Abstract
Golgi-localized γ-ear homology domain, ADP-ribosylation factor (ARF)-binding proteins (GGAs) facilitate distinct steps of post-Golgi traffic. Human and yeast GGA proteins are only ∼25% identical, but all GGA proteins have four similar domains based on function and sequence homology. GGA proteins are most conserved in the region that interacts with ARF proteins. To analyze the role of ARF in GGA protein localization and function, we performed mutational analyses of both human and yeast GGAs. To our surprise, yeast and human GGAs differ in their requirement for ARF interaction. We describe a point mutation in both yeast and mammalian GGA proteins that eliminates binding to ARFs. In mammalian cells, this mutation disrupts the localization of human GGA proteins. Yeast Gga function was studied using an assay for carboxypeptidase Y missorting and synthetic temperature-sensitive lethality between GGAs and VPS27. Based on these assays, we conclude that non-Arf-binding yeast Gga mutants can function normally in membrane trafficking. Using green fluorescent protein-tagged Gga1p, we show that Arf interaction is not required for Gga localization to the Golgi. Truncation analysis of Gga1p and Gga2p suggests that the N-terminal VHS domain and C-terminal hinge and ear domains play significant roles in yeast Gga protein localization and function. Together, our data suggest that yeast Gga proteins function to assemble a protein complex at the late Golgi to initiate proper sorting and transport of specific cargo. Whereas mammalian GGAs must interact with ARF to localize to and function at the Golgi, interaction between yeast Ggas and Arf plays a minor role in Gga localization and function.
INTRODUCTION
The Golgi-localized, γ-ear homology domain, ARF-binding protein (GGA) family of proteins facilitates the sorting and transport of proteins out of the trans-Golgi network (for review, see Boman, 2001). GGA proteins interact directly with ADP-ribosylation factor (ARF) proteins, a family of 21-kDa GTP-binding proteins that have been implicated as regulators of membrane traffic at many steps in the secretory, endocytic, and recycling pathways (Stearns et al., 1990a,b; Boman and Kahn, 1995; Gaynor et al., 1998; Yahara et al., 2001). GGA proteins interact with the activated, GTP-bound form of ARF, establishing them as effectors of ARF signaling (Boman et al., 2000; Dell'Angelica et al., 2000; Zhdankina et al., 2001).
Three GGA genes have been described in mammalian cells: GGA1, GGA2, and GGA3. Alternative splicing of GGA3 results in a long form and a short form of the GGA3 protein. The yeast Saccharomyces cerevisiae has two GGA genes, GGA1 and GGA2. The expressed yeast proteins, Gga1p and Gga2p, share 50% amino acid identity. The human and yeast GGA proteins are ∼25% identical. Although the sequence identity is low, the domain structure of GGA proteins is conserved between species. Four domains are apparent when the amino acid sequences of yeast and human GGA proteins are aligned (see Figure 1A). An N-terminal VHS domain (150 residues) resembles the VHS domain present in Vps27p, HRS, and STAM proteins (Lohi and Lehto, 1998). The 170-residue GAT domain is the most highly conserved (65% identity between human GGA proteins) and contains two predicted coiled-coil regions. The GAT domain (Dell'Angelica et al., 2000) was so named for its sequence homology to a protein named Tom1 (GGA and Tom1). A “hinge” region of variable length contains one or more clathrin-binding domains but no other significant homology to each other or other known proteins. A C-terminal ear domain (120 amino acids; 40% identity between human GGA proteins) is homologous to the ear domain of γ-adaptin.
Figure 1.
ARF-binding region of GGA proteins and effects of mutations. (A) Comparison of ARF-binding domains from yeast and human GGA proteins. GGA proteins have four domains, with the highest identity between yeast and human GGA proteins in the ARF-binding domain. Residues identical in at least three proteins are highlighted in red. Dashes indicate gaps in the alignment. The numbers indicate the first and last residues shown for each GGA protein. Asterisks indicate mutated residues. (B) Loss of ARF binding by two-hybrid analysis. Gal4AD fusions of wild-type and mutant GGA proteins were assayed for binding to Gal4BD fusions of activated ARF in the two-hybrid assay. Serial 10-fold dilutions of each strain were replica plated on SD plates lacking tryptophan and leucine (−trp-leu) and SD plates lacking histidine, tryptophan, and leucine with 25 mM 3AT. Growth on −trp-leu shows that all strains are viable. Interaction is positive when colonies grow on 3AT and turn blue in X-gal assays. Scores in the right-hand column are defined in MATERIALS AND METHODS. (C) Loss of Arf binding by using affinity chromatography. Increasing concentrations of Arf1p·GDP or Arf1p·GTPγS (as indicated) were incubated with GST fusion proteins immobilized on glutathione agarose beads: GST-Gga2p (lanes 1–6), GST-Gga2pI207N (lanes 7–12), and GST only (lanes 13–14). Retained proteins were eluted and analyzed by Coomassie staining to visualize the GST fusion proteins (bottom) or by immunoblotting to detect Arf1p (top). Arf1p was retained by GST-Gga2p, but not by mutant GST-Gga2pI207N or GST only.
Work in both mammalian cells and S. cerevisiae suggests that GGA proteins function in trafficking at the TGN. In yeast, deletion of either gene alone causes no (GGA1 deletion) or minor (GGA2 deletion) defects, whereas deletion of both genes disrupts distinct post-Golgi trafficking events (Black and Pelham, 2000; Dell'Angelica et al., 2000; Hirst et al., 2000; Costaguta et al., 2001; Zhdankina et al., 2001). Several proteins that are transported between the TGN and early or late endosomes, including carboxypeptidase Y (CPY), carboxy peptidase S, Pep12p, and Kex2p, are at least partially missorted in cells lacking both GGA1 and GGA2. For example, ∼40% of newly synthesized CPY is secreted in gga1gga2 cells (Hirst et al., 2000; Zhdankina et al., 2001). These data support a role for Gga proteins in a TGN-to-early endosome pathway, in a TGN-to-late endosome pathway, or both.
The function of mammalian GGA proteins has been deduced based on the protein interactions of each domain. Mammalian GGA proteins interact directly with a defined subset of cargo that trafficks between the TGN and lysosomes. Sortilin, LRP3, and both the cation-independent and cation-dependent forms of mannose 6-phosphate receptor (M6PR) contain an acidic cluster-dileucine motif that interacts with the VHS domain of human GGA proteins (Puertollano et al., 2001a; Takatsu et al., 2001; Zhu et al., 2001). Many other transmembrane proteins that lack this motif do not interact with GGA proteins, suggesting that GGAs are specific for a subset of transported proteins. It is not known whether a similar motif (acidic cluster-dileucine) is recognized by yeast Gga proteins, or even whether cargo is sorted via direct interaction with Gga proteins in yeast.
The hinge domain of mammalian (Puertollano et al., 2001b) and yeast (Costaguta et al., 2001) GGA proteins interacts directly with clathrin in vitro. The ear domain strengthens this interaction, and the ear domains of certain GGA proteins also interact with clathrin on their own (Costaguta et al., 2001). These findings suggest that GGA proteins can recruit clathrin to sites of vesicle budding on the TGN. Overexpression in cultured cells of a GGA3 construct lacking the hinge and ear domains causes M6PR to accumulate in the TGN, supporting a role for GGA-dependent clathrin recruitment in the packaging of these cargo into vesicles (Puertollano et al., 2001a). Thus, it is likely that both yeast and mammalian GGA proteins are monomeric clathrin adaptors that function at the TGN.
The ear domain of human GGA proteins interacts with γ-synergin (Takatsu et al., 2000), a Golgi-localized protein partner of γ-adaptin that has an unknown function (Page et al., 1999). Thus, the function of the GGA ear was accurately predicted by its homology to γ-adaptin. However, γ-synergin has no obvious homolog in yeast, and the ear domains of yeast and human GGA proteins are not well conserved; hence, the role of the ear domain in yeast remains unclear.
In mammalian cells, endogenous GGA1, GGA2, and GGA3 localize predominantly to the trans-Golgi region (Boman et al., 2000; Dell'Angelica et al., 2000; Hirst et al., 2000; Poussu et al., 2000; Takatsu et al., 2000). Three lines of evidence show that TGN localization of GGAs requires interaction between the GAT domain and activated ARF proteins. First, treatment with brefeldin A causes rapid translocation of GGA proteins to the cytosol in a time frame indistinguishable from that of ARF itself (Boman et al., 2000; Dell'Angelica et al., 2000; Hirst et al., 2000; Poussu et al., 2000). Second, the interaction between the GAT domain and ARF is strong enough to direct a green fluorescent protein (GFP) fusion of GAT onto the Golgi, even in the absence of the VHS, hinge, and ear domains (Dell'Angelica et al., 2000). Third, and most convincingly, point mutations within the ARF-binding domain that abolish ARF interaction also cause loss of Golgi localization (Puertollano et al., 2001b). Together, these data suggest that GTP-bound ARF recruits mammalian GGA proteins from the cytosol onto the late Golgi membrane by interacting with the GAT domain.
The function of GGA proteins in mammalian cells is also intimately tied to activated ARF. First, expression of the constitutively active ARF1Q71L in mammalian cells causes dramatic expansion of the Golgi apparatus (Zhang et al., 1994). Overexpression of GGA1 or GGA3 in these same cells prevents Golgi expansion (Boman et al., 2000). This alteration of ARF1 function indicates a functional interaction between ARF1 and GGAs in vivo. Second, as described above, mutations within the GAT domain of GGA3 disrupt its Golgi localization. These same mutations prevent GGA3 overexpression from displacing adaptor complexes or fragmenting the Golgi apparatus (Puertollano et al., 2001b), which usually results from GGA overexpression in mammalian cells. Although these mutations in GGA3 might disrupt other interactions, current evidence supports the model that loss of ARF interaction disrupts GGA function in mammalian cells.
Together, these data suggest that GGA proteins are ARF-dependent, monomeric clathrin adaptors that facilitate the sorting of specific cargo at the TGN into vesicles destined for endosomes. Much of the data comparing yeast and mammalian GGA function assumes that the mechanisms of GGA function are well conserved between these organisms. However, the mechanism of yeast GGA function has not been analyzed. In particular, it is not known whether Gga function or localization in yeast depends on its binding to Arf (via the GAT domain) or other proteins (via the VHS and/or hinge and ear domains).
In this article, we show that yeast Gga1p and Gga2p do not require Arf for their localization or function, although in vivo binding to Arf is detectable. We propose that yeast Ggas are targeted to the Golgi membrane through interactions with Golgi-localized proteins other than Arf, which then stabilize the Gga–Arf interaction.
MATERIALS AND METHODS
Materials
Unless otherwise specified, chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Fair Lawn, NJ). Yeast and bacterial media reagents were purchased from Difco (Detroit, MI). Restriction and other enzymes were purchased from Promega (Madison, WI) or Invitrogen (Carlsbad, CA). TA cloning vector pGEMT-easy was purchased from Promega. Unique Site Elimination Mutagenesis kit was purchased from CLONTECH (Palo Alto, CA).
Yeast Methods
Yeast were grown under standard conditions (Sherman et al., 1974) at 30°C unless otherwise noted. Transformations into yeast were performed using the LiOAc method, with herring sperm DNA as a carrier (Ito et al., 1983; Schiestl and Gietz, 1989).
Yeast Strains
Yeast strains used in this study are listed in Table 2. Diploid strains YAB538 and YAB539 were generated by mating YAB531 (gga1::TRP1) and YAB532 (gga2::HIS3) (Zhdankina et al., 2001). Tetrad dissections generated several gga1gga2 strains used for trafficking assays and generation of triple knockout strains (YAB538 T6a, YAB538 T2d, YAB539 T3d; Zhdankina et al., 2001). Strain YAB650 (gga1gga2apm1) was generated by mating and tetrad dissections of YAB539 T3d and Piper1878. Strain YAB658 (gga1gga2apm3) was generated by mating and tetrad dissections of YAB538 T2d and Piper1022. Strain YAB667 (gga1gga2vps1) was generated by transforming plasmid pCKR3A, digested with SacI and XbaI, into strain YAB538 T6a and selecting for LEU auxotrophy. Strains YAB679 and YAB680 (gga1gga2vps27 in SEY6210 background) were generated by mating and tetrad dissections of ABC114 T6b and vps27 strain JUY68. Strain YAB677 (gga1gga2vps27 in S288C background) was generated by transforming plasmid pKJH2, digested with BamHI and PstI, into strain YAB538 T6a and selecting for LEU auxotrophy. Gene deletions were confirmed by Western blotting for Gga1p and Gga2p, and by phenotype for VPS27 (class E compartment), APM3 (ALP processing), and VPS1 (CPY secretion). Multiple isolates of gga1gga2apm1 were analyzed. All other strains were generated by transformation.
Table 2.
Yeast strains used in this study
| Yeast strain | Genotype | Source |
|---|---|---|
| BY4704 | MATa ade2Δ∷hisG his3Δ200 leu2Δ0 lys2Δ0 met15Δ0 trp1Δ63 | ATCC 200868 |
| BY4735 | MATalpha ade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 | ATCC 200897 |
| BY4742 | MATalpha his3Δ200 leu2Δ0 lys2Δ0 ura3Δ0 | ATCC |
| SEY6210 | MATalpha ura3-52 leu-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 | Robinson et al., 1988 |
| Y190 | MATa gal4 gal80 his 3 trp1-901 ade2-101 ura3-52 leu2-3,-112 URA3∷GAL–lacZ, LYS2∷GAL(UAS)–HIS3 cyh′ | Durfee et al., 1993 |
| YAB538 T6a | MATalpha ade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 gga1Δ∷TRP1 gga2Δ∷HIS3 | Zhdankina et al., 2001 |
| YAB538 T2d | MATa ade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 gga1Δ∷TRP1 gga2Δ∷HIS3 | This study |
| YAB539 T3d | MATa ade2Δ∷hisG his3Δ200 leu2Δ0 lys2Δ0 met15Δ0 trp1Δ63 gga1Δ∷TRP1 gga2Δ∷HIS3 | This study |
| ABC114 T6b | YAB538 T6a backcrossed to SEY6210, gga1Δ∷TRP1 gga2Δ∷HIS3 | This study |
| YAB508 | Y190 Gal4AD-GGA3 + Gal4BD-ARF3Q71L | This study |
| YAB616 | Y190 Gal4BD-Arf2p Gal4AD-Gga1p | This study |
| YAB617 | Y190 Gal4AD-Gga1L203Q + Gal4BD-Arf2Q71L | This study |
| YAB618 | Y190 Gal4AD-Gga2 + Gal4BD-Arf2Q71L | This study |
| YAB650 | apm1Δ∷KAN gga1Δ∷TRP1 gga2Δ∷HIS3 | This study |
| YAB658 | apm3Δ∷URA3 gga1Δ∷TRP1 gga2Δ∷HIS3 | This study |
| YAB667 | YAB538 T6a vps1Δ∷LEU2 | This study |
| YAB672 | Y190 Gal4AD-GGA3 L149Q + Gal4BD-ARF3Q71L | This study |
| YAB677 | YAB538 T6a vps27∷LEU2 | This study |
| YAB679 | vps27Δ∷LEU2 gga1Δ∷TRP1 gga2Δ∷HIS3 | This study |
| YAB680 | vps27Δ∷LEU2 gga1Δ∷TRP1 gga2Δ∷HIS3 | This study |
| YAB701 | Y190 Gal4AD-GGA1 + Gal4BD-ARF3Q71L | This study |
| YAB702 | Y190 Gal4AD-GGA1 L182Q + Gal4BD-ARF3Q71L | This study |
| YAB703 | Y190 Gal4AD-Gga2I207N + Gal4BD-Arf2Q71L | This study |
| JUY68 | SEY6210 vps27∷LEU2 | Rob Piper |
| Piper1878 | BY4742 apm1∷KAN | Rob Piper |
| Piper1022 | SEY6210 apm3∷URA3 | Rob Piper |
| GPY2385 | SEY6210 gga1∷TRP1 gga2∷HIS3 | Costaguta et al., 2001 |
| YCS41 | SEY6210 vps28∷LEU2 | Scott Emr |
| YCS195 | SEY6210 gga1∷TRP1 gga2∷HIS3 vps28∷LEU2 | Scott Emr |
Plasmid Construction
Plasmids used in this study are listed in Table 1. Oligonucleotide primers used for polymerase chain reaction (PCR) amplification are listed in Table 3. All constructs generated by PCR amplification were verified by automated sequencing (Advanced Genetic Analysis Center, University of Minnesota).
Table 1.
Plasmids used in this study
| Plasmid name | Construct | Source | If PCR, Oligos Used |
|---|---|---|---|
| pAB169 | pGAD-GGA1 · N | Boman et al., 2000 | |
| pAB202 | pBG4D-hARF3(Q71L) | Boman et al., 2000 | |
| pAB318 | pcDNA3-HA-hGGA1ΔVHS | Boman et al., 2000 | |
| pAB348 | pcDNA3-HA-hGGA1 | Boman et al., 2000 | 797 and 701 |
| pAB354 | pACT2-hGGA3 | Boman et al., 2000 | |
| pAB361 | pACT2-Ggalp(1–330) | Zhdankina et al., 2001 | |
| pAB362 | pACT2-Ggalp(1–330, L203Q) | This study | |
| pAB363 | pcDNA3-HA-hGGA3 | Boman et al., 2000 | 795 and 780 |
| pAB374 | pACT2-Gga2p(1–326) | Zhdankina et al., 2001 | |
| pAB382 | pGEX5X-Gga2p(1–326) | Zhdankina et al., 2001 | |
| pAB418 | pACT2-hGGA1ΔN(L182Q) | This study | A025 and A026, 638 and A028 |
| pAB441 | pRS316-GGA2 | Zhdankina et al., 2001 | A037 and A038 |
| pAB443 | pcDNA3-HA-hGGA1(L182Q) | This study | A025 and A026, 797 and A028 |
| pAB454 | pBG4D-yArf2(Q71L) | Zhdankina et al., 2001 | |
| pAB456 | pRS316-GGA2(I207N) | This study | A044 and A045, A037 and A038 |
| pAB462 | pACT2-Gga2p(1–326, I207N) | This study | |
| pAB468 | pRS316-GGA2(1–326) | This study | A037 and A049 |
| pAB469 | pRS316-GGA1(L203Q) | This study | |
| pAB470 | pRS316-GGA1 | Zhdankina et al., 2001 | A041 and A048 |
| PAB472 | pGEX5X-Gga2p(1–326, I207N) | This study | |
| pAB477 | pRS316-GGA2(1–326, I207N) | This study | A037 and A049 |
| pAB493 | pRS315-GGA2(PEDL→AAAL) | This study | USE mutagenesis, A056/A060 |
| pAB503 | pACT2-hGGA3(L149Q) | This study | A092 and A093, 772 and 637 |
| pAB504 | pGFP-Gga2p(I207N) | This study | A097 and A098 |
| pAB505 | pGFP-Gga1p(L203Q) | This study | |
| pAB506 | pCDNA3-HA-hGGA3(L149Q) | This study | |
| pAB509 | pRS315-GGA2(ANKL→AAAL) | This study | USE mutagenesis, A056/A061 |
| pAB510 | pGFP-Gga1p(141–331) | This study | A107 and A108 |
| pAB511 | pGFP-Gga1p(141–331, L203Q) | This study | A107 and A108 |
| pAB512 | pGFP-Gga1p(140–557) | This study | A107 and T7 |
| pAB513 | pGFP-Gga1p(140–557, L203Q) | This study | A107 and T7 |
| pAB524 | pGFP-Gga1p(1–331) | This study | A024 and A108 |
| pAB525 | pGFP-Gga1p(1–331, L203Q) | This study | A024 and A108 |
| pAB526 | pGFP-Gga1p(1–133) | This study | |
| pAB529 | pRS316-GGA2(PEDL→AAAL) | This study | |
| pAB530 | pRS316-GGA2(ANKL→AAAL) | This study | |
| pAB532 | pGFP-Gga1p(326–557) | This study | A118 and T7 |
| pAB533 | pGFP-Gga1p(421–557) | This study | A119 and T7 |
| pAB543 | pGFP-Gga1p(140–430) | This study | A107 and A121 |
| pAB555 | pRS316-GGA2 (110–585) | This study | |
| pAB580 | pACT2-Gga2pANKL→AAAL | This study | |
| pAB581 | pACT2-Gga2pPEDL→AAAL | This study | |
| pAB582 | pcDNA3-hGGA1ΔVHS(L182Q) | This study | |
| pCKR3A | vps1∷LEU2 in pUC12HP | Rob Piper | |
| pCS135 | pGFP-Gga1p, URA3 2μ | Scott Emr | |
| pCS136 | pGFP-Gga2p, URA3 2μ | Scott Emr | |
| pKJH2 | vps27∷LEU2 in pUC19 | Piper et al., 1995 | |
| pMR2654 | 3HA as XbaI fragment | Kurihara et al., 1996 | |
| pNB1092 | RFP-GYP1 in pRS316 | (Du and Novick, 2001) |
Table 3.
Oligonucleotide primers used in this study
| Oligo name | Sequence |
|---|---|
| A024 | CGAGCCAGGATCCTGCAAATCTCCAGGAAGG |
| A025 | GGAGCTCTTCTGCAGGCGGGCCAGCATCTT |
| A026 | GCCCGCCTGCAGAAGAGCTCCCATCCCGAA |
| A027 | GCAGCGGATCCACCATATGGAGCCCGCGATGGAG |
| A028 | GTGGCGGGATCCCTCGAGCCCAGCCCCTCTGTTCTA |
| A037 | CGAGCCAGGATCCTGGTTGGGAAGATTGGAT |
| A038 | GCGACCTCGAGTGAGGATGGCGAATTATACTATTT |
| A041 | CGAGCCAGGATCCTCGCAAAACCTAAAATAACCTGCT |
| A044 | CGAAGAATTGAACAGGCGTGGTAAACCTGAAG |
| A045 | ACCACGCCTGTTCAATTCTTCGAGTTTTGC |
| A048 | GTGCCTGGATCCGCGAATTTTGTGTTATGGTTAAA |
| A049 | TGTCTCGAGTTAAGCAGCGTTGGAGTCACCG |
| A060 | AGGCGTGGTAAAGCTGCAGCTTTGAGGGAAGCTAAC |
| A061 | TTGAGGGAAGCTGCCGCATTAATGAAAATCATGGC |
| A092 | TTTGCTTTTCTGCAGCTTGGCTAAAAGCTTG |
| A093 | GCCAAGCTGCAGAAAAGCAAAAACCCAGATGAC |
| A097 | GCAGGTCGACATCATGTCCCATCCGCAC |
| A098 | CAGGGTACCAGTGGTTATGAGGATGGCG |
| A107 | CAGGCCGTCGACGCCAGCTACAAAGATGATCTTCA |
| A108 | CAGCGCGGTACCTTATGCCGGTTCATTCGTATCG |
| A118 | GCAGGTCGACCCCGATACGAATGAACCGGC |
| A119 | GCAGGTCGACTCGATTGTTCTACCTAATGG |
| A121 | CGAGCCGGTACCTTAACCATTAGGTAGAACAATCGAGT |
| 837 | ACATGGTGCACGATGCAC |
| 638 | GAGCCAGGGATCCCCATGGATGACACTACCTTTCCC |
| 701 | CAGCATCTTGGATTTCTC |
| 772 | CGCGAGCAGATCTTCCATATGGCGGAGGCGGAAGGG |
| 780 | GCCAGGTCTCGAGTATCAGAAGAGGATGCGGAAGCC |
| 795 | GCAGCCGAATTCATGGCGGAGGCGGAAGGG |
| 797 | GCAGCCGAATTCCGGATGGAGCCCGCGATG |
Two-Hybrid Plasmids.
Gal4 binding domain (Gal4BD) fusions of yeast and human ARFs were generated from plasmid pBG4D (a kind gift from Rob Brazas, University of California, San Francisco, CA), as described previously (Boman et al., 1999, 2000; Zhdankina et al., 2001). Gal4 activation domain (Gal4AD) fusions of yeast and human GGA proteins were generated from plasmid pACT2 (a kind gift from Steve Elledge, Baylor University, Waco, TX), as described previously (Zhdankina et al., 2001). A single point mutation within the Arf-binding domain of yeast Gga1p was fortuitously identified after PCR amplification of the GGA1 VHS/GAT domain and subcloning into the two-hybrid vector pACT2, resulting in plasmid pAB362. This single-base substitution changed leucine at position 203 to a glutamine (L203Q). Homologous mutations in yeast Gga2p (I207N), human GGA1 (L182Q), and human GGA3 (L149Q) were generated by two-step PCR mutagenesis with oligos specified in Table 3. The PCR products were generated with BamHI and XhoI sites at the 5′ and 3′ ends of the open reading frames, respectively, and subcloned into vector pACT2 to generate Gal4AD fusion proteins of mutant Gga2p (pAB462), GGA1 (pAB418), and GGA3 (pAB503). Alanine substitutions in Gga2p (PEDL→AAAL and ANKL→AAAL) were generated using Unique Site Elimination mutagenesis (CLONTECH) in plasmid pAB473 (see below). The open reading frames were then subcloned into pACT2 by using NcoI and XhoI, resulting in plasmids pAB580 and pAB581.
Yeast Expression Vectors.
Plasmids for expression of Gga1p (pAB470) and Gga2p pAB441) were described in Zhdankina et al. (2001). An NcoI site was generated at the start ATG of GGA2 in pAB441 by using two-step PCR to generate plasmid pAB471. The GGA2(NcoI) gene was then subcloned into vector pRS315 by using BamHI and XhoI to generate pAB473. Unique site elimination mutagenesis of pAB473 was used to generate PEDL→AAAL (pAB493) and ANKL→AAAL (pAB509). The mutated BamHI-XhoI fragments of pAB493 and pAB509 were then subcloned into pRS316 to generate pAB529 and pAB530, respectively. The mutated NcoI-XhoI fragments of pAB493 and pAB509 were also subcloned into pACT2, as described above. For expression of Gga1pL203Q, the mutated region from pAB362 was subcloned into pAB470, resulting in pAB469. For expression of Gga2pI207N, two-step PCR mutagenesis was used to generate the I207N mutation from plasmid pAB441. The PCR product was then subcloned into pRS316, resulting in pAB456. The amino terminal truncation of Gga2p(110–585) was generated by PCR (oligos A053 and A038) followed by subcloning the NcoI-XhoI–digested products into pAB473. Then the NotI-XhoI cassette was subcloned into pRS316 to generate pAB555. The carboxy-terminal truncation of Gga2p(1–326) was generated by PCR (oligos A037 and A049) and subcloned with BamHI and XhoI into pRS316, resulting in pAB468.
Hemagglutinin (HA)-tagged GGA Proteins.
Unique site elimination was used to generate an NheI site immediately preceding the stop codon of GGA1 and GGA2, resulting in plasmids pAB487 and pAB483. The XbaI fragment from pMR2654 (a kind gift from Mark Rose, Princeton University, Princeton, NJ) containing a triple HA repeat was inserted into the NheI-digested plasmids, resulting in plasmids pAB492 and pAB491. Orientation of the HA tag was determined by sequencing. Mutant alleles were generated by subcloning the mutated portion of the open reading frames into the HA-tagged plasmids, resulting in plasmids pAB579 (GGA1) and pAB578 (GGA2).
Glutathione S-Transferase (GST) Fusion Proteins.
All constructs were subcloned from pACT2 (described above) into pGEX5X-2 (Stratagene, La Jolla, CA) by using BamHI and XhoI sites. The following constructs were used for these studies: GST-Gga1p1–331 (pAB391), GST-Gga1p1–331, L203Q (pAB476), GST-Gga2p1–326 (pAB382), and GST-Gga2p1–326, I207N (pAB472).
GFP Fusion Proteins.
Plasmids for expression of GFP-Gga1p (pCS135) and GFP-Gga2p (pCS136) were obtained from Chris Stefan and Scott Emr (University of California, San Diego, CA). Both are pRS426-based plasmids with the CPY promoter driving expression of the GFP open reading frame. A multiple cloning site follows GFP. Plasmid pAB505 for expression of GFP-Gga1pL203Q was generated by subcloning the KpnI-KpnI fragment of pAB469 into pCS135. Plasmid pAB504 for expression of GFP-Gga2pI207N was generated by PCR amplification of the open reading frame from pAB456 to incorporate an SalI site at the 5′ end then subcloning the SalI-KpnI fragment into pCS136. The truncated forms of GFP-Gga1p were all generated by PCR amplification from either pCS135 or pAB505 then subcloned into pCS135 by using SalI-KpnI or KpnI-KpnI sites.
Mammalian Expression Vectors.
Plasmids for expression of human GGA proteins in normal rat kidney (NRK) cells were generated from pcDNA3 as described in Boman et al. (2000). GGA1L182Q was generated by two-step PCR mutagenesis with mutant oligos A025 and A026 and flanking oligos 797 and A028 then subcloned into pcDNA3-HA with EcoRI and XbaI, resulting in pAB443. GGA3L149Q was generated by subcloning the mutated region of GGA3 (HindIII-XhoI) from pAB503 into vector pAB366 (pcDNA3-HA-GGA3; Boman et al., 2000), resulting in pAB506. Truncated GGA1ΔVHS (pAB318) begins at residue 145, as described previously (Boman et al., 2000). GGA1ΔVHS,L182Q (pAB582) was generated by subcloning the BamHI-XhoI fragment of pAB418 into pcDNA3. An ATG immediately following the BamHI site acts as the start ATG.
Two-Hybrid Assays
Filter assays for β-galactosidase activity were performed as described previously (Guarente, 1983) with 1 mg/ml X-gal as the substrate. Colony color was scored every hour for 3 h then allowed to develop overnight. Strong positives (+++) turned blue within 1 h, moderate positives (++) turned blue within 3 h, weak positives (+) turned blue overnight, and negatives (−) remained white throughout the development period. Assays for histidine auxotrophy were performed by replica plating yeast strains on SD-trp-leu plates and 3AT plates (SD plates lacking tryptophan, leucine, and histidine, and containing 25 mM 3-amino triazole) and scoring growth after 3–4 d.
GST-Affinity Chromatography
Bacterially expressed Arf1pQ71L was purified as described previously (Randazzo et al., 1995). Bacterially expressed GST fusion proteins were purified on glutathione agarose beads as described previously (Boman et al., 1999). The beads were washed three times with TND (25 mM Tris pH 7.5, 100 mM NaCl, and 1 mM dithiothreitol) and once with binding buffer (20 mM HEPES pH 7.5, 1 mM EDTA, 100 mM NaCl, 0.5 mM MgCl2, 1 mM dithiothreitol, 50 μg/ml bovine serum albumin, and 1% Triton); 30 μl of bead volume was used for each binding reaction. Bacterially expressed yeast Arf1pQ71L (6 μM) was incubated for 30 min at 30°C in binding buffer with 10 μM guanosine-5′-O-(3-thio)triphosphate (GTPγS) or GDP. GST fusions were mixed with 100 μl of 0.6, 1.8, or 6 μM Arf1p for 30 min. The beads were then washed three times with 1 ml of binding buffer containing 10 μM nucleotide. Bound proteins were eluted from the beads with an equal volume of 2× Laemmli sample buffer and run on a 12% SDS-PAGE gel. The final wash was also analyzed to ensure complete washing. Duplicate gels were either stained with Coomassie brilliant blue or developed by immunoblotting, by using a polyclonal rabbit antiserum (R23; Kahn et al., 1991) to detect yeast Arf1p.
Antibodies and Immunoblotting
Immunoblotting was performed as described previously (Cavenagh et al., 1996; Boman et al., 1999). Antibodies were used at the following dilutions, as indicated for each experiment: 12CA5 (to HA epitope; BabCo, Berkeley, CA), 1:10,000; R89675 (to Gga1p), 1:10,000; R89013 (to Gga2p), 1:10,000; R23 (to Arf1p, a gift from R. Kahn, Emory University, Atlanta, GA), 1:2000; 10A5-B5 (to yeast CPY; Molecular Probes, Eugene, OR), 1:5000; and anti-ALP (a gift from S. Nothwehr, University of Missouri, Columbia, MO), 1:5000. Bound antibodies were detected with horseradish peroxidase-linked secondary antibodies and enhanced chemiluminescence detection reagents (Amersham Biosciences, Piscataway, NJ). Films were scanned using a flatbed scanner and figures were prepared using Adobe PhotoShop 5.0 (Adobe Systems, Mountain View, CA).
Affinity Purification of Antibodies to Gga1p and Gga2p.
For Gga1p, antibodies were adsorbed onto a nitrocellulose strip to which GST-Gga1p was transferred. After washing, bound antibodies were stripped with 0.2 M glycine, pH 2.8. For Gga2p, antibodies were mixed with GST-Gga2p bound to glutathione agarose, washed, and eluted with 0.2 M glycine. The efficacy of the affinity purification was analyzed by immunoblotting (our unpublished data).
Yeast Fractionation
Yeast cells (0.15 g) were suspended in 100 μl of fractionation buffer (0.4 M sucrose, 25 mM KPO4 pH 7.0, and 2 mM EDTA) and 5 μl of protease inhibitor cocktail for yeast (Sigma-Aldrich). Cells were lysed by vortexing with glass beads for 2× 3 min, on ice between. Mixture was centrifuged for 5 min at 3000 × g to remove nuclei and unbroken cells; the supernatant from this step is the lysate. The lysate was centrifuged at 100,000 × g for 30 min at 4°C to generate supernatant (S100) and pellet (P100) fractions. Equal fractions of lysate, S100, and P100 were loaded on 12% acrylamide gels and immunoblotted as described above.
Secretion Assays
CPY Pulse-Chase Assay.
Processing and sorting of CPY was assayed as described previously (Vater et al., 1992; Zhdankina et al., 2001) by using anti-CPY antisera (provided by Tom Stevens, University of Oregon, Eugene, OR; or Elizabeth Jones, Carnegie Mellon University, Pittsburgh, PA). Films were scanned on a flatbed scanner and processed using Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA).
CPY Colony Immunoblotting.
Serial dilutions of yeast cultures were replica plated on YPD plates and incubated at 30°C for 12–24 h. Nitrocellulose filters (0.45-μm BA85; Schleicher & Schuell, Keene, NH) were overlaid for an additional 12 h at 30°C. Cells were thoroughly rinsed from the filters with distilled H2O. Immunoblotting with CPY monoclonal antibodies (1:1500; Molecular Probes) or Arf1p and alkaline phosphatase polyclonal antibodies (to control for cell lysis) was performed as described above.
Staining with FM 4-64.
Cultures were grown in YPD to an optical density of 0.5–0.8. FM 4-64 (10 mM stock in dimethyl sulfoxide; Molecular Probes) was added to 500 nM; cells were incubated at 30°C for 30 min. Cells were washed with water, resuspended in YPD lacking FM 4-64, and incubated an additional 30 min at 30°C. Cells were concentrated 10-fold by brief centrifugation, mounted with AntiFade (Molecular Probes), and observed immediately. To assay for temperature sensitivity, strain YAB679 was shifted to 37°C for 2 h before labeling. Labeling with FM 4-64 and chase incubations were performed at 37°C. Cells were observed and photographed on a E600 microscope (Nikon, Melville, NY) equipped with a Spot II digital camera and MetaMorph 3.6 software (Universal Imaging, Downingtown, PA). Images were processed using Adobe Photoshop 5.0.
Indirect Immunofluorescence Microscopy
pcDNA3-based plasmids for expression of GGA1, GGA1L182Q, GGA3, and GGA3L149Q with an HA epitope tag at the amino terminus were transiently transfected into NRK cells by using FuGENE 6 (Roche Applied Science, Indianapolis, IN). The cells were analyzed by indirect immunofluorescence, as described previously (Boman et al., 2000) by using antibodies to GGA1 (R79709), the HA epitope (12CA5; BabCo), or mannosidase II (53FC3; Covance Research Products, Richmond, CA).
GFP Fluorescence Microscopy
For analysis of GFP-tagged proteins by fluorescence microscopy, mid-log cultures were diluted with 0.5 volumes of GFP KILL buffer (1 M Tris pH 8.0 and 5% sodium azide; Urbanowski and Piper, 1999). Samples were mixed with an equal volume of AntiFade (Molecular Probes) and observed within 1 h. Cells were photographed as described for FM 4-64 staining.
RESULTS
Mutations in GAT Domain Eliminate Interaction with ARF
The Arf-binding domain of human and yeast GGA proteins was previously mapped by truncation analysis to a highly conserved region within the GAT domain (Boman et al., 2000; Zhdankina et al., 2001). Several residues within this domain are conserved in all known GGA proteins (Figure 1A, highlighted residues). To disrupt binding to ARF without disrupting overall protein folding, selected point mutations within this region were generated and tested for binding to ARF by using the two-hybrid assay. To compare between species, homologous mutations were generated in both human and yeast GGA proteins. We changed leucine at position 203 of Gga1p to glutamine (Gga1pL203Q), isoleucine 207 of Gga2p to asparagine (Gga2pI207N), leucine 182 of GGA1 to glutamine (GGA1L182Q), and leucine 149 of GGA3 short form to glutamine (GGA3L149Q). Additional mutations in the GAT domain of Gga2p were also generated: residues 212–214 were changed to alanines (referred to as Gga2pPEDL → AAAL) and residues 219 and 220 were changed to alanines (referred to as Gga2pANKL → AAAL). None of these mutations affect protein expression or stability, because similar levels of protein were detected by Western blotting in yeast strains that expressed wild-type or mutant forms of each protein as either Gal4AD fusions or untagged constructs (our unpublished data). These mutations are not predicted to disrupt the coiled-coils in the GAT domain, determined using the COILS program (Lupas et al., 1991).
Wild-type and mutant human GGA1 and GGA3 as Gal4AD fusions were tested for binding to ARF3Q71L as a C-terminal Gal4BD fusion. As described previously (Boman et al., 2000), wild-type GGA1 and GGA3 interact strongly with ARF3Q71L, shown herein by growth on medium lacking histidine and supplemented with 3-amino triazole (3AT; Figure 1B) and by blue color development in the β-galactosidase assay (X-gal; Figure 1B). In contrast, GGA1L182Q and GGA3L149Q show no interaction by growth on 3AT plates or in the X-gal assay (Figure 1B). These results showed that our mutations abolished detectable binding of mammalian GGAs to ARF. We then tested wild-type and mutant yeast Gga1p and Gga2p as Gal4AD fusions for binding to Arf2pQ71L as a C-terminal Gal4BD fusion. Arf2pQ71L was used for these experiments because Arf1pQ71L is lethal in this construct (Zhdankina et al., 2001). As expected (Zhdankina et al., 2001), wild-type yeast Gga1p and Gga2p interact with Arf2p, shown herein by growth on 3AT plates and blue color development in the X-gal assay (Figure 1B). Our mutations effectively abolished this interaction. Gga1pL203Q and Gga2pI207N showed no detectable binding to Arf2p; strains grew much slower on 3AT plates and remained white in the X-gal assay, even after extended times. The overall folding of these mutant proteins was unperturbed, because these mutants remained active for binding to a novel Gga-binding protein (our unpublished data).
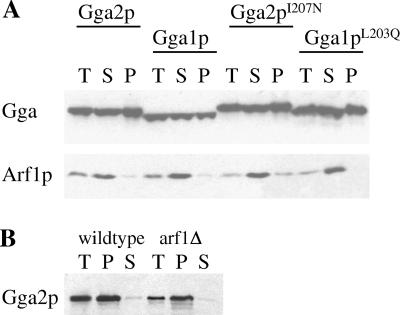
To confirm that the loss of signal in our two-hybrid assays was due to loss of affinity for Arf, we analyzed the interaction between Arf1p and Gga2p or Gga2pI207N by affinity chromatography (Figure 1C). GST alone was used as a negative control. Bacterially expressed Arf1p was incubated with GTPγS or GDP then mixed with purified GST fusion proteins on glutathione agarose beads. Retained Arf1p was detected by immunoblotting (top). Equal amounts of GST-Gga2p, GST-Gga2pI207N, or GST were present in each reaction, as shown by Coomassie staining (bottom). Arf1p·GTPγS, but not Arf1p·GDP, bound to GST-Gga2p; the amount of Arf1p retained by Gga2p increased linearly with the concentration of Arf1p in the binding reaction. Identical experiments were performed with GST-Gga1p, but no binding was detected at these concentrations (our unpublished data). This suggests a lower affinity of Arf1 for Gga1p than for Gga2p. Because of the unique nucleotide-binding properties of Arf proteins (Zhu et al., 2000), we do not know the actual concentration of GTP-bound Arf1p in these samples and we cannot determine a Kd value for the binding of Arf1p. However, we can detect as little as 3 ng of Arf1p by immunoblotting, which is 10-fold less than present in lane 4 (GST-Gga2p, 0.6 μM Arf1p·GTPγS) and 100-fold less than lane 6 (GST-Gga2p, 6 μM Arf1p·GTPγS) as determined by densitometry. Strikingly, we could not detect any binding of Arf1p·GTPγS to GST-Gga2pI207N or GST alone, even with 6 μM Arf1p. This suggests that the I207N mutation reduced the binding to Arf1p by at least 99% in vitro. Based on the two-hybrid and in vitro binding experiments, we concluded that these conserved residues in the GAT domain are critical for interaction with GTP-bound ARF proteins.
ARF Interaction Is Required for Localization of Mammalian GGA Proteins
We next tested the mammalian GGA mutants for localization in vivo. Plasmids encoding HA-tagged wild-type or mutant GGA1 or GGA3 were transfected into normal NRK fibroblasts and localized by indirect immunofluorescence (IIF) by using antibodies to GGA1 (Boman et al., 2000) or the HA epitope (12CA5). The Golgi apparatus was double labeled using antibodies to mannosidase II (Figure 2, b, d, and f) or β-COP (Figure 2h). Transfected wild-type GGA1 localized predominantly to the trans-Golgi region (Figure 2a), as expected. In contrast, GGA1L182Q was distributed throughout the cytosol, with no detectable Golgi staining (Figure 2c). The staining shown in Figure 2, a and c, used antibodies against GGA1 at a dilution that fails to detect endogenous protein, thus this staining detected only the transfected proteins. Similar results were seen with antibodies against the HA epitope (our unpublished data). At higher concentrations of GGA1 antibody, the endogenous GGA1 was detected at the Golgi region (our unpublished data), suggesting that the Golgi localization of endogenous GGA1 was not altered by overexpression of GGA1L182Q. Similarly, transfected wild-type GGA3 localized to the trans-Golgi region (Figure 2e), whereas GGA3L149Q was distributed throughout the cytosol (Figure 2g). Double labeling with antibodies to ARF (1D9; our unpublished data), mannosidase II (Figure 2d), or β-COP (Figure 2h) showed that ARF localization and Golgi structure were unaffected by these mutant GGA proteins. These results suggest that GGA1L182Q and GGA3L149Q fail to interact with ARF in vivo as well as in vitro, and that interaction with ARF is required for normal GGA localization to the Golgi membrane in mammalian cells.
Figure 2.
Localization of wild-type and mutant human GGA proteins in NRK fibroblasts. Cells were double labeled by indirect immunofluorescence to visualize transfected GGA proteins (left) and Golgi markers mannosidase II or β-COP (right). Wild-type GGA1 and GGA3 are localized to the Golgi region, whereas mutants GGA1L182Q and GGA3L149Q localized throughout the cytosol.
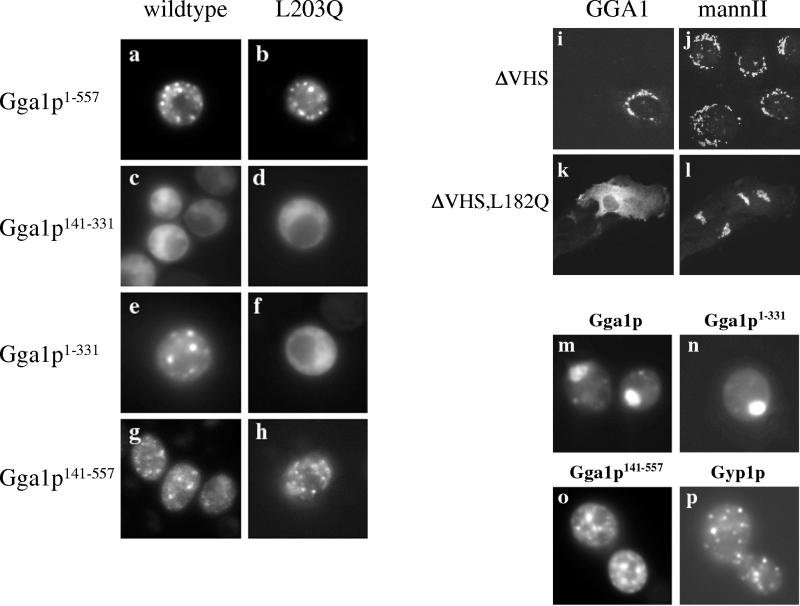
These data are consistent with a recent report (Puertollano et al., 2001b) that different mutations in the GAT domain of GGA3 disrupt ARF interaction and Golgi localization. Also consistent with previous work, we found that removal of the VHS or ear domains does not alter GGA localization in NRK cells (Figure 9; and our unpublished data), suggesting an exclusive role for the GAT/Arf-binding domain in Golgi localization of mammalian GGA proteins. Our new results identify a distinct residue (L182 in GGA1, L149 in GGA3) in the GAT domain that is essential for interaction with ARF in vivo.
Figure 9.
Localization of truncated GFP-Gga1p. (a–h) Strains expressing wild-type or mutant versions of the indicated constructs were analyzed by GFP fluorescence. Punctate staining, characteristic of Golgi localization, was observed in a, b, e, g, and h. Cytosolic staining was observed in c, d, and f. (i–l) Localization of wild-type and mutant human GGA1ΔVHS. NRK cells were transiently transfected with pcDNA3-based vectors for expressing GGA1ΔVHS (residues 145–639, i–j) or mutant GGA1ΔVHS, L182Q (k and l). Cells were double-labeled for IIF with antibodies to GGA1 (R79709, i and k) and mannosidase II (j and l). GGA1ΔVHS localizes to the Golgi region, whereas GGA1ΔVHS, L182Q is cytosolic. (m–p) Localization of yeast Gga1p constructs in a class E mutant strain. The indicated constructs were expressed in a strain deleted of VPS27. Full-length GFP-Gga1p (m) and GFP-Gga1p1–331 (n) localize to the class E compartment. In contrast, GFP-Gga1p141–557 (o) localizes to distinct puncta throughout the cytoplasm in addition to limited staining near the vacuole. The early Golgi protein Gyp1p, expressed as an RFP fusion, localizes exclusively to Golgi puncta (p).
Mutant Yeast GGA Proteins Retain Golgi Localization
Based on the above-mentioned results for mutant human GGAs and the high conservation of the ARF-binding domain between human and yeast orthologs, we expected the homologous mutations in yeast Gga1p and Gga2p to disrupt their localization and function. Surprisingly, this was not the case. To investigate whether Ggas and Arf interact in vivo, we analyzed the localization of wild-type and mutant Gga proteins by fluorescence microscopy and subcellular fractionation.
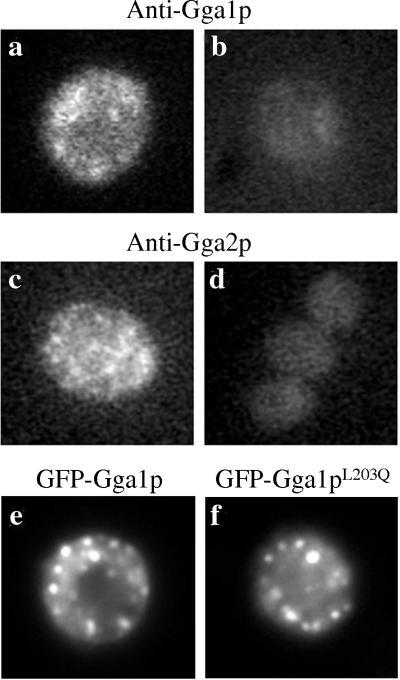
Antibodies specific to each Gga protein (Zhdankina et al., 2001) were initially used for indirect immunofluorescence of yeast cells. Affinity-purified antibodies to Gga1p and Gga2p showed faint but distinct puncta throughout the yeast cytoplasm, consistent with Golgi staining (Figure 3, a and c). Yeast strains deleted of both GGA genes failed to stain with either antibody, indicating specificity (Figure 3, b and d). Endogenous Gga1p and Gga2p did not colocalize with an HA-tagged form of the early Golgi protein Och1p (Harris and Waters, 1996; our unpublished data). Technical difficulties have hampered our attempts to colocalize Ggas with late Golgi markers. Because our detection of endogenous proteins was weak, we next localized GFP-tagged Gga proteins. GFP-Gga1p and GFP-Gga2p fully complement the trafficking defects of gga1gga2 cells (Stefan, personal communication; Figure 7). These GFP-constructs were each expressed in wild-type strain BY4735. Western blots with antibodies to Gga1p or Gga2p indicated that the GFP-Ggas are expressed at significantly higher levels than endogenous Gga1p and Gga2p (our unpublished data). When analyzed by fluorescence microscopy, we detected a distinct punctate fluorescence pattern for GFP-Gga1p (Figure 3e) that was very similar to, but significantly brighter than, the endogenous Gga1p signal. GFP-Gga2p gave similar staining, plus high levels of cytosolic staining not seen with endogenous Gga2p (our unpublished data). We therefore used GFP-Gga1p for the studies described below. GFP-Gga1p did not colocalize with a red fluorescent protein (RFP)-tagged form of the early Golgi protein Gyp1p (Du and Novick, 2001; our unpublished data), consistent with our IIF results. In vps27Δ cells, which accumulate TGN, endosomal, and vacuolar components in the class E compartment, GFP-Gga1p but not RFP-Gyp1p localizes to this compartment (Figure 9, m and p). These data suggest that Gga proteins are localized to the late Golgi, consistent with their function and with a recent report using HA-tagged Gga1 in a class E mutant strain (Hirst et al., 2001). Surprisingly, the L203Q mutation in GFP-Gga1p did not alter the staining pattern (Figure 3f), suggesting that the Golgi localization of Gga1p did not require Arf interaction. Identical results were observed with GFP-Gga2pI207N (our unpublished data). These data were in stark contrast to their mammalian counterparts.
Figure 3.
Localization of Gga1p and Gga2p in yeast. Top and middle, indirect immunofluorescence of Gga1p or Gga2p in wild-type strain BY4704 (a and c) or gga1gga2 strain YAB538 T6a (b and d). Bottom, GFP fluorescence of GFP-Gga1p or mutant GFP-Gga1pL203Q in wild-type strain BY4735.
Figure 7.
Synthetic ts phenotype of gga1gga2vps27 is fully complemented by mutant Gga2p. Plasmids encoding wild-type or mutant Gga1p or Gga2p were transformed into gga1gga2vps27 strain YAB680. GFP fusions of Gga1p are expressed at high copy, all others at low copy. Serial dilutions of wild-type, YAB680, and each transformed strain were replica plated on YPD and incubated at 30 or 37°C. Complementation of the ts growth defect is indicated by restored growth at 37°C.
ARF proteins (except ARF6) cycle on and off their target membranes in concert with GTP binding and hydrolysis; GTP-bound ARF is membrane associated, and GDP-bound ARF is cytosolic. However, after cell lysis and centrifugation, the majority of yeast and mammalian ARF is found in a soluble fraction. Mammalian GGA proteins behave similarly (Boman et al., 2000; Hirst et al., 2000), consistent with ARF-dependent membrane association. We analyzed yeast Ggas by subcellular fractionation to determine whether yeast Gga proteins fractionate with membranes or cytosol, and to determine whether mutations in the Arf-binding domain altered the fractionation profile. Lysates from stains expressing endogenous or HA-tagged Gga1p or Gga2p were centrifuged at 100,000 × g to generate soluble (S100) and membrane (P100) fractions, which were analyzed on immunoblots with antibodies against endogenous Gga1p or Gga2p (our unpublished data) or the HA tag (Figure 4A), with identical results. Duplicate gels were probed with R23 antibodies against Arf1p. As expected, Arf1p was found almost exclusively in the S100 fraction (Figure 4A). In contrast, yeast Gga1p and Gga2p partitioned equally between the soluble and membrane fractions (Figure 4A, left), strongly suggesting that yeast Gga proteins are bound to membranes by interactions with proteins other than, or in addition to, Arf. In addition, we found that the expression levels of HA-Gga1p and HA-Gga2p are nearly identical. Each is expressed from its own promoter, suggesting that endogenous Gga1p and Gga2p expression levels are also similar. This differs from a previous report (Costaguta et al., 2001) stating that Gga1p is expressed at significantly lower levels than Gga2p.
Figure 4.
Immunoblots of yeast lysate fractions. (A) Fractionation of wild-type and mutant Gga proteins. Total lysates (T) from strains expressing HA-tagged Gga2p, Gga1p, Gga2pI207N, or Gga1pL203Q were centrifuged at 100,000 × g to generate supernatant (S) and pellet (P) fractions. Duplicate gels were probed with antibodies to the HA tag (top) or Arf1p (bottom). Arf1p was predominantly in the soluble fraction, whereas all Gga proteins fractionated equally between the soluble and pellet fractions. (B) Fractionation of Gga2p in the absence of Arf1p. Fractions from wild-type or arf1Δ strain TT104 were prepared as in A, and probed with antibodies to Gga2p. Gga2p was predominantly in the pellet fraction in both strains.
In the same experiments, untagged (our unpublished data) or HA-tagged mutants Gga1pL203Q and Gga2pI207N also partitioned equally between the soluble and membrane fractions (Figure 4A, right), confirming that Arf is not the primary mediator of Golgi localization for yeast Gga proteins. In some of our fractionation experiments, the wild-type and mutant Gga proteins partitioned exclusively into the membrane fraction. We cannot yet explain why this was so, except to note that the wild-type and mutant Ggas behaved identically in any given experiment.
To independently test a role for Arf in localizing Ggas to membranes, Gga2p was analyzed in yeast cells devoid of Arf1p (arf1Δ strain TT104; Stearns et al., 1990a). Consistent with our mutant analysis, Gga2p fractionated with membranes in the absence of Arf1p (Figure 4B). This strain expresses low levels of Arf2p (10% that of Arf1p; Stearns et al., 1990a), unlikely to account for all membrane localization of Gga2p. We conclude that Arf is not required for recruiting Gga proteins to membranes in yeast.
GGA Mutants Complement CPY Sorting Defects
Yeast strains deleted of GGA1 and GGA2 have been analyzed for defects in trafficking of several proteins, including CPY (Boman, 2001). To determine whether defects in binding to Arf disrupt the trafficking functions of Gga proteins, we tested whether Gga1pL203Q or Gga2pI207N could complement the CPY sorting defect of gga1gga2 strains. The wild-type and mutant alleles of GGA1 and GGA2 were subcloned into low copy vectors under control of their endogenous promoters and transformed into gga1gga2 yeast strain YAB538 T6a (Table 2). All constructs were expressed at levels similar to the endogenous Gga proteins, as determined by Western blotting (our unpublished data).
As expected, the gga1gga2 strain was defective in sorting of CPY, as shown by pulse-chase analysis of newly synthesized CPY (Figure 5A) and colony immunoblot (Figure 5B). Also as expected, wild-type Gga2p fully complemented the defects in both assays (Figure 5, A and B). Surprisingly, the mutant Gga2pI207N also fully complemented these defects, as shown by pulse-chase analysis (Figure 5A) and colony immunoblotting (Figure 5B). We concluded that either Arf interaction is not required for Gga2p function, or these mutants can still bind Arf in vivo. Expression of Gga1p complemented the CPY-processing and -sorting defects only partially (Figure 5, A and B). Our first indication of a functional defect in our mutant Gga proteins was seen for Gga1pL203Q, which partially complemented the CPY processing defect in the pulse-chase assays (Figure 5A) but failed to complement the CPY sorting defect (Figure 5B). There was minor variability in the extent of partial complementation by Gga1pL203Q (compare Figure 5, A and B), but it is always less than wild-type Gga1p. This suggests that the sorting function of Gga1p is partially disrupted by the mutation that blocks Arf binding.
Figure 5.
CPY sorting is complemented by Arf-binding domain mutants in yeast. (A) Pulse-chase analysis of newly synthesized CPY in wild-type, gga1gga2, and gga1gga2 harboring low copy plasmids of Ggas. Time course is indicated in minutes. Expression of Gga2p or Gga2pI207N (right) fully complemented the CPY processing defect in gga1gga2 strains. Expression of Gga1p or Gga1pL203Q (middle) partially complemented this defect. P1, unglycosylated endoplasmic reticulum form of CPY; P2, glycosylated Golgi form; M, fully processed mature (vacuolar) form. (B) Detection of secreted CPY by colony immunoblotting. Serial dilutions of yeast cultures were replica plated onto YPD plates. A signal on colony immunoblots indicates that CPY is secreted from the cell, suggesting that CPY is missorted. The CPY sorting defect of gga1gga2 strains (ΔΔ) was complemented by wild-type Gga2p and mutant Gga2pI207N. Wild-type Gga1p partially complemented the CPY sorting defect, whereas mutant Gga1pL203Q failed to complement.
Although the homologous mutations in human GGA1 and GGA3 disrupt ARF interaction in vivo, this single mutation might not fully eliminate Arf binding in the yeast Gga2p mutants. To address this possibility, we made two clusters of alanine substitution mutations in the Arf-binding domain of Gga2p. We replaced three residues of a conserved PEDL sequence (P212A, E213A, and D214A), designated Gga2pPEDL → AAAL, or two residues in a conserved ANKL sequence (N219A and K220A), designated Gga2pANKL → AAAL. The latter includes the asparagine residue mutated by Puertollano et al. (2001b) to disrupt ARF–GGA3 interaction. Neither mutant Gga2pPEDL → AAAL nor Gga2pANKL → AAAL interacted with Arf2p in the two-hybrid assay (Figure 1B). However, both mutants still complemented the CPY defect when assayed by either pulse chase or colony immunoblot (our unpublished data), showing that they were functional in yeast. These data suggest that reduction of Arf interaction does not affect the ability of Gga2p to sort CPY in yeast.
Genetic Interaction between GGAs and VPS27
As a complementary approach to understand the function of Gga proteins in yeast, we took advantage of the nonlethal phenotype of GGA gene deletions. Specifically, we tested for synthetic interactions between GGA deletions and genes that function in distinct post-Golgi trafficking pathways: APM1, APM3, VPS1, and VPS27. APM1 encodes the medium (μ) subunit of the adaptor protein (AP)-1 clathrin adaptor complex, which is thought to function in anterograde or retrograde traffic between the TGN and early endosome (Hirst and Robinson, 1998). However, we note that deletion of APM1 does not eliminate AP-1 function, because a related protein, Apm2p, can substitute in the AP-1 complex (Yeung et al., 1999). APM3 encodes the medium (μ) subunit of the AP-3 clathrin adaptor complex, which mediates a direct TGN-to-vacuole pathway (Cowles et al., 1997; Stepp et al., 1997). Deletion of APM3 eliminates AP-3 function. VPS1 encodes yeast dynamin, a protein involved in the scission of clathrin-coated vesicles from donor membranes (Vater et al., 1992). Vps1p mediates both TGN-to-endosome and TGN-to-vacuole traffic, and deletion causes missorting of vacuolar proteins via the plasma membrane (Nothwehr et al., 1995). VPS27 is a class E gene (Raymond et al., 1992) that regulates exit from the prevacuolar compartment (PVC) and entry into intralumenal vesicles (Piper et al., 1995; Urbanowski and Piper, 2001). Strains were constructed with triple gene deletions (Table 2) and analyzed for growth defects at 30 or 37°C. Deletion of either apm3 or vps1 in the gga1gga2 knockout strain showed no synthetic growth defects (Figure 6A, strains YAB658 and YAB667). Deletion of apm1 in the gga1gga2 knockout produced a very slight growth defect at 37°C (Figure 6A, strain YAB650). Furthermore, these three triple-knockout strains showed no synthetic defects in trafficking of alkaline phosphatase, CPY, or Vps10p (our unpublished data).
Figure 6.
(A) Synthetic ts growth phenotype of gga1gga2vps27 strains. Strains were tested for synthetic growth defects at 30 or 37°C. Strains were streaked onto YPD plates and incubated for 2 d. Deletion of the AP-1 medium subunit (apm1), the AP-3 medium subunit (apm3), or the dynamin homolog VPS1 (vps1) showed no synthetic growth phenotypes with gga1gga2. In contrast, deletion of VPS27 in two different gga1gga2 strain backgrounds was (ts for growth. Strains: wild-type (BY4704), gga1gga2 (YAB538 T6a), gga1gga2apm1 (YAB650), gga1gga2apm3 (YAB658), gga1gga2vps1 (YAB667), gga1gga2vps27 in S288C background (YAB677), gga1gga2vps27 in SEY6210 background (YAB679), and vps27 (JUY68). (B) Synthetic ts growth phenotype of gga1gga2vps28 strain. As in Figure 6A, strains were streaked onto YPD plates and incubated for 2 d at 30 or 37°C. Strains: wild-type (SEY6210), gga1gga2 (GPY2385), vps27 (JUY68), vps28 (YCS41), gga1gga2vps27 (YAB680), and gga1gga2vps28 (YCS195). All strains grew at 30°C. Deletion of GGA1, GGA2, and VPS28 is ts for growth, similar to deletion of GGA1, GGA2, and VPS27. (C) Class E compartment forms at 30 and 37°C in gga1gga2vps27 strain. Cells grown at 30 or 37°C were stained with FM 4-64 to visualize the vacuole and prevacuolar compartment. FM4-64 fluorescence (left) and differential interference contrast images (right) are shown. Wild-type cells show vacuolar staining with no enlarged PVC (our unpublished data). In contrast, the gga1gga2vps27 cells had enlarged PVC, indicated by bright staining adjacent to the vacuole, at both 30 and 37°C.
In contrast, cells deleted of GGA1, GGA2, and VPS27 were temperature sensitive (ts) for growth in two different strain backgrounds (Figure 6A, strains YAB677 and YAB679). The slight differences in strain background are currently unexplained. This ts growth defect was reversible after at least 3 d at the restrictive temperature, indicating growth inhibition rather than lethality (our unpublished data). This tight and reversible ts growth phenotype in the SEY6210 background was found in multiple isolates of the genotype, including strain YAB680 shown in Figure 6B. To determine whether the ts phenotype was related to the VHS domain of Vps27p, we tested another class E gene for genetic interaction with GGA deletion. Deletion of GGA1, GGA2, and VPS28 in the SEY6210 background was synthetically ts for growth (Figure 6B). This suggests that the synthetic phenotypes are due to the function of class E genes rather than the VHS domain of Vps27p.
A notable defect of class E mutants (such as vps27) is the enlargement of the PVC, or the class E compartment. Using FM 4-64 as a marker for the vacuole and PVC, we tested for PVC enlargement in the triple knockout strain to determine whether GGA deletion prevented the formation of this compartment. Both vps27 (our unpublished data) and gga1gga2vps27 strains (Figure 6C) contained characteristic class E compartments at 30 and 37°C, suggesting that the PVC can form in the absence of GGA gene function. We also noted that the vacuolar morphology was altered in these strains, with a bubble-like appearance. This is consistent with a vacuolar phenotype of gga1gga2 strains reported by others (Hirst et al., 2001; Mullins and Bonifacino, 2001) and not observed in our previous experiments with a different strain background.
GGA Mutants Complement gga1gga2vps27 Phenotype
To determine whether binding to Arf was required for Gga1p or Gga2p to complement the synthetic ts phenotype of the gga1gga2vps27 strain, the wild-type and mutant alleles of GGA1 and GGA2 were transformed into yeast strain YAB680. Transformants were streaked onto YPD and incubated at 30 or 37°C for 2 d. The ts phenotype was fully complemented by expression of plasmid-borne wild-type GGA2 (Figure 7). The three mutant alleles Gga2pI207N, Gga2pPEDL→AAAL, and Gga2pANKL→AAAL also fully complemented the growth defect at 37°C when expressed in this strain (Figure 7). Thus, these mutants seemed to complement all Gga trafficking functions. We concluded that Gga2p function is not dependent on Arf, or that these mutants do not disrupt Gga2p-Arf binding in vivo. As in the CPY assay, expression of Gga1p partially complemented the ts growth defect (Figure 7). High copy expression of GFP-Gga1p fully complemented the ts growth defect (Figure 7), showing that the partial complementation by Gga1p could be overcome by higher expression (see DISCUSSION). In contrast, expression of mutant Gga1pL203Q failed to complement the ts growth defect; only very slow growth was restored (Figure 7). This suggests that Arf binding is important for Gga1p function. At high expression levels, GFP-Gga1pL203Q partially complemented the ts growth defect (Figure 7). This suggests that the mutation reduced, but did not eliminate, Gga1p function.
N- and C-Terminal Domains Confer Golgi Localization of Yeast GGA Proteins
Because interaction with Arf was not required for Golgi localization of Gga proteins, we made a series of truncation constructs of GFP-Gga1p (Figure 8) to test the VHS, hinge, and ear domains for roles in Gga localization. All constructs that included the GAT domain were made as wild-type and L203Q mutants to analyze the role of Arf interaction in the truncated proteins. We used Gga1p for these experiments because the L203Q mutation reduces function of the full-length protein. All proteins were expressed at levels similar to full-length GFP-Gga1p, as determined by Western blotting (our unpublished data). Each GFP fusion protein was localized in wild-type strain BY4735 by fluorescence microscopy. Full-length wild-type GFP-Gga1p and mutant GFP-Gga1pL203Q are shown for comparison (Figure 9, a and b).
Figure 8.
Schematic of truncation constructs of GFP-Gga1p and Gga2p.
A construct expressing the yeast GAT domain alone (GFP-Gga1p141–331) was localized in the cytosol (Figure 9c), in stark contrast to the GAT domain of mammalian GGAs. A similar construct as a Gal4AD fusion protein interacts with Arf2pQ71L in the two-hybrid assay (Zhdankina et al., 2001), yet Arf interaction is not sufficient to localize the yeast GAT domain to membranes in vivo. The mutant GFP-Gga1p141–331,L203Q also localized to the cytosol (Figure 9d). Similarly, the VHS domain alone was cytosolic (our unpublished data). Importantly, a construct expressing the VHS/GAT domains (GFP-Gga1p1–331) localized to the Golgi and was indistinguishable from full-length GFP-Gga1p (Figure 9e). When this same construct carried the L203Q mutation, GFP-Gga1p1–331,L203Q was found exclusively in the cytosol (Figure 9f). We made two conclusions from these results. First, we concluded that binding to Arf contributes to stable association of Gga1p with membranes and that the L203Q mutation indeed disrupts interaction with Arf in vivo. Second, we concluded that the VHS domain confers weak Golgi localization, which is stabilized by GAT binding to Arf.
A construct expressing the GAT/hinge/ear domains (GFP-Gga1p141–557) localized to Golgi-like puncta (Figure 9g). This suggests that the hinge and/or ear domains also contribute to stable association with membranes. This construct carrying the L203Q mutation (GFP-Gga1p141–557,L203Q) also localized to Golgi-like puncta (Figure 9h), suggesting that the hinge and/or ear domains interact with some Golgi component with high enough affinity to drive the GFP reporter onto the Golgi, regardless of Arf interaction. Consistent with this observation, a hinge/ear construct of Gga1p also seems membrane associated (our unpublished data). In contrast, comparable constructs of human GGA1 confirm that only the GAT domain confers Golgi localization in mammalian cells. IIF of GGA1ΔVHS in NRK cells shows Golgi localization indistinguishable from full-length GGA1, whereas GGA1ΔVHS,L182Q is entirely cytosolic (Figure 9, i–l).
Interestingly, the GFP-Gga1p141–557 construct lacking the VHS domain seemed to stain more puncta than did full-length Gga1p, suggesting that the VHS domain may restrict Gga1p to distinct Golgi cisternae (likely the TGN). To test for altered localization of the truncated constructs, each was expressed in a vps27Δ strain (Figure 9, m–p). Full-length GFP-Gga1 and GFP-Gga1p1–331 localized exclusively to the class E compartment, suggesting that removal of the hinge/ear domain did not alter the localization of Gga1p. In contrast, GFP-Gga1p141–557 localized to spots throughout the cytoplasm in addition to some accumulation near the vacuole (Figure 9o). The class E compartment formed in these cells, as determined by FM 4-64 staining (our unpublished data). Early Golgi cisternae do not collapse into the class E compartment; RFP-Gyp1p localized to puncta in vps27Δ cells (Figure 9p). These data suggest that removal of the VHS domain caused mislocalization of Gga1p to other organelles such as early Golgi. We concluded that at least three domains on Gga proteins interact with Golgi-associated proteins to recruit and stabilize Gga proteins at the TGN and that Arf interaction occurs in vivo but is neither sufficient nor required for Gga localization.
Both VHS and Hinge/Ear Domains Are Required for GGA Function
To determine whether the VHS and hinge/ear domains are required for Gga function, we tested truncations of Gga2p and GFP-Gga1p (diagrammed in Figure 8) for complementation of the CPY sorting defect of gga1gga2 or ts growth defect of gga1gga2vps27 strains. Each construct was expressed and stable, as determined by Western blotting (our unpublished data). In contrast to the GAT domain mutants, deletion of the VHS domain or the hinge and ear domains eliminated the function of Gga2p and GFP-Gga1p. A construct expressing the VHS and GAT domains of Gga2p (Gga2p1–326) failed to complement the gga1gga2vps27 ts growth defect (Figure 10) or the CPY sorting defect when assayed by pulse-chase or colony immunoblot (our unpublished data). In all three assays, the strains expressing Gga2p1–326 were indistinguishable from strains lacking Gga2p entirely. This indicates that the hinge and/or ear domains are essential for Gga2p function. Similarly, a construct expressing the GAT, hinge, and ear domains of Gga2p (Gga2p110–585) failed to complement the gga1gga2vps27 ts growth defect (Figure 10) or the CPY sorting defect (our unpublished data). These results indicate that the VHS domain is essential for Gga2p function.
Figure 10.
VHS and ear domains are required for Gga function in yeast. Indicated constructs of Gga2p or GFP-Gga1p were transformed into gga1gga2vps27 strain YAB680. Serial dilutions were replica plated on YPD and grown at 30 or 37°C for 2 d. None of the truncated proteins complemented the ts growth defect, indicating that the VHS and ear domains are required for Gga function in yeast.
Identical results were obtained using truncations of GFP-Gga1p to complement the gga1gga2vps27 growth defect (Figure 10). Neither GFP-Gga1p1–331 nor GFP-Gga1p141–557 complemented the defect. Not surprisingly, a construct expressing only the GAT domain (GFP-Gga1p141–331) also failed to complement the gga1gga2vps27 growth defect. Together, these data show that the VHS, hinge, and ear domains are important not only for localization of Ggas to the Golgi but also for the function of the Ggas at the Golgi.
DISCUSSION
Our main focus of this work was to analyze the functional significance of Gga–Arf interaction in yeast. Because of the high homology between human and yeast GAT domains, particularly in the ARF-binding domain, we expected that the function of this domain would be conserved between human and yeast GGA proteins. Likewise, we expected that mutations within this domain would have similar effects on the localization and function of yeast and human GGA proteins. Surprisingly, our data refute this hypothesis and show strong differences between human and yeast GGA proteins. In mammalian cells, ARF interaction is necessary and sufficient for recruitment of GGAs to the Golgi. In yeast, the VHS, GAT, and hinge/ear domains all contribute to Golgi localization; when expressed individually, only the hinge/ear domain is sufficient for Golgi localization. Although we can detect interaction with Arf in vivo, this interaction is not required for localization or function of yeast Gga proteins.
Mutant GGA Proteins
We are confident that the mutations we generated in the GAT domain eliminate binding to activated ARF. First, our two-hybrid assays show loss of interaction with all mutations, and affinity chromatography confirms the loss of affinity between Gga2p and Arf1p. Second, the mutations in human GGA1 and GGA3 cause a complete shift from the Golgi to the cytosol, consistent with a recent report describing a mutation in the GAT domain of GGA3 (Puertollano et al., 2001b). Third, in the context of a truncated GFP-Gga1p (but not in full-length GFP-Gga1p), the L203Q mutation causes a complete shift from the Golgi to the cytosol. Our results extend those in a recent report (Puertollano et al., 2001b) characterizing a mutation in human GGA3 that eliminates ARF–GGA interaction. Herein, we identified at least two additional amino acids in the ARF-binding domain that are also essential for the ARF–GGA interaction. Notably, Puertollano et al. (2001b) showed that mutation of the aspartate in the GGA3 PEDL sequence (D189) had no effect on ARF interaction. Because our PEDL→AAAL mutation eliminates Arf2p binding, we conclude that the proline or glutamate residues must be important for ARF binding. The growing number of residues within the GAT domain now reported as essential for interaction with ARF proteins is interesting, particularly that alteration of any one residue eliminates interaction with ARF. Perhaps the affinity of the GGA–ARF interaction is low, and elimination of any contributing element reduces the affinity below detection by two-hybrid analysis. Alternatively, the mutations may alter the structure of the GAT domain sufficiently to disrupt interaction. Structures of this domain and cocrystal structures of ARF and GGAs will surely reveal much about the interaction.
Differences between Yeast and Human GGA Proteins
In mammalian cells, ARF interaction is necessary for recruitment of GGAs to the Golgi. In yeast, our mutants have minimal effects on both localization and function, suggesting that Arf interaction is not required. The two most surprising results are that Gga1pL203Q remained Golgi localized and that all Gga2p mutants are fully functional. One possible explanation for our data is that yeast Ggas interact with Arf in vivo, and the mutations described herein reduce the affinity for Arf but do not fully disrupt the interaction. We note that the ARF-GGA interaction is significantly weaker in yeast than in mammalian cells, because the GAT domain of yeast Gga1p is not targeted to the Golgi. However, the GAT domain does contribute to Golgi localization: VHS/GAT is Golgi localized, whereas VHS alone or VHS/GATL203Q is cytosolic. The strength of the interactions between Ggas and other proteins (via the VHS and hinge/ear domains) could stabilize the Gga–Arf interaction at the Golgi and allow signaling from activated Arf. We favor a model in which interaction between Gga and Arf is stabilized by other protein interactions, hence reducing the effect of the mutations in vivo. We conclude that the role of Arf–Gga interaction in yeast is not to recruit Ggas to the Golgi, but rather to alter the structure of Ggas to allow sequential interactions with clathrin or other components.
Unfortunately, we cannot detect expression of mammalian GGAs in yeast. We have tried multiple promoters, fusion constructs, and truncation constructs; only the two-hybrid constructs are expressed at a detectable level. Furthermore, we cannot detect yeast Ggas in mammalian cells after either plasmid transfection or protein microinjection. Hence, we cannot test for complementation of the yeast phenotypes by wild-type or mutant human GGAs.
Differences between Gga1p and Gga2p
Previous reports concluded that Gga1p and Gga2p are functionally redundant, because expression of either Gga1p or Gga2p can complement the defects of gga1gga2 strains (Costaguta et al., 2001; Hirst et al., 2000, 2001; Zhdankina et al., 2001). However, our data reveal differences between Gga1p and Gga2p. Three mutant alleles of Gga2p function fully in all trafficking-related assays, whereas the function of mutant Gga1p is reduced. Also, full complementation of gga1gga2 defects by Gga1p requires high expression levels. There are several possible explanations for these differences. First, Gga1p and Gga2p may perform distinct functions in yeast and only Gga1p function is dependent upon interaction with Arf. This scenario is unlikely, because all gga1gga2 defects described to date can be rescued by expression of either Gga1p or Gga2p. Second, the different effect of the mutations may reflect differences in affinities for ARF. We feel this is the best explanation of our data. Our two-hybrid and affinity chromatography analyses indicate that the interaction between Gga1p and Arfs is weaker than that between Gga2p and Arfs. Whether these differences in affinity are a true reflection of affinity in vivo is not known. If the mutations reduce affinity for Arf, this reduction could ablate Gga1p–Arf interaction in vivo, but not fully ablate Gga2p–Arf interaction. Perhaps we would see an effect of the Gga2p mutants if we lowered the level of Gga2p expression. Third, Gga1p may interact with different proteins than Gga2p. Future screens to identify binding partners will address this possibility. Fourth, Gga1p may interact with the same proteins as Gga2p, but with different affinities. Perhaps these interactions are stronger with Gga2p than with Gga1p, thus stabilizing the Gga2p–Arf interaction and masking any effects of the mutants. High expression of Gga1p could force interaction with these proteins, thus complementing the double deletion. Fifth, the level of expression of Gga2p may be higher than that of Gga1p, as noted previously (Hirst et al., 2000; Costaguta et al., 2001; Zhdankina et al., 2001). However, our results with HA-tagged Gga1p and Gga2p argue that the expression levels are in fact very similar. Finally, the L203Q mutation may reduce interaction with another, unknown partner of Gga proteins that is involved in Gga function. Although we cannot rule out this possibility, the current evidence strongly favors Gga–Arf interaction via the GAT domain.
Roles of VHS Domain
The VHS domain is required for function; a construct lacking the VHS domain fails to rescue the ts phenotype of gga1gga2vps27. This conclusion is consistent with two recent reports (Hirst et al., 2001; Mullins and Bonifacino, 2001) showing that the VHS domain is required for CPY sorting. If the yeast and mammalian VHS domains of GGA proteins have similar functions then the VHS domain of Gga1p and Gga2p should interact with cargo that is sorted into Gga-dependent pathways. The mammalian GGA proteins interact with an acidic cluster-dileucine motif in transmembrane cargo receptors that traffic from the TGN to endosomes (Nielsen et al., 2001; Puertollano et al., 2001a; Zhu et al., 2001). The closest yeast homolog to these cargoes is the CPY receptor Vps10p. We cannot detect direct interaction between the Vps10p cytoplasmic tail and Gga proteins by two-hybrid analysis (our unpublished data). It will be important to identify the VHS binding partners in yeast and to test whether the yeast VHS domain functions to recognize and sort cargo. Because the VHS domain of Vps27p cannot substitute for the Gga VHS domain (Hirst et al., 2001), it will also be interesting to identify essential residues within the GGA VHS domain required for function.
We also show that the VHS domain contributes to the Golgi localization of Gga proteins, likely through interaction with other Golgi-associated proteins. This contribution is seen most definitively with the VHS/GAT construct of GFP-Gga1p. This construct (GFP-Gga1p1–331) is localized to Golgi-like puncta, whereas addition of the L203Q mutation causes exclusively cytosolic staining. It is possible that the L203Q mutation alters folding of the VHS/GAT construct such that interactions with the VHS domain are disrupted. We do not favor this explanation, because the L203Q mutation has only minor effects on Gga1p function. If L203Q altered VHS folding, we would expect the total loss of function phenotype of the VHS deletion. Hence, we conclude that both the VHS domain and the GAT domain interact weakly with Golgi-localized proteins, and that together these interactions confer Golgi localization.
It seems that the GFP-Gga1p construct lacking the VHS domain localizes to more puncta than does full-length GFP-Gga1p. There are two possibilities for this staining pattern. First, this construct may fragment the late Golgi. Second, the truncated Gga1p may mislocalize to other membranes such as early Golgi or endosomes. Our data using vps27Δ cells show that the class E compartment forms but the construct still localizes to distinct puncta, supporting the second possibility. These data suggest that the VHS domain confers specificity to the late Golgi, perhaps by binding to cargo proteins that are present at the TGN. Because the VHS/GAT construct localizes to Golgi but GAT alone does not, a genetic screen for mutants that disrupt Golgi localization of VHS/GAT may identify the VHS-binding partners.
Role of Hinge/Ear Domains
The hinge and/or ear domains are required for function; when both domains are deleted, Gga1p and Gga2p are nonfunctional. Hirst et al. (2001) reported that deletion of the ear domain partially reduced Gga function. Hence, our results suggest that the hinge is required for Gga function. Because clathrin interacts with the hinge domain and facilitates vesicle formation (Costaguta et al., 2001; Puertollano et al., 2001b), this loss of function is likely due to loss of clathrin interaction, leading to loss of vesicle formation. However, the hinge and ear domains also confer Golgi localization to yeast Gga proteins. This contrasts with the human GGA proteins, for which the hinge/ear domain is soluble. Two models could explain this result. First, clathrin is present at the TGN membrane in yeast and helps to recruit Gga proteins. Second, a trans-Golgi component interacts with the ear domain to recruit Ggas to the TGN, which in turn recruit clathrin. Many have shown that clathrin is recruited from the cytosol to the membrane by adaptor proteins (for review, see Hirst and Robinson, 1998), supporting the second model. However, the identity of these potential ear binders is unknown, and the role of the ear domain is still unclear. Perhaps the ear domain interacts with Golgi components to increase cargo specificity, stabilize the interactions during vesicle formation, or disassemble the complex after vesicle formation. As discussed for the VHS domain, it will be very informative to identify Gga ear partners.
Mammalian GGA Function
We show that ARF interaction is required for the localization of human GGA1 and GGA3 to the TGN. This is consistent with a recent report (Puertollano et al., 2001b) showing that a mutant GGA3 is cytosolic with dramatically reduced function. We found that high levels of expression of mutant GGA3 shift M6PR to the plasma membrane (our unpublished data). This was similar to the shift we observed with wild-type GGA3 overexpression (Boman et al., 2000), suggesting that mutant GGA3 in fact retains some function. There are two possible explanations for this observation. First, the mutant GGA3 may interact weakly with ARF, and high levels of expression allow a functional interaction at the TGN. However, we do not see any accumulation of mutant GGA3 at the TGN in our immunofluorescence analyses. Second, mutant GGA3 may interact with binding partners in the cytosol rather than at the TGN, sequestering these components away from their functional sites on the TGN or plasma membrane. The most likely candidate is clathrin, shown to be recruited by GGAs to the TGN (Puertollano et al., 2001b). This would be interesting, because it would indicate that GGA–clathrin interaction is not dependent upon an established ARF–GGA interaction, but that GGA and clathrin are recruited together to the TGN through interaction between GGA and ARF. If this sequestering occurs, then we would expect that clathrin-dependent endocytosis would be reduced. We have preliminary data showing that transferrin receptor accumulates on the plasma membrane in cells overexpressing GGA3, supporting this possibility. Further studies must be done to show interaction of the mutant GGA3 with clathrin in cytosol.
Genetic Interactions
Our characterization of the synthetic growth defect in gga1gga2vps27 and gga1gga2vps28 strains supports the hypothesis that Gga proteins are involved in delivery of select cargo proteins to the endosomes. In vps27 and vps28 strains, vacuolar function is maintained due to mixing of vacuolar components into the enlarged PVC, or class E compartment, allowing cell growth (Piper et al., 1995). We show that the enlarged PVC is still able to form in the gga1gga2vps27 strain, even at the restrictive temperature, indicating that not all traffic to the PVC is blocked. If some essential proteins cannot reach the PVC due to loss of Gga function then vacuolar function would be further reduced. When cells are stressed by high temperature, this reduced function may be exacerbated, leading to growth inhibition. Because both Vps27p and Gga proteins contain a VHS domain, we tested whether the synthetic interaction is related to VHS function or common to all class E VPS genes. The ts phenotype of gga1gga2vps28 strains supports the latter.
In our genetic analyses, we also tested for synthetic interactions with VPS1 and with the medium subunits of adaptor complexes AP-1 and AP-3. Others have recently reported genetic interactions between GGAs and the large subunits of AP-1 (Costaguta et al., 2001; Hirst et al., 2001). We show that deletion of the medium subunit of AP-1 does not exacerbate the gga1gga2 phenotype, except for very minor ts growth inhibition. Our result is likely due to incomplete loss of AP-1 function when the medium subunit is deleted, because Apm2p can function in AP-1 complexes (Yeung et al., 1999). We also show that VPS1 and GGAs do not display genetic interactions. It is possible that Vps1p is involved with Ggas in vesicle formation, but deletion of GGAs does not accentuate the more severe vps1 phenotype. Because a number of lipid modifying enzymes act upstream or downstream of ARF1, it will be interesting to test for genetic interactions between GGAs and these same genes.
Together, our data suggest a model in which yeast Gga proteins assemble a protein complex at the TGN that is required for proper sorting and transport of proteins to endosomes. The overall function of GGA proteins is thus conserved between yeast and humans. However, the mechanics of the interactions seem to be very different. In mammalian cells, interaction between activated ARF and GGA proteins recruits GGAs to the Golgi membrane, where interactions with cargo and clathrin can occur. In yeast, it seems that interactions between Gga proteins and cargo and/or other Golgi components drive the recruitment of Ggas to the Golgi membrane, where Arf interaction can occur. We speculate that a conformational change in Gga proteins upon Arf activation then drives the formation of a functional complex. The mutant Gga proteins with reduced Arf-binding affinity still function in yeast, because Arf and Ggas are juxtaposed at the Golgi membrane by other interactions, thus stabilizing the Arf–Gga interaction.
ACKNOWLEDGMENTS
We thank Rob Piper, Steve Nothwehr, Scott Emr, Richard Kahn, Tom Stevens, Peter Novick, Elizabeth Jones, and Mark Rose for providing plasmids, strains, and antibodies. We especially thank Rob Piper for helpful discussions and for performing the Vps10p degradation experiment with our gga1gga2vps1 strain. Thanks also to Kathy Wilson and Pat Scott for helpful comments and critical reading of the manuscript. This work was supported in part by grants to A.L.B. from Minnesota Medical Foundation and American Heart Association.
Abbreviations used:
- ARF
ADP-ribosylation factor
- Gal4AD
Gal4 activation domain
- Gal4BD
Gal4 binding domain
- GAT
GGA and Tom1
- GFP
green fluorescent protein
- Golgi-localized
GGA, Golgi-localized, γ-ear homology domain, ARF-binding protein
- GST
glutathione S-transferase
- M6PR
mannose 6-phosphate receptor
- PVC
prevacuolar compartment
- RFP
red fluorescent protein
- TGN
trans-Golgi network
- VHS
domain present in Vps27p, HRS, and STAM proteins
- VPS
vacuolar protein sorting
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0078. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0078.
REFERENCES
- Black MW, Pelham HR. A selective transport route from Golgi to late endosomes that requires the yeast GGA proteins. J Cell Biol. 2000;151:587–600. doi: 10.1083/jcb.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL. GGA proteins: new players in the sorting game. J Cell Sci. 2001;114:3413–3418. doi: 10.1242/jcs.114.19.3413. [DOI] [PubMed] [Google Scholar]
- Boman AL, Kahn RA. Arf proteins: the membrane traffic police? Trends Biochem Sci. 1995;20:147–150. doi: 10.1016/s0968-0004(00)88991-4. [DOI] [PubMed] [Google Scholar]
- Boman AL, Kuai J, Zhu X, Chen J, Kuriyama R, Kahn RA. Arf proteins bind to mitotic kinesin-like protein 1 (MKLP1) in a GTP-dependent fashion. Cell Motil Cytoskeleton. 1999;44:119–132. doi: 10.1002/(SICI)1097-0169(199910)44:2<119::AID-CM4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Boman AL, Zhang C, Zhu X, Kahn RA. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol Biol Cell. 2000;11:1241–1255. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenagh MM, Whitney JA, Carroll K, Zhang C, Boman AL, Rosenwald AG, Mellman I, Kahn RA. Intracellular distribution of Arf proteins in mammalian cells. Arf6 is uniquely localized to the plasma membrane. J Biol Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- Costaguta G, Stefan CJ, Bensen ES, Emr SD, Payne GS. Yeast GGA coat proteins function with clathrin in Golgi to endosome transport. Mol Biol Cell. 2001;12:1885–1896. doi: 10.1091/mbc.12.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, Bonifacino JS. GGAs. A family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du LL, Novick P. Yeast Rab GTPase-activating protein gyp1p localizes to the Golgi apparatus and is a negative regulator of ypt1p. Mol Biol Cell. 2001;12:1215–1226. doi: 10.1091/mbc.12.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, Chen CY, Emr SD, Graham TR. ARF is required for maintenance of yeast Golgi and endosome structure and function. Mol Biol Cell. 1998;9:653–670. doi: 10.1091/mbc.9.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Harris SL, Waters MG. Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. J Cell Biol. 1996;132:985–998. doi: 10.1083/jcb.132.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Lindsay MR, Robinson MS. GGAs: roles of the different domains and comparison with AP-1 and clathrin. Mol Biol Cell. 2001;12:3573–3588. doi: 10.1091/mbc.12.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Lui WW, Bright NA, Totty N, Seaman MN, Robinson MS. A family of proteins with γ-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J Cell Biol. 2000;149:67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, Kern FG, Clark J, Gelmann EP, Rulka C. Human ADP-ribosylation factors. A functionally conserved family of GTP-binding proteins. J Biol Chem. 1991;266:2606–2614. [PubMed] [Google Scholar]
- Lohi O, Lehto VP. VHS domain marks a group of proteins involved in endocytosis and vesicular trafficking. FEBS Lett. 1998;440:255–257. doi: 10.1016/s0014-5793(98)01401-x. [DOI] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled-coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Mullins C, Bonifacino JS. Structural requirements for function of yeast GGAs in vacuolar protein sorting, α-factor maturation, and interactions with clathrin. Mol Cell Biol. 2001;21:7981–7994. doi: 10.1128/MCB.21.23.7981-7994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001;20:2180–2190. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Conibear E, Stevens TH. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J Cell Biol. 1995;129:35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page LJ, Sowerby PJ, Lui WW, Robinson MS. γ-Synergin: an EH domain-containing protein that interacts with γ-adaptin. J Cell Biol. 1999;146:993–1004. doi: 10.1083/jcb.146.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussu A, Lohi O, Lehto VP. Vear, a novel Golgi-associated protein with VHS and γ-adaptin “ear” domains [In Process Citation] J Biol Chem. 2000;275:7176–7183. doi: 10.1074/jbc.275.10.7176. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001a;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001b;105:93–102. doi: 10.1016/s0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Weiss O, Kahn RA. Preparation of recombinant ADP-ribosylation factor. Methods Enzymol. 1995;257:128–135. doi: 10.1016/s0076-6879(95)57018-7. [DOI] [PubMed] [Google Scholar]
- Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Lawrence CW. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1974. [Google Scholar]
- Stearns T, Kahn RA, Botstein D, Hoyt MA. ADP ribosylation factor is an essential protein in Saccharomyces cerevisiae and is encoded by two genes. Mol Cell Biol. 1990a;10:6690–6699. doi: 10.1128/mcb.10.12.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Willingham MC, Botstein D, Kahn RA. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci USA. 1990b;87:1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu H, Katoh Y, Shiba Y, Nakayama K. GGA proteins interact with acidic di-leucine sequences within the cytoplasmic domains of sorting receptors through their VHS domains. J Biol Chem. 2001;276:28542–28545. doi: 10.1074/jbc.C100218200. [DOI] [PubMed] [Google Scholar]
- Takatsu H, Yoshino K, Nakayama K. Adaptor gamma ear homology domain conserved in γ-adaptin and GGA proteins that interact with γ-synergin [In Process Citation] Biochem Biophys Res Commun. 2000;271:719–725. doi: 10.1006/bbrc.2000.2700. [DOI] [PubMed] [Google Scholar]
- Urbanowski JL, Piper RC. The iron transporter Fth1p forms a complex with the Fet5 iron oxidase and resides on the vacuolar membrane. J Biol Chem. 1999;274:38061–38070. doi: 10.1074/jbc.274.53.38061. [DOI] [PubMed] [Google Scholar]
- Urbanowski JL, Piper RC. Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole. Traffic. 2001;2:622–630. doi: 10.1034/j.1600-0854.2001.20905.x. [DOI] [PubMed] [Google Scholar]
- Vater CA, Raymond CK, Ekena K, Howald-Stevenson I, Stevens TH. The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol. 1992;119:773–786. doi: 10.1083/jcb.119.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara N, Ueda T, Sato K, Nakano A. Multiple roles of Arf1 GTPase in the yeast exocytic and endocytic pathways. Mol Biol Cell. 2001;12:221–238. doi: 10.1091/mbc.12.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung BG, Phan HL, Payne GS. Adaptor complex-independent clathrin function in yeast. Mol Biol Cell. 1999;10:3643–3659. doi: 10.1091/mbc.10.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CJ, Rosenwald AG, Willingham MC, Skuntz S, Clark J, Kahn RA. Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J Cell Biol. 1994;124:289–300. doi: 10.1083/jcb.124.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdankina O, Strand NL, Redmond JM, Boman AL. Yeast GGA proteins interact with GTP-bound Arf and facilitate transport through the Golgi. Yeast. 2001;18:1–18. doi: 10.1002/1097-0061(200101)18:1<1::AID-YEA644>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zhu X, Boman AL, Kuai J, Cieplak W, Kahn RA. Effectors increase the affinity of GTP to Arf and lead to increased binding. J Biol Chem. 2000;275:13465–13475. doi: 10.1074/jbc.275.18.13465. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Doray B, Poussu A, Lehto VP, Kornfeld S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 2001;292:1716–1718. doi: 10.1126/science.1060896. [DOI] [PubMed] [Google Scholar]