpoint inhibitors in advanced cutaneous squamous cell carcinoma: a systemic review and meta-analysis. Skin Res Technol. 2023;29:e13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye H, Liao W, Pan J, Shi Y, Wang Q. Immune checkpoint Blockade for cancer therapy: current progress and perspectives. J Zhejiang Univ-Sci B. 2025;26:203–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke KP, Chaudhri A, Freeman GJ, Sharpe AH. The B7:CD28 family and friends: unraveling coinhibitory interactions. Immunity. 2024;57:223–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-Gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni L, Lu J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018;7:4509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 24.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. [DOI] [PubMed] [Google Scholar]
- 25.McNutt L-A. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–3. [DOI] [PubMed] [Google Scholar]
- 26.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. [DOI] [PubMed] [Google Scholar]
- 27.Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-γ in tumor progression and regression: a review. Biomark Res. 2020;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaidi MR, Merlino G. The two faces of Interferon-γ in cancer. Clin Cancer Res. 2011;17:6118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J, Wu M, Liu Z. Dysregulation in IFN-γ signaling and response: the barricade to tumor immunotherapy. Front Immunol. 2023;14:1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-γ: its role in promoting cancer immunoevasion. Int J Mol Sci. 2017;19:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wawrzyniak P, Hartman ML. Dual role of interferon-gamma in the response of melanoma patients to immunotherapy with immune checkpoint inhibitors. Mol Cancer. 2025;24:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genova C, Tasso R, Rosa A, Rossi G, Reverberi D, Fontana V, et al. Prognostic role of soluble and extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Cells. 2023;12:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attallah AM, El-Far M, Zahran F, Shiha GE, Farid K, Omran MM, et al. Interferon-gamma is associated with hepatic dysfunction in fibrosis, cirrhosis, and hepatocellular carcinoma. J Immunoass Immunochem. 2016;37:597–610. [DOI] [PubMed] [Google Scholar]
- 34.Chang MJ, Stamos DB, Urtis C, Bowers NL, Schmalz LM, Deyo LJ, et al. Mutational profile of blood and tumor tissue and biomarkers of response to PD-1 inhibitors in patients with cutaneous squamous cell carcinoma. Cancers. 2025;17:1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.