Abstract
Immunotherapies have thus far proved of limited efficacy against glioblastoma. Failures can be attributed to a host of immunosuppressive mechanisms that are either directly employed by the tumor or are instead a convenient feature of the intracranial environment. This review aims to categorize glioblastoma immune-evasive tendencies, provide an update on our understanding of etiologies, and describe newer approaches to improving therapeutic responses.
Keywords: glioblastoma, immune checkpoint blockade, immunosuppression, T cell exhaustion
Key Points:
Glioblastoma employs multiple methods of immune-evasion and immunosuppression.
Brain tumors proffer unique immunosuppressive mechanisms due to its central nervous system location.
Glioblastoma is the most aggressive and most common malignant primary brain tumor in adults, with an average survival of less than 21 months following diagnosis.1 The 1-year survival rate is just 41.4% and 5-year survival is a dismal 5.4%.2–4 More than 90% of glioblastomas recur following treatment,5 and median survival following recurrence is only 3-9 months.6
Glioblastoma accounts for 57% of all gliomas and 48% of all primary malignant central nervous system (CNS) tumors. Standard of care remains maximally safe resection along with radiotherapy plus concomitant/adjuvant temozolomide.7 This treatment paradigm has remained largely unchanged in the two decades since the publication of the Stupp protocol.7
While immunotherapies such as checkpoint blockade have become a mainstay of treatment for a range of solid tumors, successes have been limited in glioblastoma.8,9 Failures can be attributed in large part to the profound immune dysfunction elicited by these tumors, both at a local and systemic level.10,11
This review will systematically describe the various immunosuppressive measures employed by glioblastoma (Figure 1). Mechanisms will be attributed and described within the context of 4 domains: the tumor cell (tumor-intrinsic), tumor microenvironment (TME), tumor location within the CNS (CNS-imposed), or peripheral to the tumor/tumor extrinsic (systemic). Intrinsic to the tumor, active mechanisms for immune evasion are augmented by notable tumor heterogeneity, which can be further exacerbated by the selective pressures imposed by therapy.12–17 Locally, within the TME, glioblastomas foster evasion of T cell recognition, dysfunctional lymphocyte activity, and a disrupted cytokine milieu.18–23 Active mechanisms for immune evasion are aided by notable tumor heterogeneity, which can be further exacerbated by the selective pressures imposed by therapy.12–17 Glioblastoma’s intracranial location presents unique challenges to immune access and avails of unique interactions, such as those between glial cells and neurons. Systemic alterations evoked by glioblastoma can include lymphopenia, lymphoid organ atrophy, sequestration of T cells, systemic T cell dysfunction, and altered hematopoiesis.10,24,25 These systemic immune derangements are perhaps particularly surprising given that glioblastoma remains almost exclusively confined within the CNS. Ultimately, however, this combination of local and systemic immunosuppression promotes glioblastoma immune escape and severely limits the efficacy of immune-based treatment platforms.18,26,27
Figure 1.
Overview of immunosuppressive mechanisms. At the tumor-intrinsic level, glioblastomas are markedly heterogeneous at even the single cell level and exhibit changes to gene expression or metabolic profiles that permit them to evade or counter the immune response. Within the tumor microenvironment, myeloid populations alter the tenor of the immune response, contributing to T cell exhaustion and an immunosuppressive milieu. The central nervous system (CNS) itself also provides safe harbor to tumors, creating challenges for antigen presentation and immune entry and forcing immune interactions with CNS-specific cell populations (microglia, neurons) that can restrict immune responsivity. Systemically, glioblastoma, and other intracranial tumors elicit such changes as lymphopenia, lymphoid organ involution, altered hematopoiesis, and T cell sequestration, despite being confined within the brain. Created in https://BioRender.com.
Tumor Intrinsic Factors
Glioblastoma can pass through the “cancer immunoediting” cycle, where it undergoes elimination by immune cells, achieves equilibrium, and escapes an immune system attack via self-and immune-editing.18,28 Self-editing at the tumor cell level can constitute a mode of tumor-intrinsic immune evasion. Common historical examples in the case of glioblastoma can include the upregulation of programmed cell death ligand 1 (PD-L1),29–31 downregulation of major histocompatibility complex (MHC) molecules,32,33 and various metabolic and epigenetic alterations34–36 (Figure 2).
Figure 2.
Tumor-intrinsic mechanisms of glioblastoma immune-evasion and suppression. Tumor cell-intrinsic mechanisms include antigen modifications, MHC downregulation, and antigenic and transcriptional heterogeneity that allow tumor cells to evade the immune system. Checkpoint upregulation and DNA methylation add further layers of suppression. Therapeutic approaches aim to modify gene expression or metabolism, target immune checkpoints, or sidestep heterogeneity. Created in https://BioRender.com.
A well-recognized and tumor cell-intrinsic immunosuppressive strategy is the upregulation of PD-L1, which subsequently binds to the immune checkpoint PD-1 on T cells, limiting their function.37 Therapies targeting the PD-1/PD-L1 axis, that is immune checkpoint blockade, represent some of the most successful immunotherapeutic strategies in solid tumors to date.9 While anti-PD1 has failed to date in clinical trials in glioblastoma,38 some more recent studies suggest that applications in the neoadjuvant setting may still bear fruit.8 Cloughesy et al. for instance, observed improved immune parameters and a significant extension in overall survival (417 vs 228.6 days, HR 0.39, P =.04) when pembrolizumab was administered to patients with recurrent glioblastoma in the neoadjuvant, rather than adjuvant, setting.8
Interestingly, treatments incorporating anti-PD-1 have enjoyed tremendous success against brain metastatic melanoma,39 suggesting that the CNS location is not a hindrance per se: it remains unclear whether the antibodies require brain access or may simply act systemically on T cells. Failures against glioblastoma then appear to result from features unique to these tumors. These can include an especially immunosuppressive microenvironment, limited T cell infiltration, and overall T cell dysfunction (particularly severe exhaustion),40–45 all factors we will discuss in the following sections of the review. Additionally, T cells may develop adaptive resistance to checkpoint blockade therapy, upregulating alternative immune checkpoints, such as immunoglobulin mucin-3 (TIM-3).46 TIM-3 serves a similar function to PD-1 in restricting T cell activity and may even induce T cell death following binding of exposed phosphatidyl serine on the tumor cell surface. As a result, anti-TIM-3 has been found to augment PD-1 blockade therapy to increase survival in patients with solid tumors, combating this adaptive resistance mechanism.46 A clinical trial of anti-TIM-3 in combination with anti-PD-1 and stereotactic radiosurgery for recurrent glioblastoma is currently underway (NCT03961971).
MHC downregulation on tumor cells has historically been viewed as a cell-intrinsic mechanism of immune escape with varying relevance to glioma.32,33,47 The loss of MHC and accompanying antigen presentation theoretically hides tumor cells from T cells and permits their unchecked outgrowth. Mutations leading to low or absent expression of β2-microglobulin (β2m), a crucial component to the MHC structure, have in particular been identified as harbingers of tumor immune-evasion.32,33,48 Recent studies, in contrast, have also shown low β2m expression to actually be associated with favorable prognosis in gastric cancer and glioblastoma.49,50 While elimination of tumor cells by CD8 + T-cells is thought to be hindered by MHC downregulation, there remain alternative mechanisms by which the immune system can attack tumor cells that do not rely on T cells.51 For instance, the loss of MHC class I can activate NK-mediated innate immunity and promote tumor cell expression of natural killer groups 2 member D (NKG2D) ligands (NKG2DL), which are typically upregulated following DNA damage and cellular.52 The presence of such ligands can mark cells for destruction by NK cells in antigen-independent fashion.
NKG2DL may also seemingly mark tumors cells for destruction by CD8 + T cells in both MHC and antigen-independent fashion. In a true paradigm shift, it was recently revealed that MHC-I-negative glioma and melanoma cells remain susceptible to CD8 + T cell killing through the NKG2D/NKG2DL axis.53 Importantly, MHC-I-negative tumor cell killing by CD8 + T cells was antigen-agnostic, though dependent on prior antigen-specific T cell receptor activation by antigen presenting cells (APCs) or even local MHC-I-positive tumor cells. These findings challenge the notion that loss of tumor MHC-I is synonymous with immune evasion.
The NKG2D/NKG2DL axis retains relevance here for other reasons as well. Soluble NKG2DL (ie, MICA or MICB) may be released by tumor cells and prove to be immunosuppressive in this context, competing for NKG2D and limiting the detection of tumor cells by NKG2D + immune cells.54,55 Additionally, MICA and MICB may be transferred from the tumor cell surface to inhibit immune cell tumor-binding and activity.34–36 Thus, therapies aimed at binding or removing soluble NKG2DL (such as soluble NKG2D), or at bringing NKG2D + T cells into better contact with tumor, may improve antitumor T cell function and/or counter tumor-imposed immunosuppressive mechanisms.
Despite its relatively high cellular and antigenic heterogeneity, glioblastoma possesses a low tumor mutational burden of ~1.5 mutations/megabase1 with few coding mutations. As a result, there are relatively few neoantigens proffered for generating targeted adaptive immune responses. Likewise, those neoantigens present tend not to be homogenously expressed. Targeted therapies may successfully eliminate cells expressing the chosen target but be thwarted by outgrowth of antigen-negative variants. A classic example of this is found amidst therapies targeting the tumor-specific variant of the epidermal growth factor receptor (EGFRvIII) on glioblastoma. EGFRvIII is expressed on 30% of glioblastomas, and on 37%-86% of the cells when present.56 EGFRvIII-targeted therapies, such as EGFRvIII CAR-T cells, have exhibited limited efficacy, and even successful targeting of EGFRvIII + cells has seen antigen negative variants continue to grow.20,57–59
Further confounding therapy, treatments can also often increase intratumoral heterogeneity, as treatment-induced selective pressures can lead to hypermutation and the outgrowth of target-loss variants in the case of targeted therapies.15–17 This is seen following alkylating chemotherapy, such as with temozolomide, where treatment induces mutations and genomic changes leading to further chemo-resistance and immune evasion.16,17,60 Tumor cells may also self-edit in response to targeted therapies, downregulating the expression of immunogenic antigens and fostering subsequent immune escape.1
Altering the metabolome is another tumor cell-intrinsic means for escaping immune-based platforms. In addition to perhaps providing tumors cells themselves a survival advantage, such alterations may serve to create a hostile environment for immune cells, fostering, that is, hypoxia and nutrient depletion that can lead to immune dysfunction. Metabolic alterations specific to glioblastoma include those in oxidative phosphorylation (OXPHOS), the pentose phosphate pathway (PPP), fatty acid biosynthesis, and more.61 Fatty acid metabolism can promote tumor growth, and current work aims to target fatty acid oxidation with drugs such as Acyl-CoA binding proteins (ACBP, DBI) to hinder glioma growth.62,63 The OXPHOS and PPP pathways play critical roles in tumor development through their influence on glycolysis, with OXPHOS inducing glioblastoma differentiation.64 Glioma cells also frequently express indoleamine 2,3 dioxygenase, an enzyme that metabolizes the amino acid tryptophan, a process known to play roles in both enhancing tumorigenicity and recruiting immunosuppressive Tregs.65
A variety of epigenetic modifications, such as DNA methylation, can also help tumors evade the immune system by altering the expression of genes related to self-renewal and cell death.66 Other epigenetic changes in subsets of glioblastoma can include mutations in complexes such as SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A-like protein 1 (SMARCAL1), normally involved in regulating chromatin structure and transcription. Such mutations can drive changes in chemokine expression and inflammatory cell recruitment to influence treatment resistance.67–70 Ultimately, by modulating the expression of inflammasome components, tumors are able to manipulate the immune milieu and promote their survival.
In summary, there are several tumor-intrinsic features that may permit glioblastoma IDO IDO immune-evasion, including PDL1 upregulation, MHC downregulation, intratumoral heterogeneity, metabolic alterations, and epigenetic modifications (Figure 2). Such tumor cell-intrinsic changes are now the focus of a number of therapeutic platforms. For instance, metabolism-targeting agents include those inhibiting the OXPHOS pathway, with the compound gboxin aiming to inhibit the production of ATP in tumor cells and thus prevent proliferation.71 As described above, immune checkpoint blockade targets receptors or ligands that limit CD8 + T-cell activation, with canonical targets to date including PD-1/PD-L172,73 and CTLA-4.74 Additional targets have included TIM3,75 LAG-3,76,77 and TIGIT,73,78 amongst others.
The most straight-forward attempts to side-step tumor heterogeneity simply employ multitarget strategies. For instance, multipeptide or neoantigen vaccines are designed to target multiple tumor-specific or tumor-associated antigens and may be customized to a patient’s own tumor antigen expression profiles.79–83 Tandem CARs targeting both IL-13Ra2 and/or EGFRvIII have likewise been developed and tested in clinical trials.84,85 Concurrently, Boolean logic-gated CAR-T cells are beginning to be developed. These strategies equip CAR T cells with the capacity to respond only when certain combinations of targets are or are not expressed, in an effort to limit immune responses to normal tissues expressing tumor-associated (ie, not tumor-specific) antigens.86
Tumor Microenvironment Factors
The TME of glioblastoma is generally considered to be immunologically “cold” given a relative lack of T cell infiltration and fairly immunosuppressive milieu (Figure 3). The latter contributes a substantial degree of local T cell dysfunction within the TME that proves to be a significant barrier to effective antitumor immune responses. Most broadly, T cell dysfunction can be divided into the following 5 nonmutually exclusive categories: senescence, tolerance, anergy, exhaustion, and ignorance.44
Figure 3.
Glioblastoma tumor microenvironment-induced immune-evasion and immune-suppression includes mechanisms for locally eliciting various modes of T cell dysfunction (regulatory T cell-induced tolerance, exhaustion), much of which is aided by the activity of prominent populations of infiltrating myeloid cells. Created in https://BioRender.com.
T cell senescence is typically characterized by the loss of costimulatory markers and shortened telomeres resulting from chronic proliferation and stimulation.87–89 Larger immune senescence may be marked as well by thymic involution, which can occur naturally with aging, but is also found in the context of chronic inflammation and leads to decreased T cell output.90
Tolerance is evolutionarily designed to limit T cell responses to self-antigens and is frequently therefore adaptive. It can either be central (ie, thymic deletion of autoreactive T cells) or peripheral (ie, Treg-imposed restrictions to autoreactive T cell responses) and is generally intended to completely curb cytotoxicity. In autoimmune diseases, however, tolerance may fail to properly induce T-cell unresponsiveness.90 In the context of cancer, tolerizing mechanisms may instead be usurped by tumors to restrict responses to shared or even neoantigens. Glioblastoma cells, for instance, can overexpress FasL in order to delete T-effector cells peripherally, as well as to recruit regulatory T cells (Tregs) with the help of microglia, tumor-associated macrophages (TAMS), dendritic cells, and immunosuppressive cytokine secretion.23,91–97 Tregs, in turn, serve as a means for propagating peripheral tolerance via direct contact-dependent inhibition of T cell responses or via the production of cytokines such as IL-10 and TGFB98–100 and the inhibition of IL-2 production.101,102 In the context of glioblastoma, Tregs become disproportionately represented among the CD4 compartment,23,90 thus contributing to both local and systemic immunosuppression. Increased CCL2 within the TME has been shown to recruit both Tregs and myeloid-derived suppressor cells.23,94,103 Therapeutic strategies aimed at countering tolerance frequently focus on Tregs or their functional implications.
Anergy describes a fairly specific mode of T-cell inactivation after antigen binding and can be characterized by a lack of delayed-type hypersensitivity reactions upon secondary exposure to antigens. Clonal anergy follows insufficient costimulation from APCs leading to suboptimal antigen exposure, thus impairing T cell proliferation and preventing effective antigen recognition, respectively.91,104–109
Ignorance occurs when functional T cells remain inappropriately antigen-naïve, such as when targets are situated within immune “privileged” or “distinct” locations (such as the CNS), or conversely, when T cells become sequestered away from APC and/or targets and are not able to access their target antigen.10,11,110–114 The T cell sequestration in bone marrow observed with glioblastoma and other intracranial tumors is a quintessential example.10
T cell exhaustion is a programmed hyporesponsive (but not nonresponsive) state that occurs often following chronic antigen exposure of appropriately primed T cells (ie, nonautoreactive) within the TME. It is characterized by the upregulation of various canonical and noncanonical immune checkpoint receptors (ie, PD-1, TIM3) on the T cell surface. Immune checkpoint receptor-ligand binding between T cells and tumor cells or APC elicits subsequent alterations to T cell metabolism and function and limits their capacity to clear antigen-expressing targets.115,116 The result is the persistence of the target in a “stalemate” with the immune system.
Of the above modes of T cell dysfunction within the TME, exhaustion has become the most prominently studied of late, likely due to the frequent therapeutic focus on immune checkpoints. Likewise, T cell exhaustion is especially severe in glioblastoma.11 Exhausted T cells are now divided into 2 subgroups: progenitor exhausted T cells (Tex_prog) and terminally exhausted T cells (Tex_term). Tex_prog (PD-1+SLAMF6+TIM3-) can proliferate but have less cytotoxic potential, and Tex_term (PD-1hiSLAMF6-TIM3+) are cytotoxic but nonproliferative, with higher expression of inhibitory receptors.117 The master transcription factor regulator, thymocyte selection-associated high mobility group box factor (TOX), is expressed within exhausted T-cells, with levels increasing as exhaustion progresses.117 TOX promotes chromatin remodeling at the promoters of genes driving T-cell exhaustion.118 However, alone, it is insufficient to induce exhaustion and requires other functional contributors, such as PD-1 and SLAMF6.119
Recent studies have suggested that the classical definitions of exhaustion may be less relevant within glioblastoma. One such study, for instance, revealed unique transcriptional profiles among glioma-infiltrating lymphocytes, finding that clonally expanded T cells within the TME expressed lower levels of canonical exhaustion markers and instead terminally differentiated into a GZMK+ effector population with less cytotoxic capabilities.120 Another study has highlighted a novel role for the receptor TNFR2 in marking the progression from Tex_prog to Tex_term within the glioblastoma TME, with blockade of the receptor prolonging survival in murine models of glioma.121 Both studies advance novel phenotypes that may redefine the face of T cell dysfunction in the intracranial compartment.
While T cells are an expected focus of discussions surrounding immune dysfunction within the TME, more prevalent contributors within the glioblastoma TME are the various myeloid cell populations present. Tumor-associated macrophages and other myeloid cell populations play prominent roles in creating an immunosuppressive and/or pro-tumor TME. Tumor-associated macrophages found in tumors can be either microglia- or monocyte-derived and are self-renewing. Microglia-derived TAMs may be more prevalent in newly diagnosed tumors, whereas monocyte-derived TAMs may predominate amidst recurrence.122 Altogether, they typically make up more than half of the cells within the glioblastoma TME, and they contribute significantly to immunosuppression via the secretion of immune-modulating cytokines,123–125 such as transforming growth factor beta (TGFB), IL-10, IL-6, IL-1b, and others.126 Likewise, numerous recent studies, including by our own group, suggest myeloid populations rather than tumor cells as the direct source of T cell exhaustion within the TME.42,120,127,128 Altogether, these studies implicate antigen presentation by CD163+ or HMOX1+ myeloid cells as an initial event bringing them into contact with T cells, with secondary interactions involving, that is, IL-10 or SPP1 furthering the exhausted phenotype.
Additional myeloid-derived populations of relevance within the glioblastoma TME include infiltrating tumor-associated neutrophils (TANs). Neutrophils may play multiple roles that both support tumor growth directly while simultaneously limiting immune responsivity. For instance, TANs may release osteopontin, which stimulates the maintenance of stem-like glioblastoma cells.129 These, in turn, can inhibit T cytotoxicity and promote proliferation in glioma stem cells through the activation of 3-phosphoinositide-dependent protein kinase 1.130–133 Conversely, others have more recently identified a novel population of skull bone marrow-derived TANs that appear to possess APC-like features and can activate T cell cytotoxicity through antigen presentation on MHC II.134 Thus, our newer understanding of neutrophils suggests increasing complexity surrounding their roles within the TME.
Current therapies aimed at modifying the TME most frequently address either T cell exhaustion or myeloid-induced immunosuppression (Figure 3). Regarding the former, approaches such as immune checkpoint blockade (discussed above) aim to target T cell exhaustion directly, while other therapies, such as CAR-T-cells, are increasingly being built with an eye toward conferring resistance to exhaustive mechanisms within the TME. Exhaustion-resistant CAR-T cells can be developed by genetically editing CAR-T cells, for example, via overexpression of specific genes. One such gene is BATF3, which has been shown to be associated with memory T cell features and improved cytotoxicity.135 Other strategies include “armoring” CARs with cytokine support. Cotreatment with or coproduction of IL-15, for instance, can promote self-renewal of progenitor exhausted CAR-T cells, thus replenishing the cytotoxic T-cell compartment.136 Other cytokine supports, such as IL-2 and IL-33,137 have been shown to improve T cell polyfunctionality. Epigenetically focused therapies for exhaustion can target hypermethylation with DNA methyltransferase inhibitors. DNA methyltransferase inhibitors can be used to upregulate immune signaling, including a Type 1 interferon response.138 Examples include the enhancer of zeste 2 polycomb repressive complex 2 subunit139 inhibitors, as well as hyperacetylation with histone deacetylase140 and bromo- and extra-terminal domain inhibitors.139,140
Therapies targeting myeloid cells to date have most typically aimed at impacting macrophage polarization. Such therapies include colony stimulating factor 1 receptor (CSF1R) blockade, which has been shown to deplete microglia and monocyte-derived TAMs122 and to alter macrophage polarization toward more pro-inflammatory phenotypes.141 Some groups have employed anti-CSF1R preclinically along with checkpoint blockade to elicit modest success against murine glioma.142 Additional strategies for repolarization of TAMs have utilized CD40 agonists to improve dendritic cell T cell priming and TAM activation.126,143,144 Likewise, inhibition of signal transducers and activation of transcription 3 (STAT3) within the TME may play a variety of antitumor roles, including restricting the immunosuppressive capabilities of infiltrating myeloid cells.145–148 A Phase I trial of STAT3 inhibition in patients with glioblastoma was recently completed149 (NCT02977780).
Looking to the future, the recent studies highlighted above delineating the importance of myeloid populations in driving T cell exhaustion may support newer strategies to block myeloid—T cell interactions within the TME.42,120,127,128 Likewise, as newer roles for neutrophils are uncovered, novel approaches for impacting TAN recruitment or cytotoxicity may be justified. For instance, the discovery of a pro-inflammatory dendritic-like neutrophil population in the skull marrow insinuated a role for the CXCR4 antagonist AMD3100 for promoting their egress and recruitment.134
CNS Factors
Glioblastoma is unique among solid malignancies insomuch as it typically remains confined to the intracranial compartment. The CNS provides its own set of distinct challenges from an immunotherapy perspective (Figure 4). Historically, the CNS had been viewed as immune privileged: Harkening back to the 1940s, Peter Medawar demonstrated the absence of rejection when allogeneic skin grafts were implanted within the brain. What is less frequently recalled, however, is that Medawar’s studies did actually observe rejection if the same skin grafts were previously grafted outside of the brain, making the notion of immune privilege somewhat less absolute.1,150,151
Figure 4.
Central nervous system (CNS)-specific factors influencing glioblastoma immune-evasion and immune-suppression. The brain represents an immunologically “distinct” site, with unique aspects to antigen presentation and immune access. Microglia are CNS-resident cells that play an important immunomodulatory role. Glioblastoma also both alters and is influenced by surrounding neural circuits. Created in https://BioRender.com.
The blood-brain barrier (BBB) is also typically advanced as a feature of an immune-restrictive CNS, limiting access to both drugs and immune cells. However, the BBB deteriorates in glioblastoma,152 and disruption likely allows many therapies to enter the brain space in some capacity.153–156 Furthermore, an increasing participation of the brain with the immune system is now recognized. In more recent studies, for instance, Louveau et al. observed that meningeal lymphatics are an essential aspect of immune cell migration from the brain to the draining lymph nodes (dLNs).157 The glymphatic system (glial-lymphatic) was identified as a mechanism of clearance from the brain where cerebrospinal fluid (CSF) could drain to venous perivascular spaces.110,157–160 Functional lymphatic vessels have also been found in the meninges, allowing CSF to travel to cervical lymph nodes.133–136 More recent research has shown that dural sinuses can also accumulate CNS antigens in the CSF, allowing APC-T cell interactions and promoting effector immune function.161 The elucidation of these various CNS antigen drainage and presentation mechanisms has paved the way for newer therapies for several CNS pathologies such as Alzheimer’s157,162 and stroke,163 incorporating novel therapeutic delivery routes, engineered particle delivery, and more.164
Although not as isolated as perhaps once thought,165 the “immunologically distinct” brain still offers challenges to effective immunity against glioblastoma. Surrounding cell types unique to the intracranial compartment, such as microglia, astrocytes, and neurons, all can contribute to an immune-restrictive TME. While microglia and TAM were discussed above, tumor-associated astrocytes have also been shown to possess immunosuppressive activity, secreting cytokines such as IL-10 and TGFB.166 Likewise, neurons have been shown to play a role in gliomagenesis, supporting tumor progression and infiltration.167 The Monje group has recently shown that cholinergic neuron stimulation increases glioma proliferation, while neuron–tumor interactions promote tumor growth through glutamatergic and GABAergic neurons.168 Furthermore, gliomas can alter neural circuits to promote proliferation by tumor cells169,170 and promote hyperexcitability with the secretion of glutamate and neuroligin-3 (NLGN3).169,171,172 Such activity suggests a crosstalk occurs between gliomas and neurons to promote tumor survival.169 Recently, it has also been shown that radiotherapy can enhance tumor–neuron connections in a manner that actually contributes to therapeutic resistance, while virus-mediated ablation of these connections can instead decrease tumor proliferation.173
Therapies designed to address CNS-imposed limitations can employ such strategies as better delivery routes premised in newer understanding of antigen egress and presentation, or can perhaps further open the BBB to allow more efficient delivery (Figure 4). Regarding delivery routes, intrathecal and intraventricular injections have recently been used to deliver cellular therapies to the brain, allowing better access to the CSF and intracranial spaces.85,174 Intratumoral injections have also been used for therapy administration: one recent clinical trial used this route to deliver a modified poliovirus (PVSRIPO) into recurrent glioblastomas.175
Regarding BBB opening, focused ultrasound has been used in patients to transiently open the BBB, permitting classically nonbrain penetrant therapies better intracranial access.176 Laser interstitial thermal therapy has also been found to open the BBB in mouse models, thus proffering both tumor cell kill and subsequent improved therapeutic delivery.177–179 With regards to brain-specific cell targeting, in addition to the virus-induced ablation of neuron–tumor connections mentioned earlier, targeting microglia via, that is, inhibition of nuclear receptor subfamily 4 group A member 2 (NR4A2) has been shown to synergize with immune checkpoint blockade.180 Additional examples of novel strategies include nanoparticles designed to deliver various molecules such as small interfering RNA or chemotherapy with differing routes for administrations such as intranasal or intertumoral injections.
Systemic Factors
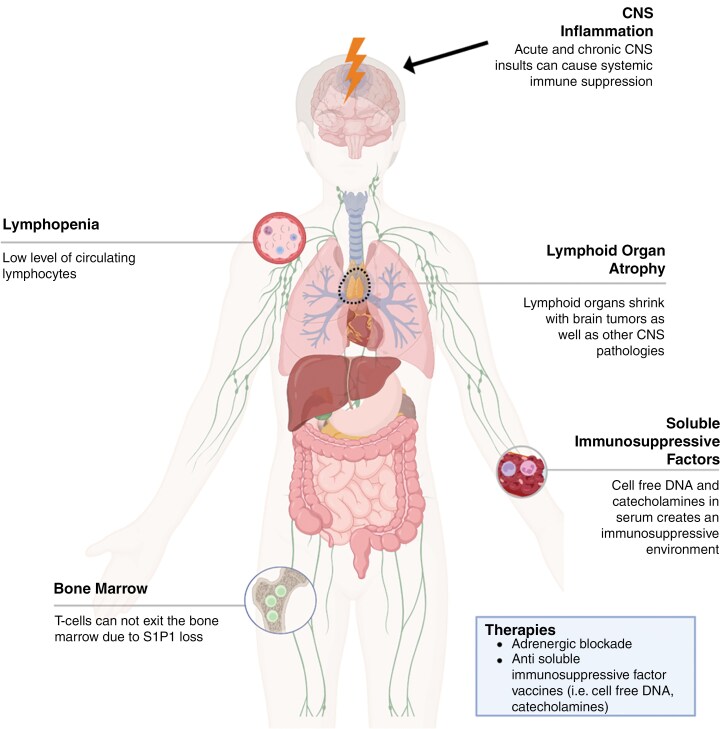
Various examples of systemic immune derangements have been documented in the context of glioblastoma and other intracranial tumors10,25,181–185 (Figure 5). Early work by Brooks and Roszman in patients with glioblastoma first identified systemic immune dysfunction in the form of lymphopenia, impaired antibody production, and weakened lymphocyte function.183,184,186–188 Regarding lymphocytes, studies have shown reduced counts and function of both CD4 + and CD8 + T cells,189 as well as defects in T cell development.190 IL-2 activity and signaling is diminished, leading to T cell proliferative defects.189,191 Glioblastoma elaborates anti-inflammatory cytokines, such as TGFB, IL-10, and PGE2,22,192,193 which serve to further suppress IL-2 secretion by T cells, reduce IFN-y production, downregulate MHC expression/presentation, suppress Th1 cytokine synthesis, inhibit APC capacities, and suppression proinflammatory cytokine production. Tregs are also elevated both locally and systemically in patients with glioblastoma and serve to limit cellular immunity and promote immune escape.23,65,194,195
Figure 5.
Glioblastoma and central nervous system-driven systemic immune derangements include lymphopenia, lymphoid organ atrophy, and bone marrow sequestration. The upstream mechanisms driving these systemic changes are an active area of investigation. Created in https://BioRender.com.
More recent work in this area has revealed that peripheral immunosuppression in glioblastoma patients and mouse models is characterized by T cell lymphopenia, splenic and thymic involution, sequestration of T cells within the bone marrow, and the presence of potent immunosuppressive factors in circulation.10,25,181 The role of serum-derived soluble factors as mediators of systemic immunosuppression in glioblastoma has been previously established.25 Interestingly, systemic immune derangements, including peripheral lymphopenia mediated by serum-derived factors, are not unique to the glioblastoma setting and have also been described in other brain injuries, including stroke and brain viral infections.25,196 Immunosuppressive factors in the serum of mice with intracranial pathologies are large in molecular weight and nonsteroidal in nature.25 Notably, “systemic immune derangements” have been described across various intracranial pathologies, including brain tumors, demyelinating diseases, stroke, and traumatic brain injury.25,181,197,198 These derangements include T cell lymphopenia and dysfunction, lymphoid organ atrophy/involution, and naïve T cell sequestration in the bone marrow,10,181,197,199–201 suggesting a common mode of CNS-insult-driven immunosuppression.
Importantly, while much of the work described above was performed in treatment-naive patients and thus highlights tumor-imposed deficits, various components to standard of care therapy for glioblastoma further contribute to an environment of systemic immune dysfunction. While temozolomide assuredly contributes to lymphopenia, for instance, a perhaps even larger source of immune dysfunction is the typical dosing regimens of steroids such as dexamethasone, intended to curb cerebral edema. While such benefits are well documented, they come at a collateral cost to antitumor immune responses, restricting the number and function of lymphoid cells and hampering immunotherapeutic success.202,203 It is a growing trend among immunotherapeutic clinical trials for cancers in general to place limits on the maximal dose of steroids that may be employed.
Several hypotheses exist as to the proximal causes of diminished systemic immunity amidst intracranial processes. One focuses on increased sympathetic activity, which appears to follow neuroinflammation and can impose adrenergic stress on immune cells.204,205 One study showed that beta blockade in combination with immunotherapy was able to increase survival in a brain tumor model, as well as in lung cancer and melanoma brain metastases models. Likewise retrospective clinical data revealed extended survival in patients with glioblastoma or brain metastases who were prescribed beta blockade.205
Another potential mediator of systemic immunosuppression in serum may be cell-free (CF) DNA. Analysis of CF-DNA, especially that of circulating tumor DNA, is an active area of investigation; as such, “liquid biopsies” can potentially be noninvasive sources of prognostic biomarkers. However, the functional role of CF-DNA in immunity and its immune-modulatory roles in cancer and other intracranial pathologies remains poorly understood. Ayasoufi et al. have shown heightened levels of CF-DNA in serum of mice with glioblastoma,206 which has also been observed in patients.207,208 Furthermore, they demonstrated that CF-DNA isolated from the serum of mice with gliomas suppresses T cell function.206 Roth et al. also recently showed that the presence of CF-DNA in the circulation of mice and humans poststroke conferred T cell immunosuppression indirectly through monocyte sensing of nucleic acids by Absent In Melanoma 2 (AIM2).196 Distinct immune-modulatory roles of CF-DNA in various context are likely mediated through cell-type specific intracellular nucleic sensing mechanisms.
Overall, this common systemic immunosuppression seen across various CNS diseases and pathologies hints at a common mechanism, with several hypotheses as to the key drivers. Reversing systemic immunosuppression in the setting of glioblastoma will be an important step in improving global host immune dysfunction in a manner that better permits immunotherapeutic success (Figure 5). Strategies to address systemic immune derangements may well then synergize with and license systemically focused immune-stimulating therapies, such as checkpoint blockade.205
Conclusion
Immunotherapies for glioblastoma and other intracranial tumors continue to face unique challenges. These challenges exist at the level of the tumor cell itself, the TME, the CNS more generally, and even systemically. While we have discussed these 4 domains in isolation for the purpose of organization and clarity, they are by no means mutually exclusive and are ultimately overlapping and interconnected facets dictating glioblastoma’s limited response to treatment. Likewise, while glioblastoma’s intracranial locale poses specific immune-related considerations, the tumor exhibits particularly severe capacities for restricting immune function that make it more formidable than other brain-situated lesions, such as metastases. Glioblastoma presents a notable challenge to immunotherapy, proffering few neoantigens and eliciting marked local and systemic immune dysfunction. The latter includes such barriers as regulatory T cells-induced tolerance, severe T cell exhaustion, an immunosuppressive and myeloid-heavy TME, glial–neuronal interactions, lymphopenia, lymphoid organ involution, altered hematopoiesis, and T cell sequestration. These barriers ultimately serve to hinder T cell number, access, and function, all 3 necessary components to immunotherapeutic success. In such combination, the sum total of these immune obstacles is unique to glioblastoma, and immunotherapeutic approaches must include means for clearing some if not all of these hurdles. Alternative delivery routes, combinatorial approaches, epigenetic and metabolic manipulation, innovative cellular therapeutic modifications, and myeloid- or regulatory T cell-targeting approaches are just a few examples of strategies to be explored and expanded.
Contributor Information
Bhairavy J Puviindran, Brain Tumor Immunotherapy Program, Duke University, Durham, North Carolina; Department of Biomedical Engineering, Duke University, Durham, North Carolina.
Shannon Wallace, Brain Tumor Immunotherapy Program, Duke University, Durham, North Carolina; Department of Biomedical Engineering, Duke University, Durham, North Carolina.
Katayoun Ayasoufi, Preston Robert Tisch Brain Tumor Center, Duke University, Durham, North Carolina; Department of Neurosurgery, Duke University, Durham, North Carolina.
Emily Lerner, Medical Science Training Program, Duke University, Durham, North Carolina; Brain Tumor Immunotherapy Program, Duke University, Durham, North Carolina; Department of Biomedical Engineering, Duke University, Durham, North Carolina.
Peter E Fecci, Preston Robert Tisch Brain Tumor Center, Duke University, Durham, North Carolina; Department of Neurosurgery, Duke University, Durham, North Carolina; Brain Tumor Immunotherapy Program, Duke University, Durham, North Carolina; Department of Biomedical Engineering, Duke University, Durham, North Carolina.
Supplement sponsorship
This article appears as part of the supplement “Immunotherapy for Brain Tumors,” sponsored by the Wilkins Family Chair in Neurosurgical Brain Tumor Research.
Conflict of interest statement
The authors declare no competing interests with regards to the current work.
References
- 1. Sampson JH, Gunn MD, Fecci PE, Ashley DM.. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS.. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4(9):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weller M, Cloughesy T, Perry JR, Wick W.. Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro-Oncology. 2012;15(1):4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nahm J, Sinha M, Schumann EH, et al. Overall survival in patients with recurrent glioblastomas with combination chemotherapy and tumor treating fields (TTF). J Clin Oncol. 2023;41(16):e14057–e14057. [Google Scholar]
- 7. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 8. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hodges TR, Ott M, Xiu J, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. 2017;19(8):1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 14. Muscat AM, Wong NC, Drummond KJ, et al. The evolutionary pattern of mutations in glioblastoma reveals therapy-mediated selection. Oncotarget. 2018;9(8):7844–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barthel FP, Johnson KC, Varn FS, et al. ; GLASS Consortium. Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576(7785):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim H, Zheng S, Amini SS, et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015;25(3):316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Cazzato E, Ladewig E, et al. Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48(7):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gavin P, Dunn PEF, William T.. Curry. Cancer immunoediting in malignant glioma. Neurosurgery. 2012;71(2):201–223. [DOI] [PubMed] [Google Scholar]
- 19. Lert F. Advances in HIV treatment and prevention: should treatment optimism lead to prevention pessimism? AIDS Care. 2000;12(6):745–755. [DOI] [PubMed] [Google Scholar]
- 20. Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roszman TL, Brooks WH, Steele C, Elliott LH.. Pokeweed mitogen-induced immunoglobulin secretion by peripheral blood lymphocytes from patients with primary intracranial tumors. Characterization of T helper and B cell function. J Immunol. 1985;134(3):1545–1550. [PubMed] [Google Scholar]
- 22. Wrann M, Bodmer S, de Martin R, et al. T cell suppressor factor from human glioblastoma cells is a 12.5-kd protein closely related to transforming growth factor-beta. EMBO J. 1987;6(6):1633–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. [DOI] [PubMed] [Google Scholar]
- 24. Ayasoufi K, Wolf DM, Namen SL, et al. Brain resident memory T cells rapidly expand and initiate neuroinflammatory responses following CNS viral infection. Brain Behav Immun. 2023;112:51–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ayasoufi K, Pfaller CK, Evgin L, et al. Brain cancer induces systemic immunosuppression through release of non-steroid soluble mediators. Brain. 2020;143(12):3629–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dunn GP, Old LJ, Schreiber RD.. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. [DOI] [PubMed] [Google Scholar]
- 27. Dunn GP, Old LJ, Schreiber RD.. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. [DOI] [PubMed] [Google Scholar]
- 28. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD.. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. [DOI] [PubMed] [Google Scholar]
- 29. Masood AB, Batool S, Bhatti SN, et al. Plasma PD-L1 as a biomarker in the clinical management of glioblastoma multiforme-a retrospective cohort study. Front Immunol. 2023;14:1202098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xue S, Song G, Yu J.. The prognostic significance of PD-L1 expression in patients with glioma: a meta-analysis. Sci Rep. 2017;7(1):4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee AH, Sun L, Mochizuki AY, et al. Neoadjuvant PD-1 blockade induces T cell and cDC1 activation but fails to overcome the immunosuppressive tumor associated macrophages in recurrent glioblastoma. Nat Commun. 2021;12(1):6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Facoetti A, Nano R, Zelini P, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11(23):8304–8311. [DOI] [PubMed] [Google Scholar]
- 33. Zagzag D, Salnikow K, Chiriboga L, et al. Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab Invest. 2005;85(3):328–341. [DOI] [PubMed] [Google Scholar]
- 34. Delle Donne R, Iannucci R, Rinaldi L, et al. Targeted inhibition of ubiquitin signaling reverses metabolic reprogramming and suppresses glioblastoma growth. Commun Biol. 2022;5(1):780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Won WJ, Deshane JS, Leavenworth JW, Oliva CR, Griguer CE.. Metabolic and functional reprogramming of myeloid-derived suppressor cells and their therapeutic control in glioblastoma. Cell Stress. 2019;3(2):47–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao J, Chen AX, Gartrell RD, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reardon DA, Omuro A, Brandes AA, et al. OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro-Oncology. 2017;19(3):iii21–iii21. [Google Scholar]
- 39. Tawbi HA, Forsyth PA, Algazi A, et al. Combined Nivolumab and Ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee J, Nicosia M, Hong ES, et al. Sex-biased t-cell exhaustion drives differential immune responses in glioblastoma. Cancer Discov. 2023;13(9):2090–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mohme M, Schliffke S, Maire CL, et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clin Cancer Res. 2018;24(17):4187–4200. [DOI] [PubMed] [Google Scholar]
- 42. Ravi VM, Neidert N, Will P, et al. T-cell dysfunction in the glioblastoma microenvironment is mediated by myeloid cells releasing interleukin-10. Nat Commun. 2022;13(1):925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davidson TB, Lee A, Hsu M, et al. Expression of PD-1 by T cells in malignant glioma patients reflects exhaustion and activation. Clin Cancer Res. 2019;25(6):1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE.. T-cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res. 2018;24(16):3792–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Woroniecka K, Chongsathidkiet P, Rhodin KE, et al. T cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7(1):10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S.. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. [DOI] [PubMed] [Google Scholar]
- 48. Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Busch E, Ahadova A, Kosmalla K, et al. Beta-2-microglobulin mutations are linked to a distinct metastatic pattern and a favorable outcome in microsatellite-unstable stage IV gastrointestinal cancers. Front Oncol. 2021;11:669774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang F, Zhao YH, Zhang Q, et al. Impact of beta-2 microglobulin expression on the survival of glioma patients via modulating the tumor immune microenvironment. CNS Neurosci Ther. 2021;27(8):951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mehling M, Simon P, Mittelbronn M, et al. WHO grade associated downregulation of MHC class I antigen-processing machinery components in human astrocytomas: does it reflect a potential immune escape mechanism? Acta Neuropathol. 2007;114(2):111–119. [DOI] [PubMed] [Google Scholar]
- 52. Gasser S, Orsulic S, Brown EJ, Raulet DH.. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lerner EC, Woroniecka KI, D’Anniballe VM, et al. CD8(+) T cells maintain killing of MHC-I-negative tumor cells through the NKG2D-NKG2DL axis. Nat Cancer. 2023;4(9):1258–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Senger DR, Davis GE.. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3(8):a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mazzone M, Dettori D, de Oliveira RL, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136(5):839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD.. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57(18):4130–4140. [PubMed] [Google Scholar]
- 57. Padfield E, Ellis HP, Kurian KM.. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front Oncol. 2015;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Migliorini D, Dietrich P-Y, Stupp R, et al. CAR T-cell therapies in glioblastoma: a first look. Clin Cancer Res. 2018;24(3):535–540. [DOI] [PubMed] [Google Scholar]
- 59. O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao J, Ma X, Gao P, et al. Advancing glioblastoma treatment by targeting metabolism. Neoplasia. 2024;51:100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kant S, Kesarwani P, Prabhu A, et al. Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dynamic nutrient microenvironment. Cell Death Dis. 2020;11(4):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Duman C, Di Marco B, Nevedomskaya E, et al. Targeting fatty acid oxidation via Acyl-CoA binding protein hinders glioblastoma invasion. Cell Death Dis. 2023;14(4):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stanke KM, Wilson C, Kidambi S.. High expression of glycolytic genes in clinical glioblastoma patients correlates with lower survival. Front Mol Biosci. 2021;8:752404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18(22):6110–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dawson MA, Kouzarides T.. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. [DOI] [PubMed] [Google Scholar]
- 67. Gusyatiner O, Hegi ME.. Glioma epigenetics: from subclassification to novel treatment options. Semin Cancer Biol. 2018;51:50–58. [DOI] [PubMed] [Google Scholar]
- 68. Hassan A, Mosley J, Singh S, Zinn PO.. A comprehensive review of genomics and noncoding RNA in gliomas. Topics Magnetic Resonance Imaging : TMRI. 2017;26(1):3–14. [DOI] [PubMed] [Google Scholar]
- 69. Pan D, Kobayashi A, Jiang P, et al. A major chromatin regulator determines resistance of tumor cells to T cell–mediated killing. Science. 2018;359(6377):770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359(6377):801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shi Y, Lim SK, Liang Q, et al. Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma. Nature. 2019;567(7748):341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zeng Y-F, Wei X-Y, Guo Q-H, et al. The efficacy and safety of anti-PD-1/PD-L1 in treatment of glioma: a single-arm meta-analysis. Front Immunol. 2023;14:1168244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang T, Kong Z, Ma W.. PD-1/PD-L1 immune checkpoint inhibitors in glioblastoma: clinical studies, challenges and potential. Hum Vaccin Immunother. 2021;17(2):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen D, Varanasi SK, Hara T, et al. CTLA-4 blockade induces a microglia-Th1 cell partnership that stimulates microglia phagocytosis and anti-tumor function in glioblastoma. Immunity. 2023;56(9):2086–2104.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim JE, Patel MA, Mangraviti A, et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res. 2017;23(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Harris-Bookman S, Mathios D, Martin AM, et al. Expression of LAG-3 and efficacy of combination treatment with anti-LAG-3 and anti-PD-1 monoclonal antibodies in glioblastoma. Int J Cancer. 2018;143(12):3201–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lim M, Ye X, Piotrowski AF, et al. Updated safety phase I trial of anti-LAG-3 alone and in combination with anti-PD-1 in patients with recurrent GBM. J Clin Oncol. 2020;38(15_suppl):2512–2512. [Google Scholar]
- 78. Raphael I, Kumar R, McCarl LH, et al. TIGIT and PD-1 immune checkpoint pathways are associated with patient outcome and anti-tumor immunity in glioblastoma. Front Immunol. 2021;12:637146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hotchkiss KM, Batich KA, Mohan A, et al. Dendritic cell vaccine trials in gliomas: Untangling the lines. Neuro Oncol. 2023;25(10):1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hotchkiss KM, Cho EJ, Khasraw M.. A first-in-human peptide vaccine targeting H3K27M; encouraging early findings in 8 adults with diffuse midline glioma. Neuro Oncol. 2024;26(1):5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Johanns TM, Garfinkle EAR, Miller KE, et al. Integrating multisector molecular characterization into personalized peptide vaccine design for patients with newly diagnosed glioblastoma. Clin Cancer Res. 2024;30(13):2729–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Latzer P, Zelba H, Battke F, et al. A real-world observation of patients with glioblastoma treated with a personalized peptide vaccine. Nat Commun. 2024;15(1):6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immunoreactivity and clinical outcome following vaccination with glioma-associated antigen peptides in children with recurrent high-grade gliomas: results of a pilot study. J Neurooncol. 2016;130(3):517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schmidts A, Srivastava AA, Ramapriyan R, et al. Tandem chimeric antigen receptor (CAR) T cells targeting EGFRvIII and IL-13Rα2 are effective against heterogeneous glioblastoma. Neurooncol Adv. 2023;5(1):vdac185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Choi BD, Gerstner ER, Frigault MJ, et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N Engl J Med. 2024;390(14):1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tousley AM, Rotiroti MC, Labanieh L, et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature. 2023;615(7952):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Akbar AN, Henson SM, Lanna A.. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37(12):866–876. [DOI] [PubMed] [Google Scholar]
- 88. Hayflick L, Moorhead PS.. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25(3):585–621. [DOI] [PubMed] [Google Scholar]
- 89. Watson JD. Origin of concatemeric T7DNA. Nat New Biol. 1972;239(94):197–201. [DOI] [PubMed] [Google Scholar]
- 90. Lamas A, Lopez E, Carrio R, Lopez DM.. Adipocyte and leptin accumulation in tumor-induced thymic involution. Int J Mol Med. 2016;37(1):133–138. [DOI] [PubMed] [Google Scholar]
- 91. Xu L, Xiao H, Xu M, et al. Glioma-derived T cell immunoglobulin-and mucin domain-containing molecule-4 (TIM4) contributes to tumor tolerance. J Biol Chem. 2011;286(42):36694–36699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Heimberger AB, Kong L-Y, Abou-Ghazal M, et al. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin Neurosurg. 2009;56:98–106. [PubMed] [Google Scholar]
- 93. Choi BD, Fecci PE, Sampson JH.. Regulatory T cells move in when Gliomas say “I DO.”. Clin Cancer Res. 2012;18(22):6086–6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Andaloussi AE, Lesniak MS.. An increase in CD4+ CD25+ FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-Oncology. 2006;8(3):234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hall ED, Travis MA.. Attenuation of progressive brain hypoperfusion following experimental subarachnoid hemorrhage by large intravenous doses of methylprednisolone. Exp Neurol. 1988;99(3):594–606. [DOI] [PubMed] [Google Scholar]
- 96. Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G.. Ex vivo isolation and characterization of CD4+ CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193(11):1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Maloy KJ, Erdmann I, Basch V, et al. Intralymphatic immunization enhances DNA vaccination. Proc Natl Acad Sci USA. 2001;98(6):3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G.. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. J Exp Med. 2002;196(2):247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jonuleit H, Schmitt E, Kakirman H, et al. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196(2):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173(11):6526–6531. [DOI] [PubMed] [Google Scholar]
- 101. Ermann J, Szanya V, Ford GS, et al. CD4(+)CD25(+) T cells facilitate the induction of T cell anergy. J Immunol. 2001;167(8):4271–4275. [DOI] [PubMed] [Google Scholar]
- 102. Thornton AM, Shevach EM.. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chang AL, Miska J, Wainwright DA, et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5671–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abe BT, Shin DS, Mocholi E, Macian F.. NFAT1 supports tumor-induced anergy of CD4+ T cells. Cancer Res. 2012;72(18):4642–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Martinez GJ, Pereira RM, Äijö T, et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 2015;42(2):265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fathman CG, Lineberry NB.. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7(8):599–609. [DOI] [PubMed] [Google Scholar]
- 108. Beverly B, Kang S-M, Lenardo MJ, Schwartz RH.. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4(6):661–671. [DOI] [PubMed] [Google Scholar]
- 109. Chiodetti L, Choi S, Barber DL, Schwartz RH.. Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol (Baltimore, MD: 1950). 2006;176(4):2279–2291. [DOI] [PubMed] [Google Scholar]
- 110. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Laman JD, Weller RO.. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol. 2013;8(4):840–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Heimberger AB, Sampson JH.. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol. 2011;13(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B.. Immune microenvironment of gliomas. Lab Invest. 2017;97(5):498–518. [DOI] [PubMed] [Google Scholar]
- 114. Lohr J, Ratliff T, Huppertz A, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 2011;17(13):4296–4308. [DOI] [PubMed] [Google Scholar]
- 115. Wherry EJ, Ha S-J, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. [DOI] [PubMed] [Google Scholar]
- 116. Patsoukis N, Bardhan K, Chatterjee P, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Beltra JC, Manne S, Abdel-Hakeem MS, et al. Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. 2020;52(5):825–841.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Khan O, Giles JR, McDonald S, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571(7764):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Miller BC, Sen DR, Al Abosy R, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20(3):326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang AZ, Mashimo BL, Schaettler MO, et al. Glioblastoma-infiltrating CD8+ T cells are predominantly a clonally expanded GZMK+ effector population. Cancer Discov. 2024;14(6):1106–1131. [DOI] [PubMed] [Google Scholar]
- 121. Hoyt-Miggelbrink A, Polania JW, Wachsmuth L, et al. TNFR2 loss leads to decreased TOX expression in T cells without affecting TIM3 and improves responses to tumor and chronic LCMV. bioRxiv. 2024;603311. [Google Scholar]
- 122. Pombo Antunes AR, Scheyltjens I, Lodi F, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021;24(4):595–610. [DOI] [PubMed] [Google Scholar]
- 123. Ye XZ, Xu SL, Xin YH, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol. 2012;189(1):444–453. [DOI] [PubMed] [Google Scholar]
- 124. Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11):1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yi L, Xiao H, Xu M, et al. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. 2011;232(1-2):75–82. [DOI] [PubMed] [Google Scholar]
- 126. Wang Z, Zhong H, Liang X, Ni S.. Targeting tumor-associated macrophages for the immunotherapy of glioblastoma: navigating the clinical and translational landscape. Front Immunol. 2022;13:1024921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Waibl Polania J, Hoyt-Miggelbrink A, Tomaszewski WH, et al. Antigen presentation by tumor-associated macrophages drives T cells from a progenitor exhaustion state to terminal exhaustion. Immunity. 2024;58(1):232–246.e6. [DOI] [PubMed] [Google Scholar]
- 128. Kilian M, Sheinin R, Tan CL, et al. MHC class II-restricted antigen presentation is required to prevent dysfunction of cytotoxic T cells by blood-borne myeloids in brain tumors. Cancer cell. 2023;41(2):235–251.e9. [DOI] [PubMed] [Google Scholar]
- 129. Shah SS, Yagnik G, Nguyen AT, et al. Pro-tumoral Effects of Intra-tumoral Neutrophils in the Glioblastoma Microenvironment. Neurosurgery. 2019;66(Supplement_1):310–314. [Google Scholar]
- 130. Lu J, Xu Z, Duan H, et al. Tumor-associated macrophage interleukin-β promotes glycerol-3-phosphate dehydrogenase activation, glycolysis and tumorigenesis in glioma cells. Cancer Sci. 2020;111(6):1979–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang Y, Yu G, Chu H, et al. Macrophage-associated PGK1 phosphorylation promotes aerobic glycolysis and tumorigenesis. Mol Cell. 2018;71(2):201–215.e7. [DOI] [PubMed] [Google Scholar]
- 132. Xuan W, Lesniak MS, James CD, Heimberger AB, Chen P.. Context-dependent glioblastoma–macrophage/microglia symbiosis and associated mechanisms. Trends Immunol. 2021;42(4):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Simonds EF, Lu ED, Badillo O, et al. Deep immune profiling reveals targetable mechanisms of immune evasion in immune checkpoint inhibitor-refractory glioblastoma. J ImmunoTher Cancer. 2021;9(6):e002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lad M, Beniwal AS, Jain S, et al. Glioblastoma induces the recruitment and differentiation of dendritic-like “hybrid” neutrophils from skull bone marrow. Cancer Cell. 2024;42(9):1549–1569.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. McCutcheon SR, Swartz AM, Brown MC, et al. Transcriptional and epigenetic regulators of human CD8+ T cell function identified through orthogonal CRISPR screens. Nat Genet. 2023;55(12):2211–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lee J, Lee K, Bae H, et al. IL-15 promotes self-renewal of progenitor exhausted CD8 T cells during persistent antigenic stimulation. Front Immunol. 2023;14:1117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Brog RA, Ferry SL, Schiebout CT, et al. Superkine IL-2 and IL-33 armored CAR T cells reshape the tumor microenvironment and reduce growth of multiple solid tumors. Cancer Immunol Res. 2022;10(8):962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chiappinelli KB, Strissel PL, Desrichard A, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2015;162(5):974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lue JK, Prabhu SA, Liu Y, et al. Precision targeting with EZH2 and HDAC inhibitors in epigenetically dysregulated lymphomas. Clin Cancer Res. 2019;25(17):5271–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Eckschlager T, Plch J, Stiborova M, Hrabeta J.. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18(7):1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Przystal JM, Becker H, Canjuga D, et al. Targeting CSF1R Alone or in Combination with PD1 in Experimental Glioma. Cancers (Basel). 2021;13(10):2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. van Hooren L, Vaccaro A, Ramachandran M, et al. Agonistic CD40 therapy induces tertiary lymphoid structures but impairs responses to checkpoint blockade in glioma. Nat Commun. 2021;12(1):4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med. 2020;71:47–58. [DOI] [PubMed] [Google Scholar]
- 145. Fan QW, Cheng CK, Gustafson WC, et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell. 2013;24(4):438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ferguson SD, Srinivasan VM, Heimberger AB.. The role of STAT3 in tumor-mediated immune suppression. J Neurooncol. 2015;123(3):385–394. [DOI] [PubMed] [Google Scholar]
- 147. Hussain SF, Kong LY, Jordan J, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67(20):9630–9636. [DOI] [PubMed] [Google Scholar]
- 148. Yu H, Kortylewski M, Pardoll D.. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41–51. [DOI] [PubMed] [Google Scholar]
- 149. Groot J, Ott M, Wei J, et al. A first-in-human Phase I trial of the oral p-STAT3 inhibitor WP1066 in patients with recurrent malignant glioma. CNS Oncol. 2022;11(2):CNS87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 151. Goldmann J, Kwidzinski E, Brandt C, et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80(4):797–801. [DOI] [PubMed] [Google Scholar]
- 152. Long DM. Capillary ultrastructure and the blood-brain barrier in human malignant brain tumors. J Neurosurg. 1970;32(2):127–144. [DOI] [PubMed] [Google Scholar]
- 153. Watkins S, Robel S, Kimbrough IF, et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Vajkoczy P, Menger MD.. Vascular microenvironment in gliomas. J Neurooncol. 2000;50(1-2):99–108. [DOI] [PubMed] [Google Scholar]
- 155. Schlager C, Korner H, Krueger M, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530(7590):349–353. [DOI] [PubMed] [Google Scholar]
- 156. Owens T, Bechmann I, Engelhardt B.. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008;67(12):1113–1121. [DOI] [PubMed] [Google Scholar]
- 157. Louveau A, Herz J, Alme MN, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21(10):1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Eide PK, Vatnehol SAS, Emblem KE, Ringstad G.. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep. 2018;8(1):7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Rustenhoven J, Drieu A, Mamuladze T, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184(4):1000–1016.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Antila S, Chilov D, Nurmi H, et al. Sustained meningeal lymphatic vessel atrophy or expansion does not alter Alzheimer’s disease-related amyloid pathology. Nat Cardiovasc Res. 2024;3:474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Keuters MH, Antila S, Immonen R, et al. The impact of VEGF-C-induced dural lymphatic vessel growth on ischemic stroke pathology. Transl Stroke Res. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. [DOI] [PubMed] [Google Scholar]
- 165. Fecci PE, Heimberger AB, Sampson JH.. Immunotherapy for primary brain tumors: no longer a matter of privilege. Clin Cancer Res. 2014;20(22):5620–5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Henrik Heiland D, Ravi VM, Behringer SP, et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun. 2019;10(1):2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Huang-Hobbs E, Cheng Y-T, Ko Y, et al. Remote neuronal activity drives glioma progression through SEMA4F. Nature. 2023;619(7971):844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Drexler R, Drinnenberg A, Gavish A, et al. Cholinergic neuronal activity promotes diffuse midline glioma growth through muscarinic signaling. bioRxiv. 2024;614235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Krishna S, Choudhury A, Keough MB, et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature. 2023;617(7961):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Venkatesh HS, Johung TB, Caretti V, et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell. 2015;161(4):803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Venkatesh HS, Tam LT, Woo PJ, et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature. 2017;549(7673):533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Tetzlaff SK, Reyhan E, Bengtson CP, et al. Characterizing and targeting glioblastoma neuron-tumor networks with retrograde tracing. bioRxiv. 2024;585565. [DOI] [PubMed] [Google Scholar]
- 174. Bagley SJ, Logun M, Fraietta JA, et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: phase 1 trial interim results. Nat Med. 2024;30(5):1320–1329. [DOI] [PubMed] [Google Scholar]
- 175. Desjardins A, Gromeier M, HerndonJE, 2nd, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Anastasiadis P, Gandhi D, Guo Y, et al. Localized blood-brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions-controlled focused ultrasound. Proc Natl Acad Sci U S A. 2021;118(37):e2103280118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Salehi A, Paturu MR, Patel B, et al. Therapeutic enhancement of blood-brain and blood-tumor barriers permeability by laser interstitial thermal therapy. Neurooncol Adv. 2020;2(1):vdaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3(4):971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Patel B, Yang PH, Kim AH.. The effect of thermal therapy on the blood-brain barrier and blood-tumor barrier. Int J Hyperthermia. 2020;37(2):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ye Z, Ai X, Yang K, et al. Targeting microglial metabolic rewiring synergizes with immune-checkpoint blockade therapy for glioblastoma. Cancer Discov. 2023;13(4):974–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lorrey SJ, Waibl Polania J, Wachsmuth LP, et al. Systemic immune derangements are shared across various CNS pathologies and reflect novel mechanisms of immune privilege. Neurooncol Adv. 2023;5(1):vdad035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Gustafson MP, Lin Y, New KC, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Brooks WH, Netsky MG, Normansell DE, Horwitz DA.. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136(6):1631–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Brooks WH, Roszman TL, Mahaley MS, Woosley RE.. Immunobiology of primary intracranial tumours. II. Analysis of lymphocyte subpopulations in patients with primary brain tumours. Clin Exp Immunol. 1977;29(1):61–66. [PMC free article] [PubMed] [Google Scholar]
- 185. Dix AR, Brooks WH, Roszman TL, Morford LA.. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100(1-2):216–232. [DOI] [PubMed] [Google Scholar]
- 186. Brooks WH, Caldwell HD, Mortara RH.. Immune responses in patients with gliomas. Surg Neurol. 1974;2(6):419–423. [PubMed] [Google Scholar]
- 187. Brooks WH, Roszman TL, Rogers AS.. Impairment of rosette-forming T lymphocytes in patients with primary intracranial tumors. Cancer. 1976;37(4):1869–1873. [DOI] [PubMed] [Google Scholar]
- 188. Roszman TL, Brooks WH, Elliott LH.. Immunobiology of primary intracranial tumors. VI. Suppressor cell function and lectin-binding lymphocyte subpopulations in patients with cerebral tumors. Cancer. 1982;50(7):1273–1279. [DOI] [PubMed] [Google Scholar]
- 189. Elliott L, Brooks W, Roszman T.. Role of interleukin-2 (IL-2) and IL-2 receptor expression in the proliferative defect observed in mitogen-stimulated lymphocytes from patients with gliomas. J Natl Cancer Inst. 1987;78(5):919–922. [PubMed] [Google Scholar]
- 190. Morford LA, Elliott LH, Carlson SL, Brooks WH, Roszman TL.. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol. 1997;159(9):4415–4425. [PubMed] [Google Scholar]
- 191. Ausiello CM, Palma C, Maleci A, et al. Cell mediated cytotoxicity and cytokine production in peripheral blood mononuclear cells of glioma patients. Eur J Cancer. 1991;27(5):646–650. [DOI] [PubMed] [Google Scholar]
- 192. de Martin R, Haendler B, Hofer-Warbinek R, et al. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987;6(12):3673–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Fontana A, Hengartner H, de Tribolet N, Weber E.. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984;132(4):1837–1844. [PubMed] [Google Scholar]
- 194. Himes BT, Geiger PA, Ayasoufi K, et al. Immunosuppression in glioblastoma: current understanding and therapeutic implications. Front Oncol. 2021;11:770561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wainwright DA, Sengupta S, Han Y, Lesniak MS.. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. 2011;13(12):1308–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Roth S, Cao J, Singh V, et al. Post-injury immunosuppression and secondary infections are caused by an AIM2 inflammasome-driven signaling cascade. Immunity. 2021;54(4):648–659.e8. [DOI] [PubMed] [Google Scholar]
- 197. Solti I, Kvell K, Talaber G, et al. Thymic atrophy and apoptosis of CD4+CD8+ Thymocytes in the Cuprizone model of multiple sclerosis. PLoS One. 2015;10(6):e0129217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ritzel RM, Doran SJ, Barrett JP, et al. Chronic alterations in systemic immune function after traumatic brain injury. J Neurotrauma. 2018;35(13):1419–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Chiu NL, Kaiser B, Nguyen YV, et al. The volume of the spleen and its correlates after acute stroke. J Stroke Cerebrovasc Dis. 2016;25(12):2958–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Vahidy FS, Parsha KN, Rahbar MH, et al. Acute splenic responses in patients with ischemic stroke and intracerebral hemorrhage. J Cereb Blood Flow Metab. 2016;36(6):1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Sahota P, Vahidy F, Nguyen C, et al. Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int J Stroke. 2013;8(2):60–67. [DOI] [PubMed] [Google Scholar]
- 202. Swildens KX, Sillevis Smitt PAE, van den Bent MJ, French PJ, Geurts M.. The effect of dexamethasone on the microenvironment and efficacy of checkpoint inhibitors in glioblastoma: a systematic review. Neurooncol Adv. 2022;4(1):vdac087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J ImmunoTher Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Eng JW, Kokolus KM, Reed CB, et al. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63(11):1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Selena JL, Lucas PW, John BF, et al. Intracranial tumors elicit systemic sympathetic hyperactivity that limits immunotherapeutic responses. bioRxiv. 2023;565368. [Google Scholar]
- 206. Ayasoufi K, Wolf D, Tritz Z, et al. IMMU-26. Heightened levels of circulating cell-free DNA contribute to peripheral immunosuppression during GBM progression. Neuro-Oncology. 2022;24(Supplement_7):vii136–vii137. [Google Scholar]
- 207. Bagley SJ, Nabavizadeh SA, Mays JJ, et al. Clinical utility of plasma cell-free DNA in adult patients with newly diagnosed glioblastoma: a pilot prospective study. Clin Cancer Res. 2020;26(2):397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Nabavizadeh SA, Ware JB, Guiry S, et al. Imaging and histopathologic correlates of plasma cell-free DNA concentration and circulating tumor DNA in adult patients with newly diagnosed glioblastoma. Neurooncol Adv. 2020;2(1):vdaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]