Abstract
Prophylaxis is an effective method for preventing dental caries and periodontal diseases. This in-vitro study evaluated changes in the roughness of the immature enamel surface following different prophylaxis methods. The crowns of 35 extracted third molars were sectioned buccolingually to obtain buccal and lingual halves. Seventy samples were mounted in acrylic blocks and randomly allocated to seven groups (n = 10 per group): Group 1 (Golchai paste/Rubber cup), Group 2 (Morvabon paste/rubber cup), Group 3 (Sina paste/Rubber cup), Group 4 (Rubber cup-only), Group 5 (Brush-only), Group 6 (Golchai paste/brush), and Group 7 (Golchai paste—double amount/ higher speed rubber cup). Prophylaxis was performed for 3 s using a handpiece at 3000 rpm with 200 g pressure. The untreated half of each sample served as a control. A non-contact profilometer was utilized to evaluate the surface roughness. Statistical analyses were conducted employing ANOVA, Tukey’s post-hoc test, paired t-tests, and Wilcoxon tests (α = 0.05). ANOVA indicated a significant difference in surface roughness among groups (p-value = 0.008). Pairwise comparison showed that Golchai paste reduced surface roughness when applied with a rubber cup but increased it when used with a brush, with a statistically significant difference between the two methods. However, Golchai/rubber cup group was comparable to Morvabon/rubber cup and Sina/rubber cup groups. Within-group comparisons showed a significant reduction in surface roughness in Golchai/rubber cup group (p-value = 0.010), while Brush-only group exhibited a significant increase (p-value = 0.028). Among the tested methods, the combination of Golchai paste and a rubber cup produced the smoothest enamel surface. In contrast, using Golchai paste with a brush resulted in the roughest surface. Doubling the paste amount and increasing the handpiece speed did not significantly alter roughness. Based on these in-vitro findings, the use of a prophylaxis brush is not recommended for immature or newly erupted teeth, as it resulted in increased surface roughness. Future in-vivo studies are warranted to further investigate these effects in clinical settings.
Keywords: Dental Prophylaxis, Enamel, Prophylaxis pastes, Surface roughness
Introduction
Preventive care is fundamental to modern dental practices, focused on preventing oral diseases before invasive treatments become necessary (Alshammari et al. 2024). More patients are now interested in preventive dentistry and visit dental clinics for professional oral care every three to six months (Kracher 2012; Yurdaguven et al. 2012). The removal of dental plaque and stains is essential for most preventive and operative dentistry procedures (Salami and Luz 2003). This process can reduce the total bacterial count per tooth by 72%, improving dental aesthetics and maintaining oral health (Kristoffersson et al. 1984; Goodson et al. 2004; Patil et al. 2015). Dental prophylaxis can be performed either for the purpose of cleaning or polishing, based on the patient’s clinical requirements (Sawai et al. 2015). It is often performed prior to fluoride application, the placement of fissure sealants, and enamel etching, or following the removal of orthodontic braces and scaling and root planing (Main et al. 1997; Ansari et al. 2004; Shah et al. 2019; Uguru et al. 2020). Dental practitioners often use prophylaxis paste with a rubber cup or brush to ensure a smooth enamel surface and minimize bacterial adhesion (Yurdaguven et al. 2012). However, dental prophylaxis can be performed using various methods, including hand instruments, rubber cups, brushes, dental floss, and air polishing (Policy on the Role of Dental Prophylaxis in Pediatric Dentistry 2016).
An ideal prophylactic paste should possess both cleaning and polishing properties (Yurdaguven et al. 2012). Furthermore, the abrasive particles should become less aggressive under load to minimize abrasion and surface roughness of dental hard tissues (Yurdaguven et al. 2012; Eram et al. 2024). These pastes have varying compositions; some contain fluoride, Amorphous Calcium Phosphate (ACP), or desensitizing agents, offering additional therapeutic benefits beyond cleaning (Al-Twaijri et al. 2011; Tsai et al. 2012; Neuhaus et al. 2013; Crowley et al. 2024). Thus, understanding the composition of prophylaxis paste can help clinicians select the most suitable paste for various clinical situations.
Prophylaxis pastes are classified into fine, medium, and coarse grades based on their abrasive agents (Truong et al. 2017b; da Rosa et al. 2024). Fine-grit pastes offer a gentler polishing effect, making them ideal for patients with minimal staining or sensitive teeth (Truong et al. 2017a). In contrast, coarse pastes effectively remove heavy stains but may increase surface roughness (Salami and Luz 2003). The roughness of a surface can be influenced by various factors, including the size of the abrasive particles, the method employed to apply prophylaxis paste, the time and pressure applied by the operator, and the characteristics of the surface (Warren et al. 2002; Yurdaguven et al. 2012; Sugiyama et al. 2017). Enamel maturation, a process involving progressive mineralization, typically takes 2–3 years post-eruption (Lynch 2013). Immature enamel exhibits a lower mineralization degree (approximately 45–91%) compared to mature enamel (99%), resulting in larger porosity and inter-crystallite spaces (Bonar et al. 1991; ten Bosch et al. 2000; Wada et al. 2023). These structural characteristics make it more vulnerable to wear, as confirmed by Wada et al. (Wada et al. 2023), where immature enamel showed significantly rougher wear surfaces with deep, irregular craters and multiple microcracks. Rough enamel surfaces can hinder effective cleaning, leading to plaque buildup, bacterial growth, and staining, which negatively impact the aesthetic appearance of teeth (Shah et al. 2019). Given that dental practitioners frequently encounter immature enamel during procedures like fissure sealant placement and fluoride application, and that primary or immature permanent teeth are more susceptible to abrasion, the selection of appropriate prophylactic materials and techniques is a crucial clinical decision to minimize enamel damage while ensuring effective cleaning.
Several methods are currently used to evaluate surface roughness, including scanning electron microscopy (SEM), stereo microscopy, contact profilometry, non-contact white light 3D profilometry, and atomic force microscopy (AFM), which allow precise measurement of surface roughness and microstructural alterations (Banerjee et al. 2008; Janiszewska-Olszowska et al. 2015). These techniques offer important information regarding the impact of prophylaxis pastes on enamel integrity, enabling the optimization of clinical protocols to strike a balance between effective cleaning and minimal enamel wear. The present research focuses on evaluating the immature enamel surface roughness following the application of three distinct prophylaxis pastes using rubber cups and brushing methods in an in-vitro setting. This will help clinicians select the most suitable prophylaxis paste using the proper method to minimize enamel wear.
Methods
Study design and ethical approval
The Research and Ethics Committee of Mashhad University of Medical Sciences approved the protocol of this in-vitro study, with the approval code IR.MUMS.DENTISTRY.REC.1401.082.
Sample size calculation and collection
A total of 35 extracted third molars with open apices were chosen for this study. All teeth were healthy, free of enamel cracks and caries, and were extracted for orthodontic purposes. The teeth were examined under a microscope (Kern & Sohn, Germany) at 4 × magnification to identify any structural defects. After the examination, the teeth were cleaned using ultrasonic instruments, disinfected with a 2% thymol solution, and stored in distilled water for a maximum period of two weeks until the experiment. To ensure proper preservation and prevent microbial contamination, the distilled water was changed daily.
According to the article by Tuzcel, Akkaya, and Karacaoglu (Yilmaz Tuzcel et al. 2017), the sample size for each group was calculated to be 10 using the following formula, based on a statistical power of 95% and a confidence level of 99%:
In the present equation, n is the sample size per group; and are Z-scores for significance (α) and power (1 − β), respectively; S1 and S2 are the standard deviations of the two groups; and M1 and M2 are their respective means.
Sample preparation and allocation
Initially, the teeth were sectioned buccolingually into two halves using a diamond disc (Kerr Dental, Brea, CA, USA) attached to a low-speed handpiece. Throughout the sectioning process, continuous water irrigation was maintained to prevent thermal damage to the tooth structure and ensure a precise, clean cut. This procedure resulted in a total of 70 dental surfaces for the study (Fig. 1A). Subsequently, the resulting 70 dental surfaces were then meticulously mounted in self-curing acrylic (Acropars – Marlik Company, Iran). A small amount of the acrylic resin was first dispensed into a flat-bottomed mold. Prior to complete polymerization, each sectioned tooth was carefully positioned with the enamel surface facing upward. A clean, flat glass slide was gently placed over the specimen to ensure that the enamel surface remained flat and parallel to the horizontal plane. Special attention was paid to prevent any contact between the enamel surface and the acrylic resin during setting, thereby preserving its integrity for subsequent surface analyses. After that, each enamel surface was randomly divided into a control section (unpolished) and an experimental section (prepared for prophylactic treatment). The control section was covered with PVC tape (Pro Tapes, North Brunswick, United States) (Fig. 1B). The samples were distributed randomly into seven groups, with every group made up of 10 surfaces, as detailed below:
Group 1 (Golchai—Rubber Cup): Dental prophylaxis using Golchai paste (Golchai Company, Iran) with a rubber cup (TPC Advanced Technology, CA, USA).
Group 2 (Morvabon—Rubber Cup): Dental prophylaxis using Morvabon paste (Morvabon Company, Iran) with a rubber cup.
Group 3 (Sina—Rubber Cup): Dental prophylaxis using Sina paste (Sina Company, Iran) with a rubber cup.
Group 4 (Rubber Cup Only): Dental prophylaxis using a rubber cup without paste.
Group 5 (Brush Only): Dental prophylaxis using a nylon-bristled brush (Eagle, China) without paste.
Group 6 (Golchai—Brush): Dental prophylaxis using Golchai paste with a nylon-bristled brush.
Group 7 (Golchai – Double Amount – High-Speed Rubber Cup): Dental prophylaxis using Golchai paste (double the amount used in Group 1), applied with a rubber cup at 4000 rpm.
Fig. 1.
Sample preparation: A) Each Tooth was sectioned into two halves buccolingually and then mounted in self-cure acrylic. B) Each sample was divided into a control section (covered with PVC tape) and an experimental section
In-vitro procedure
Prophylaxis was performed using an angle handpiece (NSK, Japan) at a speed of 3000 RPM for 3 s, applying a force of 200 g with a rubber cup and 17 g of prophylaxis paste in groups 1 to 3. The procedure for group 4 was identical, except that prophylaxis paste was not used. Group 5 utilized a nylon-bristled brush instead of a rubber cup and did not use prophylaxis paste. In group 6, the procedure was the same as in group 1, but a nylon-bristled brush replaced the rubber cup. In group 7, the procedure was similar to that of group 1, but it utilized double the quantity of Golchai prophylaxis paste (34 g) and raised the handpiece speed to 4000 RPM. In all groups, prior to prophylaxis, the surface of the experimental half of the tooth was left moist from storage and then coated with approximately 0.25 cc of freshly collected, unstimulated human saliva.
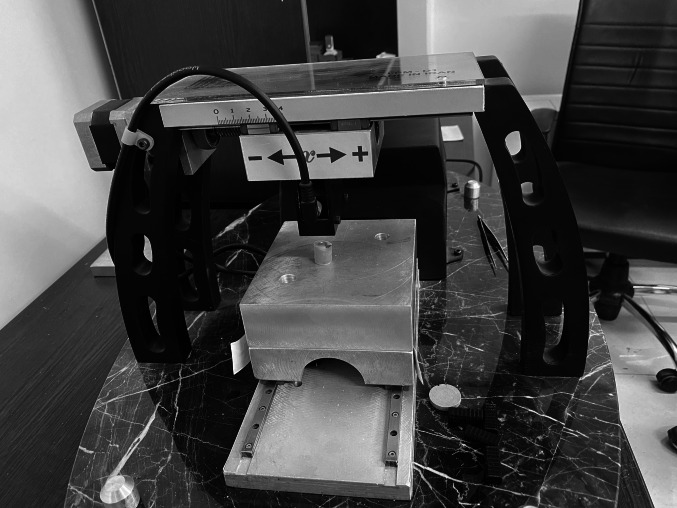
A 200-g weight was used to standardize the applied force (Fig. 2A). To secure this weight onto the handpiece, a custom holder was fabricated using acrylic and putty impression material (Speedex, Coltene, Altstätten, Switzerland) (Fig. 2A). This holder, along with the weight, was placed on the handpiece (Fig. 2B) to ensure uniform force application across all samples. Furthermore, a holder device was constructed to maintain a consistent force for all samples (Fig. 2C). The acrylic base of the sample was secured to the horizontal plate of the holding device using sticky wax (Kerr, Switzerland) to prevent movement during the prophylaxis procedure. An angle handpiece with an attached 200-g weight was suspended from the top of the holding device, ensuring that only the weight's force was applied without additional pressure. The operator moved the handpiece manually forward and backward. To prevent lateral displacement of the handpiece during operation, two vertical plates were positioned on either side to maintain alignment. Following the completion of the prophylaxis for each sample, the specimen was detached from the device and rinsed with distilled water for 10 s to remove any residual prophylaxis paste. It was then dried with an air blower for another 10 s. Next, the PVC tape covering the control half was removed, and a 2 mm × 2 mm square was then marked within the now-exposed control section and another 2 mm × 2 mm square within the adjacent experimental section with a marker. These markings indicated the area for measuring surface roughness during the profilometry analysis. To distinguish the control half in each sample, the underside of that half was marked with red lacquer (Fig. 2D).
Fig. 2.
In-vitro procedure: A) A 200-g weight, secured by a custom holder, was utilized to standardize the applied pressure. B) The custom holder with the weight was placed on the handpiece. C) A holder device was developed to maintain a consistent force across all samples. D) A 2 mm × 2 mm square was marked to define the surface roughness measurement area
Measuring surface roughness
The surface roughness of the samples was assessed using a laser surface profilometer (Fanavari Kahroba, Tehran, Iran) (Fig. 3). Surface roughness was measured separately in a 2 × 2 mm area on both sides of the midline, including the control and polished sections.
Fig. 3.
Enamel surface roughness was measured using a laser profilometry device
Randomization and blinding procedures
To randomize the study, all 70 tooth specimens were first numbered. The samples were subsequently divided into seven groups, each containing 10 samples, using random numbers generated from www.random.org. Additionally, a coin toss was used to determine whether the right or left half of each specimen would be designated for polishing or retained as the control.
Due to the visible color differences among the prophylaxis pastes, blinding of the operator during the procedure was not feasible. However, outcome evaluators responsible for assessing surface roughness and conducting statistical analyses were blinded to group allocation.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics version 20 (IBM Corp., Armonk, NY, USA). The normal distribution of the data was assessed using the Kolmogorov–Smirnov test. A one-way ANOVA was used to analyze the mean changes in surface roughness among the groups. The Tukey post-hoc test was conducted for pairwise comparisons between groups.
To compare the polished and unpolished surfaces within each group, the Paired T-test was applied to Groups 1, 2, and 4, as these data sets followed a normal distribution. For the remaining groups, the Wilcoxon test was utilized. Statistical significance was set at p-value < 0.05.
Results
The mean and standard deviation of surface roughness for both polished and non-polished surfaces in each group are presented in Table 1. The smoothest surface was recorded in the Golchai-Rubber cup group, while the roughest surface was found in the Golchai-Brush group.
Table 1.
Comparative analysis of surface roughness (mean ± standard deviation in µm) between polished and non-polished surfaces within each study group, along with the results of the One-way ANOVA and pairwise comparisons of surface roughness changes among the groups
| GROUP* | N | Non-Polished mean ± SD |
Polished mean ± SD |
Analysis Results (p -value)# |
ΔRa† mean ± SD |
|
|---|---|---|---|---|---|---|
| 1 | Golchai-R | 10 | 6.89 ± 0.70 | 4.5 ± 0.45 | Paired T-test, (p = 0.010) | −2.38 ± 0.73a |
| 2 | Morvabon-R | 10 | 6.38 ± 0.63 | 5.5 ± 0.94 | Paired T-test, (p = 0.46) | −0.92 ± 1.20ab |
| 3 | Sina-R | 10 | 6.4 ± 1.08 | 5.99 ± 0.61 | Wilcoxon test, (p = 0.721) | −0.37 ± 0.63abc |
| 4 | R-only | 10 | 6.05 ± 0.39 | 6.90 ± 0.64 | Paired T-test, (p = 0.198) | 0.85 ± 0.61bc |
| 5 | B-only | 10 | 6.0 ± 0.71 | 7.54 ± 0.79 | Wilcoxon test, (p = 0.028) | 1.54 ± 0.57c |
| 6 | Golchai-B | 10 | 6.4 ± 1.06 | 8.44 ± 0.80 | Wilcoxon test, (p = 0.093) | 2.00 ± 1.12c |
| 7 | Golchai-R Double | 10 | 6.5 ± 1.6 | 6.07 ± 0.94 | Wilcoxon test, (p = 0.57) | −0.37 ± 0.83abc |
* R = Rubber cup; B = Brush
# Values less than 0.05 represent a significant difference between the polished and non-polished surfaces
† ΔRa: Difference in surface roughness (Ra) before and after the procedure
† Negative values indicate a reduction in roughness; Positive values indicate an increase in roughness
† Values with different superscript letters are significantly different according to Tukey’s post-hoc test (p -value < 0.05)
† ANOVA test F=3.220, p -value = 0.008
The intragroup analysis of surface roughness measurements before and after the use of each prophylaxis method indicated that the Golchai-Rubber cup group exhibited significantly improved surface smoothness (p-value = 0.010). In contrast, the Brush-only group significantly increased surface roughness (p-value = 0.028). Additionally, surface roughness was comparable between polished and non-polished surfaces in the other groups.
Intergroup comparisons of the mean differences in surface roughness (ΔRa) after prophylaxis treatment indicated significant statistical variations across study groups (p-value = 0.008). According to the post-hoc analysis, the Golchai-Rubber cup group demonstrated the greatest reduction in surface roughness, with statistically significant differences from the Rubber cup-only (p-value = 0.009), the Brush-only (p-value = 0.002), and the Golchai-Brush (p-value = 0.001) groups. The Morvabon-Rubber cup group presented a moderate but statistically non-significant reduction in surface roughness compared to the Golchai-Rubber cup, while still differing from the more abrasive groups, such as the Brush-only (p-value = 0.044) and the Golchai-Brush (p-value = 0.018) groups. The Sina-Rubber cup and Golchai-Rubber cup Double groups exhibited minimal changes in surface roughness. These groups showed no significant differences compared to the other groups. In contrast, the Rubber cup-only group presented a slight increase in roughness, and the Brush-only and Golchai-Brush groups both demonstrated the highest roughness increases post-treatment. The mean changes in enamel surface roughness and the statistical differences among the prophylaxis groups are visually presented in Fig. 4.
Fig. 4.
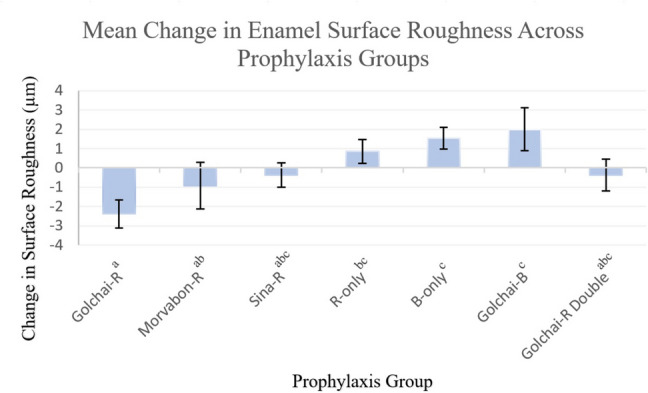
Mean Change in Enamel Surface Roughness Across Prophylaxis Groups. Bar chart illustrating mean change in surface roughness (ΔRa, µm) and standard deviation (error bars) for each prophylaxis group. Groups with different superscript letters are significantly different according to Tukey’s post-hoc test (p-value < 0.05). Abbreviations: R, Rubber cup; B, Brush
Discussion
The present study evaluates enamel surface roughness after using three different prophylaxis pastes with rubber cups and brushing techniques in an in-vitro setting, enabling comparisons relevant to clinical efficacy. Several factors generally influence the surface roughness resulting from prophylaxis procedures (Sawai et al. 2015; Mossman 2016; Eakle and Bastin 2019). These include (1) paste-related factors, such as the abrasiveness of the paste, as well as the concentration and amount of paste used; (2) operator-related factors, including handpiece speed, applied pressure, duration of application, and the type of instrument used; and (3) tooth-related factors referring to the condition of the enamel (e.g., sound or demineralized) and the surface moisture level.
Previous studies have outlined various protocols for conducting prophylaxis. The speed of the angle handpiece ranged from 1000 to 3000 RPM (Sawai et al. 2015; Camboni and Donnet 2016; Duman and Doğan 2021). Additionally, the duration of applying prophylaxis and the amount of force varied among these studies (Roulet and Roulet-Mehrens 1982; Camboni and Donnet 2016; Duman and Doğan 2021). In the current study, prophylaxis was conducted at a speed of 3000 RPM for 3 s, using a force of 200 g. Most surfaces can be polished in 2 to 5 s by using a gentle, consistent speed with a patting motion (Sawai et al. 2015). To replicate intraoral conditions more accurately, the experimental half of each specimen was coated with freshly collected, unstimulated human saliva before the polishing procedure. To examine how the quantity of prophylaxis pastes and the speed of the handpiece influence enamel roughness, the RPM was increased to 4000 in the final group, and the amount of prophylaxis paste was doubled. The surface in that group was rougher compared to the groups that used prophylaxis paste with rubber cups following standard protocols. However, this difference was not statistically significant. One possible explanation for this outcome is that, despite doubling the amount of paste applied, the excess paste was quickly spread around the tooth as the rubber cup began to rotate. Consequently, the amount of paste that remained on the tooth was similar to that in the first group. Additionally, increasing the speed of the handpiece did not significantly impact the surface roughness. However, with longer application times, it is possible that a higher speed could influence surface roughness, which requires further investigation in future studies.
Different dental substrates and restorative materials vary in their susceptibility to surface roughening during prophylaxis due to their intrinsic structural and physical properties. Yurdaguven et al. (Yurdaguven et al. 2012) assessed the impact of prophylaxis paste on enamel, composite, porcelain, and dentin by using a profilometer. They indicated that the surfaces of the composite material and dentin showed a greater level of impact, but neither surpassed the bacterial retention roughness limit of 0.2 µm. Covey et al. (Covey et al. 2011) found that conventional prophylaxis pastes significantly increased surface roughness in enamel and composite, but not in dental porcelain. Sugiyama et al. (Sugiyama et al. 2017) examined the impact of professional dental prophylaxis on composite and ceramic blocks used as CAD/CAM materials. The results showed that polishing with prophylaxis paste negatively impacted the surface characteristics of the resin composites, resulting in diminished gloss and increased roughness. In contrast, the changes observed in Celtra DUO, a zirconia-reinforced lithium silicate, were less pronounced. Therefore, it is necessary to use proper prophylaxis paste and techniques based on surface properties.
Despite the enamel surface appearing flat, it is actually not. The presence of Retzius grooves, depressions, and small defects leads to a naturally rough surface (Al-Amri et al. 2021). After the eruption of the tooth, exposure to environmental factors such as beverages, soft drinks, and acidic byproducts of bacterial species in the oral environment can increase enamel surface roughness (Fujii et al. 2011). Furthermore, restorative procedures such as enamel etching and bleaching can increase the roughness of the surface (Younis and Sulaiman 2024). Also, the enamel thickness and abrasion resistance vary among teeth, as primary teeth are more prone to abrasion than permanent teeth (Chowdary et al. 2018). In the present study, third molar teeth with open apices were selected to simulate the clinical conditions commonly encountered in pediatric dentistry, where immature enamel is often present (Ijbara et al. 2018; Wada et al. 2023). These teeth have not yet undergone post-eruptive maturation. As a result, they have more irregularities and larger inter-crystalline spaces compared to mature teeth, which leads to a relatively lower microhardness of their enamel surface (Palti et al. 2008). This makes them more vulnerable to abrasion. Therefore, dentists should consider the degree of mineralization of teeth to minimize enamel damage, particularly in pediatric patients where primary or immature permanent teeth are present.
Various methods are employed for dental prophylaxis, including hand instruments, rubber cups, brushes, dental floss, bicarbonate jet spray, and air polishing (Policy on the Role of Dental Prophylaxis in Pediatric Dentistry 2016; Gomes et al. 2018). The present study evaluated the effect of rubber cups and nylon-bristled brushes on enamel surface roughness, both with and without prophylaxis paste. The findings revealed that groups treated with rubber cups, regardless of whether paste was used, exhibited smoother surfaces compared to the brush groups. Aligning with these findings, Kimyai et al.(Kimyai et al. 2011) indicated that although air powder polishing devices have the most significant impact on surface roughness, using pumice with a rubber cup results in less roughness than when using a brush. Yildirim et al. (Yildirim et al. 2021) and Gomes et al.(Gomes et al. 2018) also confirmed the potential of rubber cups to minimize enamel damage compared to ultrasonic scalers and bicarbonate jet spray. Under the in-vitro conditions of the present study, current findings suggest that using brushes, especially with prophylaxis paste, led to increased enamel roughness. Therefore, in situations where maintaining enamel smoothness is a primary clinical priority, particularly on immature or newly erupted teeth, this approach may warrant caution. Instead, Rubber cup techniques are suggested as a safer alternative to minimize the likelihood of enamel damage during prophylaxis. While all prophylaxis methods can affect surface roughness to some extent, selecting the appropriate technique can optimize clinical outcomes and preserve enamel integrity.
The composition of prophylaxis paste may include fluoride, amorphous calcium phosphate (ACP), desensitizing agents, and abrasive components (Al-Twaijri et al. 2011; Neuhaus et al. 2013). Abrasive agents vary in texture, ranging from fine to coarse, and their use depends on the specific application. Coarse prophylaxis pastes are effective at quickly removing stains caused by tobacco, tea, red wine, and coffee; however, they can also lead to roughness and scratching of the enamel (LaCross et al. 2007). In contrast, fine particle pastes preserve the surface gloss of the teeth and reduce roughness (Monaco et al. 2020). In the present study, three commercially available prophylaxis pastes commonly used in Iran were evaluated. Morvabon paste is composed of zirconium silicate (37 µm), along with silica (6 µm), while Sina paste composition includes calcium carbonate, pumice, and aluminum oxide as abrasive agents. Due to limited technical information from manufacturers, it was essential to assess their abrasive behavior directly. All three pastes showed comparable potential for improving surface smoothness; however, the Golchai paste produced the smoothest surface. Moreover, a statistically significant difference in roughness between polished and unpolished surfaces was observed only in the Golchai/rubber cup group, unlike the Morvabon and Sina groups. Selecting an appropriate prophylaxis paste is critical due to the varying levels of abrasiveness. This consideration becomes even more important when prophylaxis is performed on tooth-colored restorative materials, which are more susceptible to surface damage than natural enamel (Warren et al. 2002; Monaco et al. 2020).
Several methods are currently used to evaluate surface roughness, including AFM, stereo microscopy, contact profilometry, non-contact white light 3D profilometry, and SEM, which allow precise measurement of surface roughness and microstructural alterations (Banerjee et al. 2008; Janiszewska-Olszowska et al. 2015). AFM allows for the detailed examination of mineralization in dental hard tissues and the mechanical properties of calcified tissues through high-resolution, three-dimensional imaging (Marshall et al. 2001; Duman and Doğan 2021). Nevertheless, it has some limitations, including a slow scanning speed, high costs, and a failure to identify undercuts (Duman and Doğan 2021). In the present study, profilometry measurements were conducted to provide a quantitative analysis. Due to the curvature of the tooth surface, contact profilometry does not yield accurate results; therefore, non-contact profilometry was employed. This device enables non-contact measurement of surface roughness with an accuracy of ± 1 µm and can detect height variations with an accuracy of 2 µm. It also offers high-speed measurement capabilities, collecting up to 1000 measurements per second. The device accommodates a maximum sample displacement of 4 cm on each axis, making it a reliable choice for measuring enamel roughness. However, a disadvantage of this method is that it does not allow for qualitative examination.
The current study has some limitations. Future research should involve a larger sample size to compare the surface roughness of mature and immature teeth. Moreover, both qualitative and quantitative methods are recommended for a more accurate evaluation of surface roughness. Although several attempts were made to obtain detailed manufacturer specifications regarding the physicochemical properties of the prophylaxis pastes, the lack of this information represents a limitation, making a direct comparison based on these properties not feasible. The choice to evaluate only Golchai paste with a brush was dictated by the limited availability of extracted teeth suitable for the study, the financial burden associated with conducting additional procedures, and its prevalent use in our national dental schools. This indicates a need for broader investigation into other paste-brush combinations. While the present study aimed to standardize the procedure by using a 200 g weight to control vertical force, the manual operation of the handpiece may have introduced variability in the horizontal force applied across specimens. This underscores the importance of incorporating automated procedures in future research to minimize operator-dependent variability. Another limitation of this study was the inability to blind the operator due to visible color differences among the prophylaxis pastes, which could introduce potential procedural bias. It is important to emphasize that these observations come from an in-vitro model. While they provide valuable insights, further in-vivo studies are essential to fully understand the clinical implications and translate these findings to dynamic oral environments, where factors like salivary pellicle formation and remineralization processes could influence outcomes. Therefore, clinical recommendations should also consider existing and future in-vivo evidence.
Conclusion
Within the limitations of this in-vitro study, the following statements can be concluded:
Among the seven prophylaxis methods investigated, only the Golchai paste applied with a rubber cup demonstrated a statistically significant reduction in enamel surface roughness, achieving the smoothest surface. The remaining six groups did not show a significant reduction.
Brushing, whether used alone or in combination with prophylaxis paste, resulted in greater enamel roughness than the rubber cup alone, though the difference was not statistically significant. Furthermore, comparison between polished and unpolished sections revealed that brushing alone significantly increased surface roughness.
These findings underscore the critical importance of selecting appropriate prophylactic materials and techniques to minimize enamel damage, particularly on immature teeth.
Acknowledgements
The current study was extracted from a thesis, and the authors wish to thank the research deputy of Mashhad University of Medical Sciences for providing support to do this work.
Author contributions
F.M. and R.S. made a substantial contribution to the concept and design of the study, supervised the study, methodology, and reviewed the final manuscript. E.L. and B.F. participated in collecting and analyzing data, interpreting data, and preparing the first draft of the manuscript. Finally, all authors have read and approved the final manuscript.
Funding
This study was funded by the Mashhad University of Medical Sciences, Mashhad, Iran (code: 4002142).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All methods were carried out following relevant guidelines and regulations. The protocol of this study was approved by the Ethics Committee of Mashhad University of Medical Sciences (code: IR.MUMS.DENTISTRY.REC.1401.082).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Amri I, Albounni R, Binalrimal S (2021) Evaluation of the effect of soft drinks on the surface roughness of dental enamel in natural human teeth. F1000Res 10:1138. 10.12688/f1000research.55556.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari RM, Aljelaly HW, Almutair TJ, Alarfaj NA, Alhamami AA, Alsomali EA, Alzahrani JS (2024) Integrating preventive care strategies in routine dental practice. Int J Community Med Public Health 11:4119–4122. 10.18203/2394-6040.ijcmph20242902 [Google Scholar]
- Al-Twaijri S, Viana G, Bedran-Russo AK (2011) Effect of prophylactic pastes containing active ingredients on the enamel-bracket bond strength of etch-and-rinse and self-etching systems. Angle Orthod 81:788–793. 10.2319/101210-598.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari G, Oloomi K, Eslami B (2004) Microleakage assessment of pit and fissure sealant with and without the use of pumice prophylaxis. Int J Paediatr Dent 14:272–278. 10.1111/j.1365-263X.2004.00565.x [DOI] [PubMed] [Google Scholar]
- Banerjee A, Paolinelis G, Socker M, McDonald F, Watson TF (2008) An in vitro investigation of the effectiveness of bioactive glass air-abrasion in the “selective” removal of orthodontic resin adhesive. Eur J Oral Sci 116:488–492. 10.1111/j.1600-0722.2008.00561.x [DOI] [PubMed] [Google Scholar]
- Bonar LC, Shimizu M, Roberts JE, Griffin RG, Glimcher MJ (1991) Structural and composition studies on the mineral of newly formed dental enamel: a chemical, x-ray diffraction, and 31P and proton nuclear magnetic resonance study. J Bone Miner Res 6:1167–1176. 10.1002/jbmr.5650061105 [DOI] [PubMed] [Google Scholar]
- Camboni S, Donnet M (2016) Tooth surface comparison after air polishing and rubber cup: a scanning electron microscopy study. J Clin Dent 27:13–18 [PubMed] [Google Scholar]
- Chowdary C, Athawale R, Srinath SK (2018) Comparative evaluation of enamel abrasivity of different commercially available dentifrices – an in vitro study. J Indian Assoc Public Health Dent 16:78–82. 10.4103/jiaphd.jiaphd_165_17 [Google Scholar]
- Covey DA, Barnes C, Watanabe H, Johnson WW (2011) Effects of a paste-free prophylaxis polishing cup and various prophylaxis polishing pastes on tooth enamel and restorative materials. Gen Dent 59:466–473; quiz 474–465 [PubMed]
- Crowley J, Abdulhameed N, Al-Obaidi R, Hussein H (2024) Effect of preventive dental products on bonding force: an in vitro study. J Int Soc Prev Community Dent 14:243–251. 10.4103/jispcd.jispcd_201_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rosa LS, Souza LFB, Pilecco RO, Kluch TAC, Binotto FS, Henriques VZ, Kleverlaan CJ, Pereira GKR, Tribst JPM (2024) Are dental prophylaxis protocols safe for CAD-CAM restorative materials? Surface characteristics and fatigue strength. Coatings 14:1510 [Google Scholar]
- Duman B, Doğan S (2021) Comparison of polishing methods used in extrinsic discolouration of primary teeth in terms of surface roughiness. 10.21203/rs.3.rs-153735/v1
- Eakle WS, Bastin KG (2019) Dental materials: clinical applications for dental assistants and dental hygienists. Elsevier Health Sciences
- Eram A, Vinay Kr R, K NC, Keni LG, Shetty DD, Zuber M, Kumar S, S P (2024) Air-abrasion in dentistry: a short review of the materials and performance parameters. J Biomed Phys Eng 14:99–110. 10.31661/jbpe.v0i0.2310-1670 [DOI] [PMC free article] [PubMed]
- Fujii M, Kitasako Y, Sadr A, Tagami J (2011) Roughness and pH changes of enamel surface induced by soft drinks in vitro-applications of stylus profilometry, focus variation 3D scanning microscopy and micro pH sensor. Dent Mater J 30:404–410. 10.4012/dmj.2010-204 [DOI] [PubMed] [Google Scholar]
- Gomes IA, Mendes HG, Filho EMM, de CRC, Nina MG, Turssi CP, Vasconcelos AJ, Bandeca MC, de Jesus Tavarez RR (2018) Effect of dental prophylaxis techniques on the surface roughness of resin composites. J Contemp Dent Pract 19:37–41. 10.5005/jp-journals-10024-2208 [DOI] [PubMed]
- Goodson JM, Palys MD, Carpino E, Regan EO, Sweeney M, Socransky SS (2004) Microbiological changes associated with dental prophylaxis. J Am Dent Assoc 135:1559–1564. 10.14219/jada.archive.2004.0082 [DOI] [PubMed] [Google Scholar]
- Ijbara M, Wada K, Tabata MJ, Wada J, Inoue G, Miyashin M (2018) Enamel microcracks induced by simulated occlusal wear in mature, immature, and deciduous teeth. BioMed Res Int 2018:5658393. 10.1155/2018/5658393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiszewska-Olszowska J, Tandecka K, Szatkiewicz T, Stępień P, Sporniak-Tutak K, Grocholewicz K (2015) Three-dimensional analysis of enamel surface alteration resulting from orthodontic clean-up–comparison of three different tools. BMC Oral Health 15:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimyai S, Savadi-Oskoee S, Ajami AA, Sadr A, Asdagh S (2011) Effect of three prophylaxis methods on surface roughness of giomer. Med Oral Patol Oral Cir Bucal 16:e110-114. 10.4317/medoral.16.e110 [DOI] [PubMed] [Google Scholar]
- Kracher CM (2012) Current concepts in preventive dentistry. In
- Kristoffersson K, Axelsson P, Bratthall D (1984) Effect of a professional tooth cleaning program on interdentally localized Streptococcus mutans. Caries Res 18:385–390. 10.1159/000260792 [DOI] [PubMed] [Google Scholar]
- LaCross I, Darby M, Stull S, Lynch C (2007) In vitro evaluation of the reciprocating disposable prophylaxis angle versus the rotating disposable prophylaxis angle in extrinsic stain removal effectiveness. Am Dental Hygienists Assoc 81:105–105 [Google Scholar]
- Lynch RJ (2013) The primary and mixed dentition, post-eruptive enamel maturation and dental caries: a review. Int Dent J 63:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main PA, Lewis DW, Hawkins RJ (1997) A survey of general dentists in Ontario, Part II: knowledge and use of topical fluoride and dental prophylaxis practices. J Can Dent Assoc 63(607):610–607 [PubMed] [Google Scholar]
- Marshall GW Jr., Chang YJ, Gansky SA, Marshall SJ (2001) Demineralization of caries-affected transparent dentin by citric acid: an atomic force microscopy study. Dent Mater 17:45–52. 10.1016/s0109-5641(00)00056-7 [DOI] [PubMed]
- Monaco C, Arena A, Scheda L, Di Fiore A, Zucchelli G (2020) In vitro 2D and 3D roughness and spectrophotometric and gloss analyses of ceramic materials after polishing with different prophylactic pastes. J Prosthet Dent 124:787 e781–787 e788. 10.1016/j.prosdent.2020.05.040 [DOI] [PubMed]
- Mossman SL (2016) Polishing protocol. Dimens Dent Hyg 14:38–40 [Google Scholar]
- Neuhaus KW, Milleman JL, Milleman KR, Mongiello KA, Simonton TC, Clark CE, Proskin HM, Seemann R (2013) Effectiveness of a calcium sodium phosphosilicate-containing prophylaxis paste in reducing dentine hypersensitivity immediately and 4 weeks after a single application: a double-blind randomized controlled trial. J Clin Periodontol 40:349–357. 10.1111/jcpe.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palti DG, Machado MA, Silva SM, Abdo RC, Lima JE (2008) Evaluation of superficial microhardness in dental enamel with different eruptive ages. Braz Oral Res 22:311–315. 10.1590/s1806-83242008000400005 [DOI] [PubMed] [Google Scholar]
- Patil SS, Rakhewar PS, Limaye PS, Chaudhari NP (2015) A comparative evaluation of plaque-removing efficacy of air polishing and rubber-cup, bristle brush with paste polishing on oral hygiene status: a clinical study. J Int Soc Prev Community Dent 5:457–462. 10.4103/2231-0762.167723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policy on the Role of Dental Prophylaxis in Pediatric Dentistry (2016) Pediatr Dent 38:43–44 [PubMed]
- Roulet JF, Roulet-Mehrens TK (1982) The surface roughness of restorative materials and dental tissues after polishing with prophylaxis and polishing pastes. J Periodontol 53:257–266. 10.1902/jop.1982.53.4.257 [DOI] [PubMed] [Google Scholar]
- Salami D, Luz MA (2003) Effect of prophylactic treatments on the superficial roughness of dental tissues and of two esthetic restorative materials. Pesqui Odontol Bras 17:63–68. 10.1590/s1517-74912003000100012 [DOI] [PubMed] [Google Scholar]
- Sawai MA, Bhardwaj A, Jafri Z, Sultan N, Daing A (2015) Tooth polishing: the current status. J Indian Soc Periodontol 19:375–380. 10.4103/0972-124X.154170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Sharma P, Goje SK, Kanzariya N, Parikh M (2019) Comparative evaluation of enamel surface roughness after debonding using four finishing and polishing systems for residual resin removal-an in vitro study. Prog Orthod 20:18. 10.1186/s40510-019-0269-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kameyama A, Enokuchi T, Haruyama A, Chiba A, Sugiyama S, Hosaka M, Takahashi T (2017) Effect of professional dental prophylaxis on the surface gloss and roughness of CAD/CAM restorative materials. J Clin Exp Dent 9:e772–e777. 10.4317/jced.53826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Bosch JJ, Fennis-le Y, Verdonschot EH (2000) Time-dependent decrease and seasonal variation of the porosity of recently erupted sound dental enamel in vivo. J Dent Res 79:1556–1559. 10.1177/00220345000790080501 [DOI] [PubMed] [Google Scholar]
- Truong K, Chen J-W, Lee S, Riter H (2017a) Changes of surface properties of composite preveneered stainless steel crowns after prophy polishing to remove stains. Pediatr Dent 39(2):17–24 [PubMed] [Google Scholar]
- Truong K, Chen JW, Lee S, Riter H (2017b) Changes of surface properties of composite preveneered stainless steel crowns after prophy polishing to remove stains. Pediatr Dent 39:17–24 [PubMed] [Google Scholar]
- Tsai WS, Placa SJ, Panagakos FS (2012) Clinical evaluation of an in-office desensitizing paste containing 8% arginine and calcium carbonate for relief of dentin hypersensitivity prior to dental prophylaxis. Am J Dent 25:165–170 [PubMed] [Google Scholar]
- Uguru N, Onyejaka N, Uguru C (2020) Professional oral prophylaxis: assessment of practice by oral health professionals in Southeastern Nigeria. Niger J Med 29:670–675. 10.4103/njm.Njm_94_20 [Google Scholar]
- Wada K, Ijbara M, Salim NA, Wada J, Iwamoto T (2023) Three-dimensional microscopic comparison of wear behavior between immature and mature enamel: an in vitro study. BMC Oral Health 23:40. 10.1186/s12903-023-02751-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DP, Colescott TD, Henson HA, Powers JM (2002) Effects of four prophylaxis pastes on surface roughness of a composite, a hybrid ionomer, and a compomer restorative material. J Esthet Restor Dent 14:245–251. 10.1111/j.1708-8240.2002.tb00170.x [DOI] [PubMed] [Google Scholar]
- Yildirim TT, Oztekin F, Keklik E, Tozum MD (2021) Surface roughness of enamel and root surface after scaling, root planning and polishing procedures: an in-vitro study. J Oral Biol Craniofac Res 11:287–290. 10.1016/j.jobcr.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz Tuzcel N, Akkaya M, Karacaoglu F (2017) A comperative evaluation of 3 different polishing methods on tooth surface roughness. J Biomed Sci 06. 10.4172/2254-609x.100046
- Younis A, Sulaiman A (2024) Effects of acid etching on color changes and surface morphology of enamel to be bleached with different techniques. Georgian Med News 349:103–109 [PubMed] [Google Scholar]
- Yurdaguven H, Aykor A, Ozel E, Sabuncu H, Soyman M (2012) Influence of a prophylaxis paste on surface roughness of different composites, porcelain, enamel and dentin surfaces. Eur J Dent 6:1–8 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.