Abstract
Background
Stroke is a leading cause of mortality and disability in Belgium and worldwide. Increasing evidence highlights air pollution as a significant stroke risk factor. Despite efforts in the past decade to mitigate air pollution in Belgium, a considerable part of the population remains exposed to concentrations exceeding the World Health Organization (WHO) Air Quality Guidelines. Therefore, quantifying the effectiveness of further pollution reduction interventions is crucial in supporting policymaking. This study applies a g-computation approach to assess the benefits of hypothetical air pollution reduction scenarios on stroke prevalence in Belgium within a multi-exposure context.
Methods
Belgian health interview survey data (2008/2013/2018, n = 27536) were linked to environmental data at the participant’s residential address. Missing data and bias related to self-reported covariates were addressed based on data from the 2018 Belgian health examination survey and a random-forest multiple imputation. A g-computation approach was used to calculate the potential impact fractions of air pollution reduction scenarios on stroke prevalence in Belgium, with regression models adjusted for socio-demographic, environmental, and lifestyle factors. Scenarios included lowering annual exposure to fine particulate matter (PM2.5) and nitrogen dioxide levels (NO2) to levels recommended by WHO, and assessing dose-response effects of reducing exposure to PM2.5, NO2, and black carbon (BC) by 20–80%.
Results
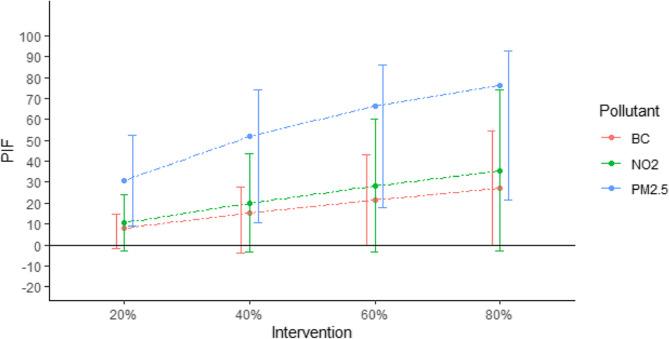
Stroke was significantly associated with PM2.5, while associations with NO2 and BC were borderline significant. Meeting WHO air quality guidelines for PM2.5 and NO2 would reduce stroke risk by 0.88% (SE: 0.24) and 0.33% (SE: 0.19), preventing 67% and 25% of stroke cases in Belgium, respectively. Results reveal a dose-response association between air pollution reduction and stroke prevalence. Reduction in air pollution exposure, ranging from 20% to 80% showed increasing potential impact fractions for stroke: PM2.5 (29%, 48%, 61%, 69%), NO2 (10%, 18%, 25%, 31%) and BC (8%, 14%, 20%, 23%).
Conclusion
This study highlights the importance of air pollution on the stroke burden and demonstrates that air pollution reduction interventions could significantly decrease the prevalence of stroke in Belgium. The g-computation approach represents a straightforward approach in epidemiology for making causal inference from observational data while also providing useful information for policymakers.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12874-025-02661-8.
Keywords: Cardiovascular diseases, Ambient air pollutants, Causal inference, Potential impact fractions, Health policy, Health impact assessment
Background
By affecting approximately 17,000 people every year, stroke is the second most common cause of death and the leading cause of disability in adults in Belgium [1]. In 2019, Belgium had an estimated 138,000 stroke survivors, reflecting a substantial impact on society, in terms of both direct healthcare costs and indirect costs, such as loss of productivity [2]. Stroke is a type of cerebrovascular disease that occurs when a part of the brain is deprived of oxygen due to a blockage in the blood supply [3]. The majority of strokes are ischemic, resulting from blood clots, whereas hemorrhagic strokes arise from burst blood vessels [4]. The risk factors of stroke include non-modifiable factors - like race and age - and modifiable factors - such as hypertension, obesity, diabetes, smoking, sedentary, and environmental pollutants [5–7]. To mitigate the impact of stroke in Belgium, the reduction of modifiable risk factors plays a crucial role. In the last decades, it has become clear that ambient exposure to air pollution is a significant risk factor for stroke [5, 8–12]. Motor vehicles are important contributors to combustion pollutants, including nitrogen dioxide (NO2), black carbon (BC), and fine particulate matter (PM10 and PM2.5). Both gaseous and PM pollutants have been associated with hospital admissions and mortality due to stroke [9]. Although epidemiological studies have certain limitations in establishing direct causal associations between air pollution and cerebrovascular disease, mainly due to potential confounding factors and measurement errors, robust biologically plausible evidence supporting this relationship has been drawn from various toxicological and longitudinal clinical studies [13, 14]. The primary determinant of the adverse health effects of air pollution appears to be combustion-derived nanoparticles that contain reactive organic and transition metal components. Inhalation of this particulate matter results in pulmonary inflammation, leading to secondary systemic effects [15] or, if it translocates from the lung into the circulation, causes direct toxic effects on the cardiovascular system [16]. Particulate matters increase the development of atherosclerosis through the induction of cellular oxidative stress and pro-inflammatory pathways.
Air pollution differs from other modifiable risk factors because it is unavoidable by changing lifestyle choices for most people, resulting in a significant overall population attributable risk even with a small risk estimate [17]. Despite considerable efforts made in the past 10 years to mitigate air pollution levels in Belgium, a significant part of the population is still exposed to concentrations exceeding the World Health Organization Air Quality Guidelines [18]. Therefore, quantifying the effectiveness of policies that further reduce those levels is becoming particularly important in supporting policymaking.
However, in environmental epidemiology, the reliance on observational data introduces significant methodological challenges in evaluating the potential health impact of air quality interventions, especially in the presence of numerous confounders. To capture the association between a risk factor exposure and a health outcome, typical approaches in epidemiological studies use logistic regression models, which estimate the differences between outcomes associated with a change in the risk factor exposure. However, these methods, which rely on stratum-specific estimates, are limited in that they do not provide insight into how changes in risk factor exposure across the entire population would impact the overall disease burden. Moreover, interpreting odds ratios from logistic regression is subtle due to the non-collapsibility issue—odds ratios often move further from 1 when additional covariates are included in the model, even without confounding [19]. A commonly used metric in epidemiology to estimate the proportion of disease cases attributable to a specific risk factor is the Population Attributable Fraction (PAF), introduced by Levin [20]. Typically based on relative risk and risk factor prevalence, it reflects the percentage of cases that could be avoided if the risk factor was eliminated. While Levin’s formulation did not account for coexisting risk factors, later methods, such as adjusted and average attributable fractions (AAF), were developed to accommodate multi-causal contexts [21–23]. Still, drawing causal inferences from PAF or AAF requires strong assumptions. For example, the excess disease cases observed among individuals exposed to air pollution may not be attributable to that exposure alone, as other co-occurring risk factors could also play a role [24]. Unfortunately, these assumptions are often inaccurately reported in research [25]. Another limitation of the PAF is that it assumes an ideal intervention that would completely eliminate the risk factor, which is often an unrealistic scenario in practice. In contrast, the Potential Impact Fraction (PIF), also known as the generalized impact fraction, estimates the proportional reduction in disease resulting from reducing the exposure to a modified level [26]. Both PAF and PIF are influenced by the prevalence of the exposure, as well as the strength of the association between the exposure and disease. In practice, the relative risk is often drawn from existing literature, and it is typically assumed that this value can be extrapolated to the study population —an assumption that may not always hold true. The application of traditional PAF or PIF measures in policymaking is, therefore, limited by the unrealistic assumption of complete risk factor elimination and by the limitations of conventional methods based on standard regression models [27, 28]. As a result, PAF-based methods frequently fall short in estimating the causal impact of complex, real-world public health interventions, particularly in high-dimensional data settings. G-computation is a method from the causal inference framework introduced by Robins J. in 1986, to respond to the challenges and limitations of traditional methods based on standard regression models [29–34]. This approach enables drawing causal inferences from observational data and estimating the impact of hypothetical interventions. The advantages of G-computation are numerous: ability to handle continuous risk factors, flexibility to model joint or dynamic interventions (where different subjects can receive different levels of the exposure under study) [35], and generation of interpretable results directly relevant to policymakers. Unlike traditional regression models, the method allows the estimation of population parameters, where the population average causal effect is estimated as the difference in the health outcome that would have been observed in the population if there had been a specific intervention as opposed to no intervention.
This study aims to demonstrate the use of a g-computation approach to assess the potential benefits of hypothetical air pollution reduction scenarios on the prevalence of stroke in Belgium in a multi-exposure context, considering lifestyle, metabolic, and environmental exposures.
Methods
Study area, study population, and data
The study area is the Belgian territory, which had a a population of 11.6 million inhabitants in 2023. The study sample consists of 27,536 participants of different waves of the Belgian Health Interview Survey (BHIS 2008, 2013 and 2018) older than 18 years, including a subset of 1184 participants who additionally participated in the Belgian Health Examination Survey in 2018 (BELHES 2018).
The BHIS is a national cross-sectional population survey carried out every five years by Sciensano, the Belgian Institute for Health, in partnership with Statbel, the Belgian statistical office [36]. Data are collected through a stratified multistage, clustered sampling design, and weighting procedures are applied to obtain results that are as representative as possible of the Belgian population [37]. In the BELHES, objective health information was collected among a random subsample of the BHIS participants. The BELHES included a short additional questionnaire, a physical examination, and the collection and analysis of blood and urine samples. Details on the data collection are available in the BELHES publication [38].
Based on the geographical coordinates of the participant’s residential address and using Geographical Information Systems (GIS), the dataset was further enriched with objective measures of the residential environment related to long-term exposure to air pollution, green space, and noise from road traffic.
Indicators for stroke and metabolic disorders
The stroke indicator was based on a self-reported variable and includes cerebral hemorrhage and cerebral thrombosis. The biases related to self-reported height, weight, diabetes, hypercholesterolemia, and hypertension were addressed using information from the BELHES and a random-forest multiple imputation [39]. The variables used to construct these indicators are displayed in Table 1.
Table 1.
Stroke and metabolic disorders indicators
| Indicator | Variable description | |
|---|---|---|
| Body mass index (BMI) | The measured imputed variable (using information from the BELHES and a random-forest multiple imputation method) was used instead of the self-reported variable. | Based on the measured height (cm) and the measured weight (kg) |
| Diabetes | Based on the measured fasted blood sugar (≥ 126 mg/dl), HbA1C (≥ 6.5%), or use of diabetes medication | |
| Hypertension | Based on the measured systolic blood pressure ≥ 140 mmHg or diastolic blood pressure > 90 mmHg or use of hypertension medication | |
| Stroke | Self-reported variable: “Suffered from stroke (cerebral hemorrhage, cerebral thrombosis) in the past 12 months” | Stroke (cerebral hemorrhage, cerebral thrombosis) |
Socio-demographic and lifestyle indicators
The following variables were used to describe each participant’s socio-demographic status: age, sex, highest educational level in the household, and birth country. To describe the participants’ lifestyle, we used the variables: level of physical activity and smoking status. The proposed levels of physical activity, based on the WHO indicator describing leisure time activity in the last 12 months [40], are: (i) ≥ 4 h sport or intensive training or (ii) < 4 h sport or light activities or (iii) sedentary behavior, defined as the complete absence of physical leisure activities. The proposed levels of smoking status are: (i) daily smoker, (ii) occasional smoker, (iii) former smoker, or (iv) never smoked.
Environmental indicators
Air pollution was assessed through the annual average exposure to BC, PM2.5, PM10, and NO2 at the residential address of the BHIS participants. Air pollution exposure was obtained as a continuous grid through the Belgian Interregional Environment Agency (IRCEL – CELINE) which supervises the national monitoring system assessing air pollutant concentrations through a dense network of stations, and estimates local exposure through interpolation, taking into account land cover data in combination with a dispersion model [41, 42]. The BHIS data of 2008, 2013, and 2018 were linked to BC exposure data of 2010, 2013, and 2018. The BHIS data from 2008, 2013, and 2018 were each linked to the air pollution data of the corresponding year, except for BC exposure data, where the 2010 data were used for the 2008 BHIS wave.
Exposure to green spaces was assessed based on CORINE Land Cover (CLC) data [43]. Vegetation coverage was obtained at the neighborhood level in a one-kilometer buffer around the respondent’s dwelling. The BHIS data of 2008, 2013, and 2018 were linked to green space data of 2006, 2012, and 2018.
Noise pollution, approached through the road traffic noise (Lden, day–evening–night noise level), was obtained from published noise maps, as required by the European Noise Directive (2002/49/EC) [44–46]. Noise data are created at the regional level and downloaded from the regional portals for environmental data [47–50]. BHIS data of 2008, 2013, and 2018 were linked to noise data of 2016 (and 2017 for Wallonia).
Statistical analyses
All variables of interest were described with their 95% confidence interval, and the missing data pattern was displayed for the merged BHIS/BELHES dataset.
Database compiling
First, the measurement error related to self-reported height, weight, hypertension, diabetes, and hypercholesterolemia in the BHIS database was corrected based on the objective information included in the BHES and using a random-forest multiple imputation method. A multiple imputation by chained equations (MICE) algorithm [51] was used to multiply impute the missing values of the merged dataset. The imputation model included a large set of variables, including all the variables from the final model (stroke, BC, PM2.5, NO2, diabetes, hypertension, hypercholesterolemia, age, sex, education, country of birth, physical activity, year, region, noise and green coverage) and additional variables related to socio-economics factors and chronic diseases (see the missing data pattern of the complete data set in additional files 1,2,3). In addition, the imputation model included variables used in the weighting procedure associated with the survey sample design (province, number of persons by household, age, and sex). All missing values of the covariates included in the imputation models were imputed in the same process. Details on the application of this correction method in the BHIS are found in a previous publication [39]. The number of iterations of the random-forest multiple imputation was set to 500, and the defined number of trees was set to 100. The algorithm’s convergence was monitored by plotting the mean and standard deviation of the synthetic values against the iteration number for the imputed BHIS data. The number of imputations was limited to 10, which was found satisfactory: using infinitely many imputations instead of 10 was estimated to reduce the variance of the estimators by at most 1%.
Health impact assessment
In a second step, a g-computation approach was used in each of the 10 completed datasets to assess the potential impact fractions (PIFs) of several air pollution reduction scenarios. In the first two scenarios, the average annual exposure to PM2.5 and NO2 was reduced to meet the updated WHO Air Quality Guideline (AQG) values of 5 µg/m3 and 10 µg/m3,, respectively as revised in 2021. It is worth noting that while these thresholds were adopted by WHO in 2021, the new European air quality directives aiming to align more closely with these guidelines were only adopted at the end of 2024. In subsequent scenarios, a dose-response function was investigated by decreasing the average annual exposure to PM2.5, NO2, and BC by varying percentages ranging from 20 to 80%. The imputation steps of the g-computation approach were:
-
I.
Modelling the air pollutant exposure and stroke association, taking into account the confounding variables.
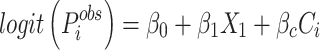 |
where
is the predicted stroke probability for individual i,
is the observed level of air pollution, and
is a vector of confounders.
-
II.
Imputing the stroke probability for each individual while manipulating the air pollution exposure by setting it to the value targeted by the hypothetical scenario.
 |
Where
is the counterfactual stroke probability for individual 𝑖,
is the hypothetical level of air pollution (modified in the counterfactual scenario), and
is a vector of confounders.
-
III.
Averaging these probabilities across the two populations: the observed population and the pseudo-population.
 |
-
IV.
Obtaining the risk difference between the observed and the pseudo-population.
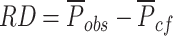 |
-
XXII.
Obtaining the PIF by dividing the risk difference by the stroke prevalence in the observed population.
 |
-
VI.
Iterating the process many times using 1000 non-parametric bootstrap samples to obtain valid confidence intervals.
Models were adjusted for important metabolic risk factors of stroke (diabetes, hypercholesterolemia and hypertension), for socio-economic factors (age, sex, country of birth, education level), lifestyle factors (smoking status, physical activity level), environmental factors (noise from the road traffic, vegetation coverage in one km buffer), region and year. Interactions were tested between each air pollutant and each of the covariates. The potential non-linearity of the association was also examined using Generalized Additive Models (GAMs) with spline smoothing. The performance of the models was assessed by randomly splitting each of the ten imputed datasets into a training dataset (70%) and a test dataset (30%) and by evaluating the Area under the curve (AUC). The ten obtained AUC values were then averaged.
The PIF of each scenario was calculated in each of the ten imputed datasets, and the results of the multiple analyses were pooled using the standard Rubin rules [52]. Final standard errors were obtained by taking the 97.5th percentile and the 2.5th percentile of the 10 thousands PIF estimates [53]. PIF was reported as a percentage, indicating the proportion of disease cases that would be avoided under the hypothetical air pollution reduction scenarios. The degree to which all the underlying assumptions required to draw a causal inference [34] (temporal ordering, exchangeability, no-interference, experimental treatment assignment, consistency, no model misspecification, no measurement error) is addressed in the Discussion section.
Statistical analyses were performed taking into account the survey sample design (weights, strata, and clusters). All analyses were fit and evaluated using the statistical software R, version 4.2.1 (R Development Core Team, 2006) and the “mice” package [54].
Results
Data description
A total of 27,536 participants of the 2008, 2013, and 2018 BHIS, older than 18 years, of which 1184 participated in the 2018 BHES, were included in the analysis. The missing data pattern of all considered variables and the description of the merged BHIS/BHES dataset are displayed in Additional files 1–4. The summary statistics of the study population after random-forest multiple imputation (estimates obtained using Rubin’s Rules) are available in Table 2. The distribution of the average annual exposure to PM2.5 and NO2 of the BHIS 2008/2013/2018 participants and the WHO air quality guideline threshold is visualized in additional file 5. The impact of each air pollutant reduction scenario on the air pollution distribution is visualized in Additional files 6, 7, and 8.
Table 2.
Description of the study population after random-forest multiple imputation (n = 27,536; estimates obtained using rubin’s rule)
| Proportion (%)[95% CI] |
Mean [SE] |
Median [IQR] |
|
|---|---|---|---|
| Socio-economic status | |||
| Age (year) | 49.08 [0.18] | 48 [31] | |
| Sex | |||
| Man | 51.7 [51.2;52] | ||
| Women | 48.3 [47.7;49] | ||
| Education level | |||
| No diploma/Prim | 9.77 [9.13;10] | ||
| Low secondary | 14 [13.54;14.45] | ||
| High secondary | 33.2 [29.8;36.8] | ||
| Higher | 43 [41.8;44.1] | ||
| Country of birth | |||
| Belgian | 84.6 [83.93;85.2] | ||
| Non belgian EU | 6.73 [6.31;7.14] | ||
| Non belgian non EU | 8.63 [8.12;9.13] | ||
| Lifestyle | |||
| Physical activity | |||
| Sport > 4 h/week | 15.6 [15.01;16.18] | ||
| Sport < 4 h/week | 54.8 [53.85;55.7] | ||
| Sedentary | 29.6 [28.61;30.18] | ||
| Smoking status | |||
| Daily smokers | 18.5 [17.8;19.2] | ||
| Occasional smokers | 3.76 [3.44;4.07] | ||
| Former smokers | 21.2[20.49;21.90] | ||
| Never smoked | 56.51 [55.9;57.4] | ||
| Alcohol consumption | 1.89 [0.01] | 2 [1] | |
| Stroke and metabolic disorders | |||
| Stroke | 0.96 [0.8;1.1] | ||
| BMI | 26.23 [0.04] | 25.51 [6.62] | |
| Diabetes | 4.56 [4.01;5.11] | ||
| Hypertension | 26.8 [25.25;28.34] | ||
| Hypercholesterolemia | 47.7 [45.54;49.85] | ||
| Environment | |||
| Black carbon exposure (µg/m³) | 1.17 [0.005] | 1.1 [0.6] | |
| PM2.5 exposure (µg/m³) | 13.67 [0.03] | 13.8 [3.6] | |
| NO2 exposure (µg/m³) | 20.13 [0.06] | 19.5 [8.03] | |
| Vegetation coverage (1 km buffer) | 40.17 [0.03] | 30.8 [55.1] | |
| Road noise (Lden) | |||
| > 55 dB | 11.6 [10.89;12.3] | ||
| < 55 dB | 88.4 [87.64;89.1] | ||
| Region | |||
| Brussel’s region | 10.6 [10.36;10.84] | ||
| Walloon’s region | 32.2 [31.6;32.9] | ||
| Flemish region | 57.1 [56.4;57.8] | ||
| Year | |||
| 2008 | 31.3 [30.27;32.32] | ||
| 2013 | 32.8 [31.74;33.8] | ||
| 2018 | 35.9 [34.82;36.97] | ||
Association between PM2.5, BC and NO2 and stroke
Results of the multivariable logistic regression models showed a significant association between stroke and PM2.5, while associations with NO2 and BC were at the limit of significance (Table 3). None of the interactions tested between air pollutants and covariates were significant, nor was there significant evidence of non-linearity in the associations assessed using GAMs. The three models for stroke (including each air pollutant separately) demonstrated a good predictive performance with AUCs of 77%, 80%, and 72%, respectively. Forest plot of the logistic regression model including PM2.5 is displayed in Fig. 1. Forest plots of the logistic regression model including NO2 and BC are shown in Additional files 9 and 10.
Table 3.
Estimates of the logistic regression models (associations between PM2.5, NO2, BC, and stroke) and potential impact fractions (PIFs) for the different air pollution reduction scenarios
| Stroke baseline prevalence 1.33% 95% CI [1.14;1.51] |
PM2.5 | NO2 | BC | |
|---|---|---|---|---|
|
OR [95% CI] (for 1 IQR increase) |
1.69 [1.06;2.69] | 1.35 [0.91;1.99] | 1.17 [0.99;1.39] | |
| WHO guideline threshold |
RD (%) [95% CI] |
−0.88 [−1.35;−0.41] | −0.33 [−0.71;0.04] | |
|
PIF (%) [95% CI] |
67.08 [19.77;86.12] | 25.21 [−4.06;54.48] | ||
| −20% |
RD (%) [95% CI] |
−0.39 [−0.68;−0.10] | −0.13 0.30;0.03] | −0.11 [−0.22;0.01] |
|
PIF (%) [95% CI] |
29.10 [5.70;48.52] | 9.63 [−4.02;22.3] | 7.60 [−1.87;15.25] | |
| −40% |
RD (%) [95% CI] |
−0.67 [−1.10;−0.22] | −0.26 [−0.56;0.05] | −0.20 [−0.41;0.01] |
|
PIF (%) [95% CI] |
48.07 [9.42;73.29] | 18.44 [−7.29;39.22] | 14.15 [−3.88;27.56] | |
| −60% |
RD (%) [95% CI] |
−0.86 [−1.35;−0.36] | −0.36 [−0.56;0.05] | −0.29 [−0.58;0.01] |
|
PIF (%) [95% CI] |
61.47 [14.78;85.69] | 25.38 [−11.6;52.36] | 20 [−5.98;37.7] | |
| −80% |
RD (%) [95% CI] |
−0.99 [−1.52;−0.45] | −0.46 [−0.98;0.06] | −0.34 [−0.75;0.01] |
|
PIF (%) [95% CI] |
69.70 [16.59;92.11] | 31.24 [−18.04;61.7] | 22.76 [−13.5;45.96] | |
RD, risk difference, PIF, potential impact fraction, WHO, World Health Organization, CI, confidence interval, IQR, interquartile range
Fig. 1.
Forest plot of the multivariate logistic regression model for the association between stroke and PM2.5 exposure. Abbreviations: HT, hypertension; Chol, hypercholesterolemia; BMI, body mass index
Potential impact fractions of air pollution reduction scenarios
Adhering to the WHO air quality guideline for PM2.5 and NO2 exposure would lead to a substantial reduction in stroke risk in Belgium: −0.88% [−1.35;−0.41] for PM2.5 and − 0.33% [−0.71;0.04] for NO2. The proportion of prevented stroke cases would be 67% for PM2.5 and 25% for NO2 (Table 2). Under these first two scenarios, the average PM2.5 reduction would be 8.7 units, and the average NO2 reduction would be 11.5 units. The conditional SD of PM2.5, given all the covariates, is 1.3 units, and the conditional SD of NO2, given all the covariates, is 4.4 units. The proportions of BHIS participants affected by the various air quality scenarios, along with the average annual exposure to air pollutants and the average decrease in µg/m³ under the different air quality interventions, are presented in Additional File 11.
Results reveal a clear dose-response association between air pollution reduction and the PIFs on stroke in Belgium (Fig. 2). Reduction in air pollution exposure, ranging from 20 to 80% showed increasing proportion of prevented stroke cases: PM2.5 (29%, 48%, 61%, 69%), NO2 (10%, 18%, 25%, 31%) and BC (8%, 14%, 20%, 23%).
Fig. 2.
Dose-response association between air pollution reduction and the potential impact fractions (PIFs expressed as percentages) on stroke in Belgium. Error bars represent the 95% confidence intervals. The intervention refers to a reduction, by varying percentages, of the annual average exposure for each BHIS participant to black carbon (BC), nitrogen dioxide (NO₂), and fine particulate matter (PM₂.₅)
PIFs related to PM2.5 reduction scenarios were all significantly different from 0, while PIFs related to BC and NO2 were at the limit of significance.
Discussion
Main findings
In this study, we used a g-computation approach combined with a random-forest multiple imputation method to estimate stroke prevalence rates under several hypothetical air pollution reduction scenarios in Belgium, allowing to answer intuitive questions such as “What would be the reduction in stroke risk in the Belgian population, if we could manipulate the annual average exposure to PM2.5, BC and NO2?”. This methodological approach enabled the assessment of the potential effects of any specific interventions, while addressing bias related to self-reported data and missing data issues in health interview surveys. In epidemiology, the selection of the measure of association is often determined by the regression model used rather than the specific question of interest. In this study, we presented a population intervention measure that estimates stroke prevalence rates had air pollution exposure been equal to the WHO air quality guidelines. This approach differs from traditional regression models that generate conditional odds ratios for one unit change in air pollution exposure. The presentation of marginal causal effects has the advantage of providing concrete and directly actionable evidence for policymakers.
Our results suggest that air pollution reduction interventions could significantly reduce the prevalence of stroke in Belgium. A major benefit was found for scenarios targeting PM2.5 exposure. Adhering to the WHO air quality guideline for PM2.5 exposure would drastically reduce the prevalence of stroke in Belgium, with 67% of avoidable cases. The reduction was less pronounced for scenarios targeting NO2 and BC exposure. Adhering to the WHO air quality guideline for NO2 exposure would lead to a reduction of stroke risk of 25%. However, only the PIFs associated with PM2.5 reduction scenarios were significantly different from zero, while the PIFs related to BC and NO2 were at the significance limit. Several recently published studies, systematic reviews, and meta-analyses support the overall results from this study, showing positive associations between stroke and PM2.5, NO2, and BC exposure [5, 9, 10, 55, 56]. In Belgium specifically, research on the long-term effects of air pollution on stroke remains limited. The few existing studies have primarily focused on short-term exposure. For example, one study reported that acute exposure to NO₂ was associated with increased hospital admissions for stroke [57]. Another study has shown that short-term exposure to PM10 and PM2.5 is linked to a higher use of medications and medical interventions for thrombo-embolic events [58]. Our findings highlight the need for more research on the long-term effects of air pollution on stroke in the Belgian population.
When comparing our results with those of the Global Burden of Disease (GBD) study, PAFs estimated in this study appear notably higher. According to the GBD study, 29∙2% of the global stroke burden was attributed to air pollution [59]. In Belgium, the GBD estimated that ambient particulate matter was responsible for 12.5% of the stroke burden in 2008, decreasing to 7.5% in 2018, measured in terms of years of healthy life lost [1]. It is worth noting that this figure is slightly higher than that observed in neighboring countries such as France, Germany, and Luxembourg, and is comparable to that of the Netherlands. In contrast, developing countries face a much higher burden, with 45% of strokes linked to particulate matter [1].
However, it must be noted that the GBD estimates cannot be directly compared to the estimates presented in this article, due to differences in data sources and methodologies. What complicates the comparison between the two sets of estimates is the difference in their underlying conceptual frameworks: while the GBD focuses on population-level disease burden, our study is grounded in individual-level causal effects. The g-computation approach used in our analysis is tailored to the available individual-level data by estimating, for each participant, the conditional probability of experiencing a stroke within the past 12 months given the covariates included in the model, and then averaging these individual probabilities across the population. In contrast, the GBD’s PAF estimates reflect the overall contribution of air pollution to stroke cases across the global population and are typically not based on a specific national dataset. Instead, they often rely on relative risk estimates derived from external studies, primarily from cohort data, which measure incident stroke cases, including both fatal and non-fatal events. In contrast, our analysis is based on prevalent stroke cases, which include only individuals who have survived a stroke. In addition, the counterfactual scenarios used in the GBD are not based on the fixed WHO air quality guideline value, but on the theoretical minimum-risk exposure level (TMREL), which refers to the distribution of exposure associated with the lowest population risk (ranging from 2.4 to 5.9 µg/m³ for PM2.5).
While these scenarios may appear ambitious and unrealistic for the current situation in Belgium, these PIFS are nonetheless valuable in informing policymakers about the magnitude of health impacts associated with air pollution and by providing a clear vision of the potential health benefits that could be achieved if air pollution levels were brought to the WHO air quality guidelines thresholds. These findings underscore the urgency and importance of implementing effective air quality improvement strategies and policies in Belgium. By comparing PIFs for different pollutants and scenarios, our results can guide the prioritization of pollution control policies and help allocate resources more effectively to target the most critical pollutants and maximize public health benefits. The achievement of the WHO air quality guidelines threshold should guide the development of long-term strategies for gradual improvement in air quality. Based on the latest scientific evidence, these guidelines aim to provide a level of protection that reduces the health risks associated with air pollution [18]. However, it is essential to acknowledge that even below the WHO air quality guidelines, adverse health effects can still occur; no “safe” threshold for air pollution exposure exists [60, 61].
Strengths and limitations
Besides the use of a causal inference technique, a significant strength of this study lies in the inclusion of important metabolic factors, such as hypertension, diabetes, hypercholesterolemia, and overweight in the statistical models. These factors are well-established as crucial modifiable factors for stroke. Moreover, this study effectively addressed potential biases related to these self-reported variables, using a random-forest multiple-imputation method and clinical information from the BELHES. This study also provided the opportunity to examine the influence of urban co-exposures such as the lack of green spaces and road traffic noise. The linkage of the BHIS data with the objective environmental factors at the participants’ residential address represents a substantial added value to this study.
We acknowledge several limitations that need to be carefully considered when interpreting the results of this study. The primary concern lies in the potential under-assessment and misclassification of stroke due to the self-reported nature of the variable “stroke”. Self-reported stroke is susceptible to bias, particularly recall bias, and does not equate to a clinical diagnosis by a physician with explicit evidence from medical imaging. Moreover, the definition of the variable stroke did not allow us to distinguish between ischemic and hemorrhagic stroke and to stratify the analysis by stroke type. It is also important to note that our PIF estimates are based on self-reported stroke incidents that occurred within the past 12 months, which, by definition, exclude fatal stroke cases. Consequently, our estimation of the impact of air pollution on stroke is based on a subset of cases, specifically stroke survivors. This may have introduced a significant selection bias, which likely resulted in underestimating the effect estimates because the complete extent of the risk associated with air pollution could not be captured.
For air pollution exposure, measurement bias can not be ruled out, as it is extrapolated from the average annual concentration of a given area to individual exposure, without taking into account the time spent at the residence or whether the individual was inside or outside. This extrapolation might lead to exposure misclassification. Ideally, personal mobility should be integrated in dynamic exposure assessments, but this is unfortunately impossible to apply for large samples, over long periods, or for retrospective studies. However, future studies could address this limitation by incorporating personal exposure monitoring in smaller sub-samples, which could help validate and refine exposure assessment models used in large epidemiological research.
If the g-computation allowed to evaluate the PIFs of several air pollution reduction scenarios, several assumptions must be met to interpret them causally. While these assumptions are not limited to causal inference methods, they are, however, often not explicitly considered in the literature. We assumed exchangeability, which implies adequate control for confounders and no unmeasured confounding factors in the exposure-outcome association. Although we included in our analysis a large set of confounders identified in the literature, we cannot exclude the existence of residual confounding. The models were adjusted for several environmental factors with long-term exposure known to be associated with stroke risk, such as exposure to noise [62, 63] and green spaces [64, 65]. However, we acknowledge that certain relevant environmental exposures could not be accounted for due to data unavailability, particularly ultrafine particles, which have been increasingly linked to cerebrovascular outcomes [66]. Additionally, certain lifestyle factors, such as alcohol consumption and dietary habits, as well as contextual factors such as area-level socio-economic status, were also not included in our models. Although alcohol consumption and nutritional habits were available in the BHIS dataset, we decided not to include them because they were highly prone to a reverse causality effect. Specifically, these factors can be influenced by the onset of stroke, making it difficult to distinguish whether these behaviors are a cause or an effect of the stroke. For example, individuals who have suffered a stroke may alter their alcohol consumption or dietary habits as a result of the health event, rather than these behaviors being causal risk factors for stroke. The assumption of “consistency” assumes that exposure levels correspond to a well-defined intervention. Violations of the consistency assumption occur when there is ambiguity in the definition of interventions to change exposure. While exposure to a defined level of PM2.5, NO2, or BC is well-defined, the exact interventions used by a community to change pollution levels may impact stroke incidence through pathways other than air pollution. For example, encouraging people to use bikes instead of vehicles for short trips improves air quality and leads to higher physical activity levels and reduced overweight in the population. These positive effects could further contribute to lower stroke incidence, making such interventions beneficial for multiple health outcomes. Ambiguity arises from the fact that there are many different approaches to decreasing air pollution exposure, and each of these approaches may have a different causal effect on the outcome. This intervention heterogeneity complicates the interpretation of causal effects derived from hypothetical exposure reductions. While we presented these reduction scenarios in this study as simplified exposure contrasts for analytical purposes, we fully acknowledge that implementing such changes in the real world is far more complex. Nonetheless, recent studies evaluating the effectiveness of specific traffic-related measures implemented in Belgium over the past decade allow us to establish a link between our hypothetical scenarios and concrete policy interventions [67]. For example, analyses of low-emission zones (LEZs)—the primary policy tool in Europe to combat traffic-related air pollution—have shown measurable reductions in air pollutant levels in several Belgian cities. In Brussels and Antwerp, the implementation of LEZs between 2018 and 2023 led to emission reductions of 31% for NO₂, 30% for PM2.5, and 62% for BC, resulting in a significant improvement in air quality, with NO₂ concentrations decreasing by more than 30% [68, 69]. In Brussels, projections further estimate that between 2022 and 2035, the LEZ will reduce average NO₂ concentrations by 24%, with the greatest impact on high-traffic roads. The most substantial reduction is expected in 2027, following the enforcement of the ban on Euro 5 diesel light vehicles [70]. A growing body of evidence from various international studies has shown that LEZs effectively improve air quality and public health, with particularly consistent effects reported for cardiovascular outcomes [71]. The effectiveness of low-traffic neighborhoods (LTNs) to reduce concentrations of air pollutants in Belgium has also been demonstrated. These neighborhoods are designed through traffic circulation plans that limit through motorized traffic using measures such as reduced speed limits and expanded space for active mobility. In Flanders, the city of Ghent provides a concrete example: in its historic city center, car traffic has been largely restricted, which resulted in an 18% reduction in NO₂ concentrations [72]. Other local and targeted measures—such as school streets, car-free days, and the greening of urban spaces—have also shown effectiveness in reducing air pollution levels in Belgium in specific locations and periods [73–75]. In addition, interventions to reduce air pollution from residential wood combustion also hold considerable potential. In Wallonia, for instance, one-third of fine PM₂.₅ emissions originate from wood heating, highlighting the importance of addressing this source as part of broader air quality improvement strategies [76]. These findings suggest that some of the hypothetical exposure reductions assessed in our study may be achievable through targeted interventions that are already being implemented in the Belgian context.
We also assume the positivity assumption, also called the “experimental treatment assignment” assumption, which means all exposure values must be experienced in every confounder subgroup [77]. This assumption is closely related to the realism of the scenario and is therefore more likely to be violated for the first two scenarios, which require changes in air pollution exposure that are almost never observed in the population. In such cases, the model is required to extrapolate beyond the empirical support of the data, estimating outcomes for combinations of covariates and exposure levels that are poorly represented in the sample. This extrapolation increases the risk of model-dependent inferences and may result in less reliable causal effect estimates. We therefore emphasize that the results for these scenarios should be interpreted with caution.
In addition, we assumed the “temporal ordering assumption”, which ensures that the exposure to air pollution occurs before the occurrence of stroke and that confounding factors precede the exposure. In the context of this study, we can reasonably assume that long-term exposure to air pollution precedes the stroke incident and that fixed variables such as age, sex, or education precede the air pollution exposure. However, for certain variables, like metabolic and lifestyle factors, their occurrence might be influenced by the stroke incident. For example, an individual might develop excess weight or engage in less physical activity after experiencing a stroke. Moreover, those metabolic risk factors could lie on the causal pathway from air pollution exposure to stroke, which could lead to an underestimation of the total effect. Ideally, longitudinal data would have strengthened our ability to assess temporal relationships and causality more robustly. However, due to the unavailability of such data, this study relied on the available cross-sectional data. The use of prevalent data does not capture changes over time in terms of exposure and outcomes, potentially leading to exposure misclassification or measurement bias. For instance, using the annual average air pollution exposure as an indicator overlooks the occurrence of spikes and within-year variations in air pollution exposure, which can impact stroke risk. Furthermore, the use of cross-sectional data in this study did not allow us to fully take advantage of the G-computation approach, whose main strength lies in its ability to account for time-varying variables—i.e., variables whose values change over time. The new BELCOHORT project, which involves setting up a population-based cohort, is very promising and will provide many new study opportunities [78]. In this cohort study, more accurate exposure summaries will be defined by reconstructing the complete exposure history for each participant, taking into account changes in individual residential information [79].
The “no-interference” assumption states that the outcome of each individual is not influenced by the exposures and outcomes of other individuals. In this study, we expect this assumption to be fully met since strokes are not contagious. Finally, we assumed a correctly specified model. Models were constructed based on a directed acyclic graph (DAG) reflecting a hypothesized causal structure, and the AUC demonstrated a good predictive performance for the three models. However, potential model misspecification or non-linearity in the association between stroke and air pollution should be further investigated using machine learning techniques.
Lastly, it is important to consider that our estimates are specific to the Belgian population and may not apply to other populations with different distributions of stroke risk factors. In particular, the distribution of air pollution exposure may vary significantly in other populations.
Conclusions
This study demonstrated the use of a g-computation approach to assess the benefits of hypothetical air pollution reduction policies on stroke in Belgium in a multi-exposure context. The g-computation-based approach to assess PIF of interventions represents a straightforward approach in epidemiology for making causal inference from observational data while also providing useful information for policymakers.
Our results indicate that implementing air quality control interventions could significantly reduce the prevalence of stroke in Belgium. It provides valuable insight and benefits for guiding policies, raising awareness, and promoting long-term efforts to improve air quality and mitigate the burden of stroke in the Belgian population. Air pollution should be recognized more widely as one of the most important modifiable risk factors for the prevention of stroke in Belgium. Since almost the entire population in Belgium is exposed to air pollution levels that exceed the WHO air quality guideline thresholds, implementing a comprehensive particulate matter control policy is imperative to reduce the burden of stroke in the country.
Supplementary Information
Supplementary Material 1. Additional file 1: missing data pattern for socio-economic and lifestyle variables of the merged dataset BHIS/BHES.
Supplementary Material 2. Additional file 2: missing data pattern for health variables of the merged dataset BHIS/BHES.
Supplementary Material 3. Additional file 3: missing data pattern for environmental variables of the merged dataset BHIS/BHES.
Supplementary Material 4. Additional file 4: description of the merged BHIS/BHES dataset.
Supplementary Material 5. Additional file 5. Distribution of the annual average exposure to PM2.5 and NO2 of the Belgian health interview survey 2008/2013/2018 participants in Belgium and WHO air quality guideline thresholds.
Supplementary Material 6. Additional file 6. Impact of air pollution reduction scenarios on the distribution of PM2.5 exposure of the Belgian health interview survey participants (2008/2013/2018). Reduction of the PM2.5 annual average exposure: A. 20%, B. 40%, C.60%. D.80%.
Supplementary Material 7. Additional file 7. Impact of air pollution reduction scenarios on the distribution of NO2 exposure of the Belgian health interview survey participants (2008/2013/2018). Reduction of the NO2 annual average exposure: A. 20%, B. 40%, C.60%. D.80%.
Supplementary Material 8. Additional file 8. Impact of air pollution reduction scenarios on the distribution of black carbon exposure of the Belgian health interview survey participants (2008/2013/2018). Reduction of the BC annual average exposure: A. 20%, B. 40%, C.60%. D.80%.
Supplementary Material 9. Additional file 9. Forest plot of the multivariate logistic regression model for the association between stroke and NO2.
Supplementary Material 10. Additional file 10. Forest plot of the multivariate logistic regression model for the association between stroke and Black carbon.
Supplementary Material 11. Additional file 11. Proportion of BHIS participants affected by the different air quality interventions and corresponding average reduction in air pollution exposure.
Acknowledgements
Not applicable.
Authors’ contributions
IP performed the analysis and wrote the manuscript. BD, JVH, EDC provided critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was conducted as part of the BELAIR-POL project (Assessing the benefits of air pollution reduction interventions on multi-morbidity and mortality in Belgium), which is supported by the Orcadia fund, managed by the King Baudouin Foundation.
Data availability
The data that support the findings of this study are not publicly available. Data are however available from the authors upon reasonable request and with specific permission ([https://www.sciensano.be/en/node/55737/health-interview-survey-microdata-request-procedure](.)). Legal restrictions make that BHIS and BHES data can only be communicated to other parties if an authorization is obtained from the sectoral committee social security and health of the Belgian data protection authority.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics committee of Ghent University Hospital, and positive advice was obtained (advice with registration number B670201734213 and advice with registration number B670201834895). Informed consent was obtained for every BHIS and BHES participant. All methods were carried out in accordance with relevant guidelines and regulations. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki/).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Burden of Disease Collaborative Network. Institute for Health Metrics and Evaluation. 2021. Global Burden of Disease Results Tool. Available from: https://vizhub.healthdata.org/gbd-results. [cited 2025 May 13].
- 2.Budig K, Harding E. Secondary prevention of heart attack and stroke in Europe- Country profile for Belgium. London: The Health Policy Partnership.; 2021. Available from: https://www.healthpolicypartnership.com/app/uploads/Secondary-prevention-of-heart-attack-and-stroke-in-Europe-Belgium.pdf.
- 3.Dong H, Yu Y, Yao S, Lu Y, Chen Z, Li G, et al. Acute effects of air pollution on ischaemic stroke onset and deaths: a time-series study in Changzhou, China. BMJ Open. 2018;8(7):e020425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJB, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American heart association/american stroke association. Stroke. 2013;44(7):2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haddad P, Kutlar Joss M, Weuve J, Vienneau D, Atkinson R, Brook J, et al. Long-term exposure to traffic-related air pollution and stroke: a systematic review and meta-analysis. Int J Hyg Environ Health. 2023;247: 114079. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman JD, Elkind MSV, Bhatnagar A, Koehler K, Balmes JR, Sidney S, et al. Guidance to reduce the cardiovascular burden of ambient air pollutants: A policy statement from the American Heart Association. Circulation. 2020;142(23):e432-47. [DOI] [PubMed] [Google Scholar]
- 7.Franklin BA, Brook R, Arden Pope C. Air pollution and cardiovascular disease. Curr Probl Cardiol. 2015;40(5):207–38. [DOI] [PubMed] [Google Scholar]
- 8.Verhoeven JI, Allach Y, Vaartjes ICH, Klijn CJM, de Leeuw FE. Ambient air pollution and the risk of ischaemic and haemorrhagic stroke. Lancet Planet Health. 2021;5(8):e542–52. [DOI] [PubMed] [Google Scholar]
- 9.Niu Z, Liu F, Yu H, Wu S, Xiang H. Association between exposure to ambient air pollution and hospital admission, incidence, and mortality of stroke: an updated systematic review and meta-analysis of more than 23 million participants. Environ Health Prev Med. 2021;26(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan S, Wang J, Jiang Q, He Z, Huang Y, Li Z, et al. Long-term exposure to PM2.5 and stroke: a systematic review and meta-analysis of cohort studies. Environ Res. 2019;177: 108587. [DOI] [PubMed] [Google Scholar]
- 11.Lee KK, Miller MR, Shah ASV. Air pollution and stroke. J Stroke. 2018;20(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulick ER, Kaufman JD, Sack C. Ambient air pollution and stroke: an updated review. Stroke. 2023;54(3):882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, et al. Adverse cardiovascular effects of air pollution. Nat Rev Cardiol. 2009;6(1):36–44. [DOI] [PubMed] [Google Scholar]
- 14.Robertson S, Miller MR. Ambient air pollution and thrombosis. Part Fibre Toxicol. 2018;15(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345(8943):176–8. [DOI] [PubMed] [Google Scholar]
- 16.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65(20):1531–43. [DOI] [PubMed] [Google Scholar]
- 17.Nawrot TS, Perez L, Künzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. 2011;377(9767):732–40. [DOI] [PubMed] [Google Scholar]
- 18.WHO. WHO Global Air Quality Guidelines. World Health Organisation, 2021. Available from: https://www.who.int/news-room/feature-stories/detail/what-are-the-who-air-quality-guidelines. [cited 2023 Mar 4].
- 19.Pang M, Kaufman JS, Platt RW. Studying noncollapsibility of the odds ratio with marginal structural and logistic regression models. Stat Methods Med Res. 2016;25(5):1925–37. [DOI] [PubMed] [Google Scholar]
- 20.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9(3):531–41. [PubMed] [Google Scholar]
- 21.Rückinger S, von Kries R, Toschke AM. An illustration of and programs estimating attributable fractions in large scale surveys considering multiple risk factors. BMC Med Res Methodol. 2009;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nusselder WJ, Looman CW. Decomposition of differences in health expectancy by cause. Demography. 2004;41(2):315–34. [DOI] [PubMed] [Google Scholar]
- 23.Eide GE. Attributable fractions for partitioning risk and evaluating disease prevention: a practical guide. Clin Respir J. 2008;2(Suppl 1):92–103. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S, Robins JM. Conceptual problems in the definition and interpretation of attributable fractions. Am J Epidemiol. 1988;128(6):1185–97. [DOI] [PubMed] [Google Scholar]
- 25.Greenland S. Concepts and pitfalls in measuring and interpreting attributable fractions, prevented fractions, and causation probabilities. Ann Epidemiol. 2015;25(3):155–61. [DOI] [PubMed] [Google Scholar]
- 26.Morgenstern H, Bursic ES. A method for using epidemiologic data to estimate the potential impact of an intervention on the health status of a target population. J Community Health. 1982;7(4):292–309. [DOI] [PubMed] [Google Scholar]
- 27.Khosravi A, Mansournia MA. Recommendation on unbiased estimation of population attributable fraction calculated in prevalence and risk factors of active pulmonary tuberculosis among elderly people in China: a population based cross-sectional study. Infect Dis Poverty. 2019;8(1): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansournia MA, Altman DG. Population attributable fraction. BMJ. 2018;360:k757. [DOI] [PubMed] [Google Scholar]
- 29.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. [DOI] [PubMed] [Google Scholar]
- 31.Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Modelling. 1986;7(9):1393–512. [Google Scholar]
- 32.Igelström E, Craig P, Lewsey J, Lynch J, Pearce A, Katikireddi SV. Causal inference and effect estimation using observational data. J Epidemiol Community Health. 2022;76(11):960–6. [Google Scholar]
- 33.Hernan M. A definition of causal effect for epidemiological research. J Epidemiol Community Health. 2004;58(4):265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernan MA, Robins JM. Causal inference: what if. Boca Raton: Chapman&Hall/CRC; 2020. [Google Scholar]
- 35.Robins JM, Hernan MA. Estimation of the causal effects of time-varying exposures. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, eds. Longitudinal data analysis. New York: Chapman & Hall/CRC Press. 2008;553–99. https://scispace.com/pdf/estimation-of-the-causal-effects-of-time-varying-exposures-hhlrh9iqsn.pdf.
- 36.Demarest S, Van der Heyden J, Charafeddine R, Drieskens S, Gisle L, Tafforeau J. Methodological basics and evolution of the Belgian health interview survey 1997–2008. Arch Public Health. 2013;71(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Health Interview Survey protocol. Available from: https://his.wiv-isp.be/SitePages/Protocol.aspx. [cited 2021 May 6].
- 38.Nguyen D, Hautekiet P, Berete F, Braekman E, Charafeddine R, Demarest S, et al. The Belgian health examination survey: objectives, design and methods. Arch Public Health. 2020;78(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelgrims I, Devleesschauwer B, Vandevijvere S, De Clercq EM, Vansteelandt S, Gorasso V, et al. Using random-forest multiple imputation to address bias of self-reported anthropometric measures, hypertension and hypercholesterolemia in the Belgian health interview survey. BMC Med Res Methodol. 2023;23(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Bruin A, Picavet HSJ, Nossikov A. Health interview surveys: towards international harmonization of methods and instruments. World Health Organization. Regional Office for Europe; 1996. xiii, 161 p. Available from: https://apps.who.int/iris/handle/10665/107328. [cited 2023 Apr 10]. [PubMed]
- 41.Janssen S, Dumont G, Fierens F, Mensink C. Spatial interpolation of air pollution measurements using CORINE land cover data. Atmos Environ. 2008;42(20):4884–903. [Google Scholar]
- 42.Lefebvre W, Vranckx S. Validation of the IFDM-model for use in urban applications. Study accomplished in the framework of the ATMOSYS project. 2013;208. https://marvin.vito.be/~hooyberh/Validation_ATMOSYS_report.pdf.
- 43.EEA.2012. CLC CORINE Land Cover 2012, Version 18.5.1.. Available from: https://land.copernicus.eu/user-corner/technical-library/clc-country-coverage-v18.5.
- 44.LEEFMILIEU BRUSSEL-BIM. 49, DOELSTELLINGEN EN METHODOLOGIE VAN DE, GELUIDSKADASTERS IN HET BRUSSELS HOOFDSTEDELIJK GEWEST. COLLECTIE FACTSHEETS, THEMA GELUID; 2018. Available from: https://document.environnement.brussels/opac_css/elecfile/Geluid_49. [cited 2020 Dec 23].
- 45.Basner M, McGuire S. WHO environmental noise guidelines for the European region. A systematic review on environmental noise and effects on sleep. Int J Environ Res Public Health. 2018;15(3): 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DIRECTIVE 2002/49/CE du Parlement européen et du Conseil du 25. juin 2002 relative à l’évaluation et à la gestion du bruit dans l’environnement. 2002 ;12–25. Report No.: Journal Officiel n° L 189. Available from: http://publications.europa.eu/resource/cellar/0354e2a3-4ee8-45a2-aa4a-090036045111.0010.04/DOC_1. [cited 2021 Jan 6].
- 47.ACOUPHEN ENVIRONNEMENT - Dec. 2008. Carte de multi-exposition Bruxelles Environnement. Cadastre du bruit des transports routier, ferroviaire, aérien, trams et métro aérien de la Région de Bruxelles-Capitale. Available from: https://document.environnement.brussels/opac_css/elecfile/IBGE_Multi_2006_1.pdf. [cited 2020 Dec 23].
- 48.https://geopunt.be.
- 49.https://geoapps.wallonie.be
- 50.Bruxelles Environnement. Rapport 2011–2014 van de staat van het leefmilieu: Exposition de la population au bruit des transports. 2011. Available from: https://environnement.brussels/lenvironnement-etat-des-lieux/rapports-sur-letat-de-lenvironnement/rapport-2011-2014/bruit-0. [cited 2021 Jan 6].
- 51.Van Buuren S. Flexible imputation for missing data Chapman & Hall/CRC; 2018. 10.1201/9780429492259.
- 52.Campion WM, Rubin D. Multiple imputation for nonresponse in surveys. J Mark Res. 1989. 10.2307/3172772. [Google Scholar]
- 53.Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med. 2018;37(14):2252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Buuren S. Package ‘mice’. 2021. Available from: https://cran.r-project.org/web/packages/mice/mice.pdf.
- 55.Tian Y, Liu H, Xiang X, Zhao Z, Juan J, Li M, et al. Ambient coarse particulate matter and hospital admissions for ischemic stroke: a national analysis. Stroke. 2019;50(4):813–9. [DOI] [PubMed] [Google Scholar]
- 56.Ljungman PLS, Andersson N, Stockfelt L, Andersson EM, Nilsson Sommar J, Eneroth K, et al. Long-term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ Health Perspect. 2019;127(10):107012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collart P, Dubourg D, Levêque A, Sierra NB, Coppieters Y. Short-term effects of nitrogen dioxide on hospital admissions for cardiovascular disease in Wallonia, Belgium. Int J Cardiol. 2018;255:231–6. [DOI] [PubMed] [Google Scholar]
- 58.Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. Long-term exposure to particulate matter air pollution is a risk factor for stroke: meta-analytical evidence. Stroke. 2015;46(11):3058–66. [DOI] [PubMed] [Google Scholar]
- 59.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016;15(9):913–24. [DOI] [PubMed] [Google Scholar]
- 60.Wilker EH, Mostofsky E, Fossa A, Koutrakis P, Warren A, Charidimou A, et al. Ambient pollutants and spontaneous intracerebral hemorrhage in greater Boston. Stroke. 2018;49(11):2764–6. [DOI] [PubMed] [Google Scholar]
- 61.Tian Y, Liu H, Wu Y, Si Y, Song J, Cao Y, et al. Association between ambient fine particulate pollution and hospital admissions for cause specific cardiovascular disease: time series study in 184 major Chinese cities. BMJ. 2019;367:l6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poulsen AH, Sørensen M, Hvidtfeldt UA, Christensen JH, Brandt J, Frohn LM et al. Concomitant exposure to air pollution, green space, and noise and risk of stroke: a cohort study from Denmark. The Lancet Regional Health – Europe. 2023;31. Available from: https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(23)00074-1/fulltext. [cited 2025 May 6]. [DOI] [PMC free article] [PubMed]
- 63.Fu W, Liu Y, Yan S, Wen J, Zhang J, Zhang P, et al. The association of noise exposure with stroke incidence and mortality: a systematic review and dose-response meta-analysis of cohort studies. Environ Res. 2022;215: 114249. [DOI] [PubMed] [Google Scholar]
- 64.El Masri J, Finge H, Afyouni A, Baroud T, Ajaj N, Ghazi M, et al. The effects of green spaces and noise exposure on the risk of ischemic stroke: a case-control study in Lebanon. Int J Environ Res Public Health. 2024;21(10): 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whyte M, Douwes J, Ranta A. Green space and stroke: a scoping review of the evidence. J Neurol Sci. 2024;457: 122870. [DOI] [PubMed] [Google Scholar]
- 66.Schraufnagel DE. The health effects of ultrafine particles. Exp Mol Med. 2020;52(3):311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pelgrims I, Pauwels A, De Clercq E. Overview of air quality measures aiming to reduce emissions from road traffic and mitigate health impact in Belgium. Bruxelles: Sciensano; 2024;58. Available from: https://www.sciensano.be/sites/default/files/overview_airquality_measures.pdf.
- 68.Bruyneel L, Cox B, Stauffer A, Vandenthoren L, Fierens F, Nawrot TS et al. Positive Impact of the Introduction of Low-Emission Zones in Antwerp and Brussels on Air Quality, Socio-Economic Disparities and Health: A Quasi-Experimental Study. Rochester, NY: Social Science Research Network; 2024. Available from: https://papers.ssrn.com/abstract=5054557. [cited 2025 Mar 17]. [DOI] [PubMed]
- 69.Louise DUPREZ, Simon DEHOUCK, EVALUATION DE LA ZONE DE BASSES ÉMISSIONS. RAPPORT 2023. Bruxelles Environnement; 2023. Available from: file:///C:/Users/InPe1097/Downloads/FR-Rapport-LEZ-2023-DEF.pdf.
- 70.Pourtois M, Thiry C. Evaluating traffic policies on NO₂ pollution in brussels: impact of the low emission zone and the good Move Plan. Faculté des bioingénieurs, Université catholique de Louvain; Prom: Bogaert, Patrick; 2024. https://thesis.dial.uclouvain.be/entities/masterthesis/2c8a717a-6bc9-4fb8-8a8c-f8afbe99f015.
- 71.Chamberlain RC, Fecht D, Davies B, Laverty AA. Health effects of low emission and congestion charging zones: a systematic review. Lancet Public Health. 2023;8(7):e559-74. [DOI] [PubMed] [Google Scholar]
- 72.IVA Mobiliteitsbedrijf i.s.m. Transport & Mobility Leuven. Evaluatie Circulatieplan Gent [Internet], Transport. & Mobility Leuven; 2018. Available from: https://stad.gent/sites/default/files/page/documents/Evaluatierapport%20Circulatieplan%20Gent.pdf.
- 73.Vandeninden B, De Clercq EM, Devleesschauwer B, Otavova M, Masquelier B, Fierens F, et al. Impact assessment of local traffic interventions on disease burden: A case study on paediatric asthma incidence in two European cities. J Transp Health. 2025;40: 101953. [Google Scholar]
- 74.Den Hondt E. Interventiestudie schoolomgeving: Impact van schoolstraat. VITO; 2020. Available from: https://www.zorg-en-gezondheid.be/sites/default/files/2022-04/Studie%20impact%20schoolstraat%20-%20Samenvatting%20algemene%20publiek.pdf.
- 75.Janhäll S. Review on urban vegetation and particle air pollution – deposition and dispersion. Atmos Environ. 2015;105:130–7. [Google Scholar]
- 76.Service public de Wallonie. Agriculture, ressources naturelles et environnement. État de l’Environnement Wallon Diagnostic environnemental de la Wallonie 2024;2024. https://environnement.wallonie.be/home/a-la-une/publications/publications/diagnostic-environnemental-de-la-wallonie-2024.html
- 77.Petersen M, Porter K, Gruber S, Wang Y, van der Laan M. Diagnosing and Responding to Violations in the Positivity Assumption. UC Berkeley Division of Biostatistics Working Paper Series. 2010. Available from: https://biostats.bepress.com/ucbbiostat/paper269. [DOI] [PMC free article] [PubMed]
- 78.Schutte N, Saelaert M, Bogaert P, De Ridder K, Van Oyen H, Van der Heyden J, et al. Opportunities for a population-based cohort in Belgium. Arch Public Health. 2022;80(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vanoli J, Mistry MN, De La Cruz Libardi A, Masselot P, Schneider R, Ng CFS, et al. Reconstructing individual-level exposures in cohort analyses of environmental risks: an example with the UK biobank. J Expo Sci Environ Epidemiol. 2024;34(6):1012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Additional file 1: missing data pattern for socio-economic and lifestyle variables of the merged dataset BHIS/BHES.
Supplementary Material 2. Additional file 2: missing data pattern for health variables of the merged dataset BHIS/BHES.
Supplementary Material 3. Additional file 3: missing data pattern for environmental variables of the merged dataset BHIS/BHES.
Supplementary Material 4. Additional file 4: description of the merged BHIS/BHES dataset.
Supplementary Material 5. Additional file 5. Distribution of the annual average exposure to PM2.5 and NO2 of the Belgian health interview survey 2008/2013/2018 participants in Belgium and WHO air quality guideline thresholds.
Supplementary Material 6. Additional file 6. Impact of air pollution reduction scenarios on the distribution of PM2.5 exposure of the Belgian health interview survey participants (2008/2013/2018). Reduction of the PM2.5 annual average exposure: A. 20%, B. 40%, C.60%. D.80%.
Supplementary Material 7. Additional file 7. Impact of air pollution reduction scenarios on the distribution of NO2 exposure of the Belgian health interview survey participants (2008/2013/2018). Reduction of the NO2 annual average exposure: A. 20%, B. 40%, C.60%. D.80%.
Supplementary Material 8. Additional file 8. Impact of air pollution reduction scenarios on the distribution of black carbon exposure of the Belgian health interview survey participants (2008/2013/2018). Reduction of the BC annual average exposure: A. 20%, B. 40%, C.60%. D.80%.
Supplementary Material 9. Additional file 9. Forest plot of the multivariate logistic regression model for the association between stroke and NO2.
Supplementary Material 10. Additional file 10. Forest plot of the multivariate logistic regression model for the association between stroke and Black carbon.
Supplementary Material 11. Additional file 11. Proportion of BHIS participants affected by the different air quality interventions and corresponding average reduction in air pollution exposure.
Data Availability Statement
The data that support the findings of this study are not publicly available. Data are however available from the authors upon reasonable request and with specific permission ([https://www.sciensano.be/en/node/55737/health-interview-survey-microdata-request-procedure](.)). Legal restrictions make that BHIS and BHES data can only be communicated to other parties if an authorization is obtained from the sectoral committee social security and health of the Belgian data protection authority.