Abstract
The food hydrocolloids κ-carrageenan and xanthan gum, used in processed foods including meat products, have unclear effects on gut health. This study investigated the effects of incorporating 1 % κ-carrageenan or xanthan gum into pork on protein digestibility, gut microbiota, oxidative stress, and gene expression using both in vitro gastrointestinal digestion/fermentation and an in vivo rodent model. In vitro, xanthan gum reduced protein digestibility (−11 %) in the simulated small intestine, thus elevating protein fermentation metabolites (up to 4-fold), but this was not observed in vivo. Consumption of a low-fiber pork diet without hydrocolloids promoted Akkermansia (29.5 % median abundance) and Tannerellaceae (24.7 %) growth in the colon, whereas κ-carrageenan increased Desulfovibrio (7.95 %) and Alistipes (6.14 %), and xanthan gum enhanced unclassified Muribaculaceae (14.8 %) and Bacteroides (12.1 %). Unexpectedly, transcriptomic analysis revealed a down-regulation of gut inflammatory pathways, accompanied by lower fecal calprotectin levels, in rats consuming pork with hydrocolloids. While κ-carrageenan notably reduced lipid oxidation in stomach contents, only xanthan gum lowered plasma and colonic oxidative stress. These findings highlight the potential of hydrocolloids to modulate dietary responses, suggesting a role in influencing gut health following high processed meat consumption.
Keywords: Akkermansia, Food additives, Gut microbiota, Pork, Protein digestibility
Graphical abstract
Highlights
-
•
Xanthan gum reduced protein digestibility during in vitro digestion.
-
•
κ-carrageenan did not affect meat protein digestibility.
-
•
Both hydrocolloids altered gut microbiota composition.
-
•
Pork consumption enhanced inflammatory pathways and calprotectin.
-
•
Xanthan gum reduced oxidative stress in colon and plasma.
1. Introduction
Food hydrocolloids like carrageenan and xanthan gum are used in processed meats to enhance texture through their emulsifying and water retention properties, especially in reduced-fat products (Gao et al., 2024; Guo et al., 2023; Udo et al., 2023). While these low-fat products are often marketed as healthier, the health implications of using these additives remain debated. Carrageenan, a sulfated polysaccharide derived from red algae, is commercially classified into kappa (κ), iota (ι), and lambda (λ) based on the number and position of sulfate groups. Xanthan gum, by contrast, is a bacterial exopolysaccharide produced by Xanthomonas campestris.
Recent studies, both in vitro and in vivo, have raised concerns about potential interactions between hydrocolloids and dietary proteins, suggesting possible negative impacts on protein digestibility. The safety of carrageenan has been controversial mainly due to confusion in differentiating food-grade carrageenan (molecular weight ∼500 kDa) from its acid-hydrolyzed, low-molecular-weight derivatives such as degraded carrageenan (20–40 kDa) and poligeenan (10–20 kDa), the latter being linked to carcinogenicity in animal models (McKim et al., 2019; Weiner et al., 2019). Food-grade carrageenan has been reported to impair protein digestibility by interacting directly with proteins and inhibiting digestive enzymes (David et al., 2020a, 2020b), and lipid digestion (Huang et al., 2023, 2024). The biological effects of carrageenan can vary substantially depending on the administration method (Mi et al., 2020). observed colitis induced by κ-carrageenan only when delivered via drinking water but not when incorporated directly into diets. These findings underline the critical role of the delivery matrix in modulating the biological effects of κ-carrageenan, warranting further investigation into its interaction mechanisms. Compared to carrageenan, the effects of xanthan gum on digestibility remain understudied, though it was reported to decrease in vitro proteolysis as well (David et al., 2020a). High cooking temperatures (>100 °C) can compromise protein digestibility (Bax et al., 2012), but the extent to which cooking temperature modulates the conformation of the additives and their interaction with the food matrix is currently unknown.
The impact of food additives with emulsifying and/or thickening properties on the intestinal microbiota composition is currently a topic of much interest (Naimi et al., 2021), as certain additives have been reported to potentially increase intestinal permeability and inflammation (Chassaing et al., 2015). As a sulfated polysaccharide, κ-carrageenan may promote the growth of sulfate-reducing bacteria, which produce sulfur metabolites like hydrogen sulfide (H2S). This compound is linked to the degradation of the protective mucus layer in the colon (Ijssennagger et al., 2015). High MW carrageenan (≥100 kDa) remained intact when exposed to human fecal microbiota, while low MW carrageenan (∼4.5 kDa) underwent degradation in seven out of eight human fecal samples (Yin et al., 2021). However, this degradation did not involve the removal of sulfate groups. Similarly, the degradation of xanthan gum by the human gut microbiota is also under study (Ostrowski et al., 2022). To the best of our knowledge, no research has yet been conducted on the influence of κ-carrageenan and xanthan gum on gut and systemic health in a dietary intervention high in red meat.
This study consists of an in vitro gastrointestinal digestion experiment and a rodent feeding study. First, an in vitro digestion model was used to simulate the human gastrointestinal digestion (enzymatic + fermentation) of processed pork with and without 1 % κ-carrageenan or xanthan gum, heated at two different core temperatures (70 °C and 100 °C). The in vitro experiment was designed to determine protein digestibility and the formation of protein fermentation metabolites. It was hypothesized that the hydrocolloids and a high-temperature cooking would impair protein digestibility, enhancing protein fermentation and leading to an increased formation of protein fermentation metabolites in the simulated colon. Second, a rodent feeding trial studied the in vivo digestion of processed pork cooked at 70 °C with and without 1 % κ-carrageenan or xanthan gum. It was hypothesized that these hydrocolloids in a high red meat diet would alter colonic microbiota composition, fecal fermentation metabolites, intestinal histology, oxidative stress, inflammation markers, and gene expression in the colonic mucosa.
2. Material and methods
2.1. Chemicals
Enzymes used for in vitro digestion (α-amylase from hog pancreas (∼50 U/mg) [Merck code 10080], mucin from porcine stomach type II [M2378], pepsin from porcine gastric mucosa (>250 U/mg solid) [P7000], lipase from porcine pancreas type II (10–400 U/mg protein) [L3126], pancreatin from porcine pancreas (8 × USP specifications) [P7545], and porcine bile extract [B8631] were acquired from Sigma-Aldrich (Diegem, Belgium). Standards (carbon disulfide (CAS 75-15-0) [180173], indole (CAS 120-72-9) [I3408], p-cresol (CAS 106-44-5) [C85751], dimethyl disulfide (CAS 624-92-0) [471569], phenol (CAS 108-95-2) [33517], glutathione (CAS 70-18-8) [G1404], HNE-DMA [H9538], HEX (CAS 66-25-1) [115606] and PROP (CAS 123-38-6) [64409]) were purchased from Merck (Diegem, Belgium). Food-grade κ-carrageenan (E407a) and xanthan gum (E415) were purchased from Voordeelkruiden (Rotterdam, NL).
2.2. In vitro gastrointestinal digestion
Pork chops were purchased from a local butchery. Bones were manually removed and meat was manually chopped and minced in a grinder (Omega T-12) equipped with a 3.5 mm plate, whereafter 2 % NaCl and 0.5 % Na5(PO4)3 were added. The mixture was divided into three batches, to which either no hydrocolloids, 1 % κ-carrageenan or 1 % xanthan gum was added. Water was incorporated (10 % meat weight), and the batches were then manually mixed for 5 min until homogeneous. Each batch was then double-packed in temperature-resistant vacuum bags and cooked in an oven (Memmert U universal heating oven, Memmert, Germany). The core temperature was registered using a digital food thermometer (ThermPro, model TP-04). Heating of the meats was maintained for 15 min after reaching a core temperature of 70 °C or 100 °C for each treatment. Meats were cooled at room temperature and homogenized in a food processor (Moulinex DP700), vacuum-packed again and stored at −80 °C until digestion was conducted.
Meats were exposed to an in vitro enzymatic digestion simulating the conditions in the mouth, stomach and small intestine. In brief, meats (1.5 g) were sequentially incubated under stirring conditions with 2 ml of simulated saliva (5 min, room temperature), 4 mL of gastric juice (2 h, 37 °C), 0.6 mL of bicarbonate buffer (1 M, pH 8.0), 4 mL of duodenal juice and 2 mL of bile juice (2 h, 37 °C). The composition of the digestive juices can be found in Table S1. After enzymatic digestion, digests were subjected to a dialysis membrane to simulate intestinal absorption (n = 4 per treatment). A sulfur-free dialysis membrane of pore size 3.5 kDa (SpectraPor®7 Dialysis Membrane Pre-treated RC Tubing, Spectrum Laboratories, Inc) was chosen to allow free amino acids and small peptides to pass through the membrane. Dialysis occurred overnight, with the dialysate solution in continuous stirring conditions and replaced after 4 h. Thereafter, the retentate was poured into a vessel and immediately frozen (−80 °C). Lastly, the retentate was exposed to a large intestinal fermentation model (for 24 h, 37 °C), using the fecal microbiota from three individual healthy volunteers as previously described (Elias Masiques et al., 2024). In brief, digestion vessels were defrosted at 37 °C for 1 h and subsequently flushed with N2 to obtain an anaerobic environment. Next, 7 mL of fecal inoculum solution were added, and 1 mL of ethane was injected into the flask as an internal gas standard for the measurement of H2S. After fermentation for 24 h at 37 °C, H2S levels were measured immediately in the headspace of the vessels, and digestion samples were transferred into small tubes and stored at −80 °C.
The enzymatic digestions were performed in quadruplicate, whereafter the large intestinal fermentation was repeated three times, each time using a different human fecal inoculum (total n = 12 per treatment). The volunteers (22–31 years old, one female and two males) had no known gastrointestinal disorders or antibiotic treatment during the last six months. A consent form was signed prior participation to the study. The Kjeldahl method (ISO, 20483:2013) was used to determine the protein content in digests before and after dialysis. Protein digestibility was calculated as the proportion of total protein that passed through the dialysis membrane.
2.3. Rat feeding study
Lard and lean samples from mixed muscles of pork were purchased as fresh as possible from a local retailer. Meats were manually chopped and minced in a grinder (Omega T-12), equipped with a 10 mm plate. Lard was also chopped and minced and was manually added to the pork at a proportion of 15 % of the meat weight, and manually homogenized. Salt (2 % w/w) was added to all meat batches, after which either none or κ-carrageenan or xanthan gum were added (1 % w/w). Next, meats were packed in vacuum bags in equal weights and cooked in the oven until a core temperature of 70 °C was reached. Meats were cooled at room temperature and stored at 4 °C. The next day, meats (65 %) were mixed with the other ingredients to obtain the experimental diets (Table 1). Diets were vacuum packed in daily portions (±120 g per cage) and stored at −20 °C.
Table 1.
Composition of experimental meat diets.
| Pork | CGN | XNT | ||
|---|---|---|---|---|
| Ingredients | ||||
| Meat Product | g/kg | 650 | 650 | 650 |
| Sucrose | g/kg | 150 | 150 | 150 |
| Corn starch | g/kg | 146 | 146 | 146 |
| Cellulose | g/kg | 21.0 | 21.0 | 21.0 |
| Mineral mix | g/kg | 13.4 | 13.4 | 13.4 |
| Vitamin mix | g/kg | 6.30 | 6.30 | 6.30 |
| Calcium carbonate | g/kg | 1.30 | 1.30 | 1.30 |
| Choline bitartrate | g/kg | 1.20 | 1.20 | 1.20 |
| Safflower oil | g/kg | 10.8 | 10.8 | 10.8 |
| Macronutrients | ||||
| Dry matter | % | 56.7 | 57.2 | 57.4 |
| Crude fat | % | 9.91 | 9.63 | 10.0 |
| Crude protein | % | 18.9 | 18.7 | 18.8 |
| Crude ash | % | 2.91 | 3.04 | 3.00 |
| SFA | % FAME | 33.1 | 32.7 | 33.0 |
| MUFA | % FAME | 43.8 | 44.2 | 43.7 |
| PUFA | % FAME | 20.5 | 20.6 | 20.2 |
| LA | % FAME | 18.2 | 18.3 | 18.3 |
| n-6 PUFA | % FAME | 19.4 | 19.5 | 19.2 |
| ALA | % FAME | 0.76 | 0.76 | 0.75 |
| n-3 PUFA | % FAME | 1.10 | 1.07 | 1.05 |
| n-6/n-3 | 17.7 | 18.3 | 18.2 | |
| Heme-Fe | mg/kg | 2.14 | 2.31 | 2.33 |
| Oxidation products | ||||
| Total TBARS | nmol/g | 45.0 | 43.8 | 44.7 |
| PCC | nmol/mg protein | 2.99 | 3.93 | 3.25 |
| PROP | μg/g | 0.40 | 0.13 | 0.93 |
| HEX | μg/g | 17.2 | 15.7 | 33.3 |
| HNE | μg/g | 0.50 | 0.41 | 1.96 |
SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; LA = linoleic acid (C18:2n-6); n-6 PUFA = n-6 polyunsaturated fatty acids (LA. C20:2n-6. C20:3n-6. C20:4n-6. C22:4n-6. C22:5n-6); ALA = α-linolenic acid (C18:3n-3); n-3 PUFA = n-3 polyunsaturated fatty acids (ALA. C20:3n-3. C20:4n-3. C20:5n-3. C22:5n-3. C22:6n-3); TBARS = thiobarbituric acid reactive substances; PCC = protein carbonyl compounds; PROP = propanal; HNE = 4-hydroxynonenal; HEX = hexanal; FAME = fatty acid methyl esters.
The rat experiment was performed following the principles of laboratory animal care and the Belgian law on the protection of animals. The experiment was approved by the Ghent University Ethical Committee (ECD 21–68) and conducted at the Core ARTH Animal Facilities of Ghent University (BOF/COR/2022/007). Thirty male Sprague-Dawley rats (±200 g) (Janvier laboratories, Le Genest-Saint-Isle, France) were housed by two rats per cage during an adaptation period of one week, in environmental conditions of 22.0 ± 0.6 °C, 75 ± 5 % humidity and 15 h daylight. During the adaptation period, rats were fed a standard laboratory diet (Ssniff R/M−N pellets) (Ssniff, Soest, Germany) and water was provided ad libitum. After the adaptation period, rats were randomly assigned to one of the three experimental diets. Diets were provided fresh each day and offered ad libitum. Body weight and feed intake were monitored every 2 days at the cage level. On day 17, rats were individually housed for fecal collection during 24 h. At the end of the experiment (21 days on experimental diets), rats were anesthetized with 5 % isoflurane gas, followed by blood collection by cardiac puncture into heparin tubes until death occurred. Immediately after blood collection, plasma and red blood cells (RBC) were separated by low-speed centrifugation and divided in different aliquots. Organs (brain, colon mucosa, duodenal mucosa, heart, kidney, liver) and fat deposits (mesenteric and retroperitoneal) were carefully rinsed with 0.9 % NaCl solution, weighted and divided in aliquots. The colon length was measured, and the tissue was subdivided. The colon mucosa of the proximal half was collected and stored as such for the analysis of oxidation parameters, whereas the colon mucosa of the distal half was stored separately for the analysis of the transcriptome. One cm in the middle of the colon was stored in a 4 % aqueous formaldehyde solution for histology. All aliquots were snap frozen in liquid nitrogen and stored at −80 °C until analysis.
2.4. Chemical analysis of meats & diets
Contents of dry matter (ISO 1442–1973), crude protein (ISO 937–1978), crude fat (ISO 1444–1973) and crude ash (ISO 5984–2002) were determined in the diets. Lipids were extracted using chloroform/methanol (2/1; v/v), and subsequently, fatty acid methyl esters (FAME) were prepared using methanolic NaOH (0.5 M) and methanolic HCl (1/5 v/v). The FAMEs were analyzed by gas chromatography (HP6890, Brussels, Belgium) (Raes et al., 2001). Nonadecanoic acid (C19:0) was used as an internal standard to quantify the fatty acids. Levels of total SFA (sum of C08:0, C10:0, C12:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0 and C22:0), MUFA (sum of C14:1, C16:1, C17:1, c9C18:1, c11C18:1, and C20:1), n-3 PUFA (C18:3n-3, C20:3n-3, C20:4n-3 and C20:5n-3), ALA (C18:3n-3), long chain (LC) n-3 PUFA (sum of C20:4n-3, C20:5n-3, C22:5n-3 and C22:6n-3), n-6 PUFA (C18:2n-6, C18:3n-6, C20:2n-6, C20:3n-6 and C20; 4n-6), LA (C18:2n-6) and LC n-6 PUFA (sum of C20:2n-6, C20:3n-6, C20:4n-6, C22:4n-6, C22:5n-6) were calculated. Heme iron was determined spectrophotometrically in the meats, and calculated for the diet (Hornsey, 1956).
2.5. Microbiota composition of human inocula and colonic contents of rats
The DNA extraction of the three human fecal inoculum solutions and colonic contents of rats was performed by means of bead beating with a PowerLyzer (Qiagen, Venlo, the Netherlands) and phenol/chloroform extraction (Vilchez-Vargas et al., 2013). The DNA extract was sent to LGC Genomics GmbH (Berlin, Germany) for Illumina amplicon sequencing of the V3-V4 region of the 16S rRNA gene of the bacterial community on the MiSeq platform with V3 chemistry with the primers 341F (5′-CCT ACG GGN GGC WGC AG -3′) and 785Rmod (5′-GAC TAC HVG GGT ATC TAA KCC-3′) (detailed analysis in Supplementary information). The total number of bacteria in the rat colonic contents were quantified by qPCR (De Vrieze et al., 2015). A table containing the amplicon sequence variant (ASV) with their taxonomic assignments was generated. The raw fastq files of the rat experiment have been deposited in the National Center for Biotechnology Information (NCBI) database (Accession number PRJNA1254314). The microbial nucleotide Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1990) was used to identify the unclassified ASVs from Tannerellaceae and Muribaculaceae which were significant after LEfSe analysis.
2.6. Fecal and cecal volatile organic compounds
Volatile organic compounds in in vitro ferments and rat feces were analyzed as described in (Elias Masiques et al., 2024, 2025). In brief, volatiles were extracted using a solid phase micro-extraction (SPME) and separated and detected by a gas chromatography-mass spectrophotometer (GC–MS) (Thermo, Finnigan). Peaks were integrated for area quantification by targeting the quantification ion as follows (m/z): indole 117; phenol 94; x-cresol 108; carbon disulfide 76; methanethiol 48; dimethyl disulfide 94; dimethyl trisulfide 126.
Branched-chain fatty acids (iso-butyrate and iso-valerate) and short-chain fatty acids (acetate, propionate, butyrate and valerate) in cecal contents of rats were measured by gas chromatography (HP 7890A, Agilent Technologies, Diegem, Belgium), equipped with a flame ionization detector and a Supelco Nukol capillary column (30 m × 0.25 mm × 0.25 μm, Sigma-Aldrich, Diegem, Belgium) as previously described (Gadeyne et al., 2016). In brief, a 10 % formic acid solution containing 2-ethyl butanoic acid as internal standard was added to the cecal content. Following centrifugation (22.000g at 4 °C), the supernatant was filtered and transferred into a glass vial, followed by injection on the GC.
Ammonia was measured spectrophotometrically at 625 nm in the same supernatant of the cecal content, with a calibration curve of NH4Cl following the reaction with phenol, sodium nitroprusside, sodium hydroxide and sodium hypochlorite (Chaney and Marbach, 1962).
2.7. Oxidative stress
Concentrations of protein carbonyl compounds (PCC) in diets and stomach contents were determined spectrophotometrically following reaction with 2,4-dinitrophenylhydrazine according to (Ganhão et al., 2010). Levels of free 4-HNE, HEX and PROP in diets and stomach content were detected by HPLC-Fluorescence (Agilent 1200 series, Waldbronn, Germany) following their derivatization with cyclohexanedione, as described previously (Van Hecke et al., 2017). For the analysis of TBARS and GSH-Px activity, phosphate buffer (pH 7; 50 mM) was added to the frozen tissues in a 1/5 ratio (w/v). Samples were homogenized by ultra-turrax, and centrifuged (10 min, 12.000g, 4 °C). Then, the supernatant was filtered through glass wool and transferred into different aliquots to immediately determine the activity of GSH-Px and TBARS. The measurement of GSH-Px activity in plasma and organ extracts occurred by the oxidation of NADPH, whereby one unit of GSH-Px activity was defined as the amount of extract needed to oxidize 1 μmol NADPH/min at 25 °C (Hernández et al., 2004). The total fraction (free + bound) of TBARS were measured in plasma, stomach content and tissue extracts, by measuring the absorbance at 532 nm following the reaction with 2-thiobarbituric acid and quantified with a standard curve with 1,1,3,3-tetramethoxypropane, as described by (Grotto et al., 2007). The concentrations of glutathione in the red blood cell (RBC) fraction were determined by HPLC using γ-glutamyl glutamate as internal standard (Degroote et al., 2012).
2.8. C-reactive protein, calprotectin and low-density lipoprotein
Quantification of plasma C-reactive protein (CRP) (RAB0097, Merck, Diegem, Belgium), fecal calprotectin (S100A8/S100A9, Sanbio KR6936, Uden, The Netherlands) and plasma low-density lipoprotein (LDL) (E-EL-R0579, Elabscience, Texas, USA) were performed using commercial ELISA kits and measured on a microplate reader (Infinite M Nano, Tecan, Grödig, Austria), according to manufacturer's instructions.
2.9. Histology
Colonic tissue samples were fixed in a formaldehyde solution and processed in an automatic tissue processor under standard conditions (Shandon, Pittsburgh, PA, USA). In brief, the tissue was cut into small and homogeneous sections to be dehydrated and embedded in paraffin wax. Next, the embedded tissue was trimmed and placed in a microscope slide. Lastly, histological staining was performed on paraffine-embedded colonic sections with periodic acid Schiff (PAS) staining to assess crypt depth and number of goblet cells per crypt. A minimum of four sections of colon tissue were assessed, with a total of 20 reads of well-oriented crypts per animal.
2.10. Transcriptomics
Total RNA was extracted from colonic mucosa tissues using the Total RNA Fatty and Fibrous Tissue kit (Bio-Rad Laboratories, California, USA) in an RNase-free environment. The RNA quantity, purity, and integrity were evaluated by nanodrop, and with the Experion automated electrophoresis station, utilizing the Experion™ RNA StdSens kit (Bio-Rad, Temse, Belgium). The RNA was stored in a −80 °C freezer until shipment to NxtGent (Ghent University, Ghent, Belgium) for sequencing. Two samples were excluded due to low quality of the RNA, checked using the Agilent DNF-472 HS RNA (15 nt) Kit on a Fragment Analyzer 5200 system (Agilent). Sequencing libraries were constructed using the QuantSeq 3’ mRNA-Seq V2 Library Prep Kit FWD for Illumina (Lexogen) and the UMI Second Strand Synthesis Module for QuantSeq FWD (Illumina) and sequenced as single-read 75 on an Aviti device (Element Biosciences). The unique molecular identifier (UMI) and spacer were removed using UMI-tools (v1.1.6) (Smith et al., 2017). Raw sequencing reads were inspected using FastQC (v0.12.1) for their quality and length (Wingett and Andrews, 2018). Putative contaminations were checked using FastQ Screen (v0.15.3). Adaptor trimming was done using cutadapt (v4.9) with added filtering of reads containing ambiguities or not passing the phred score threshold of 20 (Martin, 2011). The quality of the remaining read pairs was checked using FastQC. Trimmed reads were aligned on the rat reference genome (Rattus_norvegicus.mRatBN7.2, ENSEMBL release 113) using the splice-aware STAR (v2.7.11b) mapper (Dobin et al., 2013). UMI-based deduplication of mapped reads was done with UMI-tools (v1.1.6). Feature counting at the gene level was done using rsem-calculate-expression (RSEM v1.3.1) (Li and Dewey, 2011).
2.11. Statistical analysis
For all data, normality and homogeneity of variance were determined by Shapiro-Wilk and Levene test, respectively. For the in vitro data, protein digestibility was analyzed with a mixed ANOVA procedure (SAS Enterprise Guide 8), with the fixed effects ‘Additive’ (PAdd), ‘Temperature’ (PT°C), and their interaction term (PA × T), and post hoc comparisons were conducted using Tukey's adjustment. For the in vitro fermentation metabolites, the non-parametric Kruskal-Wallis test (SPSS Statistics 27) was used with ‘Additive’ (PAdd), ‘Temperature’ (PT°C) and the combined effect ‘Additive × Temperature’ (PC) as independent variables, and significance levels were adjusted with the Bonferroni correction for multiple tests.
For the rat trial, feed intake and body weight of rats were statistically analyzed using a repeated-measures ANOVA procedure with ‘rat ID’ as a random factor and ‘feeding day’ as a repeated effect. If data was normally distributed, a mixed model ANOVA procedure was used with the fixed effect of ‘Additive’, and the random factor ‘euthanasia day’. Tukey-adjusted post hoc tests were performed for all pairwise comparisons. When data normality and homogeneity of variance was violated, an independent samples Kruskal-Wallis test with pairwise comparisons was performed, with ‘Additive’ as independent variable. For all statistical tests, p ≤ 0.05 was considered significant.
Differences in colonic bacterial communities were identified by using LEfSe (linear discriminant analysis effect size) (http://huttenhower.sph.harvard.edu/galaxy) between dietary treatments as main class (Segata et al., 2011) with the following conditions: 1) alpha values for the factorial Kruskal-Wallis test among classes, and for the pairwise Wilcoxon test among subclasses were <0.05; 2) the threshold on the logarithmic linear discriminant analysis (LDA) score for discriminative features was set to 2.0, and 3) the strategy for multi-class analysis was one-against-all. The α-diversity of the microbiota was assessed by determining the Shannon and Inverted Simpson indexes. The β-diversity, by using Principal Coordinate Analysis (PCoA) based on the Bray-Curtis dissimilarity metric, and the Permutational Multivariate Analysis of Variance (PERMANOVA) to test statistical significance, using the Rstudio packages vegan (Oksanen et al., 2013) and ggplot2 (Wickham, 2016). Spearman correlation analysis was performed to explore the associations between discriminant bacterial genera identified through LEfSe, restricted to those with relative abundances ≥1 %, using the Rstudio packages corrplot (Wei and Simko, 2010) and circlize for the Chord diagram (Gu et al., 2014). P-values were corrected for multiple testing using the Benjamini-Hochberg procedure.
For the transcriptomics data, statistical analyses were done in R (v4.4.1) using the edgeR (v4.2.2) package for differential gene expression (Robinson et al., 2010). The preferred and more robust quasi likelihood model including ‘euthanasia day’ as batch effect was used. Pairwise comparison at the gene level was done using the F-test for a more reliable error rate. Correction of the p-values for repeated testing (PAdj) was done with the Benjamini-Hochberg method. The fold-change of all genes were used to screen for putative relevant pathways enrichments using KEGG pathway data and the GAGE (v2.54.0) package in R (Luo et al., 2009). P-values were corrected for multiple testing using the Benjamini-Hochberg procedure.
3. Results
3.1. In vitro digestion
The addition of xanthan gum reduced protein digestibility (−11 %, p = 0.001), whereas κ-carrageenan had no effect (Fig. 1). The heating treatment did not significantly influence any of the studied parameters in the digests (Table S2). Hence, data were grouped per additive irrespective of the cooking temperature (n = 24).
Fig. 1.
Protein digestibility and fermentation metabolites after in vitro digestion and fermentation of pork without (Pork, grey) or with κ-carrageenan (κ-CGN, orange) or xanthan gum (XNT, green). Each treatment was tested using fecal inocula from 3 individual donors, with 4 technical replicates per donor. As the heating condition had no significant effect, results were pooled (total n = 24 per treatment). Protein digestibility and H2S levels were analyzed following a mixed model ANOVA procedure with the fixed factor ‘Additive’. Tukey-adjusted post hoc tests were performed for pairwise comparisons. For sulfur and protein metabolites, a non-parametric Kruskal-Wallis test with pairwise comparisons was performed using the effect ‘Additive’. Significant p-values were adjusted by the Bonferroni correction for multiple tests.
Following the 24 h fermentation, most sulfur metabolites (H2S, methanethiol, dimethyl disulfide, dimethyl trisulfide, dimethyl tetrasulfide) and protein fermentation metabolites (phenol, indole) were significantly higher in in vitro ferments when xanthan gum was added to the meats (up to 4-fold, Fig. 1), whereas the addition of κ-carrageenan did not influence any fermentation marker. No differences in CS2 and x-cresol levels were observed among treatments. No major differences were observed in metabolite formation across the different inocula, except for a lower phenol formation using inoculum 1 compared to other inocula (Fig. S1).
The microbial community of the inocula can be found in Fig. S2. Across all three applied fecal inocula, Firmicutes was the predominant phylum, followed by Bacteroidota. The phylum Verrucomicrobiota, which includes the genus Akkermansia, accounted for 10 % of the relative abundance in inoculum 2, but was nearly absent in the other inocula. The Ruminococcaceae (14 %–33 %) and Lachnospiraceae (12 %–27 %) were the main bacterial families among inocula whereas Bacteroidaceae was more abundant in inoculum 1 (21 % vs. circa 8 % in the other inocula) and Prevotellaceae in inoculum 3 (30 % vs circa 2 % the other inocula). The family Desulfovibrionaceae showed a very low relative abundance in all inocula (average of 0.06 % in all groups).
3.2. Animal trial
3.2.1. Experimental diets
The proximate analysis of the experimental diets can be found in Table 1. As intended, the three diets exhibited similar results in the proximate analysis. The pork diet including κ-carrageenan contained higher levels of PCC compared to the control pork diet (+31 %), whereas levels of TBARS and PROP were similar among diets. Surprisingly, levels of HEX and 4-HNE were higher in the pork diets with xanthan gum (2 and 4-fold, respectively).
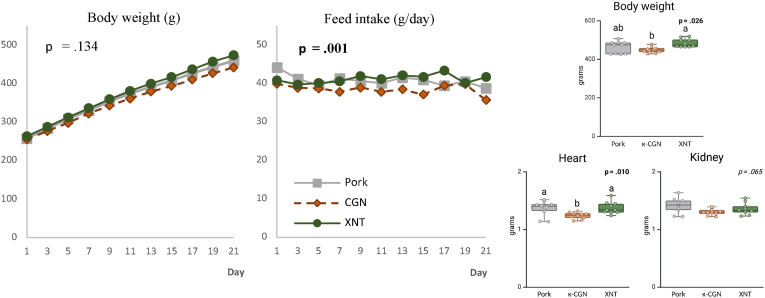
3.2.2. Animal performance
Rats on the κ-carrageenan diet had a lower feed intake compared to the rats on the other diets (circa −6 %, p < 0.001) (Fig. 2). In accordance, these rats had a lower final body weight (−7 % vs. xanthan gum, p = 0.026), lower heart weight (−9 % vs. other diets, p = 0.010) and lower kidney weight (−8 % vs. control diet, p = 0.053). No significant differences were found in the weight of other organs, fat depositions, and colon length (Table S3).
Fig. 2.
Average feed intake and body weight of rats throughout the feeding period, and final body weight, heart weight and kidney weight in rats fed pork, pork with κ-carrageenan (κ-CGN) or pork with xanthan gum (XNT), (n = 10 per treatment). For feed intake and body weight, a repeated-measures ANOVA procedure with ‘rat ID’ as a random factor and ‘feeding day’ as a repeated effect was used to test significance. For the rest, a mixed model ANOVA procedure was used with the fixed effect ‘Additive’. Tukey-adjusted post hoc tests were performed for pairwise comparisons.
3.2.3. Colon microbiota composition
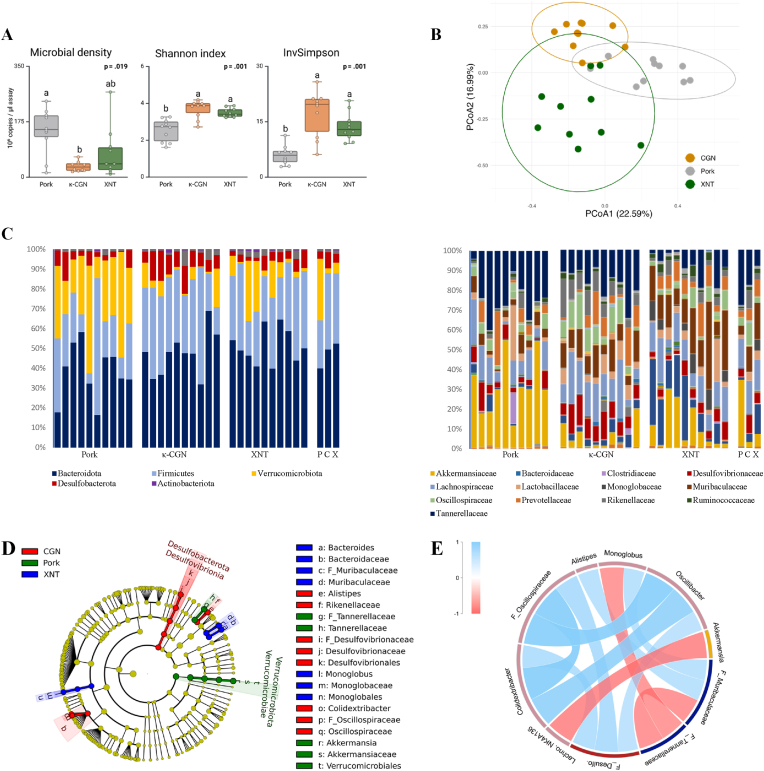
The total bacterial count, measured by qPCR, was significantly decreased in rats fed the κ-carrageenan diet compared to the control pork group (4-fold, p = 0.018), but not the xanthan gum group (p = 0.170) (Fig. 3). The α-diversity was higher in rats that consumed either of both additives, as indicated by the significantly higher levels of the Shannon (+41 %) and InvSimpson (+160 %) indices, when compared to rats that consumed the control pork. Analysis of β-diversity, which highlights differences in microbial community composition among samples, revealed significant variation across groups (R2 = 0.284, p PERMANOVA = 0.001).
Fig. 3.
Colonic microbial characterization. A Bacterial density and α-diversity (Shannon index and Inverted Simpson indices) in rats fed pork (grey), pork with κ-carrageenan (κ-CGN, orange) or pork with xanthan gum (XNT, green). B PCoA plot based on Bray-Curtis dissimilarity. C Relative microbial abundance at the phylum and family levels, showing individual animals (left bars) and treatment medians (right bars). D Cladogram from the LEfSe analysis, identifying taxa with significant variations among diets (LDA = 4). E Network analysis of bacterial genera with strong correlations (|r| ≥ 0.5), indicating positive correlations in blue and negative correlations in red. For bacterial density (median with 95 % confidence interval) an independent samples Kruskal-Wallis test with pairwise comparisons was performed, using the effect ‘Additive’ as an independent variable. Significant p-values were adjusted by the Bonferroni correction for multiple tests. For Shannon and Inverted Simpson, a mixed model ANOVA procedure was used, with the fixed effect ‘Additive’, and the random factor ‘euthanasia day’. Tukey-adjusted post hoc tests were performed for pairwise comparisons.
At the phylum level, the most predominant phyla were Bacteroidota (38 % pork vs. circa 50 % additives) and Firmicutes (23 % pork vs. circa 35 % additives). Remarkably, Verrucomicrobiota were more abundant in rats consuming the control pork diet (15-fold vs. κ-carrageenan and 6-fold vs. xanthan gum). Desulfobacteriota accounted for a median relative abundance of 8 % in the κ-carrageenan group and circa 4 % in both other groups. LEfSe analysis identified 63 discriminant taxa among the different diets (Fig. S3). A heatmap displaying the significant taxa ranked according to the LDA score is presented in Table 2. Bacterial taxa with a median relative abundance of <0.1 % were excluded. The genus Akkermansia had the highest LDA score (LDA = 5.48), exhibiting a relative abundance of 29.5 % in the pork group, which was 8-fold higher compared to the rats on the other diets (p < 0.001). Similarly, an unclassified genus from the family Tannerellaceae was more abundant in the control pork group (+64 % vs. κ--carrageenan and +272 % vs. xanthan gum, p = 0.001). In contrast, rats on the κ-carrageenan diet contained relatively higher abundances of the family Rikenellaceae (7-fold vs. pork and 3-fold vs. xanthan gum), mainly due to the genus Alistipes (p < 0.001) and Rikenellaceae RC9 gut group (p = 0.002). Rats on the κ-carrageenan diet had higher relative abundances of the families Desulfovibrionaceae (2-fold, p = 0.015) and Oscillospiraceae (4-fold, p = 0.017), including the genera Colidextribacter, Oscillibacter and an unclassified genus of the family Oscillospiraceae. This group also had higher abundances of an unclassified genus of Peptococcaceae (p = 0.005), and an unclassified genus of the order Gastranaerophilales (p = 0.017), and the genus Tuzzurella (p = 0.031), Lachnospiraceae FCS020 group (p = 0.002) and Negativibacillus (p = 0.039). In the xanthan gum group, several taxa were significantly more abundant, including an unclassified genus of the family Muribaculaceae (2-fold, p < 0.001), as well as Bacteroides (6-fold, p = 0.002), Monoglobus (400-fold, p < 0.001), Ruminococcaceae (+33 %, p = 0.011) and Lachnospiraceae NK4A136 group (2-fold, p = 0.005). In lower abundances, the group had higher levels of Ligilactobacullus (p = 0.024) and Marvinbryantia (p = 0.013).
Table 2.
Heat map on median relative abundance, ranked from highest to lowest LDA score.
Data is sorted from high to low linear discriminant analysis (LDA) score with the factor diet as main effect. Data is presented as median relative abundance (%). Taxonomic levels in bold are significant by LEfSe analysis (p < 0.05). Bacteria with abundances lower than <0.1 % are not shown.
Correlation analysis identified 26 significant associations among discriminant bacterial genera (Fig. S4). Strong correlations (|r| ≥ 0.5) are visualised in a Chord diagram to illustrate potential relationships among bacterial genera (Fig. 3). In brief, the strongest correlations showed Akkermansia to be negatively correlated to Lachnospiraceae NK4A136 (r = −0,76, p < 0.001); and F_Tannerellaceae to F_Muribaculaceae (r = −0,72, p < 0.001) and Monoglobus (r = −0,70, p < 0.001). Positive correlations were found for F_Desulfovibrionaceae and F_Oscillospiraceae (r = 0,69, p < 0.001).
A phylogenetic tree with the results from the BLAST analysis can be found in Fig. S5.
3.2.4. Fermentation metabolites
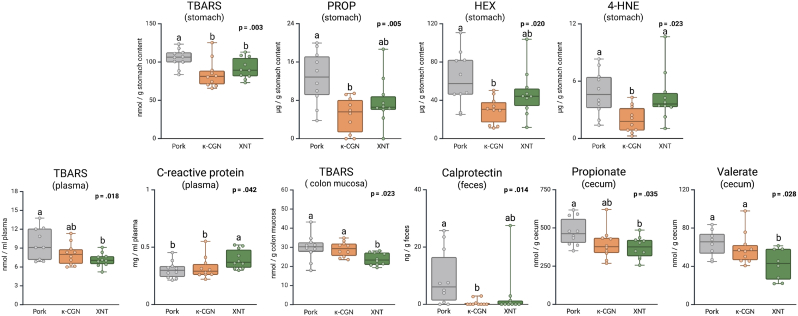
Rats fed the xanthan gum diet showed a significant reduction in cecal levels of propionate (−23 %, p = 0.035) and valerate (−33 %, p = 0.028) compared to those on the control pork and κ-carrageenan diets (Fig. 4). The levels of the other fermentation metabolites were not significantly influenced by the dietary treatments (Table S4).
Fig. 4.
Oxidative stress, inflammation markers and metabolites in blood, stomach, cecum and colon mucosa and feces in rats fed pork (grey), pork with κ-carrageenan (κ-CGN, orange) or pork with xanthan gum (XNT, green). For all parameters – except calprotectin – a mixed model ANOVA procedure was used, with the fixed effect ‘Additive’, and the random factor ‘euthanasia day’. Tukey-adjusted post hoc tests were performed for pairwise comparisons. For calprotectine, an independent samples Kruskal-Wallis test with pairwise comparisons was performed, using the effect ‘Additive’ as an independent variable. Significant p-values were adjusted by the Bonferroni correction for multiple tests.
3.2.5. Oxidative stress in rats
The stomach contents of the rats generally exhibited higher levels of TBARS (2-fold), PCC (2-fold), PROP (30-fold), 4-HNE (6-fold) and HEX (2-fold) compared to the levels found in the diets (Fig. 4). Whereas the levels of TBARS in the experimental diets were similar, the addition of both hydrocolloids decreased the formation of TBARS in the stomach content (−16 %). Rats on the κ-carrageenan diets additionally had reduced PROP (−60 %, p = 0.003), HEX (−52 %, p = 0.015) and 4-HNE (−55 % p = 0.035) levels in stomach content. No differences were found in PCC levels in stomach content.
Rats consuming the pork with xanthan gum had lower levels of TBARS in plasma (−30 %, p = 0.020) and colon mucosa (−8 %, p = 0.041) compared to rats fed control pork. Levels of TBARS and GSH-Px activity in other organs were not different between diets, nor were the glutathione levels in blood affected (Table S5).
3.2.6. Inflammation and low-density lipoprotein
The addition of xanthan gum to the pork diets increased CRP levels in plasma compared to rats fed the control pork diet (+33 %, p = 0.051), but not compared to the κ-carrageenan diet (p = 0.105). In contrast, the addition of κ-carrageenan to the diet did not alter CRP levels compared to the control pork group (Fig. 4). Fecal calprotectin levels were higher in rats on the control pork diets compared to rats consuming the pork diets including κ-carrageenan (17-fold, p = 0.017) or xanthan gum (3-fold, p = 0.084). Notably, in the xanthan gum group, one rat had exceptionally high calprotectin levels relative to the others (27 ng/g feces vs the other rats ranging between 0.04 and 2.9 ng/g feces). If excluding this rat from the analysis, the difference became statistically significant (p = 0.008). The LDL levels were not significantly altered by the addition of the additives (Fig. S6).
3.2.7. Histology
The average number of goblet cells did not differ significantly among the groups, with values of 28.3, 32.1, and 31.7, for control pork, κ-carrageenan and xanthan gum respectively (SEM = 1.45, p = 0.517). Similarly, crypt depth showed no significant differences, with mean values of 252 μm for control pork, 257 μm for κ-carrageenan, and 266 μm for xanthan gum (SEM = 4.20, p = 0.431).
3.2.8. Transcriptomics
In the initial analysis, 3325 genes were significantly different among diets (p < 0.05); however, no differences remained after Benjamini-Hochberg correction between the control pork group and the hydrocolloid-containing groups. Despite this, KEGG pathway analysis revealed 33 pathways down-regulated in rats fed pork with κ-carrageenan compared to control pork, 31 of which were associated with inflammatory processes in the colon (e.g., Th17, Th1, and Th2 cell differentiation, natural killer cell-mediated cytotoxicity, primary immunodeficiency, the intestinal immune network for IgA production, etc.). A similar outcome was observed for pork with xanthan gum versus control pork, with 34 pathways down-regulated, 33 of which were linked to inflammatory or immunological processes (Fig. 5). Interestingly, two genes were significantly down-regulated in the colonic tissue of rats fed the κ-carrageenan diet (vs. xanthan gum): the B4galnt2 (PAdj = 0.035, fold-change (FC) 4.32) and Arg2 (PAdj = 0.048, FC 3.81). In addition, several genes showed a statistical trend toward differential expression: Dmbt1 (PAdj = 0.075, FC 3.83), Stx19 (PAdj = 0.075, FC 1.86), St3gal2 (PAdj = 0.075, FC 0.59), Slc35b1 (PAdj = 0.075, FC 1.45), and Pla2g2a (PAdj = 0.075, FC 3.00) (Fig. S7).
Fig. 5.
Significant up- or down-regulated KEGG pathways in the colon of rats fed pork with κ-carrageenan (left graph), or xanthan gum (right graph) sorted by logPAdj.
4. Discussion
In this study, we used both in vitro gastrointestinal digestion and an in vivo rodent trial to investigate gut health implications following the consumption of cooked pork to which either κ-carrageenan or xanthan gum was used during processing. Protein digestibility and subsequent microbial fermentation metabolites were assessed in vitro, while the rodent model examined broader gut and systemic health outcomes after three weeks of dietary intervention.
4.1. Effects of hydrocolloids on protein digestibility
In the present in vitro digestion study, the addition of 1 % xanthan gum to pork decreased protein digestibility, whereas κ-carrageenan had no such effect. These results are consistent with Zeng et al. (2023), who demonstrated decreased digestibility in meat sausages with 0.3–0.8 % xanthan gum, and with Ben David et al. (2023) where 0.4 % of different types of carrageenan added to beef meatballs had no effects on digestibility. In contrast, David et al. (2020a) found that carrageenan and xanthan gum (both at 0.5 %) diminished proteolysis in chocolate milk. These authors hypothesized that hydrophilic polysaccharides might interact electrostatically with proteins, hereby inhibiting proteolysis. Thus, the variable effects of carrageenan observed across studies suggest a dependency on the used food matrix. In addition, Cao et al. (2022) highlighted different interactions between meat proteins and κ-carrageenan depending on the incorporation form of κ-carrageenan (dry powder or pre-hydrated suspension). When κ-carrageenan was pre-hydrated, it could disperse more uniformly in the meat protein gel than when added as powder, as was done in the present study. Adding a water suspension containing κ-carrageenan to frankfurters resulted in lower protein digestibility compared to the addition of κ-carrageenan as a powder or brine suspension (Li et al., 2023). Understanding these interactions across diverse meat matrices is key to accurately assess their impact on protein digestibility and optimizing food formulations for improved nutritional outcomes.
The reduced in vitro protein digestibility in the xanthan gum group provided more substrate for microbial fermentation, leading to a higher formation of protein fermentation metabolites. The absence of sulfur metabolite formation during fermentation of food-grade κ-carrageenan is in line with reports that high molecular weight carrageenan (≥100 kDa) resists microbial degradation (Yin et al., 2021). Although fermentation metabolites were largely unaffected during in vivo digestion, the dietary additives induced notable differences in the colonic microbial community, as discussed in the following section.
4.2. Effects of hydrocolloids on colonic microbiota composition
Dietary supplementation with hydrocolloids decreased bacterial density but increased α-diversity, as indicated by enhanced Shannon and InvSimpson indices. Akkermansia was the taxon that mostly differed between diets, with a large abundance in rats consuming the pork diet without additives. Akkermansia thrives by mucin degradation and promotes mucus layer renewal, a process generally beneficial for gut health (Ioannou et al., 2025). However, excessive proliferation of Akkermansia, especially in a fiber-deprived dietary context, can degrade the mucus barrier and potentially increase pathogen susceptibility (Desai et al., 2016; Wolter et al., 2024). Thus, maintaining an optimal balance between Akkermansia and other commensal bacteria appears crucial. Additionally, the outgrowth of Akkermansia may explain the higher bacterial density but lower diversity in rats fed pork without hydrocolloids.
An unclassified taxon from the Tannerellaceae family, matching to Parabacteroides goldsteinii, was also notably abundant in the pork diet. The BLAST analysis reported the ASV from Tannerellaceae (ASV2) showed 100 % sequence identity with Parabacteroides goldsteinii. Gaifem et al. (2024) previously reported a beneficial synergy between Parabacteroides distasonis and Akkermansia in supporting gut barrier integrity. In that experiment, these bacteria collectively accounted for a relative abundance ranging between 1 and 16 % for Akkermansia and 0.1–6 % for Parabacteroides. The coexistence of these taxa in our experiment aligns with Gaifem et al. (2024), suggesting a potentially beneficial functional relationship. Nonetheless, the two taxa seem to strongly dominate the ecosystem since together they accounted for nearly 60 % of the total bacterial community, which could result in an alteration of the microbial homeostasis. The increased presence of mucin-degrading taxa in rats on the control pork suggests enhanced mucus turnover or erosion, potentially compromising barrier integrity. This could contribute to elevated fecal calprotectin levels, reflecting low-grade gut inflammation and host immune activation.
Previous studies investigating gut microbiota responses to carrageenan predominantly administered it dissolved in drinking water or PBS, complicating comparisons due to differential outcomes dependent on administration route. Mi et al. (2020) reported that κ-carrageenan decreased Akkermansia, and increased Alistipes in the colon of rats, only when administered through drinking water in a high-fat diet, whereas dietary incorporation or low-fat diets negated these effects. In contrast, our findings show that even when κ-carrageenan was incorporated into a protein-rich food matrix such as pork, it still modulated the colonic microbiota, as evidenced by increased Desulfovibrionaceae, Alistipes, and Oscillospiraceae. In our experiment, Akkermansia did not increase alongside Desulfovibrionaceae, despite previous reports of their synergistic expansion during red meat and heme iron intake (Ijssennagger et al., 2015). This divergence may be explained by the expansion of Alistipes, which could have competed with Akkermansia for mucin degradation, as both genera are known to utilize mucosal sugars (Pereira et al., 2020). All currently known Alistipes spp. possess galactosidase enzymes (Glover et al., 2022), enabling the degradation of galactose-containing substrates, including mucin and potentially the galactose backbone of κ-carrageenan. Given their close association with the mucus layer and sensitivity to epithelial inflammation (Pereira et al., 2020), Alistipes may play a key role in early mucus destabilization. Although carrageenan itself may not be directly utilized by Desulfovibrio (Yin et al., 2021), and the formation of sulfur metabolites was not observed during in vitro fermentation, its presence may interact with the mucus layer in a way that secondarily promotes Desulfovibrionaceae growth, possibly through increased availability of sulfate released from mucins. Wolter et al. (2024) hypothesized that, under fiber-free conditions, Desulfovibrio grows by exploiting sulfate groups from mucin, facilitated via cross-feeding interactions with Bacteroides thetaiotaomicron. At the same time, the degradation of mucin glycans may allow their utilization by other mucosa-associated taxa, including butyrate-producing Clostridia from the Oscillospiraceae family (Shuoker et al., 2023), which could explain the concomitant rise in Oscillospiraceae observed in the present study. The relative contribution of mucin versus carrageenan degradation to the proliferation of Alistipes and Desulfovibrionaceae warrants further investigation.
Dietary incorporation of xanthan gum increased the abundance of Ruminococcaceae and Bacteroides, two bacterial taxa recognized for their ability to degrade xanthan gum. Ruminococcaceae can cleave xanthan gum's polysaccharide backbone, releasing oligosaccharides fermented subsequently by Bacteroides intestinalis (Ostrowski et al., 2022). The Muribaculaceae family was notably enriched in rats fed xanthan gum diets, aligning with previous findings demonstrating sensitivity of Muribaculaceae abundance to dietary fiber shifts (Rous et al., 2025). Similar results were observed (Wu et al., 2023), reporting increased Muribaculaceae abundance in mice consuming xanthan gum. The most abundant ASVs from the Muribaculaceae family (ASV13 and ASV23) closely matched Muribaculum gordoncarteri (93.2 % identity). There appears to be a consistent microbial response to the presence of xanthan gum; however, the extent to which these changes in gut microbiota influence host health in the long term remains unclear. For instance, xanthan gum exhibited a protective effect against Clostridioides difficile colonization, primarily by stabilizing gut microbiota compared to a standard chow diet (Schnizlein et al., 2020).
4.3. Effects of hydrocolloids on gut health
Unexpectedly, signs of colonic inflammation were observed in rats fed pork without hydrocolloids, which was the group that was assumed to represent the lower-risk baseline. This was evidenced by the elevated fecal calprotectin and enhanced inflammatory signaling pathways identified via KEGG analysis. This inflammation might have resulted from the overgrowth of Akkermansia and Parabacteroides, known for their mucus-degrading capacity when present in excessive abundance. It is important to note that the diets were intentionally fiber-deprived to mimic typical Western dietary patterns. Consistent with our findings, Wolter et al. (2024) showed that fiber-deprivation increases Akkermansia abundance and heightens susceptibility to infection. The κ-carrageenan and xanthan gum modulated the gut microbiota in ways that appeared to attenuate colonic inflammation. These hydrocolloids, especially xanthan gum, may have mimicked dietary fiber due to their polysaccharide nature (Nie et al., 2023), contributing to reduced colonic inflammation. Gene expression analysis revealed that xanthan gum and κ-carrageenan differentially modulated genes involved in mucosal glycosylation and immune function. Compared to xanthan gum, κ-carrageenan was associated with significantly lower expression of B4galnt2 and Arg2, and showed statistical trends for upregulation of Dmbt1, and others. B4galnt2 plays a role in mucin-type glycosylation and may influence microbial adherence (Staubach et al., 2012), while Arg2-deficient mice exacerbated colitis compared to wild-type mice (Imazu et al., 2024). Dmbt1 may play a role in intestinal mucosal protection and prevention of inflammation (Renner et al., 2007). While these differences suggest distinct host responses to the two hydrocolloids, the direction and magnitude of these changes relative to control remain to be determined.
The reduced lipid oxidation levels in stomach contents following the consumption of pork with hydrocolloids align with previous reports demonstrating antioxidant activity of κ-carrageenan and xanthan gum in meat and other food systems (Udo et al., 2023; Fu et al., 2025). Despite the superior antioxidant activity of κ-carrageenan in stomach contents, only xanthan gum lowered TBARS in colon mucosa and plasma. The reduced oxidative stress in the colonic mucosa of xanthan gum-fed rats may be attributed to their more balanced microbial composition and lower inflammatory activity, processes that are closely interrelated (Muro et al., 2024). However, the elevated plasma CRP levels in the xanthan gum group remain unexplained, underscoring the need for further investigation into systemic effects beyond the gut. Also, whether the reduced body and heart weights observed in the κ-carrageenan group resulted solely from lower feed intake, or also involve metabolic effects, remains unclear. Given prior evidence that κ-carrageenan impairs lipid digestion and absorption (Huang et al., 2023, 2024), such mechanisms cannot be excluded in the present context.
5. Conclusion
In conclusion, the food hydrocolloids κ-carrageenan and xanthan gum distinctly influenced protein digestibility, microbiota composition, inflammatory pathways, and oxidative processes in a high red-meat diet. Consumption of a low fiber pork diet without hydrocolloids promoted the growth of Akkermansia and Tannerellaceae in the colon, whereas κ-carrageenan favored Desulfovibrio and Alistipes, and xanthan gum stimulated Muribaculaceae and Bacteroides. There seemed to be an inflammatory state in the colon of rats consuming pork without hydrocolloids, whereas both additives were associated with reshaping the colonic microbiota and lowering oxidative stress markers. Differential expression of the genes B4galnt2 and Arg2 was observed in rats fed pork with κ-carrageenan and xanthan gum. Although these effects are likely influenced by the low fiber content of the experimental diets, this study provides novel insights into how κ-carrageenan and xanthan gum influence gut health and modulate gut microbiota.
CRediT authorship contribution statement
Núria Elias Masiques: Writing – original draft, Investigation, Formal analysis, Data curation, Visualization. Sam Vermeiren: Writing – review & editing, Investigation, Formal analysis. Jo De Vrieze: Writing – review & editing, Methodology. Yannick Gansemans: Writing – review & editing, Methodology. Dieter Deforce: Writing – review & editing, Methodology. Filip Van Nieuwerburgh: Writing – review & editing, Methodology. Stefaan De Smet: Writing – review & editing, Resources, Methodology, Supervision, Funding acquisition, Conceptualization. Thomas Van Hecke: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of generative ai and ai-assisted technologies in the Writing process
During the preparation of this work the author(s) used OpenAI's ChatGPT in order to improve the clarity of the introduction and discussion sections of this manuscript. After using this tool, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Funding
This work was funded by the Flanders Research Foundation (FWO) project G038620N and the Special Research Fund (BOF) BOF.BAS.2022.0016.01.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Acknowledgements
The authors acknowledge the assistance of Sabine Coolsaet and Dr. Els Vossen from LANUPRO; Prof. Robrecht Raedt, Dr. Karlijn Debusschere, Sabine De Groote and all the team from the Core ARTH Animal Facilities of Ghent University (BOF/COR/2022/007). We acknowledge the use of OpenAI's ChatGPT for editorial assistance in enhancing the clarity of the introduction and discussion sections of this manuscript.
Handling Editor: Dr. Quancai Sun
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2025.101162.
ABBREVIATIONS
- AUC
area under the curve
- BLAST
basic local alignment search tool
- CRP
c-reactive protein
- GSH-Px
glutathione peroxidase activity
- KEGG
kyoto encyclopedia of genes and genomes
- LDA
linear discriminant analysis
- LDL
low-density lipoprotein
- LEfSe
linear discriminant analysis effect size
- MW
molecular weight
- PCC
protein carbonyl compounds
- PUFA
polyunsaturated fatty acids
- RBC
red blood cells
- SCFA
short-chain fatty acids
- TBARS
thiobarbituric acid reactive substances
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bax M.-L., Aubry L., Ferreira C., Daudin J.-D., Gatellier P., Rémond D., Santé-Lhoutellier V. Cooking temperature is a key determinant of in vitro meat protein digestion rate: investigation of underlying mechanisms. J. Agric. Food Chem. 2012;60:2569–2576. doi: 10.1021/jf205280y. [DOI] [PubMed] [Google Scholar]
- Ben David M., Shani Levi C., Lesmes U. Carrageenan impact on digestive proteolysis of meat proteins in meatballs or soluble hydrolyzed collagen. Food Res. Int. 2023;174 doi: 10.1016/j.foodres.2023.113560. [DOI] [PubMed] [Google Scholar]
- Cao C., Yuan D., Kong B., Chen Q., He J., Liu Q. Effect of different κ-carrageenan incorporation forms on the gel properties and in vitro digestibility of frankfurters. Food Hydrocoll. 2022;129 doi: 10.1016/j.foodhyd.2022.107637. [DOI] [Google Scholar]
- Chaney A.L., Marbach E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962;8:130–132. doi: 10.1093/clinchem/8.2.130. [DOI] [PubMed] [Google Scholar]
- Chassaing B., Koren O., Goodrich J.K., Poole A.C., Srinivasan S., Ley R.E., Gewirtz A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., Magram Klaiman M., Shpigelman A., Lesmes U. Addition of anionic polysaccharide stabilizers modulates in vitro digestive proteolysis of a chocolate milk drink in adults and children. Foods. 2020;9:1253. doi: 10.3390/foods9091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., Wojciechowska A., Portmann R., Shpigelman A., Lesmes U. The impact of food-grade carrageenans and consumer age on the in vitro proteolysis of whey proteins. Food Res. Int. Ott. Ont. 2020;130 doi: 10.1016/j.foodres.2019.108964. [DOI] [PubMed] [Google Scholar]
- De Vrieze J., Raport L., Willems B., Verbrugge S., Volcke E., Meers E., Angenent L.T., Boon N. Inoculum selection influences the biochemical methane potential of agro-industrial substrates. Microb. Biotechnol. 2015;8:776–786. doi: 10.1111/1751-7915.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroote J., Michiels J., Claeys E., Ovyn A., De Smet S. Changes in the pig small intestinal mucosal glutathione kinetics after weaning. J. Anim. Sci. 2012;90:359–361. doi: 10.2527/jas.53809. [DOI] [PubMed] [Google Scholar]
- Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Terrapon N., Muller A., Young V.B., Henrissat B., Wilmes P., Stappenbeck T.S., Núñez G., Martens E.C. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias Masiques N., Vossen E., De Vrieze J., De Smet S., Van Hecke T. The formation of sulfur metabolites during in vitro gastrointestinal digestion of fish, white meat and red meat is affected by the addition of fructo-oligosaccharides. Food Funct. 2024;15:8729–8739. doi: 10.1039/d4fo00928b. [DOI] [PubMed] [Google Scholar]
- Elias Masiques N., De Vrieze J., Hemeryck L.Y., Vanhaecke L., De Smet S., Van Hecke T. Dietary fiber mitigates the differential impact of beef and chicken meat consumption on rat intestinal health. Food Funct. 2025 doi: 10.1039/D5FO00900F. [DOI] [PubMed] [Google Scholar]
- Fu Q., Cheng J., Shi H., Han M., Chen Q., Song S. Effects of xanthan gum and psyllium husk powder with different ratios on the emulsification and oxidative stability of low-salt myofibrillar protein emulsions prepared by ultrasound. Food Chem. X. 2025;25 doi: 10.1016/j.fochx.2025.102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeyne F., Ruyck K.D., Ranst G.V., Neve N.D., Vlaeminck B., Fievez V. Effect of changes in lipid classes during wilting and ensiling of red clover using two silage additives on in vitro ruminal biohydrogenation. J. Agric. Sci. 2016;154:553–566. doi: 10.1017/S0021859615001203. [DOI] [Google Scholar]
- Gaifem J., Mendes-Frias A., Wolter M., Steimle A., Garzón M.J., Ubeda C., Nobre C., González A., Pinho S.S., Cunha C., Carvalho A., Castro A.G., Desai M.S., Rodrigues F., Silvestre R. Akkermansia muciniphila and Parabacteroides distasonis synergistically protect from colitis by promoting ILC3 in the gut. mBio. 2024;15 doi: 10.1128/mbio.00078-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganhão R., Morcuende D., Estévez M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: influence on colour and texture deterioration during chill storage. Meat Sci. 2010;85:402–409. doi: 10.1016/j.meatsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Gao X., Pourramezan H., Ramezan Y., Roy S., Zhang W., Assadpour E., Zou J., Jafari S.M. Application of gums as techno-functional hydrocolloids in meat processing and preservation: a review. Int. J. Biol. Macromol. 2024;268 doi: 10.1016/j.ijbiomac.2024.131614. [DOI] [PubMed] [Google Scholar]
- Glover J.S., Ticer T.D., Engevik M.A. Characterizing the mucin-degrading capacity of the human gut microbiota. Sci. Rep. 2022;12:8456. doi: 10.1038/s41598-022-11819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotto D., Santa Maria L.D., Boeira S., Valentini J., Charão M.F., Moro A.M., Nascimento P.C., Pomblum V.J., Garcia S.C. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography–visible detection. J. Pharm. Biomed. Anal. 2007;43:619–624. doi: 10.1016/j.jpba.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Gu Z., Gu L., Eils R., Schlesner M., Brors B. Circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- Guo J., Cui L., Meng Z. Oleogels/emulsion gels as novel saturated fat replacers in meat products: a review. Food Hydrocoll. 2023;137 doi: 10.1016/j.foodhyd.2022.108313. [DOI] [Google Scholar]
- Hernández P., Zomeño L., Ariño B., Blasco A. Antioxidant, lipolytic and proteolytic enzyme activities in pork meat from different genotypes. Meat Sci. 2004;66:525–529. doi: 10.1016/S0309-1740(03)00155-4. [DOI] [PubMed] [Google Scholar]
- Hornsey H.C. The colour of cooked cured pork. I.—estimation of the Nitric oxide-Haem Pigments. J. Sci. Food Agric. 1956;7:534–540. doi: 10.1002/jsfa.2740070804. [DOI] [Google Scholar]
- Huang Z., Ma Y., Xie Y., Zhao D., Li C. Carrageenan in meat: improvement in lipid metabolism due to Sirtuin1-mediated fatty acid oxidation and inhibited lipid bioavailability. Food Funct. 2023;14:5404–5416. doi: 10.1039/D3FO00906H. [DOI] [PubMed] [Google Scholar]
- Huang Z., Ding M., Xie Y., Chen B., Zhao D., Li C. Kappa-carrageenan in a pork-based high-fat diet inhibited lipid bioavailability through interactions with pork protein. Int. J. Biol. Macromol. 2024;276 doi: 10.1016/j.ijbiomac.2024.133922. [DOI] [PubMed] [Google Scholar]
- Ijssennagger N., Belzer C., Hooiveld G.J., Dekker J., van Mil S.W.C., Müller M., Kleerebezem M., van der Meer R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. U. S. A. 2015;112:10038–10043. doi: 10.1073/pnas.1507645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazu N., Torisu T., Yokote A., Umeno J., Kawasaki K., Fujioka S., Matsuno Y., Nagasue T., Kawatoko S., Moriyama T., Nitahata T., Uchida Y., Aihara S., Taniguchi Y., Oda Y., Kitazono T. Arginase 2 attenuates ulcerative colitis by antioxidant effects of spermidine. J. Gastroenterol. 2024;59:682–698. doi: 10.1007/s00535-024-02104-z. [DOI] [PubMed] [Google Scholar]
- Ioannou A., Berkhout M.D., Geerlings S.Y., Belzer C. Akkermansia muciniphila: biology, microbial ecology, host interactions and therapeutic potential. Nat. Rev. Microbiol. 2025;23:162–177. doi: 10.1038/s41579-024-01106-1. [DOI] [PubMed] [Google Scholar]
- Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xu Y., Kong B., Sun F., Liu H., Zhang H., Liu Q., Cao C. In vitro gastrointestinal digestibility and peptidomic analysis of frankfurters as influenced by different forms of κ-carrageenan. Agric. Commun. 2023;1 doi: 10.1016/j.agrcom.2023.100017. [DOI] [Google Scholar]
- Luo W., Friedman M.S., Shedden K., Hankenson K.D., Woolf P.J. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinf. 2009;10:161. doi: 10.1186/1471-2105-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads, EMBnet. Journal. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- McKim J.M., Willoughby J.A., Blakemore W.R., Weiner M.L. Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: a review of the chemistry, nomenclature, and in vivo toxicology by the oral route. Crit. Rev. Food Sci. Nutr. 2019;59:3054–3073. doi: 10.1080/10408398.2018.1481822. [DOI] [PubMed] [Google Scholar]
- Mi Y., Chin Y.X., Cao W.X., Chang Y.G., Lim P.E., Xue C.H., Tang Q.J. Native κ-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020;147:284–294. doi: 10.1016/j.ijbiomac.2020.01.072. [DOI] [PubMed] [Google Scholar]
- Muro P., Zhang L., Li S., Zhao Z., Jin T., Mao F., Mao Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024;15 doi: 10.3389/fendo.2024.1390351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi S., Viennois E., Gewirtz A.T., Chassaing B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome. 2021;9:66. doi: 10.1186/s40168-020-00996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Q., Sun Y., Li M., Zuo S., Chen C., Lin Q., Nie S. Targeted modification of gut microbiota and related metabolites via dietary fiber. Carbohydr. Polym. 2023;316 doi: 10.1016/j.carbpol.2023.120986. [DOI] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F.G., Kindt R., Legendre R., Legendre P., Minchin P., O'Hara R., Simpson G., Solymos P., Henry M. Vegan: community ecology package. R package version. 2013;2.0–10 https://vegandevs.github.io/vegan/ 2013. [Google Scholar]
- Ostrowski M.P., La Rosa S.L., Kunath B.J., Robertson A., Pereira G., Hagen L.H., Varghese N.J., Qiu L., Yao T., Flint G., Li J., McDonald S.P., Buttner D., Pudlo N.A., Schnizlein M.K., Young V.B., Brumer H., Schmidt T.M., Terrapon N., Lombard V., Henrissat B., Hamaker B., Eloe-Fadrosh E.A., Tripathi A., Pope P.B., Martens E.C. Mechanistic insights into consumption of the food additive xanthan gum by the human gut microbiota. Nat. Microbiol. 2022;7:556–569. doi: 10.1038/s41564-022-01093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira F.C., Wasmund K., Cobankovic I., Jehmlich N., Herbold C.W., Lee K.S., Sziranyi B., Vesely C., Decker T., Stocker R., Warth B., von Bergen M., Wagner M., Berry D. Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nat. Commun. 2020;11:5104. doi: 10.1038/s41467-020-18928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes K., de Smet S., Demeyer D. Effect of double-muscling in Belgian Blue young bulls on the intramuscular fatty acid composition with emphasis on conjugated linoleic acid and polyunsaturated fatty acids. Anim. Sci. 2001;73:253–260. doi: 10.1017/S1357729800058227. [DOI] [Google Scholar]
- Renner M., Bergmann G., Krebs I., End C., Lyer S., Hilberg F., Helmke B., Gassler N., Autschbach F., Bikker F., Strobel-Freidekind O., Gronert-Sum S., Benner A., Blaich S., Wittig R., Hudler M., Ligtenberg A.J., Madsen J., Holmskov U., Annese V., Latiano A., Schirmacher P., Amerongen A.V.N., D'Amato M., Kioschis P., Hafner M., Poustka A., Mollenhauer J. DMBT1 confers mucosal protection in vivo and a deletion variant is associated with Crohn's disease. Gastroenterology. 2007;133:1499–1509. doi: 10.1053/j.gastro.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rous C., Cadiou J., Yazbek H., Monzel E., Desai M.S., Doré J., van de Guchte M., Mondot S. Temporary dietary fiber depletion prompts rapid and lasting gut microbiota restructuring in mice. Microbiol. Spectr. 2025;13 doi: 10.1128/spectrum.01517-24. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnizlein M.K., Vendrov K.C., Edwards S.J., Martens E.C., Young V.B. Dietary xanthan gum alters antibiotic efficacy against the murine gut microbiota and attenuates Clostridioides difficile colonization. mSphere. 2020;5 doi: 10.1128/mSphere.00708-19. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuoker B., Pichler M.J., Jin C., Sakanaka H., Wu H., Gascueña A.M., Liu J., Nielsen T.S., Holgersson J., Nordberg Karlsson E., Juge N., Meier S., Morth J.P., Karlsson N.G., Abou Hachem M. Sialidases and fucosidases of Akkermansia muciniphila are crucial for growth on mucin and nutrient sharing with mucus-associated gut bacteria. Nat. Commun. 2023;14:1833. doi: 10.1038/s41467-023-37533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T., Heger A., Sudbery I. UMI-tools: modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Res. 2017;27:491–499. doi: 10.1101/gr.209601.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubach F., Künzel S., Baines A.C., Yee A., McGee B.M., Bäckhed F., Baines J.F., Johnsen J.M. Expression of the blood-group-related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME J. 2012;6:1345–1355. doi: 10.1038/ismej.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T., Mummaleti G., Mohan A., Singh R.K., Kong F. Current and emerging applications of carrageenan in the food industry. Food Res. Int. 2023;173 doi: 10.1016/j.foodres.2023.113369. [DOI] [PubMed] [Google Scholar]
- Van Hecke T., Ho P.L., Goethals S., De Smet S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017;102:785–792. doi: 10.1016/j.foodres.2017.09.090. [DOI] [PubMed] [Google Scholar]
- Vilchez-Vargas R., Geffers R., Suárez-Diez M., Conte I., Waliczek A., Kaser V.S., Kralova M., Junca H., Pieper D.H. Analysis of the microbial gene landscape and transcriptome for aromatic pollutants and alkane degradation using a novel internally calibrated microarray system. Environ. Microbiol. 2013;15:1016–1039. doi: 10.1111/j.1462-2920.2012.02752.x. [DOI] [PubMed] [Google Scholar]
- Wei T., Simko V. Corrplot: visualization of a correlation matrix. 2010. [DOI]
- Weiner M.L., McKim J.M. Comment on “Revisiting the carrageenan controversy: do we really understand the digestive fate and safety of carrageenan in our foods?”. David S., Levi C.S., Fahoum L., Ungar Y., Meyron-Holtz E.G., Shpigelman A., Lesmes U., editors. Food Funct. 2019;10:1760–1762. doi: 10.1039/c8fo01282b. 2018, 9, 1344-1352, Food Funct. [DOI] [PubMed] [Google Scholar]
- Wickham H. Springer International Publishing; Cham: 2016. ggplot2: Elegant Graphics for Data Analysis. [DOI] [Google Scholar]
- Wingett S.W., Andrews S. FastQ Screen: a tool for multi-genome mapping and quality control. F1000Research. 2018;7:1338. doi: 10.12688/f1000research.15931.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter M., Grant E.T., Boudaud M., Pudlo N.A., Pereira G.V., Eaton K.A., Martens E.C., Desai M.S. Diet-driven differential response of Akkermansia muciniphila modulates pathogen susceptibility. Mol. Syst. Biol. 2024;20:596–625. doi: 10.1038/s44320-024-00036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Huang X., Ma W., Li M., Wen J., Chen C., Liu L., Nie S. Bioactive polysaccharides promote gut immunity via different ways. Food Funct. 2023;14:1387–1400. doi: 10.1039/D2FO03181G. [DOI] [PubMed] [Google Scholar]
- Yin Y., Li M., Gu W., Zeng B., Liu W., Zhu L., Pi X., Primerano D.A., Yu H.D., Wei H., Yu G., Wang X. Carrageenan oligosaccharides and associated carrageenan-degrading bacteria induce intestinal inflammation in germ-free mice. J. Genet. Genomics. 2021;48:815–824. doi: 10.1016/j.jgg.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Lv B., Zhu Y., Li Q., Zhang K., Li C., Zhao D., Li C. Influence of hydrophilic polysaccharide fat replacers on the in vitro digestibility of protein in emulsion-type sausage. Food Res. Int. 2023;170 doi: 10.1016/j.foodres.2023.113008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.