Abstract
Throughout history, cultural norms and stereotypes have discouraged resistance training in women. Today, as awareness and acceptance of resistance training in women has grown, supported by scientific research and advocacy, more women are achieving health and performance benefits from resistance training. This narrative review discusses the current scientific literature on sexual dimorphisms, the mechanisms underlying health and performance adaptations of resistance training in women, with implications for program design. In general, the physiological adaptations to resistance training in women are mediated largely by the neuroendocrine and immune systems, similar to in men albeit via some distinct predominant pathways involving sex hormones estrogen, testosterone, growth hormone (GH), and insulin-like growth factor- I (IGF-I). As a result, women may have unique adaptations in terms of muscle hypertrophy, substrate utilization, fatiguability, and recovery. Despite subtle physiological differences, women achieve measurable increases in strength, power and athletic performance via engaging in resistance training programs of sufficient frequency, intensity, and duration. Moreover, beyond performance, resistance training has a favorable impact on women’s health including metabolic health, body composition, bone health, cardiovascular health, mental health, self-esteem, and body image. Resistance training recommendations for men and women are highly similar and goal-dependent, with some specific caveats that need to be addressed in women. As resistance training has become regarded as a key element of programs for achieving performance and health improvements in women, additional research may further our understanding.
Keywords: Strength training, Sexual dimorphism, Hormones, Neuromuscular
Highlights
-
•
Resistance Training (RT) for Women: Historically discouraged, RT is widely recognized for its health and performance benefits for women.
-
•
Physiological Adaptations: Women’s adaptations to RT involve the neuroendocrine and immune systems, with unique effects from estrogen and testosterone.
-
•
Health Benefits: Resistance training improves strength, body composition, bone health, cardiovascular function, mental health, self-esteem, and body image.
-
•
Similar Training Principles: While men and women can follow similar RT principles, individualization is important due to some physiological differences.
-
•
Barriers to Participation: Challenges such as perceived time and effort, intimidation, and psychological factors still affect women’s participation in RT.
Nomenclature
- ACL
Anterior Cruciate Ligament
- BMD
Bone Mineral Density
- CMJ
Countermovement Jump
- CSA
Cross-Sectional Area
- GH
Growth Hormone
- GCT
Ground Contact Time
- HI-RT
High-Intensity Resistance Training
- IGF-1
Insulin-Like Growth Factor 1
- IAT
Illinois Agility Test
- kD
Kilodalton
- LI-BFRT
Low-Intensity Blood Flow Restriction Training
- MRF4
Muscle Regulatory Factor 4
- mTOR
Mechanistic Target of Rapamycin
- NSCA
National Strength and Conditioning Association
- PFD
Pelvic Floor Disorders
- PP
Peak Power
- RT
Resistance Training
- T/C Ratio
Testosterone-to-Cortisol Ratio
- WHtR
Waist-to-Height Ratio
- 1RM
One-Repetition Maximum
- y
years
- wk
weeks
1. Introduction
It has been established that the major sexual dimorphisms between men and women are related to endocrine function (i.e., primarily testosterone) and muscle fiber size profiles when appropriate matched comparisons are made.1,2 Muscle mass depositions anatomically can also carry over to differences observed in muscle strength and power. Experimentally, a key factor that is often overlooked is the comparative equity of the men and women being compared (e.g., age, training experience, sport, etc.) Observations of sexual dimorphic and end point performance variables are impacted by the compared groups (e.g., top female drug free power lifters can best many males at that same weight for strength in the major lifts). Absolute values for strength in matched comparisons typically show men to be stronger.3 However, in some cases (including lighter weight classes) relative measures of strength and power in certain exercises like the squat, deadlift, and power in the countermovement jump are more similar between male and female athletes.4 However, relative differences in bench press strength may persist due to men typically having greater muscle mass in the upper body, regardless of how the strength is expressed.4
Current literature often overlooks the unique psychological and social factors that impact women’s participation and adherence to resistance training programs. A new review is needed to incorporate recent findings and provide updated strategies for program sequencing, aimed at improving engagement and retention among women in resistance training programs. With the upcoming National Strength and Conditioning Association (NSCA) position stand expected to introduce updated guidelines, synthesizing this new information alongside existing knowledge is crucial. Moreover, while short-term studies dominate the field, long-term adaptations and the specific needs of women over long periods of training are relatively underexplored.5 This review will also clarify how the core principles of resistance training apply across genders, while advocating for individualized approaches based on specific goals, fitness levels, and sports requirements.
The purpose of this review is to integrate the latest research, identify gaps in long-term training, and provide a comprehensive guide that reinforces established principles while promoting tailored, evidence-based practices for improved resistance training sequencing. This will ensure that trainers and coaches are equipped with the most current, holistic information to optimize training outcomes for women at all levels of fitness and athletic performance.
2. Historical changes with resistance training and women
The historical path which women have taken has been challenged by a host of different societal, structural and perceptions as noted in an excellent historical review by Shurley and colleagues.6 At the dawn of the 20th century, women had very limited access to weight training facilities. Societal norms of the time dictated that physical activity for women should focus on maintaining grace and femininity, which often restricted them to calisthenics, light exercises, and other activities deemed appropriate for their gender. Strength training was widely considered a male domain, with little thought given to its potential benefits for women. Health spas and YMCAs (Young Men’s Christian Associations) started to offer women access to light exercise equipment, but these were far from the comprehensive weight training setups available to men. The exercise equipment provided to women was often limited to machines designed for toning rather than building strength, reflecting the prevailing attitudes that muscularity was undesirable for women.
2.1. Mid-20th century
By the 1950s and 1960s, there was a gradual shift in the landscape of women’s fitness. Women’s gyms began to emerge, though they typically emphasized aerobic exercises, stretching, and light resistance work using small weights or resistance bands.7 Fitness classes that gained popularity during this period, such as calisthenics, yoga, and dance, were designed to improve flexibility and cardiovascular health rather than build significant muscle mass. The 1960s saw the emergence of “aerobics” popularized by a physician in the US Air Force, Dr. Ken Cooper. Jogging and running would be a primary modality of exercise for many including women well into its peak in the 1970s.8 In educational settings, some universities and schools started to offer women’s physical education programs. However, these programs often lacked comprehensive weight training facilities, further perpetuating the idea that strength training was not suitable for women.
2.2. 1970s–1980s
The 1970s and 1980s were transformative decades for the fitness industry, characterized by the rapid rise of health clubs and commercial gyms offering a broader range of equipment. However, these facilities were largely male dominated, with equipment primarily designed for men in terms of weight and ergonomics. The fitness boom during this period also introduced aerobics to the mainstream, driven by influential figures from the 1950s on with television and video celebrities such as Jack LaLanne, Richard Simmons, and Jane Fonda, who played pivotal roles in popularizing group fitness among women and encouraging their participation in physical activity. Despite this increased engagement, the focus remained overwhelmingly on cardiovascular fitness, with aerobics classes taking center stage in women’s fitness routines. Strength training was still often seen as a male pursuit, relegated to the sidelines in most fitness programs aimed at women. Nevertheless, this era laid critical groundwork for the broader acceptance of women in fitness spaces, paving the way for the eventual integration of strength training into women’s fitness regimens.
During the 1980s, several other key figures and cultural shifts played a critical role in normalizing and popularizing weight training for women in the United States. Betty Weider, along with her husband Joe Weider, was instrumental in promoting fitness and bodybuilding for women through influential publications like Shape and Muscle & Fitness. These magazines provided women with guidance and inspiration, helping to shift public perception towards the acceptance of muscularity in women. Simultaneously, Arnold Schwarzenegger emerged as a prominent advocate for women’s participation in strength training, using his influence to challenge the long-standing barriers that had kept women away from the weight room. Rachel McLish, the first Ms. Olympia, also played a pivotal role by showcasing a level of muscularity that was aspirational yet non-threatening, helping to change perceptions about female bodybuilding during this decade.
The broader fitness boom of the 1980s further entrenched weight training in women’s fitness routines, as health clubs began offering more inclusive programs that catered to women. Media outlets, especially Shape magazine, reinforced the benefits of strength training, encouraging women to embrace a strong, toned physique as a desirable body image. This period marked a significant shift in societal attitudes, moving away from the preference for slimness and towards the acceptance of a more muscular female form. Movies of the 1980s also contributed significantly to changing perceptions about women and muscularity. “Pumping Iron II: The Women” (1985) was particularly influential, documenting the lives and challenges of female bodybuilders, and highlighting their dedication and strength. This documentary helped to challenge stereotypes and made the idea of muscular development in women more socially acceptable. Other films like “Flashdance” (1983) and “Perfect” (1985) also played a role, with their portrayal of athletic, toned female protagonists who engaged in rigorous jobs and physical training. These films, combined with the growing visibility of strong female bodies in the media, helped to further normalize and popularize weight training among women, making it an integral part of women’s fitness culture.
A significant shift also began in the mid 1970s and early 1980s, as women’s participation in lifting sports started to gain recognition. Dr. Jan Todd, a distinguished historian at the University of Texas and a prominent figure in strength sports, demonstrated her considerable influence in the field of powerlifting by appearing on “The Tonight Show Starring Johnny Carson” on February 2, 1977. This appearance on the iconic late-night television show underscored her stature as a leading pioneer, highlighting the growing mainstream acceptance of strength training. The official sport of powerlifting for women gained prominence in 1977 with the first AAU sanctioned Women's Powerlifting Meet held in New Hampshire, the first official USPF National Women's Powerlifting Championships in 1978, and the first IPF Women's Powerlifting Championship in 1980. Dr. Todd was one of the notable competitors and over her career would establish herself as probably the strongest woman in the world. Following this, women’s weightlifting was officially recognized in 1981 with the first US National Weightlifting contest in Waterloo, Iowa (with the 2nd in St. Charles, IL the following year) and the first International Weightlifting Federation (IWF) World Championships for women held in Daytona Beach in 1987. This historic event, held in Daytona Beach, Florida, marked a significant milestone, bringing women’s competitive weightlifting to the international stage.
The increasing visibility of women in these lifting sports began to influence the broader fitness culture, particularly in the realm of athletic strength and conditioning programs. Coaches with experience in competitive lifting started to incorporate more structured resistance training into the regimens of female athletes, recognizing the benefits of strength training for performance and injury prevention. Boyd Epley, the first full time strength coach in the USA at the University of Nebraska in 1969 was the founder of the NSCA but created a full array strength training program which included women’s sports.9 This period also saw the founding of the NSCA in 1978, which played a crucial role in the evolution of resistance training for both men and women. The NSCA promoted evidence-based practices and provided education and certification for strength and conditioning professionals, helping to standardize and elevate the field.10 Its influence extended to the development of training programs that included resistance training as a core component for athletes and fitness enthusiasts, thus gradually integrating it into women’s athletic strength and conditioning programs.10
As a result, the integration of resistance training into women’s fitness and athletic programs began to gain momentum, moving from the periphery to a central role in enhancing physical performance and health. The efforts of pioneering women in lifting sports, along with the professionalization of strength and conditioning through organizations like the NSCA, helped break down barriers and challenge the prevailing stereotypes that had long kept women away from resistance training. Today, resistance training is widely recognized as an essential aspect of women’s fitness, with a growing number of women participating in competitive lifting and strength training programs designed to meet their specific needs.6 This evolution reflects broader societal changes, as women increasingly embrace strength and muscularity as vital components of fitness and health.
2.3. Title IX influence in the United States
The passage of Title IX in 1972 marked a significant turning point for women’s sports in the United States. This landmark federal civil rights law prohibited sex-based discrimination in any educational program or activity receiving federal funding, leading to a dramatic increase in opportunities for women in sports. Before Title IX, fewer than 300 000 girls participated in high school sports, while today, over 3.3 million girls are involved, reflecting Title IX’s significant impact on increasing opportunities for female athletes.11 This legislation has led to a substantial rise in visibility and support for women in sports across various levels in the United States. Title IX not only opened doors for female athletes but also spurred the development of women’s sports programs across educational institutions, increasing visibility and support for female athletes at all levels. This surge in participation has had lasting effects, influencing everything from collegiate sports to the professional leagues, and has played a crucial role in reshaping societal attitudes towards women in sports.10
2.4. 1990s-present
The 1990s ushered in a new era for women’s fitness, highlighted by the emergence of specialized women’s gyms and fitness centers designed to cater specifically to female needs. These facilities provided weight training equipment that was more accessible and tailored to women, making strength training more approachable. During this period, mainstream gyms also became more inclusive, fostering environments where women could comfortably engage in resistance training alongside men. This shift was evident as gyms began offering personal training and group fitness classes focused on strength, marking a significant change in how women’s fitness was approached. Additionally, the rise in home fitness equipment provided more opportunities for women to incorporate weight training into their routines from the comfort of their homes. As societal perceptions of women’s muscularity evolved, more women began to embrace strength training, recognizing its benefits for health, athletic performance, and body composition.
The influence of the NSCA played a pivotal role in this evolution.10 As the century closed out, the NSCA, under the leadership of its former president, William J. Kraemer, organized a task force that led to the publication of its first position stand on women and resistance training in 1989.12,13 The publication of the position stand across different outlets was a landmark moment, advocating for the inclusion of resistance training in women’s fitness and athletic programs.14 It addressed the controversies and misconceptions of the time, particularly the belief that women could not be trained in the same way as men due to perceived physiological differences. The position stand emphasized that women could benefit from resistance training similarly to men and that the differences in training needs were minimal, if any. This publication not only challenged outdated notions but also provided a scientific foundation for the integration of resistance training into women’s athletic and fitness routines. The NSCA’s advocacy helped to normalize strength training for women, contributing significantly to the broader acceptance and inclusion of resistance training in women’s fitness as the 21st century approached.
2.5. The 21st century
As we moved into the 21st century, the landscape of women’s strength and resistance training underwent a remarkable transformation. The foundations laid in the late 20th century, including the increased visibility of women in competitive lifting sports, the influence of organizations like the NSCA, and the rise of specialized women’s gyms, set the stage for a new era in women’s fitness.
In the early 2000s, the momentum for women’s participation in resistance training continued to grow. The stigma surrounding women lifting weights began to diminish as more women embraced strength training for its health benefits, including improved muscle tone, bone density, and overall physical performance. This shift was fueled by a greater understanding of exercise science and the widespread dissemination of information through the internet and social media, which played a significant role in educating women about the benefits of resistance training.
The increased interest in sports participation also translated to involvement in strength and conditioning activities to prevent injuries and improve performance. Yet, the evolution of resistance training for women continues. This transformation is closely tied to the recognized sports and fitness benefits and the gradual shift in societal perceptions regarding women’s muscularity. As women’s participation in sports has increased, so too has their involvement in strength and conditioning programs. Historically, women were often excluded from these aspects of training due to misconceptions about the effects of resistance training, such as the fear of becoming “too bulky.”6 However, as the understanding of exercise science has evolved, so has the recognition of the numerous benefits that strength training offers to women. These benefits include improved athletic performance, enhanced injury prevention, and overall better health. For instance, targeted strength training has been shown to reduce the risk of common injuries like anterior cruciate ligament (ACL) tears, which are more prevalent in female athletes due to anatomical and hormonal differences.15 The increased emphasis on strength and conditioning has not only improved performance outcomes for women but has also empowered female athletes to take control of their training and health in ways that were previously unavailable to them.
Mainstream fitness culture began to celebrate strong, athletic female bodies, moving away from the traditional ideals of thinness. The rise of fitness influencers and the growing popularity of athletic wear brands that emphasized strength and empowerment further normalized the idea that women could, and should, engage in weightlifting and other forms of resistance training. This cultural shift was also reflected in the increased presence of women in roles traditionally dominated by men, such as personal trainers and strength coaches, which further encouraged women to participate in resistance training.
3. Institutional support and research
The early 21st century also saw significant advancements in research and institutional support for women’s resistance training. The NSCA and other organizations continued to advocate for evidence-based practices, and new studies consistently reinforced the benefits of strength training for women across all ages and fitness levels. Programs designed specifically for women, such as female-centric weightlifting competitions and strength training certifications, became more prevalent, further breaking down barriers to entry.
The fitness industry responded to these trends by creating more inclusive and supportive environments for women. Gyms and fitness centers began offering more diverse programming that included strength and resistance training tailored to women’s needs. Equipment manufacturers also began designing products with women in mind, making weight training more accessible and appealing.
Competitive strength sports, such as powerlifting, weightlifting, bodybuilding and fitness competitions, have seen significant increases in female participation, with women athletes breaking records and challenging stereotypes. Moreover, the professionalization of women’s strength training has continued to grow, with more women entering fields like strength coaching, exercise physiology, and sports science. Yet, sex imbalance in sporting professions remains due to complex and institutionally entrenched social attitudes.16
Today, strength and resistance training are integral components of women’s fitness routines, with participation levels higher than ever before. The concept of “strong is beautiful” has become a mainstream mantra, reflecting the widespread acceptance and celebration of female strength. Women of all ages, from teenagers to seniors, are now regularly engaging in resistance training, recognizing it as essential for health, fitness, and longevity. However, for many women not competitive athletes many barriers still appear to exist.
Still in 2018, in a study by Hurley and colleagues17 in a college setting overviewed the past history and the barriers many women perceive in participating in resistance training as historically men are about 30% more likely than women to participate in resistance training. In a college setting, they found that the biggest barrier for women is the perceived time and effort required for resistance training, which discourages regular participation. Interestingly, there was no strong link between the perceived benefits of resistance training and the barriers to it, suggesting that even when women recognize the advantages, they might still avoid it due many different perceived obstacles. The study identified several perceived obstacles to participation in resistance training among college women. The most significant barrier was the perceived time and effort required for resistance training. Women who saw these as obstacles were less likely to engage in regular resistance training. Other obstacles included concerns about looking silly, muscle soreness, and not wanting an athletic physique, a lack of encouragement from friends, not liking to exercise alone, and feeling uncomfortable or intimidated in the weight room. Additionally, factors like the lack of convenient locations to exercise, bad weather, medical problems, family obligations, and interference with other activities were noted as obstacles for consistent participation in a resistance training program. Additionally, older women were less likely to engage in resistance training, with age emerging as a key factor in lower participation rates. Still there are very little data on resistance training in women aged 45–65 year (y), despite the health benefits and their participation in training.18, 19, 20 To address these issues, the investigators suggested that educational interventions with classes teaching weight training exercises, and in some cases creating women-only training spaces for beginners may help reduce these barriers and encourage more women to take up resistance training.
Thus, despite these advances observed over the past decades, challenges remain, particularly in ensuring equal access to resources, qualified strength and conditioning professionals and facilities for training in both the athletic and commercial settings. However, the progress made over the past few decades has laid a strong foundation, and the future of women’s strength and resistance training looks promising. As societal perceptions continue to evolve, and as more women embrace the physical and mental benefits of resistance training, this area of fitness will likely continue to grow, further empowering women to achieve their full potential.
3.1. Research on women and resistance training
Research on women and resistance training has significantly advanced our understanding of the physiological differences and benefits unique to women. However, to design successful individualized programs for women in both athletics and general health, it is crucial to carefully assess past research. The evolution of resistance training for women began with a few pioneering studies in the 1970s, such as Mayhew and Gross21 work on body composition changes in young women and Brown and Wilmore22 who were one of the first studies on strength and body composition in women athletes. As the field of exercise science expanded, more research was conducted, but it is essential to critically examine this body of work. Factors such as the duration of studies, the quality of training supervision, participants’ initial fitness levels, and the specifics of program design (including acute program variables and training goals) must all be considered to glean valuable insights for contemporary practice. This review delves into these issues, aiming to extract the most relevant findings from decades of research to inform effective, evidence-based resistance training programs tailored to women today.
4. Physiological basis for adaptive changes with resistance training in women
Muscular strength is the maximum amount of force one can generate during a specific movement pattern at a specified contraction velocity. The expression of maximal strength depends on many factors including the type of muscle action (concentric, eccentric, and isometric), range of motion, contraction velocity, and a host of physiological/biomechanical attributes that enable an individual to generate high levels of force and lift an extensive amount of weight.23 In addition, maximal strength is muscle group and exercise specific and depends on the activities engaged by the trainee. Higher levels of muscular strength are strongly associated with improved force-time characteristics that contribute to many elements of athletic performance.24 Some critical physiological and biomechanical variables that contribute to muscular strength include neural drive and motor unit recruitment based on the size principle, firing rate, and temporal firing; muscle fiber type (fast-twitch, slow-twitch); muscle fiber number, arrangement, and size; energy metabolism; connective tissue support; anabolic hormone concentrations (testosterone, growth hormone superfamily, IGF-1, insulin) and signaling properties; catabolic hormone (cortisol) concentrations and signaling properties; muscle length and tendon insertion location (leverage); and stretch-shortening cycle potency (see Fig. 1).23
Fig. 1.
The areas of impact on women who participate in resistance training programs.
Differences in maximal strength between men and women have been well documented. Primarily, small differences (mostly examining grip strength) may be observed during youth but substantial sex differences are noted around pubertal ages 12–13 y (onset approximately 10 y in girls [range of 8–13 y] and 11.5 y in boys [range of 9–14 y]) and beyond with men displaying significantly larger absolute values of maximal strength.3,25 Early studies showed that women had approximately 56% of the upper-body strength of men, 72% of the lower-body strength of men, and 64% of trunk strength of men.26 A recent review has shown that women have ∼50%–60%, 60%–70%, and 60% of the upper-body, lower-body, and trunk strength of men, respectively.3 In an extensive review of the literature, Nuzzo3 reported the strength differences seen between men and women during different movements and exercises (see Table 1). It is important to note that these strength assessments were mostly concentric (with some isometric) as strength disparities between men and women are less (by ∼5%–16%) when examining eccentric strength.3 In addition, most of these studies examined untrained or lesser-trained men and women with varied backgrounds which could affect maximal strength assessment. Given notable differences in exercise participation rates (i.e., lower strength training participation in women compared to men), activity levels and types of activities engaged in (i.e., greater preference rate for strength training in men compared to women), and greater preference in men to select higher intensity exercise, more studies are needed to investigate trained populations with similar backgrounds to examine if the strength gap is narrowed.3 Nevertheless, female powerlifters have been shown to exhibit 46%–55%, 56%–61%, and 58%–64% of the maximal strength of male powerlifters for the bench press, squat, and deadlift, respectively.3
Table 1.
Differences (dynamic and isometric) in muscular strength between males and females.
| Exercise/Movement | Female % of Male Strength Range | Female % of Male Strength Estimated Mean |
|---|---|---|
| Handgrip strength | 45%–70% | ∼60% |
| Bench press/chest press | 20%–70% | ∼40%–55% |
| Shoulder press | 25%–70% | ∼50%–55% |
| Lat pulldown | 40%–63% | ∼50%–60% |
| Row | 45%–80% | ∼50%–60% |
| Upright row | 50%–60% | ∼50%–60% |
| Elbow flexion | 30%–65% | ∼55% |
| Elbow extension | 40%–60% | ∼50% |
| Wrist flexion | 50%–63% | ∼60% |
| Wrist extension | 45%–65% | ∼50% |
| Shoulder flexion, extension, abduction, adduction | 50%–65% | ∼50%–55% |
| Shoulder internal/external rotation | 45%–60% | ∼50%–55% |
| Knee extension | 40%–90% | ∼65% |
| Knee flexion | 35%–66% | ∼60% |
| Hip extension/flexion | 55%–80% | ∼60% |
| Hip abduction/adduction | 45%–90% | ∼60% |
| Hip external/internal rotation | 50%–70% | ∼60% |
| Leg press | 45%–80% | ∼65% |
| Back squat | 40%–70% | ∼60% |
| Deadlift (mostly isometric) | 45%–60% | ∼53%–58% |
| Ankle plantar flexion/dorsiflexion | 55%–85% | ∼65%–70% |
| Trunk flexion/extension | 45%–80% | ∼55%–60% |
| Neck flexion/extension | 45%–95% | ∼70% |
Modified from data presented by Nuzzo (2023).3
When maximal strength is expressed relative to body mass or lean body mass, it is defined as relative muscular strength (strength-to-mass ratio). A high strength-to-mass ratio enables high levels of force production without the addition of substantial mass gains where strength gains exceed increases in body mass. Men exhibit larger relative strength than women. For example, relative strength measures of 1.5, 2.1, and 2.5 have been shown in male powerlifters for the bench press, squat, and deadlift, respectively, versus 1.0, 1.6, and 2.0 shown in female powerlifters.3 In strength and power athletes (weightlifters, rugby, track and field), relative strength of measures of 1.36, 2.0, and 2.29 were shown in men versus 0.85, 1.31, and 1.53 shown in women for the bench press, squat, and deadlift, respectively.4 We have previously shown that with 8 weeks (wk) of intense resistance training program (3 × 6-8 RM and 10-12 RM, 2-min RI), the relative strength gap between men and women was reduced for the leg press, squat, and leg extension exercises.27 Thus, strength disparity between men and women exists in both absolute and relative measures, and this disparity exists among individuals of different training status as well as with other strength-tested exercises.28
Power is the rate of performing work. Because power is the product of force and velocity, there is a strength component to power development (strength at low-to-high velocities of movement).23 Thus, peak power is produced at the optimal interaction of force and velocity. Power is a critical component to all anaerobic sports and is trained via resistance, plyometric, speed, and agility training as well as sport-specific practice.23 For resistance training, light-to-moderate loading performed as fast as possible for many exercises leads to maximal power expression. The optimal expression of muscle power is reliant upon the correct exercise technique. Studies have shown that men exert up to ∼50% more power than women in activities such as cycling, running, skiing, jumping and swimming25,29, 30, 31 Larger differentials have been shown in upper-body (∼61%) compared to lower-body (∼44%) power between male and female athletes.4 Male athletes have shown higher upper and lower body power in both absolute and relative terms.4 Male sprinters have been shown to be faster than females by 14% in 40 m sprints and 15% in 80 m sprints, with concomitant larger force and power (19%–46%) produced during sprints.32 Male athletes have been shown to have higher countermovement jump heights and reactive strength index (RSI) than female athletes30,33 where circulating testosterone in the blood correlated highly with jump performance (r = 0.61).33 Other studies have shown positive relationships between testosterone concentrations and various measures of power performance, e.g., sprint, jumps, primarily in men.34 In competition, male athletes have shown 10%–30% better performances in strength/power events than female athletes with largest disparities seen in weightlifting events.25 Yet, few studies present relative power. When peak force is expressed relative to body mass, men and women have similar power capacities.35
A number of biomechanical, anthropometric, and physiological factors may help explain the muscle strength and power disparities. Sex differences in height, bone length, and moment arm length alter performance biomechanics creating an advantage for several movements and exercises. Culturally linked behavior and exercise differences may affect the ability of women to perform certain exercises or exercise at a high intensity (particularly with upper-body exercise). For example, men have shown greater preference for and devote more time to upper-body training while women tend to devote more training time to the lower body.3 Strength training participation rates are lower in women compared to men and men have greater preference rates for strength training compared to women.3 We have previously shown that women self-selected lighter training loads for four exercises but this self-selected load was higher when the women were trained by a personal trainer, e.g., 50% vs. 41% for the leg press, 57.4% vs. 48% for the chest press, 56% vs. 42% for the seated row, and 43% vs. 38% for the leg extension.38 Thus, different backgrounds, training experiences, and programming may contribute, in part, to the noted strength disparities between men and women.
Largely, hormonal changes at puberty and the increase in serum testosterone in males may play the most substantial role in the observed sex differences.25 Testosterone values in men are at least 15-20 × greater than women during adulthood with women ranging from 0.1 nmol/L to 1.8 nmol/L.41 Serum testosterone is significantly elevated in response to resistance exercise in men with the response dependent on several factors while smaller or no elevations may be observed in women.42 In addition, trained women have significant androgen receptor content in the vastus lateralis muscle but less than that of trained men.43 Testosterone is a highly anabolic hormone shown to improve a multitude of health- and skill-related fitness components.42,44, 45, 46 Higher testosterone production in young, healthy men offers a clear advantage for muscle strength, power, endurance, and hypertrophy increases42,46,47 and this hormonal disparity may be the single largest contributor to strength and power disparity between men and women.3 The potent influence of testosterone has been noted in men, but plays a significant role in women as well (e.g., especially when examining testosterone/anabolic-androgenic steroid use in women and hyperandrogenism).47 For example, female athletes with polycystic ovary syndrome (PCOS) have higher than normal circulating testosterone concentrations of 2 nmol/L–5 nmol/L (but lower than men) and are thought to have an advantage in competitive sports.25 There is strong heritability for serum testosterone as genetics account for 40%–70% of the variation in testosterone in men and 65% in women.48 Genomic single nucleotide polymorphisms (SNPs) associated with testosterone concentrations have been shown to correlate with muscle size and strength.48 Women with the highest concentrations of serum free testosterone were shown to perform significantly better (by 1.8%–4.5%) in several track and field events compared to women in the lowest tertile.49 The application of 10 mg/day of testosterone cream to active women aged 18 y–35 y for 10 wk (that increased total testosterone to an average of 4.3 nmol/L) increased lean muscle mass compared to a placebo.50 Thus, higher testosterone concentrations and the associated enhanced signaling properties may provide an advantage for male and female athletes.
Women have higher levels of estrogen (mostly 17β-estradiol) and progesterone. At puberty, estrogen concentrations increase to 4 × the level found in men until menopause.25 Estrogen plays prominent roles in glucose and fat metabolism, immune function, bone strength, cardiovascular function, and fertility. For example, women oxidize more lipids and fewer carbohydrates during endurance exercise compared with men likely due to the effects of estrogen.51 The effects may depend on the responses of both hormones (e.g., the estradiol/progesterone ratio) in total as progesterone may counteract some of the effects of estradiol especially during the mid-luteal phase.52 However, estradiol’s role in skeletal muscle anabolism is less clear as it appears to help maintain muscle mass but does not have close to the anabolic effects as testosterone. Estrogen deficiency has been implicated in the development of sarcopenia, and estrogen replacement therapy has been shown to provide small (∼5%) improvements in strength while maintaining lean muscle mass in postmenopausal women.53 It does appear to have a protective effect from post-exercise pro-inflammatory response and skeletal muscle damage.51,53 Estrogen and progesterone vary throughout the menstrual cycle where both hormones are low during the early follicular and early luteal phases, high estrogen and low progesterone seen during the late follicular phases, and increasing estrogen and progesterone during the mid-luteal phase with declines in both during the late luteal phase.54 Circulating estrogen concentrations of women during the early follicular phase of the menstrual cycle are similar to men; however, women have cyclically higher concentrations of estrogen throughout other phases of the cycle.51
Other critical hormones to muscle tissue remodeling and adaptation are the superfamily of growth hormone (GH) isoforms, IGF-1, and the catabolic hormone cortisol, as well as exerkines, e.g., signaling molecules released during exercise which have endocrine, autocrine, and paracrine functions that affect health and performance adaptations. Each of these hormones operates in a complex signaling network at the systemic and local levels. These hormones have been reviewed extensively.42,44,45,51,55 Although these hormones (as well as receptors, binding proteins, intra-cellular signaling proteins, and receptor-binding interaction effects) are responsive to the stress of acute resistance exercise and chronic training, they do not appear to explain much of the differences in muscle size and strength in men and women.25 Several studies have shown either similar acute systemic responses in men and women (i.e., cortisol) or similar or augmented 22-kD GH responses in men (with higher resting concentrations in women)2,56, 57, 58 and higher IGF-1 (and IGFBP3) in women with a similar acute resistance exercise response.56 We have previously shown significant elevations in GH molecular weight isoforms less than 60 kD in untrained women, strong resistance-trained women (> 6 y of experience, 1 RM squat/body mass of 1.7), and after 6 months of resistance training,56,59,60 and previously untrained women had higher values than men at rest and showed significant exercise-induced responses across all GH molecular weight isoforms.61 In addition, differences exist in the GH molecular weight variants between strong and weak women as lower molecular weight variants may be less responsive to larger amounts of resistance exercise in stronger women.62 Although the GH superfamily, IGF-1, and cortisol responses are critical to tissue remodeling and adaptation to training in women, it appears the more substantial effects of testosterone signaling in men outweigh these other anabolic/catabolic hormonal effects in explaining gender differences in performance.
Men have greater absolute lean body mass, muscle mass, and muscle cross-sectional area (CSA) than women. In a review from Nuzzo,3 it was shown that women have ∼70% of the lean body mass of men, 60% of the upper body mass, and 67% of the lower-body mass of men. Specifically, women have ∼50%–63% of the biceps brachii and triceps brachii muscle CSA as men, but 65%–80% of the muscle CSA in the quadriceps and hamstrings compared to men.3 Despite sex differences in muscle size, it appears neural drive to skeletal muscle is similar between men and women. Muscle fiber number varies between muscles as some studies show similar estimated fiber number in men and women for muscles such as the vastus lateralis and triceps brachii but other studies show greater fiber number in men in the biceps brachii and tibialis anterior.63 However, in trained populations such as male and female bodybuilders, estimated biceps brachii fiber number was not statistically different indicating that elite female lifters may have higher fiber numbers than lesser-trained females.64
Skeletal muscle fiber type is another consideration. Resistance training leads to hypertrophy of all fiber types with more hypertrophy seen in type II fibers.65 Men and women display typical IIX to IIA fiber type transitions seen with resistance training with changes taking place within 2 wk–4 wk when the stimulus is sufficient.5,27,65 Studies have shown that men have a significantly larger type II fiber area and a greater type IIA/I fiber area ratio than women,5 those fibers critical to strength and power expression. The majority of these studies have examined untrained or recreationally trained men and women. Women have been shown to have smaller, similar, or larger type 1 fiber area compared to men.3,5,63 Type I muscle fibers occupy a greater area percentage of muscle mass in women, yielding a higher Type II/I fiber area ratio in men compared to women.3,5 A recent meta-analysis showed that men exhibit greater cross-sectional areas for all muscle fiber types, greater area percentages for Type II muscle fibers, and greater Type II/I fiber ratios than women, while women exhibit greater distribution and area percentages for Type I fibers and have a greater Type I/II fiber area ratio than men.5 These studies have largely examined the vastus lateralis, gastrocnemius, multifidus, triceps and biceps brachii muscles. Genetics and activity level are two key promoters of fiber-type adaptations to training. Thus, the possibility that men typically train with higher frequency and intensity (and recruit fast-twitch fibers more regularly via the size principle) may contribute to these findings.3
Following resistance exercise, satellite cells proliferate, differentiate, fuse with existing myofibers, and donate myonuclei to hypertrophying muscle as part of the repair/remodeling process. Training-induced changes in satellite cell number and myonuclei are positively related to muscle hypertrophy.66 In some studies, men have been shown to have higher satellite cell content, myonuclei, and myonuclear domain in Type II fibers compared to women,67 and the acute satellite cell response to resistance exercise may be augmented in men.68 Other studies have shown men to have greater myonuclei number in Type I fibers as well but similar myonuclear domain in Type I and II fibers in men and women.66 In previously untrained women engaging in resistance training, satellite cell proliferation only occurred in higher responders and the percent change in satellite cell number moderately correlated mean muscle CSA in all trainees.69 These responses may be largely driven by hormonal differences as testosterone, estrogen, and progesterone have been shown to play roles in the regulation of myogenesis.68 In fact, one study compared 10 wk of resistance training in women who used and did not use oral contraceptives and showed that oral contraceptive users increased type I fiber size by ∼8.8% and type II fiber size by ∼20% whereas non-users increased type I fiber size by ∼6.4% and type II fiber size by ∼16.6%, respectively.70 These changes were accompanied by greater increases in satellite cell content and muscle regulatory factor 4 (MRF4) mRNA in oral contraceptive users.70 However, other studies have showed similar adaptations between oral contraceptive users and non-users.71
A widely recognized anabolic signaling pathway in skeletal muscle is the mammalian (or mechanistic) target of rapamycin (mTOR) pathway (primarily mTORC1). This pathway is highly sensitive to hormones such as insulin, IGF-1, androgens, growth factors, mechanosensing proteins, and amino acids72 mTOR pathway signaling is critical to muscular adaptations to training and is sensitive to the training stimulus in mediating increases in protein synthesis. Resistance exercise increases phosphorylation of several mTORC1 pathway intermediates 30 min to 5 h post exercise with a timeline corresponding to increased protein synthesis in men and women.46,72 In direct comparison, similar levels of phosphorylation of mTOR1 pathway intermediates has been shown between men and women 3 h post resistance exercise.72 Thus, it appears mTORC1 pathway signaling is a key regulator of protein synthesis in men and women following resistance exercise.
In highly trained populations, some unique observations have been made. Male bodybuilders demonstrate larger type I and II areas than female bodybuilders.64 Type I and II fiber type distribution was similar between male and female bodybuilders.64 A study examining elite female Olympic weightlifters showed that these athletes displayed the highest Type IIA fiber concentrations reported in the vastus lateralis possibly suggesting that the caliber of the athletes and the years of training influence fiber type distribution.73
The optimal expression of muscle strength depends on the physical properties of tendons. On average, women have been shown to have weaker tendons relative to men and attenuated training adaptations.74,75 For example, trained male distance runners were shown to have greater patellar and Achilles tendon CSA and patellar tendon stiffness than trained female distance runners.74,75 Miller et al. and colleagues also showed differences between men and women at rest by 45% and following exercise (unilateral leg ergometer kicking for 60 min at 67% workload maximum) by 53% where men had higher patellar tendon collagen fractional synthetic rate.76 No effects of menstrual cycle were observed. The authors suggested that estrogen and/or progesterone may alter the exercise-induced increase in collagen synthesis after an acute bout of exercise.76
Women show a high level of strength improvement and muscle adaptability to resistance training.65 Of interest, a recent case study documented the training of a 71-year-old female powerlifting champion who did not begin resistance training until the age of 63; thus showing remarkable improvements during an advanced age.77 Although absolute strength, power, and hypertrophy increases are greater in men, women have been shown to have similar relative strength and hypertrophy increases in lower-body strength, similar relative hypertrophy in the upper body, but higher relative upper-body strength increases in comparison to men.3 A meta-analysis showed 2%–26% increases in muscle hypertrophy, 3%–110% increases in upper-body strength, and 4%–140% increases in lower-body strength in women following a range of durations from 6 wk to 2 y of resistance training.78 The larger relative increase in upper-body strength in women shown in some studies likely reflects the initial training status and lower relative upper-body strength seen in women.3,78
The training programs, and implementation/supervision of training, is critical to the magnitude of adaptation. It is well known that a variety of resistance training programs can increase maximal strength in women (provided they target appropriate levels of progressive overload, specificity, and variation) especially since most studies examined previously untrained or recreationally trained women.78 We have shown large increases in 1 RM strength in women using periodized, total-body or regional splits, high-intensity (3 RM –10 RM and more) resistance training for multiple sets with varied rest intervals of a variety of multi- (Olympic lifts, basic strength, ballistics) and single-joint exercises for periods of 8 wk–36 wk.62,37, 79, 80, 81, 82, 83 For example, we showed > 20% increases in 1 RM squat, bench press, and high pull following 24 wk of RT.79 Squat jump and bench throw power, functional performance (box lift), and muscle endurance (squat, sit-ups, push-ups) increased highly as well.79 In another 24-wk study we showed 1 RM squat increases of 35%, bench press ∼29%, jump squat power of 19%, and 7%–17% increases in arm and thigh muscle CSA.81 This periodized approach produced superior strength improvements compared to single-set training,80,82 superior improvements compared to non-periodized training in female athletes,62 produced substantial performance enhancement alone and when combined with endurance training,37 and produced significant performance enhancement when combined with sprint/plyometric training, e.g., 1 RM squat, bench press, vertical jump height, and broad jump.83 Thus, a number of biomechanical, anthropometric, and physiological factors explain the muscle strength and power disparities between men and women but it appears hormonal differences (primarily testosterone) may be the largest contributing factor.
5. Implications for resistance training program design
In 1989, the NSCA published a consensus position stand on strength training in female athletes.12,13 The position stand did an excellent job providing the historical context of women and resistance training as well as describing socio-psychological and physiological differences in men and women of information known at the time with respect to strength, body composition, hormonal, muscle size, and fiber type differences.12 The panel of experts concluded that “training programs for female athletes should be organized around the sound principles of training as used by their male counterparts.“13 They highlighted the recommendation with 8 key points: 1) training should begin in junior high school (middle school) and high school; 2) training staff should be sensitive to the needs (i.e., menstrual cycle) of female athletes; 3) female athletes should continue to have strength training workouts during the competitive season; 4) female athlete should be encouraged to approach training with high levels of arousal; 5) training should focus on multi-joint free weight exercises; 6) female athletes should be training with an overall higher level of intensity and volume than previously thought; 7) total body should be trained with emphasis on the upper body; and 8) plyometrics should be monitored during the menstrual cycle.13 They also concluded that “due to similar physiological responses, it appears that males and females should train for strength in the same basic way, employing similar methodologies, programs and types of exercises.“13
In modern day, training principles for men and women still remain virtually the same with a few caveats (see Table 2). In fact, the latest position stand from the American College of Sports Medicine on Progression Models in Resistance Training for Healthy Adults gave resistance training recommendations for novice, intermediate and advanced trainees for increasing and maximizing muscle strength, hypertrophy, power, endurance, and motor performance that targeted both men and women.84 A recent meta-analysis has failed to identify any differences in the effects of sex on improvements in strength or hypertrophy during resistance training.85 This information will also serve the basis of the new NSCA position stand on resistance training in women; a seminal document extending the information presented in the original NSCA position stand.86 The comprehensive position statement is expected to help strength and conditioning practitioners improve sports performance, reduce injury risk, and enhance overall female athlete health and well-being through up-to-date research. Despite the fact that training recommendations between men and women are similar, there are some points that need to be addressed. As mentioned previously, it is appropriate to expand the focus on upper body strength training given the larger deficits seen between men and women in upper body strength compared to lower body strength.3 Thus, expanding the exercise selection, volume, or intensity may be appropriate especially for novice to intermediate-trained women.
Table 2.
Strength and power resistance training recommendations/considerations for female athletes.
|
Injury reduction risk is a significant part of the strength and conditioning program. Women have higher injury rate risks for certain types of injuries. For example, ACL injuries are more likely to occur in female than male athletes.87 During cutting tasks, ∼60% of female demonstrate a biomechanical deficit that could put them at a higher risk of injury.88 Women have several anatomical and physiological attributes that make them more susceptible to non-contact ACL injuries.87 Women have greater valgus stress (outward angulation) placed on the knee joint which increases susceptibility to knee injury. As knees may be viewed as the weaker link in the kinetic chain, proper instruction and training should focus on strengthening the trunk, hips, and lower extremities. It is recommended that integrated training methods consisting of resistance, corrective exercise, balance, speed and agility, and plyometric training be implemented to target specific deficits in the female body.89 Resistance training has been shown to prevent ACL injury in female athletes.90,91 Pre-screening or mobility/functional movement testing can reveal improper landing, squatting, jumping, and cutting maneuvers that make women more vulnerable to injury and training can be designed to emphasize these issues and correct deficits.
Another issue more prevalent in women is pelvic floor disorders (PFD). Weakness and loss of functionality of pelvic floor muscles can lead to a variety of conditions such as urinary incontinence, fecal incontinence, pelvic organ prolapse, pelvic pain and sexual dysfunction.92 High BMI and participation in strenuous exercise that involves high-impact landing, running, or jumping increases the risk of PFD.92 Pelvic floor muscle training should be considered an essential component of resistance training programs for women. It consists of a cycle of repetitive pelvic floor contractions and relaxations.93 Training can progress from gentle contractions of 6 s–10 s in the supine or seated position to more intense contractions for 3 s–6 s in different body positions (e.g., standing, quadruped, hip hinging).93 A general workout can include 1–3 different exercises performed for 1 sets–3 sets of 6 reps–10 reps with proper breathing and adequate rest intervals between reps and sets.
Another consideration is the menstrual cycle and subsequent hormonal fluctuations. As estrogen concentrations rise from the early to the late follicular phase, and again (as well as progesterone) in the mid-luteal phase, potential responses to the hormone flux and their interactions (antagonism) may be considered. Protein catabolism is highest during the mid-luteal phase than the early follicular phase when progesterone concentrations rise.52 In addition, estrogen has been shown to assist in skeletal muscle repair and recovery and impact energy metabolism and substrate utilization (e.g., greater fat oxidation).94 The structure of estradiol allows it to interact with plasma membrane bilayers to increase membrane stability, act like an antioxidant, and reduce lipid peroxidation and subsequent inflammation and muscle damage.52 At rest, energy expenditure is up to 11.5% greater during the luteal phase while estradiol and progesterone are elevated.94 Estradiol increases plasma glucose uptake during exercise via increased GLUT4 expression and translocation, increases glycogen synthase activity and has a glycogen sparing effect, increases fat oxidation rate during moderate-intensity exercise, and appears to help support muscle mass and strength during training possibly due to enhanced satellite cell activity.52 These effects help explain, in part, noted differences in recovery rates and substrate utilization during exercise in women compared to men.94 However, menstrual cycle differences are less consistent metabolically. Studies have shown similar strength, power, and endurance performance despite the phase of the menstrual cycle. The practical performance impact of the menstrual cycle is difficult to decipher because women with menstrual disturbances self-select into using hormonal contraceptives to reduce the negative impact. Thus, although menstrual cycle is a potential consideration, it may require an individualized approach to see how each female athlete responds to training at different phases and deals with other side effects.
6. Health benefits beyond performance
Beyond performance, resistance training offers numerous underappreciated health benefits for women, both physically and mentally.95 There is both established and emerging evidence, that resistance exercise supports many aspects of healthy aging including bone health, muscle health, mobility, cognitive function, metabolic health, cardiovascular health and cancer survivorship.95 While strength and performance improvements of resistance training are strongly associated with intensity and load, many of the health benefits of resistance training can be achieved at varying intensities and frequencies.95,96
The health benefits of resistance training for women cumulate in lower morbidity and mortality.95, 96, 97, 98, 99 Population-level research shows that although only 1 in 5 women (19.9%) report engaging in any regular muscle-strengthening exercise.96(averaging less than 1 session per week), performing muscle-strengthening exercise is associated with mortality reductions of 15%–46%.96, 97, 98, 99 Prospective population-level research based on 412 413 U.S. adults (age [44 ± 17] y) from 1997 through 2019 demonstrated that women who perform muscle-strengthening exercises have a mortality risk reduction of 19% (HR: 0.81; 95% CI: 0.76–0.85).96 Interestingly, resistance exercise appears even more beneficial for women as compared to men as observed mortality reductions in women of 19% exceeded the 11% seen in men.96
A 2022 meta-analysis of 6 studies demonstrated similar mortality reductions where compared with undertaking no resistance training, undertaking any amount of resistance training reduced the risk of all-cause mortality by 15% (RR of 6 studies = 0.85; 95% CI = 0.77, 0.93).97 This meta-analysis demonstrated a nonlinear dose-response between resistance training and the risk of all-cause mortality where a maximum risk reduction of 27% was observed at around 60 min per week of resistance training (RR = 0.74; 95% CI = 0.64, 0.86), with diminished mortality risk reductions at higher volumes.97
Highest mortality reductions associated with performing strength training were seen in linking the National Health Interview Survey to death certificate data in the National Death Index. In this cohort, only 9.6% of older adults age 65 and older (n = 30 162) performed strength training according to guidelines of 2 × per week.99 Older adults who performed resistance training had 46% lower odds of all-cause mortality than those who did not (adjusted odds ratio: 0.64; 95% CI: 0.57, 0.70; p < 0.001)- even after adjustment for past medical history and health behaviors.99 Mortality reductions observed with resistance training are independent of time spent performing aerobic exercise as shown in the Women’s Health Study.98 Over 12 y of follow-up, a moderate amount of time (1 min/wk–145 min/wk) of strength training was associated with 19%–29% lower all-cause mortality (independent of aerobic activity) across n = 28 879 women ([62.2 ± 6.8] y; BMI = [26.8 ± 5.3] kg·m−2).98
Taken together, several large population-level studies show a significant reduction in overall mortality for women who perform any amount of resistance exercise, especially those who meet guidelines of 2 × per week. Reductions in overall mortality can be attributed to improved health and disease-specific morbidly and mortality. In terms of cardiovascular mortality, undertaking any amount of resistance training reduced the risk of cardiovascular disease mortality by 19% (RR of 4 studies = 0.81; 95% CI = 0.66, 1.00) compared with undertaking no resistance training.97 Resistance training enhances cardiovascular health contributing to reduced cardiovascular mortality by reducing resting blood pressure, decreasing low-density lipoprotein cholesterol and triglycerides, and increasing high-density lipoprotein cholesterol.100
The role of resistance training and cancer has been addressed in a number of studies. Among several health benefits, the benefits of resistance exercise on cancer survival and quality of life are worth noting. Research has consistently shown the benefits of resistance training on cancer-related fatigue, muscle preservation, pain reduction, and quality in women with cancer.101, 102, 103, 104, 105, 106, 107 In patients undergoing cancer therapy, where muscle atrophy and strength decreases impair functional ability and quality of life, evidence supports resistance training as the most effective means to preserve muscle mass and strength.106,107 Protocols ranging from 12 wk to 52 wk of an intensity of 40%–80% 1 RM report varying outcomes.107 Moreover, as pain associated with cancer is one of the most debilitating symptoms, resistance exercise modulates pain reduction pathways relieving cancer pain through release of pain-relieving substances including beta-endorphins, anti-inflammatory cytokines, and endocannabinoids.105
In addition to quality-of-life benefits, resistance exercise has shown an encouraging impact on cancer-related survival. Women in the Aerobics Center Longitudinal Study who participated in resistance exercise during cancer survival had 33% a lower risk for all-cause mortality.108 Other research showed that compared with undertaking no resistance training, undertaking any amount of resistance training reduces the risk of cancer mortality by 14% (RR of 5 studies = 0.86; 95% CI = 0.78, 0.95),97 although other research did not find this association.109 Differences appear related to study design and factors related to the disease. For example, while many evaluations of resistance training in cancer are observational, a randomized controlled trial showed that adding exercise to standard chemotherapy may improve breast cancer outcomes including disease-free survival in women.110 Additionally, some factors were associated with stronger effects, including women who were overweight/obese, had stage II/III cancer, certain tumor types (including estrogen receptor-positive tumors, human epidermal growth factor receptor 2-positive tumors), therapies (including those who received taxane-based chemotherapies, and ≥ 85% of their planned chemotherapy.110
6.1. Mechanisms underlying health benefits
Mechanisms accountable for the positive effects of resistance exercise on health and survival relate to the physiological mechanisms of adaptions to resistance training described earlier. The disease-preventing benefits of resistance exercise relate to signaling cascades triggered by ‘exerkines’ -including autocrine, paracrine, and endocrine agents released in response to acute and chronic exercise.111 In addition to strength and performance benefits, exerkines trigger subsequent adaptions in the body’s tissues with relevance in the prevention and treatment of several diseases.111 The concomitant health effects in multiple target tissues simultaneously relate to the tissue-specific expression patterns of exerkine receptors.111 The resistance exercise-mediated health benefits relate to molecular signaling through exerkine target receptors at distinct tissues such as skeletal muscle, cardiac muscle, adipose, and liver tissue.111 Exerkines mediate structural and functional tissue adaption and inter-organ communication mediating the preventive and therapeutic value of exerkine signaling in various diseases.111
Among known impacts, exerkine signaling via the endocrine response of resistance exercise appears to reduce the risk for breast cancer development in women. Estradiol, the ovarian hormone that regulates the female reproductive system is now known to play an important protective role in skeletal muscle signaling and recovery with resistance exercise.111, 112, 113, 114, 115 Excess estradiol can be a risk factor for some cancer types in women. Yet the protective benefits of resistance exercise on cancer development and progression in women appear related to reduced expression and binding to cell receptors that attenuate uncontrolled proliferation of breast tissue116,117 and alterations in estrogenic action by hydroxylated estrogens, 16-Hydroestrone (C-16a) and C-2-hydroxylated estrogens.118 Anticancer molecular mechanisms may also relate to the metabolic profile of other hormones, reductions in systemic inflammation, inflammatory pathways (including the function of natural killer cells, decreases in acute-phase biomarkers of systemic inflammation and CRP levels), increased insulin sensitivity increased antioxidant actions, and improved immune surveillance with direct effects on the tumor.118,119 Mechanisms may also relate to changes in circulating concentrations of growth factors (including insulin-like growth factor-1 (IGF-1) concentration, insulin-like growth factor binding protein-3 (IGFBP-3) concentration, and IGF-1:IGFBP-3 ratio influencing tumor progression.119 Changes in these growth factors regulate the release of secondary factors from other organ sites such as the bone marrow, skeletal muscle, or liver which may alter ligand availability in the microenvironment of the tumor influencing tumorigenesis and metastasis.120 Moreover, evidence of the systemic benefits of resistance exercise may also include the nervous system which is involved in the initial and progressive process of carcinogenesis by inducing biochemical, physiological, and cellular modifications involved in the 'hallmarks' of cancer.121
Among other notable health benefits of resistance training for women are the metabolic and bone health benefits and pain reduction. Resistance training plays a role in the prevention and management of type 2 diabetes by decreasing visceral fat, reducing HbA1c, increasing the density of glucose transporter type 4, and improving insulin sensitivity.100 Resistance training is also known to promote bone development, with studies showing 1%–3% increase in bone mineral density.100 For example, a meta-analysis of 24 clinical trials demonstrated that resistance training significantly increased femoral neck BMD and lumbar spine BMD in postmenopausal women.122 Resistance training also has research supporting pain reduction in women,123,124 including those with arthritis and fibromyalgia.100,125 For example, 21 wk of progressive strength training improved perceived fatigue, depression, neck pain/neuromuscular function, and subjectively perceived symptoms in premenopausal women with fibromyalgia.125
In summary, the potential benefits of resistance training on women’s health are numerous resulting in reduced morbidity and mortality and improved quality of life. As most population-level research has studied only resistance training frequency, further research into the elements of program design, (progressive overload, variation, intensity, volume, type, and specificity), related to the performance benefits,126 is needed to further understand the full scope of the health benefits. Regardless, research has demonstrated clear health benefits related to resistance exercise with mechanisms related to acute responses and adaptations to training.
7. Mental health benefits
In addition to physical health, resistance training provides several mental health benefits for women, contributing to overall well-being and enhancing quality of life.123 In terms of general mental well-being, resistance exercise can also play a positive role in boosting confidence, self-worth, self-efficacy, self-perceptions, and self-esteem as strength goals are achieved.127, 128, 129 Positive benefits have been observed across stages of life including youth,127, 128, 129 post-partum,130 and older women.131 In the evaluation of mental health benefits of resistance training in women, it had the most favorable effect on mental health and body pain,123 with benefits extending across dimensions of emotional role function, social function, and physical role function.123
Similarly for mental health conditions, research has shown that resistance training reduces symptoms of anxiety and depression in both healthy and clinical populations,132, 133, 134 across ages.135,136 Even short-term resistance training (2 sessions per week for 6-wk) has been shown to reduce worry symptoms in female patients with generalized anxiety disorder.132 In young adult women, an 8 wk program significantly reduced anxiety symptoms.135 Similarly, in older women, resistance training (12 wk, 3 × /wk, 3 sets, 8 reps–12 reps, progressive, full body) reduced depressive and anxiety symptoms, regardless of age, muscular strength, and cognition function ([68 ± 8] y).136 Overall, meta-analysis confirms that resistance training significantly improves anxiety symptoms among both healthy participants and participants with a physical or mental illness, regardless of age, sex, and program design.134
Resistance training has demonstrated consistent benefits for reducing depression and depressive symptoms in women. Resistance exercise training was associated with a significant reduction in depressive symptoms in a meta-analysis of 33 clinical trials including 1 877 participants.135 Another meta-analysis of over 200 studies compared exercise modalities as a “treatment” for depression.137 Strength training was well-tolerated and more effective than other exercise modalities (walking, jogging and yoga were also effective).137 Resistance exercise appeared equally effective for people with and without comorbidities and with different baseline levels of depression.137 Interestingly, the effects of resistance exercise on reducing depression appeared larger for women than men.137 Program design (i.e., appropriate intensity and/or load may play a role in outcomes) may be an important factor in achieving the mental health benefits of resistance exercise for women, as 8 wk of exercise with bands may not be sufficient to improve mental functioning or quality of life in women.138
7.1. Mechanisms underlying health benefits
Mechanisms underlying the mental health benefits139,140 (anxiolytic and antidepressant effects) of resistance exercise may be attributable to several pathways.140 For example, they may involve monoamine neurotransmitters, immune-serotonin pathways, neurotrophic factors, neuroanatomical structure and function, neurovisceral integration, neuroplasticity, inflammation, oxidative stress, endocrine interactions, and heart-brain cross-talk.133,139,140 More specifically, mechanisms may relate to adaptive changes in neurotransmitter activity and receptor regulation including Serotonin (5-hydroxytryptamine, 5-HT) norepinephrine,139 gamma-aminobutyric acid (GABA) (an inhibitory neurotransmitter) and adenosine, (an inhibitory neuromodulator that affects synaptic dopamine and glutamate transmission),139 hypothalamic-pituitary-adrenal axis (HPA-axis) including cortisol and atrial natriuretic peptide (ANP),139 and chronic inflammatory processes including interleukin-1β and oxidative stress.139,140
Neuroplasticity, or the ability of the nervous system to adapt to stimuli through reorganization of structure, function, and connection appears to play a key role in the antidepressant mechanisms of training.140 It has been established, that stress-related mental disorders are partially associated with brain morphological abnormalities, such as reduced hippocampal volume.141 The hippocampus, embedded in the temporal lobe of the brain, is involved in learning, memory, and decision-making.36,39 Due to its vulnerability to health and disease, interventions to prevent and reverse hippocampal atrophy are being sought.36 Exercise is one such intervention through increasing regional cerebral blood flow and neurotrophic growth factors, and stimulating the neurogenesis.139 Exercise training, including resistance exercise training in women, has been shown to increase hippocampal volume,40,142 improving the structure and function of this region of the brain. Mechanisms are likely related to the regulation of brain-derived neurotrophic factor (BDNF) which is involved in the consolidation of memories, synaptic plasticity, and associative learning.139
Mental health benefits of resistance exercise extend to cognitive function, including academic performance, school-aged youth,143,144 attention and conflict,145 and alleviating cognitive decline and Alzheimer’s disease symptoms in older women.146 Of note, resistance exercise frequency, intensity, and duration are important factors for recovering cognitive decline in older women by not only improving symptoms but also attenuating the progression of neurodegeneration in Alzheimer’s disease.146 Mechanisms appear to relate to reducing Aβ deposition and plaques, neurofibrillary tangles, and neuroinflammation, as well as for increasing levels of neurotrophic factors and neurogenesis, leading to improvements in memory deficits and cognitive decline.146
8. Conclusion
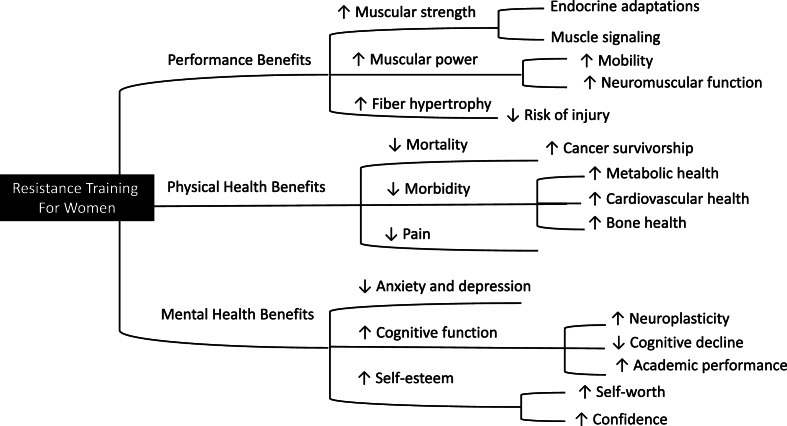
Resistance training has emerged as a critical element of fitness for women, offering a wide array of benefits that extend beyond traditional goals of strength and performance enhancement (see Fig. 2). Regular resistance exercise leads to significant improvements in muscular strength, power, and overall athletic performance, comparable to those observed in men, albeit through different physiological mechanisms involving sex hormones such as estrogen, testosterone, and IGF-1. Moreover, women experience unique adaptations in muscle hypertrophy, substrate utilization, and recovery, contributing to these outcomes.
Fig. 2.
Benefits and adaptations from resistance training participation for women.
Understanding the underlying mechanisms of these adaptations is crucial for optimizing training programs tailored to women’s specific needs. These include neuroendocrine responses and immune system involvement, which together drive the health benefits of resistance training.
Beyond physical performance, resistance training offers numerous health benefits, particularly in metabolic health, bone density, cardiovascular function, and mental health. Notably, in areas such as mortality risk reduction and mental health, the benefits of resistance exercise in women appear to exceed those in men. Regular participation in resistance training is associated with lower risks of all-cause mortality, cardiovascular disease, and mental health disorders, underscoring its vital role in promoting long-term health and well-being for women of all ages.
8.1. Future directions
While substantial progress has been made in understanding the benefits of resistance training for women, several areas require further exploration. Future research should focus on the long-term effects of resistance training, particularly how sustained exercise over decades impacts health outcomes in older women. Additionally, more studies are needed to explore the optimal training variables—such as frequency, intensity, and volume—that maximize benefits for different age groups and fitness levels.
Investigating the psychological and social factors influencing women’s participation in resistance training is another critical area. Understanding these factors could help design more effective programs that enhance engagement and adherence, particularly among populations with historically low participation rates.
Moreover, research should examine the intersection of resistance training with other health interventions, such as dietary modifications and aerobic exercise, to determine the most effective combinations for improving overall health outcomes. The potential for resistance training to mitigate the effects of chronic diseases, such as cancer and cardiovascular disease, also warrants deeper investigation, particularly regarding the molecular mechanisms involved.
Finally, as societal norms continue to evolve, it will be essential to study the changing perceptions of women’s strength and muscularity and their impact on fitness trends. Addressing these research gaps will further enhance the role of resistance training in women’s health and performance, paving the way for more personalized and effective interventions.
CRediT authorship contribution statement
William J. Kraemer: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Formal analysis, Conceptualization. Maren S. Fragala: Writing – review & editing, Writing – original draft, Visualization, Validation, Formal analysis, Conceptualization. Nicholas A. Ratamess: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors have no conflict of interest to declare in this paper’s content or topic. During the preparation of this work, the authors utilized ChatGPT's image generation tool to create depictions of women performing resistance exercises. This approach was chosen to illustrate key concepts without using images of real human subjects, ensuring ethical considerations, privacy protection, and compliance with publication guidelines. The generated figures serve to visually support the concepts presented without the need for actual participant photography or consent. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Acknowledgment statement
The authors would like to express their gratitude to our colleagues whose invaluable contributions and dedication have significantly influenced the understanding and importance of this topic. We deeply appreciate their collaborative efforts and the foundational work they have contributed to the many studies that have advanced this critical area of research.
Footnotes
Peer review under the responsibility of Editorial Board of Sports Medicine and Health Science
References
- 1.Staron R.S., Hagerman F.C., Hikida R.S., et al. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48(5):623–629. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer W.J., Gordon S.E., Fleck S.J., et al. Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int J Sports Med. 1991;12(2):228–235. doi: 10.1055/s-2007-1024673. [DOI] [PubMed] [Google Scholar]
- 3.Nuzzo J.L. Narrative review of sex differences in muscle strength, endurance, activation, size, fiber type, and strength training participation rates, preferences, motivations, injuries, and neuromuscular adaptations. J Strength Cond Res. 2023;37(2):494–536. doi: 10.1519/JSC.0000000000004329. [DOI] [PubMed] [Google Scholar]
- 4.Bartolomei S., Grillone G., Di Michele R., Cortesi M. A comparison between male and female athletes in relative strength and power performances. J Funct Morphol Kinesiol. 2021;6(1):17. doi: 10.3390/jfmk6010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuzzo J.L. Sex differences in skeletal muscle fiber types: a meta-analysis. Clin Anat. 2024;37(1):81–91. doi: 10.1002/ca.24091. [DOI] [PubMed] [Google Scholar]
- 6.Shurley J., Felkar V., Greviskes L.C., Todd J.S. Historical and social considerations of strength training for female athletes. Strength Cond J. 2019;42(4):22–35. doi: 10.1519/SSC.0000000000000478. [DOI] [Google Scholar]
- 7.Latham A. The history of a habit: jogging as a palliative to sedentariness in 1960s America. Cult Geogr. 2015;22(1):103–126. doi: 10.1177/1474474013491927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper K.H. The history of aerobics (50 years and still counting) Res Q Exerc Sport. 2018;89(2):129–134. doi: 10.1080/02701367.2018.1452469. [DOI] [PubMed] [Google Scholar]
- 9.Shurley J.P., Todd J.S. “The strength of Nebraska”: boyd Epley, Husker Power, and the formation of the strength coaching profession. J Strength Cond Res. 2012;26(12):3177–3188. doi: 10.1519/JSC.0b013e31823c4690. [DOI] [PubMed] [Google Scholar]
- 10.Shurley J.P., Todd J., Todd T. University of Texas Press; 2019. Strength Coaching in America: A History of the Innovation that Transformed Sports. [Google Scholar]
- 11.Associations NFoSHS. 2022-23 high school athletics participation Survey. 2023. September 8, 2023. https://www.nfhs.org/sports-resource-content/high-school-participation-survey-archive/
- 12.Holloway J.B., Gater D., Ritchie M., et al. Strength training for female athletes: a position paper: Part I. Strength Cond J. 1989;11(4):43–51. doi: 10.1519/0744-0049(1989)011<0043:stffaa>2.3.co;2. [DOI] [Google Scholar]
- 13.Holloway J.B., Gater D., Ritchie M., et al. Strength training for female athletes: a position paper: Part II. Strength Cond J. 1989;11(5):26–36. doi: 10.1519/0744-0049(1989)011<0029:stffaa>2.3.co;2. [DOI] [Google Scholar]
- 14.National Strength and Conditioning Association N.S.C.A. Position stand on resistance training for female athletes. National Strength Cond J. 1991;13(2):36–39. doi: 10.1519/SSC.0b013e3181b9c34c. [DOI] [Google Scholar]
- 15.Bradsell H., Frank R.M. Anterior cruciate ligament injury prevention. Ann Jt. 2022;7:1. doi: 10.21037/aoj-2020-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Malley L., Greenwood S. Female coaches in strength and conditioning – why so few? Strength Cond J. 2018;40(6):40–48. doi: 10.1519/SSC.0000000000000401. [DOI] [Google Scholar]
- 17.Hurley K.S., Flippin K.J., Blom L.C., Bolin J.E., Hoover D.L., Judge L.W. Practices, perceived benefits, and barriers to resistance training among women enrolled in college. Int J Exerc Sci. 2018;11(5):226–238. doi: 10.70252/ZRMT3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burrup R., Tucker L.A., Jd L.E.C., Bailey B.W. Strength training and body composition in middle-age women. J Sports Med Phys Fit. 2018;58(1-2):82–91. doi: 10.23736/S0022-4707.17.06706-8. [DOI] [PubMed] [Google Scholar]
- 19.Isenmann E., Kaluza D., Havers T., et al. Resistance training alters body composition in middle-aged women depending on menopause - a 20-week control trial. BMC Womens Health. 2023;23(1):526. doi: 10.1186/s12905-023-02671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santagnello S.B., Martins F.M., de Oliveira Junior G.N., et al. Resistance training-induced gains in muscle strength and power mediate the improvement in walking speed in middle-aged women who are breast cancer survivors. J Strength Cond Res. 2024;38(4):773–782. doi: 10.1519/JSC.0000000000004020. [DOI] [PubMed] [Google Scholar]
- 21.Mayhew J.L., Gross Gross P.M. Body composition changes in young women with high resistance weight training. Res Q. 1974;45(4):433–440. [PubMed] [Google Scholar]
- 22.Brown C.H., Wilmore J.H. The effects of maximal resistance training on the strength and body composition of women athletes. Med Sci Sports. 1974;6(3):174–177. [PubMed] [Google Scholar]
- 23.Kraemer W.J., Ratamess N.A., Newman T.H. Human Kinetics; 2024. Developing the Athlete: An Applied Sport Science Roadmap for Optimizing Performance. [Google Scholar]
- 24.Suchomel T.J., Nimphius S., Stone M.H. The importance of muscular strength in athletic performance. Sports Med. 2016;46(10):1419–1449. doi: 10.1007/s40279-016-0486-0. [DOI] [PubMed] [Google Scholar]
- 25.Hunter S.K., Edward F. Adolph Distinguished Lecture. Age and sex differences in the limits of human performance: fatigability and real-world data. J Appl Physiol (1985) 2024;136(4):659–676. doi: 10.1152/japplphysiol.00866.2023. [DOI] [PubMed] [Google Scholar]
- 26.Laubach L.L. Comparative muscular strength of men and women: a review of the literature. Aviat Space Environ Med. 1976;47(5):534–542. [PubMed] [Google Scholar]
- 27.Staron R.S., Karapondo D.L., Kraemer W.J., et al. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol (1985) 1994;76(3):1247–1255. doi: 10.1152/jappl.1994.76.3.1247. [DOI] [PubMed] [Google Scholar]
- 28.Bishop P., Cureton K., Collins M. Sex difference in muscular strength in equally-trained men and women. Ergonomics. 1987;30(4):675–687. doi: 10.1080/00140138708969760. [DOI] [PubMed] [Google Scholar]
- 29.Alegre L.M., Lara A.J., Elvira J.L., Aguado X. Muscle morphology and jump performance: gender and intermuscular variability. J Sports Med Phys Fit. 2009;49(3):320–326. [PubMed] [Google Scholar]
- 30.Sole C.J., Suchomel T.J., Stone M.H. Preliminary scale of reference values for evaluating reactive strength index-modified in male and female NCAA Division I athletes. Sports. 2018;6(4):133. doi: 10.3390/sports6040133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiecek M., Szymura J., Maciejczyk M., Cempla J., Szygula Z. Effect of sex and menstrual cycle in women on starting speed, anaerobic endurance and muscle power. Phys Int. 2016;103(1):127–132. doi: 10.1556/036.103.2016.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Nuell S., Illera-Dominguez V., Carmona G., et al. Sex differences in thigh muscle volumes, sprint performance and mechanical properties in national-level sprinters. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0224862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardinale M., Stone M.H. Is testosterone influencing explosive performance? J Strength Cond Res. 2006;20(1):103–107. doi: 10.1519/R-16864.1. [DOI] [PubMed] [Google Scholar]
- 34.Bezuglov E., Ahmetov I.I., Lazarev A., et al. The relationship of testosterone levels with sprint performance in young professional track and field athletes. Physiol Behav. 2023;271 doi: 10.1016/j.physbeh.2023.114344. [DOI] [PubMed] [Google Scholar]
- 35.Comfort P., McMahon J.J., Lake J.P., Ripley N.J., Triplett N.T., Haff G.G. Relative strength explains the differences in multi-joint rapid force production between sexes. PLoS One. 2024;19(2) doi: 10.1371/journal.pone.0296877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand K.S., Dhikav V. Hippocampus in health and disease: an overview. Ann Indian Acad Neurol. 2012;15(4):239–246. doi: 10.4103/0972-2327.104323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendrickson N.R., Sharp M.A., Alemany J.A., et al. Combined resistance and endurance training improves physical capacity and performance on tactical occupational tasks. Eur J Appl Physiol. 2010;109(6):1197–1208. doi: 10.1007/s00421-010-1462-2. [DOI] [PubMed] [Google Scholar]
- 38.Ratamess N.A., Faigenbaum A.D., Hoffman J.R., Kang J. Self-selected resistance training intensity in healthy women: the influence of a personal trainer. J Strength Cond Res. 2008;22(1):103–111. doi: 10.1519/JSC.0b013e31815f29cc. [DOI] [PubMed] [Google Scholar]
- 39.Fogwe L.A., Reddy V., Mesfin F.B. Neuroanatomy, Hippocampus. StatPearls. 2024 [PubMed] [Google Scholar]
- 40.Erickson K.I., Voss M.W., Prakash R.S., et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Handelsman D.J., Hirschberg A.L., Bermon S. Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr Rev. 2018;39(5):803–829. doi: 10.1210/er.2018-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraemer W.J., Ratamess N.A. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–361. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- 43.Vingren J.L., Kraemer W.J., Hatfield D.L., et al. Effect of resistance exercise on muscle steroid receptor protein content in strength-trained men and women. Steroids. 2009;74(13-14):1033–1039. doi: 10.1016/j.steroids.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Kraemer W.J., Ratamess N.A., Nindl B.C. Recovery responses of testosterone, growth hormone, and IGF-1 after resistance exercise. J Appl Physiol (1985) 2017;122(3):549–558. doi: 10.1152/japplphysiol.00599.2016. [DOI] [PubMed] [Google Scholar]
- 45.Kraemer W.J., Ratamess N.A., Hymer W.C., Nindl B.C., Fragala M.S. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise. Front Endocrinol. 2020;11:33. doi: 10.3389/fendo.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratamess N.A. second ed. Wolters-Kluwer Lippincott-Williams and Wilkins; 2022. The ACSM’s Foundations of Strength Training and Conditioning. [Google Scholar]
- 47.Bhasin S., Hatfield D.L., Hoffman J.R., et al. Anabolic-androgenic steroid use in sports, health, and society. Med Sci Sports Exerc. 2021;53(8):1778–1794. doi: 10.1249/MSS.0000000000002670. [DOI] [PubMed] [Google Scholar]
- 48.Guilherme J., Semenova E.A., Borisov O.V., et al. Genomic predictors of testosterone levels are associated with muscle fiber size and strength. Eur J Appl Physiol. 2022;122(2):415–423. doi: 10.1007/s00421-021-04851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bermon S., Garnier P.Y. Serum androgen levels and their relation to performance in track and field: mass spectrometry results from 2127 observations in male and female elite athletes. Br J Sports Med. 2017;51(17):1309–1314. doi: 10.1136/bjsports-2017-097792. [DOI] [PubMed] [Google Scholar]
- 50.Hirschberg A.L., Elings Knutsson J., Helge T., et al. Effects of moderately increased testosterone concentration on physical performance in young women: a double blind, randomised, placebo controlled study. Br J Sports Med. 2020;54(10):599–604. doi: 10.1136/bjsports-2018-100525. [DOI] [PubMed] [Google Scholar]
- 51.Fragala M.S., Kraemer W.J., Denegar C.R., Maresh C.M., Mastro A.M., Volek J.S. Neuroendocrine-immune interactions and responses to exercise. Sports Med. 2011;41(8):621–639. doi: 10.2165/11590430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Oosthuyse T., Strauss J.A., Hackney A.C. Understanding the female athlete: molecular mechanisms underpinning menstrual phase differences in exercise metabolism. Eur J Appl Physiol. 2023;123(3):423–450. doi: 10.1007/s00421-022-05090-3. [DOI] [PubMed] [Google Scholar]
- 53.Pellegrino A., Tiidus P.M., Vandenboom R. Mechanisms of estrogen influence on skeletal muscle: mass, regeneration, and mitochondrial function. Sports Med. 2022;52(12):2853–2869. doi: 10.1007/s40279-022-01733-9. [DOI] [PubMed] [Google Scholar]
- 54.McNulty K.L., Elliott-Sale K.J., Dolan E., et al. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: a systematic review and meta-analysis. Sports Med. 2020;50(10):1813–1827. doi: 10.1007/s40279-020-01319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraemer W.J. Endocrine responses to resistance exercise. Med Sci Sports Exerc. 1988;20(5 Suppl):S152–S157. doi: 10.1249/00005768-198810001-00011. [DOI] [PubMed] [Google Scholar]
- 56.Hatfield D.L., Kraemer W.J., Volek J.S., et al. Hormonal stress responses of growth hormone and insulin-like growth factor-I in highly resistance trained women and men. Growth Hormone IGF Res. 2021;59 doi: 10.1016/j.ghir.2021.101407. [DOI] [PubMed] [Google Scholar]
- 57.Kraemer W.J., Staron R.S., Hagerman F.C., et al. The effects of short-term resistance training on endocrine function in men and women. Eur J Appl Physiol Occup Physiol. 1998;78(1):69–76. doi: 10.1007/s004210050389. [DOI] [PubMed] [Google Scholar]
- 58.Linnamo V., Pakarinen A., Komi P.V., Kraemer W.J., Hakkinen K. Acute hormonal responses to submaximal and maximal heavy resistance and explosive exercises in men and women. J Strength Cond Res. 2005;19(3):566–571. doi: 10.1519/R-15404.1. [DOI] [PubMed] [Google Scholar]
- 59.Hymer W.C., Kraemer W.J., Nindl B.C., et al. Characteristics of circulating growth hormone in women after acute heavy resistance exercise. Am J Physiol Endocrinol Metab. 2001;281(4):E878–E887. doi: 10.1152/ajpendo.2001.281.4.E878. [DOI] [PubMed] [Google Scholar]
- 60.Kraemer W.J., Nindl B.C., Marx J.O., et al. Chronic resistance training in women potentiates growth hormone in vivo bioactivity: characterization of molecular mass variants. Am J Physiol Endocrinol Metab. 2006;291(6):E1177–E1187. doi: 10.1152/ajpendo.00042.2006. [DOI] [PubMed] [Google Scholar]
- 61.Pierce J.R., Martin B.J., Rarick K.R., et al. Growth Hormone and insulin-like growth factor-I molecular weight isoform responses to resistance exercise are sex-dependent. Front Endocrinol. 2020;11:571. doi: 10.3389/fendo.2020.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kraemer W.J., Hakkinen K., Triplett-Mcbride N.T., et al. Physiological changes with periodized resistance training in women tennis players. Med Sci Sports Exerc. 2003;35(1):157–168. doi: 10.1097/00005768-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 63.Sale D.G., MacDougall J.D., Alway S.E., Sutton J.R. Voluntary strength and muscle characteristics in untrained men and women and male bodybuilders. J Appl Physiol (1985) 1987;62(5):1786–1793. doi: 10.1152/jappl.1987.62.5.1786. [DOI] [PubMed] [Google Scholar]
- 64.Alway S.E., Grumbt W.H., Gonyea W.J., Stray-Gundersen J. Contrasts in muscle and myofibers of elite male and female bodybuilders. J Appl Physiol (1985) 1989;67(1):24–31. doi: 10.1152/jappl.1989.67.1.24. [DOI] [PubMed] [Google Scholar]
- 65.Staron R.S., Leonardi M.J., Karapondo D.L., et al. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol (1985) 1991;70(2):631–640. doi: 10.1152/jappl.1991.70.2.631. [DOI] [PubMed] [Google Scholar]
- 66.Abou Sawan S., Hodson N., Babits P., Malowany J.M., Kumbhare D., Moore D.R. Satellite cell and myonuclear accretion is related to training-induced skeletal muscle fiber hypertrophy in young males and females. J Appl Physiol (1985) 2021;131(3):871–880. doi: 10.1152/japplphysiol.00424.2021. [DOI] [PubMed] [Google Scholar]
- 67.Horwath O., Moberg M., Larsen F.J., Philp A., Apro W., Ekblom B. Influence of sex and fiber type on the satellite cell pool in human skeletal muscle. Scand J Med Sci Sports. 2021;31(2):303–312. doi: 10.1111/sms.13848. [DOI] [PubMed] [Google Scholar]
- 68.Beaudry K., De Lisio M. Sex-Based differences in muscle stem cell regulation following exercise. Exerc Sport Sci Rev. 2024;52(3):87–94. doi: 10.1249/JES.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 69.Smith M.A., Sexton C.L., Smith K.A., et al. Molecular predictors of resistance training outcomes in young untrained female adults. J Appl Physiol (1985) 2023;134(3):491–507. doi: 10.1152/japplphysiol.00605.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oxfeldt M., Dalgaard L.B., Jorgensen E.B., et al. Molecular markers of skeletal muscle hypertrophy following 10 wk of resistance training in oral contraceptive users and nonusers. J Appl Physiol (1985) 2020;129(6):1355–1364. doi: 10.1152/japplphysiol.00562.2020. [DOI] [PubMed] [Google Scholar]
- 71.Dalgaard L.B., Jorgensen E.B., Oxfeldt M., et al. Influence of second generation oral contraceptive use on adaptations to resistance training in young untrained women. J Strength Cond Res. 2022;36(7):1801–1809. doi: 10.1519/JSC.0000000000003735. [DOI] [PubMed] [Google Scholar]
- 72.Duplanty A.A., Budnar R.G., Luk H.Y., et al. Effect of acute alcohol ingestion on resistance exercise-induced mTORC1 signaling in human muscle. J Strength Cond Res. 2017;31(1):54–61. doi: 10.1519/JSC.0000000000001468. [DOI] [PubMed] [Google Scholar]
- 73.Serrano N., Colenso-Semple L.M., Lazauskus K.K., et al. Extraordinary fast-twitch fiber abundance in elite weightlifters. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0207975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Westh E., Kongsgaard M., Bojsen-Moller J., et al. Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports. 2008;18(1):23–30. doi: 10.1111/j.1600-0838.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 75.Magnusson S.P., Hansen M., Langberg H., et al. The adaptability of tendon to loading differs in men and women. Int J Exp Pathol. 2007;88(4):237–240. doi: 10.1111/j.1365-2613.2007.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller B.F., Hansen M., Olesen J.L., et al. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol (1985) 2007;102(2):541–546. doi: 10.1152/japplphysiol.00797.2006. [DOI] [PubMed] [Google Scholar]
- 77.Fuchs C.J., Trommelen J., Weijzen M.E.G., et al. Becoming a world champion powerlifter at 71 years of age: it is never too late to start exercising. Int J Sport Nutr Exerc Metabol. 2024;34(4):223–231. doi: 10.1123/ijsnem.2023-0230. [DOI] [PubMed] [Google Scholar]
- 78.Roberts B.M., Nuckols G., Krieger J.W. Sex differences in resistance training: a systematic review and meta-analysis. J Strength Cond Res. 2020;34(5):1448–1460. doi: 10.1519/JSC.0000000000003521. [DOI] [PubMed] [Google Scholar]
- 79.Kraemer W.J., Mazzetti S.A., Nindl B.C., et al. Effect of resistance training on women’s strength/power and occupational performances. Med Sci Sports Exerc. 2001;33(6):1011–1025. doi: 10.1097/00005768-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 80.Kraemer W.J., Ratamess N., Fry A.C., et al. Influence of resistance training volume and periodization on physiological and performance adaptations in collegiate women tennis players. Am J Sports Med. 2000;28(5):626–633. doi: 10.1177/03635465000280050201. [DOI] [PubMed] [Google Scholar]
- 81.Kraemer W.J., Nindl B.C., Ratamess N.A., et al. Changes in muscle hypertrophy in women with periodized resistance training. Med Sci Sports Exerc. 2004;36(4):697–708. doi: 10.1249/01.mss.0000122734.25411.cf. [DOI] [PubMed] [Google Scholar]
- 82.Marx J.O., Ratamess N.A., Nindl B.C., et al. Low-volume circuit versus high-volume periodized resistance training in women. Med Sci Sports Exerc. 2001;33(4):635–643. doi: 10.1097/00005768-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 83.Ratamess N.A., Kraemer W.J., Volek J.S., et al. The effects of ten weeks of resistance and combined plyometric/sprint training with the Meridian Elyte athletic shoe on muscular performance in women. J Strength Cond Res. 2007;21(3):882–887. doi: 10.1519/R-50512.1. [DOI] [PubMed] [Google Scholar]
- 84.American College of Sports M. American College of Sports Medicine Position Stand Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 85.Swinton P.A., Schoenfeld B.J., Murphy A. Dose-response modelling of resistance exercise across outcome domains in strength and conditioning: a meta-analysis. Sports Med. 2024;54(6):1579–1594. doi: 10.1007/s40279-024-02006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dabbs N.C., Binkley H., Bott C., et al. Strength and conditioning of female athletes: position statement from the National Strength and Conditioning Association. J Strength Cond Res. 2025 In Press. [Google Scholar]
- 87.Hewett T.E., Myer G.D., Ford K.R., Paterno M.V., Quatman C.E. Mechanisms, prediction, and prevention of ACL injuries: cut risk with three sharpened and validated tools. J Orthop Res. 2016;34(11):1843–1855. doi: 10.1002/jor.23414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pappas E., Shiyko M.P., Ford K.R., Myer G.D., Hewett T.E. Biomechanical deficit profiles associated with ACL injury risk in female athletes. Med Sci Sports Exerc. 2016;48(1):107–113. doi: 10.1249/MSS.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Myer G.D., Sugimoto D., Thomas S., Hewett T.E. The influence of age on the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a meta-analysis. Am J Sports Med. 2013;41(1):203–215. doi: 10.1177/0363546512460637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noyes F.R., Barber Westin SD. Anterior cruciate ligament injury prevention training in female athletes: a systematic review of injury reduction and results of athletic performance tests. Sport Health. 2012;4(1):36–46. doi: 10.1177/1941738111430203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mancino F., Kayani B., Gabr A., Fontalis A., Plastow R., Haddad F.S. Anterior cruciate ligament injuries in female athletes: risk factors and strategies for prevention. Bone Jt Open. 2024;5(2):94–100. doi: 10.1302/2633-1462.52.BJO-2023-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu J.M., Vaughan C.P., Goode P.S., et al. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014;123(1):141–148. doi: 10.1097/AOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rebullido T.R., Stracciolini A. Pelvic floor dysfunction in female athletes: is relative energy deficiency in sport a risk factor? Curr Sports Med Rep. 2019;18(7):255–257. doi: 10.1249/JSR.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 94.Cabre H.E., Gould L.M., Redman L.M., Smith-Ryan A.E. Effects of the menstrual cycle and hormonal contraceptive use on metabolic outcomes, strength performance, and recovery: a narrative review. Metabolites. 2024;14(7) doi: 10.3390/metabo14070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abou Sawan S., Nunes E.A., Lim C., McKendry J., Phillips S.M. The health benefits of resistance exercise: beyond hypertrophy and big weights. Exerc Sport Mov. 2023;1(11) doi: 10.1249/ESM.0000000000000001. [DOI] [Google Scholar]
- 96.Ji H., Gulati M., Huang T.Y., et al. Sex differences in association of physical activity with all-cause and cardiovascular mortality. J Am Coll Cardiol. 2024;83(8):783–793. doi: 10.1016/j.jacc.2023.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shailendra P., Baldock K.L., Li L.S.K., Bennie J.A., Boyle T. Resistance training and mortality risk: a systematic review and meta-analysis. Am J Prev Med. 2022;63(2):277–285. doi: 10.1016/j.amepre.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 98.Kamada M., Shiroma E.J., Buring J.E., Miyachi M., Lee I.M. Strength training and all-cause, cardiovascular disease, and cancer mortality in older women: a cohort study. J Am Heart Assoc. 2017;6(11) doi: 10.1161/JAHA.117.007677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kraschnewski J.L., Sciamanna C.N., Poger J.M., et al. Is strength training associated with mortality benefits? A 15year cohort study of US older adults. Prev Med. 2016;87:121–127. doi: 10.1016/j.ypmed.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 100.Westcott W.L. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep. 2012;11(4):209–216. doi: 10.1249/JSR.0b013e31825dabb8. [DOI] [PubMed] [Google Scholar]
- 101.Steindorf K., Schmidt M.E., Klassen O., et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol. 2014;25(11):2237–2243. doi: 10.1093/annonc/mdu374. [DOI] [PubMed] [Google Scholar]
- 102.Martinez-Vizcaino V., Cavero-Redondo I., Reina-Gutierrez S., et al. Comparative effects of different types of exercise on health-related quality of life during and after active cancer treatment: a systematic review and network meta-analysis. J Sport Health Sci. 2023;12(6):726–738. doi: 10.1016/j.jshs.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cramp F., James A., Lambert J. The effects of resistance training on quality of life in cancer: a systematic literature review and meta-analysis. Support Care Cancer. 2010;18(11):1367–1376. doi: 10.1007/s00520-010-0904-z. [DOI] [PubMed] [Google Scholar]
- 104.Mavropalias G., Sim M., Taaffe D.R., et al. Exercise medicine for cancer cachexia: targeted exercise to counteract mechanisms and treatment side effects. J Cancer Res Clin Oncol. 2022;148(6):1389–1406. doi: 10.1007/s00432-022-03927-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang Y., Angeletti P.C., Hoffman A.J. Investigating the physiological mechanisms between resistance training and pain relief in the cancer population: a literature review. J Cancer Ther. 2023;14(2):80–101. doi: 10.4236/jct.2023.142008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lakicevic N., Ficarra S., Ortega-Gomez S., et al. One more rep! The case for resistance training in young cancer survivors. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1284052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Padilha C.S., Marinello P.C., Galvao D.A., et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv. 2017;11(3):339–349. doi: 10.1007/s11764-016-0592-x. [DOI] [PubMed] [Google Scholar]
- 108.Hardee J.P., Porter R.R., Sui X., et al. The effect of resistance exercise on all-cause mortality in cancer survivors. Mayo Clin Proc. 2014;89(8):1108–1115. doi: 10.1016/j.mayocp.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saeidifard F., Medina-Inojosa J.R., West C.P., et al. The association of resistance training with mortality: a systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26(15):1647–1665. doi: 10.1177/2047487319850718. [DOI] [PubMed] [Google Scholar]
- 110.Courneya K.S., Segal R.J., McKenzie D.C., et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46(9):1744–1751. doi: 10.1249/MSS.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 111.Walzik D., Wences Chirino T.Y., Zimmer P., Joisten N. Molecular insights of exercise therapy in disease prevention and treatment. Signal Transduct Targeted Ther. 2024;9(1):138. doi: 10.1038/s41392-024-01841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chidi-Ogbolu N., Baar K. Effect of estrogen on musculoskeletal performance and injury risk. Front Physiol. 2018;9:1834. doi: 10.3389/fphys.2018.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paroo Z., Dipchand E.S., Noble E.G. Estrogen attenuates postexercise HSP70 expression in skeletal muscle. Am J Physiol Cell Physiol. 2002;282(2):C245–C251. doi: 10.1152/ajpcell.00336.2001. [DOI] [PubMed] [Google Scholar]
- 114.Enns D.L., Tiidus P.M. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40(1):41–58. doi: 10.2165/11319760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 115.Hansen M. Female hormones: do they influence muscle and tendon protein metabolism? Proc Nutr Soc. 2018;77(1):32–41. doi: 10.1017/S0029665117001951. [DOI] [PubMed] [Google Scholar]
- 116.Tian J.M., Ran B., Zhang C.L., Yan D.M., Li X.H. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Braz J Med Biol Res. 2018;23(3):1–7. doi: 10.1590/1414-431X20175612. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garcia-Chico C., Lopez-Ortiz S., Penin-Grandes S., et al. Physical exercise and the hallmarks of breast cancer: a narrative review. Cancers. 2023;15(1):324. doi: 10.3390/cancers15010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spanoudaki M., Giaginis C., Karafyllaki D., et al. Exercise as a promising agent against cancer: evaluating its anti-cancer molecular mechanisms. Cancers. 2023;15(21):5135. doi: 10.3390/cancers15215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Betof A.S., Dewhirst M.W., Jones L.W. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. 2013;30:S75–S87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koelwyn G.J., Quail D.F., Zhang X., White R.M., Jones L.W. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;17(10):620–632. doi: 10.1038/nrc.2017.78. [DOI] [PubMed] [Google Scholar]
- 121.Torres-Juarez K.V., Queiroga F.L., Romero-Romero L.P. The nervous system as a regulator of cancer hallmarks: insights into therapeutic implications. Cancers. 2022;14(18):4372. doi: 10.3390/cancers14184372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao R., Zhao M., Xu Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: a meta-analysis. Osteoporos Int. 2015;26(5):1605–1618. doi: 10.1007/s00198-015-3034-0. [DOI] [PubMed] [Google Scholar]
- 123.Hart P.D., Buck D.J. The effect of resistance training on health-related quality of life in older adults: systematic review and meta-analysis. Health Promot Perspect. 2019;9(1):1–12. doi: 10.15171/hpp.2019.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nestler K., Witzki A., Rohde U., Ruther T., Tofaute K.A., Leyk D. Strength training for women as a vehicle for health promotion at work. Dtsch Arztebl Int. 2017;114(26):439–446. doi: 10.3238/arztebl.2017.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hakkinen A., Hakkinen K., Hannonen P., Alen M. Strength training induced adaptations in neuromuscular function of premenopausal women with fibromyalgia: comparison with healthy women. Ann Rheum Dis. 2001;60(1):21–26. doi: 10.1136/ard.60.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kraemer W.J., Ratamess N.A., French D.N. Resistance training for health and performance. Curr Sports Med Rep. 2002;1(3):165–171. doi: 10.1249/00149619-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 127.Collins H., Booth J.N., Duncan A., Fawkner S., Niven A. The effect of resistance training interventions on ‘the self’ in youth: a systematic review and meta-analysis. Sports Med - Open. 2019;5:29. doi: 10.1186/s40798-019-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holloway J.B., Beuter A., Duda J.L. Self-efficacy and training for strength in adolescent girls. J Appl Soc Psychol. 1988;18(8, Pt 2):699–719. doi: 10.1111/j.1559-1816.1988.tb00046.x. [DOI] [Google Scholar]
- 129.Lubans D.R., Aguiar E.J., Callister R. The effects of free weights and elastic tubing resistance training on physical self-perception in adolescents. Psychol Sport Exerc. 2010;11:497–504. doi: 10.1016/j.psychsport.2010.06.009. [DOI] [Google Scholar]
- 130.LeCheminant J.D., Hinman T., Pratt K.B., et al. Effect of resistance training on body composition, self-efficacy, depression, and activity in postpartum women. Scand J Med Sci Sports. 2014;24(2):414–421. doi: 10.1111/j.1600-0838.2012.01490.x. [DOI] [PubMed] [Google Scholar]
- 131.Seguin R.A., Eldridge G., Lynch W., Paul L.C. Strength training improves body image and physical activity behaviors among midlife and older rural women. J Ext. 2013;51(4) [PMC free article] [PubMed] [Google Scholar]
- 132.Herring M.P., Jacob M.L., Suveg C., Dishman R.K., O’Connor P.J. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21–28. doi: 10.1159/000327898. [DOI] [PubMed] [Google Scholar]
- 133.Herring M.P., Meyer J.D. Resistance exercise for anxiety and depression: efficacy and plausible mechanisms. Trends Mol Med. 2024;30(3):204–206. doi: 10.1016/j.molmed.2023.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gordon B.R., McDowell C.P., Lyons M., Herring M.P. The effects of resistance exercise training on anxiety: a meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 2017;47(12):2521–2532. doi: 10.1007/s40279-017-0769-0. [DOI] [PubMed] [Google Scholar]
- 135.Gordon B.R., McDowell C.P., Hallgren M., Meyer J.D., Lyons M., Herring M.P. Association of efficacy of resistance exercise training with depressive symptoms: meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatr. 2018;75(6):566–576. doi: 10.1001/jamapsychiatry.2018.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cunha P.M., Werneck A.O., Nunes J.P., et al. Resistance training reduces depressive and anxiety symptoms in older women: a pilot study. Aging Ment Health. 2022;26(6):1136–1142. doi: 10.1080/13607863.2021.1922603. [DOI] [PubMed] [Google Scholar]
- 137.Noetel M., Sanders T., Gallardo-Gomez D., et al. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2024;384 doi: 10.1136/bmj-2023-075847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Damush T.M., Damush J.G., Jr. The effects of strength training on strength and health-related quality of life in older adult women. Gerontol. 1999;39(6):705–710. doi: 10.1093/geront/39.6.705. [DOI] [PubMed] [Google Scholar]
- 139.Bendau A., Petzold M.B., Kaminski J., Plag J., Strohle A. Exercise as treatment for “Stress-related” mental disorders. Curr Neuropharmacol. 2024;22(3):420–436. doi: 10.2174/1570159X22666230927103308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kandola A., Ashdown-Franks G., Hendrikse J., Sabiston C.M., Stubbs B. Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. 2019;107:525–539. doi: 10.1016/j.neubiorev.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 141.Logue M.W., van Rooij S.J.H., Dennis E.L., et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244–253. doi: 10.1016/j.biopsych.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim Y.S., Shin S.K., Hong S.B., Kim H.J. The effects of strength exercise on hippocampus volume and functional fitness of older women. Exp Gerontol. 2017;97:22–28. doi: 10.1016/j.exger.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 143.Robinson M.L., Mirza A., Gallagher N., et al. Limitations of molecular and antigen test performance for SARS-CoV-2 in symptomatic and asymptomatic COVID-19 contacts. J Clin Microbiol. 2022;60(7) doi: 10.1128/jcm.00187-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fraile-Martinez O., Garcia-Montero C., Fraile-Martinez M., et al. A comprehensive study of the academic benefits and practical recommendations to include resistance training programs in institutional education. Front Psychol. 2024;15 doi: 10.3389/fpsyg.2024.1387162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu-Ambrose T., Nagamatsu L.S., Graf P., Beattie B.L., Ashe M.C., Handy T.C. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Azevedo C.V., Hashiguchi D., Campos H.C., et al. The effects of resistance exercise on cognitive function, amyloidogenesis, and neuroinflammation in Alzheimer’s disease. Front Neurosci. 2023;17 doi: 10.3389/fnins.2023.1131214. [DOI] [PMC free article] [PubMed] [Google Scholar]