Abstract
Skin ageing is a multifactorial process influenced by both intrinsic (genetic and metabolic) and extrinsic (environmental) factors, leading to noticeable changes such as wrinkles, loss of elasticity, and pigmentation disorders. Recent advancements in regenerative medicine have highlighted the potential of exosomes, small extracellular vesicles, in mediating cellular communication and promoting rejuvenation processes in skin tissues. Exosomes are secreted by various cell types and are rich in bioactive molecules such as proteins, lipids, and nucleic acids, which are crucial for modulating physiological responses. Exosomes derived from mesenchymal stem cells (MSCs), adipose-derived stem cells (ADSCs), and other sources have shown promising results in enhancing skin cell proliferation and collagen synthesis and reducing oxidative stress, thereby mitigating both intrinsic and extrinsic skin ageing. Therefore, this review explores the mechanisms through which exosomes exert their effects, including the modulation of signalling pathways involved in cell growth, anti-inflammatory responses, and matrix remodelling. We also explore innovative delivery systems for exosome-based therapies, such as microneedling and hydrogels, which enhance the penetration and efficacy of these vesicles in skin applications. However, despite their potential, the clinical application of exosome-based therapies faces challenges such as scalability of production, standardization of purification methods, and understanding of long-term effects. This comprehensive investigation emphasised the potential of exosomes in the fields of dermatology and regenerative medicine in combating skin ageing.
Keywords: Skin ageing, Stem cells, Exosomes, Oxidative stress, Rejuvenation
Introduction
The skin, the largest organ of the body, serves as a critical barrier against environmental aggressors and plays a vital role in maintaining homeostasis [1]. However, skin ageing is an inevitable biological process characterised by a decline in structural integrity and physiological function. Intrinsic ageing, driven by genetic and metabolic factors, manifests as fine lines, dryness, and a loss of elasticity [2]. Extrinsic ageing, predominantly caused by ultraviolet (UV) radiation, pollution, and lifestyle factors, exacerbates these effects by inducing collagen degradation, oxidative stress, and inflammation [3]. Together, these processes contribute to visible signs of ageing, such as wrinkles, sagging, and pigmentation disorders, which not only affect appearance but also compromise the protective functions of skin tissue [4] (Fig. 1).
Fig. 1.
Skin structure and aging skin. The normal skin structure comprises three distinct layers, including the epidermis, the dermis, and the subcutaneous tissue. The epidermis, as the outermost layer of skin tissues, consists of the stratum corneum, keratin-forming cells, and melanocytes, serving crucial roles in protection and barrier function. Beneath it lies the dermis, which contains fibroblasts, collagen, macrophages, and capillaries, contributing to the skin elasticity, strength, and nutrient supply of the skin. The subcutaneous layer consists of loose connective tissue and adipocytes, modulating energy storage, and insulation. External factors such as ultraviolet radiation, air pollution, and temperature fluctuations can accelerate skin aging. This process leads to the production of senescent melanocytes, a reduction in collagen, dermal fibroblasts, blood vessels, and subcutaneous fat, culminating in hyperpigmentation, wrinkles, diminished nutrient supply, repair capacity, fullness, and thermal retention of the skin
Exosomes, which are nanoscale extracellular vesicles (EVs), are secreted by diverse arrays of cells and encapsulate a rich assortment of bioactive molecules, including proteins, lipids, and nucleic acids [5]. These vesicles facilitate intercellular communication by transferring their cargo to target cells, thereby modulating a wide range of physiological and pathological processes [6]. Exosomes are enriched with specific proteins, such as tetraspanins (CD63, CD81), heat shock proteins (HSP70, HSP90), and signalling molecules that are crucial for their biogenesis and function [7]. Owing to their ability to modulate cellular behaviour, exosomes hold significant promise as therapeutic agents in regenerative medicine [8]. Exosomes have been investigated for potential applications in treating cardiovascular diseases, neurological disorders, cancers, and wound healing [9] (Fig. 2).
Fig. 2.
The history of exosome identification and application. In 1983, Johnstone et al. identified vesicles released from multivesicular bodies in reticulocytes, leading to their formal designation as exosomes in 1987. The field advanced in 1996 when Raposo et al. described antigen-presenting exosomes from B lymphocytes carrying MHC class II molecules and adhesion factors. Zitvogel et al. demonstrated dendritic cell-derived exosomes with similar functionality in 1998. Valadi et al. established exosome roles in RNA transfer and intercellular communication in 2007. Clinical translation commenced in 2008 through the landmark ESCORT trial, representing the first clinical application of engineered exosomes for targeted therapy. This milestone enabled critical pathology studies, including the 2011 finding that Staphylococcus aureus-derived vesicles induce atopic dermatitis-like inflammation. The vesicular transport mechanisms elucidated by Rothman, Südhof, and Schekman earned them the 2013 Nobel Prize. Since 2016, exosome research has expanded into regenerative medicine, immunomodulation, and targeted drug delivery, with an emerging focus on their potential in alleviating skin aging
The interplay between exosomes and skin ageing is a burgeoning area of research [10]. Exosomes derived from different cell types, such as mesenchymal stem cells (MSCs), fibroblasts, and even plant cells, have shown potential in modulating the ageing process of skin cells [11]. Exosomes can increase cell proliferation, reduce oxidative stress, and promote collagen synthesis, thereby mitigating the effects of both intrinsic and extrinsic ageing [12]. The ability of exosomes to deliver bioactive molecules directly to target cells offers a novel mechanism to rejuvenate ageing skin and restore its function (Fig. 3). The therapeutic potential of exosomes in combating skin ageing lies in their stability, biocompatibility, and capacity for targeted delivery [13]. Unlike traditional stem cell therapies, exosomes pose a lower risk of immune rejection and tumorigenesis [14]. Their small size allows for efficient penetration through the skin barrier, and their natural origin ensures minimal toxicity [15]. These properties make exosomes attractive candidates for developing anti-ageing treatments.
Fig. 3.
Processes of exosome biogenesis, secretion, and uptake. Exosomes are derived from almost all cell types and are crucial mediators of intercellular communication. The production of exosomes involves a continuous and temporally orchestrated biological process. The biogenesis of exosomes begins with the invagination of the cell membrane to form early endosomes, which mature into multivesicular bodies (MVBs). These MVBs can either fuse with lysosomes for degradation and recycling or merge with the cell membrane to release exosomes via budding. Exosomes are internalized by the recipient cells, possibly by three main mechanisms, including endocytosis, membrane fusion, and receptor binding, initiating a cascade of cellular events. Exosomes are enriched with a diverse array of conserved components in their membranes, including adhesion molecules, biogenesis-related proteins, membrane transport and fusion proteins, tetraspanning proteins (CD63, CD9), MHC, and other receptors. Exosomes carry specific bioactive molecules, including DNA, miRNAs, lncRNAs, circRNAs, amino acids, metabolites, and proteins. The delivery of these bioactive molecules modulates signaling pathways in recipient cells, enhances cellular repair mechanisms, and reduces UV-induced oxidative stress and inflammatory responses, holding significant potential for maintaining skin homeostasis and decelerating skin aging
This review aims to elucidate the mechanisms by which exosomes exert their anti-ageing effects on the skin, categorise the different types of exosomes on the basis of their cellular origin, and discuss their therapeutic potential and limitations. By providing a comprehensive overview of the current research, this paper highlights the value of exosome-based therapies in the fields of dermatology and regenerative medicine.
The mechanism of exosomes in skin regeneration and rejuvenation
This section explores the specific molecular pathways and interactions through which exosomes contribute to skin health, emphasising the various sources of exosomes and their unique therapeutic properties. Exosomes are derived from diverse sources, including MSCs, fibroblasts, and adipose-derived stem cells (ADSCs), each of which provides distinct advantages for skin regeneration. We begin with exosomes derived from human umbilical cord MSCs (hucMSCs), which have demonstrated considerable potential in reducing signs of skin ageing and enhancing skin repair.
HucMSC-exos
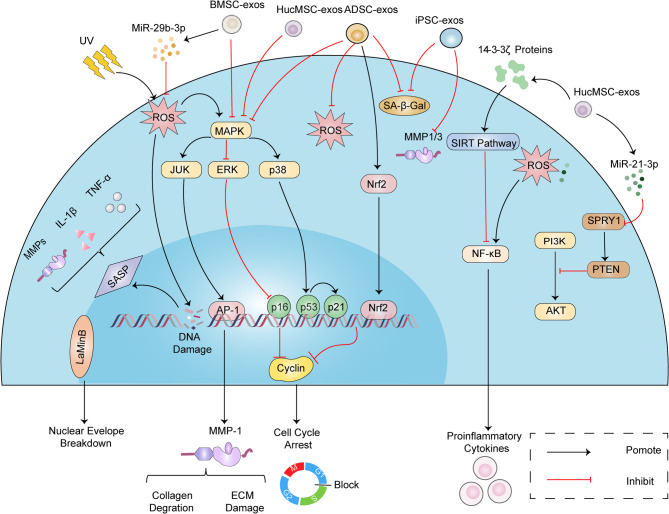
HucMSCs are derived mostly from Wharton’s Jelly of the umbilical cord, which is a gel-like substance composed mainly of mucopolysaccharides and is also rich in MSCs [16]. Compared with other types of MSCs, HucMSCs have the advantages of easy extraction, low cost, noninvasive collection procedures, high cell yield, and low immunogenicity. HucMSCs can effectively promote skin wound healing, reduce apoptosis, decrease ROS production, and enhance cell motility, which can effectively attenuate skin photoageing. For example, hucMSC-derived exosomes (hucMSC-exos) increased cell proliferation and type I collagen and decreased matrix metalloproteinase-1 (MMP-1) levels in irradiated fibroblasts [17]. Liu et al. revealed the anti-ageing potential of hucMSC-exos, which inhibited UVB-induced skin ageing by increasing the proliferation and migration of normal HaCaT cells, reducing cell apoptosis and senescence, increasing the expression of type I collagen, and decreasing the expression of MMP-1 [18]. Additionally, Vu et al. reported that EV subpopulations released by hucMSCs primed with transforming growth factor-β (TGF-β) upregulated the mRNA and levels of extracellular matrix (ECM) genes (COL I, COL III, elastin, HAS II, and HAS III) in fibroblasts. Thus, EVs from UCMSCs protect the skin from ageing by promoting the production of ECM proteins [19]. Liang et al. reported that hucMSC-exos repaired human skin fibroblasts (HSFs) that were damaged by oxidative stress from H2O2 [20]. UV radiation induces the production of reactive oxygen species (ROS) through photochemical reactions. The ROS inactivate protein tyrosine phosphatases (PTPs), leading to the net phosphorylation of receptor tyrosine kinases (RTKs), and then activate downstream mitogen-activated protein kinases (MAPKs), including ERK, p38, and JNK. The activation of these MAPKs is crucial for the expression of c-Fos and c-Jun, which combine to form the transcription factor activator protein 1 (AP-1). AP-1 subsequently promotes the expression of matrix metalloproteinases (MMPs), resulting in collagen loss and fragmentation of the ECM, thereby accelerating skin ageing. Inhibition of the MAPK/AP-1 pathway can reduce the expression of MMPs, thereby alleviating skin ageing [21]. The activation of NF-κB leads to the expression of inflammatory cytokines TNF-α, IL-1β, and IL-6, which further activate the MAPK and JNK pathways, thereby promoting the expression of MMPs and resulting in collagen degradation and skin ageing [22]. HucMSC-exos inhibited the MAPK, JNK, and NF-κB signalling pathways, which resulted in increased p21 expression and decreased lamin B1 expression, ultimately mitigating cell damage and ageing caused by oxidative stress. These results suggested that hucMSC-exos could improve skin photodamage.
MiRNAs in hucMSC-exos
miRNAs are a class of short non-coding RNAs with lengths of 20–24 nucleotides (nt) that regulate gene expression in multicellular organisms by inhibiting mRNA degradation and translation [23]. Studies have shown that specific miRNAs are dysregulated after skin injury and can effectively promote matrix remodelling and re-epithelialization, as well as inhibit epithelial-mesenchymal transition [24]. HucMSC-exos can serve as efficient and secure vectors for the precise delivery of miRNAs, consequently modulating the gene expression and biological functions of recipient cells. ERK is a member of the MAPK family. The persistent activation of ERK1/2 can disrupt the nuclear envelope localization of progerin and inhibit the nuclear envelope budding process, thereby preventing the lysosomal degradation of chromatin and telomeres, promoting genomic and telomeric stability, and consequently alleviating cell apoptosis and skin ageing [25]. Stimulation of the PI3K/Akt pathway can foster cell proliferation and migration, thereby diminishing apoptosis and consequently facilitating skin repair and regeneration [26]. It has been reported that hucMSC-exos can stimulate fibroblast proliferation and migration and promote EC angiogenesis [27]. The microRNA miR-21-3p, which was highly enriched in hucMSC-exos, acted as a key mediator to exert the above effects by inhibiting the expression of PTEN and SPRY1.
Protein in hucMSC-exos
The 14-3-3ζ protein is an isoform of the highly conserved 14-3-3 family. They bind specifically to phosphorylated serine or threonine peptides and are involved in various signalling pathways that regulate essential cellular activities such as metabolism, cell cycle progression, growth, survival, apoptosis, and gene transcription [28]. Additionally, 14-3-3ζ proteins act as molecular chaperones, preventing protein aggregation under stress conditions, highlighting their crucial role in ageing regulation and potential as targets for age-delaying therapies [29]. The activation of SIRT1 can mitigate oxidative stress, thereby suppressing cell apoptosis and enhancing cell proliferation, thus contributing to the alleviation of skin ageing [30]. Wu et al. reported that the subcutaneous injection of hucMSC-exos in vivo exhibited antioxidant and anti-inflammatory effects against UV-induced DNA damage and apoptosis [31]. Further studies revealed that hucMSC-exos-treated skin keratinocytes released 14-3-3ζ protein, which regulated the SIRT1-dependent antioxidant pathway, inhibited oxidative stress, and promoted autophagy activation. This reduction in cytotoxicity from UV and H2O2 exposure suggested that hucMSC-exos could be novel therapeutic agents for treating skin photodamage and ageing caused by UV radiation.
HucMSC-exos in combination with other materials
Some low-molecular-weight peptides are used as antioxidants to prevent skin ageing, which may be related to the presence of aromatic amino acids and histidine [32]. Combining exosomes with these antioxidative peptides can enhance their efficacy and application in combating skin ageing [33]. Hydrolysed collagen oligopeptides (HCOPs) are antioxidants that enzymatically hydrolyse forms of collagen to reduce skin ageing, sagging, dryness, and wrinkle formation, thus exerting anti-ageing effects on the skin [34]. Zhu et al. reported that cells treated with hucMSC-exos and HCOPs showed enhanced proliferation and migration capabilities, reduced production of ROS and senescence-associated β-galactosidase activity, and increased expression of type I and III collagen [35]. hucMSC-exos + HCOPs decreased the expression of MMP-1, MMP-3, MMP-9, interleukin-1 β (IL-1β), and tumour necrosis factor-α (TNF-α) and reduced the expression of p16, p21, and p53. This study highlighted the positive effect of hucMSC-exos in alleviating photoageing when used in conjunction with certain materials.
Microneedling (MN) is a transdermal drug delivery technique that enhances transdermal drug absorption by creating tiny channels in the skin to rapidly open skin channels [36]. Additionally, MNs can promote repair mechanisms through mechanical stimulation, increase the local drug concentration, and reduce degradation rates, ultimately effectively reducing side effects [37]. MNs with exosomes increase skin permeability by creating microchannels, allowing exosomes to penetrate the skin barrier and reach the dermis, thereby promoting repair and regeneration [38]. Haliclona sp. is a marine sponge that produces spicules, which are sharp and rod-like siliceous structures [39]. The marine sponge Haliclona sp. Spicules (SHSs) can physically disrupt the skin to form microchannels that facilitate drug penetration and can be retained in the skin, thus enhancing the transdermal absorption of biomolecules. Zhang et al. discovered that the combined use of SHSs and hucMSC-exos reduced the number of microwrinkles, alleviated histological changes, and promoted the expression of ECM components [40]. Skin irritation tests revealed that the combination of hucMSC-exosomes and SHSs caused mild skin irritation in guinea pigs, which resolved quickly. This indicated that the therapeutic effects of hucMSC-exosomes combined with SHSs against UV-induced photoaging in mice represent a safe and effective approach.
ADSC-exos
Adipose tissue consists of the stromal vascular fraction (SVF), mature adipocytes, and ECM [41]. ADSCs are a type of MSC isolated from SVFs and are abundant, readily available, and easy to isolate [42]. ADSC-derived exosomes (ADSC-exos) can mimic the ability of ADSCs to perform innovative cell-free therapies and are safer therapeutic agents than ADSCs. ADSC-exos are used as tools for promoting tissue regeneration and repair, which involves the inhibition of apoptosis and senescence, immune regulation, anti-inflammatory effects, and maintenance of tissue homeostasis [43].
ADSC-exos show therapeutic potential for treating skin ageing because of their ability to promote tissue regeneration, alleviate pigmentation loss, and reduce wrinkles in the clinic. Liang et al. established a photoaged skin model by exposing Sprague‒Dawley rats to UVB radiation and administered ADSC-Exos as a treatment. ADSC-exos at a single therapeutic dose for 7 consecutive days were injected into the photoaged skin [44]. In the treated photoaged skin, there was a decrease in epidermal thickness, the proportion of the stratum corneum within the epidermis, and the mRNA expression of type III collagen, MMP-1, and MMP-3. Additionally, there was an increase in dermal thickness and type I collagen mRNA expression, indicating that ADSC-Exos can suppress skin ageing. Additionally, ADSC-exos mitigated human dermal fibroblast (HDF) senescence and promoted migration [45]. The binding of TGF-β to its receptor initiates the activation of Smad proteins, thereby promoting the migration of fibroblasts and the synthesis of extracellular matrix (ECM) proteins and consequently facilitating skin repair and regeneration [46]. ADSC-exos enhanced type I collagen expression, decreased ROS and SA-β-Gal activity, and suppressed the expression of the senescence-associated proteins p53, p21, and p16, thus effectively preventing cellular ageing. At the HDF level, ADSC-exos inhibited UVB-induced cellular DNA damage, ROS, and MMP-1 overexpression by regulating the Nrf2 and MAPK/ activator protein-1 (AP-1) pathways, increasing type I collagen expression by activating the TGF-β/Smad pathway, thus alleviating cellular photoageing [47].
MiRNAs in ADSC-exos
Autophagy plays a protective role against UV-induced skin photoageing by regulating organelle homeostasis and reducing oxidative stress, thereby decelerating the photoageing process. The activation of autophagy has been shown to increase the intracellular levels of ROS, MMP-1, type I procollagen, and DNA damage in skin fibroblasts exposed to UVB irradiation [48]. Autophagic flux refers to the dynamic process of material degradation and recycling during autophagy. With ageing, the rate of cellular waste production increases, whereas autophagic flux decreases, leading to the accumulation of intracellular protein aggregates, reduced clearance of dysfunctional organelles, and heightened inflammation, which collectively accelerate the biological ageing process [49]. Thus, the efficiency of autophagic flux and the status of autophagy-related proteins directly influence cellular responses to damage and repair mechanisms, thereby impacting the skin ageing process.
Research has demonstrated that miR-1246-overexpressing exosomes isolated from ADSCs significantly reduce the expression of GSK3β and p62 and increase autophagy flux and the expression of autophagy-related proteins, such as LC3II [50]. Moreover, OE-EXs markedly reversed the UVB irradiation-induced increases in the levels of ROS, MMP-1, type I collagen, and DNA damage in HSFs. The injection of OE-EXs reversed the wrinkles, epidermal hyperplasia, and reduction in collagen fibres caused by photoageing in the photoaged Kunming mouse model. Hence, OE-EXs ameliorated UVB-induced skin photoageing by activating autophagy through GSK3β. Modulating the autophagic process may provide new insight for preventing and repairing UV-damaged skin.
LncRNAs in ADSC-exos
LncRNA H19 is a commonly reported long-stranded ncRNA that regulates cell proliferation and invasion through multiple pathways, and its aberrant expression has been associated with multiple critical biological functions, including tissue development, the immune response, and stem cell differentiation [51]. The aberrant expression and significant biological functions of lncRNA H19 have made it a diagnostic and therapeutic target for various diseases, particularly in tumours, as well as in certain skin conditions, including psoriasis, scars, and melanoma [52]. Nrf2 regulates the expression of antioxidant genes (such as SOD, GPx, and NQO1) by binding to the antioxidant response element (ARE), thereby reducing oxidative stress and increasing the cell’s antioxidant capacity, which in turn can alleviate skin ageing [53]. With respect to skin ageing, Gao et al. reported that lncRNA H19-overexpressing exosomes (H19-exos) reduce MMP production, DNA damage, and ROS levels while promoting type I procollagen synthesis in UVB-exposed HSFs [54]. In UVB-irradiated mice, H19-exos reversed epidermal thickening and collagen degradation. Thus, H19-exos could treat UV-induced skin photoageing by upregulating SIRT1.
BMSC-exos
Bone marrow stem cells (BMSCs) are pluripotent stem cells derived from bone marrow that can modulate inflammation and repair damaged tissues via paracrine functions [55]. The directional differentiation of BMSCs into adipogenic and osteogenic lineages is associated with the development of various diseases, including osteoporosis, impaired wound healing, and tissue regeneration disorders [56, 57]. Senescence or functional disturbances in BMSCs can result in the loss of their regenerative capacity. BMSC-derived exosomes (BMSC-exos) promote angiogenesis and osteogenesis [58]. Currently, BMSC-exos have been confirmed to reverse skin photoageing by repairing tissue damage. For example, the addition of BMSC-exos to UVB-irradiated HDFs restored cell viability; inhibited the activation of the MAPK/AP-1 signalling pathway; decreased the secretion of ROS and lactate dehydrogenase, TNF-α, IL-6, and IL-1β; inhibited the expression of MMP-1 and MMP-3; and promoted the expression of type I collagen [59]. These results showed that BMSC-exos protected HDFs from UVB-induced inhibition of cell viability, activation of cellular oxidative stress, and inflammatory responses and consequently reduced skin photoageing. Another study revealed that BMSC-exos-miR-29b-3p promoted the migration of UVB-irradiated HDFs; reduced oxidative stress, apoptosis, and MMPs; and increased type I collagen, thereby inhibiting photoageing [60].
iPSC-exos
Human induced pluripotent stem cells (iPSCs) are stem cells that have been reprogrammed from adult cells to an embryonic stem cell-like state, with unlimited expansion potential and the ability to differentiate into most somatic cell types. iPSCs are useful tools for in vitro disease modelling, drug screening, cellular therapies, personalised medicine, and regenerative therapies [61]. Furthermore, iPSCs have been widely used in clinical studies of diseases, such as heart, liver, and neurological disorders, and spinal cord injuries [62]. iPSC-derived exosomes (iPSC-exos) have regenerative properties that promote cell renewal and tissue repair and can effectively reverse the ageing state of fibroblasts for skin-related anti-ageing therapy [63].
The iPSC-exos stimulated the proliferation and migration of HDFs under normal conditions. Pretreatment with iPSC-exos inhibited the damage to HDFs and the overexpression of MMP-1 and MMP-3 caused by UVB irradiation [64]. The iPSC-Exos also increased the expression level of type I collagen in the photoaged HDFs. In addition, iPSC-exos significantly reduced the expression levels of SA-β-Gal, MMP-1, and MMP-3 and restored type I collagen expression in senescent HDFs. These results indicate the therapeutic potential of iPSC-exos for treating skin ageing (Fig. 4).
Fig. 4.
Stem cell-derived exosomes in skin aging. Exosomes from various stem cell sources, such as human umbilical cord MSCs (hucMSC-exos), adipose-derived stem cells (ADSC-exos), bone marrow stem cells (BMSC-exos), and induced pluripotent stem cells (iPSC-exos), contribute to skin regeneration and anti-aging through diverse mechanisms. These exosomes enhance collagen synthesis, reduce oxidative stress, and modulate key signaling pathways like MAPK, NF-κB, and TGF-β/Smad. Stem cell-derived exosomes deliver miRNAs and proteins that regulate cell proliferation, apoptosis, and autophagy, thereby promoting the repair and rejuvenation of skin tissue. The combined use of exosomes with materials such as hydrolyzed collagen oligopeptides and microneedles further amplifies their therapeutic effects, offering promising strategies for combating photoaging and maintaining skin homeostasis.
adipose-derived stem cells-derived exosomes (ADSC-exos); protein kinase b (AKT); activator protein 1 (AP-1); bone marrow stem cells-derived exosomes (BMSC-exos); cyclin; extracellular matrix (ECM); extracellular signal-regulated kinase (ERK); human umbilical cord mesenchymal stem cells-derived exosomes (HuMSC-exos); induced pluripotent stem cells-derived exosomes (iPSC-exos); c-jun n-terminal kinase (JNK); matrix metalloproteinase (MMP); nuclear factor kappa-b (NF-κB); nuclear factor erythroid 2-related factor 2 (Nrf2); phosphoinositol-3 kinase (PI3K); phosphatase and tensin homolog (PTEN); reactive oxygen species (ROS); senescence-associated β-galactosidase (SA-β-Gal); sirtuin pathway (SIRT Pathway); ultraviolet (UV)
HDF-exos
Fibroblasts are important skin cells in the dermis and can synthesise and degrade fibrous and amorphous ECM proteins, produce collagen, and promote wound healing [65]. HDF-derived exosomes (HDF-exos) are nanoscale EVs secreted by dermal fibroblasts that regulate matrix metabolism, reduce inflammatory responses, and promote cell proliferation. As the skin ages, a decline in fibroblast function and collagen synthesis leads to visible signs of ageing, such as wrinkles and loss of elasticity. HDF-exos can restore the function of fibroblasts and slow the ageing process of the skin.
Recent studies have highlighted the anti-ageing properties of exosomes derived from 3D-cultured HDF spheroids (3D HDF-XOs) [66]. Hu et al. demonstrated that 3D HDF-XOs suppress UVB-induced MMP-1 expression, restore type I procollagen levels, activate the TGF-β pathway, and reduce skin inflammation and senescence by downregulating TNF-α [66]. These findings suggest that 3D HDF-XOs have significant potential for preventing and treating cutaneous ageing. Consistent with these findings, Park et al. reported that exosomes derived from human foreskin fibroblasts BJ 5ta (BJ-5ta exos) reduced the production of ROS, decreased oxidative stress, and prevented ageing induced by UV rays in HSFs [67]. In SKH‑1 hairless mice subjected to UVB irradiation, BJ-5ta exos inhibited transepidermal water loss and wrinkle formation and increased the expression levels of type I collagen and elastin in the dorsal skin caused by UVB, consequently combating skin photoageing. To further extend the therapeutic potential of exosomes, Sanada et al. reported that dermal stem/progenitor cells (DSPCs) might maintain skin homeostasis by increasing fibroblast production of type I collagen through the secretion of exosomes containing ANP32B [68]. DSPC-derived exosomes (DSPC-exos) possibly contribute to preserving the skin equilibrium to potentiate the rejuvenation of ageing skin.
Milk-exos
Milk is rich in casein and whey proteins and is effective in boosting the immune system. Compared with other types of exosomes, milk-derived exosomes (milk-exos) are more abundant and readily available. Milk-exos are rich in proteins and lipids, thus promoting fibroblast and collagen production and reducing inflammation [69], and therefore have unique potential for skincare and anti-ageing applications. By activating the Wnt/β-catenin pathway, skin cell proliferation and migration can be enhanced, and apoptosis can be reduced, aiding in skin repair and regeneration [70]. Han et al. reported that treatment with colostrum exosomes could inhibit the production of intracellular ROS caused by UV-induced photoageing and reduce the generation of the protective skin-darkening pigment melanin, thereby lowering the risk of hyperpigmentation disorders due to excessive melanin formation [71]. Bovine colostrum-derived exosomes also suppressed the expression of MMP and increased cell proliferation and collagen production. Lu et al. reported that purified milk-exos were absorbed by skin cells in vitro, upregulated the levels of hydration factors such as filaggrin (FLG), aquaporin 3 (AQP3), and CD44 in keratinocytes; increased hyaluronidase (HAS2) activity in fibroblasts, and restored the production of type I collagen and type III collagen, which in turn maintained dermal hydration and reduced the formation of facial wrinkles [72].
Plant-exos
Plant-derived exosomes (plant-exos) are usually extracted from edible vegetables, fruits, and herbs. Compared with mammalian cell-derived exosomes, plant-exos have multiple advantages, including high yield, low immunogenicity and toxicity, and high biocompatibility and stability [73]. Plant-exos exhibit anti-inflammatory, antioxidant, and cellular homeostasis maintenance effects [74]. In particular, exosomes extracted from fruits, such as blueberries, mandarin oranges, and apples, attenuate oxidative stress damage. Plant-exos can increase skin cell viability and migration, promote angiogenesis, and reduce ROS production, thereby promoting skin wound healing. Plant-exos have been shown to extensively regulate various skin-related diseases, such as skin pigmentation and diabetic ulcer healing [75].
Polyphenols, which are abundant in apples, have anti-inflammatory and antioxidant effects. Apple-derived nanovesicles (ADNVs) demonstrated anti-inflammatory activity in classically activated THP-1-derived macrophages by regulating miR-146a [76]. Trentini et al. confirmed that ADNVs reduce the degradation of the ECM in dermal fibroblasts by inhibiting TLR4 activity to downregulate the NF-κB pro-inflammatory pathway, thus increasing collagen synthesis and reducing the production of MMPs [77]. Thus, ADNVs with a 2% hyaluronic acid formulation and MeHa patches possessed the ability to combat skin ageing.
Ginseng, a common medicinal plant, has been established as a medicinal supplement with anti-inflammatory, stress-resistant, antioxidant, and anti-ageing effects [78]. This evidence has aroused interest in exploring the roles and mechanisms of ginseng root-derived exosome-like nanoparticles (GrDENs) in combating skin photoageing. In HaCaT cells irradiated with UVB, GrDENs reduced the generation of ROS and downregulated the mRNA expression of proapoptotic genes, ageing-related genes (MMP2 and MMP3), proinflammatory genes (COX-2 and IL-6), and the cellular senescence biomarker p21, possibly through the inhibition of AP-1 signalling [78]. This study indicated that GrDENs could effectively protect against skin damage caused by UV radiation and oxidative stress.
Olea europaea leave (OLEX) has antioxidant, anti-inflammatory, and anti-ageing properties, and is commonly used in skin care products to increase skin cell viability by inhibiting fibroblast apoptosis [79]. Olea europaea leaf-derived nanovesicles (OLELNVs) possess significant anti-photoageing properties and enhance skin and cellular absorption, making them a popular material for combating photoageing and reducing the accumulation of senescent cells. Wang et al. demonstrated that OLELNVs have high dose effects, are skin friendly, and have noncytotoxic properties that inhibit cellular ageing [80]. They incorporated OLELNVs into crosslinked hyaluronic acid (HA) and tannic acid (TA) hydrogels, forming the OLELNVs@HA/TA hydrogel system. In a UVB-damaged HaCaT and HDF-α cell model, OLELNVs reduced ROS, IL-6, MMP-1, and MMP-3 levels, decreased SA-β-Gal activity, restored SOD activity, and increased type I collagen activity, mitigating UV-induced cellular damage and ageing. The OLELNVs@HA/TA hydrogel system protected the skin structure, reduced cellular senescence, enhanced antioxidant defence, and promoted skin repair and regeneration in a UVB-induced skin photoageing mouse model (Fig. 5; Table 1).
Fig. 5.
Role of exosomes from other cellular sources on photoaged skin. Exosomes from human dermal fibroblasts (HDF-exos), milk (milk-exos), and plants (plant-exos) offer promising anti-aging benefits for skin. HDF-exos restore fibroblast function, enhance collagen synthesis, and activate the TGF-β pathway, effectively reducing wrinkles and improving elasticity. Milk-exos, abundant and accessible, decrease oxidative stress, promote collagen production, and enhance hydration by upregulating factors like filaggrin and aquaporin 3. Plant-exos, especially those from apples and ginseng, provide strong antioxidant and anti-inflammatory effects, reducing ROS and promoting skin repair. Olea europaea leaf-derived nanovesicles (OLELNVs) further combat UV-induced damage and enhance collagen synthesis. Together, these exosomes offer innovative strategies for rejuvenating aging skin and maintaining dermal health
acidic leucine-rich nuclear phosphoprotein 32 family member b (ANP32B); activator protein 1 (AP-1); apple-derived nanovesicles (ADNVs); human foreskin fibroblasts BJ 5ta (BJ-5ta); collagen (COL); cyclooxygenase-2 (COX-2); dermal stem/progenitor cells-derived exosomes (DSPC-exos); extracellular matrix (ECM); ginseng root-derived exosome-like nanoparticles (GrDENs); hyaluronan synthase 2 (HAS2); human dermal fibroblasts-derived exosomes (HDF-exos); interleukin-6 (IL-6); mitogen-activated protein kinase (MAPK); milk-derived exosomes (milk-exos); matrix metalloproteinases 1/8/9 (MMP-1/8/9); matrix metalloproteinase-1 (MMP-1); nuclear factor kappa-b (NF-κB); Olea europaea leaf-derived nanovesicles (OLELNVs); reactive oxygen species (ROS); senescence-associated β-galactosidase (SA-β-Gal); superoxide dismutase (SOD); toll-like receptor 4 (TLR4); wnt/β-catenin signaling pathway (Wnt/β-catenin)
Table 1.
Exosomes, contents, and related signaling pathways in reversing aging
| Source | Content | Recipient cells | Pathway | Roles and Functions | Ref |
|---|---|---|---|---|---|
| HucMSC-exos | Not applicable | HaCaT | Inhibit MAPK, JNK, and NF-κB | Reduce TNF-α, IL-1β, IL-6, and MMPs, increase IL-10 and collagen I, promote photoaged cell proliferation, and reduce apoptosis. | [18] |
| HucMSC-exos | Not applicable | HDFs | Not applicable | Increase collagen and elastin, promote fibroblast migration. | [19] |
| HucMSC-exos | Not applicable | HDFs | Inhibit MAPK, JNK, and NF-κB | Increase p21 protein, decrease lamin B1 protein, reduce TNF-α, CXCL12, and IL-6, and reduce oxidative stress-induced cell damage and senescence. | [20] |
| HucMSC-exos | miR-21-3p | HDFs and endothelial cells | Activate PI3K/Akt and ERK1/2 | Inhibit PTEN and SPRY1, promote angiogenesis, and promote cell proliferation and migration. | [27] |
| HucMSC-exos | 14-3-3ζ proteins | HaCaT | Activate SIRT1 | Reduce ROS, p-p65, TNF-α, increase proliferating cell nuclear antigen, reduce DNA damage, reduce skin inflammation and promote skin cell regeneration, and alleviate UV radiation-induced skin damage. | [31] |
| HucMSC-exos | HCOPs | HDFs | Not applicable | Reduce p16, p21, and p53, decrease ROS, SA-β-gal, MMP-1, MMP-3, MMP-9, IL-1β, and TNF-α, increase collagen types I and collagen types III, and promote cell proliferation and migration. | [35] |
| ADSC-exos | Not applicable | HDFs | Not applicable | Increase type I collagen, reduce MMP-1 and MMP-3, increase fibroblast proliferation, reduce the abnormal proliferation of basal layer cells, decrease epidermal layer, and increase dermal layer thickness of photoaged skin. | [44] |
| ADSC-exos | Not applicable | HDFs | Not applicable | Inhibit senescence-associated proteins p53, p21, and p16, increase type I collagen, decrease ROS and A-β-Gal, and promote cell proliferation and migration. | [45] |
| ADSC-exos | Not applicable | HDFs | Inhibit MAPK, activate Nrf2, TGF-β/Smad | Increase type I procollagen, decrease ROS and MMP-1 overexpression, and reduce apoptosis. | [47] |
| ADSC-exos | miR-1246 | HDFs | Not applicable | Inhibit p62, decreases ROS, MMP-1, GSK3β, increase type I procollagen, autophagy flux, and autophagy-related proteins (LC3II), and reduce wrinkles, epidermal hyperplasia, and collagen fiber reduction in photoaged mice. | [50] |
| ADSC-exos | lncRNA H19 | HDFs | Activate SIRT1 | Inhibit MMPs, DNA damage, and ROS, increase type I collagen, and reduce epidermal thickening and collagen degradation in photoaged mice. | [54] |
| BMSC-exos | miR-29b-3p | HDFs | Inhibit MAPK/AP-1 | Increase type I collagen, reduce ROS, lactate dehydrogenase, TNF-α, IL-6, IL-1β, MMP-1, and MMP-3, and increase type I collagen. | [59, 60] |
| iPSC-exos | Not applicable | HDFs | Not applicable | Decrease SA-β-Gal, MMP-1, and MMP-3, increase type I collagen, and increase cell proliferation and migration. | [64] |
| 3D HDF-XOs | Not applicable | HDFs | Activate TGF-β | Decrease MMP1, and TNF-α, increase type I procollagen, increase cell proliferation and migration, and reduce wrinkles in photoaged mice. | [66] |
| BJ-5ta exos | Not applicable | Foreskin fibroblasts | Inhibit MAPK/AP-1, activate Nrf2, TGF-β1/Smad | Reduce ROS, SA-β-Gal, and p16, increase type I collagen and elastin, inhibit the decrease of SOD, inhibit the increase of γH2AX, p53/21 and cleaved PARP, and reduce photoaging epidermal water loss, wrinkle formation in photoaged mice. | [67] |
| DSPC-exos | ANP32B | HDFs | Not applicable | Increase type I collagen. | [68] |
| Milk-exos | Not applicable | HDFs | Activate Wnt/β-catenin | Decrease MMPs, increase collagen, and increase cell proliferation and migration. | [71] |
| Milk-exos | Not applicable | Keratinocytes and HDFs | Not applicable | Increase type I/III collagen, filaggrin, aquaporin 3, CD44, hyaluronidase, promote fibroblast migration, increase skin moisture and elasticity, and reduce skin wrinkles. | [72] |
| ADNVs | Not applicable | HDFs | Inhibit NF-κB | Decrease TLR-4, IL-1, MMP1, MMP8, and MMP9, increase collagen, and decrease extracellular matrix degradation. | [77] |
| OLELNVs | miR168a-5p | HaCaT and HDF-α cells | Inhibit NF-κB | Reduce ROS, IL-6, SA-β-Gal, MMP-1, and MMP-3, increase type I collagen, elastin fibers, and SOD, promote cell growth and migration, and reduce wrinkles in photodamaged mouse skin. | [80] |
In summary, MSC-exos derived from hucMSCs, ADSCs, BMSCs, and iPSC-exos play a vital role in skin cell proliferation, repair, and collagen production, contributing to improved skin elasticity and reduced wrinkle formation. Additionally, MSC-exos exhibit immunomodulatory functions and secrete multiple bioactive factors with anti-inflammatory and antioxidant effects, making them valuable for skin rejuvenation [81]. While MSC-exos have been extensively studied in clinical trials for various conditions, including cancer, diabetes, skin damage, and ageing, the safety profile of long-term application remains a concern, necessitating stringent quality control measures [82]. On the other hand, plant-exos sourced from edible vegetables, fruits, and herbs contain bioactive molecules, providing antioxidant capacity and anti-inflammatory properties that are beneficial for skin health [83]. Plant-exos show promise in neutralising harmful free radicals, reducing chronic inflammation, and nourishing the skin with multiple nutrients and bioactive compounds, but the lack of extensive clinical trials limits their widespread application [84]. The advantages of plant-exos lie in their high productivity, cost-effectiveness, pharmacological activity, biocompatibility, and good skin absorption [85]. However, challenges such as complicated extraction processes and limited research data hinder their full potential in skin ageing treatments (Table 2).
Table 2.
The comparisons of MSC-exos and plant-exos
| Classification | MSC-exos | Plant-exos |
|---|---|---|
| Define | Exosomes derived from MSCs with self-renewal, multidirectional differentiation potential, and paracrine regulation. | Exosomes released by plant cells, possessing bioactive molecules and signaling substances produced by plant cells. |
| Source | hucMSCs, ADSCs, BMSCs, and iPSC-exos. | Edible vegetables, fruits, and herbs. |
| Characteristic marker | CD29, CD44, CD73, CD90, and CD105, MHC, not express CD45. | Phosphatidic acid, phosphatidylglycerol, phosphatidylcholine, phosphatidylinositol, phosphatidylethanolamine. |
| Role and function in skin | Map the basic functions of MSC, promote skin cell proliferation and repair, differentiate into fibroblasts and other skin cells, improve the production of collagen and elastin, improve skin elasticity and reduce wrinkles, paracrine secrete multiple bioactive factors, with anti-inflammatory and antioxidant effects. | Neutralize harmful free radicals, reduce chronic inflammation, and nourish the skin with antioxidant capacity, anti-inflammatory properties, and multiple nutrients and bioactives. |
| Clinical Trials | A large number of clinical trials are already underway in areas such as autoimmune diseases, graft-versus-host disease, cancer, diabetes, skin damage, and skin aging. | Little clinical trials are available. |
| Safety | Low immunogenicity, relatively low risk of teratogenicity and tumorigenicity, but still need to strictly control its quality and application dose. | Relatively few studies and data are available on clinical applications, and the potential risks in humans need to be further evaluated, while cellular and animal level safety is relatively high. |
| Advantages in skin aging | Diverse sources, strong multidirectional differentiation potential, immunomodulatory function, low immunogenicity, cell proliferation, and collagen production promotion. | High productivity, low cost, pharmacological activity, biocompatibility, good absorption, and distribution. |
| Disadvantages in skin aging | Molecular heterogeneity and differentiation potentials differ in different MSC sources, undetermined safety of long-term application, and need ethics and supervision. | Complicated extraction processes, limited exploration, and rare data in clinical applications. |
Clinical studies of exosomes in combating skin ageing
ADSC-EVs
These studies follow a logical and depth-oriented progression in the exploration of stem cell-derived exosomes for anti-ageing skin treatments. Svolacchia et al. initially demonstrated the safety and efficacy of ADSC-EVs in skin repair and anti-ageing, establishing a foundational understanding [86]. Nguyen et al. advanced this research by examining ADSC-EVs loaded with specific substances such as nicotinamide adenine dinucleotide (NAD+), nicotinamide riboside (NR), and resveratrol (Res), highlighting their role in reducing UVB-induced ROS at the cellular level [87]. Finally, Park et al. integrated ADSC-exos with microneedling technology in a clinical trial, resulting in significant improvements in skin structure and appearance [88]. Each study builds upon previous studies, moving from basic validation to enhanced cellular effects and culminating in comprehensive clinical efficacy.
Platelet-exos
Platelet-derived exosomes (platelet-exos) are nanoscale vesicles released by platelets that are endowed with multifaceted bioactive properties that modulate cellular behaviour and function. In the context of skin ageing, platelet-exos exhibit significant potential through their anti-inflammatory, regenerative, barrier-enhancing, and antioxidative mechanisms [89, 90]. Specifically, platelet-exos can mitigate chronic inflammation, promote fibroblast proliferation and collagen synthesis, enhance skin barrier function to reduce moisture loss, and neutralise free radicals to alleviate oxidative stress-induced cellular damage. These attributes position platelet-exos as promising tools for delaying skin ageing and improving overall skin health. Proffer et al. conducted a prospective clinical trial to compare the effectiveness and biosafety of topical platelet-exos for treating skin rejuvenation [91]. Fifty-six nonpregnant participants aged 40–85 years adhered to a standardised skincare routine over the six-week study and were finally checked with skin photographs, texture and morphology, and plastic surgeon evaluation. This method effectively improved the clinical parameters of facial photodamage and skin ageing. Therefore, this study emphasised the use of exosomes as high-value mediators to resist skin ageing.
PMSC-exos
Placental MSC (PMSC)-derived exosomes exhibit significant potential in regenerative medicine, particularly in combating skin ageing. These exosomes exert their biological effects through the secretion of growth factors, anti-inflammatory agents, and antioxidants, collectively reducing apoptosis and oxidative stress [92]. PMSC-derived exosomes (PMSC-exos) effectively mitigate oxidative stress-induced cellular senescence, thereby delaying the ageing process of skin cells [93]. The high content of epidermal growth factor (EGF) and other growth factors within PMSC-exos promotes skin cell proliferation and differentiation, accelerating tissue repair and regeneration [94]. Additionally, the anti-inflammatory properties of these exosomes enhance skin barrier function and skin rejuvenation. Chernoff et al. treated 40 patients with different combinations of exosome infusion and CaHA injection. Exosomes were used for topical treatment and combined with a series of steps to promote absorption, including sea salt exfoliation, nitric oxide-generating serum, and 3 MHz ultrasound. All patients experienced improvements in skin tone, quality, and texture. Notably, the combination of exosome treatment with CaHA injection led to more rapid and pronounced improvements in skin quality than either treatment alone. In conclusion, the use of exosomes as skin primers prior to CaHA injection can enhance the effects of biostimulation and promote skin ageing [95].
Milk-exos
Additionally, Lu et al. administered milk-exos twice daily to the facial areas of 31 female subjects over a 28-day period [72]. After exposure to ultraviolet light, milk exposure promoted fibroblast migration and restored the expression of type I and type III collagen, thereby maintaining skin hydration and reducing wrinkles in human facial skin. Follow-up examinations revealed no phototoxicity, photosensitization, skin irritation, or allergic reactions, thus validating the nontoxicity and safety of milk-exos on the skin (Table 3).
Table 3.
Clinical studies of exosomes in reversing skin aging
| Agent catalogues | Patients | Therapeutic regimens | Key results | Adverse events | Ref |
|---|---|---|---|---|---|
| ADSC-exos (suspension extracted from Skin-B® pretreated adipose tissue) | 72 female participants aged 34–68 years, with no skin inflammation pathology. | 5 mL ADSC-exos suspension, dermal injections, evaluated at D0 (pre-treatment), D30 (30 d post-treatment)) and D90 (90 d post-treatment). | Participant skin stability, softness, hydration, and satisfaction were lowest at D0 and highest at D30, and were higher at D90 than D0 but decreased from D30. | Not applicable. | [86] |
| Loaded ADSC-EVs (1 µg 1.21 × 109 particles ADSC-Evs, 2 µg NR, 1 µg NAD+, 1 µg Res in 0.1 mL of 0.9% sodium chloride per dose) | 3 participants aged 30–40 years, with no history of skin allergy or skin abnormalities. | Loaded ADSC-Evs, applied to the back of the hand once a day for 8 w, and examined every other week. | Skin texture was improved, skin pigmentation and redness were reduced, and pore volume was reduced. Skin hydration increased by 19%, elasticity by 104%, and pore volume decreased by 51%, with reduced melanin content. | Not applicable. | [87] |
| HACS (2 mL normal saline solution + ASCE + Derma Signal Skin Rejuvenation Lyophilized Vial) | 28 participants aged ≥ 40, with aging facial skin, with no history of keloid scarring, acute inflammation and so on. | HACS, was applied to the face, followed by microneedling to a depth of 1 mm, 3 treatments every 3 w for 6 w, and was followed up for 6 w after the last intervention. | Effectively improved facial skin wrinkles, elasticity, hydration, and pigmentation. | Minor symptoms including transient erythema, edema, and petechiae were observed, spontaneously resolving within 1 w. | [88] |
| HPE (key ingredient containing platelet-derived exosomes intensive repair serum) | 56 participants aged 40–85 years, with mild to moderate overall facial wrinkles and moderate overall fine lines. | HPE was applied to the face, and followed up for 6 w. | Significant improvement in skin erythema, hyperpigmentation, brightness, and color uniformity at 6 w. | 16.1% of participants reported dry skin. | [91] |
| PMSC-exos + CaHA | 35 females and 5 males aged 34–72 years. | Group 1: 20 participants, PMSC-exos with biostimulatory infusion. Group 2: 5 participants, neck injections of PMSC-exos + CaHA (1:1). Group 3: 5 participants, facial injections of PMSC-exos + CaHA (1:4). Group 4: 5 participants, facial CaHA (1:1) injections. | Improvements in skin wrinkles, pores, skin evenness, pigmentation, vascular distribution, and texture were seen in patients receiving PMSC-exos dermal injections only and CaHA injections only, with enhanced and faster improvement seen when PMSC-exos + CaHA was used. | Not applicable. | [95] |
| Milk-exos (Milk-exos were diluted with water to a concentration of 60 µg/mL) | Healthy Chinese female participants aged 26–45 years. | Milk-exos, applied morning and evening for 28 d. | Distribution of the skin condition assessed on day 14 and 28, the skin moisture content increased by 4.64% and 5.6%, the area of wrinkles decreased by 9.37% and 5.27%, and the number of wrinkles decreased by 9.59% and 4.99%. At day 28, F3/F4 and R2 values increased by 6.33% and 7.24%. | Not applicable. | [72] |
Challenges and future perspectives
This review comprehensively summarises the roles and mechanisms of exosomes derived from various cell types in combating skin ageing. Multiple studies have explored the therapeutic potential of these exosomes in promoting skin regeneration, reducing oxidative stress, and alleviating photoageing. As a cell-free alternative, exosomes exhibit advantages over traditional stem cell therapies, such as stability, biocompatibility, and reduced risks of immune rejection and tumour formation. We also emphasise the combination of exosomes with innovative delivery systems such as microneedles and hydrogels to increase their efficacy and application in skin regeneration.
Despite these promising results, several limitations and challenges need to be addressed to fully realise the clinical potential of exosome-based therapies for skin ageing. Incomplete isolation and purification of exosomes can interfere with their biological function studies because the presence of other exosomes or impurities in the sample may lead to erroneous signal transduction [96]. For example, when investigating exosome-mediated intercellular communication, the presence of other vesicles can mask the true role of exosomes, leading to inaccurate conclusions. Additionally, as potential biomarkers, the purity and characteristics of exosomes directly impact detection accuracy. Insufficient sample purity may result in false-positive or false-negative biomarker outcomes, thereby affecting disease diagnosis and prognosis assessment [97]. In clinical applications, the purity and characteristics of exosomes are also directly related to their safety and efficacy [98]. If samples contain other harmful components or impurities, adverse reactions may be triggered, or the therapeutic effectiveness of exosomes may be compromised, influencing patient treatment outcomes and safety.
Currently, the main techniques for EV isolation include ultracentrifugation, density gradient centrifugation, size-exclusion chromatography (SEC), ultrafiltration, polymer precipitation, and immunocapture [99]. For example, Bellotti et al. utilised SEC for initial separation, where exosomes pass through a membrane of a specific pore size on the basis of differences in particle size, while larger microvesicles and apoptotic bodies are retained. Ma et al. employed immunocapture technology, which uses specific surface markers on exosomes to bind with antibodies, capturing exosomes from the mixture and further reducing contamination from other vesicles. Exosome isolation and purification face numerous challenges. A significant limitation is the low yield and purity of the exosomes obtained through current isolation methods [100]. Existing methods possibly yield exosomes with low purity and yield, making it difficult to balance both aspects. Some isolation techniques, such as ultracentrifugation, SEC, and immunoaffinity capture, might produce heterogeneous exosome populations, which may affect the reproducibility and efficacy of treatments [101]. Ultracentrifugation, size-exclusion chromatography, and immunocapture typically cause heterogeneous exosome populations, potentially affecting treatment reproducibility and efficacy [102]. Ultracentrifugation provides high purity but has a low yield and is time-consuming [103]. The composition and characteristics of exosomes from various sources, such as cell culture supernatants and body fluids, differ significantly, leading to inconsistent isolation conditions and outcomes. Furthermore, during large-scale exosome preparation, differences in isolation conditions and personnel between batches may result in variations in exosome purity and properties. Future research should focus on developing more efficient and standardised isolation protocols to ensure the consistent production of high-purity exosome preparations on a large scale. Specifically, improving existing isolation techniques, such as optimising ultracentrifugation speeds and times, employing more precise filtration and separation media, or developing novel isolation methods such as microfluidics and nanotechnology, could significantly increase exosome yield and purity. Combining techniques such as ultracentrifugation with ultrafiltration, ultracentrifugation with SEC, ultracentrifugation with immunocapture, and polymer precipitation with SEC can compensate for the limitations and drawbacks of individual purification methods, thereby increasing the purity and specificity of exosomes [104, 105].
The introduction of exogenous exosomes for anti-ageing therapy may trigger immune reactions. In particular, plant-exos, with their unique proteins or carbohydrates, may be recognised as foreign by the human immune system, leading to allergic reactions or other immune-related side effects [84]. The biocompatibility of exosomes is influenced by their source and preparation process. Impurities such as plant cell fragments may be introduced during the extraction of plant exosomes, potentially causing skin inflammation reactions or other adverse responses [106]. Furthermore, exosomes carry multiple biomolecules, which may trigger intricate mechanisms in recipient cells, such as gene expression regulation and the modulation of cell signalling pathways, leading to unpredictable side effects in the skin [107]. Exosomes may be absorbed non-specifically by tissues in the body, resulting in immune reactions in non-targeted areas beyond the skin layers. The long-term metabolism, distribution, and accumulation of exosomes in the human body are not fully clear, and prolonged use of exosomes may lead to chronic toxicity (Table 3).
To address these issues, reducing the immunogenicity and targeting ability of exosomes is important. Firstly, using the patient own exosomes for treatment can reduce immune recognition and attackSecondly, modifying the surface of exosomes, such as by coating with polyethylene glycol (PEG) or nanocomposites, can block immune escape signals [84]. Targeting molecules, such as specific antibodies or peptides, can be modified on exosome surfaces to enable them to specifically recognise and bind to skin cells. Optimising the delivery system of exosomes, such as the use of superparamagnetic nanocarriers, can enhance their stability and targeting in the body [108]. Targeted gene editing to control the specific protein expression profile and abundance of exosomes, including receptors and immune molecules, can effectively enhance anti-ageing effects and minimise off-target side effects. Additionally, automated and high-throughput isolation equipment would further improve production efficiency and reduce costs, thereby promoting the widespread clinical application of exosomes.
Another critical area requiring further investigation is understanding the mechanisms underlying exosome-mediated skin regeneration. Although some studies have elucidated the roles of specific miRNAs, proteins, and signalling pathways in exosome function, the complex interactions among these factors and their cumulative effects on skin cells remain incompletely understood. Advanced molecular techniques, such as single-cell RNA sequencing and proteomics, can provide deeper insights into the molecular mechanisms of exosome-mediated anti-ageing effects. Moreover, exploring the role of exosomes in modulating the skin microenvironment, including interactions with immune cells and the ECM, may reveal novel therapeutic targets and strategies. Specifically, future research could employ multi-omics integrative analysis to systematically uncover the roles of exosomes in intercellular communication and elucidate their functions under various physiological and pathological conditions. Furthermore, various model-based studies should be strengthened to validate the mechanisms discovered in vitro and further investigate the role of exosomes in the overall biological system. Thus, we can more fully understand the mechanism by which exosomes promote skin regeneration and provide a theoretical basis for developing more effective treatment strategies.
Combining exosomes with novel delivery systems such as microneedles and hydrogels offers a promising approach to enhancing their therapeutic efficacy. These systems can increase the local concentration and stability of exosomes, facilitate their penetration through the skin barrier, and reduce the required dosage and potential side effects. For example, microneedle technology can create microchannels on the skin surface, directly delivering exosomes to deeper skin layers and significantly improving their bioavailability. Hydrogels can serve as carriers for exosomes, providing sustained release and prolonging their action time in the skin. Additionally, nanoparticles, liposomes, and other advanced delivery systems have shown potential in exosome delivery. However, the long-term safety and efficacy of these combination approaches need thorough evaluation in clinical settings. Specifically, large-scale animal experiments and human clinical trials are necessary to verify the safety, efficacy, and controllability of these delivery systems. Moreover, developing scalable and cost-effective manufacturing processes is crucial for the widespread adoption of these advanced delivery systems in clinical practice. By optimising production processes and reducing production costs, the commercialization of these technologies can be facilitated, benefiting more patients.
The clinical translation of exosome-based therapies for skin ageing also faces several challenges. Currently, most studies are limited to preclinical models and lack robust clinical trials to validate the efficacy and safety of these therapies in humans. The heterogeneity of human skin and individual differences in response to treatment further complicate the clinical application of exosome-based therapies. Rigorous, larger-sample, and longer-term follow-up clinical trials are needed to establish the therapeutic potential and safety of exosome-based treatments. Specifically, clinical trials should consider participants of different ages, sexes, skin types, and health conditions to ensure the broad applicability of the results. Additionally, trial designs should include evaluations of both short-term and long-term effects of exosome treatments and monitoring of potential side effects and adverse reactions. To increase the quality and credibility of clinical trials, standardised evaluation metrics and detection methods should be established to enable comparisons and integration across different studies. Furthermore, a regulatory framework for exosome-based products must be developed to ensure their safe and effective use in clinical practice. Regulatory agencies should formulate detailed guidelines and standards covering the production, quality control, storage, and transportation of exosomes to ensure product consistency and safety.
The necessity and ethical concerns surrounding the use of anti-ageing exosomes have raised numerous issues, as skin ageing is not a life-threatening condition [109]. Informed consent is challenging because of the intricate biological mechanisms and potential long-term impacts associated with exosome therapy. Researchers and clinicians should thoroughly explain to patients the origin, roles, mechanisms, and risks of exosomes, including the ethical controversies surrounding exosomes sourced from stem cells and the risk of iatrogenic tumours, to ensure that patients truly comprehend and consent to treatment [110]. Privacy and data security also present challenges, as exosome research may involve patient personal health and genetic information, necessitating strict protection against leakage and misuse [111].
The potential risks of misuse and commercialization of anti-ageing treatment involving the use of exosomes highlight the necessity of establishing regulatory strategies. Challenges exist in regulating and ensuring quality control in the production, purification, and application of exosomes because of the lack of standardised protocols and supervision frameworks. This results in significant variations in the quality and safety of exosomes across different research studies and clinical trials. The global supervision of exosomes is complex and fragmented, with inconsistent supervision requirements and approval processes in different countries and regions. The long-term safety and precise efficacy of exosomes in anti-ageing treatments remain incompletely understood, posing challenges for supervision agencies in approving exosome-related products. Furthermore, as a burgeoning field in biomedical research, exosome supervision involves interdisciplinary considerations, potentially leading to the lack of sufficient experience and expertise in developing relevant policies and standards.
First, it is essential to establish a stringent quality control system across various stages, including cell culture, exosome isolation and purification, storage, and transportation. This ensures the standardization and normalization of the production process to maintain consistent purity and activity of the exosomes. Furthermore, comprehensive monitoring of the entire process of clinical trials for anti-ageing interventions is crucial to ensure scientifically sound and ethically compliant trial designs, ensuring the safety and rights of participants and preventing the misuse or disclosure of patient information. Moreover, it is imperative to define a regulatory framework that categorises exosomes on the basis of their source, function, and application field, enabling the formulation of corresponding regulatory strategies. Collaboration between different countries and regions can facilitate the development of unified regulatory standards for exosomes.
Conclusion
Exosome-based therapies hold substantial promise in the realm of anti-ageing, harnessing the potential of nanosized vesicles derived from a variety of sources, including hucMSCs, ADSCs, BMSCs, iPSCs, fibroblasts, milk, and plants. These vesicles are replete with a plethora of bioactive molecules, including proteins, lipids, and RNAs. Through the targeted delivery of growth factors, cytokines, and antioxidants, exosomes can significantly augment cell proliferation, enhance collagen synthesis, and mitigate oxidative stress, thus offering a robust platform for cellular rejuvenation. The inherent stability, biocompatibility, and precision targeting capabilities of exosomes render them superior to conventional anti-ageing treatments, effectively minimising associated risks such as immune rejection and tumorigenesis. Despite their potential, the path towards clinical adoption of exosome-based therapies is lined with challenges, including the need for standardised isolation protocols, an enriched understanding of their molecular mechanisms and stringent clinical validation. As research progresses, exosome-based interventions are poised to redefine therapeutic strategies in regenerative medicine and dermatology, providing efficacious and safe solutions for the management of skin ageing. This innovative approach could establish a new paradigm for the treatment of age-related dermal concerns, offering a groundbreaking alternative to traditional therapeutic modalities.
Acknowledgements
Not applicable.
Abbreviations
- AP-1
Activator protein-1
- ADSCs
Adipose-derived stem cells
- ADSC-exos
ADSC-derived exosomes
- ADSC-EVs
ADSC-derived extracellular vesicles
- ADNVs
Apple-derived nanovesicles
- AQP3
Aquaporin 3
- AP-1
Activator protein 1
- BMSC-exos
BMSC-derived exosomes
- BMSCs
Bone marrow stem cells
- DSPCs
Dermal stem/progenitor cells
- DSPC-exos
DSPC-derived exosomes
- EGF
Epidermal growth factor
- exos
Exosomes
- BJ-5ta exos
Exosomes derived from human foreskin fibroblasts BJ 5ta
- 3D HDF-XOs
Exosomes from 3D-cultured HDF spheroids
- ECM
Extracellular matrix
- EVs
Extracellular vesicles
- FLG
Filaggrin
- MMPs
Matrix metalloproteinases
- GrDENs
Ginseng root-derived exosome-like nanoparticles
- HDF-exos
HDF-derived exosomes
- hucMSC-Exos
hucMSC-derived exosomes
- HDFs
Human dermal fibroblasts
- HSFs
Human skin fibroblasts
- hucMSCs
Human umbilical cord MSCs
- HCOPs
Hydrolyzed collagen oligopeptides
- iPSCs
Induced pluripotent stem cells
- IL-1β
Interleukin-1 beta
- iPSC-exos
iPSC-derived exosomes
- H19-exos
lncRNA H19-overexpressing exosomes
- SHSs
Marine sponge Haliclona sp. Spicules
- MMP
Matrix metalloproteinase
- MSCs
Mesenchymal stem cells
- MNs
Microneedlings
- milk-exos
Milk-derived exosomes
- OE-EXs
miR-1246-overexpressing exosomes
- NAD+
Nicotinamide adenine dinucleotide
- NR
Nicotinamide riboside
- OLELNVs
Olea europaea leaf-derived nanovesicles
- OLEX
Olea europaea leave
- PMSC
Placental MSC
- plant-exos
Plant-derived exosomes
- MAPKs
Mitogen-activated protein kinases
- platelet-exos
Platelet-derived exosomes
- PMSC-exos
PMSC-derived exosomes
- ROS
Reactive oxygen species
- Res
Resveratrol
- TA
Tannic acid
- TGF-β
Transforming growth factor-β
- TNF-α
Tumor necrosis factor-α
- PTPs
Protein tyrosine phosphatases
- RTKs
Receptor tyrosine kinases
- UV
Ultraviolet
Author contributions
All authors contributed equally to this work.
Funding
This work was supported by the Tongji Hospital Foundation (2021A09); and Horizontal project funding (2024068, 2024100).
Data availability
Not applicable.
Declarations
Declarations
The authors declare that they have not use AI-generated work in this manuscript.
Ethics & AI statement
The authors declare that no AI-generated content has been used in this manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chen Liang, Yi Yi and Jia Li are co-first authors and contributed equally to this work.
Contributor Information
Xue Chen, Email: chenxue_tongji@outlook.com.
Wei Cao, Email: cweitj@163.com.
Qi Zhang, Email: zhangqi06172@163.com.
References
- 1.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and MicroRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 2.Böhm M, Stegemann A, Paus R, Kleszczyåski K, Maity P, Wlaschek M, et al. Endocrine controls of skin aging. Endocr Rev. 2025;46:349–75. 10.1210/endrev/bnae034 [DOI] [PubMed] [Google Scholar]
- 3.Franco AC, Aveleira C, Cavadas C. Skin senescence: mechanisms and impact on whole-body aging. Trends Mol Med. 2022;28:97–109. 10.1016/j.molmed.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 4.Wahhab R, Sanders M, Kokikian N, Ni C, Vandiver AR. Clinical consequences of age-related skin barrier dysfunction. Part I. Structural, molecular and physiologic changes with cutaneous aging. J Am Acad Dermatol. 2024;S0190–9622:02752–X. 10.1016/j.jaad.2024.08.044 [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang GH, Lee YB, Kang D, Choi E, Nam Y, Lee KH, et al. Overcome the barriers of the skin: exosome therapy. Biomater Res. 2021;25:22. 10.1186/s40824-021-00224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y, et al. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol Res. 2021;166:105490. 10.1016/j.phrs.2021.105490 [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Wang C, Chen S, Wei C, Ji J, Liu Y, et al. Identification of blood-derived Exosomal tumor RNA signatures as noninvasive diagnostic biomarkers for multi-cancer: a multi-phase, multi-center study. Mol Cancer. 2025;24:60. 10.1186/s12943-025-02271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Feng H, Zeng H, Wu Y, Zhang Q, Yu J, et al. Exosomes: the emerging mechanisms and potential clinical applications in dermatology. Int J Biol Sci. 2024;20:1778–95. 10.7150/ijbs.92897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu Y, Ntege EH, Sunami H. Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan. Regen Ther. 2022;21:73–80. 10.1016/j.reth.2022.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajialiasgary Najafabadi A, Soheilifar MH, Masoudi-Khoram N. Exosomes in skin photoaging: biological functions and therapeutic opportunity. Cell Commun Signal. 2024;22:32. 10.1186/s12964-023-01451-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha T, Mehrotra S, Gupta P, Kumar A. Exosomal MiRNA combined with anti-inflammatory hyaluronic acid-based 3D bioprinted hepatic patch promotes metabolic reprogramming in NAFLD-mediated fibrosis. Biomaterials. 2025;318:123140. 10.1016/j.biomaterials.2025.123140 [DOI] [PubMed] [Google Scholar]
- 14.Yue M, Hu S, Sun H, Tuo B, Jia B, Chen C, et al. Extracellular vesicles remodel tumor environment for cancer immunotherapy. Mol Cancer. 2023;22:203. 10.1186/s12943-023-01898-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14:85. 10.1186/s13045-021-01096-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Zhang D, Yu Y, Wang L, Zhao M. Umbilical cord-derived mesenchymal stem cell secretome promotes skin regeneration and rejuvenation: from mechanism to therapeutics. Cell Prolif. 2024;57:e13586. 10.1111/cpr.13586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellistasari EY, Kariosentono H, Purwanto B, Wasita B, Riswiyant RCA, Pamungkasari EP, et al. Exosomes derived from secretome human umbilical vein endothelial cells (Exo-HUVEC) ameliorate the Photo-Aging of skin fibroblast. Clin Cosmet Investig Dermatol. 2022;15:1583–91. 10.2147/CCID.S371330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S-J, Meng M-Y, Han S, Gao H, Zhao Y-Y, Yang Y, et al. Umbilical cord mesenchymal stem cell-Derived exosomes ameliorate HaCaT cell Photo-Aging. Rejuvenation Res. 2021;24:283–93. 10.1089/rej.2020.2313 [DOI] [PubMed] [Google Scholar]
- 19.Vu DM, Nguyen V-T, Nguyen TH, Do PTX, Dao HH, Hai DX, et al. Effects of extracellular vesicles secreted by TGFβ-Stimulated umbilical cord mesenchymal stem cells on skin fibroblasts by promoting fibroblast migration and ECM protein production. Biomedicines. 2022;10:1810. 10.3390/biomedicines10081810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng Y, Li C, Liang Y, Jiang Y, Zhang H, Ouyang J, et al. Umbilical cord Mesenchymal-Stem-Cell-Derived exosomes exhibit Anti-Oxidant and antiviral effects as Cell-Free therapies. Viruses. 2023;15:2094. 10.3390/v15102094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong L, Barber T, Aldinger J, Bowman L, Leonard S, Zhao J, et al. ROS generation is involved in titanium dioxide nanoparticle-induced AP‐1 activation through p38 MAPK and ERK pathways in JB6 cells. Environ Toxicol. 2022;37:237–44. 10.1002/tox.23393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solá P, Mereu E, Bonjoch J, Casado-Peláez M, Prats N, Aguilera M, et al. Targeting lymphoid-derived IL-17 signaling to delay skin aging. Nat Aging. 2023;3:688–704. 10.1038/s43587-023-00431-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Au Yeung CL, Co N-N, Tsuruga T, Yeung T-L, Kwan S-Y, Leung CS, et al. Exosomal transfer of stroma-derived miR21 confers Paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. 10.1038/ncomms11150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuboi A, Fujimoto K, Kondo T. Spatiotemporal remodeling of extracellular matrix orients epithelial sheet folding. Sci Adv. 2023;9:eadh2154. 10.1126/sciadv.adh2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Ma L, Lu D, Zhao G, Ren H, Lin Q, et al. Nuclear envelope budding Inhibition slows down progerin-induced aging process. Proc Natl Acad Sci. 2024;121:e2321378121. 10.1073/pnas.2321378121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao Z, Xue K, Chen J, Zhang Y, Zhang G, Zheng Z, et al. Biliverdin improved angiogenesis and suppressed apoptosis via PI3K/Akt-mediated Nrf2 antioxidant system to promote ischemic flap survival. Free Radic Biol Med. 2024;225:35–52. 10.1016/j.freeradbiomed.2024.09.042 [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Rao S-S, Wang Z-X, Cao J, Tan Y-J, Luo J, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169–84. 10.7150/thno.21234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rial SA, Shishani R, Cummings BP, Lim GE. Is 14-3-3 the combination to unlock new pathways to improve metabolic homeostasis and β-Cell function?? Diabetes. 2023;72:1045–54. 10.2337/db23-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan X, Huang T, Wang S, Yang Z, Song W, Zeng Y, et al. The adaptor protein 14-3-3zeta modulates intestinal immunity and aging in drosophila. J Biol Chem. 2023;299:105414. 10.1016/j.jbc.2023.105414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen S-Y, Ng S-C, Chiu Y-T, Tai P-Y, Chen T-J, Chen C-J, et al. Enhanced SIRT1 activity by Galangin mitigates UVB-Induced senescence in dermal fibroblasts via p53 acetylation regulation and activation. J Agric Food Chem. 2024;72:23286–94. 10.1021/acs.jafc.4c05945 [DOI] [PubMed] [Google Scholar]
- 31.Wu P, Zhang B, Han X, Sun Y, Sun Z, Li L, et al. HucMSC exosome-delivered 14-3-3ζ alleviates ultraviolet radiation-induced photodamage via SIRT1 pathway modulation. Aging. 2021;13:11542–63. 10.18632/aging.202851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguirre-Cruz G, León-López A, Cruz-Gómez V, Jiménez-Alvarado R, Aguirre-Álvarez G. Collagen hydrolysates for skin protection: oral administration and topical formulation. Antioxidants. 2020;9:181. 10.3390/antiox9020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei B, Wei M, Huang H, Fan T, Zhang Z, Song X. Mesenchymal stem Cell-Derived exosomes: A promising therapeutic strategy for Age‐Related diseases. Cell Prolif. 2025;58:1–21. 10.1111/cpr.13795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pu S-Y, Huang Y-L, Pu C-M, Kang Y-N, Hoang KD, Chen K-H, et al. Effects of oral collagen for skin Anti-Aging: A systematic review and Meta-Analysis. Nutrients. 2023;15:2080. 10.3390/nu15092080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu H, Guo X, Zhang Y, Khan A, Pang Y, Song H, et al. The combined Anti-Aging effect of hydrolyzed collagen oligopeptides and exosomes derived from human umbilical cord mesenchymal stem cells on human skin fibroblasts. Molecules. 2024;29:1468. 10.3390/molecules29071468IF. : 4.2 Q2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical Cord-Derived mesenchymal stem Cell-Derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomed. 2020;15:5911–26. 10.2147/IJN.S249129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia T, Zhu Y, Li K, Hao K, Chai Y, Jiang H, et al. Microneedles loaded with cerium-manganese oxide nanoparticles for targeting macrophages in the treatment of rheumatoid arthritis. J Nanobiotechnol. 2024;22:103. 10.1186/s12951-024-02374-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan M, Liu K, Jiang T, Li S, Chen J, Wu Z, et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J Nanobiotechnol. 2022;20:147. 10.1186/s12951-022-01354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunathilake V, Bertolino M, Bavestrello G, Udagama P. Immunomodulatory Activity of the Marine Sponge, Haliclona (Soestella) sp. (Haplosclerida: Chalinidae), from Sri Lanka in Wistar Albino Rats: Immunosuppression and Th1-Skewed Cytokine Response. Baczek T, editor. J Immunol Res. 2020;2020:1–11. 10.1155/2020/7281295 [DOI] [PMC free article] [PubMed]
- 40.Zhang K, Yu L, Li F-R, Li X, Wang Z, Zou X, et al. Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of Photoaging. Int J Nanomed. 2020;15:2859–72. 10.2147/IJN.S249751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11:312. 10.1186/s13287-020-01831-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y, et al. Exosomes from Adipose-Derived stem cells: the emerging roles and applications in tissue regeneration of plastic and cosmetic surgery. Front Cell Dev Biol. 2020;8:574223. 10.3389/fcell.2020.574223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song Y, You Y, Xu X, Lu J, Huang X, Zhang J, et al. Adipose-Derived mesenchymal stem Cell‐Derived exosomes biopotentiated extracellular matrix hydrogels accelerate diabetic wound healing and skin regeneration. Adv Sci. 2023;10:e2304023. 10.1002/advs.202304023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao R, Monlezun DJ, Kimbrough T, Burkett BJ, Samai A, Martin-Schild S. Antiepileptic Drug Management in Acute Ischemic Stroke: Are Vascular Neurologists Utilizing Electroencephalograms? An Observational Cohort Study. Woldeamanuel YW, editor. Biomed Res Int. 2020;2020:6406395. 10.1155/2020/6250531 [DOI] [PMC free article] [PubMed]
- 45.Guo J-A, Yu P-J, Yang D-Q, Chen W. The Antisenescence Effect of Exosomes from Human Adipose-Derived Stem Cells on Skin Fibroblasts. Maioli M, editor. Biomed Res Int. 2022;2022:1034316. 10.1155/2022/1034316 [DOI] [PMC free article] [PubMed]
- 46.Kim HD, Chang YB, Suh HJ, Han SH, Jang EY, Sun EM, et al. Enhancement of skin longevity through TGF-β/Smad signaling pathway modulation by Rohu fish (Labeo rohita) collagen peptides in chronologically aged hairless mice. Food Biosci. 2025;63:105841. 10.1016/j.fbio.2025.105841 [Google Scholar]
- 47.Gao W, Wang X, Si Y, Pang J, Liu H, Li S, et al. Exosome derived from ADSCs attenuates ultraviolet B-mediated Photoaging in human dermal fibroblasts. Photochem Photobiol. 2021;97:795–804. 10.1111/php.13370 [DOI] [PubMed] [Google Scholar]
- 48.Jeong D, Qomaladewi NP, Lee J, Park SH, Cho JY. The role of autophagy in skin fibroblasts, keratinocytes, melanocytes, and epidermal stem cells. J Invest Dermatol. 2020;140:1691–7. 10.1016/j.jid.2019.11.023 [DOI] [PubMed] [Google Scholar]
- 49.Ho CY, Dreesen O. Faces of cellular senescence in skin aging. Mech Ageing Dev. 2021;198:111525. 10.1016/j.mad.2021.111525 [DOI] [PubMed] [Google Scholar]
- 50.Gao W, Yuan L, Zhang Y, Huang F, Ai C, Lv T, et al. miR-1246-overexpressing exosomes improve UVB-induced Photoaging by activating autophagy via suppressing GSK3β. Photochem Photobiol Sci. 2024;23:957–72. 10.1007/s43630-024-00567-w [DOI] [PubMed] [Google Scholar]
- 51.Behera J, Kumar A, Voor MJ, Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 2021;11:7715–34. 10.7150/thno.58410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia Y, Pei T, Zhao J, Wang Z, Shen Y, Yang Y, et al. Long noncoding RNA H19: functions and mechanisms in regulating programmed cell death in cancer. Cell Death Discov. 2024;10:76. 10.1038/s41420-024-01832-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Jiang Z, Li X. New insights into crosstalk between Nrf2 pathway and ferroptosis in lung disease. Cell Death Dis. 2024;15:841. 10.1038/s41419-024-07224-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao W, Zhang Y, Yuan L, Huang F, Wang Y. Long Non-coding RNA H19-Overexpressing exosomes ameliorate UVB-Induced Photoaging by upregulating SIRT1 via sponging miR-138. Photochem Photobiol. 2023;99:1456–67. 10.1111/php.13801 [DOI] [PubMed] [Google Scholar]
- 55.Yang Q, Su S, Liu S, Yang S, Xu J, Zhong Y, et al. Exosomes-loaded electroconductive nerve dressing for nerve regeneration and pain relief against diabetic peripheral nerve injury. Bioact Mater. 2023;26:194–215. 10.1016/j.bioactmat.2023.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Xu R, Li B, Xin Z, Ling Z, Zhu W, et al. LncRNA NEAT1 controls the lineage fates of BMSCs during skeletal aging by impairing mitochondrial function and pluripotency maintenance. Cell Death Differ. 2022;29:351–65. https://www.nature.com/articles/s41418-021-00858-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou C, Zhang B, Yang Y, Jiang Q, Li T, Gong J, et al. Stem cell-derived exosomes: emerging therapeutic opportunities for wound healing. Stem Cell Res Ther. 2023;14:107. 10.1186/s13287-023-03345-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu G, Cheng P, Liu T, Wang Z, BMSC-Derived Exosomal. miR-29a promotes angiogenesis and osteogenesis. Front Cell Dev Biol. 2020;8:608521. 10.3389/fcell.2020.608521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan T, Huang L, Yan Y, Zhong Y, Xie H, Wang X. MAPK/AP-1 signaling pathway is involved in the protection mechanism of bone marrow mesenchymal stem Cells-Derived exosomes against Ultraviolet-Induced Photoaging in human dermal fibroblasts. Skin Pharmacol Physiol. 2023;36:98–106. 10.1159/000529551 [DOI] [PubMed] [Google Scholar]
- 60.Yan T, Huang L, Yan Y, Zhong Y, Xie H, Wang X. Bone marrow mesenchymal stem cell-derived exosome miR-29b-3p alleviates UV irradiation-induced Photoaging in skin fibroblast. Photodermatol Photoimmunol Photomed. 2023;39:235–45. 10.1111/phpp.12827 [DOI] [PubMed] [Google Scholar]
- 61.Poetsch MS, Strano A, Guan K. Human induced pluripotent stem cells: from cell origin, genomic stability, and epigenetic memory to translational medicine. Stem Cells. 2022;40:546–55. 10.1093/stmcls/sxac020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aboul-Soud MAM, Alzahrani AJ, Mahmoud A. Induced pluripotent stem cells (iPSCs)—Roles in regenerative therapies, disease modelling and drug screening. Cells. 2021;10:2319. 10.3390/cells10092319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cerneckis J, Cai H, Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduct Target Ther. 2024;9:112. 10.1038/s41392-024-01809-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh M, Lee J, Kim YJ, Rhee WJ, Park JH. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int J Mol Sci. 2018;19:1715. 10.3390/ijms19061715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shin J-W, Kwon S-H, Choi J-Y, Na J-I, Huh C-H, Choi H-R, et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20:2126. 10.3390/ijms20092126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu S, Li Z, Cores J, Huang K, Su T, Dinh P-U, et al. Needle-Free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin Photoaging. ACS Nano. 2019;13:11273–82. 10.1021/acsnano.9b04384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park AY, Lee J, Jang Y, Kim Y-J, Lee J, Kim S-Y, et al. Exosomes derived from human dermal fibroblasts protect against UVB–induced skin Photoaging. Int J Mol Med. 2023;52:120. 10.3892/ijmm.2023.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanada A, Yamada T, Hasegawa S, Ishii Y, Hasebe Y, Iwata Y, et al. Enhanced type I collagen synthesis in fibroblasts by dermal stem/progenitor Cell-Derived exosomes. Biol Pharm Bull. 2022;45:872–80. 10.1248/bpb.b21-01084 [DOI] [PubMed] [Google Scholar]
- 69.Kocic H, Langerholc T, Kostic M, Stojanovic S, Najman S, Krstic M, et al. The regenerative potential of Donkey and human milk on the Redox-Sensitive and proliferative signaling pathways of skin fibroblasts. Gon Alves RV, editor. Oxid Med Cell Longev. 2020;2020:5618127. 10.1155/2020/5618127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan W, Fu S, Wang Y, Hu B, Ding G, Zhang L, et al. Metabolomic and transcriptomic analyses revealed potential mechanisms of Anchusa Italica retz. In alleviating cerebral ischemia–reperfusion Injury via Wnt/β-catenin pathway modulation. Nat Prod Bioprospect. 2025;15:11. 10.1007/s13659-024-00495-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han G, Kim H, Kim DE, Ahn Y, Kim J, Jang YJ, et al. The potential of bovine Colostrum-Derived exosomes to repair aged and damaged skin cells. Pharmaceutics. 2022;14:307. 10.3390/pharmaceutics14020307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu L, Bai W, Wang M, Han C, Du H, Wang N, et al. Novel roles of bovine milk-derived exosomes in skin antiaging. J Cosmet Dermatol. 2024;23:1374–85. 10.1111/jocd.16112 [DOI] [PubMed] [Google Scholar]
- 73.Ou X, Wang H, Tie H, Liao J, Luo Y, Huang W, et al. Novel plant-derived exosome-like nanovesicles from catharanthus roseus: preparation, characterization, and immunostimulatory effect via TNF-α/NF-κB/PU.1 axis. J Nanobiotechnol. 2023;21:160. 10.1186/s12951-023-01919-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yi Q, Xu Z, Thakur A, Zhang K, Liang Q, Liu Y, et al. Current Understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. 2023;190:106733. 10.1016/j.phrs.2023.106733 [DOI] [PubMed] [Google Scholar]
- 75.Tan M, Liu Y, Xu Y, Yan G, Zhou N, Chen H, et al. Plant-Derived exosomes as novel nanotherapeutics contrive Glycolysis Reprogramming-Mediated angiogenesis for diabetic ulcer healing. Biomater Res. 2024;28:35. 10.34133/bmr.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trentini M, Zanotti F, Tiengo E, Camponogara F, Degasperi M, Licastro D, et al. An Apple a day keeps the Doctor away: potential role of MiRNA 146 on macrophages treated with exosomes derived from apples. Biomedicines. 2022;10:415. 10.3390/biomedicines10020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trentini M, Zanolla I, Zanotti F, Tiengo E, Licastro D, Dal Monego S, et al. Apple derived exosomes improve collagen type I production and decrease MMPs during aging of the skin through downregulation of the NF-κB pathway as mode of action. Cells. 2022;11:3950. 10.3390/cells11243950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi W, Cho JH, Park SH, Kim DS, Lee HP, Kim D, et al. Ginseng root-derived exosome-like nanoparticles protect skin from UV irradiation and oxidative stress by suppressing activator protein-1 signaling and limiting the generation of reactive oxygen species. J Ginseng Res. 2024;48:211–9. 10.1016/j.jgr.2024.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Oliveira NM, Lopes L, Chéu MH, Soares E, Meireles D, Machado J. Updated mineral composition and potential therapeutic properties of different varieties of Olive leaves from Olea Europaea. Plants. 2023;12:916. 10.3390/plants12040916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z, Yuan J, Xu Y, Shi N, Lin L, Wang R, et al. Olea Europaea leaf exosome-like nanovesicles encapsulated in a hyaluronic acid / tannic acid hydrogel dressing with dual defense-repair effects for treating skin Photoaging. Mater Today Bio. 2024;26:101103. 10.1016/j.mtbio.2024.101103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Padinharayil H, Varghese J, Wilson C, George A. Mesenchymal stem cell-derived exosomes: characteristics and applications in disease pathology and management. Life Sci. 2024;342:122542. 10.1016/j.lfs.2024.122542 [DOI] [PubMed] [Google Scholar]
- 82.Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14:66. 10.1186/s13287-023-03287-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sha A, Luo Y, Xiao W, He J, Chen X, Xiong Z, et al. Plant-Derived Exosome-like nanoparticles: A comprehensive overview of their composition, biogenesis, isolation, and biological applications. Int J Mol Sci. 2024;25:12092. 10.3390/ijms252212092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Langellotto MD, Rassu G, Serri C, Demartis S, Giunchedi P, Gavini E. Plant-derived extracellular vesicles: a synergetic combination of a drug delivery system and a source of natural bioactive compounds. Drug Deliv Transl Res. 2024. 10.1007/s13346-024-01698-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dad HA, Gu T-W, Zhu A-Q, Huang L-Q, Peng L-H. Plant Exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29:13–31. 10.1016/j.ymthe.2020.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Svolacchia F, Svolacchia L, Falabella P, Scieuzo C, Salvia R, Giglio F, et al. Exosomes and signaling nanovesicles from the nanofiltration of preconditioned adipose tissue with Skin-B® in tissue regeneration and antiaging: A clinical study and case report. Med (B Aires). 2024;60:670. 10.3390/medicina60040670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen DDN, Vu DM, Vo N, Tran NHB, Ho DTK, Nguyen T, et al. Skin rejuvenation and Photoaging protection using adipose-derived stem cell extracellular vesicles loaded with exogenous cargos. Ski Res Technol. 2024;30:e13599. 10.1111/srt.13599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park G, Kwon HH, Seok J, Yang SH, Lee J, Park BC, et al. Efficacy of combined treatment with human adipose tissue stem cell-derived exosome‐containing solution and microneedling for facial skin aging: A 12‐week prospective, randomized, split‐face study. J Cosmet Dermatol. 2023;22:3418–26. 10.1111/jocd.15872 [DOI] [PubMed] [Google Scholar]
- 89.Chen D, Tang Q, Song W, He Y. Platelet-derived exosomes alleviate tendon stem/progenitor cell senescence and ferroptosis by regulating AMPK/Nrf2/GPX4 signaling and improve tendon-bone junction regeneration in rats. J Orthop Surg Res. 2024;19:382. 10.1186/s13018-024-04869-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei K, Yu L, Li J, Gao J, Chen L, Liu M, et al. Platelet-derived exosomes regulate endothelial cell inflammation and M1 macrophage polarization in coronary artery thrombosis via modulating miR-34a-5p expression. Sci Rep. 2024;14:17429. 10.1038/s41598-024-67654-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Proffer SL, Paradise CR, DeGrazia E, Halaas Y, Durairaj KK, Somenek M, et al. Efficacy and tolerability of topical platelet exosomes for skin rejuvenation: Six-Week results. Aesthetic Surg J. 2022;42:1185–93. 10.1093/asj/sjac149 [DOI] [PubMed] [Google Scholar]
- 92.Chen W, Zhu J, Lin F, Xu Y, Feng B, Feng X, et al. Human placenta mesenchymal stem cell-derived exosomes delay H2O2-induced aging in mouse cholangioids. Stem Cell Res Ther. 2021;12:201. 10.1186/s13287-021-02271-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yao Q, Chen W, Yu Y, Gao F, Zhou J, Wu J, et al. Human placental mesenchymal stem cells relieve primary sclerosing cholangitis via upregulation of TGR5 in Mdr2 –/– mice and human intrahepatic cholangiocyte organoid models. Res [Internet]. 2023;6:207. 10.34133/research.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding C, Zou Q, Wu Y, Lu J, Qian C, Li H, et al. EGF released from human placental mesenchymal stem cells improves premature ovarian insufficiency via NRF2/HO-1 activation. Aging. 2020;12:2992–3009. 10.18632/aging.102794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chernoff G. Combining topical dermal infused exosomes with injected calcium hydroxylapatite for enhanced tissue biostimulation. J Cosmet Dermatol. 2023;22:15–27. 10.1111/jocd.15695 [DOI] [PubMed] [Google Scholar]
- 96.Gorgzadeh A, Nazari A, Ali Ehsan Ismaeel A, Safarzadeh D, Hassan JAK, Mohammadzadehsaliani S, et al. A state-of-the-art review of the recent advances in exosome isolation and detection methods in viral infection. Virol J. 2024;21:34. 10.1186/s12985-024-02301-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garg S, Garg G, Patel P, Kumar M, Thakur S, Sharma N, et al. A complete sojourn on exosomes: potential diagnostic and therapeutic agents. Pathol - Res Pract. 2024;264:155674. 10.1016/j.prp.2024.155674 [DOI] [PubMed] [Google Scholar]
- 98.Wen Z, Zhang W, Wu W. The latest applications of exosome-mediated drug delivery in anticancer therapies. Colloids Surf B Biointerfaces. 2025;249:114500. 10.1016/j.colsurfb.2025.114500 [DOI] [PubMed] [Google Scholar]
- 99.Logozzi M, Orefice NS, Di Raimo R, Mizzoni D, Fais S. The importance of detecting, quantifying, and characterizing exosomes as a new diagnostic/prognostic approach for tumor patients. Cancers (Basel). 2023;15:2878. 10.3390/cancers15112878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai JJ, Chau ZL, Chen S, Hill JJ, Korpany KV, Liang N, et al. Exosome processing and characterization approaches for research and technology development. Adv Sci. 2022;9:e2103222. 10.1002/advs.202103222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–707. 10.7150/thno.41580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dilsiz N. A comprehensive review on recent advances in exosome isolation and characterization: toward clinical applications. Transl Oncol. 2024;50:102121. 10.1016/j.tranon.2024.102121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teixeira-Marques A, Monteiro-Reis S, Montezuma D, Lourenço C, Oliveira MC, Constâncio V, et al. Improved recovery of urinary small extracellular vesicles by differential ultracentrifugation. Sci Rep. 2024;14:12267. 10.1038/s41598-024-62783-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P, Exosome. A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917–34. 10.2147/IJN.S264498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2022;9:811971. 10.3389/fbioe.2021.811971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bai C, Liu J, Zhang X, Li Y, Qin Q, Song H, et al. Research status and challenges of plant-derived exosome-like nanoparticles. Biomed Pharmacother. 2024;174:116543. 10.1016/j.biopha.2024.116543 [DOI] [PubMed] [Google Scholar]
- 107.Babaei S, Fadaee M, Abbasi-kenarsari H, Shanehbandi D, Kazemi T. Exosome-based immunotherapy as an innovative therapeutic approach in melanoma. Cell Commun Signal. 2024;22:527. 10.1186/s12964-024-01906-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Essola JM, Zhang M, Yang H, Li F, Xia B, Mavoungou JF, et al. Exosome regulation of immune response mechanism: pros and cons in immunotherapy. Bioact Mater. 2024;32:124–46. 10.1016/j.bioactmat.2023.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saleem M, Shahzad KA, Marryum M, Singh S, Zhou Q, Du S, et al. Exosome-based therapies for inflammatory disorders: a review of recent advances. Stem Cell Res Ther. 2024;15:477. 10.1186/s13287-024-04107-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li D, Li D, Wang Z, Li J, Shahzad KA, Wang Y, et al. Signaling pathways activated and regulated by stem cell-derived exosome therapy. Cell Biosci. 2024;14:105. 10.1186/s13578-024-01277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blindenbach J, Kang J, Hong S, Karam C, Lehner T, Gürsoy G. SQUiD: ultra-secure storage and analysis of genetic data for the advancement of precision medicine. Genome Biol. 2024;25:314. 10.1186/s13059-024-03447-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.