Abstract
The Staphylococcus hyicus lip gene was cloned in Lactococcus lactis. Pancreatic insufficiency was induced by ligation of the pancreatic duct in pigs. In pigs who had undergone pancreatic ligation, the coefficient of fat absorption was higher after consumption of lipase-expressing L. lactis (91.9% ± 3.7%) than that after consumption of the inactive control strain (78.4% ± 2.4%).
The efficiency of pancreatic enzyme formulations used to treat pancreatic insufficiency is often unsatisfactory. Researchers are therefore trying to find new sources of lipases and new delivery vehicles (10). It was shown in a previous study that Lactococcus lactis was able to secrete the bacterial lipase of Staphylococcus hyicus (5). The aim of this study was to determine the effectiveness of genetically engineered L. lactis cells expressing the bacterial lipase of S. hyicus to help lipid digestion in pigs with induced pancreatic insufficiency.
L. lactis and thermoresistant spores of Bacillus subtilis were grown and enumerated as previously described (3, 6). Chloramphenicol and erythromycin (Merck) at a concentration of 10 μg/ml, rifampin at 50 μg/ml, streptomycin (Research Organics Inc.) at 1,000 μg/ml, and nisin powder (2.5% nisin content; ICN International) at 0.5 μg/ml were used. Basic methods for L. lactis genetic modification have been described previously (5). The L. lactis subsp. cremoris strain NZ9000 used in this study was derived from strain MG1363 and contained the genes pepN::nisRnisK (4). To obtain streptomycin- and rifampin-resistant L. lactis (strains JIM7017 and JIM7600), the strains were plated successively onto M17 agar containing 1,000 μg of streptomycin/ml and then onto agar containing 50 μg of rifampin/ml. Strain JIM7017 was the control strain, devoid of the lip gene.
Expression of the S. hyicus lip gene in L. lactis.
pJIM2093 (5) was amplified by PCR with the oligonucleotides 5′-AATCTTTTGTACCC ATGGTGAGTGCCTC-3′ and 5′-GGGGGCGTGGCAGGCC ATG GATTCG-3′. The bases in boldface indicate the mutations inserted in order to create an NcoI site (in italics) and to delete the signal peptide encoding sequence. The PCR fragment was digested by NcoI and ligated. The resulting plasmid, pJIM2095, carried the lip gene without its signal peptide encoding sequence. It was transformed in L. lactis NZ9000 (4) to yield JIM7600.
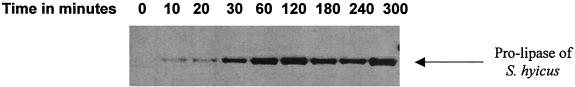
Lipase expression was induced with a nisin concentration optimal for heterologous protein expression in L. lactis (5). An overnight culture was used to inoculate fresh M17 medium. When an optical density at 600 nm of 0.3 was reached, we added the nisin and followed lipase expression in the cell extracts by Western blotting of the proteins (carried out as previously described [8]) with an anti-lipase serum (5) (Fig. 1). The lipase production was maximal 2 h after nisin induction and was still stable 5 h after induction. We found that lipase represented about 15% of the proteins of the cell (data not shown; scanning gel stained with Coomassie blue).
FIG. 1.
Western blot of total cell extract proteins from the lactococcal strain expressing the S. hyicus lipase. Samples were loaded at different times after nisin induction (in minutes).
Pancreatic duct ligation.
To perform pancreatic duct ligation (PDL), pigs were deprived of food for 24 h before surgery. After a right lateral laparotomy under halothane anesthesia, two ligatures were placed close to each other around the pancreatic duct at about 0.5 cm from the duodenum (1). The duct was then cut between the ligatures. After surgery, the pigs were housed in individual cages and their dietary intake was progressively restored to two daily meals of 800 g each (300 g of food mixed with 500 ml of water) at 10 am and 4 pm.
Fate of L. lactis in the digestive tract.
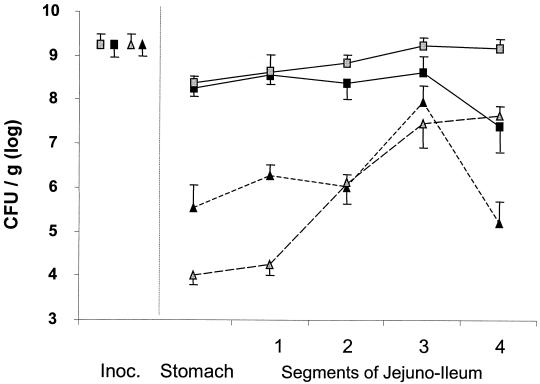
Conventional pigs as well as the pigs with PDL receive 500 ml of a concentrated culture of L. lactis prepared as follows. A 3-liter overnight culture was centrifuged for 10 min at 6,000 × g at 30°C. The bacterial pellet was resuspended in 500 ml of fresh M17 medium mixed with B. subtilis spores and 1.5 mg of nisin. The final mixture obtained, mixed with the powdered diet, contained 9.4 × 109 ± 0.4 L. lactis cells/g and 9.4 × 109 ± 0.3 B. subtilis spores/g. Two hours after the beginning of their meal, the animals were anesthetized with halothane. After a right lateral laparotomy, each nonempty part of the small intestine containing the diet was ligated. The duodenum was always empty. The contents of the intestine between the jejunum and the ileum were removed. The stomach and cecum contents were homogenized, and samples were collected. Before slaughtering the pigs, we verified that the pancreatic tissue of the pigs receiving ligation was atrophied and that the pancreatic duct was dilated. The fecal samples were weighed, homogenized, and diluted (1/10) in sterile water. L. lactis and B. subtilis counts were measured from these dilutions for each sample. Pigs with or without PDL received the control L. lactis strain JIM7017 (Fig. 2). The survival of L. lactis in the stomach and the upper parts of the jejunum was low for the two groups of pigs but higher after PDL. In the mid-jejuno-ileum (parts 2 and 3), its survival did not differ between pigs with or without PDL.
FIG. 2.
Comparison between transit of B. subtilis spores and L. lactis cells in the digestive tracts of pigs with or without PDL. Grey symbols, pigs without PDL (n = 6); black symbols, pigs with PDL (n = 6); squares, thermoresistant spores of B. subtilis; triangles, L. lactis JIM7017; Inoc., inoculum of spores and L. lactis. The duodenum was always empty, and samples were not collected from it. The jejuno-ileum was divided into four equal-length segments, and the contents were collected. Vertical bars represent standard errors of the means.
Treatment of induced pancreatic insufficiency with L. lactis expressing the S. hyicus lip gene.
The treatment of induced pancreatic insufficiency with L. lactis expressing S. hyicus lip was carried out on four pigs with PDL (Table 1; PDL occurred at day zero). The pigs were adapted to receive a high-fat diet (containing 15% lipids). To determine the efficiency of L. lactis expressing the S. hyicus lipase (JIM7600), the four pigs with PDL were fed the high-fat diet as two daily meals of 300 g each mixed with 500 ml of a concentrated culture of L. lactis JIM7600 or a negative L. lactis control (JIM7017). These concentrated cultures were prepared as described above. The pigs served as their own controls. They received both strains for 4 days each, interrupted by a 10-day washout period (strains at days 21 to 24 and then at days 35 to 38). The stools were collected twice per day over each 4-day period of bacterial treatment. Stools were immediately frozen. Fecal and dietary fats were measured (7), and the coefficient of fat absorption (CFA) was calculated as the difference between ingested and excreted fats, which yielded the percentage of ingested fat (2). Results are expressed as means with their standard errors. Values were compared by analysis of variance using SAS software. The CFA in normal pigs was 97% ± 2%. CFAs from the results obtained during the two treatment periods are shown in Table 1. The CFA was enhanced in all animals when the pigs ingested the strain producing lipase (91.9% ± 3.7%) compared to when they ingested the inactive strain (78.4% ± 2.4%), and the difference between both periods was significant (P < 0.01).
TABLE 1.
CFA determined at different phases of the experimental schedule
| Animala | CFA (%) according to the treatmentb
|
|||
|---|---|---|---|---|
| Days 21 to 24
|
Days 35 to 38
|
|||
| L. lactis treat- ment strain | Digesti- bility (%) | L. lactis treat- ment strain | Digesti- bility (%) | |
| 3254 | JIM7600 (lipase) | 95.7 | JIM7017 (control) | 85.4 |
| 94342 | JIM7600 (lipase) | 80.9 | JIM7017 (control) | 74.2 |
| 93291 | JIM7017 (control) | 76.9 | JIM7600 (lipase) | 95.3 |
| 930307 | JIM7017 (control) | 77.0 | JIM7600 (lipase) | 95.9 |
The average pig weights were 47 and 67 kg, respectively, at surgery and at time of slaughter.
All the animals received a diet containing 15% lipids.
The aim of the present study was to test the hypothesis that lactic acid bacteria expressing an heterologous lipase were efficient in reducing induced pancreatic insufficiency. We induced exocrine pancreatic insufficiency in pigs through PDL. A recent experimental study in dogs suggested that an acid- and protease-resistant lipase of bacterial origin might improve fat absorption (10, 11). Since lactococci are rapidly destroyed by bile acids and proteolytic secretions (6, 9), we hypothesized that lactococci genetically modified to have a high production of a bacterial lipase could be used as a vector instead of the pure enzyme. It was previously shown that S. hyicus lipase could be produced in L. lactis (5). In this study, we improved the lipase production by removing the initial peptide signal encoding sequence necessary for secretion.
In pigs with or without PDL, the majority of ingested L. lactis cells lost their viability in the upper jejuno-ileum. It was previously shown that dead L. lactis released its cellular content in the digestive tracts of mice (6). We have shown that in the four pigs with PDL, L. lactis producing lipase could correct steatorrhea when ingested with a high-lipid meal. The CFA showed significant improvement over the treatment period compared to that obtained under the period of treatment with lipase-devoid L. lactis. We conclude that lactic acid bacteria, such as L. lactis modified to express lipase, could be used as a new vector to deliver lipase in the gastrointestinal tract. This is potentially a major advancement in the treatment of pancreatic insufficiency in humans. The cost and safety of such a treatment also seems promising.
Acknowledgments
We are grateful to T. Corring for helpful advice, to C. Gibard for lipid analyses in food and feces, to F. Cointpas and F. Gérard, to D. Besnard and M.-G. Brachet for pig surgery and animal management, and to P. Rapine for technical assistance.
REFERENCES
- 1.Corring, T. C., A. Aumaitre, and A. Rerat. 1972. Permanent fistulization of the exocrine pancreas in the pig. Application: response of pancreatic secretion to feeding. Ann. Biol. Anim. Biochim. Biophys. 12:109-124. [PubMed] [Google Scholar]
- 2.Corring, T. C., C. Juste, C. Simoes-Nunes, and D. Bourdon. 1979. Effect of biliary secretion on digestion in the pig. Ann. Biol. Anim. Biochim. Biophys. 19:1123-1130. [Google Scholar]
- 3.Corthier, G., C. Delorme, S. D. Ehrlich, and P. Renault. 1998. Use of luciferase genes as biosensors to study bacterial physiology in the digestive tract. Appl. Environ. Microbiol. 64:2721-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drouault, S., G. Corthier, S. D. Ehrlich, and P. Renault. 2000. Expression of the Staphylococcus hyicus lipase in Lactococcus lactis. Appl. Environ. Microbiol. 66:588-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drouault, S., G. Corthier, S. D. Ehrlich, and P. Renault. 1999. Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. Appl. Environ. Microbiol. 65:4881-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folch, J., and M. Lees. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 8.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1998. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J. Bacteriol. 180:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marteau, P., M. Minekus, R. Havenaar, and J. H. Huis in't Veld. 1997. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J. Dairy Sci. 80:1031-1037. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki, A., A. Mizumoto, R. Rerknimitr, M. G. Sarr, and E. P. DiMango. 1999. Effect of bacterial or porcine lipase with low- or high-fat diets on nutrient absorption in pancreatic-insufficient dogs. Gastroenterology 116:431-437. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki, A., A. Mizumoto, M. G. Sarr, and E. P. DiMagno. 1997. Bacterial lipase and high-fat diets in canine exocrine pancreatic insufficiency: a new therapy of steatorrhea? Gastroenterology 112:2048-2055. [DOI] [PubMed] [Google Scholar]