Summary
Growing evidence suggests that reactive astrocytes can acquire different functional subtypes, playing critical roles in neurological disorders. Human induced pluripotent stem cell (hiPSC)-derived three-dimensional (3D) CNS models have been proposed to study reactive astrocytes. Still, lack of reproducibility and immature/activated astrocyte phenotypes typical of these models limit their utility to address neuroinflammation. Here, we establish a robust hiPSC-derived 3D neuroinflammation model, exploring neurospheroid (iNSpheroid) differentiation in perfusion stirred-tank bioreactors to obtain neurons and glia co-cultures. iNSpheroids were challenged with TNF-α, IL-α, and C1q (TIC) cocktail. Transcriptome analysis revealed the upregulation of inflammatory modulators (e.g., CCL2 and TNAIP3) associated with TNF and NF-kB signaling. Secretome analysis showed increased secretion of inflammation-related cytokines (e.g., CCL2 and CXCL8) in TIC-stimulated iNSpheroids. Astrocytes displayed an impaired capacity for glutamate-glutamine recycling compared to the unstimulated control, indicating functional impairment. Together, these results demonstrate that astrocytes within iNSpheroids are functional and recapitulate canonical astrogliosis events, hallmarks of neuroinflammation.
Subject areas: Biological sciences, Neuroscience, Cell biology, Stem cells research, Sequence analysis, Omics
Graphical abstract

Highlights
-
•
Scalable and homogeneous 3D iNSpheroid model containing functional astrocytes
-
•
Astrocytes display transcriptional, proteomic, and functional responses to TIC-stimuli
-
•
TNF and NF-κB activation trigger the secretion of key immunomodulators such as CXCL8
-
•
Impaired glutamate uptake and glutamine secretion under inflammatory conditions
Biological sciences; Neuroscience; Cell biology; Stem cells research; Sequence analysis; Omics
Introduction
Astrocytes play crucial roles in central nervous system (CNS) homeostasis, providing metabolic support to neurons, regulating connectivity of neural circuits, controlling blood flow as an integral part of the blood-brain barrier, and playing an essential role in CNS innate immunity.1,2 In pathological contexts such as trauma, infection, and neurodegenerative diseases, astrocytes undergo reactive astrogliosis, which comprises morphological, phenotypical, and functional changes.3 Reactive astrogliosis can contribute to the restoration of CNS homeostasis or limit its recovery, leading to neuronal death and chronic neuroinflammation (Reviewed in4). The outcome of reactive astrogliosis varies depending on a multitude of factors, including the type and duration of the insult, the degree of functional impairment, and the relative abundance of different astrocyte subpopulations. The molecular mechanisms that trigger and sustain the different reactive astrogliosis states, and how differentially activated astrocytes modulate the surrounding microenvironment, remain largely unknown, particularly in a human context. Recent reports defined a neurotoxic reactive astrocyte subtype, which has a potent neurotoxic effect on neurons and oligodendrocytes.5 These astrocytes are induced by interleukin-1 alpha (IL-1α), tumor necrosis factor alpha (TNF-α), and the classical complement component, C1q (TIC combination), all secreted by microglia.5 This 3-factor combination was necessary and sufficient to induce neuroinflammation in rodent models and in two-dimensional (2D) cultures of human astrocytes.5,6,7,8 The TIC-induce reactive astrocyte subtype is characterized by the activation of specific signaling cascades associated with inflammation, such as the IL-6, TNF-α, CXCL10 pathways, leading to the loss of critical physiological functions (e.g., glutamate recycling).9 Studying human astrocytes becomes particularly critical since they differ from mouse astrocytes in morphological complexity, transcriptomic profile, functional diversity, and reactivity.10,11,12,13 Protocols to differentiate astrocytes from neural progenitor cells (NPC)-derived from human-induced pluripotent stem cells (hiPSC-NPCs) use a cocktail of patterning agents to mimic embryonic development,14,15,16,17,18 overexpression of transcription factors19,20 or use serum-based media.15,21 However, most of these methods do not account for tissue context and maintain astrocytes in monocultures and/or on extracellular matrices that do not recapitulate the soft brain extracellular matrix. Co-cultures of hiPSC-derived neurons and astrocytes in 3D settings have been proposed to study astrocyte reactivity within human neuronal networks22; however, these co-culture strategies fail to recapitulate cell-to-cell interactions during differentiation, which are crucial for cell patterning.23 As an alternative, we and others have shown that three-dimensional (3D) differentiation of hiPSCs and hNPCs recapitulates neurodevelopmental pathways and interactions between the different neural lineages, depicting features of the complex CNS microenvironment.24,25,26,27,28 While cerebral organoids can comprise additional lineages and, to some extent, simulate regional patterning, they are highly heterogeneous, enriched in immature cell populations, and contain heterologous ECM components.29 By differentiating hiPSC-NPCs in 3D, in perfusion stirred-tank bioreactors (STBs), we obtained consistent differentiation restricted to the three neural lineages. We have shown that these differentiated 3D cultures, named hiPSC-derived neurospheroids (iNSpheroids) are composed of neurons, astrocytes, oligodendrocytes, embedded in autologous ECM secreted by the cells in culture.30 These iNSpheroids can recapitulate pathological hallmarks of a neuroinflammatory lysosomal storage disease.27
We hypothesized that iNSpheroids could be employed as a human cell model of neuroinflammation and recapitulate the main functions of astrocytes in a complex 3D CNS microenvironment. We employed transcriptome, secretome, and functional analysis to depict the inflammatory response of iNSpheroids to the TIC prototypical stimulus. We demonstrate that the challenged human iNSpheroids modulated inflammatory gene cascades, activating the secretion of cytokines and chemokines, and leading to the impairment of astrocytic functions. This study highlights the human 3D iNSpheroid model as a throughput platform to better understand neuroinflammation under different inflammatory stimuli and diseases. Furthermore, employing this model can help shed light on the role of astrocyte-mediated inflammation in a complex CNS heterotypic model, enabling the study of microenvironment remodeling, resourcing to a multi-level analysis of biological networks. Additionally, this model may be used to study disease-mediated inflammation and develop novel therapeutic strategies for targeting astrocytes.
Results
Gene and protein profiling of the iNSpheroids reveal typical human astrocytic markers
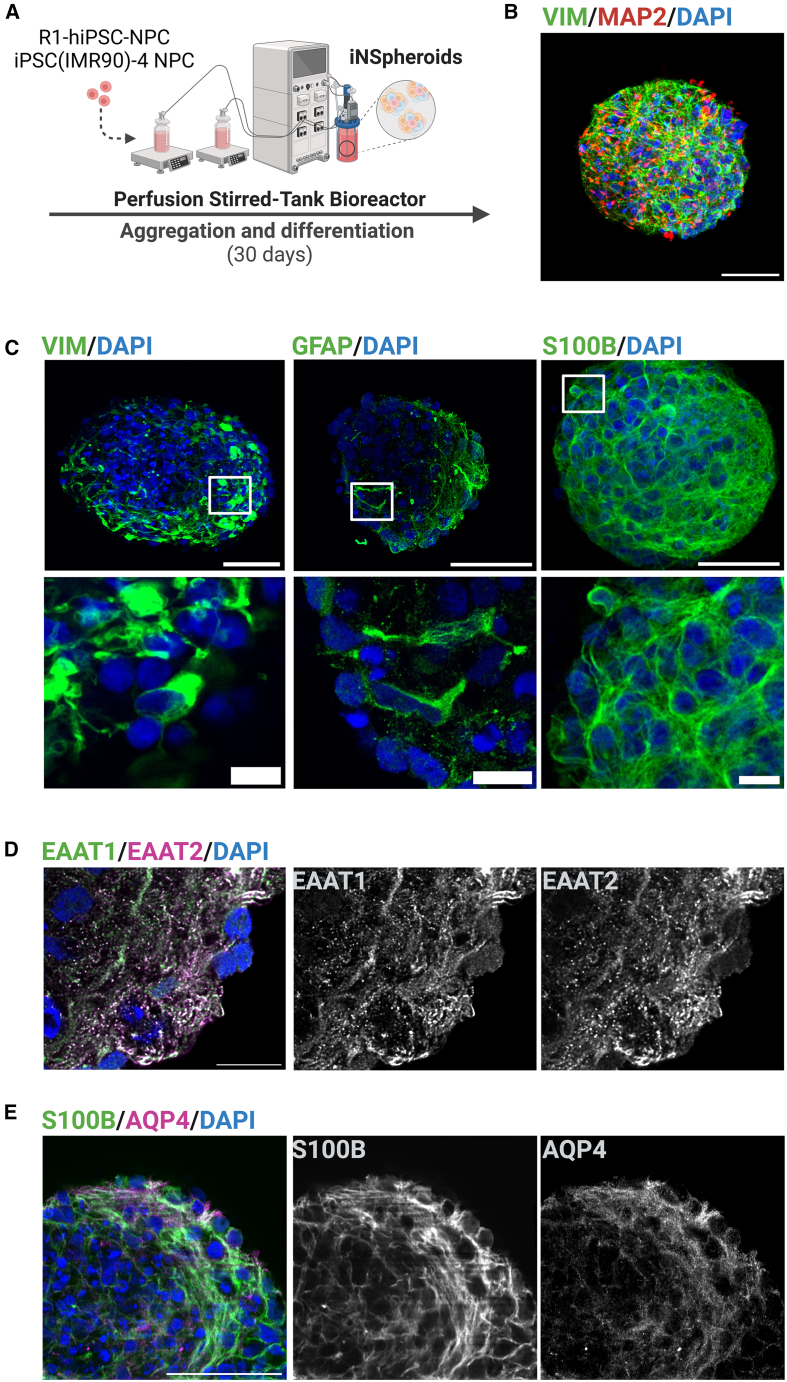
We applied a 3D culture strategy previously established by our group for the differentiation of hiPSC-NPCs into neurons and glial cells,27,30,31 using two hiPSC lines, R1-hiPSC130 and iPSC (IMR90)-4 cell line (Figure 1A). Briefly, hiPSC-NPCs were inoculated as a single cell suspension in stirred-tank bioreactors operated under perfusion and aggregated for 7 days, in the presence of epidermal growth factor (EGF) and basic fibroblast growth factor (FGF) (Figure S1A). From day 7 onwards, iNSpheroid differentiation was induced by replacing the growth factors with neurotrophic factors (Figure S1A). Differentiated iNSpheroids, harvested on day 30, showed high viability and typical differentiation into neurons and glia (Figures 1B and S1A–S1C). The neuronal network within the 3D iNSpheroid structure was identified by the expression of the somatodendritic protein MAP2, which was juxtaposed with the expression of the presynaptic vesicular protein synaptophysin (SYN) (Figure S1B), concomitant with the higher expression of neuron-specific genes, GAD1, VGLUT1, and SYN2 (Figure S1C). hiPSC-derived astrocytes within iNSpheroids revealed the expression of typical proteins, such as GFAP (glial fibrillary acidic protein) and VIM (vimentin) (Figure 1C), but also mature astrocyte markers, glutamate receptors, EAAT1 and EAAT2 (Excitatory amino acid transporter 1 and 2, respectively) (Figure 1D), S100B (S100 calcium-binding protein B) and AQP4 (aquaporin-4) (Figure 1C–1E). S100B and AQP4 were also significantly increased at the gene expression level by day 30 of culture (Figure S1C).
Figure 1.
Immunofluorescence profiling of hiPSC-derived astrocytes within in the iNSpheroids to confirm the expression of canonical markers
(A) Schematic representation of the 3D differentiation protocol. NPC generated from two hiPSC lines (iPS(IMR90)-4 and R1-hiPSC1) were aggregated into neurospheres and differentiated in stirred-tank bioreactors for 30 days. Immunofluorescence image of hiPSC-derived astrocytes (B) (vimentin, VIM, green) and neurons (microtubule-associated protein 2, MAP2, red), (C) astrocyte identity structural markers proteins VIM, glial fibrillary acidic protein (GFAP) and S100 calcium-binding protein B (S100B) in green, and functional markers, (D) EAAT1 (excitatory amino acid transporter 1, green and white) and EAAT2 (excitatory amino acid transporter 1, magenta and white) and (E) AQP4 (aquaporin-4, magenta and white). Cell nuclei were counterstained with DAPI (blue). Scale bar, 50 μm (B, C upper panel and E), 10 μm (C lower panel and D). Magnification 40X and zoom-in 2X. Representation of maximum intensity projection of 10 z-stacks, with the zoom-in showing a single z-optical slice. See also: Figure S1 and Table S2. Representative images of 3 or more iNSpheroids from iPS (IMR90)-4 cell line (n = 3).
Human induced pluripotent stem cell-derived astrocytes within the 3D iNSpheroids respond to a defined inflammatory stimulus
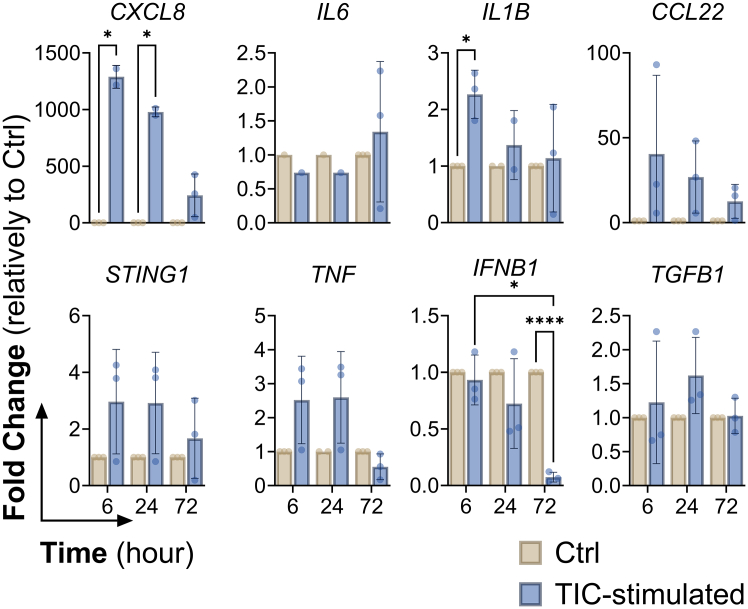
Next, we addressed astrocyte maturity within the 3D iNSpheroids, after 30 days of culture, and their ability to respond to inflammatory stimuli (Figure 2A). Transcriptomic analysis of iNSpheroids on day 30 of differentiation revealed astrocytic transcriptional signatures (Figures 2B–2D). The dataset obtained from the different independent experiments revealed good correlation and low inter-sample variability, as demonstrated by the Pearson correlation coefficient (PCC) higher than 0.95 (Figure S2A). Based on previously published astrocytic gene annotations,6,32 we identified the expression of several genes related to astrocyte identity (e.g., VIM, S100B and ALDOC), glutamate function (namely, GLUL, GLUD1, SLC1A3, SLC1A2) (Figure 2B), and APOE- and lipid metabolism known to be enriched in the brain and mainly expressed in astrocytes (e.g., APOE, and LRP1) (Figure 2C). Based on the classification used in previously published datasets,6,33 we could also identify a set of genes related to both immature (Figure 2D, upper panel) and mature astrocyte phenotypes (Figure 2D, lower panel). Several of these genes were also identified at the proteome level, by whole proteome analysis, namely GLUL, SLC1A2, SLC1A3, S100B, ALDOC, and APOE (Table S2), further supporting the presence of mature astrocytes within the iNSpheroids. After 30 days of iNSpheroids differentiation, neuroinflammation was induced by exposing the cultures to TIC, for 3 days. Cell viability and aggregate diameter were maintained in TIC-stimulated cultures compared to unstimulated control (Figures S2B and S2C). Bulk RNA sequencing of iNSpheroids after 1 day of TIC-stimulation did not reveal significant differences in gene expression profile. However, after 3 days of TIC-stimulation, an upregulation of reactive astrocytes-associated genes, namely GFAP and VCAM1 (NF-kB dependent gene), as well as cytokines and chemokines (CXCL5, IL32, CCL2, and CXCL6), and members of the TNF/NF-kB pathways (e.g., TNAIP3, UBD, CEBPD, and NFKB2), in comparison to the unstimulated control (Figures 2E and S2D). Additionally, we validated the transcriptional modulation of genes associated with reactive astrocytes after 3-, 24-, and 72-h post-TIC stimuli by RT-qPCR (Figures S3A and S3B). Complement 3 (C3) and SERPINA3 were modulated in a time-dependent manner, being upregulated as soon as 3 h after stimulation. To address TNF and NF-kB pathway involvement in iNSpheroid inflammatory responses we evaluated the gene expression of effector genes of these pathways in a time-course manner (6-, 24-, and 72-h post-TIC stimulation, Figure 3). Short-term exposure (6 h) to the TIC combination resulted in the upregulation of chemokine-coding genes (CXCL8 and CCL22), as well as IL1B. However, this response was substituted by an overall decreased expression, after 72 h.
Figure 2.
Transcriptomic profiling of the iNSpheroids before (day 30) and after 3-day challenge with TNF-α, IL-1α, and C1q (TIC) stimulus
(A) Schematic representation of the experimental design. After 30 days of differentiation, iNSpheroids within stirred-tank bioreactors (STBs) were either collected for bulk transcriptomic analysis or used for studying TIC-induced inflammation. These neuroinflammatory stimuli were maintained for 3 days. Along this stimulation period, cells were harvested at different timepoints (3, 6-, 24-, 48-, and 72-h post-stimuli) for transcriptome analysis, either by bulk RNAseq or RT-qPCR. RNA-Seq expression levels in transcripts per million (TPM) of (B) pan-astrocyte, (C) APOE metabolism-related, (D) immature and mature astrocytic genes at day 30 (day 0 of TIC challenge). Dots correspond to 4 independent experiments. Data represented as mean ± standard deviation (SD) (n = 4, R1-hiPSC1). Data points correspond to four independent experiments using the R1-hiPSC1 line. (E) Bar graph of significantly modulated genes between 0- and 72-h post-stimulation (total of 96 genes). The data shown represents four independent experiments with the R1-hiPSC1 line. Significantly modulated genes identified by performing Wald test to generate p-values and Log2 fold changes (Log2FC). Genes with an adjusted p-value < 0.05 and absolute Log2FC > 1 were called as differentially expressed genes for each comparison. Log2FC values were color coded from green (downregulation) to pink (upregulation). See also Figures S2A and S2D.
Figure 3.
TNF-α, IL-1α, and C1q (TIC) stimulus induces targeted transcriptomic modulation, especially after 6- and 24-h
Gene expression analysis of inflammatory-related genes. Control or TIC-stimulated iNSpheroids were analyzed using RT-qPCR. Data shown corresponds to three independent experiments with iPSC (IMR90)-4. Data is represented as mean ± SD, and asterisks indicate significant difference (∗p < 0.05, ∗∗∗∗p < 0.0001). One-way analysis of variance (ANOVA) followed by the Bonferroni or Tukey post hoc test was used.
TIC-challenged astrocytes undergo morphological and functional alterations, typical of activation
At the functional level, astrocytes acquire features of activation, such as morphofunctional alterations, the secretion of immunomodulatory proteins, and metabolic functions.34 Visual inspection suggested increased expression of intermediate filaments, namely GFAP and VIM, as well as morphological changes consistent with hypertrophy, including larger-appearing cell somas and thickened processes (Figures 4A and 4B).
Figure 4.
TIC-treated iNSpheroids present morphological alterations and higher secretion of inflammatory cytokines and chemokines, after 72-h of treatment
(A) Immunofluorescence images of 8 μm cryosections of iNSpheroids, with and without TIC stimulation after 72 h, demonstrating the expression of (A) VIM (gray) and (B) GFAP (gray) counterstained with DAPI (blue). Both VIM and GFAP morphology suggest the presence of hypertrophic astrocytes (enlarged soma and processes) emphasized by the zoom in images. Magnification 40X and scale bar = 50 and 10 μm (whole iNSpheroid image and zoom-in, respectively). Representative images of 3 or more iNSpheroids from iPS (IMR90)-4 cell line (n = 3). (C) Cytokine array membranes were used to analyze soluble proteins in the supernatant of control and TIC-stimulated iNSpheroids after 72 h of treatment. Each spot represents a cytokine for which the ratio of expression (arbitrary units) was obtained between the cytokine and the positive control present in the membrane. Graphically represented proteins correspond to the colored rectangles highlighted in the membranes (from left to right): TNF-α (yellow), CCL2 (blue), CXCL8 (green) and IL-1α (red). Data corresponds to the average mean pixel density of four independent experiments with R1-hiPSC1 cell line. (D) Quantitative analysis of IL-6, CCL5, CXCL10, CXCL8 and CCL2 concentration in culture media from control and TIC-stimulated iNSpheroids, at 3-, 24-, and 72-h post-stimulation. Data corresponds to six independent experiments using R1-hiPSC1 (n = 4) and iPSC (IMR90)-4 (n = 2) lines. Data is shown as mean ± SD, and asterisks indicate significant difference (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). Unpaired Student’s t test or two-way ANOVA followed by the two-stage linear step-up procedure of Benjamini–Hochberg method were used, depending on the number of groups under comparison.
At the secretome level, an antibody array was used to address the presence of a broad spectrum of cytokines and chemokines in the human iNSpheroids culture supernatant at 72 h post-TIC stimulation (Table S3; Figures 4C and 4D). The secretion of IL-1α, CXCL8, TNF-α, and CCL2 was significantly increased by the TIC stimulation, compared with the unstimulated control (Figure 4C). To quantitatively analyze the secreted proteins, these and other targets known to be modulated during neuroinflammation were assessed by ELISA (Figure 4D). For IL-6, CXCL10, CXCL8, and CCL2 the secretion increased after 24 h of treatment, in comparison with the control condition where it was barely detectable. CCL2 was the highest modulated cytokine with a fold increase of 6.0 and 7.7, respectively, for 24 and 72 h post TIC-stimuli.
The GLU-GLN shuttle has been reported as being impaired in several pathological conditions where reactive astrocytes are present.35,36 As an example, IL-1β and TNF-α, two proinflammatory cytokines that are typically elevated in neurodegenerative disease states, induce neuronal death and apoptosis in vitro by increasing intra- and extracellular GLU levels. To assess GLU uptake by the astrocytes, the iNSpheroids were cultured in a GLU-rich (3 mM) or GLN-free medium after 24 or 72 h of TIC stimulus. The culture supernatants were analyzed to assess GLU uptake throughout four days (Figures 5A and S4A). When iNSpheroids were exposed to the TIC combination for 24 h, there was a tendency for reduced GLU uptake by astrocytes (Figure S4B), which became significant when exposed to TIC for 72 h, showing a significant reduction during the 96 h of GLU exposure (Figure 5B). GLU consumption in the untreated control resulted in higher GLN secretion, whereas the lower GLU uptake in the TIC-stimulated condition led to a lower GLN secretion. (Figure 5C). We could confirm that the detected GLN was being produced by the hiPSC-astrocytes within the iNSpheroids through the GLU-GLN shuttle, by the addition of MSO, an irreversible inhibitor of GS37 (Figures S4C and S4D).
Figure 5.
Glutamate uptake is impaired in hiPSC-derived astrocytes, in the iNSpheroids exposed to TIC cocktail
(A) Schematic representation of the experimental procedure. After three days of TIC-stimulation, iNSpheroids were harvested, plated in PLOL-coated vessels, and cultured in GLU-rich (3 mM) and GLN-depleted medium. For the following 4 days, supernatant was recovered every 24-h to analyze GLU and GLN concentration.
(B and C) Concentration of GLU uptake and GLU secretion throughout the four days of culture. Data is represented as mean ± SD (n = 5, three independent experiments for R1-hiPSC1 line and two for iPSC (IMR90)-4 line), and asterisks indicate significant difference (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). See also Figure S3. The statistical test two-way ANOVA followed by the two-stage linear step-up procedure of the Benjamini–Hochberg method was used.
Discussion
hiPSC-derived CNS 3D cell models have become an essential complementary tool to animal models for understanding neuroinflammatory phenomena in the human CNS.32,38,39 In this study, we employed the human iNSpheroid model to demonstrate that hiPSC-derived astrocytes can respond to a prototypical inflammatory stimulus. Given that this bioprocess generates a high number of very homogeneous iNSpheroids with functional neurons and glia, a new avenue for drug screening and development opens by increasing the throughput needed to feed preclinical trials. Several reports have shown the effective differentiation of astrocytes in 2D and 3D,6,15,21,33 demonstrating their lineage commitment and maturation by pinpointing targets at the gene expression level. However, modeling the human brain in vitro involves a trade-off between approaching CNS complexity and robustness for downstream quantifiable readouts. For instance, postmortem tissue or primary neural cells may be used to study the brain with limited availability and low throughput. On the other end of the spectrum, hiPSC-derived 3D models offer unlimited and scalable processes preserving the genetic content of the donor. Here, we show the large production of homogeneous iNSpheroids containing astrocytes, that conserve the expression of several astrocytic gene and protein markers related to cell identity and function, namely GLUL, SLC1A2, SLC1A3, S100B, ALDOC, and APOE,40,41,42,43 identified by whole transcriptome and proteome analysis. Immunostaining for identity proteins, GFAP, VIM and S100B demonstrated a complex astrocytic network with a homogeneous distribution of glutamate receptors, EAAT1 and EAAT2, in the iNSpheroid structure. These results, together with the ability to perform glutamate clearance and glutamine secretion, demonstrate the potential of our co-differentiation process for obtaining mature astrocytes from different hiPSC lines.
A prototypical inflammatory cytokine cocktail shown to be secreted by LPS-stimulated microglia in a mouse model, and composed of TNF-α, IL-1α, and C1q, was used to induce neuroinflammation.5 This cocktail has been shown to promote several transcriptomic, morphological, and functional changes in astrocytes, acquiring a neurotoxic-astrocytic phenotype. Several genes have been reported as hallmarks of neuroinflammation, such as IL1B, IL6, and TNF, and for astrocyte activation, the increased expression of intermediate filaments GFAP and VIM, as well as C3 and SERPINA3.5,12,44,45,46,47 In our study, whole transcriptome analysis of the TIC-stimulated human iNSpheroids revealed modulation of several genes involved in TNF and NF-kB pathways (e.g., TNAIP3, CEBPD, and NFKB2), cytokine regulation (e.g., IL32 and CCL2) and astrocyte reactivity (e.g., GFAP) after 72 h of stimulation, in line with previous reports on 2D in vitro cultures7,36 and mice models.5
Targeted gene expression analysis was carried out to further understand the modulations occurring through the TNF and NF-κB pathway. A time-dependent modulation of inflammation-related genes, such as CXCL8, CCL22, TNF, and IL1B, and other genes associated with astrocyte pan-reactivity, such as SERPINA3 and C3, was observed. Under neuroinflammatory conditions, reactive astrocytes have a crucial role related to the recruitment of resident (microglia) and peripheral immune cells to the CNS, by secreting inflammatory mediators.48,49 Secretome of TIC-stimulated iNSpheroids revealed high secretion levels of TNF-α, IL-1α, CXCL8, CCL2, and CXCL10 after 72 h of stimulation. TNF-α and IL-1α were highly detected in the conditioned media from stimulated cells, which could be both an effect of the protein added from the TIC cocktail and/or cell secretion. The secretion of TNF-α, IL-1α, CXCL8, CCL2, and CXCL10 is in line with the activation of the TNF and NF-κB pathways observed in our transcriptomic data. CCL2 and CXCL8 are produced mainly by activated glial cells in the CNS, and their upregulation has been reported in several neurological diseases, facilitating transmigration of circulating immune cells, namely monocytes and leukocytes.50,51,52 IL-6 and CXCL10 concentrations in the TIC-stimulated condition increased in the first 24 h, which was expected for an acute inflammatory response. On the other hand, CCL5 levels were only observed as increased in the later timepoint (72-h post-TIC stimulation). The fact that CCL5 was not significantly secreted earlier, as the other targets, might be related to the tight control of this cytokine expression in the brain since it has a crucial role in several neuron-astrocyte functions, such as glutamate release.53
Astrocytes are essential for the formation, maintenance, and permeability of the blood-brain-barrier (BBB) by providing secreted factors that promote adequate association between the BBB cells and the formation of strong tight junctions (reviewed in54). It has been shown that the BBB disruption during neuroinflammation is largely associated with the release of numerous inflammatory mediators (e.g., IL-1, IL-6, TNF-α) by glial cells (reviewed in55,56,57). In our transcriptomics dataset, we found the gene for adhesion molecule VCAM-1 upregulated. This molecule has been reported to be expressed in astrocytes and has a crucial function in modulating BBB permeability for T cell entry into the CNS parenchyma, and is required for the manifestation of neurological disease.58,59
Altogether, the secretome profile supports the transcriptomic data demonstrating the activation of inflammatory pathways responsible for the induction of cytokine and chemokine secretion that will regulate the activation and recruitment of circulating immune cells,60,61,62 being potential targets for the modulation of neuroinflammation. Studies in animal models have tried to target several of these cytokines and chemokines ligands to reduce neuroinflammation after trauma (reviewed in63,64). As an example of these efforts is the use of a murine CXCL10-specific monoclonal antibody to treat mice with spinal cord injury, showing a pronounced reduction in neuroinflammation, decreased inflammatory cell infiltration,65,66,67 apoptosis,68 and increased vascularization69 in the CNS. Using the same approach but in a multiple sclerosis model, CXCL10 neutralizing antibody demonstrated decreased inflammation, reduced infiltrating CD4+ T cells and macrophages, and increased remyelination.65,70
Astrocytes capacity to continuously uptake extracellular neurotransmitter GLU, from the synaptic cleft through glutamate transporters (SCL1A2/EAAT-1 and SLC1A3/EAAT-2), is a crucial homeostasis function that avoids neuronal glutamate excitotoxicity (Rothstein et al., 1996). GLU that is taken up by astrocytes is then amidated into GLN via GS and will be secreted to the extracellular space, which will allow for neuron uptake and, therefore, used to replenish neuronal glutamate and GABA pool.71,72 This concept is based on the finding that GS, the enzyme that converts GLU to GLN, is exclusively localized in astrocytes.73 Several reports using human in vitro models have shown that the long-term exposure of astrocytes to TNF-α promotes an impairment in GLU uptake function.36,74,75,76,77Our results show that after three days of TIC stimulation, a significant impairment of GLU uptake is observed, which results in a lower secretion of GLN. Nonetheless, with only 24 h of stimulation, no significant impairment was observed, in line with results previously reported for short-term stimulation with TNF-α alone for hiPSC-derived astrocytes.36 To further dissect the astrocytic population function, a single-cell resolution approach could be taken into consideration. Given that reactive astrocytes have been reported to have potentially toxic effects on neurons5 the impairment of GLU uptake by the TIC stimuli, may lead to neuronal dysfunction and death.
Reactive astrocytes have been associated with several CNS pathologies, namely neurodegenerative diseases and aging. Hence, there is a great need to dissect the role of the different CNS cell populations in response to different stimuli, with increased interest in higher throughput systems, able to feed preclinical trials’ needs. In vitro human neural models have been shedding a light on the pathophysiology of these diseases; however, the majority is still highly heterogeneous, relies on animal-derived components and have limited scalability. Here we describe our highly homogeneous, controlled, and scalable iNSpheroid platform, able to recapitulate astrocytic functional features under physiological and neuroinflammation stimulation. Further, the high and homogeneous number of iNSpheroids produced can increase throughput and feed screening-intensive experiments, gaining significant relevance for translational investigation.
Limitations of the study
Although we have demonstrated hallmarks of neuroinflammation in our iNSpheroids and impairment of a core astrocytic function, all methodologies used throughout the work collected bulk information on the iNSpheroids. Our data may suggest that the astrocytic population is leading the inflammatory response by functional assessment and literature review; future studies should focus on using single-cell resolution technologies. We employed two different hiPSC lines to ensure the reproducibility of our findings. Nonetheless, to capture the heterogeneity inherent to the human population, further studies involving a wide variety of hiPSC lines will be required to ensure representation of the potential variability in inflammatory and cytokine responses observed across genetically diverse individuals.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Catarina Brito (anabrito@ibet.pt).
Materials availability
This study did not generate new unique materials.
Data and code availability
-
•
Data have been deposited at the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) and Proteome Xchange Consortium via PRIDE (Vizcaíno et al., 2016). GEO: GSE243254 (RNA-seq) and Proteome Xchange: PXD043835 (SWATH-MS).
-
•
This study does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this article is available from the lead contact upon request.
Acknowledgments
We gratefully acknowledge Dr Tomo Šaric (University of Cologne, Germany) for the supply of hNPCs. This work was supported by Fundação para a Ciência e a Tecnologia (FCT)/Ministério da Educação, Ciência e Inovação, through the Research Unit UID/04462: iNOVA4Health – Program in Translational Medicine, the Associate Laboratory LS4FUTURE (LA/P/0087/2020), and the project AstroReact (PTDC/BTM-ORG/29580/2017). C.M.G. (UI/BD/151253/2021) and B.P. (UI/BD/154873/2023) PhD fellowships funded by FCT, Portugal. MS data were obtained by UniMS – Mass Spectrometry Unit, ITQB NOVA & iBET, Oeiras, Portugal. Illustrations were created in biorender.com.
Author contributions
Conceptualization, D.S. and C.B.; methodology, C.M.G. and D.S.; formal analysis, C.M.G. and D.S.; investigation, C.M.G., D.S., C.C.G., B.P., and G.S; writing – original draft, C.M.G., D.S., and C.B.; writing – review and editing, C.M.G., D.S., C.C.G., B.P., G.S, P.M.A., and C.B.; resources, P.M.A. and C.B.; funding acquisition, D.S., P.M.A., and C.B.; supervision, D.S. and C.B.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-nestin, Rabbit | Merk Millipore | Cat# AB5922; RRID:AB_91107 |
| Anti-βIII-tubulin, Mouse | Merk Millipore | Cat# MAB1637; RRID:AB_2210524 |
| Anti-synaptophysin, Mouse | Merk Millipore | Cat# MAB5258; RRID:AB_2313839 |
| Anti-vimentin, Rabbit | Abcam | Cat# ab16700; RRID:AB_443435 |
| Anti-MAP2, Chicken | Abcam | Cat# ab92434; RRID:AB_2138147 |
| Anti-GFAP, Rabbit | Merk Millipore | Cat# AB5804; RRID:AB_2109645 |
| Anti-S100B, Rabbit | Invitrogen | Cat# PA5-87474; RRID:AB_2804180 |
| Anti-AQP4, Mouse | Santa Cruz Biotechnology | Cat# sc-32739; RRID:AB_626695 |
| Anti-EAAT1, Sheep | Bio-Techne | Cat# AF6048, RRID:AB_2044669 |
| Anti-EAAT2, Mouse | Merk Millipore | Cat# MAB2262; RRID:AB_10615610 |
| Alexa Fluor 488 goat anti-mouse IgG | Thermo Fisher Scientific | Cat# A-11001; RRID:AB_2534069 |
| Alexa Fluor 555 donkey anti-rabbit IgG | Thermo Fisher Scientific | Cat# A-31572; RRID:AB_162543 |
| Alexa Fluor 647 goat anti-chicken IgY | Thermo Fisher Scientific | Cat# A32933; RRID:AB_2762845 |
| Chemicals, peptides, and recombinant proteins | ||
| SB431542 | STEMCELL™Technologies | 72234 |
| LDN193184 | STEMCELL™Technologies | 72147 |
| mTeSR™1 | STEMCELL™Technologies | 85850 |
| Corning® Matrigel hESC-Qualified Matrix, hESC-qualified matrix | Corning® | 11573560 |
| Corning® Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrix | Corning® | 356230 |
| DMEM/F-12, GlutaMAX™ Supplement | Gibco™ | 31331093 |
| N2 SUPPLEMENT 50ML | Life Technologies | 17502001 |
| B-27® Supplement (50X), serum free | Gibco™ | 17504-044 |
| Neurobasal® Medium | Gibco™ | 21103-049 |
| DMEM/F-12, no glutamine | Gibco™ | 21331-020 |
| GlutaMAX™ Supplement | Gibco™ | 35050061 |
| B-27™ Supplement (50X), minus vitamin A | Gibco™ | 12587010 |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco™ | 17504044 |
| MEM Non-Essential Amino Acids Solution (100X) | Gibco™ | 11140035 |
| 2-Mercaptoethanol (50 mM) | Gibco™ | 31350010 |
| Insulin from bovine pancreas | SIGMA-ALDRICH | I6634 |
| Poly-L-ornithine | SIGMA-ALDRICH | P3655 |
| Laminin from Engelbreth-Holm-Swarm murine sarcoma basement membrane | SIGMA-ALDRICH | L2020 |
| PBS,DULB (1X)+CA,+MG | ThermoFisher | 14040091 |
| D-(+)-GLUCOSE, POWDER | SIGMA-ALDRICH | G7021 |
| Animal-Free Recombinant Human FGF-basic (154 aa) | PeproTech® | 167AF-100-18B |
| hEGF, EGF, recombinant, expressed in E. coli | E9644 | SIGMA-ALDRICH |
| Trypsin-EDTA (0.05%), phenol red | Gibco™ | 25300062 |
| Fetal Bovine Serum, qualified, E.U.-approved, South America origin | Gibco™ | 10270-106 |
| Y-27632 dihydrochloride-10 mg | TOCRIS | TO-1254 |
| Putrescine dihydrochloride | SIGMA-ALDRICH | P5780 |
| Progesterone | SIGMA-ALDRICH | P8783 |
| Sodium selenite | SIGMA-ALDRICH | S5261 |
| apo-Transferrin human | SIGMA-ALDRICH | T1147 |
| L-Ascorbic Acid Phosphate Magnesium Salt n-Hydrate | FujiFilm Wako | 013-12061 |
| TNF-α | Cell Signaling Technology | 8902SF |
| IL-1α | SIGMA-ALDRICH | I3901 |
| C1q | MyBioSource | MBS143105 |
| Paraformaldehyde | SIGMA-ALDRICH | 158127 |
| CRYOSTOR(R) Cell Cryopreservation Media | SIGMA-ALDRICH | C2874 |
| Sucrose, Molecular Biology | Merk | 573113 |
| Triton™ X-100 | SIGMA-ALDRICH | T8787 |
| ProLong™ Gold Antifade Mountant | Invitrogen™ | P36934 |
| DAPI (4′,6-Diamidino-2-Phenylindole, Dilactate) | Invitrogen™ | D3571 |
| Fluorescein diacetate | SIGMA-ALDRICH | F7378 |
| Propidium iodide | Sial | P4864 |
| L-Glutamic acid | SIGMA-ALDRICH | G8415 |
| Critical commercial assays | ||
| Human CXCL8 Quantikine ELISA Kits | R&D Systems | D8000C |
| Human IL-6 Quantikine ELISA Kits | R&D Systems | D6050 |
| Human CCL5 Quantikine ELISA Kits | R&D Systems | DRN00B |
| Human CXCL10 Quantikine ELISA Kits | R&D Systems | DIP100 |
| Human CCL2 Quantikine ELISA Kits | R&D Systems | DCP00 |
| Human Inflammation Antibody Array - Membrane (40 Targets) | Abcam | ab134003 |
| Deposited data | ||
| SWATH-MS data | Proteome Xchange Consortium via PRIDE | PXD043835 |
| Bulk RNA-sequencing data | Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) | GSE243254 |
| Experimental models: Cell lines | ||
| Royan iPSC clone 1/R1-hiPSC1/RIi001-A | Lab of Prof. Tomo Šarić. University of Cologne | CVCL_C888 |
| iPSC(IMR90)-clone 4 line | WiCell | CVCL_C437 |
| Oligonucleotides | ||
| See Table S4 | ||
| Software and algorithms | ||
| Prism GraphPad v9.0.1 | https://www.graphpad.com/scientific-software/prism/ | Prism GraphPad |
| ImageJ (Fiji) | https://imagej.net/ij/ | |
| RStudio | https://posit.co/products/open-source/rstudio/ | |
Experimental model and study participant details
Human induced pluripotent stem cell (hiPSC) culture
Two hiPSC lines were used in this work: Royan iPSC clone 1, also known as R1-hiPSC1 or RIi001-A,78 and iPSC(IMR90)-clone 4 line or WISCi004-B (iPSC(IMR90)-4, WiCell Research Institute). The RIi001-A cells have been reprogrammed from fibroblasts isolated from a male donor, as annotated in the Human pluripotent stem cell registry (hPSCreg; RRID:CVCL_C888). The iPSC(IMR90)-4 cells have been reprogrammed from fibroblasts isolated from a female donor, as annotated in the hPSCreg (RRID:CVCL_C437). The iPSC(IMR90)-4 line was expanded on Growth Factor Reduced Matrigel Matrix (BD Biosciences), in mTeSR1 medium (STEMCELL™Technologies) under feeder-free culture conditions. Complete medium exchange was performed every day. Cells were maintained under humidified atmosphere with 5% CO2, at 37 °C.
Method details
Generation of NPCs from hiPSC
R1-hiPSC1-derived NPCs were derived as previously described30 and kindly provided by Dr Tomo Saric. For iPSC(IMR90)-clone 4, cells were plated at 0.8x105 cell/cm2 in mTeSR1 and allowed to adhere for 24 hours. Afterwards, cells were culture in DualSMADi culture medium: N2B27 medium supplemented with SB431542 at 1 mM and LDN193189 at 10 mM (both from STEMCELL™Technologies). N2B27 medium is composed by ½ DMEM/F-12 and ½ Neurobasal, N2-supplement (1x), B27 supplement without vitamin A (0.5x), GlutaMAX (1x), Penicillin-Streptomycin (1%), MEM Non-Essential Amino Acids Solution (1%) and 2-mercaptoethanol (50 μM) (all from Gibco™), and Insulin (20 μg/mL) (Sigma-Aldrich). A 100% medium exchange was performed daily, for 10 days. On day 6 or day 10, single cells were plated on poly-L-ornithine-laminin (PLOL)-coated surfaces. PLOL coating was prepared by performing a 3-hour incubation at 37 °C with 0.16 mg/mL solution of poly-L-ornithine in PBS (with Ca2+ and Mg2+), followed by a washing step and a 3-hour incubation at 37 °C with 1 μg/mL solution of laminin in PBS (with Ca2+ and Mg2+). Cells were maintained in DMEM/F12 media with Glutamax (Life Technologies) supplemented with 1% N2 supplement (Life Technologies), 0.1% B27 supplement (Life Technologies), 1.6 μg/mL glucose (Sigma-Aldrich), 20 μg/mL insulin (Sigma-Aldrich), 20 ng/mL rhu-bFGF (Peprotech).79 The iPSC(IMR90)-4 line was maintained on PLOL-coated surfaces in a NPC expansion medium (EM), composed of DMEM/F12 media with Glutamax (Life Technologies) supplemented with 1% N2 supplement (Life Technologies), 0.1% B27 supplement (Life Technologies), 1.6 μg/mL glucose (Sigma-Aldrich), 20 μg/mL insulin (Sigma-Aldrich), 20 ng/mL rhubFGF (Peprotech) and 20 ng/ml rhu-EGF (Sigma-Aldrich). Medium was changed every other day until confluent cell culture was obtained.
NPCs derived from both hiPSC lines were passaged at 90-100% confluence (typically every 4-5 days). Cells were dislodged by 0.05% Trypsin-EDTA (1-2 min), which was neutralized with DMEM supplemented with 10% FBS (Life Technologies). Cells were sedimented by centrifugation, resuspended in NPC EM and plated on PLOL-coated T-flasks, at a cell density of 3 x 104 cell/cm2. A 50% media exchange was performed on day 2 of culture. Cell concentration and viability were determined by the trypan blue exclusion method in a Fuchs-Rusenthal hemocytometer. Cells were maintained under humidified atmosphere, in a multi-gas cell incubator (Sanyo), with 5% CO2 and 3% O2, at 37 °C.
Differentiation of hiPSC-derived iNSpheroids
hiPSC-NPC were expanded in 2D and harvested as described in the previous section before differentiated in STBs as described previously by our team.30,31 The hiPSC-NPC cell suspension was passed through a 70 μm nylon strainer (Millipore) and diluted to a cell density of 4x105 cell/mL in NPC aggregation medium (AM). AM composition was like NPC EM, except for the reduced concentration of EGF and bFGF (5 ng/mL), and the supplementation with a ROCK inhibitor (5 μM, Y-27632). Cells were inoculated into a software-controlled stirred-tank DASGIP® Bioblock bioreactor system (Eppendorf), as described previously. Culture conditions were set to maintain cells under 3 % dissolved oxygen (15 % of air with 21 % of oxygen), pH 7.4, 37 °C, and a stirring rate of 70 rpm. To control the aggregate size and avoid aggregate fusion, the stirring rate gradually increased up to 90 rpm with 10 rpm steps, based on visual inspection of the culture. After 72 hours of culture, perfusion operation mode was activated, with a dilution rate of 0.33 day-1 under gravimetric control. After 7 days, differentiation medium (DM) perfusion was initiated and maintained for further 23 days (a total of 30 days of culture in STB) to obtain differentiated hiPSC-derived iNSpheroids. DM was prepared by supplementing DMEM/F12 with Glutamax with 2 % B27 supplement, 1.6 μg/mL glucose, 10 μg/mL insulin, 10 μg/mL putrescin, 63 ng/mL progesterone, 50 μg/mL apotransferrin, 50 ng/mL sodium selenium (all from Sigma-Aldrich) and 200 mM ascorbic acid (Wako).
In vitro astrogliosis induction
Reactive astrogliosis was induced in iNSpheroids (day 30 of culture) by exposure to human recombinant TNF-α (30ng ml−1, Cell Signaling Technology, 8902SF), IL-1α (3ng ml−1, Sigma, I3901), and C1q (400ng ml−1, MyBioSource, MBS143105) diluted in DM. The cultures were maintained in batch mode, without culture medium exchange for 72 hours. iNSpheroids and cell supernatants were collected and analyzed at 3h, 6h, 24h, 48h and 72h, according to the experimental design (Figure 1A).
Immunofluorescence microscopy
iNSpheroids were either plated on PLOL-coated glass coverslips and allowed to adhere for 6-8 hours or transferred to a tube, for either whole mount or cryo-sectioning. Afterwards, the samples were fixed in 4% paraformaldehyde (PFA) + 4% sucrose in PBS for 20 min at room temperature (RT) and washed three times with PBS. Samples for cryosections were dehydrated overnight with 30% sucrose in PBS at 4°C and subsequently transferred to a cryomold with OCT and snap-frozen until further processing. For immunostaining, block and permeabilization were performed simultaneously with 0.125% fish skin gelatine (FSG) and 0.1% or 0.5% Triton X-100 in DPBS, for 20 min at RT. Primary antibodies were then incubated overnight at 4 °C, diluted in 0.125% FSG + 0.1% TritonX-100 in PBS. Cells were washed three times with PBS and incubated for 1 hour with secondary antibodies diluted in the same solution. Primary and secondary antibodies were used as in Table S4. Cell nuclei were counterstained with DAPI diluted in DPBS for 5 min (1:1000, Life Technologies), after which were washed three times in DPBS. Coverslips were mounted in ProLong™ Gold Antifade Mountant (Invitrogen™). Images were acquired on a Zeiss LSM 880-point scanning confocal microscope controlled with the Zeiss Zen 2.3 (black edition) software. Images were processed using ImageJ software and only linear manipulations were performed.80
Viability assay
For cell viability assessment, iNSpheroids were incubated with 20 μg/mL fluorescein diacetate (FDA) in PBS, for labeling of viable cells, and with 10 μg/mL propidium iodide (PI) in PBS, a membrane-impermeable DNA-dye that stains non-viable cells. Samples were observed using a fluorescence microscope (DMI6000B, Leica).
RT-qPCR
Total RNA was extracted with High Pure RNA Isolation Kit (Roche) or RNeasy Mini Kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. RNA was quantified in a NanoDrop 2000c (Thermo Scientific) and used for cDNA synthesis. Reverse transcription was performed using High Fidelity cDNA Synthesis Kit (Roche), using Anchored-oligo(dT)18 Primer (Roche) or with the Sensiscript RT Kit (Qiagen), for low RNA abundant samples, using a mix between Anchored-oligo(dT)18 Primer and random hexamers (Roche). qPCRs were performed in triplicates using LightCycler 480 SYBR Green I Master Kit (Roche) and the primers listed in Table S5. The reactions were performed with LightCycler 480 Instrument II 384-well block (Roche). Quantification cycle values (Cq’s) and melting curves were determined using LightCycler 480 Software version 1.5 (Roche). All data were analyzed using the 2-ΔΔCt method for relative gene expression analysis.81 Changes in gene expression were normalized using the housekeeping gene RPL22 (ribosomal protein L22), GADPH (Glyceraldehyde 3 Phosphate Dehydrogenase), and HPRT1 (Hypoxanthine Phosphoribosyltransferase 1) as internal controls. Statistical analysis was carried out using GraphPad Prism 9 software.
RNA-sequencing
iNSpheroids from day 0 (pre-challenge), day 1 and day 3 (after TIC-challenge) from both control (unstimulated) and TIC-stimulated conditions were analyzed, including four independent experiments per experimental condition. Samples were harvested from the STB and sedimented by centrifugation at 300 x g, for 5 min. The supernatant was discarded, and the resulting cell pellet washed twice with PBS. Cell pellets were snap-frozen in liquid nitrogen. The frozen samples were shipped on dry ice to Genewiz (Leipzig, Germany) for RNA sequencing analysis. The RNA sequencing workflow after RNA isolation included initial PolyA selection-based mRNA enrichment, mRNA fragmentation, and random priming with subsequent first- and second-strand complementary cDNA synthesis. End-repair 5′ phosphorylation and adenine nucleotide (dA)–tailing was performed. Lastly, adaptor ligation, polymerase chain reaction (PCR) enrichment, and Illumina NovaSeq technology–based sequencing with 2× 150–base pair (bp) read length were carried out. Sequence reads were trimmed to remove possible adapter sequences and nucleotides with poor quality using Trimmomatic v.0.36. The trimmed reads were mapped to the Homo sapiens GRCh38 reference genome available on ENSEMBL using the STAR aligner v.2.5.2b. Unique gene hit counts were calculated by using featureCounts from the Subread package v.1.5.2. The hit counts were summarized and reported using the gene_id feature in the annotation file. Only unique reads that fell within exon regions were counted. After extraction of gene hit counts, the gene hit counts table was used for downstream differential expression analysis. To assess the presence of astrocytic genes prior to TIC stimulation (day 0), a strategy for identifying gene expression using transcripts per million (TPM) present in the gene hit counts table was carried out, Phenotypical and functional annotation of the astrocytic population was done according to previous reports6,39. Using DESeq2, a comparison between unstimulated and TIC-stimulated samples, within each timepoint (day 1 or day 3) was performed. The Wald test was used to generate p-values and log2 fold changes. Genes with an adjusted p-value < 0.05 and absolute Log2FC> 1 were called as differentially expressed genes for each comparison.
Sample preparation for mass spectrometry
iNSpheroids were harvested from the STBs and sedimented by centrifugation at 300 x g, for 5 min. The supernatant was discarded, and the resulting cell pellet was washed twice with PBS. Cells were lysed in Triton X-100 lysis buffer (50 mM Tris, 5 mM EDTA, 150 mM NaCl, 1% Triton X-100; all from Sigma-Aldrich), and 1x complete protease inhibitors cocktail (Roche), for 45 min at 4°C, with periodic agitation. Total protein was quantified with the Micro BCA™ Protein Assay Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. Proteins were extracted and precipitated in methanol-chloroform, as previously described.30 Briefly, one volume of protein solution and 4 volumes of methanol were centrifuged at 9 000 x g for 10 s, mixed with 2 volumes of chloroform, and centrifuged again. For phase separation, 3 volumes of water were added to the samples, which were homogenized by vigorous vortex, and centrifuged at 9 000 x g for 1 min. The upper phase was discarded, and 3 volumes of methanol were added. Samples were gently mixed and centrifuged at 9 000 x g for 2 min to pellet precipitated protein. The supernatant was discarded, and the protein precipitate dried at 60 °C before solubilization in 0.1% of RapiGest SF Surfactant (Waters), overnight, at 4 °C, with continuous agitation; when protein precipitates were still detected, an additional step of solubilization was performed. Protein was quantified using the Bradford assay.82 For in-solution digestion, samples were reduced in 5 mM of dithiothreitol (DTT), for 30 min, at 60 °C; alkylated in 15 mM iodoacetamide (IAA), for 30 min in dark, and incubated at 100 °C, 5 min; digested with trypsin (Promega; 1.2 μg/100 μg protein), at 37 °C, overnight. Trypsin was inactivated by acidification in 0.5 % trifluoroacetic acid, at 37 °C, for 45 min. Samples were centrifuged at 16 000 x g for 10 min, supernatants were collected into new tubes and dried using a Savant™ Universal SpeedVac™ concentrator (Thermo Fisher Scientific).
Spectral library generation by information-dependent acquisition (IDA)
A total of twenty-four samples corresponding to timepoint 0-, 24- and 72-hours post-stimuli, control and TIC-stimulated, were subjected to information-dependent acquisition (IDA) analysis by Nano-liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS) analysis on an ekspert™ NanoLC 425 cHiPLC system coupled with a TripleTOF 6600 with a NanoSpray III source (Sciex, Framingham, MA, USA). Samples from the same condition and timepoint were pooled for subsequent analysis (n = 4). Peptides were sprayed into the MS through an uncoated fused-silica PicoTip™ emitter (360 μm O.D., 20 μm I.D., 10 ± 1.0 μm tip I.D., New Objective, Oullins, France). The source parameters were set as follows: 15 GS1, 0 GS2, 30 CUR, 2.5 keV ISVF, and 100 °C IHT. A reversed-phase nanoLC-MS/MS with a trap and elute configuration, using a Nano cHiPLC Trap column (Eksigent, USA, 200 μm × 0.5 mm, ChromXP C18-CL, 3 μm, 120°Å) and NanoLC column (Eksigent, USA, 75 μm × 15 cm, ChromXP 3C18-CL-120, 3 μm, 120°Å) was performed. Water with 0.1% (v/v) formic acid (solvent A) and 0.1% formic acid in acetonitrile (solvent B) was used. The trap column was loaded with each sample at a flow rate of 2 μL/min for 10 min using 100% (v/v) solvent A Peptide separation was performed at 300 nL/min applying a gradient (v/v) of solvent B as follows: 0–1 min, 5%; 1–91 min, 5–30%; 91–93 min, 30–80%; 93–108 min, 80%; 108–110 min, 80–5%; and 110–127 min, 5%. Each sample pool was subjected to IDA using three different MS m/z ranges, which were calculated using the SWATH Variable Window Calculator V1.0 (Sciex, Framingham, US) based on a reference sample. was subjected to two IDA runs. The mass range for the MS scan was set to m/z 400–622.9, 621.9–790.9, and 789.9–2,000. The 50 most intense precursors were selected for subsequent MS/MS scans of 150–1,800 m/z, in high sensitivity mode, for 40 ms, using a total cycle time of 2.3 s. The selection criteria for parent ions included a charge state between +2 and +5 and counts above a minimum threshold of 125 counts per second Ions were excluded from further MS/MS analysis for 12 s. Fragmentation was performed using rolling collision energy with a collision energy spread of five. The spectral library was created by combining all IDA raw files using ProteinPilot™ software (v5.0 ABSciex) with the Paragon algorithm and with the following search parameters: Homo sapiens from Uniprot/SwissProt database; trypsin digestion; iodoacetamide cysteine alkylation; TripleTOF 6600 equipment; and biological modifications as ID focus. After a false discovery rate (FDR) analysis, only proteins with FDR < 1% were included in the reference spectral library.
Protein quantification by sequential window acquisition of all theoretical fragment ion spectra-mass spectrometry (SWATH-MS)
Each sample was analyzed in triplicate (3 μg per analysis) by sequential window acquisition of all theoretical fragment ion spectra (SWATH)-MS, using the instrument setup described for the IDA runs. The mass spectrometer was operated in a cyclic data independent acquisition (DIA) as previously established.30 SWATH-MS data were acquired with SWATH acquisition method, using a set of 64 overlapping variable SWATH windows covering the precursor mass range of 400–2,000 m/z. The SWATH variable windows were calculated using the SWATH Variable Window Calculator V1.0 (Sciex, Framingham, MA, USA) based on a reference sample. A 10 ms survey scan (400–2,000 m/z) was acquired at the beginning of each cycle, and the subsequent SWATH windows were collected from 400 to 2,000 m/z for 50 ms, resulting in a cycle time of 3.26 s. Rolling collision energy with a collision energy spread of 5 was used. The spectral alignment and targeted data extraction of DIA samples were performed using PeakView v.2.2 (Sciex, Framingham, MA, USA), with the spectral library as reference. For data extraction the following parameters were used: Six peptides/protein, six transitions/peptide, peptide confidence level of >95% (corresponding to FDR < 1%), FDR threshold of 1%, excluding shared peptides, and extracted ion chromatogram (XIC) window of 6 min and width set at 20 ppm. Data were directly exported to Markerview 1.3.1 (Sciex, Framingham, MA, USA) and normalized using total area sums to obtain the final quantification values. A total of 3,239 proteins were quantified under these conditions. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE83 partner repository with the dataset identifier PXD043835.
Proteome data analysis
Protein differential expression analysis was performed using the R package “DEP” (version 1.14.0).84 Pairwise comparisons for TIC-stimulated versus control samples were performed for the 72 hours timepoint at thresholds of FDR < 0.1 and fold-change >1.5.
Antibody arrays
Cell culture supernatants were collected at 72 hours post-stimulus and centrifuged at 1000 x g for 2 min. Both control and TIC-stimulated samples were evaluated for the presence of 40 human cytokine proteins, using an inflammation membrane antibody array (Abcam; ab134003) according to the manufacturer’s instructions. Spot intensity of each protein was determined by Protein Array Analyzer for ImageJ software. Raw densitometry data was further processed by subtracting the background using negative controls in each membrane, and the positive controls signals before proceeding to analysis. Additionally, unstimulated, and stimulated membranes were normalized against a basal medium control (either with or without TIC, respectively) and represented as mean pixel density, in arbitrary units (AU).
ELISA
Cell culture supernatants were collected at 3, 6, 24, 72 hours from both control and TIC-stimulated iNSpheroids. The culture medium was aliquoted, snap frozen in liquid nitrogen, and stored at –80 °C. Human CXCL8 (D8000C), IL-6 (D6050), CCL5 (DRN00B), CXCL10 (DIP100) and CCL2 (DCP00) Quantikine ELISA Kits from R&D Systems were used according to the manufacturer protocol. The culture supernatants were assayed undiluted, within the linear range of each of the ELISA kits; the concentration of each protein was determined using standard curves, as indicated by the kits.
Glutamine depletion assay
iNSpheroids were harvested from the bioreactor after 1 and 3 days of TIC-stimulation and plated on PLOL-coated glass coverslips and allowed to adhere for 6-8 h, at a cell density of 1.5 × 106 cell/cm2. For glutamine synthetase (GS) inhibition experiments, iNSpheroids were cultured for 16 h, in DM, with or without 20 μM methionine sulphoximine (MSO, Sigma-Aldrich, M5379), an irreversible glutamine synthetase inhibitor. Afterwards, the medium was completely replaced by a glutamine-free (Gibco™ DMEM/F-12, no glutamine, 21331020) or a glutamate-rich (3 mM of L-Glutamic acid, Sigma, G8415) medium and cultures were followed-up for 4 days, with collection of supernatants every 24 h. Samples were centrifuged, and the soluble fractions frozen at –20 °C until analysis. Glutamine (GLN) and glutamate (GLU) concentrations in the cell culture supernatants were quantified using Cedex Bio Analyzer 7100 (Roche). For calculation of specific GLU and GLN concentrations, cell concentration was determined by nuclei quantification in NucleoCounter® NC-200™ after cell lysis.
Quantification and statistical analysis
Data are expressed as mean ± standard deviation (SD), with statistical analyzes conducted in GraphPad Prism v9.0.1 (GraphPad Software, San Diego, CA). When comparing only two experimental groups, the unpaired Student t test was used for data with normal distribution; if otherwise, the Mann-Whitney test was used. When comparing three or more groups, a one-way analysis of variance (ANOVA) followed by the Bonferroni or Tukey post hoc test was used for data with normal distribution. To compare different groups with two independent variables, we used a two-way ANOVA followed by the two-stage linear step-up procedure of Benjamini–Hochberg method. Statistical significance was considered for p < 0.05.
Published: August 5, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.113246.
Contributor Information
Daniel Simão, Email: dsimao@ibet.pt.
Catarina Brito, Email: anabrito@itqb.unl.pt.
Supplemental information
References
- 1.Allen N.J., Eroglu C. Cell Biology of Astrocyte-Synapse Interactions. Neuron. 2017;96:697–708. doi: 10.1016/j.neuron.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovannoni F., Quintana F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020;41:805–819. doi: 10.1016/j.it.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escartin C., Guillemaud O., Carrillo-de Sauvage M.A. Questions and (some) answers on reactive astrocytes. Glia. 2019;67:2221–2247. doi: 10.1002/glia.23687. [DOI] [PubMed] [Google Scholar]
- 4.Lazic A., Balint V., Ninkovic D.S., Peric M., Stevanovic M. Reactive and Senescent Astroglial Phenotypes as Hallmarks of Brain Pathologies. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.S., Peterson T.C., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbar L., Jain T., Zimmer M., Kruglikov I., Sadick J.S., Wang M., Kalpana K., Rose I.V.L., Burstein S.R., Rusielewicz T., et al. CD49f Is a Novel Marker of Functional and Reactive Human iPSC-Derived Astrocytes. Neuron. 2020;107:436–453.e12. doi: 10.1016/j.neuron.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labib D., Wang Z., Prakash P., Zimmer M., Smith M.D., Frazel P.W., Barbar L., Sapar M.L., Calabresi P.A., Peng J., et al. Proteomic Alterations and Novel Markers of Neurotoxic Reactive Astrocytes in Human Induced Pluripotent Stem Cell Models. Front. Mol. Neurosci. 2022;15 doi: 10.3389/fnmol.2022.870085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guttenplan K.A., Weigel M.K., Prakash P., Wijewardhane P.R., Hasel P., Rufen-Blanchette U., Münch A.E., Blum J.A., Fine J., Neal M.C., et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature. 2021;599:102–107. doi: 10.1038/s41586-021-03960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R., Xue G., Hölscher C. The role of the TNFα-mediated astrocyte signaling pathway in epilepsy. Acta Epileptologica. 2021;3:1–9. [Google Scholar]
- 10.Oberheim Bush N.A., Nedergaard M. Do Evolutionary Changes in Astrocytes Contribute to the Computational Power of the Hominid Brain? Neurochem. Res. 2017;42:2577–2587. doi: 10.1007/s11064-017-2363-0. [DOI] [PubMed] [Google Scholar]
- 11.Kelley K.W., Nakao-Inoue H., Molofsky A.V., Oldham M.C. Variation among intact tissue samples reveals the core transcriptional features of human CNS cell classes. Nat. Neurosci. 2018;21:1171–1184. doi: 10.1038/s41593-018-0216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Sloan S.A., Clarke L.E., Caneda C., Plaza C.A., Blumenthal P.D., Vogel H., Steinberg G.K., Edwards M.S.B., Li G., et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Pan L., Pembroke W.G., Rexach J.E., Godoy M.I., Condro M.C., Alvarado A.G., Harteni M., Chen Y.W., Stiles L., et al. Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat. Commun. 2021;12:3958. doi: 10.1038/s41467-021-24232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perriot S., Mathias A., Perriard G., Canales M., Jonkmans N., Merienne N., Meunier C., El Kassar L., Perrier A.L., Laplaud D.A., et al. Human Induced Pluripotent Stem Cell-Derived Astrocytes Are Differentially Activated by Multiple Sclerosis-Associated Cytokines. Stem Cell Rep. 2018;11:1199–1210. doi: 10.1016/j.stemcr.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TCW J., Wang M., Pimenova A.A., Bowles K.R., Hartley B.J., Lacin E., Machlovi S.I., Abdelaal R., Karch C.M., Phatnani H., et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017;9:600–614. doi: 10.1016/j.stemcr.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krencik R., Zhang S.C. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat. Protoc. 2011;6:1710–1717. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos R., Vadodaria K.C., Jaeger B.N., Mei A., Lefcochilos-Fogelquist S., Mendes A.P.D., Erikson G., Shokhirev M., Randolph-Moore L., Fredlender C., et al. Differentiation of Inflammation-Responsive Astrocytes from Glial Progenitors Generated from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017;8:1757–1769. doi: 10.1016/j.stemcr.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roybon L., Lamas N.J., Garcia A.D., Yang E.J., Sattler R., Lewis V.J., Kim Y.A., Kachel C.A., Rothstein J.D., Przedborski S., et al. Human Stem Cell-Derived Spinal Cord Astrocytes with Defined Mature or Reactive Phenotypes. Cell Rep. 2013;4:1035–1048. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tchieu J., Calder E.L., Guttikonda S.R., Gutzwiller E.M., Aromolaran K.A., Steinbeck J.A., Goldstein P.A., Studer L. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat. Biotechnol. 2019;37:267–275. doi: 10.1038/s41587-019-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canals I., Ginisty A., Quist E., Timmerman R., Fritze J., Miskinyte G., Monni E., Hansen M.G., Hidalgo I., Bryder D., et al. Rapid and efficient induction of functional astrocytes from human pluripotent stem cells. Nat. Methods. 2018;15:693–696. doi: 10.1038/s41592-018-0103-2. [DOI] [PubMed] [Google Scholar]
- 21.Voulgaris D., Nikolakopoulou P., Herland A. Generation of Human iPSC-Derived Astrocytes with a mature star-shaped phenotype for CNS modeling. Stem Cell Rev. Rep. 2022;18:2494–2512. doi: 10.1007/s12015-022-10376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cvetkovic C., Patel R., Shetty A., Hogan M.K., Anderson M., Basu N., Aghlara-Fotovat S., Ramesh S., Sardar D., Veiseh O., et al. Assessing Gq-GPCR–induced human astrocyte reactivity using bioengineered neural organoids. J. Cell Biol. 2022;221 doi: 10.1083/jcb.202107135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiura S., Cha J.M., Yanagawa F., Zorlutuna P., Bae H., Khademhosseini A. Dynamic three-dimensional micropatterned cell co-cultures within photocurable and chemically degradable hydrogels. J. Tissue Eng. Regen. Med. 2016;10:690–699. doi: 10.1002/term.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quadrato G., Nguyen T., Macosko E.Z., Sherwood J.L., Min Yang S., Berger D.R., Maria N., Scholvin J., Goldman M., Kinney J.P., et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sloan S.A., Darmanis S., Huber N., Khan T.A., Birey F., Caneda C., Reimer R., Quake S.R., Barres B.A., Paşca S.P. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron. 2017;95:779–790.e6. doi: 10.1016/j.neuron.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayó-Puxan N., Terrasso A.P., Creyssels S., Simão D., Begon-Pescia C., Lavigne M., Salinas S., Bernex F., Bosch A., Kalatzis V., et al. Lysosomal and network alterations in human mucopolysaccharidosis type VII iPSC-derived neurons. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-34523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madhavan M., Nevin Z.S., Shick H.E., Garrison E., Clarkson-Paredes C., Karl M., Clayton B.L.L., Factor D.C., Allan K.C., Barbar L., et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods. 2018;15:700–706. doi: 10.1038/s41592-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian X., Song H., Ming G.-L. Brain organoids: advances, applications and challenges. Development. 2019;146 doi: 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simão D., Silva M.M., Terrasso A.P., Arez F., Sousa M.F.Q., Mehrjardi N.Z., Šarić T., Gomes-Alves P., Raimundo N., Alves P.M., Brito C. Recapitulation of Human Neural Microenvironment Signatures in iPSC-Derived NPC 3D Differentiation. Stem Cell Rep. 2018;11:552–564. doi: 10.1016/j.stemcr.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simão D., Arez F., Terasso A.P., Pinto C., Sousa M.F., Brito C., Alves P.M. Perfusion Stirred-Tank Bioreactors for 3D Differentiation of Human Neural Stem Cells. Methods Mol. Biol. 2016;1502:129–142. doi: 10.1007/7651_2016_333. [DOI] [PubMed] [Google Scholar]
- 32.Wulansari N., Darsono W.H.W., Woo H.J., Chang M.Y., Kim J., Bae E.J., Sun W., Lee J.H., Cho I.J., Shin H., et al. Neurodevelopmental defects and neurodegenerative phenotypes in human brain organoids carrying Parkinson’s disease-linked DNAJC6 mutations. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abb1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundin A., Delsing L., Clausen M., Ricchiuto P., Sanchez J., Sabirsh A., Ding M., Synnergren J., Zetterberg H., Brolén G., et al. Human iPS-Derived Astroglia from a Stable Neural Precursor State Show Improved Functionality Compared with Conventional Astrocytic Models. Stem Cell Rep. 2018;10:1030–1045. doi: 10.1016/j.stemcr.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kany S., Vollrath J.T., Relja B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Landeghem F.K.H., Weiss T., Oehmichen M., von Deimling A. Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J. Neurotrauma. 2006;23:1518–1528. doi: 10.1089/neu.2006.23.1518. [DOI] [PubMed] [Google Scholar]
- 36.Trindade P., Loiola E.C., Gasparotto J., Ribeiro C.T., Cardozo P.L., Devalle S., Salerno J.A., Ornelas I.M., Ledur P.F., Ribeiro F.M., et al. Short and long TNF-alpha exposure recapitulates canonical astrogliosis events in human-induced pluripotent stem cells-derived astrocytes. Glia. 2020;68:1396–1409. doi: 10.1002/glia.23786. [DOI] [PubMed] [Google Scholar]
- 37.Ghoddoussi F., Galloway M.P., Jambekar A., Bame M., Needleman R., Brusilow W.S.A. Methionine sulfoximine, an inhibitor of glutamine synthetase, lowers brain glutamine and glutamate in a mouse model of ALS. J. Neurol. Sci. 2010;290:41–47. doi: 10.1016/j.jns.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Choi H., Kim H.J., Yang J., Chae S., Lee W., Chung S., Kim J., Choi H., Song H., Lee C.K., et al. Acetylation changes tau interactome to degrade tau in Alzheimer’s disease animal and organoid models. Aging Cell. 2020;19 doi: 10.1111/acel.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.E., Liu F., Dai Y., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218 doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose C.F., Verkhratsky A., Parpura V. Astrocyte glutamine synthetase: pivotal in health and disease. Biochem. Soc. Trans. 2013;41:1518–1524. doi: 10.1042/BST20130237. [DOI] [PubMed] [Google Scholar]
- 41.Mahmoud S., Gharagozloo M., Simard C., Gris D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells. 2019;8 doi: 10.3390/cells8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang D., Wang X., Zhang L., Fang Y., Zheng Q., Liu X., Yu W., Chen S., Ying J., Hua F. Lipid metabolism and storage in neuroglia: role in brain development and neurodegenerative diseases. Cell Biosci. 2022;12:106–116. doi: 10.1186/s13578-022-00828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haroon E., Miller A.H., Sanacora G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology. 2017;42:193–215. doi: 10.1038/npp.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarke L.E., Liddelow S.A., Chakraborty C., Münch A.E., Heiman M., Barres B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA. 2018;115:E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raghavendra V., Tanga F.Y., DeLeo J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 46.Albrecht D.S., Forsberg A., Sandström A., Bergan C., Kadetoff D., Protsenko E., Lampa J., Lee Y.C., Höglund C.O., Catana C., et al. Brain glial activation in fibromyalgia – A multi-site positron emission tomography investigation. Brain Behav. Immun. 2019;75:72–83. doi: 10.1016/j.bbi.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J.-S., Kam T.I., Lee S., Park H., Oh Y., Kwon S.H., Song J.J., Kim D., Kim H., Jhaldiyal A., et al. Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathol. Commun. 2021;9:78. doi: 10.1186/s40478-021-01180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guttikonda S.R., Sikkema L., Tchieu J., Saurat N., Walsh R.M., Harschnitz O., Ciceri G., Sneeboer M., Mazutis L., Setty M., et al. Fully defined human pluripotent stem cell-derived microglia and tri-culture system model C3 production in Alzheimer's disease. Nat. Neurosci. 2021;24:343–354. doi: 10.1038/s41593-020-00796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayden M.S., Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lund B.T., Ashikian N., Ta H.Q., Chakryan Y., Manoukian K., Groshen S., Gilmore W., Cheema G.S., Stohl W., Burnett M.E., et al. Increased CXCL8 (IL-8) expression in Multiple Sclerosis. J. Neuroimmunol. 2004;155:161–171. doi: 10.1016/j.jneuroim.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Russo R.C., Garcia C.C., Teixeira M.M., Amaral F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expet Rev. Clin. Immunol. 2014;10:593–619. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 52.El Khoury J., Luster A.D. Mechanisms of microglia accumulation in Alzheimer’s disease: therapeutic implications. Trends Pharmacol. Sci. 2008;29:626–632. doi: 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Pittaluga A. CCL5–Glutamate Cross-Talk in Astrocyte-Neuron Communication in Multiple Sclerosis. Front. Immunol. 2017;8:1079. doi: 10.3389/fimmu.2017.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabezas R., Avila M., Gonzalez J., El-Bachá R.S., Báez E., García-Segura L.M., Jurado Coronel J.C., Capani F., Cardona-Gomez G.P., Barreto G.E. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front. Cell. Neurosci. 2014;8 doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Argaw A.T., Zhang Y., Snyder B.J., Zhao M.L., Kopp N., Lee S.C., Raine C.S., Brosnan C.F., John G.R. IL-1β Regulates Blood-Brain Barrier Permeability via Reactivation of the Hypoxia-Angiogenesis Program. J. Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 56.Osipova E.D., Semyachkina-Glushkovskaya O.V., Morgun A.V., Pisareva N.V., Malinovskaya N.A., Boitsova E.B., Pozhilenkova E.A., Belova O.A., Salmin V.V., Taranushenko T.E., et al. Gliotransmitters and cytokines in the control of blood-brain barrier permeability. Rev. Neurosci. 2018;29:567–591. doi: 10.1515/revneuro-2017-0092. [DOI] [PubMed] [Google Scholar]
- 57.Brandl S., Reindl M. Blood–Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241612699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss J.M., Berman J.W. Astrocyte expression of monocyte chemoattractant protein-1 is differentially regulated by transforming growth factor beta. J. Neuroimmunol. 1998;91:190–197. doi: 10.1016/s0165-5728(98)00183-0. [DOI] [PubMed] [Google Scholar]
- 59.Gimenez M.A.T., Sim J.E., Russell J.H. TNFR1-dependent VCAM-1 expression by astrocytes exposes the CNS to destructive inflammation. J. Neuroimmunol. 2004;151:116–125. doi: 10.1016/j.jneuroim.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Erta M., Quintana A., Hidalgo J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts T.K., Eugenin E.A., Lopez L., Romero I.A., Weksler B.B., Couraud P.O., Berman J.W. CCL2 disrupts the adherens junction: implications for neuroinflammation. Lab. Invest. 2012;92:1213–1233. doi: 10.1038/labinvest.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Q., Wang G., Zhang F. Role of Peripheral Immune Cells-Mediated Inflammation on the Process of Neurodegenerative Diseases. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.582825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Proudfoot A.E.I. Chemokine receptors: multifaceted therapeutic targets. Nat. Rev. Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dhaiban S., Al-Ani M., Elemam N.M., Maghazachi A.A. Targeting Chemokines and Chemokine Receptors in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. J. Inflamm. Res. 2020;13:619–633. doi: 10.2147/JIR.S270872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu M.T., Keirstead H.S., Lane T.E. Neutralization of the Chemokine CXCL10 Reduces Inflammatory Cell Invasion and Demyelination and Improves Neurological Function in a Viral Model of Multiple Sclerosis. J. Immunol. 2001;167:4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez R., Glaser J., Liu M.T., Lane T.E., Keirstead H.S. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp. Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez R., Hickey M.J., Espinosa J.M., Nistor G., Lane T.E., Keirstead H.S. Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regen. Med. 2007;2:771–783. doi: 10.2217/17460751.2.5.771. [DOI] [PubMed] [Google Scholar]
- 68.Glaser J., Gonzalez R., Sadr E., Keirstead H.S. Neutralization of the chemokine CXCL10 reduces apoptosis and increases axon sprouting after spinal cord injury. J. Neurosci. Res. 2006;84:724–734. doi: 10.1002/jnr.20982. [DOI] [PubMed] [Google Scholar]
- 69.Glaser J., Gonzalez R., Perreau V.M., Cotman C.W., Keirstead H.S. Neutralization of the chemokine CXCL10 enhances tissue sparing and angiogenesis following spinal cord injury. J. Neurosci. Res. 2004;77:701–708. doi: 10.1002/jnr.20204. [DOI] [PubMed] [Google Scholar]
- 70.Fife B.T., Kennedy K.J., Paniagua M.C., Lukacs N.W., Kunkel S.L., Luster A.D., Karpus W.J. CXCL10 (IFN-γ-Inducible Protein-10) Control of Encephalitogenic CD4+ T Cell Accumulation in the Central Nervous System During Experimental Autoimmune Encephalomyelitis. J. Immunol. 2001;166:7617–7624. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- 71.Höltje M., Hofmann F., Lux R., Veh R.W., Just I., Ahnert-Hilger G. Glutamate Uptake and Release by Astrocytes Are Enhanced by Clostridium botulinum C3 Protein. J. Biol. Chem. 2008;283:9289–9299. doi: 10.1074/jbc.M706499200. [DOI] [PubMed] [Google Scholar]
- 72.Schousboe A., Scafidi S., Bak L.K., Waagepetersen H.S., McKenna M.C. Glutamate Metabolism in the Brain Focusing on Astrocytes. Adv. Neurobiol. 2014;11:13–30. doi: 10.1007/978-3-319-08894-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norenberg M.D., Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- 74.Fine S.M., Angel R.A., Perry S.W., Epstein L.G., Rothstein J.D., Dewhurst S., Gelbard H.A. Tumor Necrosis Factor α Inhibits Glutamate Uptake by Primary Human Astrocytes. J. Biol. Chem. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- 75.Hu S., Sheng W.S., Ehrlich L.C., Peterson P.K., Chao C.C. Cytokine Effects on Glutamate Uptake by Human Astrocytes. Neuroimmunomodulation. 2000;7:153–159. doi: 10.1159/000026433. [DOI] [PubMed] [Google Scholar]
- 76.Carmen J., Rothstein J.D., Kerr D.A. Tumor necrosis factor-α modulates glutamate transport in the CNS and is a critical determinant of outcome from viral encephalomyelitis. Brain Res. 2009;1263:143–154. doi: 10.1016/j.brainres.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hyvärinen T., Hagman S., Ristola M., Sukki L., Veijula K., Kreutzer J., Kallio P., Narkilahti S. Co-stimulation with IL-1β and TNF-α induces an inflammatory reactive astrocyte phenotype with neurosupportive characteristics in a human pluripotent stem cell model system. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-53414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Totonchi M., Taei A., Seifinejad A., Tabebordbar M., Rassouli H., Farrokhi A., Gourabi H., Aghdami N., Hosseini-Salekdeh G., Baharvand H. Feeder- and serum-free establishment and expansion of human induced pluripotent stem cells. Int. J. Dev. Biol. 2010;54:877–886. doi: 10.1387/ijdb.092903mt. [DOI] [PubMed] [Google Scholar]
- 79.Koch P., Opitz T., Steinbeck J.A., Ladewig J., Brüstle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. USA. 2009;106:3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Livak K.J., Schmittgen T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 82.Kielkopf C.L., Bauer W., Urbatsch I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020;2020:102269. doi: 10.1101/pdb.prot102269. [DOI] [PubMed] [Google Scholar]
- 83.Vizcaíno J.A., Csordas A., Del-Toro N., Dianes J.A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016;44 doi: 10.1093/nar/gkw880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X., Smits A.H., van Tilburg G.B., Ovaa H., Huber W., Vermeulen M. Proteome-wide identification of ubiquitin interactions using UbIA-MS. Nat. Protoc. 2018;13:530–550. doi: 10.1038/nprot.2017.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data have been deposited at the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) and Proteome Xchange Consortium via PRIDE (Vizcaíno et al., 2016). GEO: GSE243254 (RNA-seq) and Proteome Xchange: PXD043835 (SWATH-MS).
-
•
This study does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this article is available from the lead contact upon request.