Abstract
Background
Cervical disc herniation (CDH) is a common musculoskeletal disorder characterized by chronic neck pain, impaired proprioception, kinesiophobia, and functional limitations, often requiring multimodal conservative care. Myofascial techniques, including Instrument-Assisted Soft Tissue Mobilization (IASTM) and percussion massage therapy (PMT), have emerged as supportive physiotherapy interventions. This randomized controlled trial compared the effects of IASTM and PMT on pain, disability, kinesiophobia, and proprioceptive function in individuals with CDH.
Methods
In this double-blinded RCT, 57 participants with CDH were randomly allocated to Conventional Therapy (CT), CT + PMT, or CT + IASTM (n = 19 each). Interventions were delivered three times per week for 3 weeks. PMT was applied with a percussion massage device (33–40 Hz) for 3 min to each target muscle group (trapezius, levator scapulae, cervical paravertebral) using longitudinal strokes. IASTM used stainless-steel tools on trapezius, splenius, and suboccipital muscles, with sweep and fan techniques at 30°–60°, for 9 min per session. Primary outcomes were pain (VAS) and disability (NDI); secondary outcomes included kinesiophobia (TSK) and joint position sense (JPS). Between-group differences were analyzed using ANCOVA with baseline values as covariates.
Results
All groups showed significant within-group improvements across all outcomes (p < 0.001). Compared to CT, both PMT and IASTM produced greater improvements in pain, kinesiophobia, and JPS (p < 0.001). VAS-rest reductions were − 4.00 ± 0.89 (d = 4.49) for IASTM, − 3.38 ± 1.95 (d = 1.74) for PMT, and − 2.13 ± 1.49 (d = 1.43) for CT. VAS-activity decreased by − 4.89 ± 1.44 (d = 3.41) for IASTM and − 3.89 ± 1.84 (d = 2.11) for PMT. NDI improved by − 11.47 ± 4.23 (d = 2.71) in IASTM, − 12.11 ± 6.86 (d = 1.76) in PMT, and − 6.63 ± 5.47 (d = 1.21) in CT, all exceeding the MCID threshold of 7.5 points. JPS-flexion improved by − 3.80 ± 1.61 (d = 2.36) in IASTM, − 3.67 ± 1.34 (d = 2.73) in PMT, and − 1.09 ± 0.84 (d = 1.29) in CT. Similar patterns occurred for extension, right rotation, and left rotation. Overall, IASTM and PMT yielded comparable improvements, suggesting similar clinical efficacy.
Conclusions
IASTM and PMT provide added benefits over conventional therapy alone in managing CDH, especially in reducing pain and kinesiophobia and enhancing proprioception. Both can be effectively integrated into conservative rehabilitation programs targeting sensorimotor deficits in CDH.
Trial registration
Prospectively registered in the ClinicalTrials.gov registry (NCT06903000) on 24/03/2025.
Keywords: Cervical disc herniation, Instrument-assisted soft tissue mobilization, Percussion massage therapy, Joint position sense, Kinesiophobia
Background
Cervical disc herniation (CDH) involves displacement of the nucleus pulposus through the annulus fibrosus into the spinal canal, often leading to radiculopathy, cervicogenic headache, or myelopathy due to neural compression or irritation. While pain is the primary symptom, disc protrusion may also cause postural dysfunction and kinesiophobia, depending on severity and location [1]. The etiology of neck pain is multifactorial, including proprioceptive deficits, cervical extensor muscle weakness, and biomechanical alterations [2, 3]. Notably, impaired cervical proprioception has been identified as a key contributor to sensorimotor dysfunction in individuals with neck pain [4, 5].
Cervical proprioception is critical for eye-head coordination and motor control. In musculoskeletal conditions, factors like pain, swelling, trauma, and fatigue can impair proprioceptive input [6, 7]. Sensorimotor dysfunctions in individuals with neck pain may cause tissue damage and perpetuate pain through peripheral and central mechanisms. Thus, optimizing proprioception and alleviating pain are central to effective treatment strategies [4, 8]. Current clinical guidelines support the conservative management for chronic neck pain, incorporating strengthening exercises, motor control and proprioceptive training, stretching, and manual therapy such as mobilization and manipulation [9, 10]. In a previous study involving patients with CDH, conventional physiotherapy included hot pack application, transcutaneous electrical nerve stimulation (TENS), stretching and strengthening of cervical and scapular muscles, and proprioceptive training [11]. While exercise is known to improve pain and proprioception, evidence also supports the use of manual therapies, particularly myofascial techniques, to further enhance proprioceptive function, reduce kinesiophobia, and alleviate pain [11, 12].
Among recent soft tissue interventions, Instrument-Assisted Soft Tissue Mobilization (IASTM) has emerged as a widely used myofascial technique in musculoskeletal rehabilitation [13, 14]. Based on Cyriax’s deep friction massage principles, IASTM involves specially designed tools that improve flexibility and range of motion (ROM) by increasing tissue temperature, breaking adhesions, and promoting collagen realignment [15, 16]. Its application in individuals with mechanical neck pain has shown benefits in ROM, muscle strength, pain reduction, and functional improvement [17]. Unlike traditional manual therapy, which depends solely on the therapist’s hands, IASTM employs rigid, ergonomically designed tools to precisely target fascial restrictions, apply uniform pressure, and induce controlled microtrauma. These mechanical advantages may enhance fibroblast activity, stimulate collagen realignment, and trigger localized inflammation more consistently and effectively than conventional techniques [14, 18, 19]. Vibration therapy (VT) is another modality gaining prominence in musculoskeletal care. It may be administered as whole-body or localized vibration, with percussion massage therapy (PMT) being the most common form of localized VT [20–22]. PMT directly stimulates targeted soft tissues and is associated with physiological benefits such as pain relief, increased blood flow, improved joint mobility, enhanced lymphatic drainage, modulation of the Golgi tendon reflex, and wound healing. However, evidence on its effectiveness in the cervical region remains limited [23, 24]. Devices like Hypervolt (Hyperice, CA, USA) deliver high-frequency mechanical stimuli (up to 53 Hz) using interchangeable heads, providing consistent amplitude and depth. These battery-powered tools enhance mechanoreceptor activation and reflex relaxation, offering more reproducible stimulation than manual techniques [25, 26]. Despite their growing use in sports and rehabilitation, scientific evidence supporting their effectiveness remains limited. The lack of standardized protocols and limited clinical research highlights the need for further studies, especially in cervical applications [22, 25–27].
Although IASTM and PMT are widely applied myofascial techniques, their clinical effects have not been directly compared in individuals with CDH. While both methods share similar mechanisms such as enhancing tissue mobility, stimulating mechanoreceptors, and modulating muscle tone, they differ in terms of application method, intensity, and sensory stimulation. IASTM involves controlled pressure applied with specially designed instruments, whereas PMT delivers rapid mechanical vibrations at various frequencies through interchangeable heads. The growing clinical interest in the effects of these techniques on pain, disability, kinesiophobia, and proprioception, along with the similarities in their therapeutic targets, suggests a need for further research in this field.
Beyond pain and proprioception, psychosocial and functional factors such as disability and kinesiophobia significantly influence prognosis and rehabilitation outcomes in individuals with CDH. Disability reflects limitations in daily function, while kinesiophobia involves fear-avoidance behaviors linked to pain or injury. Both are associated with poor adherence, reduced physical performance, and delayed recovery [28, 29]. Thus, incorporating these factors alongside pain and proprioceptive assessments is crucial for a comprehensive understanding of CDH and for optimizing rehabilitation strategies [30, 31].
This study aimed to investigate the comparative effects of IASTM and PMT on pain, disability, kinesiophobia, and cervical proprioception in individuals with CDH. We hypothesized that both techniques would be more effective than conventional physiotherapy alone by enhancing sensory input and neuromuscular control. Given their shared therapeutic mechanisms, no significant difference was expected between IASTM and PMT.
Methods
Study design and participants
This study employed a parallel-group, double-blinded (participant and outcomes assessor) randomized controlled trial design with an equal (1:1:1) allocation ratio. The research was conducted at the Fizyoformer Center for Healthy Living. Ethical approval was granted by the Non-Interventional Research Ethics Committee of Istanbul Medipol University (Approval No: E-10840098-202.3.02-1924). All participants gave written and verbal informed consent prior to inclusion, and the study adhered strictly to the principles outlined in the Declaration of Helsinki. Additionally, the trial was prospectively registered at ClinicalTrials.gov (Identifier: NCT06903000). The study conformed to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for randomized trials.
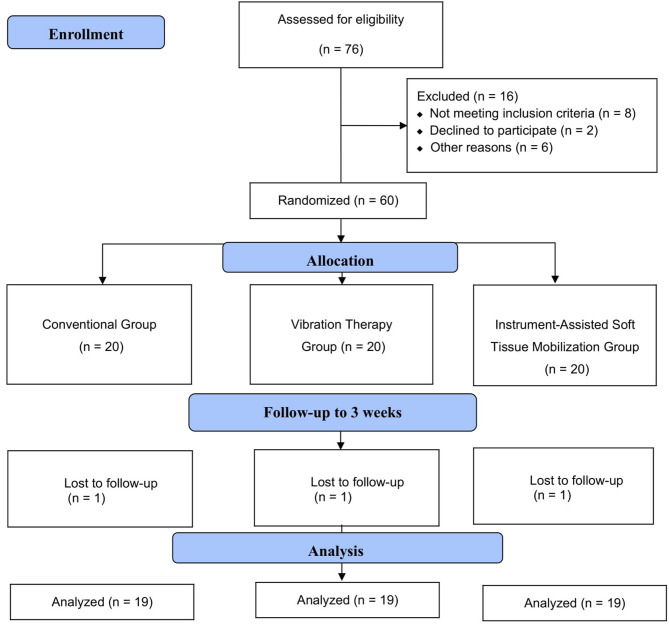
Patients included in this study were diagnosed with CDH by a neurologist following a comprehensive neurological evaluation, physical examination, and radiographic imaging. Eligible participants were between 30 and 60 years of age, had experienced neck pain for at least three weeks, reported a pain intensity of ≥ 4 on the Visual Analog Scale (VAS), and demonstrated restricted cervical ROM. The diagnosis of CDH was confirmed by magnetic resonance imaging (MRI), and only cases with mild to moderate disc protrusion or extrusion without significant nerve root or spinal cord compression were included. Participants were required to have at least one clinical symptom associated with CDH, such as localized neck pain, pain radiating to the shoulder or arm, or tingling/numbness in the upper limb. Both unilateral and bilateral pain presentations were eligible. Individuals with signs or symptoms of radiculopathy were excluded in order to ensure a more homogeneous sample focused solely on localized cervical involvement. Exclusion criteria included individuals with additional orthopedic or neurological disorders beyond cervical involvement, a history of cervical trauma, prior physiotherapy or any other alternative treatments within the last six months, or intolerance to PMT or IASTM. During the recruitment phase, a total of 76 individuals diagnosed with CDH were screened for eligibility. Eight patients were excluded for not fulfilling the inclusion criteria. Among the remaining candidates, three declined to participate due to preference for alternative treatment, two withdrew for personal reasons, two were unable to attend due to transportation difficulties, and one was excluded due to language barriers. Consequently, 60 eligible participants were randomly allocated into three intervention arms: Conventional Therapy (CT), PMT, and IASTM, with 20 participants in each group. During the follow-up period, one participant from each group discontinued the intervention, yielding a final sample of 57 participants (n = 19 per group) included in the statistical analysis (Fig. 1).
Fig. 1.
Design and flow chart of the study.
Randomization and blinding
Randomization was conducted using a computer-generated randomization list via randomizer.org by an independent researcher not involved in participant recruitment, assessment, or intervention procedures. Participants (n = 60) were randomly assigned in a 1:1:1 ratio to one of three groups: CT, VT, or IASTM, with 20 participants per group. Simple randomization without stratification was applied. Allocation concealment was ensured using sequentially numbered, opaque, sealed envelopes prepared by the independent researcher.
Although complete blinding of participants was not feasible due to the nature of the interventions, group allocation was not disclosed, and participants were informed only that they would receive one of several evidence-based physiotherapy programs. This approach minimized performance bias.
All interventions (CT, PMT, and IASTM) were administered by the same licensed physiotherapist, who had more than five years of clinical experience in musculoskeletal rehabilitation and held certifications in both IASTM and vibration therapy techniques. To ensure consistency and standardization, the same therapist delivered all treatment sessions for each intervention arm. Outcome measurements were performed independently by a physiotherapist with ten years of clinical and research experience, who was blinded to group allocation. Data entry and statistical analysis were carried out by another independent researcher, holding a PhD in physiotherapy, who was also blinded to the intervention groups.
Conventional therapy group
Participants assigned to the CT group received a standardized physiotherapy program delivered four sessions per week over three consecutive weeks (total: 12 sessions). Each session lasted approximately 45–50 min and was conducted under the direct supervision of a licensed physiotherapist with over five years of clinical experience in musculoskeletal rehabilitation, ensuring protocol adherence and participant safety.
At the beginning of each session, superficial moist heat was applied to the cervical region using a hydrocollator pack maintained at 70–75 °C for 20 min. The participant was positioned supine with slight cervical flexion, and a towel barrier was used to prevent thermal injury.
Immediately following heat application, Conventional Transcutaneous Electrical Nerve Stimulation (TENS) was administered for 20 min over the most symptomatic cervical areas using a dual-channel unit (100 Hz, 100 µs). Two 5 × 5 cm self-adhesive electrodes were placed bilaterally over the upper trapezius and C4–C7 paraspinal muscles. Stimulation intensity was increased to produce a strong but comfortable paresthesia without visible muscle contraction, following established TENS protocols. 20-minute TENS aimed to modulate pain through large-fiber afferent activation and nociceptive inhibition.
Post-electrotherapy, participants performed Active Range of Motion (AROM) exercises (flexion, extension, right/left lateral flexion) in upright sitting with feet flat on the floor and thoracic spine stabilization. Movements were carried out to the pain-free end range and held for 2 s, repeated 2 sets×10 reps per direction, with a 60-second rest between sets.
The strengthening component comprised isometric cervical muscle contractions in the same planes. Participants applied gentle pressure via the head against the therapist’s hand (or stable surface), achieving a submaximal, pain-free contraction. Each contraction lasted 6 s, followed by 4 s relaxation. Exercises were performed as 2 sets×10 reps per direction, with a 60-second rest between sets.
Percussion massage therapy group
In addition to receiving the full CT protocol described above, the intervention was administered by the same physiotherapist using a Hypervolt device (Hyperice, CA, USA) to ensure consistency and standardization across all sessions. PMT was applied as an adjunct to the CT protocol. The treatment involved a percussion massage device operating at a moderate intensity (level 2; frequency range: 33–40 Hz), targeting the anatomical course between the origin and insertion of the trapezius, levator scapulae, and cervical paravertebral muscles. Each muscle group received three minutes of treatment using the flat head attachment to provide safe and uniform stimulation. The device was applied longitudinally along the muscle fibers in a straight path, moving from proximal to distal and then back to proximal, with each stroke lasting approximately five seconds. Interventions were performed three times per week for a total duration of three consecutive weeks (Fig. 2).
Fig. 2.
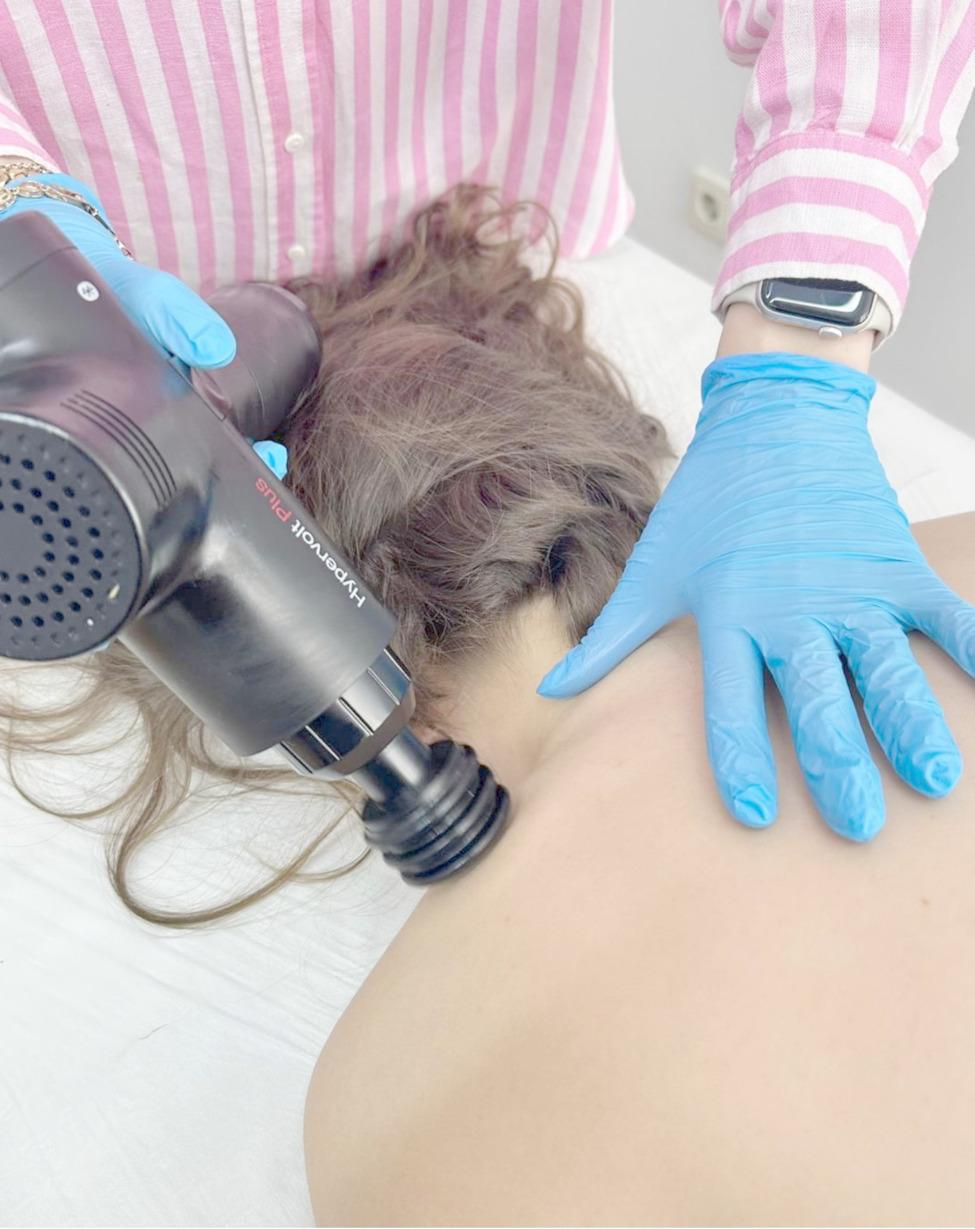
Vibration therapy applied with percussion massage device.
Instrument-assisted soft tissue mobilization group
Similar to the PMT group, participants in the IASTM group also received the full CT protocol in addition to their specific intervention. Participants in the IASTM group underwent treatment three times per week for a period of three weeks. The intervention was administered using ergonomically designed stainless-steel instruments of varying sizes, specifically shaped to conform to the anatomical contours of the cervical region. Treatment was directed toward the superficial and deep fascial layers of the upper, middle, and lower portions of the trapezius, as well as the splenius and suboccipital muscles spanning the C1 to T1 vertebral segments. Prior to the intervention, a thin layer of neutral emollient was applied to the skin surface to minimize friction and ensure smooth instrument glide. Mobilization techniques were aligned with the orientation of muscle fibers and followed standardized IASTM protocols, including sweep and fan methods. These techniques were applied at angles ranging from 30° to 60°, depending on the anatomical target and intended therapeutic effect. Each technique was performed for approximately 8 to 10 repetitions, with a total session duration of nine minutes (Fig. 3). Patients were informed that small red spots, referred to as petechiae, bruising, or mild pain might occur in the treated area. In cases of significant discomfort, ice was applied to the region for 15 to 20 min following the treatment [32, 33].
Fig. 3.
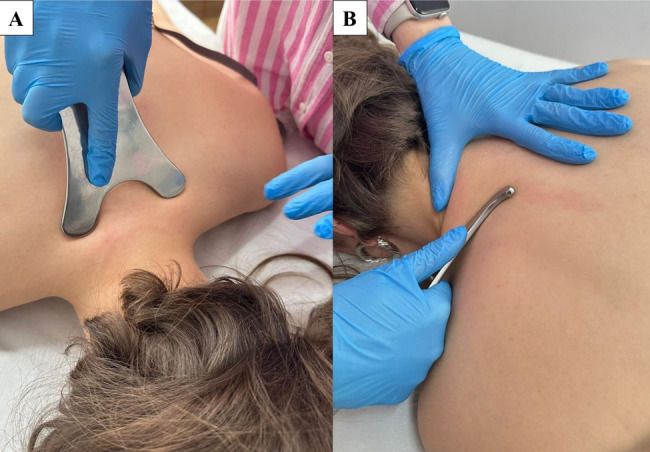
Instrument-Assisted Soft Tissue Mobilization: (A) sweep and (B) fan techniques.
Outcome measures
Evaluations of treatment outcomes for all participants were performed both before the intervention and at the end of the three-week treatment period.
Primary outcomes
Pain
Pain intensity was assessed using the VAS, which consists of a 10-centimeter horizontal line anchored by two extremes: “0” representing no pain and “10” indicating the worst imaginable pain. Participants were asked to indicate their current level of pain by placing a mark along the line. The pain score was then quantified by measuring the distance in centimeters from the left endpoint to the patient’s mark using a standard ruler [34]. The VAS has demonstrated excellent test–retest reliability (ICC > 0.90) and strong construct validity in musculoskeletal populations [35, 36].
Disability
Cervical disability was assessed using the NDI, a widely validated instrument designed to measure pain intensity and functional limitations associated with neck disorders. The scale consists of 10 items covering various domains of daily functioning, including pain intensity, personal care, lifting, reading, headaches, concentration, work, sleep, driving, and recreational activities. Each item is scored on a 6-point scale ranging from 0 (no disability) to 5 (maximum disability), with higher total scores indicating greater functional impairment. The NDI is considered a reliable and sensitive measure for evaluating both the severity and impact of cervical pathologies on daily living [37]. The NDI has demonstrated excellent internal consistency (Cronbach’s α = 0.87–0.94) and strong test–retest reliability (ICC = 0.89) in patients with cervical spine conditions, and is sensitive to clinical change [38, 39].
Secondary outcomes
Kinesiophobia
Kinesiophobia was evaluated using the TSK, a 17-item self-report instrument originally developed by Kori et al. in 1990 to assess fear of movement and re-injury. The Turkish version of the TSK has been culturally adapted and validated, demonstrating good internal consistency (Cronbach’s α = 0.78) and excellent test–retest reliability (ICC = 0.81) in musculoskeletal populations [40]. Each item is scored on a 4-point Likert scale ranging from 1 (“strongly disagree”) to 4 (“strongly agree”). Items 4, 8, 12, and 16 are reverse-coded prior to computing the total score. The final score ranges from 17 to 68, with higher scores reflecting more severe levels of kinesiophobia [31].
Joint position sense
The Laser Pointer Assisted Angle Repetition Test (LI-YATT) was employed to assess JPS, a key component of proprioceptive function. The procedure was conducted according to the standardized protocol described by Revel et al. [41]. Participants were seated at a fixed distance of 100 cm from a target board measuring 90 × 80 cm. A laser pointer was securely mounted on the participant’s head using an adjustable strap and aligned to project directly at the center of the board, defined as the reference (origin) point.
Participants were initially instructed to visually align the laser with the central target, then perform a full cervical flexion movement. Upon returning to a neutral head position, they attempted to relocate the original target without visual guidance. During the proprioceptive assessment, participants repeated the procedure with their eyes closed, ceasing the movement once they believed the laser was aligned with the center. The discrepancy between the initial and final laser positions was recorded in centimeters (cm), representing the joint position error [42]. The LI-YATT method has demonstrated high intra-rater reliability (intraclass correlation coefficient [ICC] = 0.85–0.96) and inter-rater reliability (ICC = 0.80–0.91) in patients with neck pain [41, 43]. Furthermore, it has shown strong construct validity when compared with three-dimensional motion analysis systems and is sensitive in detecting sensorimotor deficits in both clinical and research contexts [44].
Statistical analysis
The sample size was determined a priori using G*Power 3.1.9.7 software. Based on a minimal clinically important difference (MCID) of 7.5 points and a standard deviation (SD) of 7 for the Neck Disability Index (NDI), the effect size (Cohen’s f) was estimated at approximately 0.76. To achieve 90% statistical power (1–β = 0.90) at a significance level of α = 0.05 for a one-way ANOVA across three groups, a minimum of 18 participants per group was required [45, 46]. To account for potential dropouts and ensure adequate statistical power, a total of 60 participants (n = 20 per group) were enrolled.
Statistical analyses were conducted using a per-protocol approach, including only participants who completed the assigned intervention and both pre- and post-treatment assessments. Data analysis was performed using SPSS (Version 22, IBM, Chicago, IL). The normality of the distribution of numerical variables was assessed using the Shapiro–Wilk test. Baseline characteristics and demographic data were compared across groups using one-way ANOVA for continuous variables and Chi-square tests for categorical variables. Within-group changes were assessed using paired sample t-tests. Analysis of covariance (ANCOVA), with baseline values as covariates, was employed to evaluate the effects of interventions on primary and secondary outcomes three weeks post-intervention. For variables not normally distributed, between-group comparisons were analyzed with the Kruskal–Wallis test, and within-group changes were evaluated using the Wilcoxon signed-rank test. Statistical significance was set at p < 0.05 with Bonferroni correction applied for multiple comparisons [47]. Furthermore, clinical effects of the interventions were compared against previously reported MCID thresholds, which represent patient-derived scores indicating clinically meaningful changes impacting treatment decisions [48].
Results
A total of 57 participants (n = 19 per group) completed the study and were included in the final analysis. Baseline characteristics were summarized in Table 1. There were no statistically significant differences among the groups in terms of gender, height, weight, BMI, VAS-rest, VAS-activity, or JPS-right rotation (p > 0.05). However, significant differences were observed for age (p < 0.001), NDI (p = 0.019), TSK (p = 0.029), JPS-extension (p = 0.006), and JPS-left rotation (p = 0.010). These variables were treated as covariates in ANCOVA analyses to account for baseline variability in the between-group comparisons.
Table 1.
Baseline demographic and clinical characteristics of participants across intervention groups
| Conventional group (n = 19) Mean ± SD |
PMT group (n = 19) Mean ± SD |
IASTM group (n = 19) Mean ± SD |
p | |
|---|---|---|---|---|
| Age (year) | 45.89 ± 2.45 | 44.42 ± 3.02 | 37.11 ± 7.27 | 0.000 b |
| Gender, F/M (F%) | 10/9 (53%) | 14/5 (74%) | 10/9 (53%) | 0.312a |
| Height (cm) | 170.16 ± 12.03 | 165.84 ± 8.66 | 171.00 ± 8.63 | 0.236b |
| Weight (kg) | 71.53 ± 6.45 | 70.74 ± 7.29 | 68.11 ± 14.45 | 0.551b |
| BMI | 25.10 ± 4.47 | 25.99 ± 4.39 | 23.16 ± 3.81 | 0.118b |
| VAS-Resting | 7.05 ± 1.34 | 6.41 ± 1.87 | 6.60 ± 1.30 | 0.426b |
| VAS-Activity | 7.60 ± 0.77 | 6.88 ± 1.18 | 7.65 ± 0.68 | 0.144c |
| NDI | 20.26 ± 5.10 | 24.89 ± 6.73 | 24.37 ± 3.77 | 0.019 b |
| TSK | 50.05 ± 9.33 | 48.58 ± 10.25 | 42.42 ± 7.46 | 0.029 b |
| JPS-Flexion | 7.23 ± 0.89 | 7.73 ± 1.01 | 8.26 ± 1.92 | 0.075b |
| JPS-Extension | 8.31 ± 1.26 | 8.18 ± 1.32 | 6.80 ± 1.91 | 0.006 b |
| JPS-Rot Right | 7.51 ± 1.13 | 7.06 ± 1.50 | 8.18 ± 4.36 | 0.459b |
| JPS-Rot Left | 8.25 ± 0.90 | 6.34 ± 1.35 | 8.19 ± 3.29 | 0.010 b |
IASTM: Instrument-Assisted Soft Tissue Mobilization, PMT: Percussion Massage Therapy VAS: Visual Analog Scale, NDI: Neck Disability Index, TSK: Tampa Scale for Kinesiophobia, JPS: Joint Position Sense, rot: rotation, BMI: Body Mass Index; F: Female; M: Male. aChi-square Test, bOne-way analysis of variance (ANOVA), cKruskal–Wallis Test, *p < 0.05. Significant baseline differences were observed in age, TSK, NDI, JPS-Extension, and JPS-Rot Left. These variables were statistically adjusted for in subsequent analyses using ANCOVA
Following the three-week intervention, all groups (CT, PMT, and IASTM) demonstrated statistically significant within-group improvements in all measured outcomes, including pain intensity, disability, kinesiophobia, and proprioception (p < 0.001 for all comparisons; Tables 2 and 3).
Table 2.
Within-group changes and between-group comparisons in VAS, TSK, and NDI scores
| Variable | Group | Baseline | After 3 weeks | Within-group score change | p*a, b | Cohen’s d | ANCOVA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (95% CI) |
Mean ± SD (95% CI) |
Mean ± SD (95% CI) | F | p† | η²ₚ | Bonferroni | |||||
| Group | p** | ||||||||||
| VAS-Resting | CT |
7.05 ± 1.34 (6.40–7.69) |
4.91 ± 0.60 (4.62–5.20) |
-2.13 ± 1.49 (-2.85/-1.42) |
< 0.001 a | 1.43 | 23.33 | < 0.001 | 0.43 | CT-PMT | < 0.001 |
| PMT |
6.41 ± 1.87 (5.51–7.31) |
3.03 ± 1.53 (2.29–3.76) |
-3.38 ± 1.95 (-4.32/-2.44) |
< 0.001 a | 1.74 | CT-IASTM | < 0.001 | ||||
| IASTM |
6.60 ± 1.30 (5.98–7.23) |
2.60 ± 0.97 (2.13–3.06) |
-4.00 ± 0.89 (-4.43/-3.58) |
< 0.001 a | 4.49 | IASTM-PMT | 0.457 | ||||
| VAS-Activity | CT |
7.60 ± 0.77 (7.23–7.97) |
4.99 ± 1.08 (4.47–5.51) |
-2.61 ± 1.22 (-3.20/-2.02) |
< 0.001 b | 2.13 | 14.56 | < 0.001 | 0.35 | CT-PMT | 0.001 |
| PMT |
6.88 ± 1.18 (6.31–7.45) |
2.99 ± 1.79 (2.13–3.86) |
-3.89 ± 1.84 (-4.78/-3.00) |
< 0.001 b | 2.11 | CT-IASTM | < 0.001 | ||||
| IASTM |
7.65 ± 0.68 (7.32–7.97) |
2.75 ± 1.10 (2.22–3.28) |
-4.89 ± 1.44 (-5.59/-4.20) |
< 0.001 b | 3.41 | IASTM-PMT | 1.00 | ||||
| NDI | CT |
20.26 ± 5.10 (17.81–22.72) |
13.63 ± 2.48 (12.44–14.83) |
-6.63 ± 5.47 (-9.27/-4.00) |
< 0.001 a | 1.21 | 0.93 | 0.400 | 0.03 | --- | --- |
| PMT |
24.89 ± 6.73 (21.65–28.14) |
12.79 ± 2.46 (11.60–13.98) |
-12.11 ± 6.86 (-15.41/-8.80) |
< 0.001 a | 1.76 | --- | --- | ||||
| IASTM |
24.37 ± 3.77 (22.55–26.19) |
12.89 ± 2.81 (11.54–14.25) |
-11.47 ± 4.23 (-13.51/-9.43) |
< 0.001 a | 2.71 | --- | --- | ||||
| TSK | CT |
50.05 ± 9.33 (45.56–54.55) |
42.16 ± 7.20 (38.69–45.63) |
-7.89 ± 6.98 (-11.26/-4.53) |
< 0.001 a | 1.13 | 34.28 | < 0.001 | 0.48 | CT-PMT | < 0.001 |
| PMT |
48.58 ± 10.25 (43.64–53.52) |
27.26 ± 5.94 (24.40–30.13) |
-21.32 ± 9.78 (-26.03/-16.60) |
< 0.001 a | 2.18 | CT-IASTM | < 0.001 | ||||
| IASTM |
42.42 ± 7.46 (38.82–46.02) |
25.26 ± 7.42 (21.69–28.84) |
-17.16 ± 6.91 (-20.49/-13.83) |
< 0.001 a | 2.48 | IASTM-PMT | 1.00 | ||||
CT: Conventional Therapy, PMT: Percussion Massage Therapy, IASTM: Instrument-Assisted Soft Tissue Mobilization, VAS: Visual Analog Scale, NDI: Neck Disability Index, TSK: Tampa Scale for Kinesiophobia, SD: Standard Deviation. *aPaired sample t-test was used for normally distributed variables. *bWilcoxon signed-rank test was used for non-normally distributed variables. †ANCOVA was performed with baseline values as covariates. η²ₚ: artial eta squared, representing the proportion of variance in post-treatment scores explained by group differences, after adjusting for baseline. **Bonferroni correction was applied for multiple comparisons (significance set at p < 0.05). While ANCOVA revealed significant overall group effects, Bonferroni-adjusted post-hoc comparisons did not identify statistically significant differences between all group pairs. ANCOVA was not applied to certain outcomes due to violations of test assumptions or inadequate data homogeneity
Table 3.
Within-group changes and between-group comparisons of JPS in flexion, extension, and right/left rotation
| Variable | Group | Baseline | After 3 weeks | Within-group score change | p*a, b | Cohen’s d | ANCOVA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (95% CI) |
Mean ± SD (95% CI) |
Mean ± SD (95% CI) | F | p† | η²ₚ | Bonferroni | |||||
| Group | p** | ||||||||||
| JPS-Flexion | CT |
7.23 ± 0.89 (6.81–7.66) |
6.15 ± 0.72 (5.80–6.50) |
-1.09 ± 0.84 (-1.49/-0.68) |
< 0.001 a | 1.29 | 27.79 | < 0.001 | 0.45 | CT-PMT | < 0.001 |
| PMT |
7.73 ± 1.01 (7.25–8.22) |
4.06 ± 0.98 (3.59–4.54) |
-3.67 ± 1.34 (-4.32/-3.02) |
< 0.001 a | 2.73 | CT-IASTM | < 0.001 | ||||
| IASTM |
8.26 ± 1.92 (7.33–9.19) |
4.46 ± 1.53 (3.73–5.20) |
-3.80 ± 1.61 (-4.57/-3.02) |
< 0.001 a | 2.36 | IASTM-PMT | 1.00 | ||||
| JPS-Extension | CT |
8.31 ± 1.26 (7.71–8.92) |
7.12 ± 0.61 (6.83–7.42) |
-1.19 ± 1.32 (-1.82/-0.55) |
0.001 a | 0.90 | 24.41 | < 0.001 | 0.41 | CT-PMT | < 0.001 |
| PMT |
8.18 ± 1.32 (7.54–8.82) |
4.90 ± 0.80 (4.52–5.28) |
-3.28 ± 1.41 (-3.96/-2.60) |
< 0.001 a | 2.33 | CT-IASTM | < 0.001 | ||||
| IASTM |
6.80 ± 1.91 (5.88–7.72) |
3.87 ± 2.10 (2.86–4.89) |
-2.93 ± 1.57 (-3.69/-2.17) |
< 0.001 a | 1.86 | IASTM-PMT | 0.937 | ||||
| JPS-Rot Right | CT |
7.51 ± 1.13 (6.96–8.06) |
6.68 ± 0.96 (6.22–7.14) |
-0.83 ± 1.00 (-1.31/-0.35) |
0.002 a | 0.83 | 4.65 | 0.014 | 0.09 | CT-PMT | 0.875 |
| PMT |
7.06 ± 1.50 (6.34–7.79) |
6.02 ± 1.18 (5.45–6.59) |
-1.04 ± 1.48 (-1.76/-0.33) |
0.007 a | 0.70 | CT-IASTM | 0.012 | ||||
| IASTM |
8.18 ± 4.36 (6.07–10.28) |
5.63 ± 2.68 (4.34–6.92) |
-2.54 ± 3.11 (-4.05/-1.04) |
0.002 a | 0.82 | IASTM-PMT | 0.176 | ||||
| JPS-Rot Left | CT |
8.25 ± 0.90 (7.82–8.68) |
6.88 ± 0.80 (6.50–7.27) |
-1.37 ± 1.19 (-1.94/-0.79) |
< 0.001 a | 1.15 | 2.96 | 0.06 | 0.08 | --- | --- |
| PMT |
6.34 ± 1.35 (5.69–6.99) |
5.41 ± 1.37 (4.75–6.07) |
-0.93 ± 2.11 (-1.95/0.09) |
0.070a | 0.44 | --- | --- | ||||
| IASTM |
8.19 ± 3.29 (6.60–9.77) |
5.54 ± 2.93 (4.13–6.95) |
-2.65 ± 2.50 (-3.85/-1.45) |
< 0.001 a | 1.06 | --- | --- | ||||
CT: Conventional Therapy, PMT: Percussion Massage Therapy, IASTM: Instrument-Assisted Soft Tissue Mobilization, VAS: Visual Analog Scale, NDI: Neck Disability Index, TSK: Tampa Scale for Kinesiophobia, SD: Standard Deviation. *aPaired sample t-test was used for normally distributed variables. *bWilcoxon signed-rank test was used for non-normally distributed variables. †ANCOVA was performed with baseline values as covariates. η²ₚ: artial eta squared, representing the proportion of variance in post-treatment scores explained by group differences, after adjusting for baseline. **Bonferroni correction was applied for multiple comparisons (significance set at p < 0.05). While ANCOVA revealed significant overall group effects, Bonferroni-adjusted post-hoc comparisons did not identify statistically significant differences between all group pairs. ANCOVA was not applied to certain outcomes due to violations of test assumptions or inadequate data homogeneity
The most substantial reduction in VAS-rest was observed in the IASTM group (–4.00 ± 0.89; d = 4.49), followed by PMT (–3.38 ± 1.95; d = 1.74) and CT (–2.13 ± 1.49; d = 1.43). A similar trend was found in VAS-activity scores, with IASTM showing the greatest improvement (–4.89 ± 1.44; d = 3.41). Between-group comparisons via ANCOVA indicated significant effects for VAS at rest (F = 23.33, p < 0.001, η² = 0.43), favoring the PMT and IASTM interventions over CT. Post hoc tests indicated that both IASTM and PMT were significantly more effective than CT (p < 0.001), with no statistically significant difference between IASTM and PMT (p > 0.05).
NDI scores also showed significant within-group improvements in all three groups (p < 0.001), although no significant between-group differences were detected (F = 0.93, p = 0.400, η² = 0.03), indicating similar efficacy in disability reduction.
TSK scores significantly decreased in all groups, with greater reductions observed in the PMT (–21.32 ± 9.78; d = 2.18) and IASTM (–17.16 ± 6.91; d = 2.48) groups compared to CT (–7.89 ± 6.98; d = 1.13). ANCOVA confirmed a significant group effect (F = 34.28, p < 0.001, η² = 0.48).
In proprioception outcomes, JPS-flexion improved in all groups, with the IASTM group showing the most notable change (–3.80 ± 1.61; d = 2.36), followed by PMT (–3.67 ± 1.34; d = 2.73) and CT (–1.09 ± 0.84; d = 1.29). ANCOVA revealed a significant between-group effect (F = 27.79, p < 0.001, η² = 0.45). Similarly, JPS-extension improved most in the PMT group, with ANCOVA confirming group differences (F = 24.41, p < 0.001, η² = 0.41); post hoc analysis indicated that both IASTM and PMT outperformed CT. In JPS-right rotation, the IASTM group exhibited the greatest improvement (–2.54 ± 3.11; d = 0.82). ANCOVA showed a significant group effect (F = 4.65, p = 0.014, η² = 0.09), and post hoc comparisons favored IASTM over CT (p = 0.012). Although JPS-left rotation improved in the IASTM group (–2.65 ± 2.50; d = 1.06), ANCOVA did not reveal a statistically significant between-group difference (F = 2.96, p = 0.060, η² = 0.08).
All three interventions resulted in statistically significant improvements in pain, disability, kinesiophobia, and proprioception. Pain intensity, measured by the VAS, decreased well beyond the MCID of 1.5–2.5 cm, confirming that the reductions were not only statistically but also clinically meaningful [49]. Likewise, changes in NDI scores exceeded the MCID threshold of 7.5 points, indicating meaningful functional improvement in neck-related disability [45]. Improvements in TSK and JPS, particularly in flexion and extension directions, were also substantial, with effect sizes supporting their potential for clinical relevance. These findings support the potential of IASTM and PMT as effective and clinically meaningful approaches for the conservative management of CDH, particularly in addressing sensorimotor dysfunctions that are inadequately targeted by conventional physiotherapy alone.
Discussion
In this study, three different intervention approaches CT, PMT, and IASTM resulted in significant improvements across a range of multidimensional clinical parameters, including pain intensity, neck disability, kinesiophobia, and JPS. Overall, both IASTM and PMT demonstrated greater clinical effectiveness compared to conventional treatment, although no significant differences were observed between the two myofascial techniques. These findings suggest that myofascial-based interventions could serve as effective therapeutic strategies for neuromuscular rehabilitation, particularly in the management of pain (VAS-Resting and VAS-Activity), NDI, TSK, and improvement of JPS.
Cervical pain is a multifactorial condition and remains a significant public health issue in modern societies. It is a significant musculoskeletal disorder that can noticeably affect quality of life [50]. This outcome aligns with the growing body of evidence supporting the effectiveness of both IASTM and vibration stimulation in managing musculoskeletal pain. A recent systematic review and meta-analysis of randomized controlled trials showed that IASTM significantly improved patient-reported pain outcomes across a range of musculoskeletal conditions [19]. Several studies suggest that treatment of musculoskeletal injuries with IASTM can alleviate pain and improve functional capacity [17, 51–53]. Another potential mechanism underlying pain relief is enhanced circulation. A study by Portillo Soto et al. comparing the effects of IASTM and massage indicated that both interventions raised skin temperature, a marker of increased blood flow, which in turn supported nutrient and oxygen delivery to tissues. The associated reduction in pain may result from improved clearance of metabolic waste products through enhanced circulation [54].
Prior research has demonstrated that cervical muscle vibration activates sensory pathways that inhibit nociceptive transmission, resulting in short-term pain relief in individuals with chronic neck pain [11]. A 10-session PMT protocol at 35–50 Hz increased trapezius and levator scapulae pressure-pain thresholds and reduced self-reported pain in chronic non-specific neck pain [24]. Similarly, Preiss et al. emphasized the efficacy of VT in reducing pain symptoms in patients with chronic neck pain [55]. In terms of underlying mechanisms, vibration delivers repetitive sensory input to mechanoreceptors in both muscles and skin, potentially modulating pain through gate-control processes and improving circulation to tense or ischemic tissues [11, 56]. Increased regional circulation and segmental neuromodulation are frequently proposed as key contributors to vibration-induced analgesia, although further research is warranted to elucidate these pathways fully. In the present study, the substantial pain reductions observed with both IASTM and PMT support their use as effective therapeutic options in managing neck pain, often yielding superior outcomes to exercise alone, particularly in the short term [11]. Our findings regarding the pain-relieving effect of PMT are in agreement with the literature. We postulate that improved blood flow and decreased muscle tension, along with the relaxation of fascial structures induced by PMT, collectively contribute to pain reduction.
PMT may deliver oscillatory stimulation that simultaneously activate muscle spindles and cutaneous receptors, potentially modulating nociceptive transmission through segmental gating mechanisms and enhancing circulation in tense or ischemic tissues. While both interventions may ultimately contribute to improvements in sensorimotor integration, IASTM could achieve these effects primarily via structural and localized tissue adaptations, whereas PMT might exert its influence predominantly through rhythmic sensory entrainment and more widespread afferent activation. These mechanisms may be particularly relevant in the context of CDH, where the restoration of proprioceptive acuity and the potential reduction of pain-related muscle guarding may play an important role in facilitating functional recovery [11, 17].
Chronic neck pain frequently triggers a fear-avoidance behavior cycle, wherein heightened pain levels lead to increased kinesiophobia, subsequently limiting physical activity and perpetuating functional disability [28]. Supporting this, a prior study demonstrated that adding VT to a conventional rehabilitation protocol including modalities such as heat application, TENS, and range of motion exercises significantly improved pain levels, functional capacity, and proprioception in patients with CDH. Furthermore, a concurrent reduction in kinesiophobia was noted among patients whose proprioceptive function had improved, highlighting a potential mediating role of proprioception in reducing fear of movement [30].
Preiss et al. also reported that VT contributed to proprioceptive enhancement in individuals suffering from chronic neck pain [55]. In a study, the addition of PMT to a standard neck rehabilitation program in patients with CDH was found to improve pain and proprioception, while also significantly reducing kinesiophobia. The authors suggested that this reduction in fear of movement was likely mediated by enhanced proprioceptive feedback [11]. Similarly, in a study evaluating the immediate effects of cervical vibration, a 5-minute session was shown to acutely enhance JPS in the cervical region [57].
Although the number of studies directly examining the effect of IASTM on proprioception is limited, IASTM has been reported, in individuals with chronic neck pain, to enhance proprioceptive input via augmented afferent stimulation and to yield concurrent clinical improvements in pain and JPS [58, 59].
Conventional physiotherapy alone was also effective in reducing pain, improving proprioception and range of motion, and decreasing disability. The notable improvements observed in the conventional group in our trial further corroborate the well-established role of exercise in restoring function, consistent with prior literature on structured physical therapy for neck pain and disability [11, 29, 60, 61]. In support of this, Shewail et al. reported that IASTM in individuals with chronic neck pain was associated with reductions in pain severity, improvements in functional capacity, and increases in pressure-pain thresholds [62]. In our study, however, between-group differences in NDI favored the IASTM and PMT groups, suggesting that these modalities may provide additional benefit when used adjunctively. Overall, our findings align with previous reports indicating that incorporating IASTM or PMT into conventional rehabilitation programs can further improve functional outcomes in patients with cervical pain [11, 62, 63]. The superior pain modulation afforded by these approaches may enhance participation in daily activities relative to exercise therapy alone, thereby accelerating reductions in disability and fear of movement.
Several limitations must be considered when interpreting our findings. Firstly, the intervention and follow-up durations were relatively short, with outcomes assessed only after a 3-week treatment period. Consequently, it remains unclear whether the observed improvements and the equivalence between IASTM and vibration would be sustained over the long term. Given that CDH is a chronic and often recurrent condition, the short intervention period of 3 weeks may not fully capture the long-term trajectory of pain modulation, proprioceptive recovery, and functional improvement. In chronic musculoskeletal disorders, neural and structural adaptations often require prolonged, sustained interventions, and short-term gains may diminish without continued therapy. Future longitudinal studies are necessary to determine whether these interventions provide lasting relief and whether improvements in proprioception and reduced kinesiophobia can be maintained.
Additionally, all groups in our study received active treatment, which limits comparisons against placebo or no-treatment conditions. While ethical considerations precluded the inclusion of a non-intervention control group, future studies may consider incorporating sham IASTM or sham vibration conditions to better isolate the specific effects of each technique. Another limitation is the lack of computerized assessment tools for JPS measurement, which could have offered more precise quantification of proprioceptive improvements.
Moreover, we did not stratify participants based on the severity and level of disc herniation, and CDH levels were not documented. Future research should target participants at specific stages of disc herniation to investigate whether treatment efficacy varies by pathology severity. In addition, studies should investigate the optimal parameters of PMT, such as frequency, duration, and applicator type, to identify standardized protocols that maximize proprioceptive enhancement and pain relief for patients with CDH. Future research should aim to develop validated treatment regimens that consistently reduce pain and improve proprioceptive control, facilitating evidence-based integration of these techniques into clinical practice.
Conclusion
This randomized controlled trial demonstrated that supplementing IASTM or PMT to conventional physiotherapy resulted in superior clinical outcomes in individuals with CDH. Both interventions significantly reduced pain and kinesiophobia while enhancing JPS and functional status. Although CT alone yielded beneficial effects, the integration of these adjunctive modalities provided greater sensorimotor improvements. These findings support the incorporation of myofascial-based interventions into standard rehabilitation protocols for optimizing outcomes in patients with cervical spine disorders.
Acknowledgements
The authors are grateful to all the participants for their participation in this study.
Author contributions
B.M.; Investigation, Supervision, Conceptualization, Methodology, Writing- Reviewing and Editing. E.D, SG; Data curation, Writing- Original draft preparation, Visualization, Investigation, Writing- Reviewing and Editing.
Funding
This research received no external funding.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All participants signed written informed consent forms, and the study was conducted according to the principles of the Declaration of Helsinki. The study protocol received approval from the non-interventional ethics committee at Istanbul Medipol University (File number: E-10840098-202.3.02-1924). All participants provided written informed consent.
Consent for publication
Written consent for publication has been obtained from the patient shown in fıgure 2 and Fig. 3.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yaşa ME, Ün Yıldırım N, Demir P. The effects of a 6-Week balance training in addition to conventional physiotherapy on pain, postural control, and balance confidence in patients with cervical disc herniation: a randomized controlled trial. Somatosens Mot Res. 2021;38:60–7. 10.1080/08990220.2020.1845136. [DOI] [PubMed] [Google Scholar]
- 2.Mousavi-Khatir R, Talebian S, Maroufi N, Olyaei GR. Effect of static neck flexion in cervical flexion-relaxation phenomenon in healthy males and females. J Bodyw Mov Ther. 2016;20:235–42. 10.1016/j.jbmt.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Li J, Qiu G, Wei J, Qiu Y, An Y, et al. Incidence and risk factors of axial symptoms after cervical disc arthroplasty: a minimum 5-year follow-up study. J Orthop Surg Res. 2016;11:103. 10.1186/s13018-016-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng B, Yang L, Li Y, Liu T, Liu Y. Cervical proprioception impairment in neck Pain-Pathophysiology, clinical evaluation, and management: A narrative review. Pain Ther. 2021;10:143–64. 10.1007/s40122-020-00230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J-J, Xu M-L, Zeng H-Z, Zheng L-D, Zhu S-J, Jin C, et al. The Biomechanical effect of preexisting different types of disc herniation in cervical hyperextension injury. J Orthop Surg Res. 2021;16:527. 10.1186/s13018-021-02677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark NC, Röijezon U, Treleaven J. Proprioception in musculoskeletal rehabilitation. Part 2: clinical assessment and intervention. Man Ther. 2015;20:378–87. 10.1016/j.math.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Stanton TR, Leake HB, Chalmers KJ, Moseley GL. Evidence of impaired proprioception in chronic, idiopathic neck pain: systematic review and Meta-Analysis. Phys Ther. 2016;96:876–87. 10.2522/ptj.20150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCaskey MA, Schuster-Amft C, Wirth B, Suica Z, de Bruin ED. Effects of proprioceptive exercises on pain and function in chronic neck- and low back pain rehabilitation: a systematic literature review. BMC Musculoskelet Disord. 2014;15:382. 10.1186/1471-2474-15-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanpied PR, Gross AR, Elliott JM, Devaney LL, Clewley D, Walton DM, et al. Neck pain: revision 2017. J Orthop Sports Phys Therapy. 2017;47:A1–83. 10.2519/jospt.2017.0302. [DOI] [PubMed] [Google Scholar]
- 10.Corp N, Mansell G, Stynes S, Wynne-Jones G, Morsø L, Hill JC, et al. Evidence-based treatment recommendations for neck and low back pain across europe: A systematic review of guidelines. Eur J Pain. 2021;25:275–95. 10.1002/ejp.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz Menek M, Dansuk E, Tayboga UI. Effect of local vibration therapy on pain, joint position sense, kinesiophobia, and disability in cervical disc herniation: A randomized controlled trial. J Clin Med. 2024;13:4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Röijezon U, Clark NC, Treleaven J. Proprioception in musculoskeletal rehabilitation. Part 1: basic science and principles of assessment and clinical interventions. Man Ther. 2015;20:368–77. 10.1016/j.math.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Cheatham SW, Baker RT, Behm DG, Stull K, Kolber MJ. Mechanical percussion devices: A survey of practice patterns among healthcare professionals. Int J Sports Phys Ther. 2021;16:766–77. 10.26603/001c.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheatham SW, Baker RT, Larkins LW, Baker JG, Casanova MP. Clinical practice patterns among health care professionals for Instrument-Assisted soft tissue mobilization. J Athl Train. 2021;56:1100–11. 10.4085/1062-6050-047-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markovic G. Acute effects of instrument assisted soft tissue mobilization vs. foam rolling on knee and hip range of motion in soccer players. J Bodyw Mov Ther. 2015;19:690–6. 10.1016/j.jbmt.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Baker RT, Nasypany A, Seegmiller JG, Baker JG. Instrument-Assisted soft tissue mobilization treatment for tissue extensibility dysfunction. Int J Athletic Therapy Train. 2013;18:16–21. 10.1123/ijatt.18.5.16. [Google Scholar]
- 17.Mylonas K, Angelopoulos P, Billis E, Tsepis E, Fousekis K. Correction to: Combining targeted instrument-assisted soft tissue mobilization applications and neuromuscular exercises can correct forward head posture and improve the functionality of patients with mechanical neck pain: a randomized control study. BMC Musculoskelet Disord. 2021;22:385. 10.1186/s12891-021-04243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelopoulos P, Mylonas K, Tsepis E, Billis E, Vaitsis N, Fousekis K. The effects of Instrument-Assisted soft tissue mobilization, tissue flossing, and kinesiology taping on shoulder functional capacities in amateur athletes. J Sport Rehabil. 2021;30:1028–37. 10.1123/jsr.2020-0200. [DOI] [PubMed] [Google Scholar]
- 19.Tang S, Sheng L, Wei X, Liang M, Xia J, Chen J. The effectiveness of instrument-assisted soft tissue mobilization on pain and function in patients with musculoskeletal disorders: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2025;26:257. 10.1186/s12891-025-08492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menek MY, Menek B. Effects of percussion massage therapy, dynamic stretching, and static stretching on physical performance and balance. J Back Musculoskelet Rehabil. 2024;37:183–93. 10.3233/BMR-230069. [DOI] [PubMed] [Google Scholar]
- 21.Rittweger J. Vibration as an exercise modality: how it May work, and what its potential might be. Eur J Appl Physiol. 2010;108:877–904. 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 22.Konrad A, Glashüttner C, Reiner MM, Bernsteiner D, Tilp M. The acute effects of a percussive massage treatment with a hypervolt device on plantar flexor muscles’ range of motion and performance. J Sports Sci Med. 2020;19:690–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong WJ, Grinnell DC, Warren GS. The acute effect of whole-body vibration on the vertical jump height. J Strength Cond Res. 2010;24:2835–9. 10.1519/JSC.0b013e3181e271cc. [DOI] [PubMed] [Google Scholar]
- 24.Dueñas L, Zamora T, Lluch E, Artacho-Ramírez MA, Mayoral O, Balasch S, et al. The effect of vibration therapy on neck myofascial trigger points: A randomized controlled pilot study. Clin Biomech (Bristol Avon). 2020;78:105071. 10.1016/j.clinbiomech.2020.105071. [DOI] [PubMed] [Google Scholar]
- 25.Davis HL, Alabed S, Chico TJA. Effect of sports massage on performance and recovery: a systematic review and meta-analysis. BMJ Open Sport Exerc Med. 2020;6:e000614. 10.1136/bmjsem-2019-000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sams L, Langdown BL, Simons J, Vseteckova J. The effect of percussive therapy on musculoskeletal performance and experiences of pain: A systematic literature review. Int J Sports Phys Therapy. 2023;18:309–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheatham SW, Stull KR, Kolber MJ. Comparison of a vibration roller and a nonvibration roller intervention on knee range of motion and pressure pain threshold: A randomized controlled trial. J Sport Rehabil. 2019;28:39–45. 10.1123/jsr.2017-0164. [DOI] [PubMed] [Google Scholar]
- 28.Weremczuk MA, Kostka JS, Piekarski J, Otocka-Kmiecik A, Pikala M, Adamczewski T, et al. Upper limb muscle strength and neuromuscular coordination and other factors as determinants of kinesiophobia in people with cervical and cervicothoracic spine dysfunction. Sci Rep. 2025;15:11067. 10.1038/s41598-025-86109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbosa JC, da Luz BV, da Silva BFP, Marques AP, Saragiotto BT, Comachio J, et al. Effectiveness of telerehabilitation exercise programme on disability and pain in patients with chronic Non-Specific neck pain: randomised controlled trial Assessor-Blinded. Musculoskelet Care. 2025;23:e70119. 10.1002/msc.70119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S-K, Chen H-Y, You J-Y, Bau J-G, Lin Y-C, Kuo L-C. Outcomes of active cervical therapeutic exercise on dynamic intervertebral foramen changes in neck pain patients with disc herniation. BMC Musculoskelet Disord. 2022;23:728. 10.1186/s12891-022-05670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupuis F, Cherif A, Batcho C, Massé-Alarie H, Roy J-S. The Tampa scale of kinesiophobia: A systematic review of its psychometric properties in people with musculoskeletal pain. Clin J Pain. 2023;39:236–47. 10.1097/AJP.0000000000001104. [DOI] [PubMed] [Google Scholar]
- 32.Baker RT, Nasypany A, Seegmiller JG, Baker JG. Instrument-Assisted soft tissue mobilization treatment for tissue extensibility dysfunction. 2013. 10.1123/ijatt.18.5.16
- 33.Gulick DT. Influence of instrument assisted soft tissue treatment techniques on myofascial trigger points. J Bodyw Mov Ther. 2014;18:602–7. 10.1016/j.jbmt.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Kose G, Hepguler S, Atamaz F, Oder G. A comparison of four disability scales for Turkish patients with neck pain. J Rehabil Med. 2007;39:358–62. 10.2340/16501977-0060. [DOI] [PubMed] [Google Scholar]
- 35.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), Numeric Rating scale for pain (NRS pain), McGill pain Questionnaire (MPQ), Short-Form McGill pain Questionnaire (SF-MPQ), Chronic pain Grade scale (CPGS), Short Form-36 Bodily pain scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S240–252. 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 36.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8:1153–7. 10.1111/j.1553-2712.2001.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 37.Aslan E, Karaduman A, Yakut Y, Aras B, Simsek IE, Yaglý N. The cultural adaptation, reliability and validity of neck disability index in patients with neck pain: a Turkish version study. Spine (Phila Pa 1976). 2008;33:E362–365. 10.1097/BRS.0b013e31817144e1. [DOI] [PubMed] [Google Scholar]
- 38.MacDermid JC, Walton DM, Avery S, Blanchard A, Etruw E, McAlpine C, et al. Measurement properties of the neck disability index: a systematic review. J Orthop Sports Phys Ther. 2009;39:400–17. 10.2519/jospt.2009.2930. [DOI] [PubMed] [Google Scholar]
- 39.Vernon H, Mior S. The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–15. [PubMed] [Google Scholar]
- 40.Yilmaz ÖT, Yakut Y, Uygur F, Ulu N. Tampa Kinezyofobi Ölçeği’nin Türkçe versiyonu ve test-tekrar test güvenirliği. 2011.
- 41.Revel M, Andre-Deshays C, Minguet M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch Phys Med Rehabil. 1991;72:288–91. [PubMed] [Google Scholar]
- 42.Michiels S, De Hertogh W, Truijen S, November D, Wuyts F, Van de Heyning P. The assessment of cervical sensory motor control: a systematic review focusing on measuring methods and their clinimetric characteristics. Gait Posture. 2013;38:1–7. 10.1016/j.gaitpost.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Kristjansson E, Dall’Alba P, Jull G. A study of five cervicocephalic relocation tests in three different subject groups. Clin Rehabil. 2003;17:768–74. 10.1191/0269215503cr676oa. [DOI] [PubMed] [Google Scholar]
- 44.de Vries J, Ischebeck BK, Voogt LP, van der Geest JN, Janssen M, Frens MA, et al. Joint position sense error in people with neck pain: A systematic review. Man Ther. 2015;20:736–44. 10.1016/j.math.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Young BA, Walker MJ, Strunce JB, Boyles RE, Whitman JM, Childs JD. Responsiveness of the neck disability index in patients with mechanical neck disorders. Spine J. 2009;9:802–8. 10.1016/j.spinee.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 47.Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1:98–101. 10.1111/1467-8721.ep10768783. [Google Scholar]
- 48.Cook CE. Clinimetrics corner: the minimal clinically important change score (MCID): A necessary pretense. J Man Manip Ther. 2008;16. 10.1179/jmt.2008.16.4.82E. :E82-83. [DOI] [PMC free article] [PubMed]
- 49.Modarresi S, Lukacs MJ, Ghodrati M, Salim S, MacDermid JC, Walton DM, et al. A systematic review and synthesis of psychometric properties of the numeric pain rating scale and the visual analog scale for use in people with neck pain. Clin J Pain. 2021;38:132–48. 10.1097/AJP.0000000000000999. [DOI] [PubMed] [Google Scholar]
- 50.Kazeminasab S, Nejadghaderi SA, Amiri P, Pourfathi H, Araj-Khodaei M, Sullman MJM, et al. Neck pain: global epidemiology, trends and risk factors. BMC Musculoskelet Disord. 2022;23:26. 10.1186/s12891-021-04957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laudner K, Compton BD, McLoda TA, Walters CM. Acute effects of instrument assisted soft tissue mobilization for improving posterior shoulder range of motion in collegiate baseball players. Int J Sports Phys Ther. 2014;9:1–7. [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Sung DJ, Lee J. Therapeutic effectiveness of instrument-assisted soft tissue mobilization for soft tissue injury: mechanisms and practical application. J Exerc Rehabil. 2017;13:12–22. 10.12965/jer.1732824.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal S, Bedekar N, Shyam A, Sancheti P. Comparison between effects of instrument-assisted soft tissue mobilization and manual myofascial release on pain, range of motion and function in myofascial pain syndrome of upper trapezius - A randomized controlled trial. Hong Kong Physiother J. 2024;44:57–67. 10.1142/S1013702524500069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Portillo-Soto A, Eberman LE, Demchak TJ, Peebles C. Comparison of blood flow changes with soft tissue mobilization and massage therapy. J Altern Complement Med. 2014;20:932–6. 10.1089/acm.2014.0160. [DOI] [PubMed] [Google Scholar]
- 55.Preiss S, Taube W, Helmstädter S, Bentz L, Beinert K. Good vibes for the brain - Placebo versus real vibration in patients with chronic neck pain: A randomized cross-over study. Musculoskelet Sci Pract. 2024;74:103210. 10.1016/j.msksp.2024.103210. [DOI] [PubMed] [Google Scholar]
- 56.Needs D, Blotter J, Cowan M, Fellingham G, Johnson AW, Feland JB. Effect of localized vibration massage on popliteal blood flow. J Clin Med. 2023;12:2047. 10.3390/jcm12052047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beinert K, Preiss S, Huber M, Taube W. Cervical joint position sense in neck pain. Immediate effects of muscle vibration versus mental training interventions: a RCT. Eur J Phys Rehabil Med. 2015;51:825–32. [PubMed] [Google Scholar]
- 58.Unuvar BS, Gercek H, Aytar A, Aytar A. Immediate effects of Kinesio tape and Instrument-Assisted soft tissue mobilization on pain and proprioception in chronic neck pain: A randomized controlled trial. J Chiropr Med. 2024;23:93–101. 10.1016/j.jcm.2024.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gercek H, Unuvar BS, Umit Yemisci O, Aytar A. Acute effects of instrument assisted soft tissue mobilization technique on pain and joint position error in individuals with chronic neck pain: a double-blind, randomized controlled trial. Somatosens Mot Res. 2023;40:25–32. 10.1080/08990220.2022.2157388. [DOI] [PubMed] [Google Scholar]
- 60.Saleem F, Arshad M, Anwar S, Panaet EA, Tohănean DI, Alexe C-I, et al. Myokinetic stretching exercise versus Post-Isometric relaxation combined with traction in patients with cervical Radiculopathy-A randomized clinical trial. Life (Basel). 2025;15:721. 10.3390/life15050721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee M-Y, Kim S-G, Lee H-Y. The effect of cervical stabilization exercise on active joint position sense: A randomized controlled trial. J Back Musculoskelet Rehabil. 2016;29:85–8. 10.3233/BMR-150601. [DOI] [PubMed] [Google Scholar]
- 62.Shewail F, Abdelmajeed S, Farouk M, Abdelmegeed M. Instrument-assisted soft tissue mobilization versus myofascial release therapy in treatment of chronic neck pain: a randomized clinical trial. BMC Musculoskelet Disord. 2023;24:457. 10.1186/s12891-023-06540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cleland JA, Childs JD, McRae M, Palmer JA, Stowell T. Immediate effects of thoracic manipulation in patients with neck pain: a randomized clinical trial. Man Ther. 2005;10:127–35. 10.1016/j.math.2004.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.