Abstract
Membrane targeting of G-protein αβγ heterotrimers was investigated in live cells by use of Gα and Gγ subunits tagged with spectral mutants of green fluorescent protein. Unlike Ras proteins, Gβγ contains a single targeting signal, the CAAX motif, which directed the dimer to the endoplasmic reticulum. Endomembrane localization of farnesylated Gγ1, but not geranylgeranylated Gγ2, required carboxyl methylation. Targeting of the heterotrimer to the plasma membrane (PM) required coexpression of all three subunits, combining the CAAX motif of Gγ with the fatty acyl modifications of Gα. Gα associated with Gβγ on the Golgi and palmitoylation of Gα was required for translocation of the heterotrimer to the PM. Thus, two separate signals, analogous to the dual-signal targeting mechanism of Ras proteins, cooperate to target heterotrimeric G proteins to the PM via the endomembrane.
INTRODUCTION
Heterotrimeric G proteins transduce signals from cell-surface receptors to intracellular effectors. To do this, G proteins must associate with the cytoplasmic face of the plasma membrane (PM). The Gα, Gβ, and Gγ subunits of G proteins are synthesized in the cytosol on free polysomes and must be posttranslationally modified to traffic to the PM. Three types of posttranslational modifications are known to occur on subunits of G proteins (for review, see Wedegaertner et al., 1995). α-Subunits can be myristoylated and/or palmitoylated, whereas the Gγ subunits contain a CAAX motif similar to those of the Ras and Rho families of monomeric GTPases. The CAAX motif is modified by a well-characterized, three-step process yielding a prenylated and carboxyl methylated C-terminus (Clarke, 1992). The Gβ subunit is unmodified but remains tightly associated with a Gγ subunit. The various contributions of myristoylation, palmitoylation, and CAAX-box processing of individual G-protein subunits to PM association of the trimer have not been thoroughly investigated.
For Ras and Rho family small GTPases, two signals cooperate to target the monomeric GTPase to the PM (Hancock et al., 1990, 1991; Choy et al., 1999; Michaelson et al., 2001). Processing of the CAAX box is necessary and sufficient to target newly synthesized small GTPases to the cytoplasmic leaflet of endoplasmic reticulum (ER), where AAX proteolysis and carboxyl methylation take place. Final PM targeting, however, requires a second signal within the same polypeptide. This second signal consists of either a cluster of basic residues (polybasic region) or one or more palmitoylated cysteine residues immediately upstream of the CAAX box. Mutation of the second signal results in retention of the GTPase on endomembrane.
The targeting of mammalian G-protein α subunits has been extensively studied. Binding of the Gβγ dimer promotes stable membrane association of Gαs and Gαq subunits (Evanko et al., 2000). Mutations that disrupt the binding of Gα to Gβγ also disrupt membrane association of these Gα subunits, suggesting that the palmitoylation of Gα alone is insufficient for stable membrane association. Palmitoylation of Gαs and Gαz and their association with Gβγ act cooperatively (Iiri et al., 1996; Morales et al., 1998; Fishburn et al., 1999, 2000; Evanko et al., 2000). This suggests a model for Gα localization that involves a dual signal, analogous to that defined for Ras.
Previous analyses of Gα targeting leave unanswered the question of the targeting of Gβγ to the PM. The observation that heterotrimeric G proteins can be mislocalized by ectopic targeting of Gβγ (Fishburn et al., 2000) suggests that final localization is dictated by Gβγ. If Gα follows Gβγ, then understanding the intrinsic targeting of the latter is critical. The CAAX motifs of Gγ can be either farnesylated (Gγ1) or geranylgeranylated (most other Gγ subunits) (Wedegaertner et al., 1995). Analysis of the sequences of the mammalian γ subunits reveals no obvious PM-targeting second signal similar to those found in Ras proteins. If the model established for Ras and Rho proteins also applies to the Gγ subunits, then it would be expected that the Gγ subunit would localize on endomembrane. Among the possible explanations for PM localization of Gβγ are the existence of a previously uncharacterized second signal in the Gγ polypeptide and the contribution of such a signal by Gα subunits after assembly of the trimeric complex on endomembrane.
To distinguish between these possibilities, we expressed Gγ and/or Gα subunits tagged with green fluorescent protein (GFP) or spectral mutants of GFP with and without coexpression of Gβ and analyzed the localization of the fusion proteins in living cells. Our results demonstrate that the CAAX processing of Gγ targets this subunit, in complex with Gβ, to the ER and that translocation from endomembrane to the PM requires both expression and acylation of Gα. Association of Gα with Gβγ does not serve merely to stabilize PM association but rather occurs initially on Golgi and is critical for translocation to PM. Thus, PM targeting of G proteins, like Ras proteins, requires two signals, but whereas the Ras signals are on the same polypeptide, they are on different subunits for heterotrimeric G proteins. In addition, we found that carboxyl methylation was necessary for stable membrane association of farnesylated Gγ1 but not geranylgeranylated Gγ2.
MATERIALS AND METHODS
Cell Culture and Transfection
COS-1 and MDCK cells were obtained from American Type Tissue Collection, Manassas, VA. These cells were grown in DMEM containing 10% fetal bovine serum (Cellgro, Herndon, VA) at 5% CO2 and 37°C. Spontaneously immortalized murine embryonic fibroblasts (MEFs) from both prenylcysteine carboxyl methyltransferase (pcCMT)–null (CMT−/−) mice and CMT+/+ littermates were generated as we have described (Bergo et al., 2001) and cultured in DMEM with 15% fetal bovine serum (Colorado Serum Company, Denver, CO), nonessential amino acids, β-mercaptoethanol, and l-glutamine at 5% CO2 and 37°C. For all microscopy, cells were plated, transfected, and imaged in the same 35-mm culture dish that incorporated a No. 1.5 glass coverslip–sealed 15-mm cutout on the bottom (MatTek, Ashland, MA). Transfections of COS-1 and MDCK cells were performed 1 day after plating at 50% confluence using SuperFect according to the manufacturer's instructions (Qiagen, Hilden, Germany). MEFs were transfected using Lipofectamine Plus according to the manufacturer's instructions (Invitrogen, San Diego, CA). In some experiments, 50 μM 2-bromopalmitate (2-BP) (Sigma-Aldrich, St. Louis, MO) was added at the time of transfection. Unless otherwise noted, for coexpression, a 1:2:2-μg plasmid DNA ratio of γ:β:α was used. Control transfections omitting β and γ contained an equivalent amount of vector DNA. Transiently transfected cells were analyzed 1 day after transfection.
Plasmids
The plasmids pCMV-Gαi, pCMV-Gαi1Q204L, pCMV-Gαi2, pCMV-Gαi2Q205L, pCMV-Gαq, pCMV-Gαsshort (pCMV-Gαss), pCMV-GαssQ213L, and pCMV-Gαslong (pCMV-Gαsl) were generous gifts of Dr. Susanne Mumby, University of Texas (Dallas, TX). Plasmid pcDNA-Gαi2 was obtained from the Guthrie Institute (Sayre, PA). Gαi2 was subcloned into pCFP-N1 (Clonetech, Cambridge, UK) for production of Gαi2-cyan fluorescent protein (CFP). The plasmids pEV-Gγ1, pEV-Gγ2, and pEV-Gβ1 were gifts of Dr. N. Gautam, Washington University, St. Louis, MO. Gγ subunits were subcloned into pEGFP-C3 (Clonetech) for production of GFP-Gγ fusion proteins and into pYFP-C1 (Clonetech) for production of yellow fluorescent protein (YFP)-Gγ. The β-subunit was subcloned into pcDNA3.1+. The 11-amino-acid tails of the Gγ subunits were produced by PCR amplification using primers bracketing the C-terminal 11 amino acids of each subunit and were then cloned into pEGFP-C3. The C3S mutation of pcDNA-Gαi2 was produced by PCR amplification using primers that included the appropriate Cys-to-Ser mutation at position 3 (counting from the initial methionine that is cleaved off in myristoylated proteins), followed by cloning into pcDNA3.1+. This mutant was also subcloned into pCFP-N1 (Clonetech) for production of Gαi2C3S-CFP. Gα expression levels were similar for both pCMV and pcDNA vectors, as was the effect on GFP-Gγ localization.
Fluorescence Microscopy
Live cells were examined 24 h after transfection with a Zeiss Axioscope epifluorescence microscope (63× PlanApo 1.4 NA objective) (Zeiss, Oberkochen, Germany) equipped with a Princeton Instruments cooled CCD camera and MetaMorph digital imaging software (Universal Imaging, West Chester, PA) or a Zeiss 510 laser scanning confocal microscope (100× PlanApo 1.4 NA objective). Digital images were processed with Adobe Photoshop 6.0 (Adobe, San Jose, CA).
RESULTS
Gγ Subunits Are Targeted to the ER
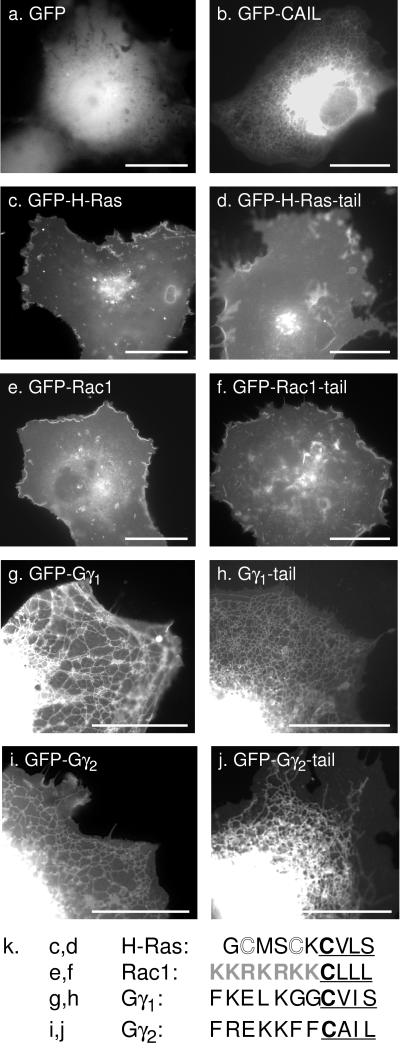
GFP is a hydrophilic protein that localizes homogeneously throughout the cytosol and nucleoplasm (Figure 1a). Addition of a four-amino-acid CAAX motif, such as the CAIL sequence of Gγ2, to the C terminus of GFP (GFP-CAAX) changed its localization dramatically to an endomembrane pattern that included ER, nuclear envelope, and Golgi but excluded PM (Figure 1b). Thus, as we have previously demonstrated, the CAAX motif alone is an efficient endomembrane targeting signal (Choy et al., 1999). For the Ras and Rho families of GTPases, sequences within the C-terminal 10–20 amino acids (the hypervariable region) are sufficient to give GFP a pattern of membrane expression identical to that of the full-length GFP-tagged protein (Choy et al., 1999; Michaelson et al., 2001). GFP-tagged H-Ras (GFP-H-Ras) and GFP extended with the last 10 amino acids of H-Ras (GFP-H-Ras tail) both localize to PM and Golgi (Figure 1, c and d). Similarly, GFP extended with the last 11 amino acids of Rac1 (GFP-Rac tail) gave a pattern of membrane localization indistinguishable from that of GFP-tagged full-length Rac1 (Figure 1, e and f). Thus, for Ras and Rho family proteins, the final 10–20 amino acids contain all the necessary information for membrane localization.
Figure 1.
Gγ subunits are targeted by their CAAX sequence to endomembrane but lack intrinsic secondary PM targeting signals. COS-1 cells were transfected with plasmids directing expression of GFP alone (a), GFP fused to the Gγ2 CAAX sequence, CAIL (b), GFP-H-Ras (c), GFP-H-Ras tail (d), GFP-Rac1 (e), GFP-Rac1 tail (f), GFP-Gγ1 (g), GFP-Gγ1 tail (h), GFP-Gγ2 (i), or GFP-Gγ2 tail (j) and imaged 24 h later alive by digital epifluorescence microscopy using a high-resolution cooled CCD camera. Bars, 10 μm. Amino acid sequence comparison of the C-terminal hypervariable regions of H-Ras, Rac1, Gγ1, and Gγ2 (k). The CAAX motif is underlined, the palmitoylation sites of H-Ras are shown in outline font, and the polybasic region of Rac1 is shaded.
In contrast, GFP fused to full-length Gγ1 or to the C-terminal 11 amino acids of Gγ1 (GFP-Gγ1, GFP-Gγ1-tail) and expressed in COS-1 cells (Figure 1, g and h) or MDCK cells (not shown) localized to the ER and Golgi in a pattern identical to that observed for GFP-CAAX (Figure 1a). Similar results were obtained (Figure 1, i and j) using GFP fused to full-length Gγ2 or to the C-terminal 11 amino acids of Gγ2 (GFP-Gγ2, GFP-Gγ2-tail). Thus, unlike the Ras and Rho family proteins, Gγ polypeptides lack a second signal for PM targeting. Analysis of the amino acid sequences of the C-termini of these molecules (Figure 1k) revealed that whereas H-Ras has sites for palmitoylation near the CAAX motif and Rac1 has a polybasic region adjacent to the CAAX motif, neither Gγ1 nor Gγ2 has analogous sequences. As with the Ras and Rho family proteins (Choy et al., 1999; Michaelson et al., 2001), neither farnesylation alone (Gγ1) nor geranylgeranylation alone (Gγ2) is sufficient to target GFP to the PM. We conclude that the intrinsic membrane targeting of G-protein γ subunits is for ER and Golgi and that an extrinsic factor(s) must therefore be required for translocation of these proteins from endomembrane to PM.
Coexpression of Gβ and Gα Targets GFP-Gγ to the PM
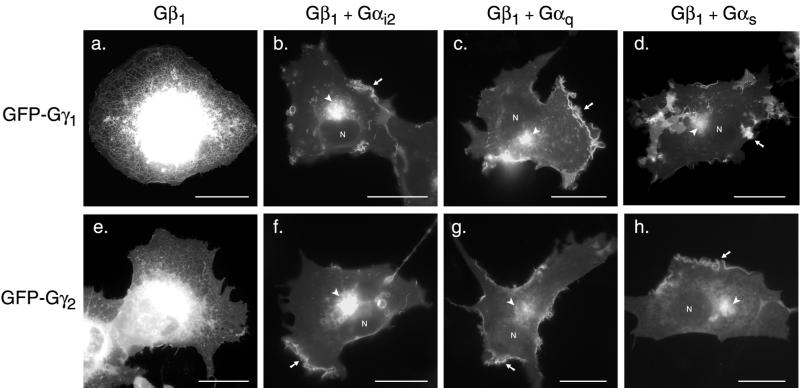
We next tested the effect of coexpression of Gβ and Gα subunits on GFP-Gγ localization. GFP-Gγ1 was coexpressed in COS-1 cells with either Gβ1 alone or with Gβ1 and a variety of Gα subunits (Figure 2). Gβ subunits form tight complexes with Gγ subunits. GFP-Gγ1 co-overexpressed with Gβ1 (Figure 2a) showed the same ER pattern as seen with GFP-Gγ1 expressed alone (Figure 1). Thus, Gβ subunits do not alter the intrinsic targeting of Gγ1 to the endomembrane. In contrast, when GFP-Gγ1 was co-overexpressed with Gβ1 and with the Gα i2 (Figure 2b), Gαq (Figure 2c), or Gαs (Figure 2d), a predominantly PM and Golgi pattern was observed that was identical to that observed for GFP-H-Ras at steady state (Figure 1c). The same results were obtained with MDCK cells (not shown). As with farnesylated GFP-Gγ1, geranylgeranylated GFP-Gγ2 coexpressed with Gβ1 alone (Figure 2e) showed the same ER/Golgi pattern as seen with the Gγ2 subunit expressed alone (Figure 1). Coexpression of Gβ and each Gα subunit with GFP-Gγ2 (Figure 2, f–h) resulted in PM and Golgi localization. Thus, neither the targeting of GFP-Gγ alone to the ER nor the heterodimer to the Golgi and PM was affected by the length of the polyisoprene lipid that modified Gγ. Coexpression of GFP-Gγ2 with Gβ1 and with constitutively active mutants of Gα, which are unable to bind to Gβγ, did not promote PM localization of GFP-Gγ (not shown). We conclude that heterotrimer formation is required for PM targeting and that sequences within the Gα subunit act in trans with the Gγ CAAX motif to deliver the trimer as a complex to the PM. Moreover, the appearance at steady state of GFP-Gγ on the Golgi as well as the PM, a pattern identical to that of GFP-H-Ras that transits the Golgi en route to the PM (Choy et al., 1999; Apolloni et al., 2000), suggests that heterotrimer formation occurs on the Golgi. These results are in agreement with a previous study (Evanko et al., 2001) that showed that PM localization of Gγ3 was facilitated by interaction with the Gα and Gβ subunits. However, our results suggest that the role of heterotrimer formation is not simply to add affinity for the PM but rather to permit protein trafficking from endomembrane to PM.
Figure 2.
Gα subunits provide a PM-targeting second signal for Gβγ. COS-1 cells were transfected with GFP-Gγ1 (a–d) or GFP-Gγ2 (e–h) and cotransfected with Gβ1 alone (a, e), Gβ1 and Gαi2 (b, f), Gβ1 and Gαq (c, g), or Gβ1 and Gαs (d, h) and imaged as in Figure 1. Arrows indicate PM, arrowheads indicate Golgi, and the positions of nuclei are marked (N). a and e, The nuclear envelope and Golgi are purposely overexposed to reveal the peripheral reticulum of the ER. Bars, 10 μm.
Palmitoylation of Gα Is Necessary for PM Localization of the Trimer
The PM and Golgi localization of GFP-Gγ coexpressed with Gβ1 and Gα (Figure 2) is very similar to the localization pattern seen with GFP-H-Ras (Figure 1c). H-Ras is palmitoylated on Golgi membranes (Apolloni et al., 2000), and palmitoylation is required for trafficking of H-Ras from the endomembrane to the PM, as demonstrated by inhibition of palmitoylation with 2-BP (Webb et al., 2000; Michaelson et al., 2001) or expression of GFP-H-RasC181,184S, which lacks palmitoylation sites (Choy et al., 1999) (Figure 3, a–c). All three of the Gα subunits tested, Gαs, Gαq, and Gαi2, are palmitoylated: Gαs is singly palmitoylated, Gαq is doubly palmitoylated, and Gαi2 is myristoylated and palmitoylated (Wedegaertner et al., 1995). To test whether the palmitate modification of the Gα subunit functions like that of H-Ras in providing the second signal required for PM targeting, we tested the ability of unpalmitoylated Gα subunits to promote PM trafficking of Gβγ. GFP-Gγ2 was coexpressed with Gβ1 and Gαi2 in the presence or absence of 2-BP. Whereas coexpression of GFP-Gγ2 with Gβ1 and Gαi2 resulted in PM localization (Figure 3d), GFP-Gγ2 remained endomembrane-associated in the presence of 2-BP (Figure 3e). Similar results were obtained using Gαs and Gαq in the presence and absence of 2-BP (not shown). To distinguish an effect on Gα binding of Gβγ from an effect on heterotrimer trafficking, we determined whether unpalmitolyated Gα could bind Gβγ. A palmitoylation-deficient mutant of the Gαi1 subunit has previously been shown to interact normally with Gβγ subunits (Degtyarev et al., 1994). We confirmed that palmitoylation-deficient Gαi2C3S can interact with Gβγ by demonstrating that this Gα, when coexpressed with GFP-Gγ2 and Gβ1, was efficiently ADP-ribosylated by pertussis toxin (not shown), a modification that requires heterotrimer formation. Coexpression of GFP-Gγ2 and Gβ1 with a palmitoylation-deficient Gαi2C3S resulted in retention of GFP-Gγ2 on the endomembrane (Figure 3f). Similar results were obtained with GFP-Gγ1 (Figure 3, g–i). Thus, palmitoylation of the Gα subunit functions like acylation of H-Ras to provide a second signal for engagement of a transport pathway from the endomembrane to the PM. Interestingly, whereas the dual signals of CAAX processing and acylation occur in cis on H-Ras, they are in trans on heterotrimeric G proteins. More important, these data show that interaction between Gα and Gβγ does not stabilize independent binding to PM of palmitoylated Gα and prenylated Gβγ, as previously thought (Evanko et al., 2001), but rather that association occurs even in the absence of palmitoylation and that PM targeting occurs after trimer formation on endomembrane.
Figure 3.
Gα palmitoylation acts as a second signal for PM targeting of GFP-Gγ. COS-1 cells were transfected with GFP-H-Ras (a, b), GFP-H-Ras with mutated palmitoylation sites (c), GFP-Gγ2 (d–f), or GFP-Gγ1 (g–i). The Gβ1 subunit was coexpressed with GFP-Gγ2 or GFP-Gγ1 as indicated (d–i) along with either Gαi2 (d, e, g, h) or palmitoylation-deficient Gαi2C3S (f, i). An inhibitor of palmitoylation, 2-BP, was added in b, e, and h. Bars, 10 μm.
Gα Interacts with Gβγ on Golgi
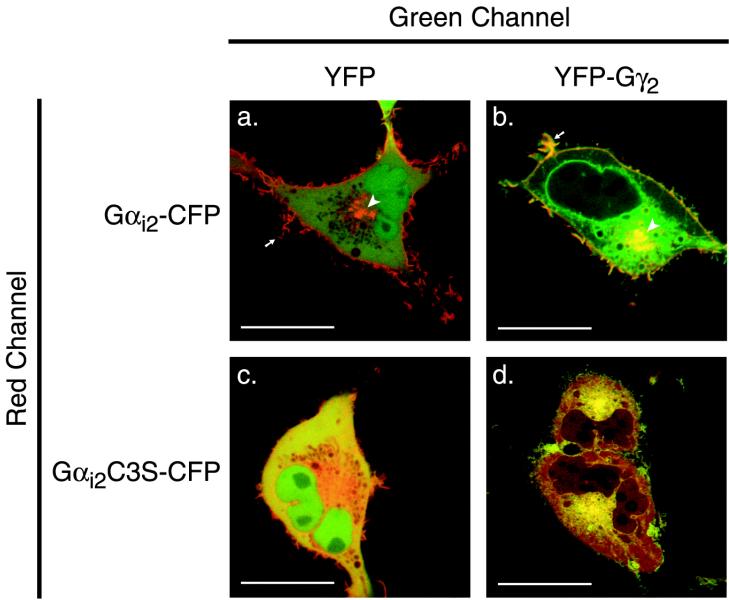
To confirm directly that Gα interacts with Gβγ on endomembrane, we tagged with CFP the C-termini of Gαi2 and Gαi2C3S and coexpressed these fusion proteins with or without Gβ1 and Gγ2 tagged at the N-terminus with YFP. Gαi2-CFP expressed with Gβ1 and YFP localized on both the PM and Golgi (Figure 4a). The PM localization is most likely a consequence of association with endogenous Gγ. When Gαi2-CFP was coexpressed with Gβ1 and YFP-Gγ2, the two tagged subunits colocalized on PM and Golgi, but only YFP-Gγ2 was observed on ER (Figure 4b). This observation suggests that whereas CAAX-processed Gβγ traffics from cytosol to ER and then onto Golgi and PM, association with Gα takes place on the Golgi, a compartment on which palmitoyltransferase activity resides (Apolloni et al., 2000). Gαi2C3S-CFP expressed with Gβ1 and YFP was largely cytosolic, although some of the fusion protein was enriched in the paranuclear region around the Golgi area (Figure 4c). When Gαi2C3S-CFP was coexpressed with Gβ1 and YFP-Gγ2, the palmitoylation-deficient Gα was recruited to the Golgi region in association with YFP-Gγ2, but neither of the fusion proteins was observed on the PM (Figure 4d). Thus, unpalmitoylated Gα associated with Gβγ on the Golgi, but in the absence of palmitoylation, neither subunit was translocated to the PM.
Figure 4.
Gα and Gβγ colocalize on Golgi. COS-1 cells were cotransfected with Gαi2-CFP (a and b) or Gαi2C3S-CFP (c and d) and either YFP plus Gβ1 (a and c) or YFP-Gγ2 plus Gβ1 (b and d). Dual color images of living cells were imaged 24 h after transfection with a Zeiss 510 LSM. The CFP channel is assigned red, the YFP channel is assigned green, and colocalization is indicated by yellow pseudocolor. Arrows indicate PM ruffles, and the arrowhead indicates Golgi. Bars, 10 μm.
Carboxyl Methylation Is Necessary for Endomembrane Targeting of Farnesylated but Not Geranylgeranylated Gγ Subunits
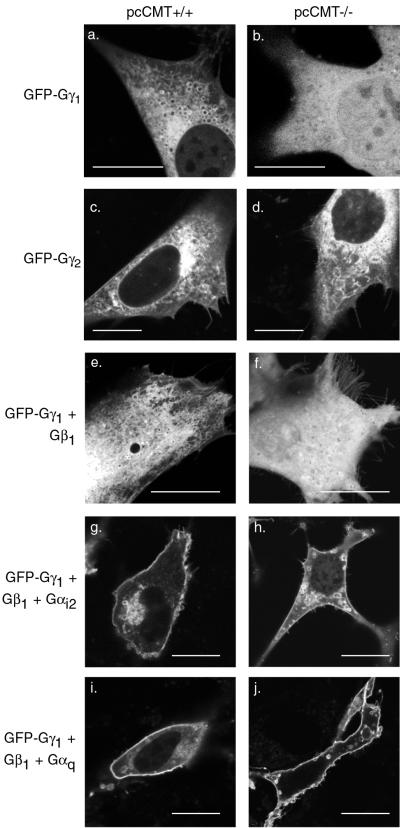
Membrane localization of farnesylated Ras proteins is dependent not only on prenylation but also on carboxyl methylation of the CAAX motif (Choy et al., 1999; Bergo et al., 2001). However, in vitro analysis of the association of prenylated peptides with liposomes suggested that the added hydrophobicity of the 20-carbon geranylgeranyl modification found on most Gγ subunits may be sufficient for membrane association in the absence of carboxyl methylation (Silvius and l'Heureux, 1994). To test the role of carboxyl methylation in the localization of farnesylated Gγ1 and geranylgeranylated Gγ2 on endomembrane, we expressed GFP-tagged Gγ1 and Gγ2 in spontaneously immortalized MEFs derived from mouse embryos null for pcCMT (CMT−/−) or their wild-type littermates (CMT+/+). Laser scanning confocal microscopy was used to analyze MEFs. The morphology of the endomembrane system of MEFs (revealed by observing live cells expressing GFP-CAAX; data not shown) differed from that observed in established cell lines such as COS-1 or MDCK in that, whereas the ER of COS-1 cells consisted of a well-defined nuclear envelope and peripheral reticulum, the endomembrane system of MEFs appeared polymorphic, with reticulum interspersed with numerous cytoplasmic vesicles. This pattern was also observed when GFP-tagged Gγ1 or Gγ2 was expressed in CMT+/+ cells (Figure 5, a and c). An identical pattern was observed when GFP-Gγ2 was expressed in CMT−/− cells (Figure 5d), indicating that geranylgeranylated Gγ2 did not require carboxyl methylation for stable association with the endomembrane. In contrast, GFP-Gγ1 was observed in the cytosol and nucleoplasm of CMT−/− cells (Figure 5b), indicating that carboxyl methylation was necessary for stable membrane association of this farnesylated molecule. Thus, although geranylgeranylated Gγ subunits are substrates for carboxyl methylation (Philips et al., 1995), this modification is not required for stable association with endomembrane.
Figure 5.
Endomembrane targeting of farnesylated GFP-Gγ1, but not geranylgeranylated GFP-Gγ2, requires pcCMT. GFP-Gγ1 (a, b, e–j) or GFP-Gγ2 (c and d) were transfected into CMT+/+ (a, c, e, g, and i) or CMT−/− (b, d, f, h, and j) MEFs alone (a–d) or with either Gβ1 only (e and f), or Gβ1 and Gαi2 (g and h), or Gβ1 and Gαq (i and j) and imaged alive 24 h later by LSM. GFP-Gγ1 remained in the cytosol and nucleoplasm in CMT−/− cells when expressed alone or coexpressed only with Gβ1 but was localized to PM when coexpressed with Gβ1 and either Gα subunit. Bars, 10 μm.
Having determined that carboxyl methylation is required for stable association of Gγ1 with endomembrane, we next tested whether carboxyl methylation of farnesylated Gγ1 is required for the Gα-mediated delivery of the trimer to the PM. GFP-Gγ1 was coexpressed with Gβ1 alone or with Gβ1 and Gαi2 in CMT+/+ and CMT−/− cells. GFP-Gγ1 coexpressed with Gβ1 alone was localized to internal membranes in CMT+/+ cells (Figure 5e) but was cytosolic in CMT−/− cells (Figure 5f), similar to results obtained with GFP-Gγ1 expressed alone (Figure 5, a and b). Nevertheless, GFP-Gγ1 coexpressed with Gβ1 and Gαi2 was localized to the PM in both CMT+/+ and CMT−/− cells (Figure 5, g and h). Similar results were obtained with Gαq (Figure 5, i and j). This indicates that the more stable endomembrane association of Gβγ1 dimers mediated by carboxyl methylation is not required for heterotrimer formation and trafficking to the PM. Thus, co-overexpression of Gα rescues the trafficking defect of farnesylated but unmethylated Gγ1. Whether unmethylated Gγ1 becomes associated with Gα subunits that have reached the PM by virtue of association with endogenous Gγ or whether unmethylated Gγ1, despite markedly diminished affinity for endomembrane, can associate with Gα on the Golgi before transport to the PM remains unresolved.
DISCUSSION
The CAAX motif, shared by Ras and Rho family proteins and the Gγ subunits of heterotrimeric G proteins, signals for prenylation that targets the protein to the ER, where it encounters the Rce1 protease (Schmidt et al., 1998) and pcCMT (Dai et al., 1998). Whereas N-Ras and H-Ras then traffic by vesicular transport to the PM via the Golgi, K-Ras4B takes an alternative, as yet uncharacterized path (Choy et al., 1999; Apolloni et al., 2000). The signal in Ras for engagement of each of these pathways is contained in the so-called “second signal” that lies adjacent to the CAAX motif and consists of either cysteines that are sites of palmitoylation (N-Ras and H-Ras) or a polybasic domain (K-Ras4B). The trafficking of Rho family GTPases is more complex, because several members of this family bind to RhoGDIα, a ubiquitously expressed chaperone that has the capacity to retain C-terminally processed Rho proteins in the cytosol. Thus, for the Rho proteins, a combination of CAAX motif processing, a second signal, and affinity for RhoGDIα determines their final membrane localization (Michaelson et al., 2001).
Heterotrimeric G proteins reside in the PM in an inactive, GDP-bound, trimeric form until association with an activated receptor triggers nucleotide exchange and dissociation of Gα from Gβγ. The mechanisms that target newly synthesized Gα subunits to the PM have been explored in some depth. A combination of palmitoylation and association with Gβγ is necessary for stable PM association of Gα (Morales et al., 1998; Fishburn et al., 1999, 2000; Evanko et al., 2000, 2001). However, if Gβγ is necessary for proper targeting of Gα to the PM, what targets Gβγ? Recent evidence suggests that the Gβγ3 dimer is found predominantly on internal membranes in the absence of Gα (Evanko et al., 2001). The conclusion that these authors drew from this observation was that Gβγ interaction with Gα serves to stabilize the otherwise transient PM association of Gα. Our study presents evidence that the role of Gβγ is not to stabilize independent PM association of Gα but rather to act cooperatively with Gα to target the entire trimer from the Golgi to PM.
We demonstrate that the intrinsic targeting of Gβγ is to the ER and Golgi, and only when complexed with Gα is there further trafficking to the PM. GFP-tagged Gγ expressed alone or coexpressed with Gβ appeared predominantly on the ER, whereas GFP-tagged Gγ coexpressed with Gβ and Gα appeared on the PM and Golgi. When palmitoylation of Gα is prevented, either by mutation of the palmitoylated cysteine residue to serine or by treatment with 2-BP, Gβγ accumulates on ER, and the heterotrimer remains on the Golgi. This result would not be expected if association of Gα and Gβγ occurred initially at the PM. However, it is the result that would be expected if G protein heterotrimers behave like H-Ras, which at steady state appears in the PM and Golgi but is retained in the ER if palmitoylation is blocked (Choy et al., 1999; Michaelson et al., 2001). We conclude that a combination of targeting elements within Gβγ (the CAAX motif) and Gα (palmitoylation and/or myristoylation) acts cooperatively in trans to target the entire trimer to the PM. Accordingly, nascent trimer formation must occur on endomembrane before translocation of the complex to the PM. Coexpression of Gα and Gβγ tagged with resolvable spectral mutants of GFP revealed colocalization on Golgi and PM but not ER, suggesting that heterotrimer formation occurs on Golgi.
This division of the two targeting signals into different subunits may have evolved to ensure that only a complete trimer (which represents an inactive signaling unit) can reach the PM. Because Gβγ signaling requires only release from Gα on receptor-mediated nucleotide exchange, it is imperative that nascent Gβγ is able to reach the PM only in association with GDP-bound Gα. If Gβγ alone, like Ras, could reach the PM in the absence of GDP-bound Gα, there would be nothing to stop the Gβγ subunits from prematurely engaging their downstream effectors even in the absence of receptor activation. Thus, it is possible that the two signals in the cis mechanism that targets monomeric GTPases to PM have been modified for the heterotrimeric G proteins into a two signal in trans mechanism to avoid premature Gβγ signaling.
Although no protein palmitoyltransferase has been characterized at the molecular level, an important conclusion that can be deduced from the data presented here is that at least one enzyme that palmitoylates Gα is localized in an endomembrane compartment, most likely Golgi. Although such a conclusion is contrary to the prevailing view that places Gα palmitoyltransferase in the PM (Dunphy et al., 1996; Evanko et al., 2001), Gα palmitoyltransferase activity has, in fact, been detected in Golgi fractions (Dunphy et al., 1996). Moreover, the Golgi has been implicated as the compartment in which the enzyme that palmitoylates H-Ras resides (Apolloni et al., 2000). Similarly, in vitro palmitoyltransferase activity for the neuronal plasticity protein GAP-43 was found in Golgi (McLaughlin and Denny, 1999).
Prenylcysteine carboxyl methylation is a modification of the CAAX motif that has been well conserved from yeast to humans, although its precise role in protein targeting and GTPase signaling remains uncertain. Whereas carboxyl methylation of yeast GTPases is not required for growth (Hrycyna et al., 1991), disruption of the CMT gene by homologous recombination (Bergo et al., 2001) has revealed that the gene is required for mouse development (embryonic lethal day 10.5). Ras proteins are mislocalized in CMT−/− cells (Bergo et al., 2000). It has been suggested that the elimination of the negative charge of the carboxy terminus of prenylated proteins accomplished by carboxyl methylation adds to the hydrophobicity of the C terminus and that the additional hydrophobicity is of much greater consequence to proteins modified by the 15-carbon farnesyl polyisoprene than to those modified by the 20-carbon geranylgeranyl lipid (Silvius and l'Heureux, 1994). Our study directly tests this hypothesis in live cells by examining the localization of GFP-Gγ1 and GFP-Gγ2 in CMT−/− MEFs (Bergo et al., 2001). In these cells, farnesylated GFP-Gγ1 was unable to associate stably with endomembranes, even though geranylgeranylated GFP-Gγ2 localized normally on ER. The conservation through evolution of two different CAAX prenyl transferases suggests distinct biological roles for the farnesyl and geranylgeranyl modifications. Our data suggest that whereas geranylgeranylation imparts a relatively high affinity for membranes independent of carboxyl methylation, farnesylation affords only a weak affinity that is then modulated by carboxyl methylation.
Because Gαi2 is myristoylated even in the absence of palmitoylation and palmitoylation-deficient Gαi2C3S failed to cooperate with processed Gγ to target heterotrimers to the PM, we conclude that myristoylation alone is not able to act as a second signal for PM targeting. This observation has implications for transducin, because Gαt is modified only with a myristoyl group. The combination of the myristoylated Gαt and the farnesylated Gγ1 would be predicted to yield a heterotrimer whose PM targeting may be inefficient and whose endomembrane association is dependent on carboxyl methylation. This is in agreement both with the relatively high amount of transducin found in the soluble fraction of retinal preparations and with the observation that unmethylated Gβγ1 associates with membranes only poorly (Fukada et al., 1994; Matsuda et al., 1994). This weak association of the transducin trimer and its subunits with cellular membranes is in sharp contrast to the relatively stable membrane interactions of most other fully processed heterotrimers studied. It is interesting to speculate that the relatively weak membrane targeting signals and unique requirement for carboxyl methylation, a modification that is reversible under physiological conditions, of transducin play a role in the biology of the visual signaling pathway.
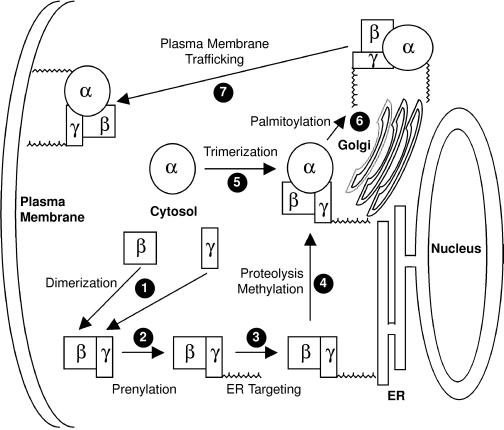
Together, our data support a model for G protein trafficking (Figure 6) analogous to that described for Ras (Choy et al., 1999). Prenylation of the CAAX motif of Gγ directs Gβγ to the ER, where the prenyl-CAAX sequence is cleaved and carboxyl methylated, the latter modification contributing significantly to the affinity of farnesylated Gγ1 for the endomembrane. Nascent Gα then associates with Gβγ on the Golgi, where it is palmitoylated, a modification that serves as a signal for further transport to the PM. This model is consistent with previous data on Gα membrane association but adds a trafficking dimension largely overlooked in previous studies of G proteins.
Figure 6.
Model of G-protein trafficking to the PM. All three G protein subunits are synthesized in the cytosol on free polysomes. Gβ and Gγ immediately dimerize on the basis of their high affinity for each other (1). Whether this occurs before or after Gγ prenylation is uncertain. Prenylation of Gγ (2) drives Gβγ to the cytosolic face of the ER (3), where it encounters the CAAX protease and carboxyl methyltransferase (4). Fully processed Gβγ is then delivered to the cytosolic face of the Golgi, where it recruits Gα (5), which is then acylated by a Golgi resident acyl transferase (6). Acylation then allows the G protein heterotrimer to be transported as a holoenzyme to the PM via a route (7) that may be the classic secretory pathway.
ACKNOWLEDGMENTS
We thank Susanne Mumby and Narasimhan Gautam for providing plasmids. This work was supported by grants from the National Institutes of Health (NIH) (AI-36224 and GM-55279 to M.R.P. and CA-09161 to D.M.), the Burroughs Wellcome Foundation (M.R.P), the University of California Tobacco-Related Disease Research Program (M.B and S.Y), and the Swedish Cancer Foundation (M.B) and a General Clinical Research Center grant from the NIH, National Center for Research Resources (M01RR-00096).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0095. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0095.
REFERENCES
- Apolloni A, Prior IA, Lindsay M, Parton RG, Hancock JF. H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol Cell Biol. 2000;20:2475–2487. doi: 10.1128/mcb.20.7.2475-2487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Gomes AQ, Seabra MC, Young SG. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J Biol Chem. 2001;276:5841–5845. doi: 10.1074/jbc.C000831200. [DOI] [PubMed] [Google Scholar]
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Young SG. Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J Biol Chem. 2000;275:17605–17610. doi: 10.1074/jbc.C000079200. [DOI] [PubMed] [Google Scholar]
- Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Dai Q, Choy E, Chiu V, Romano J, Slivka S, Steitz S, Michaelis S, Philips MR. Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J Biol Chem. 1998;273:15030–15034. doi: 10.1074/jbc.273.24.15030. [DOI] [PubMed] [Google Scholar]
- Degtyarev MY, Spiegel AM, Jones TL. Palmitoylation of a G protein alpha i subunit requires membrane localization not myristoylation. J Biol Chem. 1994;269:30898–30903. [PubMed] [Google Scholar]
- Dunphy JT, Greentree WK, Manahan CL, Linder ME. G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem. 1996;271:7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- Evanko DS, Thiyagarajan MM, Siderovski DP, Wedegaertner PB. Gbeta gamma isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Galphas and Galphaq. J Biol Chem. 2001;276:23945–23953. doi: 10.1074/jbc.M101154200. [DOI] [PubMed] [Google Scholar]
- Evanko DS, Thiyagarajan MM, Wedegaertner PB. Interaction with Gbetagamma is required for membrane targeting and palmitoylation of Galpha(s) and Galpha(q) J Biol Chem. 2000;275:1327–1336. doi: 10.1074/jbc.275.2.1327. [DOI] [PubMed] [Google Scholar]
- Fishburn CS, Herzmark P, Morales J, Bourne HR. Gbetagamma and palmitate target newly synthesized Galphaz to the plasma membrane. J Biol Chem. 1999;274:18793–18800. doi: 10.1074/jbc.274.26.18793. [DOI] [PubMed] [Google Scholar]
- Fishburn CS, Pollitt SK, Bourne HR. Localization of a peripheral membrane protein: Gbetagamma targets Galpha(Z) Proc Natl Acad Sci USA. 2000;97:1085–1090. doi: 10.1073/pnas.97.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y, Matsuda T, Kokame K, Takao T, Shimonishi Y, Akino T, Yoshizawa T. Effects of carboxyl methylation of photoreceptor G protein g-subunit in visual transduction. J Biol Chem. 1994;269:5163–5170. [PubMed] [Google Scholar]
- Hancock JF, Cadwallader K, Paterson H, Marshall CJ. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991;10:4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Hrycyna CA, Sapperstein SK, Clarke S, Michaelis S. The Saccharomyces cerevisiae STE14 gene encodes a methyl transferase that modulates C-terminal methylation of a factor and RAS proteins. EMBO J. 1991;10:1699–1709. doi: 10.1002/j.1460-2075.1991.tb07694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iiri T, Backlund PS, Jr, Jones TL, Wedegaertner PB, Bourne HR. Reciprocal regulation of Gs alpha by palmitate and the beta gamma subunit. Proc Natl Acad Sci USA. 1996;93:14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Takao T, Shimonishi Y, Murata M, Asano T, Yoshizawa T, Fukada Y. Characterization of interactions between transducin alpha/beta gamma-subunits and lipid membranes. J Biol Chem. 1994;269:30358–30363. [PubMed] [Google Scholar]
- McLaughlin RE, Denny JB. Palmitoylation of GAP-43 by the ER-Golgi intermediate compartment and Golgi apparatus. Biochim Biophys Acta. 1999;1451:82–92. doi: 10.1016/s0167-4889(99)00074-9. [DOI] [PubMed] [Google Scholar]
- Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and rhogdi binding. J Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Fishburn CS, Wilson PT, Bourne HR. Plasma membrane localization of G alpha z requires two signals. Mol Biol Cell. 1998;9:1–14. doi: 10.1091/mbc.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips MR, Staud R, Pillinger M, Feoktistov A, Volker C, Stock JB, Weissmann G. Activation-dependent carboxyl methylation of neutrophil G-protein g subunit. Proc Natl Acad Sci USA. 1995;92:2283–2287. doi: 10.1073/pnas.92.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WK, Tam A, Fujimura-Kamada K, Michaelis S. Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc Natl Acad Sci USA. 1998;95:11175–11180. doi: 10.1073/pnas.95.19.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius JR, l'Heureux F. Fluorimetric evaluation of the affinities of isoprenylated peptides for lipid bilayers. Biochemistry. 1994;33:3014–3022. doi: 10.1021/bi00176a034. [DOI] [PubMed] [Google Scholar]
- Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T-cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]