Abstract
A study of Escherichia coli O157:H7 transmission and shedding was conducted with bull calves housed in individual pens within a confined environment. For comparative purposes, the numbers and duration of E. coli O157:H7 shedding in naturally infected calves were monitored after a single purchased calf (calf 156) tested positive prior to inoculation. During the next 8 days, the calves in adjacent pens and a pen directly across a walkway from calf 156 began to shed this serotype O157:H7 strain. Five of the eight calves in this room shed this O157:H7 strain at some time during the following 8 weeks. The numbers of E. coli O157:H7 isolates shed in these calves varied from 60 to 105 CFU/g of feces, and the duration of shedding ranged from 17 to >31 days. The genomic DNAs from isolates recovered from these calves were indistinguishable when compared by using XbaI digestion and pulsed-field gel electrophoresis. Inoculation of calves with 1 liter of water containing ca. 103 to 104 CFU of E. coli O157:H7/ml resulted in shedding in 10 of 12 calves (trial 1, 4 of 4 calves; trial 2, 6 of 8 calves). The inoculated calves shed the inoculation strain (FRIK 1275) as early as 24 h after administration. The duration of shedding varied from 18 to >43 days at levels from 102 to 106 CFU/g of feces. The numbers of doses necessary to initiate shedding varied among calves, and two calves in trial 2 never shed FRIK 1275 after four doses (ca. 106 CFU per dose). Results from this study confirm previous reports of animal-to-animal and waterborne dissemination of E. coli O157:H7 and highlight the need for an effective water treatment to reduce the spread of this pathogen in cattle.
Escherichia coli O157:H7 is a human pathogen that can cause hemorrhagic colitis and in some cases hemolytic uremic syndrome (20). Although a variety of foods have been linked with outbreaks involving E. coli O157:H7 (2, 8, 33, 39), ground beef is the most frequently implicated vehicle in foodborne outbreaks (19, 20, 33). Epidemiological data indicate that waterborne and person-to-person transfer are also important modes of transmission (33). The association of E. coli O157:H7 with cattle and bovine products has focused research on slaughter practices and decontamination of carcasses in order to circumvent this zoonosis (1, 6, 7, 27).
Cattle production practices have been evaluated for an association with the presence of E. coli O157:H7 in cattle in order to identify possible intervention points that may decrease its prevalence in cattle (14, 17, 21, 38, 45). The age of cattle (12, 17), diet (5, 11, 13, 24, 30, 31), grouping of pre- and postweaned calves (15, 17, 30, 31, 38), and contaminated animal drinking water (15, 31, 38) have been identified as contributing factors to cattle shedding serotype O157:H7 strains. In addition to these risk factors, several animal vectors other than cattle have been identified and include birds, deer, flies, humans, and raccoons (19, 31, 37, 38, 41). It is unknown if the elimination of these animal vectors or the application of husbandry practices to circumvent the previously identified risk factors will significantly reduce the number of cattle shedding this human pathogen.
Most inoculation studies of cattle with E. coli O157:H7 have used high numbers (i.e., 1010 CFU) of the organism to dose calves (4, 11, 12, 22, 34) in order to assess pathogenicity and duration of shedding. Although these initial studies have provided valuable information regarding the distribution and shedding of the organism, this inoculum level is not representative of the numbers of E. coli O157:H7 isolates present in environmental samples. In bovine feces, the numbers of E. coli O157:H7 organisms range from 2.0 × 102 to 8.7 × 104 CFU/g (3, 38). Therefore, it is not surprising that shedding in cattle is sporadic and transient (11, 12, 35, 38). Other possible explanations for sporadic, low-level shedding of E. coli O157:H7 in cattle include intermittent environmental exposure to the organism and the persistence of an undetectable number of cells in the digestive tract or associated location that periodically replicate to a detectable level.
This study was undertaken to investigate water as a potential vehicle of E. coli O157:H7 transmission in cattle in a controlled environment where exposure to nonbovine animals and some environmental sources is eliminated. Cattle were dosed with water containing 103 to 104 CFU of E. coli O157:H7 per ml, and the numbers shed and the duration of shedding were monitored. During necropsy, sections from throughout the intestinal tract were collected and analyzed for the pathogen to determine if there was localization.
MATERIALS AND METHODS
Bacterial strain and inoculum.
E. coli O157:H7 strain Food Research Institute-Kaspar culture collection number 1275 (FRIK 1275) was the predominant strain recovered from a positive cattle herd in a previous study (38). The strain was stored at −70°C following growth in nutrient broth (Difco Laboratories, Detroit, Mich.) at 37°C with shaking (150 rpm) and the addition of sterile glycerol (10% final concentration). The inoculum containing FRIK 1275 was prepared with stationary-phase cells (18-h culture) grown in Trypticase soy broth (Difco). The cells were pelleted by centrifugation (8,000 × g, 20 min), resuspended in sterile tap water, and used to inoculate animal drinking water at a final concentration of 103 to 104 CFU/ml. The final concentration of FRIK 1275 in animal drinking water was determined by diluting water samples in 0.1% peptone, plating on Trypticase soy agar plates, incubating the plates at 37°C for 24 h, and enumerating the colonies.
Cattle maintenance and inoculation.
Calves were purchased from local farmers and obtained from the University Agricultural Research Station farms. Following transportation to the Livestock Laboratory at the University of Wisconsin—Madison, calves were dosed with BoviShield (Pfizer Animal Health, Exton, Pa.), Micotil 300 (Elanco Animal Health, Indianapolis, Ind.), and/or Nuflor (Schering-Plough Animal Health, Kenilworth, N.J.) as needed. Inoculation of the calves did not occur for 24 days following the last injection to allow for clearance of the antibiotic.
Weaned bull calves (79 to 168 kg in weight) were housed in individual pens within an indoor, climate-controlled containment facility (65°F, 50% relative humidity). The pens had grated floors to minimize manure accumulation and facilitate cleaning. The metal bars separating pens allowed for some contact between calves in adjacent pens. The pens and central walkway were cleaned daily, and feed and water were provided outside the pens with access through headgates in order to minimize fecal contamination and direct human-calf contact. Access to the rooms was restricted to animal caretakers and investigators. Calves were fed an alfalfa-grain blend that consisted of whole corn (30%), whole oats (15%), oat groats (15%), whole roasted soybean (14%), alfalfa meal pellets (7%), liquid molasses (4%), calcium carbonate (1%), Bovatec premix (0.7%; Hoffman-LaRoche, Inc., Nutley, N.J.), salt and trace minerals (0.3%), and vitamins A and D (0.2%). Water containers were rinsed daily to remove residual feed and filled with fresh tap water. Water was provided ad libitum except during the 12 h preceding inoculation.
The study was conducted in two rooms containing eight pens per room (two rows of four pens). In trial 1, the study of naturally infected calves was conducted in one room while inoculated and control calves were in the second room (one row of four were inoculated and the second row of four were control calves). The eight inoculated calves were housed in one room and the three control calves were held in the second room in trial 2.
Prior to inoculation, fecal samples were collected from calves and tested for E. coli O157:H7 to ensure that calves were not shedding the organism prior to inoculation. After overnight withdrawal of water, calves were provided 1 liter of water in a separate container from that used for the drinking water, with O157:H7 strain FRIK 1275 at a final concentration of 103 to 104 CFU/ml. In trial 1, four calves were provided this inoculum on three consecutive days, whereas the eight calves in trial 2 were provided a single dose or multiple doses, depending on whether they began to shed FRIK 1275 after inoculation. To allow for fecal-test results to be completed, doses in trial 2 were administered at 1-week intervals. Calves were given a maximum of four doses. In both trials, uninoculated calves were monitored for E. coli O157:H7 shedding as a control.
Sample collection and postmortem examination.
Fecal samples (ca. 30 to 50 g) were obtained by digital rectal retrieval and placed in a sterile receptacle. Feed and water samples were collected in sterile containers (Whirlpack bags or specimen cups). Samples were transported to the lab and tested within 2 h of collection. Calves were euthanatized at the end of the study with sodium pentobarbital, and the intestinal tract was removed. Segments of the intestinal tract were observed for signs of pathology, and sections (20 to 50 g) from the rumen (three samples), reticulum, omasum, abomasum, duodenum, jejunum, ileum, cranial colon, cecum, and caudal colon were removed and tested for the presence of E. coli O157:H7.
Blood was collected weekly by venous puncture of the tail vein. The serum was collected by using a Corvac serum separator tube (Sherwood Medical Company, St. Louis, Mo.), and the red blood cells were removed by centrifugation at 4,000 × g for 15 min. The serum was decanted and stored at −20°C until tested. The titers of sera from individual calves were determined for immunoglobulin G (IgG) specific for lipopolysaccharide from strain FRIK 1275 by using an indirect enzyme-linked immunosorbent assay (29).
Microbiological testing.
The presence of E. coli O157:H7 in 10-g samples of feces and feed and 100 ml of water was determined as previously described (38). Samples were enriched in modified EC broth (Difco) supplemented with novobiocin (20 μg/ml; Sigma) for 18 to 24 h at 37°C with shaking (100 rpm). Following enrichment, samples were serially diluted in 0.1% peptone (Difco) and plated at final dilutions of 10−3, 10−4, and 10−5 per ml. The dilutions were plated in duplicate on MacConkey sorbitol agar (Difco) supplemented with cefixime (50 μg/liter; Lederle Labs, Pearl River, N.Y.) and potassium tellurite (2.5 mg/liter; Sigma) (42). The plates were incubated at 42°C and examined for the presence of sorbitol-negative (i.e., white) colonies. Sorbitol-negative colonies (maximum of 10) were tested for the O157 antigen by latex agglutination (liquid suspension; Oxoid, Basingstoke, England). Additionally, isolates were confirmed biochemically to be E. coli by using an API 20E biochemical strip (bioMerieux Vitek, Inc., Hazelwood, Mo.). In trial 1, a maximum of five confirmed colonies were retained from each positive sample and stored in nutrient broth (Difco) supplemented with glycerol (10%) at −70°C until subtyping was conducted.
Enumeration of E. coli O157:H7 isolates.
Feces from inoculated and naturally infected calves were serially diluted in 0.1% peptone and spread plated on duplicate plates of MacConkey sorbitol agar plates supplemented with cefixime and potassium tellurite to determine the number of E. coli O157:H7 isolates shed per gram. Plates were incubated at 42°C for 18 to 24 h and examined for the presence of sorbitol-negative colonies, which were tested for the presence of the O157 antigen as described above. Sorbitol-negative colonies that were also O157 agglutination positive were enumerated. The ranges of CFU per gram reported were determined from 28 fecal samples collected from five naturally infected calves and 10 fecal samples from four inoculated calves. The minimum detection limit of this procedure was approximately 100 CFU/g.
Genomic subtyping.
The pulsed-field gel electrophoresis (PFGE) technique of contour-clamped homogeneous electric fields (CHEF) and XbaI were used for genomic typing of isolates. A maximum of five isolates from each positive sample from trial 1 were analyzed. XbaI (Promega Corp., Madison, Wis.) was used for digestion of genomic DNA as described previously (23, 32). Following digestion, the genomic DNA fragments were resolved by CHEF-PFGE using a CHEF-DRII apparatus (Bio-Rad Laboratories, Richmond, Calif.) at 200 V for 21 h at 14°C, and switch times ramped from 1 to 60 s. MidRange II PFG markers (New England Biolabs, Inc., Beverly, Mass.) were used as DNA size standards.
Statistical analysis.
Numbers of E. coli O157:H7 CFU per gram of feces were converted to log10 for analysis. The numbers of CFU shed as well as the duration of shedding in inoculated and naturally infected calves were analyzed using the Student t test (43). The length of shedding was the number of days from the first E. coli O157:H7-positive fecal sample to the last positive fecal or postmortem sample.
RESULTS
Transmission in naturally infected calves.
Preinoculation fecal testing of calves found that one calf (calf 156) was shedding E. coli O157:H7. Eight calves were housed in this room with pens in two rows of four and a central walkway between the rows. Although the calves were in individual pens, contact was possible between calves in adjacent pens. During the next 8 days, the calves in adjacent pens (calves 157 and 4704) and a pen across the walkway (calf 167) from calf 156 began to shed E. coli O157:H7 (Table 1). Calf 160 began to shed this organism 4 days after its water container and contents were exchanged with those of calf 157 (17 January 1998), which was shedding E. coli O157:H7. Transmission occurred even though a 100-ml water sample from the water container of calf 157 tested negative for the organism. Criteria used to determine which water containers were switched included the shedding status of the donor and recipient calves as well as the pen location of the recipient calf. When the water container and its contents from calf 160 were exchanged with those of calf 158 (29 January 1998), transmission of E. coli O157:H7 did not occur. Water samples (100 ml) from the containers of calves 158 and 160 contained E. coli O157:H7 1 and 2 days before the containers were switched, respectively. Regardless, calf 158 remained negative throughout the study even though its water tested positive (28 January 1998) and calf 157 in an adjacent pen was shedding E. coli O157:H7. During the 8-week study, five of the eight calves in room 1 shed E. coli O157:H7. These five calves shed E. coli O157:H7 for 17 to >31 days. In three of the calves (calves 167, 156, and 4704), the organism was cleared from the intestinal tract as evidenced by negative fecal and/or necropsy samples.
TABLE 1.
Transmission and shedding of E. coli O157:H7 in naturally infected dairy calves
| Calf no.b | Result(s) of fecal and water sample testing for E. coli O157:H7 on specific dates (mo/day/yr)a
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/6/98 | 1/8/98 | 1/12/98 | 1/14/98 | 1/15/98 | 1/16/98 | 1/17/98 | 1/18/98 | 1/19/98 | 1/20/98 | 1/21/98 | 1/22/98 | 1/23/98 | 1/26/98 | 1/27/98 | 1/28/98 | 1/29/98 | 1/30/98 | 2/2/98 | 2/3/98 | 2/4/98 | 2/6/98 | 2/9/98 | 2/11/98 | 2/13/98 | |
| 167 | − | − | +c | + | wd− | +, w+ | w+ | + | w− | + | w− | + | + | w+ | +, w+ | − | − | − | − | Pe− | |||||
| 166 | − | − | − | − | w− | − | − | w− | − | w− | − | − | w− | −, w− | − | w− | − | − | − | P− | |||||
| 160 | − | − | − | − | w− | − | wf | − | w− | + | w− | + | + | w+ | −, w− | wf | −, w− | w− | − | P+g | |||||
| 159 | − | − | − | − | w− | − | − | w− | − | w− | − | − | w− | −, w− | − | − | w− | − | P− | ||||||
| 158 | − | − | − | − | w− | − | − | w− | − | w− | − | − | w− | −, w+ | wf | −, w− | −, w− | w− | − | − | − | P− | |||
| 157 | − | − | − | + | w− | + | wf | w− | + | w− | + | w− | − | + | w− | −, w− | + | + | w− | + | + | + | P+h | ||
| 156 | + | + | + | + | w+ | w− | + | + | +, w− | − | w− | −, w− | − | − | w− | + | P− | ||||||||
| 4704 | − | + | + | + | w− | −, w− | w− | + | w− | − | w− | + | + | w− | −, w+ | − | + | w− | − | P− | |||||
Fecal samples collected on 11 December 1997 and 18 December 1997 tested negative for E. coli O157:H7; +, positive; −, negative.
Calves are listed in order of pen placement with calf 167 located in the first pen immediately to the right of the entrance and proceeding to the back of the room. Calf 158 was in the back of the second row of four pens on the left side of the room and calf 4704 was in the first pen to the left of the entrance.
The pen holding calf 167 was across a walkway from that of calf 156.
w, water sample (100 ml) test result.
P, postmortem examination of intestinal tract. Samples were collected from the abomasum, cecum, cranial colon, caudal colon, duodenum, ileum, jejunum, omasum, reticulum, and rumen and tested for the presence of E. coli O157:H7.
The water container and contents from calf 157 were exchanged with those of calf 160 (17 January 1998) and those of calf 160 were exchanged with those of calf 158 (20 January 2000).
Abomasum tested positive.
Reticulum, cranial colon, and caudal colon tested positive.
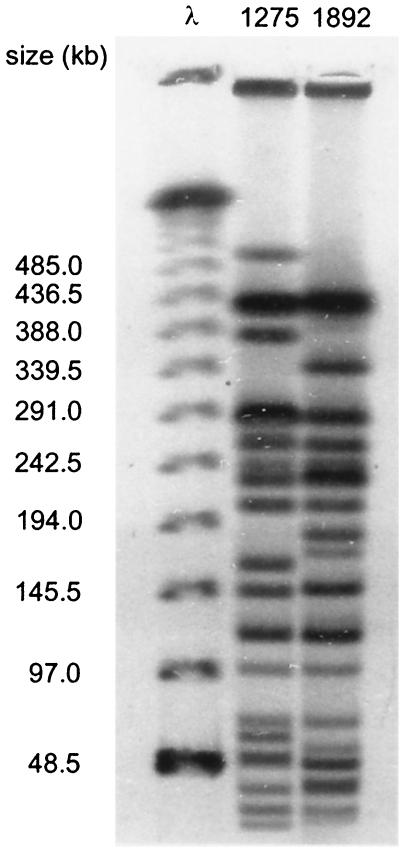
Genomic subtyping of the O157:H7 isolates recovered from the naturally infected calves using XbaI digestion and pulsed-field gel electrophoresis generated indistinguishable profiles (data not shown). Additionally, the profile of the O157:H7 isolates recovered from these naturally infected calves was distinct from that of the inoculation strain (Fig. 1). Additionally, these findings support the conclusion of transmission of a common strain rather than calves carrying an O157:H7 strain which is shed at detectable numbers after the start of the study. Because the calves originated from different herds, it is likely that multiple O157:H7 profiles would have been detected from calves because different herds usually have different O157:H7 subtypes (2, 32, 38).
FIG. 1.
XbaI restriction endonuclease digestion profile of genomic DNA from E. coli O157:H7 isolates from naturally infected (isolate FRIK 1892) and inoculated (FRIK 1275) calves. λ concatemers were used as size standards.
Waterborne inoculation.
In trial 1, control calves were housed in the same room as the inoculated calves, but in a separate row of pens where contact with the inoculated calves was not possible. Ten fecal samples from each calf tested negative for E. coli O157:H7 prior to inoculation. The four calves in trial 1 inoculated with 1 liter of water (from a container separate from that of drinking water) with strain FRIK 1275 at 103 to 104 CFU/ml began to shed the organism 24 h after administration (Table 2). In trial 1, three doses were administered on consecutive days because test results from samples collected 24 h after inoculation were not completed. The drinking water from the pens of three of the four inoculated calves tested positive during the 3-day inoculation period.
TABLE 2.
Shedding of E. coli O157:H7 (FRIK 1275) in dairy calves following waterborne inoculation on three consecutive days
| Calf no.b | Result(s) of fecal and water sample testing for E. coli O157:H7 on specific dates (mo/day/yr)a
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/26/98 | 1/27/98 | 1/28/98 | 1/29/98 | 1/30/98 | 2/2/98 | 2/3/98 | 2/4/98 | 2/9/98 | 2/11/98 | 2/16/98 | 2/18/98 | 2/19/98 | 2/22/98 | 2/24/98 | |
| 170 | − | −, wc− | − | −, w− | − | − | w− | − | − | +d | + | Pe− | |||
| 169 | − | −, w− | − | −, w− | − | − | w− | − | − | − | − | P− | |||
| 168 | − | −, w− | − | − | − | − | w− | − | − | − | − | − | − | P− | |
| 165 | − | −, w− | − | −, w+d | − | − | w− | − | +d | + | + | − | − | P− | |
| 171 | −, If | +, w+, I | +d, w+, I | +, w− | + | − | w− | + | + | + | + | P+g | |||
| 163 | −, If | +, w−, I | +, w+, I | −, w− | + | − | w− | + | + | + | + | P+h | |||
| 162 | −, If | +, w−, I | +, w+, I | −, w− | − | − | w− | + | + | + | + | + | + | P+i | |
| 161 | −, If | +, w−, I | +, w−, I | +, w− | + | + | w− | + | + | + | + | + | − | P− | |
Ten fecal samples collected from 11 December 1997 through 23 January 1998 from all calves tested negative for E. coli O157:H7; +, positive; −, negative.
Calves 170, 169, 168, and 165 were located in the four pens on the left side of the room, while calves 171, 163, 162, and 161 were in the four pens on the right side of the room.
w, water sample (100 ml) test result.
Steer (171) contamination (29 January 1998) of feed and water containers of control animals.
P, postmortem examination of intestinal tract. Samples were collected from the abomasum, cecum, cranial colon, caudal colon, duodenum, ileum, jejunum, omasum, reticulum, and rumen and tested for the presence of E. coli O157:H7.
I, inoculation with 1 liter of water containing 104 CFU (26 January 1997) and 103 CFU FRIK 1275/ml (27 and 28 January 1998).
All intestinal tract samples tested positive.
Reticulum and rumen and their contents tested positive.
Reticulum and contents tested positive.
The duration of shedding of E. coli O157:H7 in the inoculated calves ranged from 23 to >29 days. Calf 161 cleared E. coli O157:H7 in 23 days, as the last fecal sample (22 February 1998) and postmortem intestinal samples tested negative. The other three calves (calves 171, 163, and 162) were still shedding E. coli O157:H7 when postmortem samples were collected. For calf 171, all intestinal tract samples tested positive for E. coli O157:H7. In contrast, the reticulum and rumen as well as their contents tested positive for calf 163 and only the reticulum and its contents tested positive for calf 162. The reticulum was the only segment of the intestinal tract that was positive for all three calves. There were no signs of pathology in the intestinal tracts of calves even when E. coli O157:H7 was present.
Two control calves (calves 170 and 165) began shedding E. coli O157:H7 after calf 171 got loose from its pen (29 January 1998) and defecated in the water and feed containers of the control animals. These calves shed E. coli O157:H7 for only 5 to 7 days, and postmortem intestinal tract samples tested negative. All samples collected from control calves 168 and 169 tested negative.
Genomic subtyping of the O157:H7 isolates recovered from the inoculated calves in trial 1 yielded profiles indistinguishable from that of the inoculation strain FRIK 1275 but different from the strain recovered from the naturally infected calves (Fig. 1). The genomic profiles of the isolates from the two control calves were indistinguishable from FRIK 1275, indicating transmission from the inoculated calves, likely calf 171. Two isolates from calf 161 had profiles that differed from FRIK 1275 by one band (data not shown). These isolates are likely a derivative of the inoculation strain since sequential isolates from cattle and humans with slightly different genomic profiles (1- or 2-band difference) have been reported (18, 28).
In trial 2 (Table 3), the inoculated calves were housed in a room separate from that of the control calves. Six of the eight calves inoculated with water containing O157:H7 strain FRIK 1275 at 103 CFU/ml began to shed the organism. Calves 2980 and 2660 were administered four 1-liter doses of E. coli O157:H7 but never shed detectable levels of the organism in feces. Likewise, the number of doses necessary to initiate shedding in the calves varied from 1 to 4. Shedding of E. coli O157:H7 ranged from 18 to >43 days. Three calves (calves 2979, 2973, and 2705) cleared E. coli O157:H7 after 18 to 36 days as demonstrated by negative fecal and/or postmortem intestinal samples. For calves 2705 and 2973, the last three fecal samples collected (19 through 24 March 1999) and postmortem samples (25 March 1999) all tested negative for the organism. The last three fecal samples from calf 2961 also tested negative, but the duodenum sample tested positive. The opposite occurred with calf 2979, in which E. coli O157:H7 was shed for 36 days and whose feces tested positive (9 April 1999) 3 days prior to collection of postmortem samples; yet the latter samples all tested negative. Postmortem intestinal tract samples tested positive for three calves. For calf 2957, all 10 intestinal tract samples tested positive for E. coli O157:H7, while the rumen and duodenum were the only samples that tested positive for calves 2686 and 2961. Postmortem analysis of the intestinal tract found no signs of pathology, with one exception: calf 2957 exhibited epithelial hyperemia and tissue friability. No additional testing was conducted to determine the cause of this pathology. The three control calves tested negative for E. coli O157:H7 throughout the study.
TABLE 3.
Shedding of E. coli O157:H7 (FRIK 1275) in dairy calves following one to four doses in water
| Calf no.b | Result(s) of sample testing for E. coli O157:H7 on specific dates (mo/day/yr)a
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2/15/99 | 2/17/99 | 2/19/99 | 2/22/99 | 2/24/99 | 2/26/99 | 3/1/99 | 3/3/99 | 3/5/99 | 3/8/99 | 3/10/99 | 3/12/99 | 3/15/99 | 3/18/99 | 3/19/99 | 3/22/99 | 3/24/99 | 3/25/99 | 3/26/99 | 3/29/99 | 3/31/99 | 4/2/99 | 4/5/99 | 4/7/99 | 4/9/99 | 4/12/99 | |
| 2980 | − | Ic | − | − | −, I | − | − | −, I | − | − | −, I | − | − | Pd− | ||||||||||||
| 2660 | − | I | − | − | −, I | − | − | −, I | − | − | −, I | − | − | P− | ||||||||||||
| 2686 | − | I | − | − | −, Ie | − | − | −, If | − | − | −, If | − | + | + | + | + | + | − | + | − | + | + | + | P+g | ||
| 2957 | − | I | − | − | −, I | − | + | − | − | − | +, I | + | + | − | − | − | + | + | + | − | − | + | + | P+h | ||
| 2979 | − | I | − | − | −, I | − | − | −, I | + | + | + | + | + | − | − | − | + | + | + | − | + | + | + | P− | ||
| 2961 | − | I | + | + | + | + | + | + | + | + | + | + | + | − | − | − | P+i | |||||||||
| 2973 | − | I | − | − | −, I | + | + | + | + | + | + | + | + | − | − | − | P− | |||||||||
| 2705 | − | I | + | + | + | + | + | + | + | + | + | + | − | − | − | − | P− | |||||||||
| 2725 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | P− | ||||||||
| 2702 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | P− |
| 2959 | − | − | − | − | − | − | − | − | − | − | − | − | − | P− | ||||||||||||
Fecal samples collected on 2 February 1999 from all calves tested negative for E. coli O157:H7; +, positive; −, negative.
Inoculated calves were in the same room; control calves were housed in an adjoining but separate room.
I, inoculation with 1 liter of water containing 103 CFU (FRIK 1275)/ml
P, postmortem examination of intestinal tract. Samples were collected from the abomasum, cecum, cranial colon, caudal colon, duodenum, ileum, jejunum, omasum, reticulum, and rumen and tested for the presence of E. coli O157:H7.
Calf no. 2686 consumed only 0.5 liter of inoculum.
Calf no. 2686 would not consume inoculum.
Rumen sample tested positive.
All intestinal samples tested positive. Inflammation, ulceration, and tissue degradation were evident.
Duodenum sample tested positive.
Numbers of E. coli O157:H7 isolates shed in naturally infected and inoculated calves.
The numbers of E. coli O157:H7 organisms shed in naturally infected and inoculated calves were not statistically different. The naturally infected calves shed the organism at concentrations ranging from 6.0 × 101 to 6.8 × 105 CFU/g, while numbers in inoculated calves ranged from 4.3 × 102 to 1.2 × 106 CFU/g (Table 4).
TABLE 4.
Numbers of E. coli O157:H7 isolates shed in naturally infected and inoculated dairy calves housed in a confined environment
| Calf no. | No. of samplesa | Range of CFU/g |
|---|---|---|
| Naturally infectedb | ||
| 167 | 6 | 4.2 × 103-6.8 × 105 |
| 160 | 2 | 7.8 × 103-2.2 × 104 |
| 157 | 8 | 1.2 × 102-8.5 × 104 |
| 156 | 5 | 6.0 × 101-9.0 × 103 |
| 4704 | 7 | 1.7 × 102-2.2 × 105 |
| Overall range | 60-6.8 × 105 | |
| Inoculatedc | ||
| 171 | 2 | 4.3 × 102-2.1 × 105 |
| 165 | 2 | 5.9 × 104-1.2 × 106 |
| 163 | 3 | 4.7 × 102-1.6 × 103 |
| 161 | 3 | 1.5 × 103-3.5 × 104 |
| Overall range | 4.3 × 102-1.2 × 106 |
Numbers of fecal samples plated to determine the number of E. coli O157:H7 CFU/g.
Calves that shed E. coli O157:H7 but were not inoculated.
Calves that shed E. coli O157:H7 after receiving three 1-liter doses containing 103 to 104 FRIK 1275 CFU/ml of water.
Length of shedding in naturally infected and inoculated calves.
For comparative purposes, the length of shedding in naturally infected and inoculated calves was monitored by using data from animals with a defined start and end of shedding. Thus, the time (days) between the first positive fecal sample and the last E. coli O157:H7-positive fecal sample and/or negative gastrointestinal tract samples was used to define the length of shedding. There was no statistical difference in the lengths of shedding in naturally infected (17 to 30 days; n = 3) and inoculated (18 to 36 days; n = 4) animals.
DISCUSSION
Results from previous field studies (15, 21, 38) have suggested that contaminated water contributes to the dissemination of E. coli O157:H7 in cattle. The link between contaminated water and shedding in cattle is supported by PFGE-CHEF typing of genomic DNA from O157:H7 isolates from both sources (38). Herds tend to harbor a dominant and persistent O157:H7 strain (i.e., PFGE-CHEF profile) that suggests a common source. The most probable origin of E. coli O157:H7 in water troughs is cattle that contaminate the water either by feces or regurgitation. However, it is possible that there is an alternative common source of this organism and the presence of E. coli O157:H7 in water is circumstantial. This study confirmed that water containing 103 CFU of E. coli O157:H7/ml was sufficient to transmit this organism and establish shedding in bull calves.
Before studies began, feces from calves were tested to determine if they were shedding E. coli O157:H7. A single calf (calf 156) was shedding the organism, which was likely obtained from an unknown source prior to transport to the containment facility. Calf 156 had some direct contact with the calves in adjacent pens, and these calves (calves 4704 and 157) began shedding this strain of E. coli 2 and 8 days after calf 156 tested positive, respectively. Animal-to-animal transmission of E. coli O157:H7 has been demonstrated with sheep (30) and is the most probable mode of passage to calves 4704 and 157 since each had separate water and feed containers. Calf 167, located in a pen across the walkway from calf 156, began to shed E. coli O157:H7 6 days after calf 156 tested positive. Calf 156 frequently dumped the water from its container, which drained into the pen of calf 167. Calf 167 was observed licking this water from the floor of its pen; this is the likely route of exposure of this calf to E. coli O157:H7. The habit of calf 156 to dump its water container is the reason that water test results for this calf were not obtained until 4 days after calf 167 began shedding. When the water was tested, E. coli O157:H7 was isolated. Another calf (calf 160) began to shed E. coli O157:H7 after its water container and contents were exchanged with the container from a calf (calf 157) that was shedding the organism. A 100-ml water sample from the container of calf 157 tested negative; nonetheless, calf 160 began to shed E. coli O157:H7 4 days later. The calves in both pens on each side of calf 160 tested negative for the organism. In another case in which the water containers were switched between a calf shedding E. coli O157:H7 and a negative calf, transmission did not occur. These results indicate that a low level of this organism in water may be sufficient to initiate shedding in some calves. The shedding of E. coli O157:H7 organisms in these naturally infected calves occurred for 17 to >31 days, and the number of organisms shed ranged from 60 to 105 CFU/g of feces, which is comparable to previous findings (3, 35, 38). Three calves tested negative throughout the 8-week study, even though at least one calf in an adjacent pen was shedding the organism.
Waterborne inoculation of E. coli O157:H7 was demonstrated for 10 of 12 calves (trial 1, 4 of 4 calves; trial 2, 6 of 8 calves) administered 1 liter of water containing ca. 103 to 104 CFU of strain FRIK 1275/ml. FRIK 1275 was present in the feces of calves as early as 1 day after oral inoculation, which is in agreement with previous studies (4, 12, 25, 34) that reported shedding 1 to 2 days after inoculation with E. coli O157:H7 at ca. 1010 CFU. Shedding of FRIK 1275 in the inoculated calves occurred for 18 to >43 days and is similar to the length of shedding observed for the naturally infected calves (ranging from 17 to >31 days) housed under the same conditions. Additionally, the length of shedding in these calves in a confined environment is similar to that of cattle on dairy farms (3, 35, 38). The variation in the pattern and duration of shedding observed is consistent with the findings of Cray and Moon (12) and others, who found that shedding of E. coli O157:H7 varied among animals of the same age. They observed shedding for over 20 weeks in two calves and 27 weeks in one calf originally inoculated with 1010 CFU.
Results from necropsy samples were in agreement with the preceding fecal sample in 19 of the 24 calves (including inoculated, naturally infected, and control calves). In three calves, the preceding fecal sample tested positive and all of the intestinal tract samples tested negative, and the opposite occurred in two calves where the prior fecal sample(s) tested negative and at least one of the intestinal tract samples was positive. The changes in the E. coli O157:H7 status of these calves over a 2-day period can be explained by the presence of FRIK 1275 at numbers below the detection limit of the enrichment method employed (ca. 100 CFU), reintroduction of the organism from the environment, or clearance of the organism from respective calves.
In trial 1, control calves were housed in the same room as the inoculated calves, but in a separate row of pens where contact with the inoculated calves was not possible. Calves 170 and 165 (control calves) shed FRIK 1275 for 5 and 7 days, respectively. Shedding occurred approximately 2 weeks (12 to 14 days) after calf 171 contaminated the feed and water containers of several control calves with feces while out of its pen. The shorter duration of shedding in these calves may be due to the low-level exposure; however, the naturally infected calves were likely exposed to similar levels of E. coli O157:H7 but shed the organism for a minimum of 17 days. The O157:H7 isolates from the control calves had a genomic profile indistinguishable from that of FRIK 1275, demonstrating that it was transmission from the inoculated calves rather than introduction of another strain that had occurred. These results further establish the environment as an important source of E. coli O157:H7.
The dose administered in this study (ca. 106 CFU), although lower than those found in a majority of inoculation studies, is still high when considering the numbers of this organism found in environmental samples but is comparable to those found in feces (3, 38, 45). Nonetheless, this dose enabled us to determine that FRIK 1275 replicated in the bovine intestinal tract with numbers reaching a maximum of ca. 1.2 × 106 CFU/g. Moreover, the numbers of E. coli O157:H7 in naturally infected calves reached ca. 6.8 × 105 CFU/g, and in these calves, the organism was likely transmitted at lower numbers. Previous studies have found that E. coli O157:H7 replicates in the rumen fluid from fasted cattle (36) and withholding feed from calves increased their susceptibility to colonization by this organism (11). As reported in other studies (11, 12), the numbers of E. coli O157:H7 fluctuated in and among calves, with occasional spikes that resulted in numbers increasing from 6.0 × 102 to 2.0 × 105 CFU/g over 2 days. For calves that cleared E. coli O157:H7, there was a trend towards lower numbers over time, while more-constant numbers were found for calves in which the organism persisted. It is possible that the same bovine factor(s) that influence the number of E. coli O157:H7 organisms shed also affect the calf's susceptibility to colonization.
Two calves (calves 2960 and 2980) in trial 2 were inoculated four times but never shed detectable levels of FRIK 1275. Similar observations have been made in farm studies where some calves or cattle did not shed E. coli O157:H7 despite the presence and shedding of this organism in cohorts within the same pen (38). Shedding and persistence of E. coli O157:H7 is likely influenced by the O157:H7 strains and the microbial flora (44), age (12), immune status, genetic or anatomical differences, or other unidentified factors of the bovine host. The absence or diminished function of these host factors may contribute to shedding in calves 2961 and 2705 following a single 1-liter dose. Additionally, both host and O157:H7 strain factors are probably involved in the pervasive colonization noted for calves 171 and 2957, which resulted in all 12 gastrointestinal tract samples testing positive for E. coli O157:H7. These findings differ from those of other inoculation studies (4, 10, 12, 22, 40) where E. coli O157:H7 was primarily found in the rumen, reticulum, abomasum, and colon. It is not known where or if E. coli O157:H7 colonizes the alimentary tract of cattle or whether reintroduction from environmental sources accounts for its distribution and presence in the intestinal tract. Identification of the bovine factor(s) that influence shedding and colonization will undoubtedly contribute to the development of an intervention practice(s) to clear or reduce the length of shedding of E. coli O157:H7.
To evaluate one host factor, serum IgG levels for O157 lipopolysaccharide were monitored by enzyme-linked immunosorbent assay (29) to determine if there was a relationship with the duration of shedding in calves following inoculation (data now shown). Starting titers of anti-O157 IgG varied among animals and did not influence the number of doses necessary to initiate shedding or the duration of shedding. Additionally, there was not a consistent increase in the titers of antibody following inoculation at a dose of ca. 106 CFU or with shedding in naturally infected calves. Johnson et al. (26) also found little change in O157 antibody titers in calves administered 107 CFU, while at a dose of 1010 CFU there was an increase but the antibodies did not influence the reinfection of the animal with the same strain. Similarly, a horse serum albumin-O157 antigen conjugate elicits a specific systemic antibody response in BALB/c mice but does not impact intestinal colonization following oral inoculation with the pathogen (9). The isolation of E. coli including serotype O157:H7 from mesenteric lymph nodes and tonsils (12, 16) is consistent with a systemic immune response and increases in serum antibodies. However, the inability of anti-O157 IgG to prevent colonization or reinfection in cattle supports the development of an alternative approach for vaccines perhaps aimed at stimulating levels of secretory IgA.
A primary finding from this study is that contaminated water can serve as a vehicle of E. coli O157:H7 transmission in cattle, although there was variation among animals in the doses necessary to initiate shedding. The fact that two calves had a persistent and pervasive colonization by the inoculation strain suggests that a host factor(s) is involved in E. coli O157:H7 colonization or shedding in cattle.
Acknowledgments
The technical assistance of Lindsey Buswell and Barbara Cochrane is greatly appreciated. We thank the staff of the Livestock Laboratory, University of Wisconsin, particularly Terry Jobsis and Lee Sherven.
Financial support was provided by the Wisconsin Beef Council, contributions to the Food Research Institute, and the College of Agricultural and Life Sciences, University of Wisconsin—Madison.
REFERENCES
- 1.Beery, E. D., and C. N. Cutter. 2000. Effects of acid adaptation of Escherichia coli O157:H7 on efficacy of acetic acid spray washes to decontaminate beef carcass tissue. Appl. Environ. Microbiol. 66:1493-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besser, R. E., S. M. Lett, J. T. Weber, M. P. Doyle, T. J. Barrett, J. G. Wells, and P. M. Griffin. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh pressed apple cider. JAMA 269:2217-2220. [PubMed] [Google Scholar]
- 3.Besser, T. E., D. D. Hancock, L. C. Pritchett, E. M. McRae, D. H. Rice, and P. I. Tarr. 1997. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J. Infect. Dis. 175:726-729. [DOI] [PubMed] [Google Scholar]
- 4.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchko, S. J., R. A. Holley, W. O. Olson, V. P. J. Gannon, and D. M. Veira. 2000. The effect of fasting and diet on fecal shedding of Escherichia coli O157:H7 by cattle. Can. J. Anim. Sci. 80:741-744. [Google Scholar]
- 6.Castillo, A., L. M. Lucia, K. J. Goodson, J. W. Savell, and G. R. Acuff. 1999. Decontamination of beef carcass surface tissue by steam vacuuming alone and combined with hot water and lactic acid sprays. J. Food Prot. 61:146-151. [DOI] [PubMed] [Google Scholar]
- 7.Castillo, A., L. M. Lucia, D. B. Roberson, T. H. Stevenson, I. Mercado, and G. R. Acuff. 2001. Lactic acid sprays reduce bacterial pathogens on cold beef carcass surfaces and in subsequently produced ground beef. J. Food Prot. 64:58-62. [DOI] [PubMed] [Google Scholar]
- 8.Cieslak, P. R., T. J. Barrett, P. M. Griffin, et al. 1993. Escherichia coli O157:H7 infection from a manured garden. Lancet 342:367. [DOI] [PubMed] [Google Scholar]
- 9.Conlan, J. W., A. D. Cox, R. KuoLee, A. Webb, and M. B. Perry. 1999. Parenteral immunization with a glycoconjugate vaccine containing the O157 antigen of Escherichia coli O157:H7 elicits a systemic humoral response in mice, but fails to prevent colonization by the pathogen. Can. J. Microbiol. 45:279-286. [PubMed] [Google Scholar]
- 10.Cornick, N. A., S. L. Booher, T. A. Casey, and H. W. Moon. 2000. Persistent colonization of sheep by Escherichia coli O157:H7 and other E. coli pathotypes. Appl. Environ. Microbiol. 66:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cray, W. C., Jr., T. A. Casey, B. T. Bosworth, and M. A. Rasmussen. 1998. Effect of dietary stress on fecal shedding of Escherichia coli O157:H7 in calves. Appl. Environ. Microbiol. 64:1975-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diez-Gonzalez, F., T. R. Callaway, M. G. Kizoulis, and J. B. Russell. 1998. Grain feeding and the dissemination of acid-resistant Escherichia coli from cattle. Science 281:1666-1668. [DOI] [PubMed] [Google Scholar]
- 14.Elder, R. O., J. E. Keen, G. R. Siragusa, G. A. Barkocy-Gallagher, M. Koohmaraie, and W. W. Laegreid. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 97:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faith, N. G., J. A. Shere, R. Brosch, K. W. Arnold, S. E. Ansay, M.-S. Lee, J. B. Luchansky, and C. W. Kaspar. 1996. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl. Environ. Microbiol. 62:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank, G. H., R. E. Briggs, and R. A. Schneider. 1994. Characterization of Escherichia coli isolated from the tonsils of cattle. J. Clin. Microbiol. 32:256-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garber, L. P., S. J. Wells, D. D. Hancock, M. P. Doyle, J. Tuttle, J. A. Shere, and T. Zhao. 1995. Risk factors for fecal shedding of Escherichia coli O157:H7 in dairy cattle. J. Am. Vet. Med. Assoc. 207:46-49. [PubMed] [Google Scholar]
- 18.Gouveia, S., M. E. Proctor, M.-S. Lee, J. B. Luchansky, and C. W. Kaspar. 1998. Genomic comparisons and Shiga toxin production in Escherichia coli O157:H7 isolates from a day care center outbreak and sporadic cases in southeastern Wisconsin. J. Clin. Microbiol. 36:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin, P. M. 1995. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli infections of the gastrointestinal tract, p. 739-761. In M. J. Blasser, P. D. Smith, J. I. Ravdin, H. B. Greenburg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, Ltd., New York, N.Y.
- 20.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 21.Hancock, D. D., D. H. Rice, L. A. Thomas, D. A. Dargatz, and T. E. Besser. 1997. Epidemiology of Escherichia coli O157:H7 in feedlot cattle. J. Food Prot. 60:462-465. [DOI] [PubMed] [Google Scholar]
- 22.Harmon, B. G., C. A. Brown, S. Tkalcic, P. O. E. Mueller, A. Parks, A. V. Jain, T. Zhao, and M. P. Doyle. 1999. Fecal shedding and rumen growth of Escherichia coli O157:H7 in fasted calves. J. Food Prot. 62:574-579. [DOI] [PubMed] [Google Scholar]
- 23.Harsono, K. D., C. W. Kaspar, and J. B. Luchansky. 1993. Comparison and genomic sizing of Escherichia coli O157:H7 isolates by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 59:3141-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herriott, D. E., D. D. Hancock, E. D. Ebel, L. V. Carpenter, D. H. Rice, and T. E. Besser. 1998. Association of herd management factors with colonization of dairy cattle by Shiga-toxin-positive Escherichia coli O157:H7. J. Food Prot. 61:802-807. [DOI] [PubMed] [Google Scholar]
- 25.Hovde, C. J., P. R. Austin, K. A. Cloud, C. J. Williams, and C. W. Hunt. 1999. Effect of cattle diet on Escherichia coli O157:H7 acid resistance. Appl. Environ. Microbiol. 65:3233-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, R. P., W. C. J. Cray, and S. T. Johnson. 1996. Serum antibody responses of cattle following experimental infection with Escherichia coli O157:H7. Infect. Immun. 64:1879-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang, D.-H., M. Koohmaraie, W. J. Dorsa, and G. R. Siragusa. 2001. Development of a multiple-step process for the microbial decontamination of beef trim. J. Food Prot. 64:63-71. [DOI] [PubMed] [Google Scholar]
- 28.Karch, H., H. Rüssman, H. Schmidt, A. Schwarzkopf, and J. Heesemann. 1995. Long-term shedding and clonal turnover of enterohemorrhagic Escherichia coli O157 in diarrheal diseases. J. Clin. Microbiol. 33:1602-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaspar, C. W., and P. A. Hartman. 1987. Production and specificity of monoclonal and polyclonal antibodies to Escherichia coli. J. Appl. Bacteriol. 63:335-341. [DOI] [PubMed] [Google Scholar]
- 30.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1995. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl. Environ. Microbiol. 61:1363-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1997. Characterization of Escherichia coli O157:H7 and other Shiga-toxin-producing E. coli serotypes isolated from sheep. J. Clin. Microbiol. 35:892-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, M.-S., C. W. Kaspar, R. Brosch, J. Shere, and J. B. Luchansky. 1996. Pulsed-field genomic fingerprinting of Escherichia coli O157:H7 from dairy calves isolated during the United States National Dairy Heifer Evaluation Project (1991B1992). Vet. Microbiol. 48:223-230. [DOI] [PubMed] [Google Scholar]
- 33.Mead, P. S., and P. M. Griffin. 1998. Escherichia coli O157:H7. Lancet (N. Am. Ed.) 352:1207-1212. [DOI] [PubMed] [Google Scholar]
- 34.Price, S. B., C.-M. Cheng, C. W. Kaspar, J. C. Wright, F. J. DeGraves, T. A. Penfound, M.-P. Castanie-Cornet, and J. W. Foster. 2000. Role of rpoS in acid resistance and fecal shedding of Escherichia coli O157:H7. Appl. Environ. Microbiol. 66:632-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahn, K., S. A. Renwick, R. P. Johnson, J. B. Wilson, R. C. Clarke, D. Alves, S. McEwen, H. Lior, and J. Spika. 1997. Persistence of Escherichia coli O157:H7 in dairy cattle and the dairy farm environment. Epidemiol. Infect. 119:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmussen, M. A., W. C. Cray, Jr., T. A. Casey, and S. C. Shipp. 1993. Rumen contents as a reservoir of enterohemorrhagic Escherichia coli. FEMS Microbiol. Lett. 114:79-84. [DOI] [PubMed] [Google Scholar]
- 37.Rice, D. H., D. D. Hancock, and T. E. Besser. 1995. Verotoxigenic E. coli O157 colonisation of wild deer and range cattle. Vet. Rec. 137:524. [DOI] [PubMed] [Google Scholar]
- 38.Shere, J. A., K. J. Bartlett, and C. W. Kaspar. 1998. Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in Wisconsin. Appl. Environ. Microbiol. 64:1390-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilden, J., Jr., W. Young, A. M. McNamara, C. Custer, B. Boesel, M. A. Lambert-Fair, J. Majkowski, D. Vugia, S. B. Werner, J. Hollingworth, and J. G. Morris. 1996. A new route of transmission for Escherichia coli O157:H7: infection from dry fermented salami. Am. J. Public Health 86:1142-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tkalcic, S., C. A. Brown, B. G. Harmon, A. V. Jain, E. P. O. Mueller, A. Parks, K. L. Jacobsen, S. A. Martin, T. Zhao, and M. P. Doyle. 2000. Effects of diet on rumen proliferation and fecal shedding of Escherichia coli O157:H7 in calves. J. Food Prot. 63:1630-1636. [DOI] [PubMed] [Google Scholar]
- 41.Wallace, J. S., T. Cheasty, and K. Jones. 1997. Isolation of Vero cytotoxin-producing Escherichia coli O157 from wild birds. J. Appl. Microbiol. 82:399-404. [DOI] [PubMed] [Google Scholar]
- 42.Zadik, P. M., P. A. Chapman, and C. A. Siddons. 1993. Use of tellurite for the selection of verocytotoxigenic Escherichia coli O157:H7. J. Med. Microbiol. 39:155-158. [DOI] [PubMed] [Google Scholar]
- 43.Zar, J. H. 1984. Biostatistical analysis, p. 185-205. Prentice-Hall, Inc., Englewood Cliffs, N.J.
- 44.Zhao, T., M. P. Doyle, B. G. Harmon, C. A. Brown, P. O. E. Mueller, and A. H. Parks. 1998. Reduction of carriage of enterohemorrhagic Escherichia coli O157:H7 in cattle by inoculation with probiotic bacteria. J. Clin. Microbiol. 36:641-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]