Abstract
Enteroendocrine cells (EECs) are gut epithelial hormone-secreting cells, influenced by diet and the microbiome. EECs regulate local gastrointestinal functions and systemic signalling, including communication with the brain, while being linked to various diseases, yet their systemic integration remains underexplored. This review examines the metabolic and systemic roles of EECs, focusing on interactions with dietary components, the microbiome, and disease pathways, while identifying key research gaps to guide future studies.
Subject terms: Microbiology, Microbial communities
Introduction
Over the past years, enteroendocrine cells (EECs) have garnered increasing interest in research, not only for their role in the gastrointestinal (GI) tract where they reside, but also for their potential links to the central nervous system (CNS) and contribution to a wide range of disorders including inflammatory bowel diseases, metabolic and neurodegenerative diseases. This has highlighted EECs as a diverse and dynamic cell type to study. One of the primary functions of EECs is sensing and responding to nutrient intake. By producing specific hormones depending on the sensed content, EECs influence metabolic processes such as appetite regulation and digestion rate. A key facilitator of these processes is the gut-brain axis, which enables communication between the CNS and the enteric nervous system (ENS)1. Through this pathway, EECs can release hormones that regulate satiety and initiate physiological processes such as gastric emptying. These communications are complex, involving multiple neurological, immunological and endocrine factors to ensure proper functioning1.
This review will focus on the multifaceted roles of EECs in hormone regulation, their interactions with the CNS, and their response to dietary factors and the gut microbiome. It will also delve into their involvement in various (ex)-intestinal diseases and review current methodologies used to study these cells. By exploring their metabolic and systemic functions, this review aims to complement existing literature, highlight critical research gaps, and provide new perspectives for future investigations in this rapidly advancing field. For an in-depth overview of the roles EECs play in intestinal inflammation and mucosal immunity, please refer to Worthington et al.2.
Structural diversity and hormonal roles of enteroendocrine cells
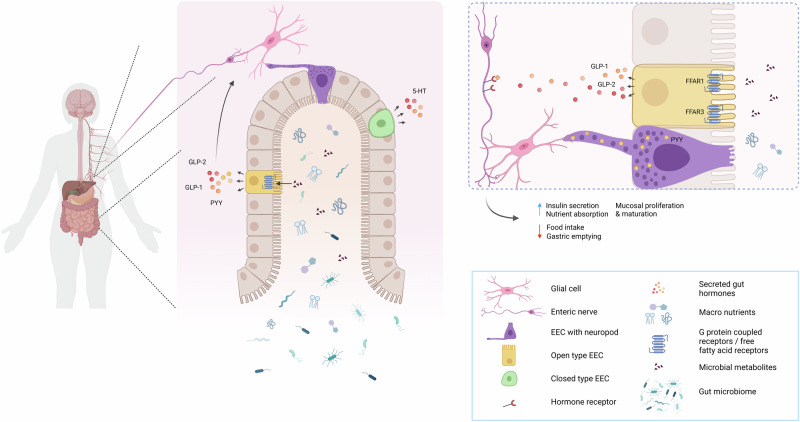
EECs are hormone-producing cells located in the epithelial layer throughout the GI tract3–6. By sensing luminal content and secreting hormones and other signalling molecules, EECs play a central role in regulating GI homeostasis and various physiological functions3,4,6. EECs are typically classified into two main types based on their structure and position: the ‘open type’, and ‘closed type’ (Fig. 1).
Fig. 1. Schematic overview of an example of EEC location and function within the gut.
The gut hormones depicted here are just an example of the many gut hormones that are secreted by EECs.
‘Open type’ EECs have microvilli that extend towards the lumen, enabling them to directly detect luminal contents. These cells are well-positioned to sense nutrients, metabolites, and other signals from the gut lumen, allowing for an immediate response, particularly in the regulation of appetite and digestion. In contrast, ‘closed type’ EECs lack microvilli and are located closer to the basal membrane, where they are activated indirectly via humoral and neural pathways5,7. This structural difference reflects the functional divergence between the two types: ‘open type’ cells are specialized in direct sensory functions, while ‘closed type’ cells likely play a greater role in integrating hormonal and neural signals from within the body to regulate more systemic functions.
Beyond their structural classification, EECs are further categorized based on the primary hormone they secrete (Table 1). For example, cells that produce serotonin (5-hydroxy-tryptamine(5-HT)), are called enterochromaffin (EC) cells and are distributed throughout the entire gut. In the stomach, EECs include histamine-producing (enterochromaffin-like (ECL)) cells, gastrin-secreting (G-) cells, somatostatin (SST)-producing D-cells, and ghrelin-producing cells (P/D1)-cells. In the duodenum, in addition to D-cells and P/D1-cells, there are secretin-producing (SCT) S-cells, glucose-dependent insulinotropic polypeptide (GIP) K-cells, motilin (M)-cells and cholecystokinin (CCK) I-cells are also present. Further down the intestine, the prevalence of EECs that produce neurotensin (NTS) N-cells and glucagon-like peptides 1 and 2 (GLP-1 and GLP-2) L-cells increases. L-cells also secrete insulin-like peptide 5 (INSL5) and peptide YY (PYY)4,6. While many of these EEC subtypes are classified as open type, a significant population in the stomach, not in direct contact with the gut lumen, is classified as closed type EECs (Fig. 1)4,5.
Table 1.
Overview of the different enteroendocrine subtypes, their location within the human body, primary secreted hormones and overall function
| Updated EEC Classification | Main location | Primary secreted hormone(s) | Function | References |
|---|---|---|---|---|
| 5-HT-positive cells (formerly EC) | Small and large intestine | Serotonin (5-HT) | Promotion of intestinal motility and secretion, mucosal immunity | 4,6,7,38 |
| Histamine-positive cells (formerly ECL) | Stomach | Histamine | Promotes gastric acid secretion | 4,6,7,38 |
| Gastrin-positive cells (formerly G) | Stomach | Gastrin | Promotes acid secretion and increases gastric motility and mucosal growth | 4,6,7,38,39 |
| Somatostatin-positive cells (formerly D) | Stomach, duodenum | Somatostatin | Inhibition of gastric acid secretion, stimulation of pancreatic exocrine functions | 4,6,7,38 |
| Ghrelin-positive cells (formerly P/D1) | Stomach, duodenum | Ghrelin | Inhibits insulin secretion, promotes food intake | 4–7,38 |
| Secretin-positive cells (formerly S) | Duodenum | Secretin | Stimulates insulin secretion, stimulates bicarbonate secretion, inhibits gastric emptying and gastric acid secretion, (pH regulation) | 4,6,7,38,152 |
| GIP-positive cells (formerly K) | Duodenum | GIP | Promotes insulin secretion | 4,6,7,38,40,41 |
| Motilin-positive cells (formerly M) | Duodenum | Motilin | Promotes gastric and intestinal motility | 4,6,7,38 |
| CCK-positive cells (formerly I) | Duodenum | CCK | Inhibition of gastric emptying, promotes pancreatic enzyme secretion, induces satiety signal | 4–7,10,38 |
| Neurotensin-positive cells (formerly N) | Small intestine | Neurotensin | Inhibits appetite and gastric motility, promotes fatty acid transport | 4,6,7,38 |
| GLP-1,2/PYY/INSL5-positive cells (formerly L) | Small and large instestine | GLP-1, GLP-2, PYY, INSL5 | Inhibition of food intake and gastric emptying, promotes insulin secretion, stimulate nutrient absorption, increases mucosal proliferation and maturation | 4–7,9,38,43,153 |
EEC enteroendocrine cell, EC enterochromaffin, ECL enterochromaffin-like, GIP glucose-dependent insulinotropic peptide, CCK cholecystokinin, GLP glucagon-like peptide, PYY peptide YY, INSL5: insulin-like peptide 5.
Neuropod formation and hormonal regulation by enteroendocrine cells
EECs are capable of forming specialized structures known as neuropods, which synapse with the nerves of the ENS, thereby creating a neuroepithelial circuit7,8. Neuropods are predominantly observed in EECs that produce PYY and CCK4,8. These hormones, along with GLP-1,-2, play crucial roles in metabolic processes and appetite regulation. Their release into the circulation is nutrient-dependent, and they act as satiety signals, reducing food intake and inhibiting gastric emptying, thereby controlling digestion rate4,9,10.
Despite being secreted by EECs, the receptors for these hormones are predominantly found in neurons, smooth muscle cells, and other tissues, rather than on the EECs themselves and their actions are mediated though binding to receptors on the afferent vagus nerve, as well as other regions of the CNS (Fig. 1)4,10–12. For example, GLP-1 and GLP-2 receptors are primarily expressed in the ENS, vagal fibres, and smooth muscle, while PYY and CCK receptors are located on neurons and smooth muscle cells in the gut. GLP-2 may also signal through specialized cells called telocytes, which have been shown to play a role in intercellular signalling and maintaining gut integrity12. These hormones are stored within secretory vesicles inside EECs. Two primary types of secretory vesicles exist: dense core vesicles (DCVs), which contain hormones and neurotransmitters and are released via the regulated secretory pathway (RSP), and constitutive vesicles (CVs), which release other proteins continuously via the constitutive secretory pathway (CSP). While the RSP requires a stimulatory signal to release its contents, the CSP operates without such signals13–15. Notably, neuropods are involved in connecting EECs to enteric glial cells (Fig. 1), as demonstrated by Bohórquez et al.16 These neuropods contain not only secretory vesicles, but also mitochondria and neurofilament structures. This suggests that neuropods may not only facilitate the transport of secretory vesicles but could be involved in the movement of other molecules necessary for proper communication within the ENS. Emerging reports also indicates that these interactions between EECs and ENS glial cells might contribute to the modulation of gut motility and local immune responses17–19, adding an extra layer of complexity to EEC-mediated gut function. This direct link between EECs and the ENS plays a pivotal role in regulating gut functions and integrating the hormonal signals with neural activity.
Enteroendocrine cells and their role in gut- nervous system communication
Enteroendocrine cells, enteric nervous system, and glial cells
The ENS spans the entire lining of the GI tract and is considered a component of the autonomic nervous system. While the ENS has the capacity to function independently of the CNS, it is intricately connected to the CNS via the vagus nerve, which facilitates continuous bidirectional communication between the two systems20,21. This communication plays a crucial role in coordinating several GI function, including peristalsis and motility (reviewed extensively in 22). The ENS consists of both neurons and glial cells, which are responsible for maintaining gut function, including immune modulation and gut-brain signalling22,23.
EECs are strategically positioned at the interface between the gut lumen and the ENS, with their specialized neuropods connecting directly to enteric glial cells16,20. This connection enables EECs to relay hormonal signals that influence both gut motility and the overall gut environment. Enteric glial cells are pivotal in maintaining intestinal homeostasis and in modulating both immunological and neurological processes. The cells detect signals from nutrient particles, microorganisms and immune cells, while also communicating directly with enteric neurons23,24, thereby orchestrating various gut responses.
Beyond their signalling role, enteric glial cells contribute to the regulation of mucosal and vascular integrity in the gut. By acting as a blood-enteric barrier, they play a protective role for enteric neurons, preventing potential damage from harmful substances in the bloodstream25,26. This protective barrier function suggests that enteric glial cells facilitate the transport of molecules between the bloodstream and the gut, further emphasizing their critical role in maintaining the structural and functional integrity of the gut.
The gut–brain axis: vagus nerve and neuroendocrine communication
Vagus nerve as a bridge between the gut and brain
The vagus nerve, the longest and most widespread autonomic nerve in the body, acts as a critical communication link between the ENS and the CNS. Originating in the brainstem, the vagus nerve innervates multiple body systems, including the heart, lungs and gut, and contains both afferent and efferent nerve fibres responsible for sensory and motor signalling27. One of its primary roles is to facilitate bidirectional communication between the gut and brain, with afferent vagal fibres transmitting information from the gut to the brain.
Within the gut, hormones such as PYY, CCK, GLP-1 and GLP-2, which are released by EECs (Fig. 1, Table 1), can bind to receptors on afferent vagus nerve fibres, influencing appetite and metabolic regulation4,10,11. This interaction is essential for regulating various physiological processes, including appetite and digestion. Research by Corp et al.28 demonstrated that CCK receptors are expressed on intestinal vagal afferent neurons, and upon activation, these receptors transmit signals to the brain via the nucleus of the solitary tract (NTS)27. In a more recent study, Kaelberer et al.29 highlighted that EECs neuropods, particularly those expressing CCK, use glutamate as a neurotransmitter to synapse directly with the vagus nerve, further facilitating gut-brain communication. This finding reveals that EECs can communicate with the CNS not only through hormone secretion but also engage in direct neurotransmission. This connection between the gut and brain via the vagus nerve underscores the importance of EECs in influencing brain function and behaviour.
The gut–brain axis and its health connections
The gut–brain axis (GBA) is a complex, bidirectional communication network, which encompasses the gut, the CNS, and several other systems including the peripheral nervous system (PNS), autonomic nervous system (ANS), ENS and the hypothalamic-pituitary-adrenal (HPA) axis30. This communication is vital for maintaining homeostasis and regulating processes such as digestion, mood, and metabolism.
One key aspect of the GBA is its regulation by the vagus nerve, which transmits signals between the gut and the brain, enabling the nervous system to respond to changes in gut function and microbiome composition (Fig. 1). When activated, the vagal afferents carry signals from the GI tract to the brain, influencing a wide range of physiological responses. Importantly, gut hormones such as PYY, CCK, GLP-1 and GLP-2, produced by EECs, contribute to this communication by acting as messengers that modulate appetite, satiety, and GI motility4,10.
Beyond the vagus nerve, the HPA axis also plays a critical role in the GBA. The HPA axis is part of the neuroendocrine system, which is activated in response to stress. When stress signals reach the gut, they can trigger the release of hormones such as cortisol, which in turn influences gut function and the overall immune response31. Notably, this pathway is also influenced by the microbiome32–34, suggesting that gut-derived signals can modulate the response of the body to stress.
One intriguing aspect of the GBA is the potential impact of gut-derived signals on mental health. Studies have shown that the gut microbiome can influence mood and cognitive function, potentially contributing to disorders like depression and anxiety. For example, a study by Homan et al. (2015) demonstrated that depletion of tryptophan, an amino acid crucial for serotonin synthesis, led to increased feelings of sadness and hopelessness in patients with remitted depression35. This highlights the potential role of tryptophan-dependent signalling in the gut-brain interaction, particularly in the context of mental health.
In this regard, EECs are of particular interest. These cells, which synthesize serotonin (5-HT) from tryptophan via the enzyme tryptophan hydroxylase 1 (TPH1), may play a significant role in regulating mood and behaviour. Research by Ye et al. (2021) found that bacterial tryptophan catabolites could activate the 5-HT pathway in EECs of zebrafish via transient receptor potential ankyrin A1 (TRPA1) receptors36. This suggests that nutrients and the gut microbiome can influence serotonin production in EECs, further linking gut health to mental health. Supporting this idea, Tian et al. (2019) found that administration of Bifidobacterium species in a stress-induced mouse model led to increased expression of TPH1 and 5-hydroxytryptophan (5HTP), a precursor of serotonin, and improved depression-like symptoms37. Given these findings, exploring the relationship between dietary molecule, the gut microbiome, EEC signalling, and brain function holds significant promise for understanding and potentially treating conditions like depression, anxiety, and other mood disorders.
Enteroendocrine cells, diet and the gut microbiome
Nutrient-driven modulation of enteroendocrine function
Dietary intake has a profound influence on EEC hormone secretion, which in turn, plays a crucial role in regulating GI homeostasis and overall metabolic function. The specific nutrient composition of a meal and eating patterns have significant effects on the secretory activity of EECs, with different dietary components eliciting varied hormonal responses5.
In the stomach, the presence of proteins, alcohol and caffeine, and even stomach distension stimulate G-cells to produce gastrin. This hormone not only promotes the secretion of pepsinogen and gastric acid but also enhances gastric motility and mucosal growth throughout the GI tract38,39. In the duodenum, K-cells produce GIP, are activated primarily by nutrient-rich meals, particularly those high in carbohydrates and fat, to secrete GIP, which in turn stimulates insulin release40,41.
Notably, recent studies have highlighted the nuanced responses of EECs seem to different types of dietary fats. For example, Thomsen et al. (1999) demonstrated that consumption of unsaturated fats, in healthy young individuals, such as those found in olive oil (unsaturated fatty acid), leads to higher expression levels and secretion of GIP and GLP-1 compared to saturated fats like butter42. These findings suggest that the type of dietary fat may modulate the endocrine function of EECs and their contribution to metabolic regulation.
Moving further along the GI tract, in the distal small intestine and colon, L-cells, are responsible for producing several important hormones, including GLP-1, GLP-2 and PYY (Fig. 1, Table 1). GLP-1 secretion is markedly enhanced by the intake of both fats and carbohydrates, while PYY production is stimulated by carbohydrates, proteins, and lipids. GLP-2 production is also influenced by nutrients such as fibres and specific fatty acids. All three hormones play a pivotal role in the inhibition of food intake and nutrient absorption and are also involved in the maintenance of glucose homeostasis and overall gut health38,43,44.
GLP-1 plays a key role in regulating glucose homeostasis by enhancing insulin secretion in response to meals, inhibiting glucagon release, and improving insulin sensitivity. Additionally, GLP-1 slows gastric emptying and promotes satiety, which contributes to its therapeutic use in managing type 2 diabetes and obesity. GLP-1 receptor agonists, such as liraglutide and semaglutide, have been developed to mimic the effects of GLP-1, improving blood glucose control and promoting weight loss45,46.
Although both are part of the glucagon-like peptide family, GLP-2 has distinct roles compared to GLP-1. GLP-2 is produced by EECs in the small intestine and plays a key role in regulating intestinal growth by promoting mucosal development, enhancing villus height, and stimulating epithelial cell proliferation, thereby maintaining intestinal integrity12. GLP-2 has therapeutic potential in conditions like short-gut syndrome (SGS), where significant portions of the intestine are lost, leading to malabsorption. Teduglutide, a GLP-2 analogue, has been shown to improve intestinal function, stimulate growth of the remaining gut tissue, and reduce the need for parenteral nutrition in SGS patients47,48.
PYY reduces food intake by promoting satiety through activation of Y2 receptors in the hypothalamus. PYY also slows gastric emptying and intestinal motility, aiding nutrient absorption. PYY secretion is stimulated by proteins, lipids, and carbohydrates. Due to its role in appetite regulation and gut function, PYY holds potential for therapeutic use in managing obesity, metabolic disorders, and improving postprandial glucose regulation49,50.
Research into sweet taste receptor signalling has also revealed its potential role in modulating EEC hormone production. A study by Gerspach et al.51 found that lactisole, a known inhibitor of sweet taste receptors, which is often used as a food additive in high sugar foods, reduced the secretion of both GLP-1 and PYY in humans, suggesting that these receptors may influence the endocrine function of EECs. Moreover, Brown et al.52 observed a synergistic effect between artificial sweeteners in diet sodas and glucose intake, resulting in enhanced GLP-1 secretion. These findings further support the idea that sweet taste receptors on EECs may contribute to regulating hormone release in response to dietary components.
In addition to influencing hormone secretion, dietary changes can also impact EECs themselves. Ye et al. (2019) demonstrated that a high-fat diet led to a phenomenon known as EEC silencing in the proximal intestine in zebrafish, in which EECs became less responsive to nutrient stimuli, such as fatty acids and glucose. This silencing was accompanied by morphological changes in EECs, with a shift from an open to a closed-type configuration. The study linked this response to endoplasmic reticulum (ER) stress and alterations in the composition of the gut microbiome. Notably, they identified a strain of Acinetobacter that could induce similar EEC silencing, further emphasizing the interplay between diet and the microbiome in modulating EEC function. These changes were reversible after a brief recovery period of 20 hours53 suggesting potential therapeutic windows for restoring EEC function in metabolic conditions. These results give interesting insights into what might also happen in metabolic diseases such as obesity and diabetes type 2 and would be very interesting for future research. Collectively, his research provides valuable insights into the mechanisms by which dietary patterns, particularly high-fat diets, can influence the functional capacity of EECs and their potential involvement in metabolic diseases such as obesity and type 2 diabetes. The interplay between diet, EEC function, and the gut microbiome presents an exciting area for future investigation, with implications for understanding the pathophysiology of metabolic disorders and developing targeted therapeutic strategies. Given the emerging role of the microbiome in mediating the effects of diet on EEC function, it is essential to explore the direct interactions between gut microbiota and EECs. As discussed in the next section, gut microbes not only influence EEC hormone secretion but also modulate EEC morphology and functionality, reinforcing the importance of microbiota in regulating gut homeostasis.
Microbial influence on enteroendocrine cells and gut hormone regulation
Building on the influence of diet on EEC function, it is crucial to consider the direct interaction between gut microbiota and EECs. EECs, particularly L-cells, not only respond to nutrients but also to luminal components, including metabolites produced by gut microbiota, which are highly shaped by the dietary composition54,55. These L-cells express various G protein-coupled receptors (GPCRs), with receptor expression patterns differing based on their anatomical location within the gut. L-cells in the proximal gut are primarily responsive to nutrients, while those in the distal gut are more likely to bind ligands produced or influenced by the gut microbiota56,57. However, metabolites such as short-chain fatty acids (SCFAs), which are produced by gut microbiota during the fermentation of dietary fibres, have a broader impact. SCFAs like acetate, propionate, and butyrate play a pivotal role in modulating both local and systemic physiological processes. They influence EECs through specific receptors, including free fatty acid receptors (FFAR2, FFAR3, and FFAR1). These receptors are expressed not only in distal gut L-cells but also in duodenal EECs. The activation of these receptors triggers the secretion of key gut hormones such GLP-1 and GLP-2 and 5-HT, which mediate important functions like bicarbonate secretion, protection of the mucosal lining from acid damage, motility, and visceral sensitivity58,59.
The influence of SCFAs on both local gut hormone release and broader systemic signalling highlights the significant role of microbiota-derived metabolites in regulating gut function, irrespective of the anatomical location of receptor expression. For example, the release of GLP-2 in response to FFAR1 and FFAR3 activation has been linked to enhanced mucosal defence and absorption, while FFAR2 activation modulates gut function through muscarinic and 5-HT4 receptor pathways, highlighting the complex signalling network involving both hormonal and neural components. Additionally, SCFAs contribute to the modulation of gut-brain communication via afferent vagal and spinal nerve pathways, linking the gut luminal environment to CNS. Thus, SCFAs and other fermentation products, by influencing enteroendocrine cells and their receptors, play a central role in maintaining gut homeostasis and contribute to the pathophysiology of disorders like functional dyspepsia and irritable bowel syndrome (IBS). Understanding these mechanisms could provide insights into therapeutic strategies targeting the gut microbiome and its fermentation products to modulate gut health and disease58,60.
Together, this highlights the essential role of the gut microbiome in regulating EEC function, a relationship supported by various studies, particularly those using mouse models60–62. Further expanding on this, research by Torres-Fuentes et al. (2019) demonstrated how gut microbiota metabolites, including SCFAs and lactate, can modulate GPCR signalling through the ghrelin receptor (GHSR-1a), a key regulator of energy balance and food intake63. Their findings suggest that microbiota-derived metabolites can attenuate ghrelin-mediated signalling, providing a novel mechanism for gut-brain communication with significant implications for metabolic regulation and potential therapeutic strategies.
Another microbiota-derived metabolite is indole, which is exclusively produced by gut bacteria from tryptophan. Chimerel et al. (2014) showed that indole affects L-cells in two distinct ways: short-term exposure acutely stimulate GLP-1 production by inhibiting K+ channels and altering the action potential of L-cells, while long-term exposure inhibits mitochondrial metabolism, leading to a reduced concentration of ATP in L-cells and subsequent reduction in GLP-1 secretion64. Another example of microbiota-driven modulation of EEC function is the study of Yano et al. (2015), who found that spore-forming microbes from both healthy human and mouse colon stimulate 5-HT production in enterochromaffin cells. This was achieved by releasing metabolites that upregulate TPH1 expression in enterochromaffin cells, thus influencing 5-HT levels in both colonic and bloodstream65.
Several commensal gut bacteria have also been identified as influencers of EEC function. Roseburia intestinalis, a butyrate-producing bacterium prevalent in the colon, significantly increased PYY secretion in L-cells when tested in a multicellular model66. Similarly, Bacteriodes thetaiotaomicron, a bacterium that resides in the colonic mucus layer, plays a key role in digesting dietary fibres and host glycans, and produces propionate, acetate and succinate67. A study by Modasia et al. (2020) found that conventionalizing germ free mice with B. thetaiotaomicron, and/or its produced SCFA metabolites, significantly restored the EEC population to a composition similar to control groups68 suggesting that metabolites from this bacterium can influence the distribution and abundance of EECs within the GI tract.
On the other hand, certain gut bacteria, such as Enterococcus faecalis, can negatively affect EEC function. E. faecalis, known for its gelatinase (GelE) secretion, alters the intestinal epithelial layer and degrade hormones like GLP-1 and PYY, potentially impairing metabolic functions69. Though direct effects of E. faecalis on EECs are still under investigation, its role in intestinal inflammation and disruption of the epithelial barrier has been demonstrated in mouse models70,71.
Akkermansia muciniphila, another gut bacterium associated with the colonic mucus layer, is one of the species influenced by dietary changes. A. muciniphila through its degradation of mucins, produces metabolites such as propionate, acetate and SCFAs, which can activate the GPCRs on L-cells56,72. Yoon et al.73 found that exposure of human L-cells and mice to A. muciniphila increased GLP-1 production through the secretion of peptide P9 by the bacterium73. The abundance of A. muciniphila is influenced by diet, with its levels increasing in response to weight loss, anorexia, or dietary fibre deprivation, and decreasing in high-fat diet models, such as those observed in type 2 diabetes and obesity72,74–79. Everard et al.76 showed that administering A. muciniphila to high-fat diet-induced mice, for four weeks, reversed type 2 diabetes and obesity in high-fat diet in mice76. In addition, A. muciniphila has been shown to positively impact L-cell numbers, further highlighting the connection between diet, microbiota, and gut homeostasis74. Overall, these findings underscore the complex interplay between diet, gut microbiota, and enteroendocrine cells, emphasizing how dietary patterns shape both microbial composition and microbial metabolites, which, in turn, modulate EEC functions and contribute to overall gut homeostasis.
Enteroendocrine cells and disease
EECs play crucial roles as chemosensors and regulators of the GI homeostasis. Given their involvement in sensing luminal contents and secreting hormones, EECs are increasingly recognized as contributors to the progression of various diseases, including IBD, IBS, metabolic diseases, neurogenerative diseases, as well as diseases of unregulated hormonal over-release like Zollinger-Ellison syndrome, a rare condition characterized by unregulated overproduction of gastrin, a hormone that stimulates the secretion of gastric acid80, and Carcinoid Syndrome, which is caused by neuroendocrine tumours, usually in the small intestine, which overproduce serotonin and other vasoactive substances81. This section elaborates on the impact of EECs in several (extra)-intestinal pathologies. An overview of these findings can also be found in Table 2.
Table 2.
Overview of the findings on how EECs and secretory products are affected in different diseases
| Disease | Findings | References |
|---|---|---|
| IBD | GLP-1 + Chromogranin A ↑, 5-HT EECs ↑, PYY ↓, Serum ghrelin ↑, EECs express IL-32, IL-32 ↑ | 83–86,154 |
| Metabolic diseases | OB: Ghrelin ↓, GLP-1 ↑ after specific diet. OB+DB2: GLP-1 ↓. DB2: CCK plasma ↓, GLP-1 vary | 81,82,86–88,91,92 |
| Schizophrenia | PPP3CA gene expression in EECs ↓ | 93,95 |
| PD | α-synuclein aggregation ↑ in EEC cell line by bacteria, GLP-1 ↓ after meal | 97,99 |
| Diseases of unregulated hormonal over-release | Gastrin ↑, 5-HT ↑, ECL ↑ | 80,81,155,156 |
EEC enteroendocrine cell, ECL enterochromaffin-like, CCK cholecystokinin, GLP glucagon-like peptide, PYY peptide YY, IL-32 Interleukin 32, 5-HT serotonin, OB Obesity, DB2 Diabetes type 2, IBD Inflammatory bowel disease, PD Parkinson's disease.
Inflammatory bowel disease and irritable bowel syndrome
Inflammatory bowel disease (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC), is characterized by chronic inflammation of the GI tract82. Emerging evidence suggests a role for EECs in the pathophysiology of IBD. Several studies have highlighted the connection between EEC activity and IBD. For example, Moran et al. (2012) observed elevated levels of GLP-1 and chromogranin A, a marker for total endocrine cells, in intestinal tissues from CD patients, which they attributed to heightened EEC activity83. Similarly, El Salhy et al. reported significant changes in gut hormone-expressing cells, with an increase in 5-HT-expressing cells in the colonic tissue of both UC and CD patients, while PYY was significantly reduced in CD patients84. These findings indicate dysregulation of EECs and their secreted hormones in IBD.
Beyond hormonal alterations, immunological changes linked to EECs have also been identified in IBD. Selleri et al. demonstrated in a human EEC line that EECs can express pro-inflammatory cytokines such as IL-32 in response to both physiological and pathological stimuli85. This observation aligns with findings by Dinarello et al., who reported elevated IL-32 expression levels in various inflammatory diseases, including CD, and found that IL-32 levels correlated with disease severity86.
ECs and their 5-HT signalling pathways may also play a pivotal role in the pathogenesis of IBS, particularly in response to early life stress (ELS)87. IBS is a common functional GI disorder, often classified as a disorder of gut-brain interaction, though its precise pathophysiology remains unclear. ELS is recognized as a major risk factor for the development of IBS, but the underlying molecular mechanisms are not fully understood. Emerging evidence suggests that an imbalance in EC-derived 5-HT signalling may contribute significantly to the development of ELS-induced IBS. In animal models mimicking ELS, such as maternal separation, there was an expansion of intestinal stem cells and their differentiation into secretory lineages, including ECs88,89. This led to EC hyperplasia, increased 5-HT production, and visceral hypersensitivity, which are indicative of the pathogenesis of IBS. ECs are also closely linked to factors like corticotropin-releasing hormone, mast cells, nerve growth factor, bile acids, and SCFAs, all of which contribute to the development of IBS87.
Metabolic diseases
Obesity is characterized by excessive fat accumulation, which is associated with increased risks of conditions like type 2 diabetes and cardiovascular disease90. Its prevalence is rising globally, with the WHO reporting that in 2022, one in eight people worldwide were affected91. Excessive food intake is considered one of the primary drivers of this obesity epidemic92,93. Given the strong association between obesity and type 2 diabetes94, the two are discussed together in this section.
Gut hormones such as ghrelin, GLP-1, PYY and CCK regulate food intake, insulin secretion and/or gastric emptying (Table 1). Disturbances in the secretion of these hormones or the activity of EECs can impair these processes, contributing to metabolic diseases such as obesity and type 2 diabetes. Ghrelin, for example, is known to stimulate appetite, with levels rising before and falling after meals95. It is of particular interest in the context of obesity to investigate whether this hormonal pathway is disrupted. Marzullo et al. observed that ghrelin levels were significantly lower in obese individuals compared to lean controls96. This finding is counterintuitive, as elevated ghrelin levels typically correlate with increased appetite. English et al. also found this discrepancy, noting that while ghrelin levels were lower in the obese group, no postprandial decrease was observed97. This suggests a potential desensitization of ghrelin secretion in response to food intake in obese individuals, possibly contributing to the chronic stimulation of appetite despite lower levels.
Furthermore, Cummings et al. demonstrated that during diet-induced weight loss, ghrelin plasma levels increased significantly, suggesting an adaptive mechanism that limits weight loss. In contrast, after gastric bypass surgery, ghrelin levels were significantly reduced, suggesting that this can be due to diminished EEC contact with nutrients, thus suppressing ghrelin production98. This reduction may contribute to the rapid weight loss seen after such surgery.
Another hormone that is involved in regulating food intake is CCK, which acts as a satiety signal. Several studies have linked polymorphisms in CCK receptors to increased food intake and obesity, indicating an impact on CCK-mediated satiety regulation99,100. Additionally, multiple studies have found lower plasma CCK levels in individuals with diabetes compared to non-diabetic controls101,102.
GLP-1, which is critical for glucose homeostasis, stimulates insulin production and inhibits glucagon production. Type 2 diabetes is primarily characterized by insulin resistance and a progressive decline in insulin secretion, leading to elevated blood glucose levels. Although clinical responses to GLP-1 vary in type 2 diabetics103, GLP-1 receptor agonists are used in the treatment of both obesity and type 2 diabetes104,105. Studies on obesity, however, have shown variable changes in the number of GLP-1-positive EECs. Aranias et al. found that high fat/low carbohydrate diets in mice and severely obese humans increased the density of GLP-1 positive EECs in the jejunum, suggesting that dietary patterns influence EEC densities106. Conversely, Osinski et al. reported a significant decrease in GLP-1 positive cells in the jejunum of obese individuals with type 2 diabetes compared to obese individuals without diabetes107. Taken together, these findings indicate that the functionality of EECs and their hormonal secretion plays a significant role in metabolic processes, potentially leading to pathological states when dysregulated.
Schizophrenia
Given that EECs use their neuropods to connect directly with enteric glial cells (Fig. 1), facilitating signalling to the ENS and eventually the CNS, it is plausible that they may play a role in neurological and psychiatric disorders. A cross-tissue transcriptome-wide association study by Uellendahl-Werth et al. (2022) identified susceptibility genes shared between IBD and schizophrenia, which were expressed in both brain and intestinal tissues. Among these genes, PPP3CA was predominantly expressed by EECs and Paneth-like cells in the intestine and by neurons in the brain108,109. PPP3CA encodes for a part of calcineurin, a protein integral to the regulation of the T-cell immune response. According to the authors, PPP3CA likely plays a dual role in signal transduction; modulating immune responses in EECs and contributing to neuronal signalling in the striatum108. This aligns with findings from other studies, such as Lin et al.110, who found that decreased predicted PPP3CA expression correlated with an increased risk of developing schizophrenia108. These findings underscore a potential role for EECs in schizophrenia through the gut-brain axis, suggesting that disruptions in EEC-associated signalling pathways could contribute to the pathophysiology of the disease.
Parkinson’s disease
PD, a neurodegenerative disorder, may also involve a significant role for EECs. A pathological hallmark of PD is Lewy bodies, which are formed by α-synuclein misfolding and aggregation111. Chandra et al. identified that EECs exhibit several neuron-like characteristics and form connections with enteric nerves. They showed that α-synuclein is expressed in the STC-1 EEC cell line, as well as in native EECs from both mouse and human intestines112. Additionally, they observed that EECs containing α-synuclein are in direct contact with α-synuclein-positive nerves, establishing a neural circuit linking the gut and the nervous system. This connection suggests that substances in the gut lumen, such as toxins and/or microbes and their products in the gut lumen, may influence α-synuclein folding in EECs, potentially initiating a pathway through which misfolded α-synuclein could spread from the gut epithelium to the brain. Indeed, Amorim Neto et al.113 demonstrated that A. muciniphila type strains could induce reactive oxygen species (ROS) production and promote α-synuclein aggregation in an EEC cell line, illustrating a potential bacterial role in PD pathogenesis. In a follow-up study114, Chandra et al. used mouse intestinal organoids expressing human α-synuclein to study the transfer of α-synuclein from epithelial cells to cocultured nodose neurons. In transgenic mice expressing human α-synuclein in the intestine, α-synuclein fibril-templating activity, the process by which misfolded α-synuclein proteins can induce other normally folded α-synuclein proteins to adopt the same misfolded structure, was detected in the intestine, vagus nerve, and dorsal motor nucleus, located in the brain stem. When the authors restricted pathological α-synuclein expression to intestinal epithelial cells in new transgenic mice, they observed fibril-templating transfer to the vagus nerve and brainstem, while vagotomy before α-synuclein induction protected the hindbrain. These findings strongly suggest that EECs could be a non-neuronal source of fibrillar α-synuclein. Nonetheless, a key question for future research is whether the disease is mainly driven by the protein traveling from the gut to the brain, or if it spreads the other way, from the brain back to the gut. Another important unanswered question is whether α-synuclein released by EECs reaches the brain by passing through the blood-brain barrier (BBB). Additionally, it is still unclear how the transfer of molecules between cells happens. It could be through general release and uptake, as reported in115–118, or through a more specific process like tunnelling nanotubes, which have been observed in neuron-to-neuron and glial cell connections but have not yet been studied in gut-to-brain signaling119–122.
Beyond α-synuclein, EECs secreted hormones such as GLP-1 may also impact PD. GLP-1 is known for its role in glucose homeostasis but can also cross the BBB and exert neuroprotective effects123. Manfready et al.124 found reduced GLP-1 secretion after food intake in PD patients compared to controls124, suggesting a potential link between GLP-1 dysregulation and PD.
To harness GLP-1’s neuroprotective properties, researchers have developed receptor agonists that prolong its half-life. Clinical trials using these agonists, originally developed for diabetes treatment, have shown promising results for PD. Treated groups exhibited reduced motor disability progression125 or even improvement126,127 compared to controls. However, a trial using NLY01, a GLP-1 receptor agonist analogue capable of crossing the BBB, did not show any improvement in PD symptoms128. For an in-depth overview, a recent review summarizes advancements in GLP-1 receptor agonists for PD treatment129. These findings highlight a novel avenue for PD treatment and suggest that EEC-derived secretory products beyond GLP-1 may also influence disease progression.
Collectively, while emerging studies point to the potential roles of EECs in the communication among the microbiome-gut-brain axis, the exact mechanisms remain poorly understood. The complexity of molecular processes, such as the ways in which EECs transfer molecules to the brain, requires further investigation. This highlights the need for more detailed research, including the development of appropriate models and methods to confirm these findings in humans. Such studies are essential to clarify the underlying mechanisms of gut-brain signalling and better understand the contribution of EECs to neurodegenerative diseases and related conditions.
Current methods used to study enteroendocrine cells
EECs have been studied using a variety of tools and models, with ongoing efforts to identify the most effective approaches. Researchers use techniques such as mass spectrometry, radioimmunoassays (RIA), and short-circuit current measurements across epithelial tissues to quantify hormone secretion and study EEC function130,131. Fluorescence Activated Cell Sorting (FACS) is another powerful tool for identifying and quantifying specific EEC subsets within mixed cell populations. By fluorescently labelling cells with markers such as PYY or GLP-1, FACS can isolate and analyse these subpopulations. It can also sort live, fluorescently labelled EECs derived from transgenic mouse models, enabling further study of specific subtypes131–133. Imaging techniques, particularly fluorescent imaging, are extensively used to visualize intracellular molecules such as calcium and the expression of hormones like PYY and CCK. Imaging also provides insights into the distribution of EEC subtypes within tissues and their morphological changes in response to treatments. This section highlights some of the commonly used methods and techniques in EEC research.
In vitro models
Several EEC cell lines, such as NCI-H716 (human), BON (human), STC-1 (mouse) and GLUTag (mouse), are widely used for EECs studies131. For example, intestinal secretin tumour cells (STC-1) are derived from mouse enteroendocrine tumours and secrete multiple gut hormones such as PYY, GLP-1 and -2, CCK and GIP, making them valuable for investigating gut hormone release134. However, a study by Kuhre et al. compared GLUTag, STC-1 and NCI-H716 cell lines with native L-cells and found that STC-1 and GLUTag cells produced more diverse peptides compared to NCI-H716. However, NCI-H716 cells secreted higher amounts of the peptides they did express. Despite this, all three cell lines exhibited limited resemblance to natural L-cells in terms of hormone expression135.
Another in vitro approach involves the use of primary cells isolated directly from tissue. Reimann et al. pioneered the use of mixed primary cultures from mouse intestinal epithelium for EEC studies, as FACS-sorted single EECs were unable to survive in culture132. Using transgenic mouse models that expressed fluorescent protein on GLP-1—and PYY-secreting EECs, researchers were able to apply advanced techniques such as electrophysiology and calcium imaging to study these cells131,132. However, a major limitation of primary cells is their short lifespan (a few days), necessitating fresh tissue samples for every experiment131.
Recently, organoid models of the GI tract have also been introduced as an innovative tool for EEC research136. Organoids are 3D cell cultures derived from stem cells that contain multiple cell types specific to the organ and are capable of self-organization137. Zeve et al. demonstrated a method for differentiating EECs within duodenal and rectal organoids derived from human intestinal stem cells. Upon stimulation with different small molecules, these EECs secreted 5-HT, CCK, GLP-1, PYY, GIP and SST6. A key advantage of organoids is their ability to undergo genetic modifications, such as gene knockouts or insertions, which make them valuable for studying specific diseases or mutations. However, a major drawback of using cell lines and stem cell-derived models, including organoids, is their complete naivety to the gut microbiome. This limitation poses significant challenges for studies investigating interactions between the gut microbiome and EECs.
Ex vivo models
Intestinal tissue samples are widely used to study hormone secretion from EECs through various ex vivo methods. One such approach is the use of Ussing chambers. In this system, a piece of tissue is mounted in a chamber that separates it into two halves. One side of the tissue is exposed to the apical environment, while the other side is exposed to the basolateral environment. This setup ensures that the solutions within the chamber are partitioned by the tissue, allowing only substances that pass through the tissue to reach the opposite side. Using this method, properties such as hormone secretion, nutrient uptake and drug transport can be analysed by measuring changes in the fluid contents or electrophysiological properties138.
An advantage of Ussing chamber system is that it requires only a small piece of tissue, making it acceptable for human studies, as samples can be obtained during routine surgical procedures. However, a significant limitation is the short viability of the tissue, which remains functional for only a few hours, greatly limiting the experimental timeframe. The Dutch organization for applied scientific research (TNO) has developed an improved alternative to the traditional Ussing chamber, known as the InTESTine™ model139. Key differences include horizontal tissue mounting, as opposed to the vertical orientation in Ussing chamber, offering better environmental control and potentially extending tissue viability.
In vivo models
Due to the before mentioned limitations in using different in vitro and ex vivo models, in vivo models are still commonly used to study EECs. Over the years, multiple studies have used a plethora of genetically altered mice to study certain aspects of EECs. A recent study by Hayashi et al. combined different reporter mouse lines to study in vivo responses of different EEC subtypes to feeding and gut motility140, giving more insight into EEC function, giving more insight in how versatile EECs are. Mellitzer et al. even generated a knockout mouse line for intestinal neurogenin 3, which is a proendocrine transcription factor responsible for the renewal of EECs, meaning that these mice do not develop EECs. This resulted in high death rates during the first week of life, and mice that did survive showed retarded growth, impaired lipid absorption and altered glucose homeostasis141. This shows how important EECs are for a healthy gut and even survival.
Besides mouse models, Drosophila and zebrafish have also been used to study EECs. Zebrafish’s intestine resembles the mammalian small intestine142 and its quick development makes it an interesting model to study the development of the ENS143, besides EEC activity36,53. Drosophila models have especially been used to study EEC molecular processes, an example of this is the study by Gao et al., which used the Drosophila model to examine how NPF/PYY modulates food intake144. For an in-depth overview, a recent review by Guo et al. compares the specification and function of EECs in Drosophila and mammals145. Caenorhabditis elegans has also been used as a model for understanding EEC metabolic functions. For example, Xia et al. showed that PARP-1 inhibition improved C. elegans survival during hyperglycaemia and significantly enhanced GLP-1 production in NCI-H716 cells, giving more insight into potential new treatments for diabetes type 2146.
Concluding remarks and future perspectives
Over the years, our understanding of EECs and their role in human health has significantly expanded. However, while much has been learned, conflicting results have emerged regarding their secreted products and their roles in diseases. Further research is essential to clarify their exact functions and to explore their potential as biomarkers or therapeutic targets, as exemplified above by GLP-1, -2 receptor agonists104,129,147,148.
Integrating cutting-edge technologies and methodologies holds immense promise for advancing EEC research. For example, coupling single-cell RNA sequencing and proteomics can uncover the heterogeneity within EEC populations, while metabolomics can provide insights into how gut-derived metabolites interact with EECs to influence systemic health. Advanced imaging techniques, such as 3D multiplex imaging149, enable spatially resolved analysis of EEC-microbiome interactions, shedding light on how these cells communicate within their native environment. The role of EECs in the neuro-enteric axis presents an exciting frontier, where the bidirectional communication with ENS and the vagus nerve could shed light on the mechanisms underlying neurodegenerative diseases, such as neurodegenerative disease, as well as mood disorders. Additionally, studying EEC contributions to metabolic regulation may reveal novel interventions for obesity, type 2 diabetes, and related conditions, particularly through their interactions with diet and microbiota-derived metabolites.
The interplay between EECs and the gut microbiome is another promising avenue. Investigating how microbial metabolites, beyond SCFAs, affect EEC function and how EEC secretions influence microbiota composition could reveal key mechanisms that connect diet, microbiota, and host physiology. Furthermore, the immune-modulatory roles of EECs in gut-associated lymphoid tissue (GALT) offer opportunities to understand their impact on inflammatory and autoimmune diseases2,150.
Emerging technologies like CRISPR/Cas9 open possibilities for precise genetic manipulation of EECs136,151, enabling studies on gene function and therapeutic engineering. Longitudinal studies tracking how EEC profiles evolve over time with aging, dietary changes, or disease progression could also identify novel biomarkers for early detection and monitoring. These insights may pave the way for personalized medicine approaches, tailoring dietary or pharmacological interventions to optimize gut health and overall well-being.
Altogether, the adage “You are what you eat” takes on new significance when considering how metabolites and diet influence not only EEC composition and secretion but also the gut microbiome, which in turn impacts EEC function53,144. Combining these aspects will provide a holistic understanding of gut physiology and aid in the development of novel biomarkers and therapies, especially those focused on dietary interventions and microbiome engineering. Through the integration of these multidisciplinary efforts, EEC research can address critical knowledge gaps, delivering innovative strategies to tackle (external)-gut-related diseases while emphasizing the central role of diet in health.
Author contributions
E.G. wrote the main manuscript text and prepared Tables 1 and 2, and Figure 1. S.E.A. edited the manuscript content. E.G. and S.E.A. revised the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carabotti, M., Scirocco, A., Antonietta Maselli, M. & Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems 28 www.annalsgastro.gr (2015). [PMC free article] [PubMed]
- 2.Worthington, J. J., Reimann, F. & Gribble, F. M. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol.11, 3–20 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Latorre, R., Sternini, C., De Giorgio, R. & Greenwood-Van Meerveld, B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol. Motil.28, 620–630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gribble, F. M. & Reimann, F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol.78, 277–299 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Gribble, F. M. & Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol.15, 226–237 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Zeve, D. et al. Robust differentiation of human enteroendocrine cells from intestinal stem cells. Nat. Commun.13, 261 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atanga, R., Singh, V. & In, J. G. Intestinal enteroendocrine cells: present and future druggable targets. Int. J. Mol. Sci.24, 8836 (2023). [DOI] [PMC free article] [PubMed]
- 8.Bohórquez, D. V. et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig.125, 782–786 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karra, E., Chandarana, K. & Batterham, R. L. The role of peptide YY in appetite regulation and obesity. J. Physiol.587, 19–25 (2009). [DOI] [PMC free article] [PubMed]
- 10.Dockray, G. J. Cholecystokinin and gut-brain signalling. Regulatory Pept.155, 6–10 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Karra, E. & Batterham, R. L. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol. Cell. Endocrinol.316, 120–128 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Kaunitz, J. D. & Akiba, Y. Control of intestinal epithelial proliferation and differentiation: the microbiome, enteroendocrine L cells, telocytes, enteric nerves, and GLP, too. Dig. Dis. Sci.64, 2709–2716 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liddle, R. A. Neuropods. CMGH7, 739–747 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loh, Y. P., Xiao, L. & Park, J. J. Trafficking of hormones and trophic factors to secretory and extracellular vesicles: a historical perspective and new hypothesis. Extracell. Vesicles Circulating Nucleic Acids4, 568–587 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park, J. J. & Loh, Y. P. How peptide hormone vesicles are transported to the secretion site for exocytosis. Mol. Endocrinol.22, 2583–2595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohórquez, D. V. et al. An enteroendocrine cell—Enteric glia connection revealed by 3D electron microscopy. PLoS ONE9, e89881 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao, M. et al. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology153, 1068–1081.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santhosh, S., Zanoletti, L., Stamp, L. A., Hao, M. M. & Matteoli, G. From diversity to disease: unravelling the role of enteric glial cells. Front. Immunol.15, 1408744 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer, N. J. & Hu, H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol.17, 338–351 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timpka, J. & Odin, P. Gastrointestinal dysfunction in Parkinson’s disease. Int. Rev. Movement Disorders1, 179–208 (2021).
- 21.Fine, R. E. In Receptors in the Evolution and Development of the Brain, pp. 183–191 (Elsevier, 2019). 10.1016/C2016-0-00013-X.
- 22.Waclawiková, B., Codutti, A., Alim, K. & El Aidy, S. Gut microbiota-motility interregulation: insights from in vivo, ex vivo and in silico studies. Gut Microbes14, 1997296 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Progatzky, F. & Pachnis, V. The role of enteric glia in intestinal immunity. Curr. Opin. Immunol.77, 102183 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seguella, L. & Gulbransen, B. D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol.18, 571–587 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savidge, T. C., Sofroniew, M. V. & Neunlist, M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab. Investig.87, 731–736 (2007). [DOI] [PubMed] [Google Scholar]
- 26.De Giorgio, R. et al. Enteric glia and neuroprotection: basic and clinical aspects. Am. J. Physiol. Gastro-intest Liver Physiol.303, 887–893 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Han, Y. et al. Vagus nerve and underlying impact on the gut microbiota-brain axis in behavior and neurodegenerative diseases. J. Inflamm. Res.15, 6213–6230 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corp, E. S., McQuade, J., Moran, T. H. & Smith, G. P. Characterization of type A and type B CCK receptor binding sites in rat vagus nerve. Brain Res.623, 161–166 (1993). [DOI] [PubMed] [Google Scholar]
- 29.Kaelberer, M. M. et al. A gut-brain neural circuit for nutrient sensory transduction. Science361, eaat5236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López-Ojeda, W. & Hurley, R. A. The vagus nerve and the brain-gut axis: implications for neuropsychiatric disorders. J. Neuropsychiatry Clin. Neurosci.36, 278–282 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Breit, S., Kupferberg, A., Rogler, G. & Hasler, G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry9, 44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bharwani, A., Mian, M. F., Surette, M. G., Bienenstock, J. & Forsythe, P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med.15, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA108, 16050–16055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt, K. et al. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl.)232, 1793–1801 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Homan, P. et al. Serotonin versus catecholamine deficiency: behavioral and neural effects of experimental depletion in remitted depression. Transl. Psychiatry5, e532 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye, L. et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe29, 179–196.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian, P., Wang, G., Zhao, J., Zhang, H. & Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutritional Biochem.66, 43–51 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Woźniak, D., Cichy, W., Przysławski, J. & Drzymała-Czyż, S. The role of microbiota and enteroendocrine cells in maintaining homeostasis in the human digestive tract. Adv. Med. Sci.66, 284–292 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Prosapio, J. G., Sankar, P. & Jialal, I. Physiology, Gastrin (StatPearls Publishing, Treasure Island (FL), 2023). [PubMed]
- 40.Holst, J. J. The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism96, 46–55 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Martin, A. M., Sun, E. W., Rogers, G. B. & Keating, D. J. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front. Physiol.10, 428 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomsen, C. et al. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am. J. Clin. Nutr.69, 1135–1143 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Posovszky, C. & Wabitsch, M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 1: Characteristics of enteroendocrine cells and their capability of weight regulation. Horm. Res. Paediatrics83, 1–10 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Amato, A., Baldassano, S. & Mulè, F. GLP2: An underestimated signal for improving glycaemic control and insulin sensitivity. J. Endocrinol.229, R57–R66 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Rakipovski, G. et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE −/− and LDLr −/− mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci.3, 844–857 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knudsen, L. B. & Lau, J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol.10, 155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burness, C. B. & McCormack, P. L. Teduglutide: a review of its use in the treatment of patients with short bowel syndrome. Drugs73, 935–947 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Sabra, H. K. et al. Efficacy and safety of glucagon-like peptide 2 in patients with short bowel syndrome: a systematic review and network meta-analysis. J. Gastrointest. Surg.28, 1194–1205 (2024). [DOI] [PubMed] [Google Scholar]
- 49.Oertel, M. et al. GLP-1 and PYY for the treatment of obesity: a pilot study on the use of agonists and antagonists in diet-induced rats. Endocr. Connect13, e230398 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guida, C. et al. PYY plays a key role in the resolution of diabetes following bariatric surgery in humans. EBioMedicine40, 67–76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerspach, A. C., Steinert, R. E., Schönenberger, L., Graber-Maier, A. & Beglinger, C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am. J. Physiol. Endocrinol. Metab.301, 317–325 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Brown, R. J., Walter, M. & Rother, K. I. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care32, 2184–2186 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye, L. et al. High fat diet induces microbiota-dependent silencing of enteroendocrine cells. Elife8, e48479 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohue-Kitano, R., Banno, Y., Masujima, Y. & Kimura, I. Gut microbial metabolites reveal diet-dependent metabolic changes induced by nicotine administration. Sci. Rep.14, 1056 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Procházková, N. et al. Gut physiology and environment explain variations in human gut microbiome composition and metabolism. Nat. Microbiol9, 3210–3225 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cani, P. D., Everard, A. & Duparc, T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharmacol.13, 935–940 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Arora, T., Vanslette, A. M., Hjorth, S. A. & Bäckhed, F. Microbial regulation of enteroendocrine cells. Med2, 553–570 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Akiba, Y. et al. Short-chain fatty acid sensing in rat duodenum. J. Physiol.593, 585–599 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes61, 364–371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nøhr, M. K. et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology154, 3552–3564 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Samuel, B. S. et al. Effects of the Gut Microbiota on Host Adiposity Are Modulated by the Short-Chain Fatty-Acid Binding G Protein-Coupled Receptor, Gpr41. www.pnas.org/cgi/content/full/ (2008). [DOI] [PMC free article] [PubMed]
- 62.Psichas, A. et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J. Obes.39, 424–429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torres-Fuentes, C. et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J.33, 13546–13559 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Chimerel, C. et al. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep.9, 1202–1208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell161, 264–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gautier, T. et al. Roseburia intestinalis modulates PYY expression in a new a multicellular model including enteroendocrine cells. Microorganisms10, 2263 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Porter, N. T., Luis, A. S. & Martens, E. C. Bacteroides thetaiotaomicron. Trends Microbiol.26, 966–967 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Modasia, A. et al. Regulation of enteroendocrine cell networks by the major human gut symbiont bacteroides thetaiotaomicron. Front Microbiol11, 575595 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LeValley, S. L., Tomaro-Duchesneau, C. & Britton, R. A. Degradation of the incretin hormone glucagon-like peptide-1 (GLP-1) by enterococcus faecalis metalloprotease GelE. mSphere5, e00585–19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steck, N. et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology141, 959–971 (2011). [DOI] [PubMed] [Google Scholar]
- 71.Maharshak, N. et al. Enterococcus faecalis gelatinase mediates intestinal permeability via protease-activated receptor 2. Infect. Immun.83, 2762–2770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ottman, N., Geerlings, S. Y., Aalvink, S., de Vos, W. M. & Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best. Pract. Res.: Clin. Gastroenterol.31, 637–642 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Yoon, H. S. et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol.6, 563–573 (2021). [DOI] [PubMed] [Google Scholar]
- 74.Everard, A. et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes60, 2775–2786 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim, E. J. et al. High levels of akkermansia muciniphilia growth associated with spring water ingestion prevents obesity and hyperglycemia in a high-fat diet-induced mouse model. Nat. Prod. Commun.17 (2022).
- 76.Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA110, 9066–9071 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Louis, S., Tappu, R. M., Damms-Machado, A., Huson, D. H. & Bischoff, S. C. Characterization of the gut microbial community of obese patients following a weight-loss intervention using whole metagenome shotgun sequencing. PLoS ONE11, e0149564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mack, I. et al. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep.6, 26742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell167, 1339–1353.e21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chatzipanagiotou, O. et al. All you need to know about gastrinoma today|Gastrinoma and Zollinger-Ellison syndrome: a thorough update. J. Neuroendocrinol.35, e13267 (2023). [DOI] [PubMed]
- 81.George, J., Ramage, J., White, B. & Srirajaskanthan, R. The role of serotonin inhibition within the treatment of carcinoid syndrome. Endocrine Oncol.3, e220077 (2023). [DOI] [PMC free article] [PubMed]
- 82.Yu, Y., Yang, W., Li, Y. & Cong, Y. Enteroendocrine cells: sensing gut microbiota and regulating inflammatory bowel diseases. Inflamm. Bowel Dis.26, 11–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moran, G. W., Pennock, J. & McLaughlin, J. T. Enteroendocrine cells in terminal ileal Crohn’s disease. J. Crohns Colitis6, 871–880 (2012). [DOI] [PubMed] [Google Scholar]
- 84.El-Salhy, M., Danielsson, Å., Stenling, R. & Grimelius, L. Colonic endocrine cells in inflammatory bowel disease. J. Intern. Med.242, 413–419 (1997). [DOI] [PubMed] [Google Scholar]
- 85.Selleri, S. et al. Induction of pro-inflammatory programs in enteroendocrine cells by the Toll-like receptor agonists flagellin and bacterial LPS. Int. Immunol.20, 961–970 (2008). [DOI] [PubMed] [Google Scholar]
- 86.Dinarello, C. A. & Kim, S.-H. IL-32, a novel cytokine with a possible role in disease. Ann. Rheum. Dis.65, iii61 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tao, E. et al. Potential roles of enterochromaffin cells in early life stress-induced irritable bowel syndrome. Front. Cell. Neurosci.16, 837166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chow, C. F. W. et al. From psychology to physicality: how nerve growth factor transduces early life stress into gastrointestinal motility disorders later in life. Cell Cycle18, 1824–1829 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong, H. L. X. et al. Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling. Nat. Commun.10, 1745 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heymsfield, S. B. & Wadden, T. A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med.376, 254–266 (2017). [DOI] [PubMed] [Google Scholar]
- 91.WHO. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2024).
- 92.Jeffery, R. W. & Harnack, L. J. Evidence implicating eating as a primary driver for the obesity epidemic. Diabetes56, 2673–2676 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Vandevijvere, S., Chow, C. C., Hall, K. D., Umali, E. & Swinburn, B. A. Increased food energy supply as a major driver of the obesity epidemic: a global analysis. Bull. World Health Organ93, 446–456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klein, S., Gastaldelli, A., Yki-Järvinen, H. & Scherer, P. E. Why does obesity cause diabetes? Cell Metab.34, 11–20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steinert, R. E. et al. Ghrelin, CCK, GLP-1, and PYY(3-36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev.97, 411–463 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marzullo, P. et al. The relationship between active ghrelin levels and human obesity involves alterations in resting energy expenditure. J. Clin. Endocrinol. Metab.89, 936–939 (2004). [DOI] [PubMed] [Google Scholar]
- 97.English, P. J., Ghatei, M. A., Malik, I. A., Bloom, S. R. & Wilding, J. P. H. Food fails to suppress ghrelin levels in obese humans. J. Clin. Endocrinol. Metab.87, 2984–2987 (2002). [DOI] [PubMed] [Google Scholar]
- 98.Cummings, D. E. et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med.346, 1623–1630 (2002). [DOI] [PubMed] [Google Scholar]
- 99.De Krom, M. et al. Common genetic variations in CCK, leptin, and leptin receptor genes are associated with specific human eating patterns. Diabetes56, 276–280 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Marchal-Victorion, S. et al. Genetic, pharmacological and functional analysis of cholecystokinin-1 and cholecystokinin-2 receptor polymorphism in type 2 diabetes and obese patients. Pharmacogenetics12, 23–30 (2002). [DOI] [PubMed] [Google Scholar]
- 101.Rushakoff, R. A. et al. Reduced postprandial cholecystokinin (CCK) secretion in patients with noninsulin-dependent diabetes mellitus: evidence for a role for CCK in regulating postprandial hyperglycemia. J. Clin. Endocrinol. Metab.76, 489–493 (1993). [DOI] [PubMed] [Google Scholar]
- 102.Bucceri, A. M., Calogero, A. E. & Brogna, A. Gallbladder and gastric emptying: relationship to cholecystokininemia in diabetics. Eur. J. Intern. Med. 13, 123–128 (2002). [DOI] [PubMed]
- 103.Calanna, S. et al. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: Systematic review and meta-analyses of clinical studies. Diabetologia56, 965–972 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Collins, L. & Costello, R. A. Glucagon-like peptide-1 receptor agonists. StatPearlshttps://www.ncbi.nlm.nih.gov/books/NBK551568/ (2024). [PubMed]
- 105.Unger, J. & Parkin, C. G. Type 2 diabetes: an expanded view of pathophysiology and therapy. Postgrad. Med.122, 145–157 (2010). [DOI] [PubMed] [Google Scholar]
- 106.Aranias, T. et al. Lipid-rich diet enhances L-cell density in obese subjects and in mice through improved L-cell differentiation. J. Nutr. Sci.4, e22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Osinski, C. et al. Type 2 diabetes is associated with impaired jejunal enteroendocrine GLP-1 cell lineage in human obesity. Int J. Obes.45, 170–183 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uellendahl-Werth, F. et al. Cross-tissue transcriptome-wide association studies identify susceptibility genes shared between schizophrenia and inflammatory bowel disease. Commun. Biol.5, 80 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu, L. & Li, Y. Involvement of intestinal enteroendocrine cells in neurological and psychiatric disorders. Biomedicines10, 2577 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin, Y., Sun, I. W., Liu, S. I., Loh, E. W. & Lin, Y. C. Tacrolimus ointment-induced relapse of schizophrenia: a case report. Int. J. Neuropsychopharmacol.10, 851–854 (2007). [DOI] [PubMed] [Google Scholar]
- 111.Angot, E. & Brundin, P. Dissecting the potential molecular mechanisms underlying α-synuclein cell-to-cell transfer in Parkinson’s disease. Parkinsonism Relat. Disord.15, S143–S147 (2009). [DOI] [PubMed] [Google Scholar]
- 112.Chandra, R., Hiniker, A., Kuo, Y. M., Nussbaum, R. L. & Liddle, R. A. α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight2, e92295 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amorim Neto, D. P. et al. Akkermansia muciniphila induces mitochondrial calcium overload and α-synuclein aggregation in an enteroendocrine cell line. iScience25, 103908 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chandra, R. et al. Gut mucosal cells transfer α-synuclein to the vagus nerve. JCI Insight8, e172192 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gustafsson, G. et al. Secretion and uptake of α-synuclein via extracellular vesicles in cultured cells. Cell Mol. Neurobiol.38, 1539–1550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xie, Y. X. et al. Lysosomal exocytosis releases pathogenic α-synuclein species from neurons in synucleinopathy models. Nat. Commun.13, 4918 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee, H. J., Patel, S. & Lee, S. J. Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci.25, 6016–6024 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Emmanouilidou, E. & Vekrellis, K. Exocytosis and spreading of normal and aberrant α-synuclein. Brain Pathol.26, 398–403 (2016). [DOI] [PMC free article] [PubMed]
- 119.Abounit, S. et al. Tunneling nanotubes spread fibrillar α‐synuclein by intercellular trafficking of lysosomes. EMBO J.35, 2120–2138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dieriks, B. V. et al. α-synuclein transfer through tunneling nanotubes occurs in SH-SY5Y cells and primary brain pericytes from Parkinson’s disease patients. Sci. Rep.7, 42984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rostami, J. et al. Human astrocytes transfer aggregated alpha-synuclein via tunneling nanotubes. J. Neurosci.37, 11835–11853 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scheiblich, H. et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell184, 5089–5106.e21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Athauda, D. & Foltynie, T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov. Today21, 802–818 (2016). [DOI] [PubMed] [Google Scholar]
- 124.Manfready, R. A. et al. Attenuated postprandial GLP-1 response in Parkinson’s disease. Front. Neurosci.15, 660942 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meissner, W. G. et al. Trial of Lixisenatide in early Parkinson’s disease. N. Engl. J. Med.390, 1176–1185 (2024). [DOI] [PubMed] [Google Scholar]
- 126.Aviles-Olmos, I. et al. Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Investig.123, 2730–2736 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Athauda, D. et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet390, 1664–1675 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McGarry, A. et al. Safety, Tolerability, and Efficacy of NLY01 in Early Untreated Parkinson’s Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Articles Lancet Neurol 23 www.thelancet.com/neurology (2024). [DOI] [PubMed]
- 129.Kalinderi, K., Papaliagkas, V. & Fidani, L. GLP-1 receptor agonists: a new treatment in Parkinson’s disease. Int. J. Mol. Sci.25, 3812 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cox, H. M. et al. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab.11, 532–542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goldspink, D. A., Reimann, F. & Gribble, F. M. Models and tools for studying enteroendocrine cells. Endocrinology159, 3874–3884 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reimann, F. et al. Glucose sensing in L cells: a primary cell study. Cell Metab.8, 532–539 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Habib, A. M. et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology153, 3054–3065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Verhoeckx, K. et al. The impact of food bioactives on health: In Vitro and Ex Vivo Models [Internet]. Cham (CH): Springer; 2015. 10.1007/978-3-319-16104-4. [PubMed]
- 135.Kuhre, R. E. et al. Peptide production and secretion in GLUTag, NCI-H716, and STC-1 cells: A comparison to native L-cells. J. Mol. Endocrinol.56, 201–211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martinez-Silgado, A. et al. Differentiation and CRISPR-Cas9-mediated genetic engineering of human intestinal organoids. STAR Protoc.3, 101639 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clevers, H. Modeling development and disease with organoids. Cell165, 1586–1597 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Clarke, L. L. A guide to ussing chamber studies of mouse intestine Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol.296, 1151–1166 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Westerhout, J. et al. A new approach to predict human intestinal absorption using porcine intestinal tissue and biorelevant matrices. Eur. J. Pharm. Sci.63, 167–177 (2014). [DOI] [PubMed] [Google Scholar]
- 140.Hayashi, M. et al. Enteroendocrine cell lineages that differentially control feeding and gut motility. Elife12, e78512 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mellitzer, G. et al. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J. Clin. Investig.120, 1708–1721 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wallace, K. N., Akhter, S., Smith, E. M., Lorent, K. & Pack, M. Intestinal growth and differentiation in zebrafish. Mech. Dev.122, 157–173 (2005). [DOI] [PubMed] [Google Scholar]
- 143.Kuil, L. E., Chauhan, R. K., Cheng, W. W., Hofstra, R. M. W. & Alves, M. M. Zebrafish: A Model Organism for Studying Enteric Nervous System Development and Disease. Front. Cell Dev. Biol.8, 629073 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gao, J. et al. Dietary L-Glu sensing by enteroendocrine cells adjusts food intake via modulating gut PYY/NPF secretion. Nat. Commun.15, 3514 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo, X., Lv, J. & Xi, R. The specification and function of enteroendocrine cells in Drosophila and mammals: a comparative review. FEBS J.289, 4773–4796 (2022). [DOI] [PubMed] [Google Scholar]
- 146.Xia, Q. et al. PARP-1 inhibition rescues short lifespan in hyperglycemic C. elegans and improves GLP-1 secretion in human cells. Aging Dis.9, 17–30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gadgaard, S. et al. Long-acting agonists of human and rodent GLP-2 receptors for studies of the physiology and pharmacological potential of the GLP-2 system. Biomed. Pharmacother.160, 114383 (2023). [DOI] [PubMed] [Google Scholar]
- 148.Reiner, J. et al. Dapiglutide, a novel dual GLP-1 and GLP-2 receptor agonist, attenuates intestinal insufficiency in a murine model of short bowel. J. Parenter. Enter. Nutr.46, 1107–1118 (2022). [DOI] [PubMed] [Google Scholar]
- 149.Cho, W., Kim, S. & Park, Y. G. Towards multiplexed immunofluorescence of 3D tissues. Mol. Brain16, 37 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Diefenbach, A., Gnafakis, S. & Shomrat, O. Innate lymphoid cell-epithelial cell modules sustain intestinal homeostasis. Immunity52, 452–463 (2020). [DOI] [PubMed] [Google Scholar]
- 151.Beumer, J. et al. Mapping prohormone processing by proteases in human enteroendocrine cells using genetically engineered organoid models. Proc. Natl Acad. Sci .USA119, e2212057119 (2022). [DOI] [PMC free article] [PubMed]
- 152.Afroze, S. et al. The physiological roles of secretin and its receptor. Ann. Transl. Med1, 29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Markovic, M. A. & Brubaker, P. L. The roles of glucagon-like peptide-2 and the intestinal epithelial insulin-like growth factor-1 receptor in regulating microvillus length. Sci. Rep.9 (2019). [DOI] [PMC free article] [PubMed]
- 154.Cekic, C. et al. Evaluation of the relationship between serum ghrelin, C-reactive protein and interleukin-6 levels, and disease activity in inflammatory bowel diseases. Hepatogastroenterology61, 1196–1200 (2014). [PubMed] [Google Scholar]
- 155.Epelboym, I. & Mazeh, H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist19, 44–50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Modlin, I. M., Lye, K. D. & Kidd, M. Carcinoid tumors of the stomach. Surg. Oncol.12, 153–172 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.