Abstract
We previously reported that TNF-α-induced apoptosis of lactotropes is estrogen dependent and predominant at proestrus. Here we observed that TNF-α (50 ng/ml) failed to induce apoptosis of anterior pituitary cells from ovariectomized rats cultured in the presence of progesterone (10−6 m). However, progesterone blocked the apoptotic effect of TNF-α in anterior pituitary cells and lactotropes cultured with 17β-estradiol (10−9 m). In addition, 17β-estradiol induced apoptosis of somatotropes and triggered the proapoptotic action of TNF-α in these cells, effects completely blocked by ICI 182 780 (10−6 m), an estrogen receptor antagonist. Progesterone reverted the permissive effect of 17β-estradiol on TNF-α-induced apoptosis of somatotropes. TNF-α induced apoptosis of somatotropes from rats killed at proestrus but not at diestrus. The antiprogestine ZK 98 299 (10−6 m) completely inhibited the protective action of progesterone on TNF-α-induced apoptosis of anterior pituitary cells, lactotropes, and somatotropes. Although progesterone can interact with glucocorticoid receptors, dexamethasone (10−6 m) had no effect on TNF-α-induced apoptosis of anterior pituitary cells, lactotropes, and somatotropes. Our results show that progesterone, by interacting with progesterone receptors, antagonizes the permissive action of estrogens on TNF-α-induced apoptosis of lactotropes and somatotropes. These observations suggest that the steroid milieu may modulate the apoptotic response of anterior pituitary cells during the estrous cycle.
Abbreviations: CL, Confidence limit; DAPI, 4′6-diamidino-2-phenylindole dihydrochloride; GR, glucocorticoid receptor; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; OVX, ovariectomized; PR, progesterone receptor; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling
TNF-α, A CYTOKINE that modulates cellular homeostasis in several tissues, modifies many biological processes such as cell proliferation, differentiation, and death (1). This cytokine and its receptors are expressed in the anterior pituitary gland (2–6). TNF-α has been observed to be involved in the control of neuroendocrine functions affecting anterior pituitary hormone secretion (3, 6, 7). We previously reported that this cytokine decreases prolactin release (8), inhibits anterior pituitary cell proliferation (8), and induces apoptosis of lactotropes and somatotropes (9). TNF-α-induced apoptosis of lactotropes is higher in cells from rats killed at proestrus than at estrus or diestrus, suggesting that the steroid environment modulates the responsiveness of anterior pituitary cells to the action of TNF-α during the estrous cycle (9).
Gonadal steroids modulate cell death in hormone-responsive tissues (9–12). Circulating levels of ovarian steroids show a characteristic pattern of variation over the estrous cycle: plasma levels of estradiol begin to rise at diestrus, reaching peak values at midproestrus, and then rapidly fall to basal values on the morning of estrus. Circulating levels of progesterone show two peaks: the first one late on the first day of diestrus and a higher second one in the evening of proestrus (13).
Cell proliferation in the anterior pituitary gland shows a cyclic pattern of variation during the estrous cycle. Lactotropes account for almost all the mitotic activity detected in the anterior pituitary (14). Proliferation of lactotropes was shown to occur predominantly on the morning of estrus (14), whereas the highest rate of apoptosis of anterior pituitary cells was observed at proestrus (15). We reported that TNF-α release is higher in anterior pituitary cells from rats killed at proestrus (16). Because the proapoptotic effect of TNF-α is predominant in lactotropes from rats killed at proestrus, we hypothesized that TNF-α acts as an auto/paracrine factor inducing apoptosis at proestrus to maintain tissue homeostasis in the anterior pituitary gland throughout the estrous cycle (9).
The proapoptotic action of TNF-α on lactotropes and its inhibitory effect on anterior pituitary cell proliferation are estrogen dependent (8, 9). To explore whether progesterone modulates the permissive effect of estradiol on TNF-α action in the anterior pituitary gland, we determined the effect of TNF-α on the apoptosis of anterior pituitary cells from ovariectomized rats, cultured in the presence of estradiol and/or progesterone. In addition, because TNF-α induces apoptosis of somatotropes (9), we investigated whether gonadal steroids modulate the apoptotic effect of TNF-α in this cell subpopulation.
Progesterone can interact with glucocorticoid receptors (GRs) (17, 18). Because GRs are expressed in almost all anterior pituitary cell types (19) and glucocorticoids were shown to antagonize the proapoptotic effect of TNF-α in several tissues (20, 21), we studied the effect of dexamethasone, a synthetic glucocorticoid, on TNF-α-induced apoptosis of anterior pituitary cells.
Materials and Methods
All drugs, media, and supplements were obtained from Sigma Chemical Co. (St. Louis, MO) except MEM Eagle (United States Biological, Swampscott, MA), fetal bovine serum (GenSa, Buenos Aires, Argentina), recombinant human TNF-α (Promega Co., Madison, WI), all terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) reagents (Roche Molecular Biochemicals, Mannheim, Germany), primary antibodies against anterior pituitary hormones (kindly donated by Dr. A. Parlow, National Hormone and Pituitary Program, Torrance, CA), and anti-guinea pig rhodamine-conjugated secondary antibody (Chemicon International; Temecula, CA) and the materials indicated below.
Animals
Adult female Wistar rats (200–250 g) were kept in controlled conditions of light (12-h light, 12-h dark cycles) and temperature (20–25 C). Rats were fed standard lab chow and water ad libitum and kept in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats were monitored by daily vaginal smears over three consecutive estrous cycles and killed at proestrus or diestrus. Groups of rats were ovariectomized (OVX) 2 wk before the experiments. Anterior pituitary glands were removed within minutes after decapitation.
Cell culture
A pool of anterior pituitary cells from six to nine OVX rats or three rats per stage of the estrous cycle was used for each culture. Anterior pituitary glands were washed several times with DMEM and cut into small fragments. Sliced fragments were dispersed enzymatically by successive incubations in DMEM supplemented with 3 mg/ml BSA containing 2.5 mg/ml trypsin (type I from bovine pancreas), 1 mg/ml DNase (deoxyribonuclease II, type V from bovine spleen), and 1 mg/ml trypsin inhibitor (type II-S from soybean), and finally dispersed by extrusion through a Pasteur pipette in Krebs buffer without Ca2+ and Mg2+. Dispersed cells were washed twice and resuspended in MEM (MEM-d-valine), which contains d-valine instead of l-valine to abrogate fibroblast proliferation, supplemented with 5 μl/ml MEM nonessential amino acids, 2 mm glutamine, 5.6 μg/ml amphotericin B, and 25 μg/ml gentamicin (MEM-d-valine-S). Cell viability as assessed by trypan blue exclusion was over 90%. Anterior pituitary cells were seeded onto coverslides in 24-well tissue culture plates (105 cells per 0.5 ml/well) for the TUNEL method, immunocytochemistry and microscopic nuclear observation, or in 96-well tissue culture plates (105 cells per 0.1 ml/well) for metabolic activity of viable cells determination. In the case of OVX rats, cells were cultured for 3 d in MEM-d-valine-S with 10% fetal bovine serum previously treated with 0.025% dextran-0.25% charcoal to remove steroids. Then cells were incubated in the same fresh medium containing vehicle (ethanol) or 10−9 m 17β-estradiol, 10−6 m progesterone (Roussel-Uclaf Laboratory, Paris, France), 10−6 m dexamethasone, 10−5 to 10−7 m ZK 98 299 (Schering AG, Berlin, Germany), and 10−6 m ICI 182 780 (Tocris, Ellisville, MO) alone or in combination for 2 d. After this period, cells were washed twice and the medium was replaced by serum-free MEM-d-valine-S, supplemented with 10 μg/ml insulin, 6.7 ng/ml sodium selenium, 5.5 μg/ml transferrin, 0.02 ng/ml triiodothyronine, and 10 μl/ml MEM vitamins (MEM-d-valine-SS) containing the tested compounds or vehicle and TNF-α (50 ng/ml) for 24 h.
Anterior pituitary cells from cycling rats were cultured for 2 d in MEM-d-valine-S with 10% fetal bovine serum/dextran-charcoal. After this period, cells were washed twice and incubated for 24 h in MEM-d-valine-SS containing TNF-α (50 ng/ml).
Metabolic activity determination
The metabolic activity of viable cells was determined by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Twenty microliters of reaction solution containing MTS (final concentration 333 μg/ml) and an electron coupling reagent (phenazine ethosulfate, final concentration 25 μm) were added to each well containing 100 μl of culture medium. After 3 h at 37 C, the OD was read in a microplate spectrophotometer at a wavelength of 495 nm. The quantity of formazan product is directly proportional to the number of living cells in culture.
Microscopic determination of DNA fragmentation by the TUNEL method
After the culture period, cells were fixed with 4% formaldehyde in PBS for 30 min and permeabilized by microwave irradiation (9). DNA strand breaks were labeled with digoxigenin-deoxyuridine 5-triphosphate using terminal deoxynucleotidyl transferase (0.18 U/μl) according to the manufacturer’s protocol. The incorporation of nucleotides into the 3′-hydroxyl end of damaged DNA was detected with an antidigoxigenin-fluorescein antibody and visualized in a fluorescence microscope (Axiophot, Carl Zeiss, Jena, Germany).
Microscopic determination of apoptotic nuclear morphology
Cells were fixed as described above, permeabilized with 0.2% Triton X-100 (vol/vol) in PBS and mounted with an antifade mounting medium, 1,4-diazabicyclo[2.2.2]octane and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) in 90% glycerol. Cell death was analyzed by nuclear chromatin morphology. Apoptotic cells were identified by the presence of nuclear condensation and/or chromatin margination or fragmentation (9).
Immunofluorescent identification of anterior pituitary cells
Anterior pituitary cells that underwent apoptosis (detected by TUNEL or nuclear staining with DAPI) were identified by indirect immunofluorescence staining (9). Briefly, cultured cells were fixed and permeabilized as described above and incubated for 30 min with 10% normal donkey serum and 10% normal sheep serum in PBS with 2% BSA. Then cells were incubated for 1 h with guinea pig antirat prolactin (National Hormone & Peptide Program-IC, 1:2500) or guinea pig antirat GH (National Hormone & Peptide Program-IC, 1:1000) in PBS containing 0.5% BSA, 1% blocking reagent (Roche Molecular Biochemicals), 1% normal donkey serum, and 1% normal sheep serum for 1 h. After rinsing, slides were incubated with donkey anti-guinea pig rhodamine-conjugated secondary antibody at 1:200 dilution in the same buffer. Control slides were incubated with normal guinea pig serum instead of primary antibody.
Statistical analysis
Viability data were expressed as mean ± se and evaluated by one-way ANOVA (followed by Dunnet or Tukey multiple comparisons tests). Differences were considered significant if P < 0.05. Each experiment was performed at least twice. The number of apoptotic cells was analyzed in duplicate slides from independent experiments. Results were expressed as the percentage ± confidence limits (CLs) of apoptotic cells of the total number of cells counted in each specific population or condition. 95% confidence intervals for proportions were analyzed by the χ2 test. Differences between proportions were considered significant if P < 0.05.
Results
Effect of TNF-α on anterior pituitary cells cultured in the presence of gonadal steroids
We previously reported that TNF-α (50 ng/ml) induces apoptosis of total anterior pituitary cells and lactotropes in an estrogen-dependent manner (9). Then we studied the effect of different concentrations of TNF-α (10–200 ng/ml) on the viability of anterior pituitary cells from OVX rats. When cells from OVX rats were cultured in the absence of 17β-estradiol, cell viability was not significantly affected by TNF-α, even at the highest concentration tested (Fig. 1A). In accordance with own previous work, we observed that TNF-α reduced the viability of anterior pituitary cells from OVX rats cultured with 17β-estradiol, this effect depending on the concentration of the cytokine (Fig. 1B). To explore whether progesterone affects the apoptotic action of this cytokine, anterior pituitary cells from OVX rats were cultured in the presence of progesterone (10−6 m). Progesterone per se did not modify the percentage of anterior pituitary cells with apoptotic nuclear morphology (Fig. 2A). TNF-α (50 ng/ml) failed to induce apoptosis of anterior pituitary cells when cells were cultured in the presence of progesterone alone (Fig. 2A).
Fig. 1.
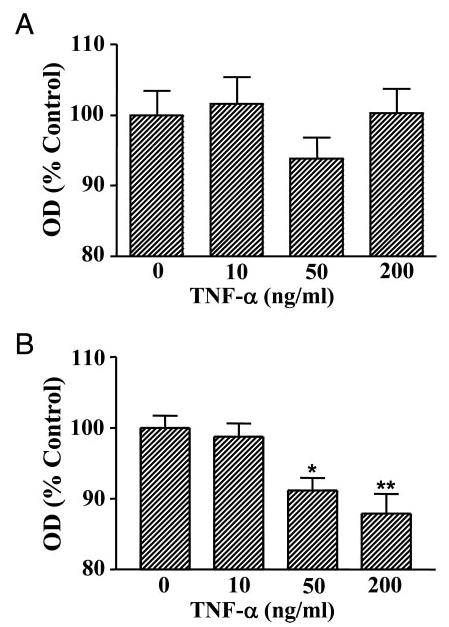
Effect of TNF-α on viability of anterior pituitary cells from OVX rats cultured in the presence of 17β-estradiol. Cells from OVX rats were cultured in the presence of vehicle (ethanol, 1 μl/liter, A) or 17β-estradiol (10−9 m, B). TNF-α was added at the specified concentrations 24 h before termination of culture. Cell viability was assessed by MTS assay. Each column represents the mean ± se of 10 wells from one of two independent experiments. *, P < 0.05; **, P < 0.01 vs. control (one-way ANOVA followed by Dunnet multiple comparisons test).
Fig. 2.
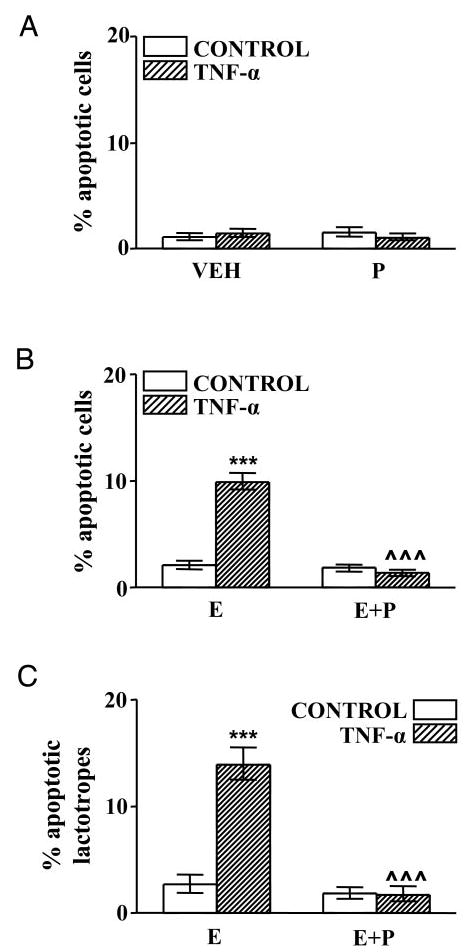
Effect of TNF-α on percentage of apoptosis in anterior pituitary cells (A and B) and lactotropes (C) from OVX rats cultured in the presence of gonadal steroids. A, Cells from OVX rats cultured with vehicle (ethanol 0.1 μl/ml, VEH) or progesterone (10−6 m, P) were incubated in the presence of TNF-α (50 ng/ml). Each column represents the percentage ± CLs of apoptotic cells (as identified by nuclear morphology of cells stained with DAPI), n > 3800 cells from two separate experiments. B and C, Cells from OVX rats cultured with 17β-estradiol (10−9 m, E) or 17β-estradiol plus progesterone (10−6 m, E+P) were incubated in the presence of TNF-α (50 ng/ml). Each column represents the percentage ± CLs of TUNEL-positive cells. B, Anterior pituitary cells (n > 5700 cells from four separate experiments). C, Lactotropes (n > 1800 cells from four separate experiments). ***, P < 0.001 vs. respective controls without TNF-α; ^^^, P < 0.001 vs. respective controls without progesterone.
To evaluate whether progesterone affects the proapoptotic action of TNF-α in anterior pituitary cells cultured with 17β-estradiol, we determined the effect of this cytokine on apoptosis of anterior pituitary cells from OVX rats cultured in the presence of both steroid hormones together. Progesterone suppressed TNF-α-induced apoptosis of anterior pituitary cells cultured in the presence of 17 β-estradiol (Fig. 2B). Also, progesterone completely reverted the permissive action of 17β-estradiol on TNF-α-induced apoptosis of lactotropes (Fig. 2C). Representative fields of anterior pituitary cells after incubation with TNF-α are shown in Fig. 3. More abundant apoptotic cells were observed when anterior pituitary cells were cultured with 17β-estradiol alone (Fig. 3, A and B) than with 17β-estradiol plus progesterone (Fig. 3, C and D).
Fig. 3.
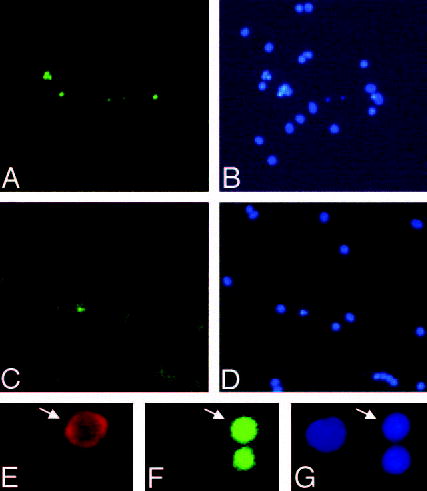
Apoptotic anterior pituitary cells. A–D, Anterior pituitary cells from OVX rats were cultured with 17β-estradiol (10−9 m, A and B) or 17β-estradiol plus progesterone (10−6 m, C and D) and incubated with TNF-α (50 ng/ml) for 24 h. DNA fragmentation was detected by the TUNEL method (A and C) and apoptotic nuclear morphology by staining with DAPI (B and D). Magnification, ×150. E–G, High-magnification micrographs showing a representative apoptotic somatotrope (arrows). E, Immunocytochemistry for GH. F, TUNEL. G, DAPI. Magnification, ×450.
Effect of gonadal steroids on TNF-α-induced apoptosis of somatotropes
Staining with TUNEL or DAPI permitted observation of the nuclear morphological features of apoptosis in somatotropes. Figure 3 shows a cell immunoreactive for GH (E) with TUNEL staining (F) and nuclear condensation (G). To explore whether 17β-estradiol modulates the apoptotic response of somatotropes to TNF-α, cells from OVX rats were cultured in the presence of 17β-estradiol. 17β-Estradiol per se increased the percentage of apoptotic somatotropes and triggered the proapoptotic effect of TNF-α in these cells (Fig. 4A). To study the specificity of estrogen action on apoptosis of somatotropes, we studied the effect of an antagonist of estrogen receptors, ICI 182 780 (22). ICI 182 780 did not modify the percentage of TUNEL-positive somatotropes when cells from OVX rats were cultured in the absence of 17β-estradiol (data not shown). However, in the presence of 17β-estradiol, ICI 182 780 significantly reduced the percentage of TUNEL-positive somatotropes and blocked TNF-α-induced apoptosis (Fig. 4B).
Fig. 4.
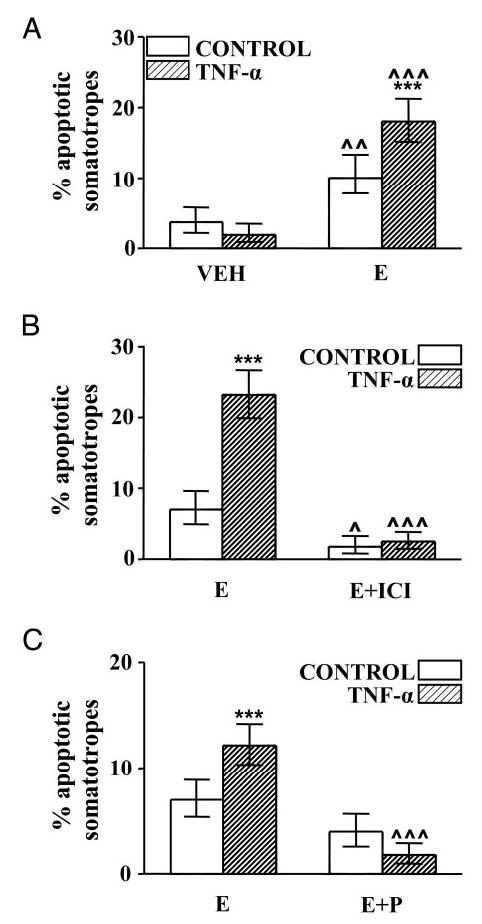
Effect of gonadal steroids on TNF-α-induced apoptosis of somatotropes from OVX rats. A, Cells from OVX rats cultured with vehicle (ethanol 1 μl/liter, VEH) or 17β-estradiol (10−9 m, E) were incubated in the presence of TNF-α (50 ng/ml). Each column represents the percentage ± CLs of apoptotic somatotropes (as identified by nuclear morphology of cells stained with DAPI), n > 500 cells from three separate experiments. ***, P < 0.001 vs. respective controls without TNF-α; ^^, P < 0.01, ^^^, P < 0.001 vs. respective controls without 17β-estradiol. B, Cells from OVX rats cultured with 17β-estradiol (10−9 m, E) or 17β-estradiol plus ICI 182 780 (10−6 m, E+ICI) were incubated with TNF-α (50 ng/ml). Each column represents the percentage ± CLs of TUNEL-positive somatotropes (n > 500 soma-totropes from two separate experiments). ***, P <0.001 vs. respective controls without TNF-α; ^, P < 0.05, ^^^, P < 0.001 vs. respective controls without ICI 182 780. C, Cells from OVX rats cultured with 17β-estradiol (10−9 m, E) or 17β-estradiol plus progesterone (10−6 m, E+P) were incubated in the presence of TNF-α (50 ng/ml). Each column represents the percentage ± CLs of TUNEL-positive somatotropes (n >800 cells from four separate experiments). ***, P <0.001 vs. respective controls without TNF-α; ^^^, P < 0.001 vs. respective controls without progesterone.
To determine whether progesterone affects the permissive action of 17β-estradiol on TNF-α-induced apoptosis of somatotropes, cells from OVX rats were cultured in the presence of 17β-estradiol alone or 17β-estradiol plus progesterone and incubated with TNF-α. Progesterone produced a nonsignificant decrease in the percentage of apoptotic somatotropes incubated with 17β-estradiol but completely reverted the apoptosis induced by TNF-α in these cells (Fig. 4C).
To evaluate whether circulating levels of ovarian steroids modulate the response of somatotropes to the proapoptotic action of TNF-α, we determined the effect of this cytokine on the percentage of TUNEL-positive somatotropes in cultures of anterior pituitary cells from rats killed on the morning of proestrus or diestrus. TNF-α-induced apoptosis was observed in somatotropes from rats killed at proestrus but not at diestrus (Fig. 5).
Fig. 5.
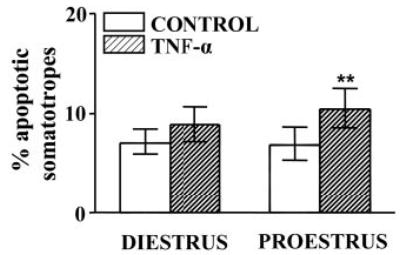
Effect of TNF-α on percentage of apoptotic somatotropes from rats killed at selected stages of the estrous cycle. Cells from rats killed at diestrus or proestrus were incubated in the presence of TNF-α (50 ng/ml). Each column represents the percentage ± CLs of TUNEL-positive somatotropes (n > 1500 cells from three to four separate experiments, each one performed with three rats per group). **, P < 0.01 vs. respective control without TNF-α.
Role of progesterone receptors in protection from TNF-α-induced apoptosis
To determine whether classical progesterone receptors are involved in the blockade of TNF-α-induced apoptosis produced by progesterone, we investigated the effect of an antiprogestine ZK 98 299 (0.1–10 μm) on TNF-α-induced apoptosis of anterior pituitary cells from OVX rats incubated with 17β-estradiol alone or 17β-estradiol plus progesterone. The presence of ZK 98 299 in the culture medium did not modify the proapoptotic effect of TNF-α on anterior pituitary cells cultured in the presence of 17β-estradiol alone (data not shown). However, in the presence of 17β-estradiol plus progesterone, ZK 98 299, at doses equal to or higher than 1 μm, completely reverted the protective action of progesterone on TNF-α-induced apoptosis (Fig. 6A). Also, ZK 98 299 (1 μm) blocked the protective effect of progesterone on estrogen-dependent apoptosis of lactotropes (Fig. 6B) and somatotropes (Fig. 6C) induced by TNF-α.
Fig. 6.
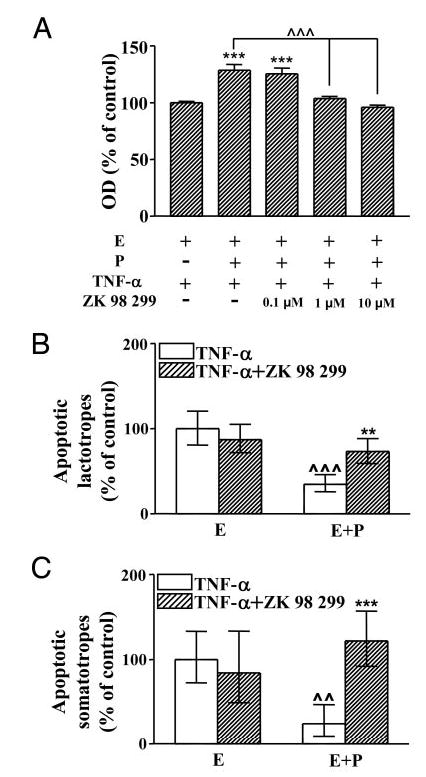
Effect of ZK 98 299 on TNF-α-induced apoptosis of anterior pituitary cells (A), lactotropes (B), and somatotropes (C) from OVX rats cultured in presence of 17β-estradiol alone or together with progesterone. A, Cells from OVX rats cultured in the presence of 17β-estradiol (10−9 m, E) or 17β-estradiol plus progesterone (10−6 m, E+P) were incubated with TNF-α (50 ng/ml) in the presence or absence of ZK 98 299 (0.1–10 μm). Cell viability was assessed by MTS assay. Each column represents the mean ± se of 10 wells from one of two independent experiments. ***, P < 0.001 vs. group without progesterone; ^^^, P < 0.001 (ANOVA followed by Tukey multiple comparisons test). B and C, Cells from OVX rats cultured with 17β-estradiol (10−9 m, E) or 17β-estradiol plus progesterone (10−6 m, E+P) were incubated with TNF-α (50 ng/ml) in the presence or absence of ZK 98 299 (1 μm). Each column represents the increase ± CLs of TUNEL-positive cells with respect to the group cultured with 17β-estradiol alone. B, Lactotropes (n > 1500 cells from two separate experiments). **, P < 0.01 vs. respective controls without ZK 98 299; ^^^, P < 0.001 vs respective controls without progesterone. C, Somatotropes (n >600 cells from four separate experiments). ***, P <0.001 vs. respective controls without ZK 98 299; ^^, P < 0.01 vs. respective controls without progesterone.
Because both progesterone (17, 18) and ZK 98 299 (23) were reported to interact with glucocorticoid receptors and considering that the activation of glucocorticoid receptors affects TNF-α-induced apoptotic pathways (20, 21), we explored the effect of dexamethasone, a synthetic glucocorticoid, on TNF-α-induced apoptosis of anterior pituitary cells. Cells from OVX rats cultured with dexamethasone (10−6 m) in the presence or absence of 17β-estradiol were incubated with TNF-α. Dexamethasone failed to affect the percentage of TUNEL-positive cells cultured in the absence of 17β-estradiol incubated either with or without TNF-α (Fig. 7A). Dexamethasone did not modify the induction of apoptosis exerted by TNF-α in the presence 17β-estradiol (Fig. 7B). Also, the pro-apoptotic effect of TNF-α on lactotropes and somatotropes cultured in the presence of 17β-estradiol was not affected by dexamethasone (Fig. 8, A and B).
Fig. 7.
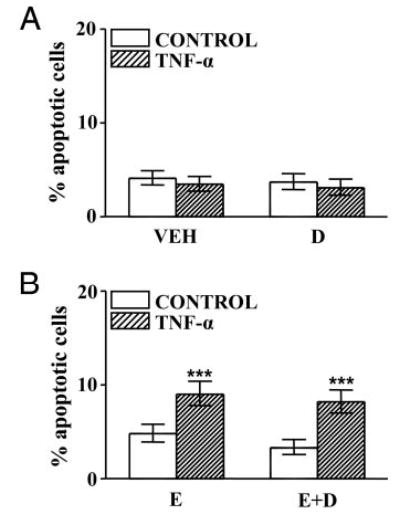
Effect of dexamethasone on TNF-α-induced apoptosis of anterior pituitary cells from OVX rats cultured with or without 17β-estradiol. A, Cells from OVX rats cultured with vehicle (ethanol 0.1 μl/ml, VEH) or dexamethasone (10−6 m, D) were incubated in the presence of TNF-α (50 ng/ml). Each column represents the percentage ± CLs of TUNEL-positive cells(n > 2200 cells from three separate experiments). B, Cells from OVX rats cultured with 17β-estradiol (10−9 m, E) or 17β-estradiol plus dexamethasone (10−6 m, E+D) were incubated in the presence of TNF-α (50 ng/ml). Each column represents the percentage ± CLs of TUNEL-positive cells (n > 2000 cells from three separate experiments). ***, P < 0.001 vs. respective controls without TNF-α.
Fig. 8.
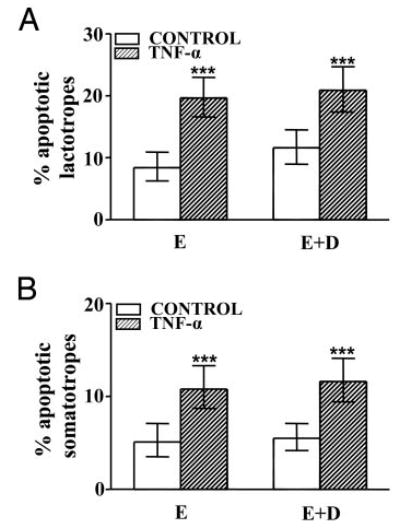
Effect of dexamethasone on percentage of apoptotic lactotropes (A) and somatotropes (B) from OVX rats cultured in presence of 17β-estradiol and TNF-α. Cells from OVX rats cultured with 17β-estradiol (10−9 M, E) or 17β-estradiol plus dexamethasone (10−6 M, E+D) were incubated in the presence of TNF-α (50 ng/ml). Each column represents the percentage ± CLs of TUNEL-positive cells. A, Lactotropes (n > 500 cells from three separate experiments). ***, P < 0.001 vs. respective controls without TNF-α. B, Somatotropes (n >700 cells from two separate experiments). ***, P < 0.001 vs. respective controls without TNF-α.
Discussion
In the present report, we show that gonadal steroids, at concentrations within the physiological range (13), modulate the response of lactotropes and somatotropes from female rats to the apoptotic action of TNF-α. 17β-Estradiol not only affects the apoptotic response of lactotropes (9) but also enables TNF-α to trigger apoptosis of somatotropes. Moreover, somatotropes undergo apoptosis in the presence of 17β-estradiol alone. The permissive action of 17β-estradiol to proapoptotic signals in lactotropes and somatotropes is antagonized by progesterone.
Cell turnover occurs in the anterior pituitary gland during the estrous cycle. Lactotropes show the highest mitotic index on the morning of estrus (14). We previously observed that TNF-α induces apoptosis of lactotropes from rats killed on the morning of proestrus but not at diestrus (9). On the morning of proestrus, when circulating levels of progesterone are still low, estrogen levels reach a peak (13). This steroid profile may sensitize the anterior pituitary gland to proapoptotic signals at this stage of the estrous cycle. On the contrary, low circulating levels of estrogens and/or high progesterone levels may impair apoptosis of anterior pituitary cells at other stages of the estrous cycle. In addition, because TNF-α release from anterior pituitary cells is higher at proestrus and stimulated by 17β-estradiol (16), the steroid milieu on the morning of proestrus could increase local production of auto/paracrine mediators, such as TNF-α, which, in turn, would lead to apoptosis of anterior pituitary cells.
An increase in apoptosis of gonadotropes has been reported on the morning of proestrus (15). In the present study, we observed that TNF-α induces apoptosis of cultured somatotropes from rats killed at proestrus but not at diestrus. Almost half of the anterior pituitary cells remain in a multipotential state, expressing different hormone mRNAs and potentially responding to several hypothalamic secretagogues (24, 25). The cyclic rise in the expression of gonadotropins during the estrous cycle coincides with the appearance of a subset of multihormonal somatotropes that share phenotypic features of gonadotropes (26). This multihormonal population has been proposed to increase in response to estrogens to support the extra needs of gonadotropin-releasing cells at proestrus (27). Also, the expression of estrogen receptor-α in somatotropes has been proposed to induce prolactin expression in this cell type (28). Therefore, because we did not dual label anterior pituitary secretory cells, we cannot rule out that GH-expressing cells undergoing apoptosis by TNF-α could be multihormonal cells.
Although estrogens have classically been recognized as potent mitogens in the anterior pituitary gland (29), a growing body of evidence shows that they also exert antiproliferative and proapoptotic actions (12, 30). In fact, our recent report shows that long-term estrogenization of OVX rats increases the percentage of apoptotic cells in the anterior pituitary gland (31). Although the mechanisms involved in the antisurvival action of estrogens in the anterior pituitary gland have not yet been studied, chronic estrogenization has been reported to increase the expression of tumor suppressor genes such as p53 in this gland (32). It has also been shown that the expression of Bax, a proapoptotic member of the Bcl-2 family, is higher at proestrus (15), when circulating estrogen levels are high (13). Therefore, estrogens seem to tightly modulate pituitary cell renewal, sensitizing cells to not only mitogenic stimuli but also proapoptotic signals, thus maintaining tissue homeostasis.
Progesterone has been reported to act as a survival factor in several reproductive tissues, including the mammary gland (33), ovary (34), and endometrium (11). Progesterone has been shown to promote cell survival by altering the expression of anti- and proapoptotic genes of the Bcl-2 family (11). Our results indicate that progesterone impairs the permissive effect of estradiol on lactotropes and somatotropes to TNF-α-induced apoptosis. Some other actions of estrogens in the anterior pituitary, such as stimulation of cell proliferation and prolactin release, are also antagonized by progesterone (35, 36). A potent antiprogestine, ZK 98 299 (37), inhibited the protective action of progesterone on TNF-α-induced apoptosis of anterior pituitary cells, suggesting that progesterone may act through progesterone receptors (PRs). However, PRs in the rat anterior pituitary seem to be confined to gonadotropes (38). Because the expression of this receptor in GH-immunoreactive cells has yet to be studied, it is possible that progesterone could act via PRs in multihormonal somatogonadotropes. Although progesterone has a relative high affinity for the GR (17) and the activation of GR suppresses TNF-α-induced apoptosis in osteoblasts (20) and several cell lines (21), we observed that the activation of GRs by dexamethasone failed to modify the sensitizing action of 17β-estradiol on TNF-α-induced apoptosis, suggesting that progesterone may not protect from apoptosis by interacting with GRs. Moreover, our results indicate that glucocorticoids do not modulate apoptosis of anterior pituitary cells.
Lactotropes have been shown to lack PRs (38), making it difficult to explain the mechanism by which progesterone antagonizes the permissive action of 17β-estradiol on TNF-α-induced apoptosis in this cell type. However, it is well recognized that paracrine signals are responsible for mediating the action of steroid and peptide hormones in the anterior pituitary gland (29). For example, it has been established that the stimulatory action of GnRH on lactotrope proliferation is mediated by angiotensin II released from gonadotropes in culture (39). Therefore, we speculate that the inhibitory effect of progesterone on TNF-α-induced apoptosis of lactotropes could be reflecting paracrine communication. Alternatively, TNF-α-induced apoptosis of anterior pituitary cells could be blocked by progesterone, at least in part, through nongenotropic mechanisms. In many tissues, it has been reported that progesterone interacts with not only classic PRs but also nonclassic membrane receptors (40, 41) that are antagonized by ZK 98 299 (42).
In conclusion, the present study shows that 17β-estradiol not only has a permissive action in the apoptotic response of lactotropes to TNF-α but also induces apoptosis of somatotropes by itself. PR activation protects anterior pituitary cells from estrogen-dependent TNF-α-induced apoptosis. Our results suggest that the steroid environment modulates the apoptotic response of anterior pituitary cells involved in the process of cell renewal during the estrous cycle.
Acknowledgments
This work was supported by grants from Agencia Nacional de Investigaciones Científicas y Tecnológicas, Consejo Nacional de Investigaciones Cientificas y Técnicas, and Universidad de Buenos Aires (Argentina), and 1RO3 Grant TW006273-01 from the Fogarty International Research Collaboration Award of the National Institutes of Health (to M.G.C. and A.S.).
References
- 1.Leong KG, Karsan A. Signaling pathways mediated by tumor necrosis factor α. Histol Histopathol. 2000;15:1303–1325. doi: 10.14670/HH-15.1303. [DOI] [PubMed] [Google Scholar]
- 2.Nadeau S, Riviest S. Regulation of the gene encoding tumor necrosis factor α (TNF-α) in rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol. 1999;58:61–77. doi: 10.1097/00005072-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanism of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi H, Fukata J, Murakami N, Usui T, Ebisui O, Muro S, Hanaoka I, Inoue K, Imura H, Nakao K. Tumor necrosis factor receptor in the pituitary cells. Brain Res. 1997;758:45–50. doi: 10.1016/s0006-8993(96)01437-0. [DOI] [PubMed] [Google Scholar]
- 5.Wolvers DAW, Marquette C, Berkenbosch F, Haour F. Tumor necrosis factor-α binding sites in rodent brain and pituitary gland. Eur Cytokine Netw. 1993;4:377–381. [PubMed] [Google Scholar]
- 6.Ray D, Melmed S. Pituitary cytokine and growth factor expression and action. Endocr Rev. 1997;18:206–228. doi: 10.1210/edrv.18.2.0297. [DOI] [PubMed] [Google Scholar]
- 7.Artz E, Páez Pereda M, Perez Castro C, Pagotto U, Renner U, Stalla GK. Pathophysiological role of the cytokine network in the anterior pituitary. Front Neuroendocrinol. 1999;20:71–95. doi: 10.1006/frne.1998.0176. [DOI] [PubMed] [Google Scholar]
- 8.Theas S, Pisera D, Duvilanski B, De Laurentiis A, Pampillo M, Lasaga M, Seilicovich A. Estrogens modulate the inhibitory effect of tumor necrosis factor-α on anterior pituitary growth and prolactin release. Endocrine. 2000;12:249–255. doi: 10.1385/ENDO:12:3:249. [DOI] [PubMed] [Google Scholar]
- 9.Candolfi M, Zaldivar V, De Laurentiis A, Jaita G, Pisera D, Seilicovich A. Tumor necrosis factor-α induces apoptosis of lactotropes from female rats. Endocrinology. 2002;143:3611–3617. doi: 10.1210/en.2002-220377. [DOI] [PubMed] [Google Scholar]
- 10.Bruckheimer EM, Kyprianou N. Dihydrotestosterone enhances transforming growth factor-β-induced apoptosis in hormone-sensitive prostate cancer cells. Endocrinology. 2002;142:2419–2426. doi: 10.1210/endo.142.6.8218. [DOI] [PubMed] [Google Scholar]
- 11.Tao XJ, Tilly KI, Maravei DV, Shifren JL, Krajewski S, Reed JC, Tilly JL, Isaacson KB. Differential expression of members of the bcl-2 gene family in proliferative and secretory human endometrium: glandular epithelial cell apoptosis is associated with increased expression of bax. J Clin Endocrinol Metab. 1997;82:2738–2746. doi: 10.1210/jcem.82.8.4146. [DOI] [PubMed] [Google Scholar]
- 12.Lee EJ, Duan WR, Jakacka M, Gehm BD, Jameson JL. Dominant negative ER induces apoptosis in GH(4) pituitary lactotrope cells and inhibits tumor growth in nude mice. Endocrinology. 2001;142:3756–3763. doi: 10.1210/endo.142.9.8372. [DOI] [PubMed] [Google Scholar]
- 13.Freeman ME 1994 The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, eds. The physiology of reproduction. 2nd ed. Chap 46. New York: Raven Press; 613–658
- 14.Hashi A, Mazawa S, Kato J, Arita J. Pentobarbital anesthesia during the proestrus afternoon blocks lactotroph proliferation occurring on estrus in female rats. Endocrinology. 1995;136:4665–4671. doi: 10.1210/endo.136.10.7664687. [DOI] [PubMed] [Google Scholar]
- 15.Yin P, Arita J. Proestrus surge of gonadotropin-releasing hormone secretion inhibits apoptosis of anterior pituitary cells in cycling female rats. Neuroendocrinology. 2002;76:272–282. doi: 10.1159/000066626. [DOI] [PubMed] [Google Scholar]
- 16.Theas MS, De Laurentiis A, Lasaga M, Pisera D, Duvilanski B, Seilicovich A. Effect of lipopolysaccharide on tumor necrosis factor and prolactin secretion from rat anterior pituitary cells. Endocrine. 1998;8:241–245. doi: 10.1385/endo:8:3:241. [DOI] [PubMed] [Google Scholar]
- 17.Carbone JP, Baldridge RC, Koszalka TR, Bongiovanni AM, Brent RL. Characterization of cytosolic glucocorticoid receptor of fetal rat epiphyseal chondrocytes. J Steroid Biochem. 1990;35:495–505. doi: 10.1016/0022-4731(90)90259-u. [DOI] [PubMed] [Google Scholar]
- 18.Sugino N, Telleria CM, Gibori G. Progesterone inhibits 20α-hydroxysteroid dehydrogenase expression in the rat corpus luteum through the glucocorticoid receptor. Endocrinology. 1997;138:4497–4500. doi: 10.1210/endo.138.10.5572. [DOI] [PubMed] [Google Scholar]
- 19.Ozawa H, Ito T, Ochiai I, Kawata M. Cellular localization and distribution of glucocorticoid receptor immunoreactivity and the expression of glucocorticoid receptor messenger RNA in rat pituitary gland. A combined double immunohistochemistry study and in situ hybridization histochemical analysis. Cell Tissue Res. 1999;295:207–214. doi: 10.1007/s004410051226. [DOI] [PubMed] [Google Scholar]
- 20.Chae HJ, Chae SW, Kang JS, Bang BG, Cho SB, Park RK, So HS, Kim YK, Kim HM, Kim HR. Dexamethasone suppresses tumor necrosis factor-α-induced apoptosis in osteoblasts: possible role for ceramide. Endocrinology. 2000;141:2904–2913. doi: 10.1210/endo.141.8.7604. [DOI] [PubMed] [Google Scholar]
- 21.Kull Jr FC. Reduction in tumor necrosis factor receptor affinity and cytotoxicity by glucocorticoids. Biochem Biophys Res Commun. 1988;153:402–409. doi: 10.1016/s0006-291x(88)81238-5. [DOI] [PubMed] [Google Scholar]
- 22.Chun TY, Gregg D, Sarkar DK, Gorski J. Differential regulation by estrogens of growth and prolactin synthesis in pituitary cells suggests that only a small pool of estrogen receptors is required for growth. Proc Natl Acad Sci USA. 1998;95:2325–2330. doi: 10.1073/pnas.95.5.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miner JN, Tyree C, Hu J, Berger E, Marschke K, Nakane M, Coghlan MJ, Clemm D, Lane B, Rosen J. A nonsteroidal glucocorticoid receptor antagonist. Mol Endocrinol. 2003;17:117–127. doi: 10.1210/me.2002-0010. [DOI] [PubMed] [Google Scholar]
- 24.Villalobos C, Nunez L, Frawley LS, Garcia-Sancho J, Sanchez A. Multi-responsiveness of single anterior pituitary cells to hypothalamic-releasing hormones: a cellular basis for paradoxical secretion. Proc Natl Acad Sci USA. 1997;94:14132–14137. doi: 10.1073/pnas.94.25.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denef C, Roudbaraki M, Van Bael A. Growth and differentiation factors derived from the N-terminal domain of pro-opiomelanocortin. Clin Exp Pharmacol Physiol. 2001;28:239–243. doi: 10.1046/j.1440-1681.2001.03421.x. [DOI] [PubMed] [Google Scholar]
- 26.Childs GV. Development of gonadotropes may involve cyclic transdifferentiation of growth hormone cells. Arch Physiol Biochem. 2002;110:42–49. doi: 10.1076/apab.110.1.42.906. [DOI] [PubMed] [Google Scholar]
- 27.Childs GV. Growth hormone cells as cogonadotropes: partners in the regulation of the reproductive system. Trends Endocrinol Metab. 2000;11:168–175. doi: 10.1016/s1043-2760(00)00252-6. [DOI] [PubMed] [Google Scholar]
- 28.Asa SL, Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr Rev. 1998;19:798–827. doi: 10.1210/edrv.19.6.0350. [DOI] [PubMed] [Google Scholar]
- 29.Denef C. Paracrine control of lactotrope proliferation and differentiation. Trends Endocrinol Metab. 2003;14:188–195. doi: 10.1016/s1043-2760(03)00057-2. [DOI] [PubMed] [Google Scholar]
- 30.Kawashima K, Yamakawa K, Takahashi W, Takizawa S, Yin P, Sugiyama N, Kanba S, Arita J. The estrogen-occupied estrogen receptor functions as a negative regulator to inhibit cell proliferation induced by insulin/IGF-1: a cell context-specific antimitogenic action of estradiol on rat lactotrophs in culture. Endocrinology. 2002;143:2750–2758. doi: 10.1210/endo.143.7.8915. [DOI] [PubMed] [Google Scholar]
- 31.Pisera D, Candolfi M, Navarra S, Ferraris J, Zaldivar V, Jaita G, Castro MG, Seilicovich A. Estrogens sensitize anterior pituitary gland to apoptosis. Am J Physiol Endocrinol Metab. 2004;287:E767–E771. doi: 10.1152/ajpendo.00052.2004. [DOI] [PubMed] [Google Scholar]
- 32.Ying C. Potential involvement of tumor suppressor gene expression in the formation of estrogen-inducible pituitary tumors in rats. Endocr J. 2000;47:1–5. doi: 10.1507/endocrj.47.1. [DOI] [PubMed] [Google Scholar]
- 33.Berg MN, Dharmarajan AM, Waddell BJ. Glucocorticoids and progesterone prevent apoptosis in the lactating rat mammary gland. Endocrinology. 2002;143:222–227. doi: 10.1210/endo.143.1.8584. [DOI] [PubMed] [Google Scholar]
- 34.Goyeneche AA, Deis RP, Gibori G, Telleria CM. Progesterone promotes survival of the rat corpus luteum in the absence of cognate receptors. Biol Reprod. 2003;68:151–158. doi: 10.1095/biolreprod.102.007898. [DOI] [PubMed] [Google Scholar]
- 35.Caronti B, Palladini G, Bevilacqua MG, Petrangeli E, Fraioli B, Cantore G, Tamburrano G, Carapella CM, Jaffrain-Rea ML. Effects of 17β-estradiol, progesterone and tamoxifen on in vitro proliferation of human pituitary adenomas: correlation with specific cellular receptors. Tumour Biol. 1993;14:59–68. doi: 10.1159/000217826. [DOI] [PubMed] [Google Scholar]
- 36.Brann DW, Rao IM, Mahesh VB. Antagonism of estrogen-induced prolactin release by progesterone. Biol Reprod. 1988;39:1067–1073. doi: 10.1095/biolreprod39.5.1067. [DOI] [PubMed] [Google Scholar]
- 37.Truss M, Bartsch J, Beato M. Antiprogestins prevent progesterone receptor binding to hormone responsive elements in vivo. Proc Natl Acad Sci USA. 1994;91:11333–11337. doi: 10.1073/pnas.91.24.11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox SR, Harlan RE, Shivers BD, Pfaff DW. Chemical characterization of neuroendocrine targets for progesterone in the female rat brain and pituitary. Neuroendocrinology. 1990;51:276–283. doi: 10.1159/000125350. [DOI] [PubMed] [Google Scholar]
- 39.Shinkai T, Ooka H. Effect of angiotensin II on the proliferation of mammotrophs from the adult rat anterior pituitary in culture. Peptides. 1995;16:25–29. doi: 10.1016/0196-9781(94)00142-s. [DOI] [PubMed] [Google Scholar]
- 40.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 41.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blackmore PF, Neulen J, Lattanzio F, Beebe SJ. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J Biol Chem. 1991;266:18655–18659. [PubMed] [Google Scholar]


