Abstract
The use of Lactococcus lactis (the most extensively characterized lactic acid bacterium) as a delivery organism for heterologous proteins is, in some cases, limited by low production levels and poor-quality products due to surface proteolysis. In this study, we combined in one L. lactis strain use of the nisin-inducible promoter PnisA and inactivation of the extracellular housekeeping protease HtrA. The ability of the mutant strain, designated htrA-NZ9000, to produce high levels of stable proteins was confirmed by using the staphylococcal nuclease (Nuc) and the following four heterologous proteins fused or not fused to Nuc that were initially unstable in wild-type L. lactis strains: (i) Staphylococcus hyicus lipase, (ii) the bovine rotavirus antigen nonstructural protein 4, (iii) human papillomavirus antigen E7, and (iv) Brucella abortus antigen L7/L12. In all cases, protein degradation was significantly lower in strain htrA-NZ9000, demonstrating the usefulness of this strain for stable heterologous protein production.
Lactococcus lactis is a gram-positive lactic acid bacterium that is widely used in the production of fermented food products, and as such, it is considered a food-grade microorganism. Experimental data and genomic analyses indicate that only a few proteins are naturally secreted in L. lactis (4, 32, 38), and a plasmid-free strain does not produce the extracytoplasmic protease PrtP (13). These features have drawn the attention of researchers to the potential use of L. lactis for secretion of proteins of biotechnological interest. Thus, L. lactis has been extensively engineered for production and export of heterologous proteins with high added value, such as antigens or enzymes (2, 6, 8-12, 20, 22, 23, 31, 35). For this purpose, several genetic tools have been developed for L. lactis, and the potential of this organism as a prokaryotic host for heterologous protein production has been confirmed (7, 9, 23, 40).
Systems that allow controlled levels of expression of foreign proteins in L. lactis may offer certain advantages over constitutive systems (8). The nisin-controlled expression (NICE) system (7, 18), based on a combination of the PnisA promoter and the nisRK regulatory genes, has proven to be highly versatile (8, 17, 18) and has already been used to express different heterologous proteins (2, 6, 10, 11, 35).
Protein export to the cell surface or into the medium is a preferred means of protein expression for several biotechnological applications (9, 23). However, poor expression and proteolytic degradation of heterologous proteins are limiting factors for stable protein production in bacteria. In Escherichia coli and Bacillus subtilis, several exported proteases that are associated with turnover of both natural and foreign proteins have been described (15, 25, 26, 30, 37). In contrast to E. coli and B. subtilis, L. lactis has a unique extracellular housekeeping protease, HtrA (high-temperature requirement), as demonstrated by construction of an L. lactis htrA-IL1403 mutant strain (previously designated htrA [33]) and confirmed by genomic analysis (4). Studies with htrA-IL1403 showed that HtrA is involved in propeptide processing, maturation of native proteins, and degradation of recombinant proteins (33). These findings obtained with L. lactis have clear applications for the development of an efficient export system for high-level production of stable heterologous proteins (31a).
In this study, we constructed L. lactis strain htrA-NZ9000, an htrA mutant of NZ9000 (Table 1) (18). Inactivation of htrA was carried out by a single crossover recombination event by using a nonreplicative plasmid harboring an internal fragment of the MG1363 htrA gene. We examined the ability of htrA-NZ9000 to stabilize the following heterologous proteins that were generally degraded in wild-type (wt) L. lactis strains: Staphylococcus aureus nuclease (Nuc), a secretion reporter (21), Staphylococcus hyicus lipase (Lip) (10, 33, 39), bovine rotavirus nonstructural protein 4 (NSP4) (1, 11), human papillomavirus antigen E7 (2), and Brucella abortus antigen L7/L12 (28, 35). As we recently found that instability and/or poor yields of heterologous proteins in L. lactis can be overcome in part by fusion to Nuc (2, 35), the NSP4, E7, and L7/L12 proteins were fused to Nuc. Genes encoding the viral or bacterial heterologous proteins and/or the Nuc fusions were placed under control of the nisin-inducible promoter PnisA and addressed for export by using the signal peptide of Usp45 (SPUsp45), the predominant L. lactis secreted protein (38). Except for native E7, these proteins showed high levels of proteolysis in the L. lactis NZ9000 wt strain and were stabilized when they were produced in htrA-NZ9000. Our results confirm the interest in combining controlled production and protein stability in one strain.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Characteristics | Reference |
|---|---|---|
| E. coli TG1 | supE hsd Δ5 thi Δ(lac-proAB) F′(traD36 proAB-lacZΔM15) | 14 |
| L. lactis MG1363 | Wild type, plasmid free | 13 |
| L. lactis NZ9000 | MG1363 (nisRK genes on the chromosome) | 18 |
| L. lactis htrA-NZ9000 | Emr, htrA disrupted by single-crossover recombination | This study |
| pRV300 | ColE1/Apr Emr | 24 |
| pRV300:htrA | pRV300 derivative carrying PCR fragment (500 bp) of the htrA gene from L lactis MG1363 (positions 47 to 547 starting at the first codon); also called pED716 | This study |
| pSEC:Nuc | pWV01/Cmr; expression vector containing a fusion between SPUsp45 and Nuc expressed under control of PmisA | 11 |
| pSEC:Lip | pWV01/Cmr: expression vector containing a fusion to SPUsp45 in which Nuc is replaced by the S. hyicus Lip gene; under control of PmisA; previously called pJIM2093 | 10 |
| pSEC:NSP4 | pWV01/Cmr; expression vector containing a fusion with SPUsp45 in which Nuc is replaced by the DNA fragment encoding NSP4; under control of PmisA | 11 |
| pSEC:LEISS:Nuc:NSP4 | pWV01/Cmr; expression vector containing a fusion with SPUsp45 in which LEISS:Nuc is fused to the DNA fragment encoding NSP4; under control of PmisA | This study |
| pSEC:E7 | pWV01/Cmr; expression vector containing a fusion with SPUsp45 in which Nuc is replaced by the DNA fragment encoding E7; under control of PmisA | 2 |
| pSEC:Nuc-E7 | pWV01/Cmr; expression vector containing a fusion with SPUsp45 in which Nuc is fused to the DNA fragment encoding E7; under control of PmisA | 2 |
| pSEC:Nuc-L7/L12 | pWV01/Cmr; expression vector containing a fusion with SPUsp45 in which Nuc is fused to the DNA fragment encoding L7/L12; under control of PmisA | 35 |
Construction of the L. lactis htrA-NZ9000 strain.
Bacterial strains and plasmids used in this work are described in Table 1. L. lactis MG1363 (13) and L. lactis NZ9000 (18) were grown in M17 medium supplemented with 0.5% glucose (GM17), and chromosomal DNA was prepared as described previously (32). An internal 500-bp htrA gene fragment was PCR amplified by using the following primers designed on the basis of the genomic DNA sequence of the L. lactis MG1363 htrA gene (E. Domakova, personal communication): htrA5′ (5′-GGTGGAGCTATCGCACTCG-3′) for the coding strand and htrA3′ (5′-GGTCACCAATAGTTAACTTGCTTG-3′) for the complementary strand. This fragment was cloned into EcoRV-cut pRV300 (nonreplicative in L. lactis [24]), resulting in pED716 (referred to as pRV300:htrA below) (Table 1), and was established in E. coli TG1 (14). Recombinant clones were screened on Luria-Bertani agar plates supplemented with 150 μg of erythromycin per ml at 37°C, and plasmid DNA was isolated as described previously (3). The insert orientation was determined by dye terminator DNA sequencing analysis (ABI PRISM BigDye terminators; Applied Biosystems), and pRV300:htrA was then established in L. lactis NZ9000 by electroporation as described previously (19). Clones were isolated on GM17 agar plates supplemented with 5 μg of erythromycin per ml at 30°C. As HtrA is essential for growth at high temperatures (39°C for L. lactis [33]), the thermosensitivity of the htrA mutants was used for primary screening. For this purpose, parallel cultures were grown in liquid medium (GM17 containing erythromycin) at 30 and 39°C. L. lactis NZ9000 and htrA-IL1403 were used as positive and negative controls, respectively. After overnight growth, 30 of 35 clones did not grow at 39°C (data not shown). Inactivation of htrA in the 30 thermosensitive clones was confirmed by PCR amplification by using genomic DNA as the template, in which one primer hybridized to the integrated plasmid and the other primer hybridized to a region outside the htrA fragment used for inactivation. The oligonucleotides used were A1 (5′-GGATGGCAAAAGCTAATATAGG-3′), A2 (5′-GGATTTGCTGTGGCTGATTTACC-3′), AE1 (5′-GGATATTCAACAGTTTCAATTCCC-3′), and AE2 (5′-GGTTTACTTTGGCGTGTTTCATTG-3′).
Production of unprocessed Nuc in L. lactis htrA-NZ9000.
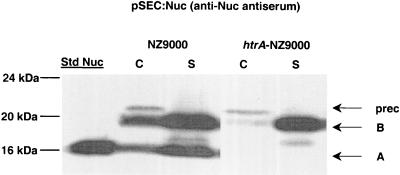
Nuc precursor (preNuc) is synthesized as a preproprotein. During secretion, preNuc matures into NucB proprotein. NucB subsequently matures into NucA by cleavage of a 21-amino-acid propeptide in L. lactis (22). Poquet et al. previously showed that NucB does not mature into NucA in L. lactis htrA-IL1403 and concluded that HtrA is responsible for NucB-to-NucA processing (33). To confirm the htrA-NZ9000 phenotype, production and maturation of NucB were examined by using plasmid pSEC:Nuc (Table 1) (11). This vector contains sequences encoding the PnisA promoter (7), the ribosome-binding site (RBSUsp45) and the signal peptide (SPUsp45) of lactococcal protein Usp45 (38), and NucB proprotein. pSEC:Nuc allowed high-level expression of Nuc due to the use of PnisA, in contrast to previous findings (Nuc was produced in strain htrA-IL403 at a relatively low level of expression due to the use of the staphylococcal native promoter and ribosome-binding site [33]). pSEC:Nuc was introduced into htrA-NZ9000. Transformants were plated on brain heart infusion agar plates containing antibiotics (5 μg of erythromycin per ml, 10 μg of chloramphenicol per ml) and nisin (1 ng per ml), incubated at 30°C overnight, and then subjected to the Nuc activity assay as described previously (21). All clones displayed a Nuc+ phenotype, confirming that Nuc was efficiently produced and secreted in L. lactis htrA-NZ9000 (21, 32). Nuc production was further analyzed by Western blotting (22, 36) by using L. lactis NZ9000(pSEC:Nuc) as a positive control (Fig. 1). Overnight cultures of both NZ9000 and htrA-NZ9000 containing pSEC:Nuc were inoculated into fresh medium at a 1:50 dilution. After 3 h of incubation (corresponding to an optical density at 600 nm of ∼0.4), cultures were induced by nisin added at a final concentration of 1 ng/ml. After 1 h of induction, protein samples were prepared as previously described and analyzed by Western blotting by using anti-Nuc antiserum (22). The cell fraction of NZ9000(pSEC:Nuc) contained three Nuc forms (preNuc, NucB, and NucA), and the supernatant contained two major forms (NucB and NucA) and a faint band, which might have resulted from export of a C-terminal degradation product generated by cytoplasmic proteases. In contrast, the htrA-NZ9000 extracts contained no NucA in either cell or supernatant fractions, thus showing the lack of propeptide cleavage in this strain. The overall amounts of Nuc forms detected were higher in the wt strain than in the htrA mutant. Nevertheless, the yields of mature NucB were highest in htrA supernatant fractions. These results show that the combination of htrA inactivation and the NICE system allows high-level production of unprocessed NucB.
FIG. 1.
Nuc production in the NZ9000 and htrA-NZ9000 strains. Protein extracts of induced exponential-phase cultures (1 ng of nisin per ml) of the L. lactis NZ9000(pSEC:Nuc) and htrA-NZ9000(pSEC:Nuc) strains were prepared from cell (lanes C) and supernatant (lanes S) fractions and were analyzed by Western blotting by using anti-Nuc antiserum. The migration positions of precursor forms (prec) and mature forms of both NucA (A) and NucB (B) are indicated by arrows. Commercial S. aureus NucA (25 ng) was used as the standard (lane Std Nuc), and molecular masses are indicated on the left.
Stable production of heterologous proteins in L. lactis htrA-NZ9000.
The heterologous proteins studied were Lip, NSP4, E7, and L7/L12. We examined production of the native and hybrid proteins (Table 1) in both strain NZ9000 and strain htrA-NZ9000. Protein samples were prepared from cell and supernatant fractions of induced cultures (1 ng of nisin per ml, 1 h of induction). Western blot analyses were performed by using appropriate antibodies. Production yields were compared, and quantification was performed when the appropriate standard was available.
(i) S. hyicus Lip.
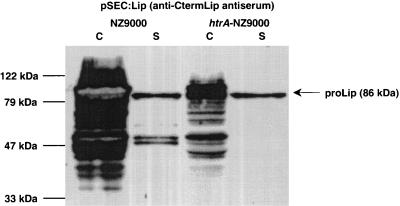
S. hyicus Lip has been well characterized and has potential applications in industry and medicine (10). Lip is reportedly secreted into the L. lactis extracellular medium in very low quantities (10) and is subject to proteolysis by HtrA during or after membrane translocation (33). Plasmid pSEC:Lip (previously called pJIM2093 [10]) was introduced into NZ9000 and htrA-NZ9000 and analyzed by Western blotting by using antiserum directed against the Lip C terminus (10). Considerable Lip degradation was observed in the NZ9000 cell fraction, as previously reported (10). In the supernatant fraction, one major band (86 kDa), corresponding to pro-Lip, plus several degradation products were detected (Fig. 2). In sharp contrast, Lip production was stabilized in the htrA-NZ9000 strain. The amounts of degradation products in the cell fraction were markedly reduced. In the supernatant fraction, a single band corresponding to pro-Lip (86 kDa) was identified (Fig. 2). The overall amounts of Lip detected were lower in the htrA mutant; however, the yields of intact secreted Lip in the supernatant were higher in the mutant strain.
FIG. 2.
S. hyicus lipase (Lip) production in the NZ9000 and htrA-NZ9000 strains. Protein extracts of induced exponential-phase cultures (1 ng of nisin ml) of the L. lactis NZ9000(pSEC:Lip) and htrA-NZ9000(pSEC:Lip) strains were prepared from cell (lanes C) and supernatant (lanes S) fractions and were analyzed by Western blotting by using antiserum directed against the Lip C terminus. The arrow indicates the position of prolipase (proLip) (86 kDa) in the cell fractions and the position of degradation products in the supernatant fractions. Molecular masses are indicated on the left.
These results confirm that degradation of Lip is due to HtrA activity (33). This hypothesis is compatible with the observation that Lip degradation occurred after translocation across the bacterial membrane (10). In conclusion, these results show that strain htrA-NZ9000 is capable of secreting and stabilizing full-size S. hyicus Lip even during high-level production.
(ii) Bovine rotavirus NSP4.
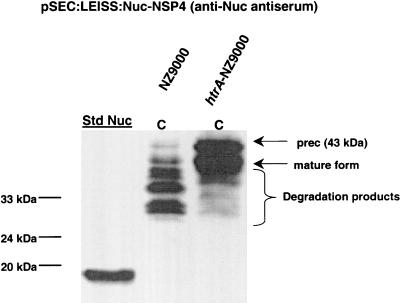
Rotavirus is the major etiologic agent of severe diarrhea in infants and young children around the world (1). Its nonstructural protein, NSP4, was previously produced in L. lactis in fusion with SPUsp45 and was detected only in the cell fraction (11). In this context, two degradation products were generated in addition to the precursor NSP4 and mature NSP4 forms (11). In this study, the precursor and mature forms were also found only in the cell fraction (data not shown) in both NZ9000 strains, but in contrast to the results obtained with a wt strain (11), the quantity of degradation products was reduced in the htrA-NZ9000(pSEC:NSP4) strain (data not shown). Since NSP4 degradation is reduced in the htrA context, we suppose that the cell-associated NSP4 mature form must be exposed to the outer surface. Trying to improve NSP4 export, we designed a fusion comprising SPUsp45, the synthetic propeptide secretion enhancer LEISSTCDA (referred to as LEISS below) (22), and the mature part of Nuc followed by NSP4 (Table 1). The resulting plasmid, pSEC:LEISS:Nuc-NSP4, was introduced into strains NZ9000 and htrA-NZ9000. LEISS:Nuc-NSP4 production was analyzed by using anti-Nuc antiserum (Fig. 3). In NZ9000(pSEC:LEISS:Nuc-NSP4), numerous degradation products were found in the cell fraction (Fig. 3). In contrast, production in htrA-NZ9000(pSEC:LEISS:Nuc-NSP4) resulted in significant protein stabilization in the cell fraction, and only minor degradation products appeared (Fig. 3). These results show that htrA-NZ9000 also stabilizes the heterologous LEISS:Nuc-NSP4 fusion. In contrast to the amounts of the two previous proteins, Nuc and Lip, the amounts of LEISS:Nuc-NSP4 are equal in the wt and htrA strains.
FIG. 3.
LEISS:Nuc-NSP4 production in the NZ9000 and htrA-NZ9000 strains. LEISS:Nuc-NSP4 production was analyzed by using anti-Nuc antiserum. Protein extracts of induced exponential-phase cultures (1 ng of nisin per ml) of the L. lactis NZ9000(pSEC:LEISS:Nuc-NSP4) and htrA-NZ9000(pSEC:LEISS:Nuc-NSP4) strains were prepared from cell (lanes C) and supernatant (lanes S) fractions. The arrows and brace indicate the positions of several degradation forms, the precursor preLEISS:Nuc-NSP4 [prec (43 kDa)], LEISS:Nuc-NSP4 (mature form) (40 kDa), and degradation products. Commercial S. aureus NucA was used as the standard (lane Std Nuc), and molecular masses are indicated on the left.
Supernatant fractions were devoid of NSP4 regardless of the strain or construction tested and the antiserum (anti-Nuc or anti-NSP4) used for detection (data not shown). As previously reported (11) and confirmed here, native NSP4 is very poorly secreted. Nevertheless, our results show that the LEISS:Nuc-NSP4 precursor is processed. Furthermore, a positive Nuc activity assay (21) also suggests that the location of Nuc-NSP4 is extracytoplasmic (data not shown). The mature form of NSP4 may be associated with the cell surface because of hydrophobic domains that prevent its release into the medium. We consider it likely that both the NSP4 and Nuc-NSP4 proteins are exported but remain cell surface associated. Taken together, the results described above show that the htrA-NZ9000 strain is an improved host for expression of heterologous proteins, conferring high protein stability even for proteins that are poorly secreted, such as NSP4.
(iii) Human papillomavirus E7.
E7 is a promising antigen candidate for development of new vaccines against cervical cancer (16, 27). Nevertheless, E7 is a labile protein (34), and this feature could be a limiting step in recombinant vaccine production (16). We recently produced E7 in L. lactis and found that a Nuc-E7 fusion resulted in higher production yields but lower secretion efficiency (the proportion of mature Nuc-E7 detected in the supernatant was ∼10%, compared to ∼95% for native Nuc [2]). However, the E7 moiety was still subject to proteolysis in a wt strain (2). Here, we tested whether secretion of Nuc-E7, as well as native E7, could be optimized in htrA-NZ9000.
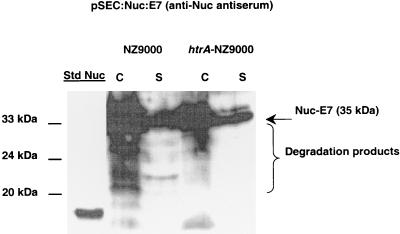
Nuc-E7 production from pSEC:Nuc-E7 (Table 1) was examined in NZ9000 and htrA-NZ9000 by using anti-Nuc antiserum (Fig. 4). In the NZ9000(pSEC:Nuc-E7) cell fraction, degradation products were detected in addition to expected precursor and mature protein forms. Similarly, the supernatant contained mature Nuc-E7 plus degradation products when anti-Nuc antiserum was used. In contrast, the htrA-NZ9000(pSEC:Nuc-E7) strain gave rise to bands corresponding to only precursor and mature forms (Fig. 4). Thus, stable production of Nuc-E7 can be achieved in this system.
FIG. 4.
Analysis of Nuc-E7 production in the NZ9000 and htrA-NZ9000 strains by using anti-Nuc antiserum. Protein extracts of induced cultures (1 ng of nisin per ml) of the L. lactis NZ9000(pSEC:Nuc-E7) and htrA-NZ9000(pSEC:Nuc-E7) strains were prepared from cell (lanes C) and supernatant (lanes S) fractions. The arrow and brace indicate the positions of Nuc-E7 (35 kDa) and degradation products. Commercial S. aureus NucA was used as the standard (lane Std Nuc), and molecular masses are indicated on the left.
Secretion of native E7 (produced from pSEC:E7 [Table 1]) by NZ9000 and htrA-NZ9000 was also examined by using anti-E7 antiserum. With both strains, E7 was efficiently secreted into the medium (secretion efficiency, ∼95%), and the total E7 production levels were equivalent, suggesting that native secreted E7 is not subject to HtrA degradation when it is efficiently released into the supernatant.
These results show that (i) stable Nuc-E7 is produced at a high level in the htrA-NZ9000 strain and is present in both cell and supernatant fractions and (ii) HtrA does not degrade native E7 but degrades Nuc-E7 hybrid protein, which is cell surface associated.
(iv) B. abortus immunodominant antigen L7/L12.
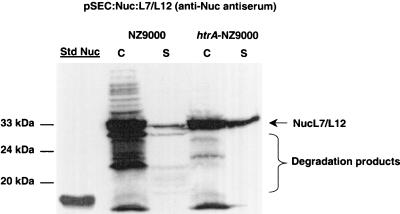
Brucellosis, a disease caused by infection with B. abortus, causes abortion and infertility in cattle (5). Ribosomal protein L7/L12 is an immunodominant antigen of B. abortus that elicits a cell-mediated immune response and confers protective immunity in mice (28, 29). It is thus a promising candidate for development of oral live vaccines against this worldwide zoonosis. Recent results obtained in our laboratory showed that the production yields of native L7/L12 were low in L. lactis (35). Although better yields were obtained when L7/L12 was fused to Nuc, the Nuc-L7/L12 fusion was subject to drastic proteolysis in a wt strain (Fig. 5). We used the previously described plasmid pSEC:Nuc-L7/L12 (Table 1) (35) to analyze Nuc-L7/L12 production in NZ9000 and htrA-NZ9000 by Western blotting using anti-Nuc antiserum (Fig. 5). Nuc-L7/L12 precursor matured in NZ9000(pSEC:Nuc-L7/L12), indicating that a normally cytoplasmic protein can be exported. However, several degradation-size products were detected in both cell and supernatant fractions, and there were very low quantities of protein in the supernatant (Fig. 5). In htrA-NZ9000(pSEC:Nuc-L7/L12), Nuc-L7/L12 was stabilized in both cell and supernatant fractions (Fig. 5). Notably, about 10-fold more Nuc-L7/L12 was detected in the supernatant fraction of htrA-NZ9000 than in the supernatant fraction of NZ9000 (Fig. 5). These results demonstrate that the htrA-NZ9000 production strain can increase both the stability and the production yield of a hybrid protein containing a cytoplasmic moiety.
FIG. 5.
Nuc-L7/L12 production in the NZ9000 and htrA-NZ9000 strains. Protein extracts of induced cultures (1 ng of nisin per ml) of the L. lactis NZ9000(pSEC:Nuc-L7/L12) and htrA-NZ9000(pSEC:Nuc-L7/L12) strains were prepared from cell (lanes C) and supernatant (lanes S) fractions and were analyzed by Western blotting by using anti-Nuc antiserum. The arrow and brace indicate the positions of mature Nuc-L7/L12 and a smear of degradation products. Commercial S. aureus NucA was used as the standard (lane Std Nuc), and molecular masses are indicated on the left.
In summary, our results demonstrate that the combination of htrA inactivation and high-level inducible expression via the NICE system (7) leads to efficient inducible production of several heterologous proteins of medical and technological interest and can stabilize heterologous proteins that were initially degraded in a wt strain. Our results suggest that proteins that are poorly released from the cell surface are more susceptible to protein degradation than proteins that are efficiently secreted. For instance, NSP4, which remains cell associated, is subject to degradation in the wt strain. In contrast, native E7 is not degraded in the wt strain for two possible reasons: either (i) it is rapidly released and may escape HtrA-mediated degradation or (ii) it is not a substrate for HtrA. The htrA-NZ9000 strain may have particular applications in the stabilization of cell wall-anchored proteins; this possibility is currently being tested (Y. Dieye and J. C. Piard, personal communication). Finally, fusion of two well-secreted proteins (as exemplified here by Nuc and E7) can result in a hybrid protein that is poorly secreted (Nuc-E7 fusion) (2), suggesting that problems in protein folding result in protein degradation. The different constructions used in this study, together with our new production strain, htrA-NZ9000, should be valuable tools for identifying which factors other than HtrA are important for protein stability and efficient release of proteins into the medium.
Acknowledgments
We are grateful to Sophie Drouault, Vincent Enouf, and Luciana Ribeiro, who kindly gave us the plasmid materials used in this work. We thank Astrid Vrang (Biotechnological Institute, Denmark), who participated in preliminary experiments with Lip. We also thank Sébastien Nouaille, Jean-Jacques Gratadoux, Yakhya Dieye, and Candice Rigoulay for scientific discussions and friendly support.
This research was supported by grants from COFECUB (Comité Français d'Etudes et de Coopération Universitaire avec le Brésil) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil).
REFERENCES
- 1.Ball, J. M., P. Tian, C. Q. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101-104. [DOI] [PubMed] [Google Scholar]
- 2.Bermúdez-Humarán, L. G., P. Langella, A. Gruss, R. Montes de Oca-Luna, and Y. Le Loir. 2002. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl. Environ. Microbiol. 68:917-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnboim, H., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boschiroli, M. L., V. Foulongne, and D. O' Callaghan. 2001. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4:58-64. [DOI] [PubMed] [Google Scholar]
- 6.Chatel, J. M., P. Langella, K. Adel-Patient, J. Commissaire, J. M. Wal, and G. Corthier. 2001. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin. Diagn. Lab. Immunol. 8:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vos, W. M. 1999. Gene expression systems for lactic acid bacteria. Curr. Opin. Microbiol. 2:289-295. [DOI] [PubMed] [Google Scholar]
- 9.Dieye, Y., S. Usai, F. Clier, A. Gruss, and J. C. Piard. 2001. Design of a protein targeting system for lactic acid bacteria. J. Bacteriol. 183:4157-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drouault, S., G. Corthier, S. D. Ehrlich, and P. Renault. 2000. Expression of the Staphylococcus hyicus lipase in Lactococcus lactis. Appl. Environ. Microbiol. 66:588-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enouf, V., P. Langella, J. Commissaire, J. Cohen, and G. Corthier. 2001. Bovine rotavirus nonstructural protein 4 produced by Lactococcus lactis is antigenic and immunogenic. Appl. Environ. Microbiol. 67:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaeng, S., S. Scherer, H. Neve, and M. J. Loessner. 2000. Gene cloning and expression of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 66:2951-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic acid streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson, T. J. 1984. Ph.D. thesis. University of Cambridge, Cambridge, England.
- 15.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 16.Jabbar, I. A., G. J. Fernando, N. Saunders, A. Aldovini, R. Young, K. Malcolm, and I. H. Frazer. 2000. Immune responses induced by BCG recombinant for human papillomavirus L1 and E7 proteins. Vaccine 22:2444-2453. [DOI] [PubMed] [Google Scholar]
- 17.Kleerebezem, M., M. M. Beerthuyzen, E. E. Vaughan, W. M. de Vos, and O. P. Kuipers. 1997. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl. Environ. Microbiol. 63:4581-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuipers, O. P., P. G. de Ruyter, M. Kleerebezen, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 19.Langella, P., Y. Le Loir, S. D. Ehrlich, and A. Gruss. 1993. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J. Bacteriol. 175:5806-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langella, P., and Y. Le Loir. 1999. Heterologous protein secretion in Lactococcus lactis: a novel antigen delivery system. Braz. J. Med. Biol. Res. 32:191-198. [DOI] [PubMed] [Google Scholar]
- 21.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1994. Direct screening of recombinants in gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J. Bacteriol. 176:5135-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1998. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J. Bacteriol. 180:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Loir, Y., S. Nouaille, J. Commissaire, L. Brétigny, A. Gruss, and P. Langella. 2001. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Appl. Environ. Microbiol. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leloup, L., S. D. Ehrlich, M. Zagorec, and F. Morel-Deville. 1997. Single-crossover integration in the Lactobacillus sake chromosome and insertional inactivation of the ptsI and lacL genes. Appl. Environ. Microbiol. 63:2117-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipinska, B., O. Fayet, L. Baird, and C. Georgopoulos. 1989. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J. Bacteriol. 171:1574-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margot, P., and D. Karamata. 1996. The wprA gene of Bacillus subtilis 168, expressed during exponential growth, encodes a cell-wall-associated protease. Microbiology 142:3437-3444. [DOI] [PubMed] [Google Scholar]
- 27.Medaglini, D., C. M. Rush, P. Sestini, and G. Pozzi. 1997. Commensal bacteria as vector for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 15:1330-1337. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira, S. C., and G. A. Splitter. 1994. Subcloning and expression of the Brucella abortus L7/L12 ribosomal gene and T-lymphocyte recognition of the recombinant protein. Infect. Immun. 62:5201-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira, S. C., J. S. Harms, M. Banai, and G. A. Splitter. 1996. Recombinant Brucella abortus proteins that induce proliferation and gamma-interferon secretion by CD4+ T cells from Brucella-vaccinated mice and delayed-type hypersensitivity in sensitized guinea pigs. Cell. Immunol. 172:262-268. [DOI] [PubMed] [Google Scholar]
- 30.Pallen, M. J., and B. W. Wren. 1997. The HtrA family of serine proteases. Mol. Microbiol. 26:209-221. [DOI] [PubMed] [Google Scholar]
- 31.Piard, J. C., R. Jimenez-Diaz, S. D. Ehrlich, V. A. Fischetti, and A. Gruss. 1997. The M6 protein of Streptococcus pyogenes and its potential as a tool to anchor biologically active molecules at the surface of lactic acid bacteria. Adv. Exp. Med. Biol. 418:545-550. [DOI] [PubMed] [Google Scholar]
- 31a.Poquet, I., A. Bolotin, and A. Gruss. 2001. Optimisation de la production de protéines hétérologues exportées chez Lactococcus lactis par inactivation de HtrA, son unique protéase de ménage de surface. Lait 81:37-47. [Google Scholar]
- 32.Poquet, I., S. D. Ehrlich, and A. Gruss. 1998. An export-specific reporter designed for gram-positive bacteria: application to Lactococcus lactis. J. Bacteriol. 180:1904-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poquet, I., V. Saint, E. Seznec, N. Simoes, A. Bolotin, and A. Gruss. 2000. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 35:1042-1051. [DOI] [PubMed] [Google Scholar]
- 34.Reinstein, E., M. Scheffner, M. Oren, A. Ciechanover, and A. Schwartz. 2000. Degradation of the E7 human papillomavirus oncoprotein by the ubiquitin-proteasome system: targeting via ubiquitination of the N-terminal residue. Oncogene 19:5944-5950. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro, L. A., V. Azevedo, Y. Le Loir, S. C. Oliveira, Y. Dieye, J. C. Piard, A. Gruss, and P. Langella. 2002. Production and targeting of the Brucella abortus antigen L7/L12 by Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 68:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Simonen, M., and I. Palva. 1993. Protein secretion in Bacillus subtilis. Microbiol. Rev. 57:109-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Asseldonk, M., G. Rutten, M. Oteman, R. J. Siezen, W. M. de Vos, and G. Simons. 1990. Cloning, expression in Escherichia coli and characterization of usp45, a gene encoding a highly secreted protein from Lactococcus lactis MG1363. Gene 95:155-160. [DOI] [PubMed] [Google Scholar]
- 39.van Oort, M. G., A. M. Deveer, R. Dijkman, M. L. Tjeenk, H. M. Verheij, G. H. de Haas, E. Wenzig, and F. Gotz. 1989. Purification and substrate specificity of Staphylococcus hyicus lipase. Biochemistry 28:9278-9285. [DOI] [PubMed] [Google Scholar]
- 40.Wells, J. M., P. W. Wilson, P. M. Norton, M. J. Gasson, and R. W. Le Page. 1993. Lactococcus lactis: high-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol. Microbiol. 8:1155-1162. [DOI] [PubMed] [Google Scholar]