Abstract
Although an important role for the mitogen-activated protein kinase (MAPK) has been established for memory consolidation in a variety of learning paradigms, it is not known if this pathway is also involved in appetitive classical conditioning. We address this question by using a single-trial food-reward conditioning paradigm in the freshwater snail Lymnaea stagnalis. This learning paradigm induces protein synthesis-dependent long-term memory formation. Inhibition of MAPK phosphorylation blocked long-term memory consolidation without affecting the sensory and motor abilities of the snails. Thirty minutes after conditioning, levels of MAPK phosphorylation were increased in extracts from the buccal and cerebral ganglia. These ganglia are involved in the generation, modulation, and plasticity of the feeding behavior. We also detected an increase in levels of MAPK phosphorylation in the peripheral tissue around the mouth of the snails where chemoreceptors are located. Although an increase in MAPK phosphorylation was shown to be essential for food-reward conditioning, it was also detected in snails that were exposed to the conditioned stimulus (CS) or the unconditioned stimulus (US) alone, suggesting that phosphorylation of MAPK is necessary but not sufficient for learning to occur.
Consolidation of long-term memory (LTM) involves signaling cascades that lead to activation of transcription and translation (Bailey et al. 1996). One of these signaling cascades is the mitogen-activated protein kinase (MAPK) signaling pathway, which has been implicated in the consolidation of LTM in a variety of learning paradigms in vertebrate and invertebrate systems (see Martin et al. 1997; Atkins et al. 1998; Berman et al. 1998; Crow et al. 1998; Selcher et al. 1999; Cammarota et al. 2000; Schafe et al. 2000; Kelly et al. 2003; Sharma et al. 2003). Although MAPK appears to play a major role in memory formation in several types of aversive classical conditioning paradigms, a role for MAPK in LTM consolidation after appetitive classical conditioning has yet to be established.
The well-described chemical conditioning of the feeding behavior of the pond snail Lymnaea stagnalis enabled us to study the role of MAPK in reward conditioning. Lymnaea can be reliably conditioned by a single pairing of a neutral chemical, amyl acetate (the conditioned stimulus [CS]) with a strong feeding stimulant, sucrose (the unconditioned stimulus [US]) (Alexander Jr. et al. 1984). In this paradigm, a single training trial induces the consolidation of a protein synthesis-dependent form of memory that can last for up to 21 d (Alexander Jr. et al. 1984; Fulton et al. 2005). The ability to study LTM formation by using a one trial learning paradigm simplifies the analysis of the temporal cascade of molecular events induced by conditioning. In addition, key molecular mechanisms of memory consolidation are conserved in the snail. For example, the transcription factors CREB and C/EBP that play an important role in transcription-dependent memory consolidation in other systems have been implicated in associative conditioning in Lymnaea (Ribeiro et al. 2003; Hatakeyama et al. 2004; Sadamoto et al. 2004). Another example is the nitric oxide-cGMP signaling pathway. This pathway has been implicated in learning and memory in a variety of learning paradigms in other species (Susswein et al. 2004) and also plays an essential role in reward learning in Lymnaea (Kemenes et al. 2002).
We show in this report that MAPK proteins can be detected in the Lymnaea central nervous system (CNS), and that MAPK activation by phosphorylation is necessary for food-reward classical conditioning. However, activation of the MAPK pathway was not restricted to snails exposed to a CS and US pairing but also occurred when the CS or the US was applied alone. In all three groups, MAPK activation was found in central ganglia containing the main interneuronal and motor circuitry for feeding (Benjamin et al. 2000) and in lip tissue containing primary chemosensory neurons (Straub et al. 2004). These results show that sensory stimulation, as well as reward classical conditioning, can cause changes in levels of phosphorylated MAPK and that learning may involve both central and peripheral activation of the MAPK signaling pathway.
Results
Detection of MAPK-like proteins in the Lymnaea's nervous system
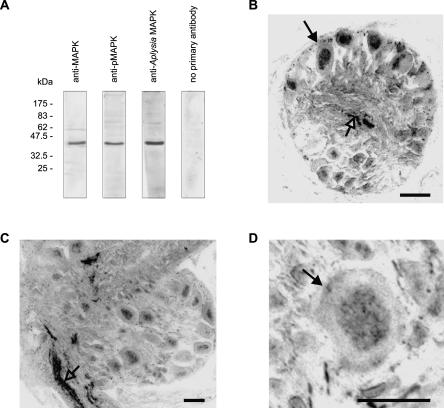
We used commercially available anti-pMAPK and anti-MAPK antibodies raised against mammalian p44 and p42 MAPK proteins for detection of MAPK-like proteins in the Lymnaea nervous system. The anti-pMAPK antibody recognizes only dually phosphorylated (activated) MAPK (pMAPK), while the anti-MAPK antibody recognizes MAPK proteins independent of their phosphorylation state (total MAPK). These antibodies have been used successfully to detect MAPK-like proteins in hemocyte protein extracts from Lymnaea (Plows et al. 2004). Consistent with the results from hemocyte extracts, although both antibodies detected two bands running very close together at ∼43 kDa in brain protein extracts, in most experiments the two bands could not be separated and migrated as a single band (Fig. 1A). We are confident that these antibodies are recognizing the Lymnaea homologs of MAPK because an antibody raised against Aplysia MAPK (Michael et al. 1998) also recognized the same band in Western blots of Lymnaea extracts (Fig. 1A).
Figure 1.
MAPK-like immunoreactivity in Lymnaea central nervous system. (A) Western blots showing immunoreactivity of anti-MAPK, anti-pMAPK, and anti-Aplysia MAPK antibodies. (B,C,D) Immunohistochemistry showing anti-pMAPK immunoreactivity in neuropile and neuronal nuclei of buccal (B) and cerebral (C,D) ganglia. (B) Section through a buccal ganglion showing neurons with nuclear staining (filled arrow) as well as some staining in the neuropile (open arrow). (C) Section through a cerebral ganglion showing staining in the nuclei of a few neurons and in a lip nerve (open arrow). (D) Section through a cerebral ganglion showing a cerebral giant cell (arrow) with nuclear staining. Bars, 50 μm.
We used the anti-pMAPK antibody in immunohistochemistry to determine the pattern of distribution of activated MAPK in the CNS. Phosphorylated MAPK was detected in the nuclei of neurons in the feeding circuitry located in the buccal ganglia (Fig. 1B) and in neurons and neuropile located in other parts of the CNS, including the lip nerves (Fig.1C). These lip nerves contain the axons of primary chemosensory neurons located in the lips and tentacles (Straub et al. 2004). Importantly, the cerebral giant cells, two symmetrical neurons that play an essential role in feeding modulation (Yeoman et al. 1994a,b) and whose gene expression is altered following food-reward conditioning (Korneev et al. 2005), showed pMAPK immunoreactivity (Fig. 1D).
MAPK phosphorylation is required for LTM formation
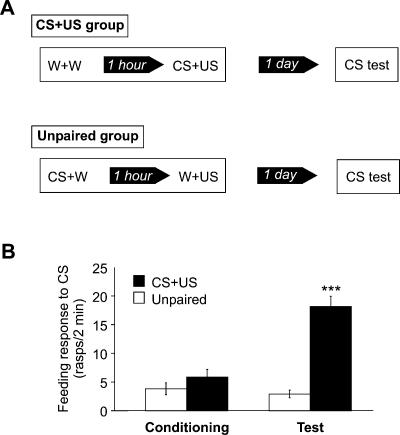
To confirm that Lymnaea is capable of associative memory consolidation after single-trial food-reward conditioning, we trained two groups of snails: a CS+US group presented with a paired presentation of amyl acetate (CS) and sucrose (US), and an unpaired group presented with the two stimuli separated by a time interval of 1 h (Fig. 2A). The feeding response of the snails (number of feeding cycles in 2 min) induced by amyl acetate was measured during training before exposure to sucrose and 1 d after training to test for LTM formation. During training, the responses of both groups were low and not significantly different (P = 0.2), whereas 1 d after training the response of the CS+US group was significantly higher than was the response of the unpaired group (P < 0.001), indicating that associative learning had occurred (Fig. 2B).
Figure 2.
Single-trial reward conditioning paradigm induces associative memory formation in Lymnaea.(A) Schematic diagram of the behavioral procedures used during food-reward classical conditioning. Two groups of snails were presented with either a single pairing of amyl acetate and sucrose (CS+US group; n = 24) or with the two stimuli presented in the same order but separated by a 1-h interval (unpaired group; n = 22). On the following day, both groups were presented again with amyl acetate. The feeding responses to amyl acetate of both groups were measured on the day of conditioning before sucrose presentation and again 1 d after. (B) A graph showing the feeding responses to amyl acetate of CS+US and unpaired group before and after conditioning. During conditioning, the responses of both groups to amyl acetate were low and not significantly different from each other (P = 0.2). In contrast, 1 d after conditioning (test), the CS+US group responded to amyl acetate with a significantly higher number of feeding movements than did the unpaired group (P < 0.001). Within-group comparisons showed that while the behavior of the CS+US group after conditioning was significantly different from its behavior before conditioning (***P < 0.001), the responses of the unpaired group on both days were not significantly different (P = 0.5).
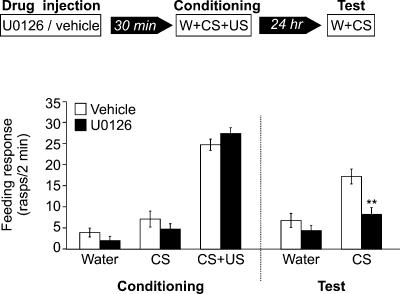
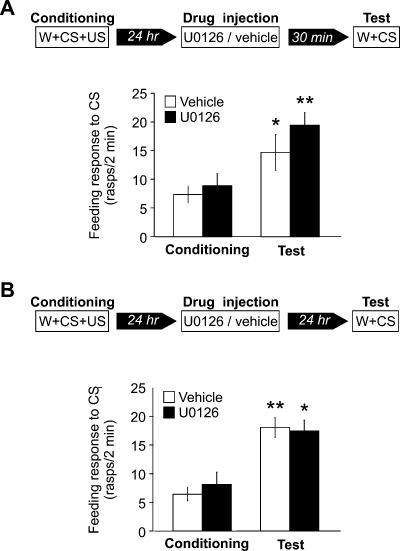
To investigate if MAPK phosphorylation is necessary for associative memory formation in Lymnaea, we used U0126, a specific inhibitor of MAPK kinase (Favata et al. 1998), to examine whether it could block the formation of the behavioral memory trace. Either vehicle or U0126 was injected into the body cavities of two groups of snails, 30 min before conditioning (Fig. 3). When tested 1 d after conditioning, the group injected with U0126 responded to amyl acetate with a significantly lower number of feeding cycles than did the vehicle-injected group (P < 0.001) (Fig. 3). The response to amyl acetate of the vehicle-injected group after conditioning was also significantly higher than was its response before conditioning (P < 0.001), indicating that learning took place. In contrast, the response to amyl acetate of the U0126-injected group after conditioning was not significantly different from its response before conditioning (P = 0.1), indicating that LTM formation was totally blocked by U0126 injection. This blocking of the memory formation does not appear to be due to the impairment of sensory or motor capabilities of the animals because the locomotor behavior of the snails after injection appeared normal and the feeding responses of vehicle- and UO126-injected groups during training were not significantly different at any point during the three-stage training procedure (water, P = 0.2; amyl acetate, P = 0.3; amyl acetate + sucrose, P = 0.2) (Fig. 3). However, this result is ambiguous for amyl acetate because during training the feeding behavior of both groups in the presence of amyl acetate was very low and not significantly different from the behavior following water presentation (vehicle, P = 0.2; U0126, P = 0.1). To rule out the possibility that amyl acetate sensory responses were blocked during the CS+US pairing and/or during the testing of the conditioned response, two further experiments were carried out. In both experiments UO126 was injected 1 d after conditioning. In the first experiment, the snails were tested 30 min after injection (Fig. 4A). During testing, both groups responded to amyl acetate with normal levels of conditioned response (Fig. 4A). No significant difference was found among the groups, showing that the sensory perception of amyl acetate was not impaired 30 min after U0126 injection. In the second experiment, the snails were tested for amyl acetate 1 d after injection, 2 d after training. Again, no significant differences were found between the groups (Fig. 4B), indicating that 1 d after U0126 injection the snails are able to perceive amyl acetate and respond to it normally with an increase in feeding rate. Taken together, these results strongly suggest that U0126 specifically impairs memory formation in Lymnaea.
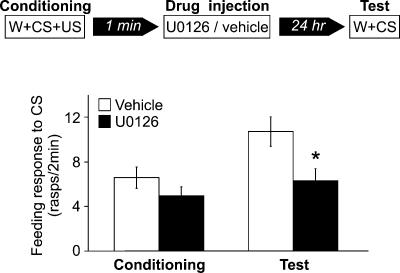
Figure 3.
Inhibition of MAPK phosphorylation blocks memory formation. Schematic diagram of experimental procedure (top) and feeding responses of two groups of snails injected with either vehicle (n = 20) or U0126, a MAPK kinase inhibitor (n = 21) (bottom). The snails were injected 30 min before conditioning, and their feeding behavior was monitored during conditioning and 1 d after conditioning during amyl acetate presentation. The groups were conditioned with a single pairing of amyl acetate and sucrose. During conditioning, the responses of both groups to water, amyl acetate, and amyl acetate mixed with sucrose, were not significantly different from each other (water, P = 0.2; amyl acetate, P = 0.3; amyl acetate + sucrose, P = 0.2). One day after conditioning, the group injected with U0126 responded to amyl acetate with a significantly lower number of feeding movements than did the vehicle-injected group. **P < 0.001.
Figure 4.
Inhibition of MAPK phosphorylation does not impair feeding related sensory and motor abilities of the snails. (A) Schematic diagram of the experimental procedure (top) and a graph showing the feeding responses to amyl acetate during conditioning and 1 d after conditioning (bottom). Snails from two groups were injected with either vehicle (n = 18) or U0126 (n = 17), 30 min before test. No significant difference was observed when the responses to amyl acetate of both groups were compared (before conditioning, P = 0.6; after conditioning, P = 0.2). However, the feeding responses to amyl acetate of both the vehicle-injected and the U0126-injected groups were significantly higher after conditioning compared with the respective responses during conditioning (vehicle, *P < 0.05; U0126, **P < 0.01), suggesting that learning occurred. (B) Schematic diagram of the experimental procedure (top) and a graph showing the feeding response to amyl acetate during conditioning and 2 d after conditioning of two groups injected 1 d after conditioning and tested 1 d after injection with either vehicle (n = 20) or U0126 (n = 19) (bottom). No significant difference was observed when the responses to amyl acetate of the two groups were compared (before conditioning, P = 0.5; after conditioning, P = 0.8). However, the feeding responses to amyl acetate of both the vehicle-injected and the U0126-injected groups were significantly higher after conditioning compared with the responses before conditioning (vehicle, **P < 0.0001; U0126, *P < 0.05), suggesting that learning occurred.
Injecting UO126 1 d after training has no effect on memory formation (Fig. 4B) unlike the injection 30 min before training (Fig. 3). This indicates that MAPK activation is required for LTM only in the early stages of memory formation either for acquisition or consolidation of the memory trace. A role in consolidation was supported by the experiment shown in Figure 5, where preventing MAPK phosphorylation immediately after CS+US pairing blocked memory formation. Two groups of snails were injected with either vehicle or U0126 immediately after conditioning. When tested 1 d after conditioning, the U0126-injected group showed a significantly lower response to amyl acetate than did the vehicle-injected group (P < 0.05) (Fig. 5).
Figure 5.
Inhibition of MAPK phosphorylation during the period of memory consolidation blocks long-term memory formation. Schematic diagram of the experimental procedure (top) and a graph showing the feeding responses to amyl acetate during conditioning and at test, 1 d after conditioning (bottom). Snails from two groups were injected immediately after conditioning with either vehicle (n = 31) or U0126 (n = 21). Before conditioning, the responses of both groups were not significantly different from each other (P = 0.2). However, 1 d after conditioning, the response of the U0126-injected group was significantly lower than was the response of the vehicle-injected group (*P < 0.05). Within-groups comparisons showed that the feeding response of the vehicle-injected group after conditioning was significantly greater than before conditioning (P < 0.01), while the responses of the U0126-injected group before and after conditioning were not significantly different from each other (P = 0.2).
It was not possible to test the effect of inhibition of MAPK phosphorylation on short-term memory formation using this conditioning paradigm due to nonspecific arousal effects caused by sucrose presentation that occur early after conditioning (M.J. Ribeiro, unpubl.).
Conditioning induces an increase in levels of MAPK phosphorylation in brain and lip tissue
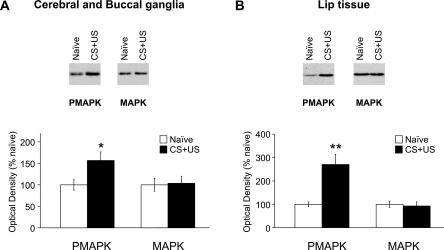
MAPK kinase inhibition impairs memory formation in Lymnaea, suggesting that conditioning induces MAPK phosphorylation. More direct evidence for this hypothesis was obtained by showing an increase in pMAPK levels 30 min after conditioning. We chose to study changes in levels of pMAPK at this early time point because we are interested in the early biochemical events that induce the formation of LTM as measured 24 h after conditioning. We used the anti-pMAPK antibody and the anti-MAPK antibody, described earlier, in Western blot analysis. We analyzed protein extracts from combined cerebral and buccal ganglia (cerebral-buccal; the feeding ganglia) and from the other ganglia of the brain combined (rest of the brain). We also analyzed protein extracts from lip tissue. The cerebral and buccal ganglia are known to be involved in the generation and modulation of the feeding behavior and are thus good candidates for brain regions involved in conditioning of feeding. Recently, conditioning-induced phosphorylation of CREB was shown to be specific for the buccal and cerebral ganglia (Ribeiro et al. 2003), and this was another important reason why we targeted these ganglia in our experiments. We chose to also analyze the lip tissue because backfilling of the lip nerves showed the existence of putative sensory neuron cell bodies in the lips (Straub et al. 2004). Analysis of this tissue is likely to give an insight into the role of the peripheral nervous system in memory formation. In this experiment, we used two groups of snails, a naive group and a conditioned CS+US group. We chose to use a naive nonstimulated control group because we wanted to compare the levels of MAPK and pMAPK of the conditioned group against an absolute base-line, and therefore we used a control group that we were sure did not learn anything. After conditioning, the two groups of snails were divided into two subgroups each: one used for Western blot analysis and the other used to test for LTM formation 24 h later. The behavioral response to the CS of the CS+US group 24 h after the single training trial was significantly higher than was the response of the naive group (CS+US: 16.8 ± 1.6 rasps/2 min; naive: 5.4 ± 1.8 rasps/2 min; P < 0.001), confirming that learning took place. The Western blot data indicated that the CS+US group had higher levels of pMAPK than did the naive group in both cerebral-buccal extracts and lip extracts, and this was confirmed statistically by densitometry (cerebral-buccal, P < 0.05; lip, P < 0.01) (Fig. 6A,B, respectively). However, the levels of pMAPK detected in extracts from the rest of the brain of the CS+US group were not significantly different from levels of the naive group (P = 0.6; data not shown). The total amount of MAPK did not differ significantly between the CS+US and naive groups (cerebral-buccal, P = 0.9; lip, P = 0.8; Fig. 6A and 6B, respectively; rest, P = 0.5; data not shown).
Figure 6.
MAPK phosphorylation levels are elevated 30 minutes after conditioning in both brain and lip tissue. (A) Western blots of cerebral-buccal extracts from naive and conditioned (CS+US) snails frozen in liquid nitrogen 30 min after conditioning. The immunoblots were probed with an anti-pMAPK antibody, stripped, and then reprobed with an anti-MAPK antibody. The corresponding densitometric analysis is shown below (n = 5). MAPK phosphorylation, but not the amount of total MAPK, is significantly increased 30 min after conditioning. *P < 0.05. (B) Western blots of lip tissue extracts from naive and conditioned (CS+US) snails frozen 30 min after conditioning. As above, the immunoblots were probed with an anti-pMAPK antibody, stripped, and then reprobed with an anti-MAPK antibody. The corresponding densitometric analysis is also shown (n = 4). Levels of MAPK phosphorylation are significantly higher 30 min after conditioning compared with naive levels. No significant difference was observed between the levels of total MAPK of conditioned and naive groups. **P < 0.01.
U0126 injection blocks conditioning-induced phosphorylation of MAPK
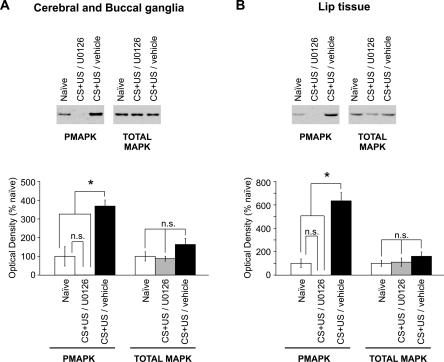
To confirm that injection of U0126 into the body cavity of the snails blocked the increase in levels of MAPK phosphorylation induced by the training procedure, we injected two groups of snails, one with U0126 and the other with vehicle, 30 min before conditioning. The snails were frozen in liquid nitrogen 30 min after conditioning. A third group of naive snails was frozen at the same time. The protein extracts from the three groups were analyzed by Western blotting using the anti-pMAPK and anti-MAPK antibodies. Snails injected with U0126 had undetectable levels of pMAPK in both cerebral-buccal extracts (Fig. 7A) and lip extracts (Fig. 7B). One-way ANOVAs of pMAPK levels revealed a source of significant difference between the three groups (cerebral-buccal: F(2,9) = 28.9, P < 0.001; lip: F(2,6) = 53.8, P < 0.001). For both types of tissue, Student-Newman-Keuls post hoc statistical analysis showed that conditioned snails injected with vehicle had significantly higher levels of pMAPK compared with that of either conditioned U0126-injected snails (P < 0.05) or naive snails (P < 0.05). These findings confirm that U0126 blocks conditioning-induced MAPK phosphorylation in Lymnaea. One-way ANOVA showed no significant differences in total MAPK levels among the naive, drug-injected, and vehicle-injected groups in cerebral-buccal extracts (F(2,9) = 2.7, P = 0.1) and lip tissue extracts (F(2,6) = 0.95, P = 0.4).
Figure 7.
Injection of U0126 blocks conditioning-induced MAPK phosphorylation in brain and lip tissues. Two groups of snails were injected with either vehicle or U0126 30 min before conditioning and frozen 30 min after conditioning. At the same time, a group of naive snails was also frozen. Levels of phosphorylated MAPK and total MAPK were monitored by Western blots. (A,B) Example of immunoblots of protein extracts from the combined cerebral and buccal ganglia (A) and lip tissue (B). The respective densitometric analysis is shown below each immunoblot example (cerebral-buccal, n = 4; lip, n = 3). In both tissues, protein extracts from the conditioned U0126-injected group contained significantly lower levels of pMAPK than did the conditioned vehicle-injected group, but levels of pMAPK of conditioned U0126-injected animals were not significantly different from naive levels. As expected, the conditioned vehicle-injected group showed significantly higher levels of pMAPK than did the naive group. No significant difference was observed in the levels of total MAPK between the three groups in both types of tissues that were analyzed (P > 0.05; *P < 0.05).
Exposure to CS or US alone increases levels of MAPK phosphorylation in brain and lip tissue
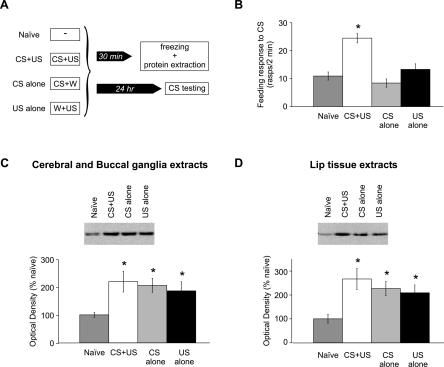
In the previous experiments, we showed that food-reward conditioning induces activation of MAPK. The next experiment was designed to establish if activation of MAPK occurred specifically after CS+US pairing or if it was due to other aspects of the training procedure such as exposure to novel stimuli (e.g., the CS) or feeding stimulation (by the US). Levels of pMAPK and total MAPK were monitored in four groups of snails: naive, CS+US, CS alone, and US alone. The CS+US group was exposed to a single paired presentation of amyl acetate and sucrose; the CS alone group was exposed to amyl acetate paired with water, and the US alone group was exposed to water paired with sucrose (Fig. 8A). Thirty minutes later, the snails were frozen in liquid nitrogen. The group of naive snails was frozen at the same time. A proportion of the snails from each group were kept alive so that the feeding responses to amyl acetate could be measured 1 d later. The behavioral results are plotted in Figure 8B. A one-way ANOVA showed a significant main effect of group (F(3,79) = 17.8; P < 0.001). Subsequent Student-Newman-Keuls post hoc comparisons revealed that the feeding response of the CS+US group was significantly higher than were the responses of the other groups (P < 0.05). The feeding responses from naive, CS alone and US alone groups did not differ significantly. These observations further emphasize the associative nature of this type of learning and are in agreement with the results obtained by Kemenes et al. (2002).
Figure 8.
Presentation of amyl acetate (the CS) alone or sucrose (the US) alone is sufficient to induce MAPK phosphorylation in the cerebral and buccal ganglia and lip tissue, although neither stimulus induces a change in the feeding response to amyl acetate. (A) Schematic diagram of the experimental procedure. The snails were divided into four groups. The naive group did not receive any stimulation. The CS+US group was presented with a single pairing of amyl acetate and sucrose. The CS alone group was presented with amyl acetate paired with water, and the US alone group was presented with water paired with sucrose. The groups were then divided into two subgroups each, one subgroup that was frozen 30 min after stimulation and another that was kept alive for behavioral testing the day after stimulation. (B) A graph showing the feeding responses to amyl acetate of the naive group (n = 21), the CS+US group (n = 21), the CS alone (n = 20), and the US alone (n = 21). *P < 0.05 relative to naive, CS alone, and US alone. (C,D) Example of immunoblot and densitometric analysis of pMAPK Western blot of cerebral-buccal extracts (C) and lip extracts (D). The levels of pMAPK of the groups CS+US (n = 10), CS alone (n = 10), and US alone (n = 10) were significantly higher than were levels of the naive group (n = 8 for cerebral-buccal extracts and n = 9 for lip extracts). There was no significant difference in pMAPK levels between any of the three stimulated groups. *P < 0.05 relative to naive.
The densitometric analyses of the pMAPK immunoblots are shown in Figure 8, C and D. In agreement with the previous results, the CS+US group had a significantly higher level of pMAPK compared with the naive level in cerebral-buccal extracts (Fig. 8C) and in lip tissue extracts (Fig. 8D). However, this increase in levels of pMAPK observed in the cerebral and buccal ganglia and lip tissue was not restricted to the CS+US group but was also detected in the CS alone and US alone groups. One-way ANOVAs of pMAPK levels revealed a significant source of difference between the groups in cerebral and buccal extracts (F(3,34) = 2.9; P < 0.05) and in lip extracts (F(3,35) = 4.4; P < 0.05). In both tissues studied, subsequent Student-Newman-Keuls post hoc comparisons revealed that the levels of pMAPK of the naive group were significantly different from levels of CS+US, CS alone, and US alone groups (P < 0.05). The levels of pMAPK of the groups CS+US, CS alone, and US alone did not differ significantly. The total MAPK levels of naive, CS+US, CS alone, and US alone groups also did not differ significantly (data not shown).
Discussion
We provide what appears to be the first evidence that MAPK is necessary for food-reward conditioning. Inhibition of MAPK activation in Lymnaea blocked conditioning without altering the sensory and motor abilities of the snails. Furthermore, we established that food-reward conditioning induced an increase in levels of activated MAPK in protein extracts from the cerebral and buccal ganglia and lip tissue around the mouth of the snails. Interestingly, activation of MAPK in these tissues was also detected after exposure to the CS alone or US alone.
The cerebral and buccal ganglia contain the central pattern generator and modulatory neurons that control the feeding behavior (Benjamin et al. 2000) and are therefore good candidates for sites of plasticity underlying conditioning of the feeding response. Furthermore, food-reward conditioning has been shown to selectively increase CREB phosphorylation in the same ganglia (Ribeiro et al. 2003). In mammalian systems, phosphorylation of CREB in neurons can occur downstream of MAPK activation (Thomas and Huganir 2004) and as these pathways seem to be highly conserved, this might also occur in Lymnaea. The lip tissue around the mouth of the snail is also a probable site of plasticity because it contains primary sensory neurons likely to be food receptors (Straub et al. 2004). Plasticity in sensory neurons plays an important role in both nonassociative and associative types of learning in Aplysia (Castellucci and Kandel 1976; Hawkins et al. 1983). The fact that in Lymnaea conditioning increases the levels of activated MAPK in the cerebral/buccal ganglia and lip tissue suggests that both the CNS and the peripheral nervous system are involved in memory formation.
Recently, by using extracellular recordings of Lymnaea lip and tentacle nerves, Straub et al. (2004) showed that the firing patterns of these sensory nerves are unaffected by food-reward conditioning. Their findings suggest that plasticity occurs at the level of synapses located in central ganglia. However, our results suggest that sensory neurons with somata in the lips undergo MAPK-dependent plasticity. These peripheral neurons project through the sensory nerves and synapse with interneurons in the cerebral ganglia (Straub et al. 2004). Therefore, activation of MAPK in the sensory neurons in the lips could lead to plasticity in the synapses between the sensory neurons and interneurons located in the cerebral ganglia. If this is the case, the firing patterns of these neurons elicited by chemical stimulation of the lips will be unaffected but the responses of their postsynaptic partners will change.
However, activation of MAPK in the lips of Lymnaea occurred not only after classical conditioning but also after amyl acetate CS or sucrose US presentation (Fig. 8D). Activation of MAPK in sensory neurons following sensory stimulation has been described before in Caenorhabditis elegans (Hirotsu et al. 2000) and in mice (Watt and Storm 2001). In both organisms, exposure to olfactory stimuli induced MAPK activation in olfactory sensory neurons. Nevertheless, in mice, the activation of MAPK induced by olfactory stimulation was unnecessary for the normal olfactory response observed in cultured sensory neurons (Watt and Storm 2001), suggesting a role in plasticity rather than olfactory perception per se. The fact that, in our study, inhibition of MAPK activation did not impair sensory perception of amyl acetate and sucrose suggests that this is also the case in Lymnaea.
Activation of MAPK in brain tissue after exposure to CS or US alone has been described before in experiments on classical conditioning. For example, control mice exposed to CS alone or US alone in conditioned taste aversion experiments (Berman et al. 1998; Swank 2000) and in contextual fear conditioning experiments (Sananbenesi et al. 2002) showed increased levels of activated MAPK compared with that of nonstimulated controls. In conditioned taste aversion, exposure to CS paired with US activated MAPK in different regions of the brain compared with exposure to CS or US alone (Swank 2000). However, in Lymnaea, our findings suggest that the main ganglia where MAPK activation is induced by CS alone or US alone are the same as where MAPK is activated due to CS+US pairing. Nevertheless, it is possible that within these ganglia, there are neurons where MAPK is activated only by CS+US pairing and not by CS or US alone. These cells would be important sites of CS/US association. Unfortunately, immunohistochemistry is not sensitive enough to detect small changes at the single cell level, and therefore, the existence of these cells could not be verified. Nevertheless, there is an alternative mechanism. It is possible that sensory stimulation alone induces MAPK activation in the same group of cells that undergo the plasticity necessary for the association between the two stimuli. Activation of MAPK by sensory stimulation might then be necessary for the animal to learn about the stimulus it just perceived. For example, animals exposed to CS alone or US alone might associate the stimulus with the visual environment where it was presented, and therefore, an associative memory might form. Activation of MAPK induced by sensory stimulation alone might thus be essential for the formation of these and other associations. In fact, in conditioned-taste aversion, MAPK activation elicited by CS presentation in the rat insular cortex is essential for the formation of LTM of the CS/US association (Berman et al. 1998). This is a well-studied example showing that molecular mechanisms triggered by the presentation of the CS alone can play a very important role in consolidation of associative memory.
As any type of sensory stimulation might be sufficient for MAPK activation, it is possible that handling alone induces MAPK activation. However, this is unlikely because activation of MAPK was only detected in the cerebral and buccal ganglia, the feeding ganglia, and not in the rest of the brain. Handling stimulation would more likely induce activation of MAPK in all parts of the brain. The selective activation of MAPK in the feeding ganglia is more likely associated with presentation of feeding stimuli, such as sucrose and amyl acetate.
MAPK can be activated by a number of different molecular pathways, and it has been suggested that in neurons MAPK might be a point of convergence integrating a wide variety of signals (Sweatt 2004). Two distinct molecular pathways that have been shown to act upstream MAPK activation in mammalian long-term potentiation, the nitric oxide-cGMP pathway and the cAMP/PKA pathway (Lu et al. 1999; Roberson et al. 1999), have also been shown to play an important role in reward conditioning in Lymnaea (Kemenes et al. 2002; G. Kemenes, unpubl.). Both these signaling pathways might act upstream of the MAPK cascade to induce long-term neuronal plasticity. Further analysis will be needed to elucidate which of these or other molecular cascades is activated specifically by the pairing of the CS and US and how these cascades interact with MAPK in the process of LTM formation.
Materials and Methods
Experimental animals
L. stagnalis were bred in our laboratory or obtained from the Free University (Amsterdam, The Netherlands). The snails were kept in groups in large holding tanks containing copper-free water at 18°C-20°C on a 12-h light/12-h dark cycle. The animals were fed lettuce three times and a vegetable-based fish food (Tetra-Phyll, TETRA Werke) twice a week.
Behavioral procedures
Reward classical conditioning was performed on individual snails by using the previously described one-trial chemical conditioning protocol (Alexander Jr. et al. 1984). A 0.004% solution of amyl acetate (CS) was paired with a 0.67% solution of sucrose (US). After a single training trial, the conditioned response is measured as the number of feeding cycles elicited by a 2-min CS presentation.
Before training, each snail was placed in an individual Petri dish containing 85 mL of water and left for 15 min to acclimatize to the new environment. Five mL of water were pipetted into the dish and the number of feeding cycles counted for 2 min to establish the level of feeding activity generated by the general disturbance caused by delivering solutions to the dish. Five milliliters of the CS were pipetted into the dish, and 2 min after, 5 mL of the US were added for 2 min (CS+US pairing), after which the snails were transferred to clean water in a holding tank. The feeding behavior of the snails was monitored throughout this 6-min training period. One day after training, prior to testing of the conditioned response with the CS, the snails were returned to individual Petri dishes containing 90 mL of clean water. Snails were allowed to acclimatize again and were then retested for a disturbance response by the addition of water, as described earlier. Five milliliters of the CS were then added to the Petri dish and the number of feeding cycles counted during the following 2 min. During both training and testing, the number of feeding cycles measured after water presentation was not significantly changed, confirming the data of Kemenes et al. (2002). This finding suggests that any differences observed in the feeding behavior after conditioning are not due to a difference in the spontaneous feeding behavior or in the behavior induced by the mechanical disturbance caused by delivering solutions to the dish.
In an initial experiment, we re-examined the ability of our snails to be trained by the one-trial chemical conditioning procedure. An unpaired group was used as the control to confirm that learning depends on the close temporal presentation of the CS and US. On the day of training, the unpaired group was exposed to amyl acetate paired with water and, 1 h after, exposed to water paired with sucrose. The CS+US (conditioned) group was exposed to amyl acetate paired with sucrose. To equalize handling between the two groups, the CS+US group was exposed to water paired with water 1 h before exposure to amyl acetate paired with sucrose (procedure summarized in Fig. 2A). The feeding response of the snails induced by amyl acetate was measured during training before exposure to sucrose and 1 d after training to test for LTM formation. During training the feeding behavior of the unpaired group was measured during amyl acetate presentation in the first part of the training, while the feeding behavior of the CS+US group was measured during amyl acetate presentation in the second part of the training. The responses of both groups during training were not significantly different from each other, suggesting that although the responses were measured in different parts of the training this did not affect the validity of the measurements. In another experiment (Fig. 8A), naive, CS alone, and US alone groups were used as controls. Here the CS+US group was exposed to amyl acetate paired with sucrose, the CS alone group was exposed to amyl acetate paired with water, and the US alone group was exposed to water paired with sucrose. The naive group was not handled or exposed to any stimulus on the day of training. Testing for amyl acetate was performed 1 d after training by using a blind protocol. In all the experiments described in this article, the snails were starved for 4 d before training and throughout the training and testing procedures.
All the behavioral data on feeding rates is expressed as mean ± standard error. The statistical analyses were performed by using parametric statistics as in previous experiments of this type (Kemenes et al. 2002; Fulton et al. 2005). When only two different groups were compared, pairwise between-group comparisons were made by using unpaired t-tests, and within-group comparisons were made by using paired t-tests. Differences between more than two groups were determined by using one-way ANOVA and subsequent Student-Newman-Keuls post hoc tests to establish differences between pairs of groups.
Application of MAPK kinase inhibitor
U0126 is a MAPK kinase inhibitor that specifically inhibits MAPK phosphorylation (Favata et al. 1998). In the experiments examining the effect of MAPK kinase inhibition, U0126 (Promega) was first dissolved in 100% methanol and, just before use, diluted in snail physiological saline (50 mM NaCl, 1.6 mM KCl, 2.0 mM MgCl2, 3.5 mM CaCl2, 10 mM Hepes at pH 7.9) (Benjamin and Winlow 1981) to a final concentration of 500 μM in 20% methanol. The solutions were kept at 43°C at all times. Injections of U0126 and vehicle (20% methanol in snail physiological saline) were performed as described previously (Kemenes et al. 2002). Two hundred microliters of U0126 or vehicle were injected into the body cavity of each snail. As the estimated volume of the hemolymph is ∼1 mL, the estimated final concentrations of U0126 and vehicle are one-sixth of the injected concentration (∼80 μM U0126 and ∼3% methanol, respectively).
Western blotting
For Western blotting studies, we used an antibody raised against Aplysia MAPK (a gift from Dr. E.R. Kandel's laboratory) (Michael et al. 1998) and two commercially available antibodies raised against mammalian p44 and p42 MAPK proteins: anti-pMAPK and anti-MAPK antibody (Cell Signaling, New England Biolabs). The anti-pMAPK antibody recognizes only dually phosphorylated (activated) MAPK (pMAPK), while the anti-MAPK antibody recognizes the MAPK proteins independent of their phosphorylation state (total MAPK).
For comparison of levels of pMAPK or total MAPK between different groups of snails, the animals were starved for 4 d before the day of training and frozen in liquid nitrogen on the fifth day with no training (naive group), 30 min after conditioning (CS+US group), 30 min after exposure to amyl acetate paired with water (CS alone group), or 30 min after exposure to water paired with sucrose (US alone group). The training procedures were as described in the previous section. Frozen snails were stored at -20°C until dissection. Each frozen snail was thawed at room temperature for 5-10 min before dissection and dissected in ice-cold snail physiological saline. The brain was carefully extracted from the snail and the cerebral and buccal ganglia immediately transferred into 50 μL of ice-cold homogenization buffer (50 mM Tris at pH 7.8, 10 mM KCl, 0.2 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 0.2 mM DTT, 2 μg/mL aprotinin, 1 μg/mL pepstatin A, 100 μg/mL PMSF, and 1 mM Benzamidine). The lips of the snail were dissected by cutting a triangle with vertices between the eyes of the snail and just below the lateral extremities of the mouth aperture. Once dissected, the lip tissue was immediately transferred into 400 μL of ice-cold homogenization buffer (same as above). For each sample, the cerebral and buccal ganglia and lip tissues from seven animals were dissected and transferred into 50 μL or 400 μL, respectively, of ice-cold homogenization buffer and homogenized at 4°C. The homogenized tissue was centrifuged at 10,000g for 10 min at 4°C. The supernatants were then frozen in liquid nitrogen and stored at -70°C. For Western blot analysis, the samples were separated on 10% SDS-polyacrylamide gels and blotted to nitrocellulose membrane. Membrane blocking and antibody incubations were as recommended by the antibody manufacturer. Enhanced chemiluminescence (Pierce) was used for signal detection, and the exposure times were adjusted so that the signals were detected in the linear range. The membranes were first probed with the anti-pMAPK antibody (1:250), stripped, and then reprobed with the anti-MAPK antibody (1:2000). The densitometry was conducted using the ImageMaster Software (Pharmacia Biotech). All the dissections, sample preparation, Western blotting, and densitometry were performed by using a blind protocol.
Statistical analyses of the optical density data were performed by using parametric methods. When only two groups were compared, the differences were determined by using unpaired t-tests. In the experiments when more than two groups were compared, differences were established by using one-way ANOVA, and where appropriate, subsequent Student-Newman-Keuls post hoc tests were performed to establish differences between pairs of groups. All the data was expressed as mean ± standard error.
Immunohistochemistry
The whole dissected CNS was fixed in 1% paraformaldehyde and 1% acetic acid in phosphate buffer (0.1 M sodium dihydrogen orthophosphate, 0.1 M disodium hydrogen orthophosphate) overnight at room temperature. The CNS was subsequently dehydrated and embedded in paraffin wax. Seven-micrometer sections were cut and mounted on microscope slides. Before immunostaining, the sections were dewaxed and rehydrated. The nonspecific binding sites were blocked with 3% BSA, 0.1% Triton X-100 in Tris-buffered saline (TBS; 0.1 M Tris-HCl at pH 7.4, 150 mM NaCl) for 1 h at room temperature. The sections were then incubated with anti-pMAPK antibody (1:50) diluted in 1% BSA and 0.1% Triton X-100 in TBS overnight at 4°C, washed three times in TBS with 0.1% Triton X-100 (10 min per wash), incubated for 1 h at room temperature in goat anti-rabbit alkaline-phosphatase-conjugated (Sigma), diluted 1:100 in 1% BSA and 0.1% Triton X-100 in TBS, and washed again three times in TBS with 0.1% Triton X-100 (10 min per wash). The color reaction was developed for 10-20 min in darkness in 50 mg/mL 5-bromo-4-chloro-3-indolyl-phosphate, 100 mg/mL nitroblue-tetrazolium chloride (both from Roche), 10 mM MgCl2 diluted in 0.2 M Tris (pH 9.5), and 0.4% Triton-X. No immunoreactivity was detected when the primary antibody was omitted (data not shown).
Acknowledgments
This work was supported by grants from the UK Biotechnology and Biological Sciences Research Council, the Portuguese Gulbenkian Foundation, Program PRAXIS XXI/Fundação para a Ciência e a Tecnologia under the Programa Gulbenkian de Doutoramento em Biologia e Medicina (M.J.R.), and the UK Medical Research Council (G.K.). We thank Dr. E.R. Kandel for his gift of the anti-Aplysia MAPK antibody.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.8305.
References
- Alexander Jr., J., Audersirk, T.E., and Audersirk, G.J. 1984. One-trial reward learning in the snail Lymnaea stagnalis. J. Neurobiol. 15: 67-72. [DOI] [PubMed] [Google Scholar]
- Atkins, C.M., Selcher, J.C., Petraitis, J.J., Trzaskos, J.M., and Sweatt, J.D. 1998. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1: 602-609. [DOI] [PubMed] [Google Scholar]
- Bailey, C.H., Bartsch, D., and Kandel, E.R. 1996. Toward a molecular definition of long-term memory storage. Proc. Natl. Acad. Sci. 93: 13445-13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin, P.R. and Winlow, W. 1981. The distribution of three wide-acting synaptic inputs to identified neurons in the isolated brain of Lymnaea. Comp. Biochem. Physiol. A 70: 293-307. [Google Scholar]
- Benjamin, P.R., Staras, K., and Kemenes, G. 2000. A systems approach to the cellular analysis of associative learning in the pond snail Lymnaea. Learn. Mem. 7: 124-131. [DOI] [PubMed] [Google Scholar]
- Berman, D.E., Hazvi, S., Rosenbum, K., Seger, R., and Dudai, Y. 1998. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J. Neurosci. 18: 10037-10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota, M., Bevilaqua, L.R., Ardenghi, P., Paratcha, G., Levi de Stein, M., Izquierdo, I., and Medina, J.H. 2000. Learning associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: Abolition by NMDA receptor blockade. Brain Res. Mol. Brain Res. 76: 36-46. [DOI] [PubMed] [Google Scholar]
- Castellucci, V. and Kandel, E.R. 1976. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science 194: 1176-1178. [DOI] [PubMed] [Google Scholar]
- Crow, T., Xue-Bian, J.-J., Siddiqi, V., Kang, T., and Neary, J.T. 1998. Phosphorylation of mitogen-activated protein kinase by one-trial and multi-trial classical conditioning. J. Neurosci. 18: 3480-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata, M.F., Horiuchi, K.Y., Manos, E.J., Daulerio, A.J., Stradley, D.A., Feeser, W.S., Van Dyk, D.E., Pitts, W.J., Earl, R.A., Hobbs, F., et al. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 173: 18623-18632. [DOI] [PubMed] [Google Scholar]
- Fulton, D., Kemenes, I., Andrew, R.J., and Benjamin, P.R. 2005. A single time-window for protein synthesis-dependent long-term memory formation after one-trial appetitive conditioning. Eur. J. Neurosci. 21: 1347-1358. [DOI] [PubMed] [Google Scholar]
- Hatakeyama, D., Sadamoto, H., and Ito, E. 2004. Real-time quantitative RT-PCR method for estimation of mRNA level of CCAAT-enhancer binding protein in the central nervous system of Lymnaea stagnalis. Acta Biol. Hung. 55: 157-161. [DOI] [PubMed] [Google Scholar]
- Hawkins, R.D., Abrams, T.W., Carew, T.J., and Kandel, E.R. 1983. A cellular mechanism of classical conditioning in Aplysia: Activity-dependent amplification of presynaptic facilitation. Science 219: 400-405. [DOI] [PubMed] [Google Scholar]
- Hirotsu, T., Saeki, S., Yamamoto, M., and Iino, Y. 2000. The Ras-MAPK pathway is important for olfaction in Caenorhabditis elegans. Nature 404: 289-293. [DOI] [PubMed] [Google Scholar]
- Kelly, A., Laroche, S., and Davis, S. 2003. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 23: 5354-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes, I., Kemenes, G., Andrew, R.J., Benjamin, P.R., and O'Shea, M. 2002. Critical time-window for NO-cGMP-dependent long-term memory formation after one-trial appetitive conditioning. J. Neurosci. 22: 1414-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneev, S.A., Starub, V., Kemenes, I., Korneeva, E.I., Ott, S.R., Benjamin, P.R., and O'Shea, M. 2005. Timed and targeted differential regulation of nitric oxide synthase (NOS) and anti-NOS genes by reward conditioning leading to long-term memory formation. J. Neurosci. 25: 1188-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y.F., Kandel, E.R., and Hawkins, R.D. 1999. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J. Neurosci. 19: 10250-10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, C.K., Michael, D., Rose, J.C., Barad, M., Casadio, A., Zhu, H., and Kandel, E.R. 1997. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron 18: 899-912. [DOI] [PubMed] [Google Scholar]
- Michael, D., Martin, K.C., Seger, R., Ning, M.M., Baston, R., and Kandel, E.R. 1998. Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia. Proc. Natl. Acad. Sci. 95: 1864-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plows, L.D., Cook, R.T., Davies, A.J., and Walker, A.J. 2004. Activation of extracellular-signal regulated kinase is required for phagocytosis by Lymnaea stagnalis haemocytes. Biochim. Biophys. Acta 1692: 25-33. [DOI] [PubMed] [Google Scholar]
- Ribeiro, M.J., Serfozo, Z., Papp, A., Kemenes, I., O'Shea, M., Yin, J.C., Benjamin, P.R. and Kemenes, G. 2003. Cyclic AMP response element-binding (CREB)-like proteins in a molluscan brain: Cellular localization and learning-induced phosphorylation. Eur. J. Neurosci. 18: 1223-1234. [DOI] [PubMed] [Google Scholar]
- Roberson, E.D., English, J.D., Adams, J.P., Selcher, J.C., Kondratick, C., and Sweatt, J.D. 1999. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J. Neurosci. 19: 4337-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadamoto, H., Sato, H., Kobayashi, S., Murakami, J., Aonuma, H., Ando, H., Fujito, Y., Hamano, K., Awaii, M., Lukowiak, K., et al. 2004. CREB in the pond snail Lymnaea stagnalis: Cloning, gene expression, and function in identifiable neurons of the central nervous system. J. Neurobiol. 58: 455-466. [DOI] [PubMed] [Google Scholar]
- Sananbenesi, F., Fischer, A., Schrick, C., Spiess, J., and Radulovic, J. 2002. Phosphorylation of hippocampal Erk-1/2, Elk-1, and p90-Rsk-1 during contextual fear conditioning: Interactions between Erk-1/2 and Elk-1. Mol. Cell. Neurosci. 21: 463-476. [DOI] [PubMed] [Google Scholar]
- Schafe, G.E., Atkins, C.M., Swank, M.W., Bauer, E.P., Sweatt, J.D., and LeDoux, J.E. 2000. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J. Neurosci. 20: 8177-8187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcher, J.C., Atkins, C.M., Trzaskos, J.M., Paylor, R., and Sweatt, J.D. 1999. A necessity for MAP kinase activation in mammalian spatial learning. Learn. Mem. 6: 478-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S.K., Sherff, C.M., Shobe, J., Bagnall, M.W., Sutton, M.A., and Carew, T.J. 2003. Differential role of mitogen-activated protein kinase in three distinct phases of memory for sensitization in Aplysia. J. Neurosci. 23: 3899-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, V.A., Styles, B.J., Ireland, J.S., O'Shea, M., and Benjamin, P.R. 2004. Central localization of plasticity involved in appetitive conditioning in Lymnaea. Learn. Mem. 11: 787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susswein, A.J., Katzoff, A., Miller, N., and Hurwitz, I. 2004. Nitric oxide and memory. Neuroscientist 10: 153-162. [DOI] [PubMed] [Google Scholar]
- Swank, M.W. 2000. Phosphorylation of MAP kinase and CREB in mouse cortex and amygdala during taste aversion learning. Neuroreport 11: 1625-1630. [DOI] [PubMed] [Google Scholar]
- Sweatt, J.D. 2004. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 14: 311-317. [DOI] [PubMed] [Google Scholar]
- Thomas, G.M. and Huganir, L. 2004. MAPK cascade signaling and synaptic plasticity. Nature Rev. Neurosci. 5: 173-183. [DOI] [PubMed] [Google Scholar]
- Watt, W.C. and Storm, D.R. 2001. Odorants stimulate the ERK/mitogen-activated protein kinase pathway and activate cAMP-response element-mediated transcription in olfactory sensory neurons. J. Biol. Chem. 276: 2047-2052. [DOI] [PubMed] [Google Scholar]
- Yeoman, M.S., Kemenes, G., Benjamin, P.R., and Elliott, C.J.H. 1994a. Modulatory role for the serotonergic cerebral giant cells in the feeding system of the snail, Lymnaea, II: Photoinactivation. J. Neurophysiol. 72: 1372-1382. [DOI] [PubMed] [Google Scholar]
- Yeoman, M.S., Vehovszky, A., Kemenes, G., Elliott, C.J.H., and Benjamin, P.R. 1994b. Modulatory role for the serotonergic cerebral giant cells in the feeding system of the snail, Lymnaea, I: Fine wire recording in the intact animal and pharmacology. J. Neurophysiol. 72: 1357-1371. [DOI] [PubMed] [Google Scholar]