Abstract
Background
Malignant hypertension (mHTN) is the most severe form of hypertension. Thrombotic microangiopathy (TMA) serves as both a complication of mHTN and a contributor to its progression by exacerbating renal damage. Proteinuria is a common manifestation of mHTN. However, the impact of proteinuria on renal prognosis in mHTN patients with TMA is unclear.
Methods
This observational cohort study included 276 mHTN-associated TMA patients based on renal biopsy from 2008 to 2023. Demographic characteristics, laboratory results, and histopathological findings were recorded and compared between the mild (< 1 g / 24 h) and significant (≥ 1 g / 24 h) proteinuria groups. Propensity score matching (PSM) was used to adjust for baseline differences. Cox regression model was employed to evaluate risk factors associated with renal prognosis.
Results
Among the 276 patients included in the study, 185 (67.0%) had significant proteinuria, while 91 (33.0%) had mild proteinuria at baseline. After PSM, 83 pairs of patients with mHTN-associated TMA were matched. Patients with significant proteinuria exhibited lower serum albumin, and higher ratio of global sclerosis compared to patients with mild proteinuria. Moreover, mHTN-associated TMA with significant proteinuria was independently associated with receiving renal replacement therapy (RRT) (adjusted hazard ratio (aHR), 1.30; 95% confidence interval (CI), 1.16–1.46; P < 0.001) compared with mild proteinuria. This association remained significant after PSM (aHR, 1.29; 95% CI, 1.13–1.47; P < 0.001). Furthermore, mHTN-associated TMA patients with significant proteinuria had a lower incidence of renal function recovery with a reduction in creatinine levels than in patients with mild proteinuria in the absence of intensive blood pressure control.
Conclusion
In mHTN-associated TMA patients, the presence of significant proteinuria serves as a strong predictor of poor renal outcome.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-025-04407-6.
Keywords: Malignant hypertension, Thrombotic microangiopathy, Significant proteinuria, Renal replacement therapy, Renal biopsy
Introduction
Malignant hypertension (mHTN) is the most severe form of hypertension, defined by very high blood pressure (BP) accompanied by retinal hemorrhages, exudates, and papilledema (grade Ⅲ/Ⅳ hypertensive retinopathy) on funduscopic examination [1]. A study from Birmingham revealed that the overall incidence rate of mHTN is 6 new cases per 100,000 individuals per year. In addition, the five-year survival rate for patients with mHTN is 90%. Among them, 10–35% of patients require dialysis okidney transplantation [2, 3]. Renal damage represents a common event in the course of mHTN [4]. Although the use of medications has further improved the prognosis, the number of patients on hemodialysis for mHTN has also increased in hemodialysis registries in the Netherlands and elsewhere in Europe [5]. Thrombotic microangiopathy (TMA), a condition characterized by endothelial injury or dysfunction, stands as one of the most classic and severe complications of mHTN [6]. The intricate relationship between mHTN and TMA highlights that they can exacerbate each other. Severe hypertension can induce endothelial damage and microangiopathic changes. Meanwhile, TMA can further intensify hypertension through mechanisms such as renal ischemia and activation of the Renin-Angiotensin-Aldosterone System (RAAS) [7]. Previous studies have confirmed that mHTN with pathological manifestations such as TMA is associated with significantly poor renal outcome and nephrosclerosis [8, 9]. However, the risk factors for renal prognosis in mHTN-associated TMA patients have rarely been comprehensively investigated.
Proteinuria has been identified as the most widely recognized and well-studied risk factor for progression to end-stage renal disease (ESRD) in various kidney diseases [10–13]. An early study has highlighted the relationship between proteinuria and renal function in mHTN [14]. A retrospective study also showed that proteinuria may be an independent predictor of poor outcomes, including death or dialysis, in patients with mHTN [15]. However, not all patients in these studies underwent renal pathological examination, and reports on the relationship between proteinuria and mHTN-associated TMA are limited.
The aim of this study was to describe the clinical and pathological characteristics of patients with mHTN-associated TMA and to identify risk factors for worse renal outcomes. The study highlighted the prognostic value of proteinuria for renal outcomes and provided insight into optimal blood pressure management in mHTN-associated TMA patients.
Methods
Study population and cohort
In this prospective cohort study, 276 hospitalized patients with renal damage related to mHTN-associated TMA were enrolled at the First Affiliated Hospital of Sun Yat-sen University from 2008 to 2023. All patients underwent screening to exclude secondary causes of TMA, including infections, autoimmune diseases, transplant-associated TMA, malignancy, pregnancy, and drug-induced causes. A diagnosis of mHTN-associated TMA was established when patients had a clinical diagnosis of mHTN combined with clinicopathological evidence of TMA, after ruling out these secondary factors. Patients with less than three months of follow-up were excluded from the study. mHTN-associated TMA patients were categorized according to the proteinuria level and divided into two different groups, including mild proteinuria group (n = 91) and significant proteinuria group (n = 185). To ensure comparability and minimize selection bias, we applied propensity-score matching (PSM) to adjust for baseline differences between the mild proteinuria and significant proteinuria groups. This study was conducted in accordance with the ethical standards of the Declaration of Helsinki. It was approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China (No. IEC [2022] 710). Written informed consent was obtained from all patients. No financial compensation was provided.
Definitions
mHTN was diagnosed based on the detection of a marked elevation of systolic blood pressure (SBP) levels of 180 mmHg or greater and/or diastolic BP (DBP) levels of 120 mmHg or greater, accompanied by grade III or IV hypertensive retinopathy according to the Keith-Wagener-Barker classification and/or evidence of imminent or progressive target organ dysfunction secondary to hypertension [16, 17]. The diagnosis of mHTN-associated TMA was confirmed based on renal pathological features, including various pathological changes such as capillary loop wrinkling, capsule thickening, significant renal artery intimal thickening, vessel wall “onion skin” appearance thickening, fibrinoid necrosis, intravascular thrombosis, ischemic glomerular changes, and tubular necrosis [6, 17, 18]. In this study, severe cardiac involvement was defined as a left ventricular ejection fraction ≤ 50% [19]. According to relevant studies and guidelines, proteinuria in mHTN patients was defined as mild proteinuria (< 1 g / 24 h) and significant proteinuria (≥ 1 g / 24 h) [15, 20–23]. In our study, systolic blood pressure (SBP) was controlled to ≤ 130 mmHg as the criterion for intensive blood pressure management, based on consultation with both international and national clinical guidelines [23, 24].
Data collection and renal histopathology
Blood and urine samples were obtained within the first 24 h of patient admission. All patients underwent comprehensive diagnostic evaluations, including chest radiography, renal ultrasound, and echocardiography. Baseline clinical and demographic data were systematically collected, including age, sex, body mass index (BMI), blood pressure readings, 24-hour proteinuria levels, platelet counts, hemoglobin levels, albumin concentrations, cholesterol levels, triglycerides, low-density lipoprotein cholesterol (LDL-c), serum creatinine, uric acid, and serum complement C3 and C4 levels. Mean arterial pressure (MAP) was defined as one-third of the SBP plus two-thirds of the diastolic blood pressure (DBP). Prescription data included statins, sulodexide, febuxostat, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARBs), sacubitril/valsartan (ARNI), and various antihypertensive medications, including α-blockers, β-blockers, and calcium channel blockers.
Percutaneous renal biopsy specimens were routinely processed according to established protocols. Renal biopsies were processed for standard light microscopy, electron microscopy, and direct immunofluorescence. Immunofluorescence was performed using fluorescein isothiocyanate-conjugated antibodies specific for human immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA), complement component 1q (C1q), complement component 3 (C3), and both kappa (κ) and lambda (λ) light chains. All biopsies were reviewed by two senior pathologists. In case of disagreement, discussions were held until consensus was reached. Light microscopic data including the number of glomeruli, the percentage of global sclerosis, segmental sclerosis and tubular atrophy/interstitial fibrosis. Additionally, vascular parameters, including hyaline degeneration, fibrinoid necrosis, “onion skin” appearance, intravascular thrombosis, and intravascular erythrocyte (RBC) fragments were also documented. Electron microscopic evaluation included identification of subepithelial, subendothelial, or mesangial deposits and assessment of the degree of endothelial cell swelling.
Study outcomes
The primary outcome of the study was renal replacement therapy (RRT), which was defined as the need for hemodialysis, peritoneal dialysis, or kidney transplantation during follow-up. All patients were followed up by nephrologists and trained nurses through office visits or telephone interviews, and the last follow-up date was June 30, 2023.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or the median (interquartile range, 25th, 75th percentile) depending on their distribution. Continuous variables were compared using the Mann-Whitney U test for non-normally distributed variables and the Student’s t-test for normally distributed variables. Categorical variables were expressed as frequencies (percentages), and analyzed with the chi-squared test or the Fisher exact test. Time to reach study outcomes was estimated using the Kaplan-Meier model, with survival comparisons between the mild proteinuria group and the significant proteinuria groups based on the log-rank test. Crude and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using univariate and multivariate Cox proportional hazards regression models. Variables that were statistically significant in the univariate analysis or considered clinically relevant to kidney outcomes were included in the multivariate analysis [25]. To adjust for the baseline differences and to minimize potential selection bias, PSM was applied between the mild proteinuria group and the significant proteinuria group. Supplementary Table 1 lists the variables included in the PSM for adjustment. A 1:1 match was performed using the greedy-matching algorithm with a caliper of 0.02 [26, 27]. A Survival analysis was used to assess the prognosis before and after PSM. All statistical analyses were performed using SPSS (version 25.0; IBM, Armonk, NY, USA), with P < 0.05 indicating a statistically significant result.
Results
Baseline demographics and characteristics
Between 2008 and 2023, a total of 276 patients underwent clinical renal biopsy and were pathologically diagnosed with mHTN-associated TMA. These patients were included in this study. A flow chart illustrating this process is shown in Supplementary Fig. 1. 91 (33.0%) patients were in the mild proteinuria group and 185 (67.0%) patients were in the significant proteinuria group. The baseline characteristics of the patients before and after PSM are shown in Table 1. Patients with significant proteinuria had lower serum albumin levels (mean ± standard deviation: 36.1 ± 5.0 vs. 38.5 ± 4.3 g/L; P < 0.001) and higher serum creatinine levels (median (interquartile range): 484.0 (296.0, 694.5) vs. 337.0 (199.0, 700.0) µmol/L; P = 0.005) than those with mild proteinuria.
Table 1.
Baseline clinical and pathological characteristics before and after propensity score matching
| Characteristic | Entire cohort | Propensity score-matched cohort | |||||
|---|---|---|---|---|---|---|---|
| Total (n = 276) | Mild proteinuria (n = 91) | Significant proteinuria (n = 185) | P-value | Mild proteinuria (n = 83) | Significant proteinuria (n = 83) | P-value | |
| Clinical characteristics | |||||||
| Age, years | 35.8 ± 8.8 | 35.7 ± 8.6 | 35.8 ± 8.9 | 0.889 | 35.8 ± 8.2 | 35.2 ± 8.2 | 0.649 |
| Male, n (%) | 247 (89.5) | 84 (92.3) | 163 (88.1) | 0.285 | 76 (91.6) | 76 (91.6) | >0.999 |
| BMI, kg/m2 | 24.8 ± 4.0 | 24.5 ± 4.1 | 24.9 ± 4.0 | 0.369 | 24.5 ± 4.0 | 24.5 ± 3.7 | 0.954 |
| Smoking, n (%) | 125 (45.3) | 40 (44.0) | 85 (45.9) | 0.755 | 38 (45.8) | 38 (45.8) | >0.999 |
| Drinking, n (%) | 81 (29.3) | 25 (27.5) | 56 (30.3) | 0.631 | 25 (30.1) | 28 (33.7) | 0.617 |
| SBP, mmHg | 217.8 ± 24.9 | 216.9 ± 22.7 | 218.2 ± 25.9 | 0.678 | 216.2 ± 22.7 | 219.9 ± 26.7 | 0.335 |
| DBP, mmHg | 136.8 ± 20.6 | 133.7 ± 19.7 | 138.3 ± 30.0 | 0.082 | 134.8 ± 19.3 | 137.0 ± 19.0 | 0.455 |
| MAP, mmHg | 163.9 ± 19.2 | 161.4 ± 17.1 | 165.1 ± 20.1 | 0.137 | 161.9 ± 17.4 | 164.6 ± 18.9 | 0.33 |
| Laboratory values | |||||||
| Platelet count, 10^9/L | 261.5 ± 85.6 | 255.2 ± 78.7 | 264.6 ± 88.8 | 0.392 | 277.7 ± 96.2 | 273.8 ± 87.1 | 0.782 |
| Hemoglobin, g/L | 107.4 ± 23.0 | 110.3 ± 24.3 | 106.0 ± 22.3 | 0.145 | 108.6 ± 23.8 | 108.9 ± 23.5 | 0.937 |
| Serum albumin, g/L | 36.9 ± 4.9 | 38.5 ± 4.3 | 36.1 ± 5.0 | <0.001 | 38.5 ± 4.3 | 36.4 ± 4.8 | 0.004 |
| Total cholesterol, mmol/L | 4.7 (4.0, 5.6) | 4.8 (4.0, 5.7) | 4.7 (4.0, 5.6) | 0.887 | 4.7 (4.0, 5.6) | 4.5 (3.7, 6.0) | 0.736 |
| Triglycerides, mmol/L | 1.8 (1.3, 2.3) | 1.6 (1.3, 1.6) | 1.8 (1.3. 2.5) | 0.05 | 1.6 (1.3, 2.1) | 1.8 (1.3, 2.4) | 0.149 |
| LDL-C, mmol/L | 3.0 (2.4, 3.6) | 3.0 (2.4, 3.7) | 2.9 (2.3, 3.6) | 0.952 | 3.0 (2.3, 3.5) | 2.9 (2.2, 3.7) | 0.961 |
| Serum creatinine, mmol/L | 447.5 (263.0, 696.8) | 337.0 (199.0, 700.0) | 484.0 (296.0, 694.5) | 0.005 | 363.0 (203.0, 703.0) | 427.0 (294.0, 620.0) | 0.17 |
| eGFR, mL/min/1.73m2 | 10.6 (6.5, 21.1) | 15.4 (6.4, 27.2) | 10.2 (6.6, 17.2) | 0.013 | 13.3 (6.4, 25.8) | 11.2 (7.0, 17.8) | 0.304 |
| Uric acid, mmol/L | 479.5 ± 132.0 | 464.9 ± 123.7 | 486.8 ± 135.7 | 0.197 | 471.8 ± 124.5 | 473.2 ± 140.7 | 0.944 |
| C3, g/L | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.1) | 1.0 (0.8, 1.2) | 0.717 | 1.0 (0.9, 1.2) | 1.0 (0.8, 1.8) | 0.197 |
| C4, g/L | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 0.635 | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.3) | 0.15 |
| Hypocomplementemia C3 (%) | 23 (8.3) | 6 (6.6) | 17 (9.2) | 0.465 | 6 (7.2) | 9 (10.8) | 0.42 |
| Ejection fraction | 60.6 ± 9.7 | 61.8 ± 9.5 | 60.1 ± 9.8 | 0.203 | 62.1 ± (9.8) | 62.0 ± (9.3) | 0.309 |
| Severe cardiac involvement (%) | 36 (13.0) | 10 (11.0) | 26 (14.1) | 0.478 | 11 (13.3) | 12 (14.5) | 0.822 |
| Medications, n (%) | |||||||
| Statin | 128 (46.4) | 38 (41.8) | 90 (48.6) | 0.28 | 34 (41.0) | 37 (44.6) | 0.638 |
| Sulodexide | 142 (51.4) | 49 (53.9) | 93 (50.3) | 0.576 | 45 (54.2) | 39 (47.0) | 0.352 |
| Febuxostat | 58 (21.0) | 15 (16.5) | 43 (23.2) | 0.195 | 14 (16.0) | 17 (20.5) | 0.55 |
| ACEI | 59 (21.4) | 17 (18.7) | 42 (22.7) | 0.444 | 17 (20.5) | 18 (21.7) | 0.849 |
| ARB/ARNI | 180 (65.2) | 63 (69.2) | 117 (63.2) | 0.326 | 57 (68.7) | 54 (65.1) | 0.621 |
| α−βλοχκερ | 168 (60.9) | 55 (60.4) | 113 (61.1) | 0.918 | 49 (59.0) | 49 (59.0) | >0.999 |
| β−βλοχκερ | 236 (85.5) | 77 (84.6) | 159 (85.9) | 0.768 | 70 (84.3) | 72 (86.7) | 0.659 |
| CCB | 266 (96.4) | 87 (95.6) | 179 (96.8) | 0.734 | 79 (95.2) | 81 (97.6) | 0.682 |
Data are median (25th, 75th percentile), mean ± standard deviation and percentage unless otherwise indicated. BMI, body mass index. SBP, systolic blood pressure. DBP, diastolic blood pressure. MAP, mean arterial pressure. LDL-C, low density lipoprotein cholesterol. eGFR, estimated glomerular filtration rate. C3, complement 3. C4, complement 4. ACEI, angiotensin-converting enzyme inhibitor. ARB, angiotensin Ⅱ receptor blocker. ARNI, sacubitril-valsartan. CCB, calcium channel blocker. Hypocomplementemia C3 was defined as a serum complement 3 concentrations below 0.75 g/L. Severe cardiac involvement was defined as a left ventricular ejection fraction less than or equal to 50%
After PSM, 83 patients with significant proteinuria were matched with 83 patients with mild proteinuria (Supplementary Fig. 1). A reassessment of the baseline characteristics after PSM showed that a satisfactory balance had been achieved between the two groups. Patients with significant proteinuria had lower serum albumin levels compared to patients with mild proteinuria (36.4 ± 4.8 vs. 38.5 ± 4.3, P = 0.004) (Table 1). No significant differences were observed between the two groups with regard to other laboratory data or drug treatment.
Renal histopathological characteristics
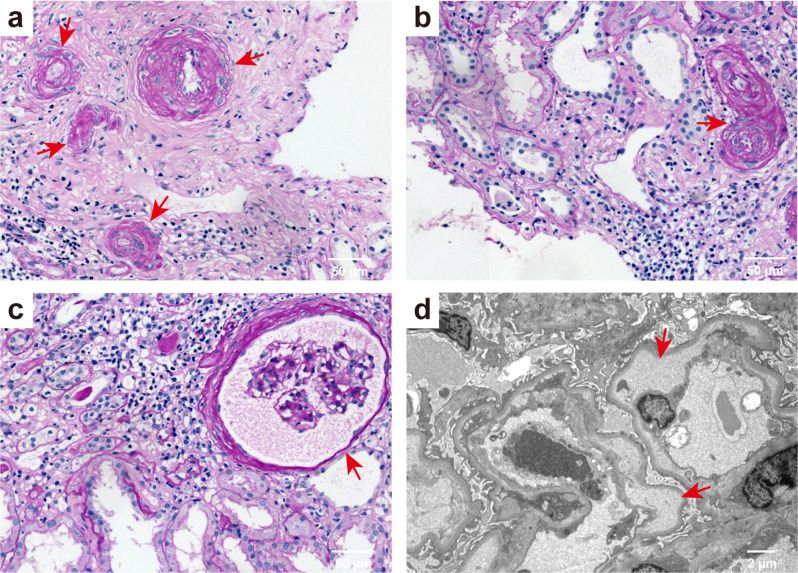
All patients with mHTN-associated TMA underwent percutaneous renal biopsy (Fig. 1). Light microscopic analysis revealed a typical set of pathological changes in mHTN-associated renal TMA, including vessel wall thickening exhibiting an “onion skin” appearance (Fig. 1a), characteristic intimal thickening, and mucus degeneration within the renal artery (Fig. 1b). Additionally, there was diffuse wrinkling of the capillary loops accompanied by capsular thickening (Fig. 1c). The electron micrograph demonstrated endothelial cell swelling and significant subendothelial widening with the presence of flocculent material beneath (Fig. 1d).
Fig. 1.
Representative light, electron microscopic, and immunofluorescence findings in mHTN patients with TMA. (a) Periodic acid-Schiff (PAS) staining showing vessel wall thickening with an “onion skin” appearance (red arrow). (b) PAS staining showing typical intimal thickening and mucus degeneration of renal artery. (c) PAS staining showing diffuse winkling of the capillary loop and capsular thickening. a-c, scale bar: 50 μm. (d) Electron micrograph showing endothelial cell swelling, and marked subendothelial widening with flocculent material underneath. Scale bar: 2 μm
The renal histopathologic findings of the patients are shown in Table 2. Compared to patients with mild proteinuria, patients with significant proteinuria had a lower number of glomeruli (22.0 (16.0, 31.0) vs. 27.0 (19.0, 35.0), P = 0.010) and a higher prevalence of global sclerosis (37.9 (20.0, 65.5) vs. 27.3 (17.2, 45.7), P = 0.011). In addition, patients with significant proteinuria exhibited a higher prevalence of fibrinoid necrosis than in patients with mild proteinuria (51 (27.6) vs. 38 (41.8), P = 0.018). After PSM, patients with significant proteinuria also had a higher prevalence of global sclerosis (47.8 (23.1, 70.4) vs. 27.3 (17.0, 45.7), P = 0.003). No significant differences were observed in the prevalence of other pathological features between the two groups.
Table 2.
Histopathologic findings of patients before and after propensity score matching
| Characteristic | Entire cohort | Propensity score-matched cohort | |||||
|---|---|---|---|---|---|---|---|
| Total (n = 276) |
Mild proteinuria (n = 91) |
Significant proteinuria (n = 185) |
P-value | Mild proteinuria (n = 83) |
Significant proteinuria (n = 83) |
P-value | |
| Global lesions, n (%) | |||||||
| Number of glomeruli, number | 24.0 (18.0, 33.0) | 27.0 (19.0, 35.0) | 22.0 (16.0, 31.0) | 0.010 | 28.0 (19.0, 35.0) | 22.0 (16.0, 32.0) | 0.054 |
| Global sclerosis (%) | 33.3 (18.9, 60.8) | 27.3 (17.2, 45.7) | 37.9 (20.0, 65.5) | 0.011 | 27.3 (17.0, 45.7) | 47.8 (23.1, 70.4) | 0.003 |
| Segmental sclerosis (%) | 0.0 (0.0, 6.3) | 0.9 (0.0, 5.0) | 0.0 (0.0, 7.1) | 0.446 | 0.0 (0.0, 5.0) | 2.2 (0.0, 7.4) | 0.247 |
| Tubular atrophy/interstitial fibrosis, n (%) | 0.311 | 0.552 | |||||
| <25% | 10 (3.6) | 6 (6.6) | 4 (2.2) | 5 (6.0) | 1 (1.2) | ||
| 25–50% | 55 (19.9) | 18 (19.8) | 37 (20.0) | 17 (20.5) | 17 (20.5) | ||
| 50–75% | 159 (57.6) | 52 (57.1) | 107 (57.8) | 31 (37.3) | 25 (30.1) | ||
| >75% | 48 (17.4) | 14 (15.4) | 34 (18.4) | 14 (16.9) | 12 (14.5) | ||
| Vascular lesions, n (%) | |||||||
| Hyaline degeneration | 148 (53.6) | 50 (54.9) | 98 (53.0) | 0.757 | 45 (54.2) | 41 (49.4) | 0.534 |
| Fibrinoid necrosis | 89 (32.2) | 38 (41.8) | 51 (27.6) | 0.018 | 37 (44.6) | 27 (32.5) | 0.111 |
| Onion skin appearance | 167 (60.5) | 57 (62.6) | 110 (59.5) | 0.611 | 49 (59.0) | 49 (59.0) | >0.999 |
| Intravascular thrombosis | 49 (17.8) | 21 (23.1) | 28 (15.1) | 0.105 | 20 (24.1) | 14 (16.9) | 0.249 |
| Intravascular RBC fragments | 27 (9.8) | 9 (9.9) | 18 (9.7) | 0.966 | 9 (10.8) | 9 (10.8) | >0.999 |
Data are median (25th, 75th percentile) and percentage unless otherwise indicated. RBC, red blood cell
Risk of significant proteinuria on the primary outcome of renal replacement therapy in patients with mHTN-associated TMA
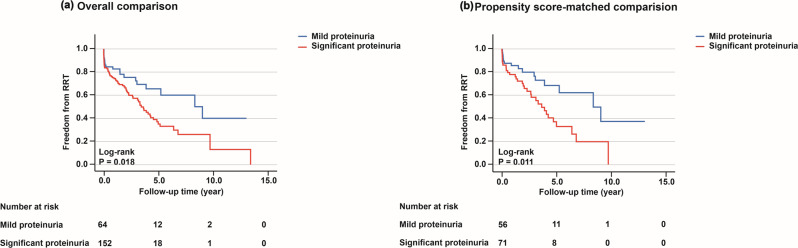
After a median follow-up period of 48.8 months (95% CI, 34.6–63.1), the primary outcome occurred in 93 patients (42.9%). The cumulative effect of patients with significant proteinuria on the hazard of RRT was higher compared to patients with mild proteinuria (overall comparison, P = 0.018; propensity score-matched comparison, P = 0.011; Fig. 2). In the crude analysis, patients with significant proteinuria exhibited a higher risk of RRT compared to patients with mild proteinuria (HR, 1.40; 95% CI, 1.15–1.70; P = 0.001). This difference remained statistically significant after adjustment for both the overall comparison (adjusted HR, 1.30; 95% CI, 1.16–1.46; P < 0.001) and the propensity score-matched comparison (adjusted HR, 1.29; 95% CI, 1.13–1.47; P < 0.001) (Table 3).
Fig. 2.
Cumulative risk of renal replacement therapy in patients with mild proteinuria versus significant proteinuria: (a) overall comparison. (b) propensity score-matched comparison. The primary outcome of this study was defined as starting renal replacement therapy. RRT, Renal Replacement Therapy
Table 3.
Association between mild proteinuria group and significant proteinuria group and study outcome within the crude analysis, multivariable analysis, and propensity score matching analysis
| Variable | HR (95% CI) for study outcome | P-value |
|---|---|---|
| The primary outcome of starting renal replacement therapy | ||
| No. of events/No. of patients at risk (%) | 93/217 (42.9) | <0.001 |
| Mild proteinuria | 20/65 (30.8) | |
| Significant proteinuria | 73/152 (48.0) | |
| Crude analysis a | 1.40 (1.15–1.70) | 0.001 |
| Multivariable analysis b | 1.30 (1.16–1.46) | <0.001 |
| Propensity score matching c | 1.29 (1.13–1.47) | <0.001 |
a The HRs from the bivariable model in all patients from the unmatched study
b The HRs from the multivariable stratified Cox proportional hazards regression model, with additional covariate adjustment
c The HR from propensity score-matched sample, constructed using 1: 1 earest neighbor matching with a 0.02 caliper
The primary outcome was defined as starting renal replacement therapy
In addition, risk factors for RRT in patients with mHTN-associated TMA are presented in Supplementary Fig. 2. In the multivariable Cox regression model, we adjusted for variables that were either statistically significant (P < 0.05) in the univariable analysis or considered clinically relevant to kidney outcomes. Patients with significant proteinuria were more likely to require RRT than patients with mild proteinuria (adjusted HR, 1.30; 95% CI, 1.16–1.46; P < 0.001). Moreover, lower platelet count (adjusted HR, 0.996; 95% CI, 0.993–0.999; P = 0.010), and higher serum creatinine (adjusted HR, 1.003; 95% CI, 1.003–1.004; P < 0.001), higher prevalence of global sclerosis (adjusted HR, 1.06; 95% CI, 1.03–1.09; P < 0.001), and a higher proportion of tubular atrophy/interstitial fibrosis ≥ 50% (adjusted HR, 3.19; 95% CI, 1.36–7.50; P = 0.008) were associated with an increased risk of RRT in patients with mHTN-associated TMA.
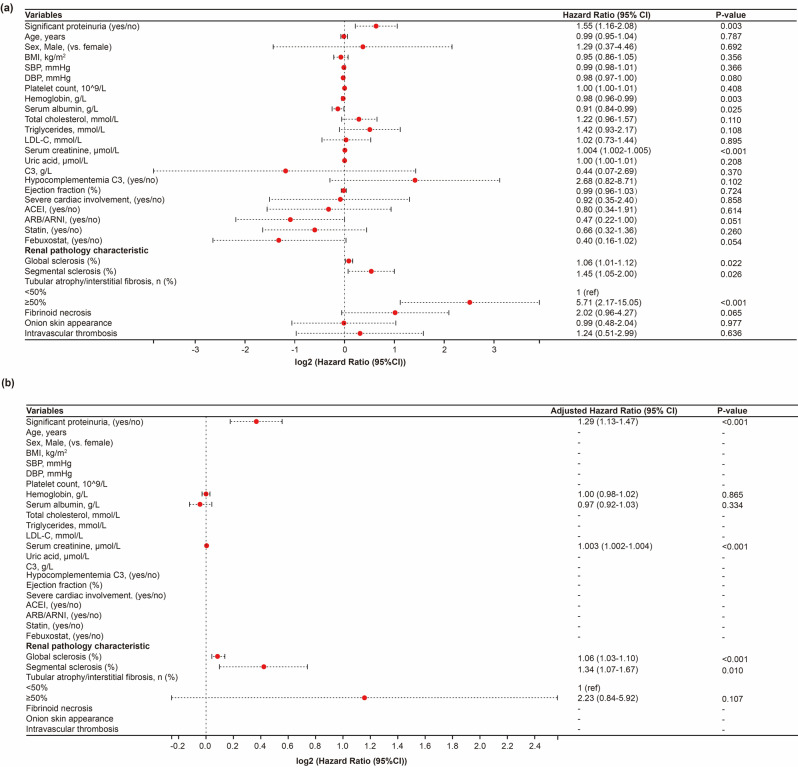
A comparable pattern was observed in the PSM cohort (Fig. 3). After the multivariable Cox regression analysis, patients with significant proteinuria (adjusted HR, 1.29; 95% CI, 1.13–1.47; P < 0.001), higher serum creatinine levels (adjusted HR, 1.003; 95% CI, 1.002–1.004; P < 0.001), and a higher prevalence of global sclerosis (adjusted HR, 1.06; 95% CI, 1.03–1.10; P < 0.001) and segmental sclerosis (adjusted HR, 1.34; 95% CI, 1.07–1.67; P = 0.010) in a renal biopsy specimen were identified as risk factors for RRT in patients with mHTN-associated TMA. No differences in medication use were observed in the PSM cohort.
Fig. 3.
Forest plot showing the result of Cox regression analysis for the primary outcome of renal replacement therapy in the propensity score-matched cohort. (a) The univariate Cox regression analysis. (b) The multivariable Cox regression analysis. *P < 0.05. HR, hazard ratio. 95% CI, 95% confidence interval. BMI, body mass index. SBP, systolic blood pressure. DBP, diastolic blood pressure. HDL-C, high density lipoprotein cholesterol. LDL-C, low density lipoprotein cholesterol. C3, complement 3. ACEI, angiotensin-converting enzyme inhibitor. ARB, angiotensin Ⅱ receptor blocker. ARNI, sacubitril-valsartan. Hypocomplementemia C3 was defined as a serum complement 3 concentrations below 0.75 g/L. Severe cardiac involvement was defined as a left ventricular ejection fraction less than or equal to 50%
Intensive blood pressure control management
To assess the benefit of intensive blood pressure control, patients were divided into two groups based on SBP at the time of discharge. The effect of proteinuria on renal prognosis was evaluated. Table 4 showed that in the SBP>130mmHg group, patients with significant proteinuria exhibited a lower incidence of renal function recovery of a 25% decrease in creatinine compared to patients with mild proteinuria (P = 0.032). Similarly, patients with significant proteinuria were less likely than those with mild proteinuria to have a favorable renal function recovery of a 50% decrease in creatinine than those with mild proteinuria (P = 0.027). However, no significant difference was observed between the two groups for the aforementioned two endpoints of renal function recovery when SBP ≤ 130 mmHg (P > 0.05).
Table 4.
Estimated event rates among intensive blood pressure control between mild proteinuria group and significant proteinuria group
| Event | SBP ≤ 130mmHg | SBP>130mmHg | ||||
|---|---|---|---|---|---|---|
| Mild proteinuria | Significant proteinuria | P-value | Mild proteinuria | Significant proteinuria | P-value | |
| Long term RRT | 7 (26.9) | 26 (43.3) | 0.151 | 13 (33.3) | 47 (51.1) | 0.062 |
| Recovery 1 | 15 (60.0) | 39 (62.9) | 0.801 | 24 (68.6) | 46 (47.4) | 0.032 |
| Recovery 2 | 5 (20.0) | 21(33.9) | 0.201 | 15 (45.5) | 24 (25.0) | 0.027 |
| Recovery 3 | 0 (0.0) | 6 (20.0) | 0.571 | 6 (30.0) | 12 (21.1) | 0.540 |
RRT: renal replacement therapy
Recovery 1: A 25% decrease in creatinine, or a decrease in creatinine to normal, or renal survival free from replacements therapy for one month
Recovery 2: A 50% decrease in creatinine, or a decrease in creatinine to normal, or renal survival free from replacements therapy for one month
Recovery 3: Free from dialysis for patient dependent on dialysis at baseline
Discussion
This observational cohort study showed that mHTN-associated TMA patients with significant proteinuria had lower serum albumin levels and higher global sclerosis scores compared to patients with mild proteinuria. In addition, patients with significant proteinuria were more likely to progress to RRT compared with the mild proteinuria group (overall comparison, HR 1.30; 95% CI 1.16–1.46; P < 0.001; propensity score-matched comparison, HR 1.29; 95% CI 1.13–1.47; P < 0.001). Furthermore, multivariable regression analysis revealed that higher serum creatinine levels were associated with an increased likelihood of requiring RRT. Moreover, the pathological changes in the kidney, including the extent of global sclerosis and segmental sclerosis were identified as unfavorable factors for RRT. Finally, our study found that significant proteinuria may be a strong risk factor for poor renal function recovery in the absence of intensive blood pressure control.
In mHTN patients, kidney complications are frequently associated with a poor prognosis. These renal issues often manifest as proteinuria, hemolysis, low platelet count, and impaired kidney function. These characteristics are very similar to the key features of TMA [28]. TMA has been recognized as a complication of mHTN, with a reported prevalence ranging from 14 to 46% [29, 30]. A study conducted in Spain showed that mHTN patients with TMA exhibited a higher incidence of kidney failure than those without TMA [2]. Recent evidence indicates that aberrant activation of the complement alternative pathway (cAP) is a hallmark of TMA pathogenesis [31, 32]. Abnormal complement levels correlate with poorer renal outcomes, suggesting that complement dysregulation may contribute to the progression of mHTN-associated TMA. Nevertheless, more the underlying mechanisms and clinical implications of mHTN complicated by TMA remain to be explored.
Although the effective treatment with antihypertensive agents could improve renal function, a significant proportion of surviving patients still require long-term dialysis or transplantation [5, 33]. Our study confirmed that 42.9% (93/217) of mHTN-associated TMA patients required RRT during the follow-up period. This highlights the importance of identifying risk factors early on and intervening promptly to improve patient outcomes. In a single-center retrospective analysis, Manuel Praga and his colleagues suggested that mean proteinuria during the follow-up period is pivotal in determining the renal outcomes of patients with mHTN [34]. A study from Birmingham also confirmed that elevated serum creatinine and proteinuria levels are significant risk factors for the presence of renal dysfunction in mHTN patients [15]. These observations were consistent with our conclusions, suggesting that patients with significant proteinuria were more likely to require RRT treatment compared to those with mild proteinuria. Therefore, persistent proteinuria can be considered as a significant predictor of adverse outcomes in mHTN-associated TMA patients.
Given that RAAS activation is a key pathogenic mechanism in mHTN and that RAAS blockers have specific anti-proteinuric properties [35], the relationship between the use of RAAS blockers and adverse outcomes associated with RRT was analyzed. It was observed that the use of ARB/ARNI was associated with a decreased likelihood of requiring RRT in mHTN-associated TMA patients. However, when included in a multivariate Cox regression analysis, ARBs were not found to be a protective factor. Nevertheless, their dual mechanism of reducing blood pressure and proteinuria levels cannot be overlooked in the recovery of renal function [36]. Our previous research showed that sacubitril/valsartan treatment was associated with a potential benefit to renal function in patients with mHTN-associated TMA compared to ACEI/ARB treatment. This effect can be attributed to the mechanistic advantages of ARNI, which inhibit the degradation of endogenous natriuretic peptides. This enhances their effects on natriuresis, diuresis, and vasodilation, while also suppressing the release of excessive renin and aldosterone [25]. These findings suggest that ARNI might be a superior therapeutic strategy for the management of this serious condition in terms of renal recovery. In addition, none of the patients in our cohort with mHTN-associated TMA received plasma replacement therapy or eculizumab therapy. Some treatments include plasma replacement or exchange, alongside emerging therapies, such as the complement C5 inhibitor eculizumab [37]. Cavero et al. showed that renal and hematological responses are significantly better with eculizumab compared to plasmapheresis. Eculizumab has been shown to improve renal survival in patients with mHTN, and these benefits appear to be independent of the severity of hypertension and complement genetic abnormalities [1]. Future studies with standardized, comprehensive data are needed to clarify the roles of plasma therapy and eculizumab in mHTN-associated TMA and their effects on renal outcomes.
Serum albumin is a widely used as clinical biomarker with prognostic value for numerous health outcomes [38]. A retrospective study revealed that hypoalbuminemia was related to a poorer renal prognosis in patients with type 2 diabetes mellitus (T2DM) and diabetic nephropathy (DN). In addition, the study found that the serum albumin level had a significant inverse correlation with proteinuria and glomerular lesions [39]. This conclusion is supported by our data. In the present study, we found that low serum albumin level was a negative factor for the renal outcome of RRT in mHTN-associated TMA patients. Furthermore, our baseline data showed that patients with mHTN-associated TMA and significant proteinuria had lower albumin levels than those with mild proteinuria. This effect remains after PSM and may be attributed to the following factors. On the one hand, the extensive damage to the glomerular filtration barrier results in massive proteinuria, which often leads to albumin loss and ultimately manifests as nephrotic syndrome [40]. On the other hand, systemic inflammation, compromised nutritional status, and endothelial dysfunction further exacerbate hypoalbuminemia [41, 42]. These observations suggested that hypoalbuminemia may serve as an independent predictor of unfavorable renal outcomes in this patient population, highlighting the need for further investigation to confirm these associations and explore potential therapeutic interventions.
Glomerulosclerosis and tubular atrophy/interstitial fibrosis are chronic, irreversible changes that are often observed in a renal biopsy specimen [43, 44]. Previous studies have indicated that chronic changes are associated with worse renal outcomes [45–47]. S. kadiri et al. demonstrated that glomerulosclerosis is a prominent feature of primary mHTN and may contribute to renal dysfunction in mHTN patients. Those with global glomerulosclerosis appeared to have the highest serum creatinine levels [48]. Previous research has also emphasized the importance of segmental glomerulosclerosis as an effective factor for predicting predictor of renal prognosis [33]. Similarly, our study found that both global glomerulosclerosis and segmental glomerulosclerosis were independent risk factors for RRT in mHTN-associated TMA patients. In addition to glomerular variables, tubular atrophy/interstitial fibrosis is another valuable predictor of worse renal prognosis in kidney disease. A study conducted in Northern India by Sanjay Vikrant et al. found that patients with IgA nephropathy and mHTN who exhibited tubular atrophy/interstitial fibrosis of at least 25% were less likely to respond favorably to treatment [49]. This finding is consistent with ours that tubular atrophy/interstitial fibrosis > 50% was independently predictive of progression to RRT in mHTN-associated TMA patients. These conclusions further confirm the value of pathological features in predicting renal prognosis.
Cardiac involvement is a recognized complication in patients with mHTN and TMA. Previous studies have reported that approximately 28% of patients with concomitant TMA and mHTN exhibit cardiac involvement, with 27% classified as severe [19, 28]. In our cohort, 13% of patients had severe cardiac involvement based on a left ventricular ejection fraction ≤ 50%, which is lower than previous reports and may reflect differences in population or assessment methods. In the present study, severe cardiac involvement was not associated with long-term renal replacement therapy risk in either the overall or PSM cohorts. Future studies with larger cohorts and standardized assessments are needed to clarify the role of cardiac involvement in renal outcomes. Including cardiac variables in prognostic models may improve risk stratification and treatment guidance for patients with mHTN-associated TMA.
Determining the optimal treatment target for blood pressure in patients with kidney disease has emerged as a key area of investigation [50]. A meta-analysis showed that intensive blood pressure control significantly reduced both cardiovascular events and proteinuria. Patients with vascular and renal disease experienced the greatest absolute benefit [51]. Nevertheless, the precise target for blood pressure to prevent deterioration of renal function in patients with mHTN, especially those with TMA, remains uncertain. A study in Australia showed that intensive blood pressure reduction appeared to protect against kidney failure events in patients with chronic kidney disease [52]. Our study found that without intensive blood pressure control, the mild proteinuria group showed better renal function recovery than the significant proteinuria group. This conclusion indirectly supports the importance of proteinuria in renal prognosis. Further studies are therefore needed to evaluate the benefits of intensive blood pressure control on renal outcomes in mHTN-associated TMA patients.
Our study has several limitations. First, while all diagnoses of TMA were established through renal histopathology, our study lacked a comprehensive diagnostic classification of the various TMA etiologies. It is important to note that we identified TMA associated with mHTN after ruling out secondary causes. However, some key parameters for the clinical diagnosis of TMA, including haptoglobin, peripheral smear, reticulocyte count, lactate dehydrogenase (LDH), and a disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13) levels, were not collected in our study. Future studies will improve data collection. Second, due to the severity and variability of kidney impairment, proteinuria was measured within 24 h of admission. However, acute kidney injury may be influenced by other factors, such as hemodynamic changes. This could lead to overestimation, even when the primary cause is malignant hypertension. Third, the median change in creatinine varied among patients in our intensified blood pressure control cohort. Some patients showed decreases, some remained stable, and a few experienced increases. Therefore, we did not report summary statistics for changes in serum creatinine by group, which may limit the interpretability of the results. Fourth, caution is warranted when generalizing these findings to patients from different ethnic backgrounds, as our study was confined to Chinese individuals with mHTN-associated TMA. Fifth, some important confounders, such as C-reactive protein (CRP), CKD history, diabetes status, liver function, and other medications, were not included in this analysis. Their impact deserves consideration in future studies to better control for potential bias. Although PSM was employed to mitigate selection bias, the reduced sample size after matching may have decreased statistical power, increasing the risk of missing true effects. In addition, the significant differences in baseline albumin levels after PSM highlight an important limitation: PSM did not fully account for unmeasured confounders that may independently influence albumin levels. Consequently, residual confounding might still be present. Finally, the 15-year study period may have included therapy changes that affected the results. The lack of complement activation, complement gene testing and tubular injury markers limited our understanding of the mechanisms. Future studies should have a longer follow-up period and include such variables to better understand mHTN-associated TMA.
Conclusion
In conclusion, this study contributes to the accumulating evidence supporting that significant proteinuria is identified as a strong prognostic factor for predicting unfavorable renal outcomes in patients of mHTN-associated TMA. Therefore, monitoring the level of proteinuria in individuals with mHTN-related TMA may provide valuable insights into the potential trajectory of renal health, enabling healthcare professionals to make more informed decisions regarding patient management and treatment strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank The First Affiliated Hospital, Sun Yat-sen University for their invaluable support and contributions to this research.
Abbreviations
- ADAMTS13
A disintegrin and metalloproteinase with thrombospondin motifs 13
- ARB
Angiotensin receptor blocker
- ACEI
Angiotensin-converting enzyme inhibitor
- BP
Blood pressure
- BMI
Body mass index
- C1q
Complement component 1q
- C3
Complement component 3
- cAP
Complement alternative pathway
- Cis
Confidence intervals
- CRP
C-reactive protein
- DBP
Diastolic blood pressure
- DN
Diabetic nephropathy
- ERSD
End-stage renal disease
- RBC
Erythrocyte
- HRs
Hazard ratios
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- LDL-c
Low-density lipoprotein cholesterol
- LDH
Lactate dehydrogenase
- mHTN
Malignant hypertension
- MAP
Mean arterial pressure
- PSM
Propensity score matching
- RRT
Renal replacement therapy
- SD
Standard deviation
- SBP
Systolic blood pressure
- T2DM
Type 2 diabetes mellitus
- TMA
Thrombotic microangiopathy
Author contributions
All authors contributed to each of the following aspects of the study: Jianbo Li and Feng He designed the research study and revised the manuscript. Wanxin Shi and Xingji Lian collected the data and wrote the paper. Wenchuan Li, Rong Lian, Shengyou Yu and Zefang Dai were responsible for data acquisition. Zhong Zhong, Yiqin Wang, and Wei Chen were responsible for data analysis and statistical analysis. All authors reviewed and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 82070752, 82470748, 82170737, 82370707), National Key Research and Development Project of China (No. 2021YFC2501302, 2022YFC2705103, 2022YFC2705100, 2022YFC2705104), Guangdong Natural Science Foundation (Grant nos. 2022B1515020106, 2023A1515012477), Key Laboratory of National Health Commission, and Key Laboratory of Nephrology, Guangdong Province, Guangzhou, China (Nos. 2002B60118 and 2020B1212060028), the Guangdong Provincial Department of Science and Technology (Grant no. 202201020273), Guangzhou Municipal Programme of Science and Technology (2024B03J1337), and the Science and Technology Projects in Guangzhou, China (No. 2023A04J0613).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University (No. IEC [2022] 710). All procedures involving human participants were performed after obtaining informed consent from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wanxin Shi and Xingji Lian contributed equally to this work.
Contributor Information
Jianbo Li, Email: lijianb5@mail.sysu.edu.cn.
Feng He, Email: eyhefeng@scut.edu.cn.
References
- 1.Cavero T, Arjona E, Soto K, et al. Severe and malignant hypertension are common in primary atypical hemolytic uremic syndrome. Kidney Int. 2019;96:995–1004. 10.1016/j.kint.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Beevers M, Beevers DG. Complications and survival of 315 patients with malignant-phase hypertension. J Hypertens. 1995;13:915–24. 10.1097/00004872-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Lane DA, Lip GY, Beevers DG. Improving survival of malignant hypertension patients over 40 years. Am J Hypertens. 2009;22:1199–204. 10.1038/ajh.2009.153. [DOI] [PubMed] [Google Scholar]
- 4.de Nattes T, Saad R, Buob D, et al. Retinal arteriolar occlusions and exudative retinal detachments in malignant hypertension: more than meets the eye. Am J Hypertens. 2021;34:30–3. 10.1093/ajh/hpaa138. [DOI] [PubMed] [Google Scholar]
- 5.Boulestreau R, van den Born BH, Lip GYH, et al. Malignant hypertension: current perspectives and challenges. J Am Heart Assoc. 2022;11:e023397doi. 10.1161/JAHA.121.023397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavero T, Aunon P, Caravaca-Fontan F, et al. Thrombotic microangiopathy in patients with malignant hypertension. Nephrol Dial Transpl. 2023;38:1217–26. 10.1093/ndt/gfac248. [DOI] [PubMed] [Google Scholar]
- 7.Raghunathan V, Sethi SK, Dragon-Durey MA, et al. Targeting renin-angiotensin system in malignant hypertension in atypical hemolytic uremic syndrome. Indian J Nephrol. 2017;27:136–40. 10.4103/0971-4065.181462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Wang Y, Yu X, et al. Potential involvement of complement activation in kidney vascular lesions of arterionephrosclerosis. Front Med (Lausanne). 2022;9:836155. 10.3389/fmed.2022.836155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakhouri F, Fremeaux-Bacchi V. Thrombotic microangiopathy in aHUS and beyond: clinical clues from complement genetics. Nat Rev Nephrol. 2021;17:543–53. 10.1038/s41581-021-00424-4. [DOI] [PubMed] [Google Scholar]
- 10.Thompson A, Carroll K, L AI, et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019;14:469–81. 10.2215/CJN.08600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherney DZI, Dekkers CCJ, Barbour SJ, et al. Effects of the SGLT2 inhibitor Dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020;8:582–93. 10.1016/S2213-8587(20)30162-5. [DOI] [PubMed] [Google Scholar]
- 12.Hou FF, Xie D, Wang J, et al. Effectiveness of mycophenolate mofetil among patients with progressive IgA nephropathy: a randomized clinical trial. JAMA Netw Open. 2023;6:e2254054. 10.1001/jamanetworkopen.2022.54054. [DOI] [PubMed] [Google Scholar]
- 13.Wahl TS, Graham LA, Morris MS, et al. Association between preoperative proteinuria and postoperative acute kidney injury and readmission. JAMA Surg. 2018;153:e182009doi. 10.1001/jamasurg.2018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillay KG, Schwartz FD, Kark RM. Proteinuria in malignant hypertension. Lancet. 1968;2:1263–4. 10.1016/s0140-6736(68)91759-5. [DOI] [PubMed] [Google Scholar]
- 15.Shantsila A, Shantsila E, Beevers DG, et al. Predictors of 5-year outcomes in malignant phase hypertension: the West Birmingham malignant hypertension registry. J Hypertens. 2017;35:2310–4. 10.1097/HJH.0000000000001446. [DOI] [PubMed] [Google Scholar]
- 16.Kitiyakara C, Guzman NJ. Malignant hypertension and hypertensive emergencies. J Am Soc Nephrol. 1998;9:133–42. 10.1681/ASN.V91133. [DOI] [PubMed] [Google Scholar]
- 17.Timmermans S, Abdul-Hamid MA, Vanderlocht J, et al. Patients with hypertension-associated thrombotic microangiopathy May present with complement abnormalities. Kidney Int. 2017;91:1420–5. 10.1016/j.kint.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Genest DS, Patriquin CJ, Licht C, et al. Renal thrombotic microangiopathy: A review. Am J Kidney Dis. 2023;81:591–605. 10.1053/j.ajkd.2022.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Allinovi M, Menale S, Querin V, et al. Potentially reversible severe cardiac involvement in thrombotic microangiopathies with malignant hypertension. J Nephrol. 2025. 10.1007/s40620-025-02334-1. [DOI] [PubMed] [Google Scholar]
- 20.Perkovic V, Barratt J, Rovin B, et al. Alternative complement pathway Inhibition with Iptacopan in IgA nephropathy. N Engl J Med. 2025;392:531–43. 10.1056/NEJMoa2410316. [DOI] [PubMed] [Google Scholar]
- 21.Lafayette R, Tumlin J, Fenoglio R, et al. Efficacy and safety of ravulizumab in IgA nephropathy: a phase 2 randomized Double-Blind Placebo-Controlled trial. J Am Soc Nephrol. 2025;36:645–56. 10.1681/ASN.0000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes Glomerular Diseases, Work G. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–276. 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Kidney Disease. Improving global outcomes CKDWG. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105:S117–314. 10.1016/j.kint.2023.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Chinese Society of N. [Guidelines for hypertension management in patients with chronic kidney disease in China (2023)]. Zhonghua Gan Zang Bing Za Zhi. 2023;39:48–80. 10.3760/cma.j.cn441217-20220630-00650. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Liu Q, Lian X, et al. Kidney outcomes following angiotensin receptor-Neprilysin inhibitor vs angiotensin-Converting enzyme inhibitor/Angiotensin receptor blocker therapy for thrombotic microangiopathy. JAMA Netw Open. 2024;7:e2432862. 10.1001/jamanetworkopen.2024.32862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Xie Y, Liang F, et al. A novel technique of reverse-sequence endoscopic nipple-sparing mastectomy with direct-to-implant breast reconstruction: medium-term oncological safety outcomes and feasibility of 24-h discharge for breast cancer patients. Int J Surg. 2024;110:2243–52. 10.1097/JS9.0000000000001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan BM. In response: using propensity score matching to balance the baseline characteristics. J Thorac Oncol. 2021;16:e46doi. 10.1016/j.jtho.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Halimi JM, Al-Dakkak I, Anokhina K, et al. Clinical characteristics and outcomes of a patient population with atypical hemolytic uremic syndrome and malignant hypertension: analysis from the global aHUS registry. J Nephrol. 2023;36:817–28. 10.1007/s40620-022-01465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin S, Cremer A, Boulestreau R, et al. Malignant hypertension: diagnosis, treatment and prognosis with experience from the Bordeaux cohort. J Hypertens. 2019;37:316–24. 10.1097/HJH.0000000000001913. [DOI] [PubMed] [Google Scholar]
- 30.van den Born BJ, Koopmans RP, Groeneveld JO, et al. Ethnic disparities in the incidence, presentation and complications of malignant hypertension. J Hypertens. 2006;24:2299–304. 10.1097/01.hjh.0000249710.21146.38. [DOI] [PubMed] [Google Scholar]
- 31.Timmermans S, Abdul-Hamid MA, Potjewijd J, et al. C5b9 formation on endothelial cells reflects complement defects among patients with renal thrombotic microangiopathy and severe hypertension. J Am Soc Nephrol. 2018;29:2234–43. 10.1681/ASN.2018020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Yang C, Zhou X, et al. Association between thrombotic microangiopathy and activated alternative complement pathway in malignant nephrosclerosis. Nephrol Dial Transpl. 2020. 10.1093/ndt/gfaa280. [DOI] [PubMed] [Google Scholar]
- 33.Tan J, Xu Y, Jiang Z, et al. Global glomerulosclerosis and segmental glomerulosclerosis could serve as effective markers for prognosis and treatment of IgA vasculitis with nephritis. Front Med (Lausanne). 2020;7:588031. 10.3389/fmed.2020.588031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez R, Morales E, Segura J, et al. Long-term renal survival in malignant hypertension. Nephrol Dial Transpl. 2010;25:3266–72. 10.1093/ndt/gfq143. [DOI] [PubMed] [Google Scholar]
- 35.Efrati S, Berman S, Goldfinger N, et al. Enhanced angiotensin II production by renal Mesangium is responsible for apoptosis/proliferation of endothelial and epithelial cells in a model of malignant hypertension. J Hypertens. 2007;25:1041–52. 10.1097/HJH.0b013e32807fb09c. [DOI] [PubMed] [Google Scholar]
- 36.Koppe L, Fouque D. The role for protein restriction in addition to Renin-Angiotensin-Aldosterone system inhibitors in the management of CKD. Am J Kidney Dis. 2019;73:248–57. 10.1053/j.ajkd.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Barbour T, Johnson S, Cohney S, et al. Thrombotic microangiopathy and associated renal disorders. Nephrol Dial Transpl. 2012;27:2673–85. 10.1093/ndt/gfs279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walther CP, Gutierrez OM, Cushman M, et al. Serum albumin concentration and risk of end-stage renal disease: the REGARDS study. Nephrol Dial Transpl. 2018;33:1770–7. 10.1093/ndt/gfx331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Zhang R, Wang Y et al. The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J Diabetes Res 2019; 2019:7825804. 10.1155/2019/7825804 [DOI] [PMC free article] [PubMed]
- 40.Butt L, Unnersjo-Jess D, Hohne M, et al. A molecular mechanism explaining albuminuria in kidney disease. Nat Metab. 2020;2:461–74. 10.1038/s42255-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 41.Heerspink HJL, Xie D, Bakris G, et al. Early response in albuminuria and Long-Term kidney protection during treatment with an endothelin receptor antagonist: A prespecified analysis from the SONAR trial. J Am Soc Nephrol. 2021;32:2900–11. 10.1681/ASN.2021030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Fang Y, Zou Z et al. Risk factors for progression of CKD with and without diabetes. J Diabetes Res 2022;9613062.10.1155/2022/9613062 [DOI] [PMC free article] [PubMed]
- 43.Tracy RE, Ishii T. What is ‘nephrosclerosis’? Lessons from the US, japan, and Mexico. Nephrol Dial Transpl. 2000;15:1357–66. 10.1093/ndt/15.9.1357. [DOI] [PubMed] [Google Scholar]
- 44.Ginley B, Jen KY, Han SS, et al. Automated computational detection of interstitial fibrosis, tubular atrophy, and glomerulosclerosis. J Am Soc Nephrol. 2021;32:837–50. 10.1681/ASN.2020050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howie AJ, Ferreira MA, Adu D. Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transpl. 2001;16:1163–9. 10.1093/ndt/16.6.1163. [DOI] [PubMed] [Google Scholar]
- 46.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 47.Kipgen D, Crosby J, Dey V, et al. The relationship between histopathological features, immunosuppression and outcome in patients undergoing native kidney biopsies. Histopathology. 2024;84:671–82. 10.1111/his.15115. [DOI] [PubMed] [Google Scholar]
- 48.Kadiri S, Thomas JO. Focal segmental glomerulosclerosis in malignant hypertension. S Afr Med J. 2002;92:303–5. [PubMed] [Google Scholar]
- 49.Jaryal A, Vikrant S. Clinical profile and outcome of IgA nephropathy from a tertiary care hospital in North India. J Assoc Physicians India. 2020;68:20–2. [PubMed] [Google Scholar]
- 50.Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812–23. 10.1681/ASN.2017020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure Lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–43. 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 52.Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure Lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949–57. 10.1503/cmaj.121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.