Abstract
Antibody discovery is crucial for developing therapeutics and vaccines as well as for understanding adaptive immunity. However, the lack of approaches to synthesize antibodies with defined sequences in a high-throughput manner represents a major bottleneck in antibody discovery. Here, we present oPool+ display, a high-throughput cell-free platform that combined oligo pool synthesis and mRNA display to rapidly construct and characterize hundreds to thousands of natively paired antibodies in parallel. As a proof-of-concept, we applied oPool+ display to probe the binding specificity of more than 300 uncommon influenza hemagglutinin-specific antibodies against 9 hemagglutinin variants through 16 screens. Over 5,000 binding tests were performed in 3 to 5 days of hands-on time with further scaling potential. Follow-up structural and functional analysis of two antibodies revealed the versatility of the human immunoglobulin gene segment D3–3 in recognizing the hemagglutinin stem. Overall, this study established an experimental platform that not only accelerates antibody characterization, but also enables unbiased discovery of recurring molecular signatures among antibodies with the same specificity.
One-sentence summary:
oPool+ display enabled rapid antibody synthesis and screening of influenza hemagglutinin-specific antibodies.
INTRODUCTION
Antibodies are central for protection against infection. Therefore, identification of antibodies that target pathogens of interest is key to improving our understanding of adaptive immunity as well as to the development of effective therapeutics and vaccines. In recent years, advances in single-cell B cell receptor sequencing (scBCR-seq) have greatly improved the capacity to discover novel antibodies (1). Thousands of natively paired monoclonal antibody sequences can be obtained from a single scBCR-seq experiment. By contrast, downstream characterization of these antibody sequences remains costly, labor intensive, and time consuming, involving cloning, expression, purification, and testing the binding activities of different antibodies individually. At the same time, protein display technologies offer a high-throughput solution for characterizing antibody binding activity (2), with antibody library construction being an essential first step. Methods for constructing antibody libraries with random heavy-light chain pairing from B cell repertoires are well-established (3, 4). However, there is a lack of approaches to synthesize custom-made antibody libraries with precise heavy-light chain pairing from a defined list of antibody sequences. This technical barrier has restricted the application of protein display technologies in antibody research, including large-scale characterization of previously discovered antibodies.
Influenza A and B viruses are major global health concerns that cause seasonal influenza epidemics. Hemagglutinin (HA) is the major glycoprotein antigen that is essential to the influenza virus life cycle (5). Its hypervariable globular head domain engages sialic acid on host cell receptors, whereas its conserved stem domain possesses the membrane fusion machinery (6, 7). Influenza A HA is further divided into two groups with a total of 19 antigenic subtypes (H1-H19). Group 1 HA includes H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17, H18, and H19, and group 2 HA includes H3, H4, H7, H10, H14, and H15. Several recurring sequence features have been observed among HA antibodies isolated from different individuals, such as IGHV1–69, IGHV1–18, IGHV6–1, and IGHD3–9 for stem antibodies, and IGHV2–70 and IGHD4–17 for head antibodies (8–13). Many of these sequence features are key determinants of broadly protective HA antibodies. Discovering the recurring sequence features of HA antibodies is therefore critical to the molecular understanding of antibody responses at the population level, which will in turn benefit the development of universal influenza vaccines and the preparedness against influenza strains with pandemic potentials. However, due to the current bottleneck in antibody characterization, only a small portion of the discovered HA antibodies has been characterized (14). In this study, we developed oPool+ display, a rapid and cost-effective cell-free platform that combined oligo pool synthesis with mRNA display to assemble and screen natively paired antibodies in a highly parallel manner. Using HA antibodies as a proof-of-concept, our results revealed IGHD3–3 as a previously overlooked recurring sequence feature of HA antibodies and demonstrated the potential of oPool+ display in rapid characterization of antibody responses.
RESULTS
Oligo pools enable high throughput assembly of a natively paired antibody library
We previously curated a dataset containing 5,561 human monoclonal HA antibodies, 1,082 (19.5%) of which are known to bind to either the head domain or the stem domain (14). Of the remaining 4,479 (80.5%) HA antibodies which lack epitope information, 292 have complete heavy chain variable (VH) and light chain variable (VL) sequences available and are not encoded by well-characterized sequence features of HA stem antibodies, namely IGHV1–69, IGHV1–18, IGHV6–1, and IGHD3–9 (8, 10–12) (fig. S1A and data file S1). These 292 HA antibodies were included in the synthesis of our natively paired antibody library. By excluding antibodies with well-characterized sequence features, we hoped to identify previously unknown recurring sequence features in HA antibodies. In addition, three known stem antibodies, namely 31.a.55, AG11-2F01, and 042-100809-2F04 (8, 15, 16), as well as 30 known HA head antibodies (7, 13, 17–24) were included as controls, bringing the total library size to 325 antibodies (Fig. 1A).
Figure 1. Oligo pool-based PCR assembly enables parallel synthesis of the natively paired HA antibody library.

(A) The overall breakdown of the HA antibody (Ab) library. (B) Design of oligos for single chain variable fragment (scFv) assembly. Each given scFv construct contains a T7 promoter and a start codon at the N-terminal as well as a FLAG tag at the C-terminal. The scFv sequences were then split into 4 fragments at the selected complementary determining region (CDR), with overlap between adjacent fragments. Through an overlap PCR, oligos of the same construct would preferably anneal to each other, ensuring the assembly of natively paired scFvs. (C) Synthesis of the natively paired HA antibody library. Synthesized oligo pools containing scFv fragments were assembled using a two-stage PCR. (D) The unassembled and assembled oligo pools were compared by agarose gel electrophoresis. “U”: unassembled oligo pool. “Rep1”: replicate 1. “Rep2”: replicate 2. The red arrow indicates the target size (800–900 bp) for full length scFvs. (E and F) The reproducibility (E) and coverage (F) of the scFv assembly using varying numbers of scFvs per PCR, ranging from 25 to 200. The Pearson correlation coefficient (R) of the occurrence frequencies of individual scFvs between the two replicates are indicated. Micro-tube icon by Servier https://smart.servier.com/ is licensed under CC-BY 3.0 Unported, available from Bio Icons.
To synthesize the natively paired antibody library in a high-throughput manner, we aimed to leverage recent advances in oligo pool synthesis. The maximum length of each oligo in commercial oligo pool synthesis is around 300 to 350 nucleotides. By contrast, the length of a single-chain variable fragment (scFv), which is the smallest format of a human antibody, is around 800 to 900 nucleotides. As a result, each given scFv sequence was split into 4 oligos with overlaps at the diverse complementary-determining regions (CDRs), namely the CDRs H1, H3, and L3 (Fig. 1B and C, data file S2). We then performed codon randomization to ensure that the overlaps among oligos for the same scFv are unique at the nucleic acid level. This would help prevent mis-annealing between oligos from different scFvs, especially if they shared similar amino acid sequences (Fig. 1B). Through a one-pot overlap polymerase chain reaction (PCR), full-length scFv sequences could then be generated with the intended native VH and VL pairing. Subsequently, this strategy was applied to design the oligo pools for our library of 325 antibodies (Fig. 1C to F, fig. S1B and C, data file S2).
The length of the assembled product was consistent with that of full-length scFvs (Fig. 1D). To thoroughly evaluate the effectiveness of our synthesis strategy, we then performed several assembly PCRs with the starting oligos of 25, 75, 100, 125, 150, and 200 scFvs each in a single tube (Fig. 1E and F, fig. S2). PacBio sequencing revealed the high reproducibility of the one-pot PCR assembly, with a Pearson correlation coefficient of 0.95 even at 200 scFvs per PCR (Fig. 1E). The one-pot PCR assembly strategy also achieved high coverage of the desired antibody sequences, with only a subtle drop from 100% (25/25) to 90% (180/200) as the complexity of the PCR increased from 25 to 200 scFvs (Fig. 1F). For our final library of all 325 scFvs, a Pearson correlation coefficient of 0.83 was observed between replicates, with a coverage of 322 of 325 (99.1%) natively paired antibodies (fig. S3). Although we opted to assemble this antibody library through 13 PCRs with 25 scFvs in each reaction, our results demonstrated the potential to further increase the throughput of our antibody library synthesis strategy by at least one order of magnitude.
mRNA display enabled rapid specificity characterization of the natively paired antibodies
To characterize antibody specificity from our natively paired scFv library, we utilized mRNA display (25–27), a well-established cell-free screening approach that allows rapid screening for protein binders. Briefly, each scFv was covalently linked to the RNA molecule that encoded it, thus providing a phenotype-genotype linkage (fig. S4A). The mRNA-displayed antibody library was then selected against seven HAs from different influenza A subtypes and influenza B strains, namely H1N1 A/Solomon Island/3/06 (H1/SI06), H1N1 A/Michigan/45/2015 (H1/MI15), H3N2 A/Singapore/INFIMH-16–0019/2016 (H3/SP16), H5N1 A/Qinghai/1A/2005 (H5/QH05), H7N9 A/Shanghai/2/2013 (H7/SH13), B/Phuket/3073/2013 (B/Phu13), and B/Lee/1940 (B/Lee40). Selections were also performed against two HA stem domain constructs that were designed based on H1N1 A/Brisbane/59/2007 HA and H3N2 A/Finland/486/2004 HA, respectively (28, 29) (Fig. 2 and fig. S4). After one round of selection, the pre- and post-selection libraries were analyzed by PacBio sequencing to quantify the enrichment of each scFv (data file S3). Pearson correlation coefficients ranging from 0.58 to 0.86 were observed between biological replicates (fig. S5), demonstrating the reproducibility of the selections. In total, 114 of the 325 scFvs were identified to target one of the HAs screened, with 52 targeting more than one HA and 11 targeting more than two HAs (data file S4). All stem antibody controls were enriched in at least one screens, whereas only 17 of the 30 head antibody controls were enriched, likely due to the considerably limited binding breadth of head antibodies. Moreover, the stem antibody controls were highly enriched in the selections against the HA stem domain constructs, whereas the head antibody controls were not, further validating that selection took place.
Figure 2. mRNA display enables rapid specificity characterization of the natively paired antibodies.

The screening results of each scFv are shown as a heatmap. The X-axis labels represent the scFv names. The Y-axis labels represent each individual screen. Screens against CR9114 IgG pre-bound HAs are indicated by “+CR9114”. Binding scores for each screen was calculated based on results from two biological replicates (Materials and Methods). Individual cutoffs were set for each screen to determine positive hits (table S4). Black circles at the top of the heatmap indicate head antibody controls, whereas empty circles indicate stem antibody controls.
We also performed seven competition screens against CR9114 (30), a broadly neutralizing HA stem antibody, for binding to H1/SI06, H1/MI15, H3/SP16, H5/QH05, H7/SH13, and B/Phu13, and B/Lee40 (Fig. 2 and fig. S6). Competition screens were performed using the same protocol as the mRNA display selections described above, except that CR9114 was pre-bound to the HAs. Whereas the CR9114 competition screen for H3/SP16 did not yield consistent PCR bands post-selection, all six other screens resulted in reproducible bands and were analyzed by PacBio sequencing (fig. S6B and C). Of these six screens, five had Pearson correlation coefficients of >0.7 between replicates (fig. S7), demonstrating their reproducibility. The results of our competition screening were in agreement with previous studies. For example, two of our stem antibody controls, AG11–2F01 and 31.a.55, competed with CR9114 in our screens, which is consistent with previous studies of these two antibodies (8, 15). Additionally, 15 of the 17 (88%) HA head antibody controls targeting at least one HA in our screens did not compete with CR9114, further supporting the effectiveness of the competition screen (data file S4). Together, we identified five antibody candidate targeting group 1 HA stem, 12 targeting group 2 HA stem, as well as 28 targeting influenza B HA stem (fig. S5 and data file S4). Of note, one of the H3 HA stem-reactive antibody candidates, AG2-G02, was concurrently identified by a machine learning approach and experimentally confirmed in another study of ours (14).
oPool+ display demonstrates robust performance in specificity characterization
To systematically evaluate the performance of oPool+ display, we used both biolayer interferometry (BLI) and enzyme-linked immunosorbent assay (ELISA) to validate the binding activities of selected antibodies (Fig. 3). In brief, 25 antibodies were selected, recombinantly expressed, and purified in fragment antigen-binding (Fab) and IgG formats. Their binding activities were then tested against all nine HA constructs used in our screens (Fig. 3A and B, fig. S8). This validation experiment substantiated the robust performance of oPool+ display. A true positive rate of 80.8% (42/52) and a true negative rate of 96.0% (166/173) was observed in BLI validation with Fabs, whereas a true positive rate of 71.7% (43/60) and a true negative rate of 96.4% (159/165) was observed in ELISA validation with IgG (Fig. 3C). Of note, both experimental validations indicated that the majority of the false positive and false negative results (10/17 for BLI, 14/23 for ELISA) were from three screens (H3 HA stem, H1/SI06, and H1/MI15). Furthermore, eight of the 25 (32.0%) scFvs had relatively low occurrence frequencies in the pre-selection library (<10−3 in both replicates), contributing to 6 of the 17 (35.3%) false positive and negative results based on BLI validation and 7 of the 23 (30.4%) false positive and negative results based on the ELISA validation. Therefore, the reliability of the screening results did not appear to decrease for scFvs with low occurrence frequencies. To examine the influences of different antibody formats on binding, we determined the dissociation constant (KD) of three H1 HA stem antibodies and six H3 HA stem antibodies that were enriched in our screen in both Fab and scFv formats (Fig. 3D, fig. S9 and S10). All nine antibodies retained binding in both formats, with no apparent correlation between the dissociation constant (KD) values. However, some of the false negatives could still be due to weakened binding of the antibody in scFv format during the screens.
Figure 3. Systematic validation reveals the robust performance of oPool+ display.
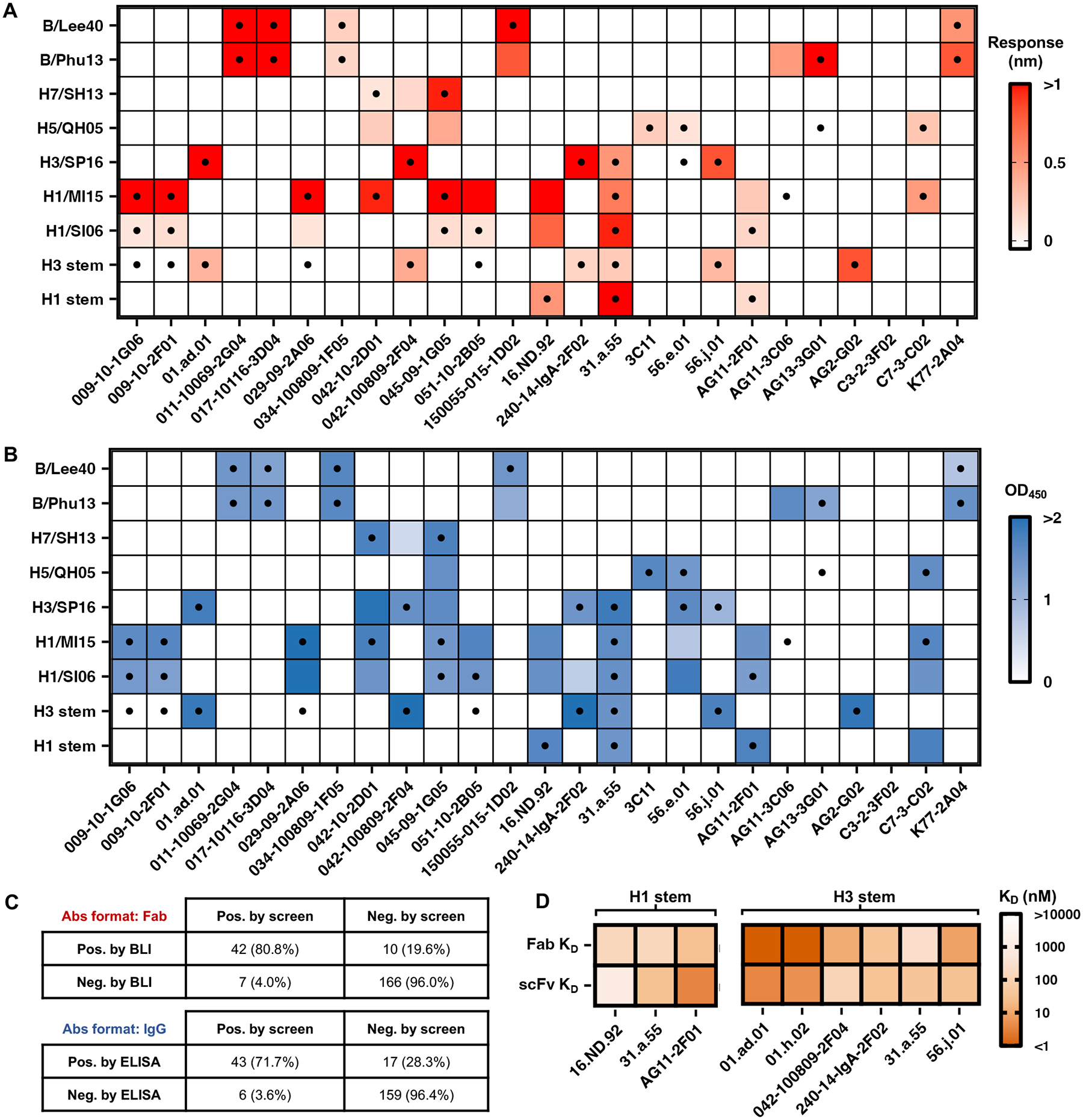
(A and B) Binding activity of 25 antibodies against different HAs was measured by BLI with antibodies in Fab format (A) and ELISA with antibodies in IgG format (B). The response signals during the association phase (A) and the average OD450 values across two technical replicates (B) are shown as heatmaps. The dots represent the positive HA binding in oPool+ display. The X-axis labels represent antibody names. The Y-axis labels represent antigen names. (C) Binary confusion matrices based on BLI and ELISA validations are shown. Pos., positive; Neg., negative. (D) Binding affinity of selected HA stem antibody candidates in both scFv and Fab format was measured by BLI. Their dissociation constants (KD) against H1 HA stem and H3 HA stem are shown as heatmaps. 31.a.55 was a positive control for binding to both H1 HA stem and H3 HA stem(8), AG11–2F01 was a positive control for binding to H1 HA stem (15), and 042-100809-2F04 was a positive control for binding to H3 HA stem (16).
We then further assessed the performance of our CR9114 competition screens using 16 of the 25 antibodies validated above (Fig. 4A and B). To quantify the competition, we first defined the CR9114 competition index as the log fold change of enrichments with versus without the presence of CR9114 during screen (data file S4, fig. S11). A higher CR9114 competition index would indicate overlapping epitopes between the scFv and CR9114, whereas a lower CR9114 competition index would indicate opposite (Fig. 4A). Experimental validation of these competition indices was then performed using BLI, where the ratio of binding responses to HA with and without CR9114 pre-bound was quantified. A Pearson correlation of 0.66 was observed between the competition indices and the validation results (Fig. 4B, fig. S12, and table S1), confirming the reliability of our CR9114 competition screens. Together, our results showed that oPool+ display enabled rapid and accurate specificity characterization of natively paired antibodies.
Figure 4. oPool+ display result infers CR9114 competition.

(A) CR9114 competition (Comp.) indices of validated antibodies. The competition indices calculated from oPool+ display are shown. High positive values indicate CR9114 competition, low positive and high negative values indicate no CR9114 competition. (B) CR9114 competition indices of validated antibodies based on BLI results are shown as scatter plot against their oPool+ display competition indices. The response of each antibody binding to the HA antigen were measured during the association step of BLI with or without prior saturation binding of CR9114. The percentage of competition is shown with the range of 0% (no competition) to 100% (complete competition). The Pearson correlation coefficient (R) between the screening and validation results is indicated. The linear fit lines are shown as red dash lines. Representative antibodies are labelled.
AG11-2F01 and 16.ND.92 have similar sequence features but distinct binding modes
One of the H1 HA stem antibodies identified from our screen was 16.ND.92, which was originally isolated from a young individual in an H5N1 influenza vaccine trial (8). 16.ND.92 was encoded by IGHV3–74/IGHD3–3/IGKV1–5 (data file S4). Coincidentally, both IGHD3–3 and IGKV1–5 were utilized by AG11–2F01, which was one of the two positive controls against H1 HA stem in our screen (Fig. 2 and fig. S5). Moreover, 16.ND.92 and AG11–2F01 shared a similar FG[V/L] motif encoded by the reading frame +3 of IGHD3–3 (fig. S13). This observation led us to hypothesize that 16.ND.92 and AG11-2F01 engaged the HA stem through similar binding modes. Consequently, we determined the cryo-EM structures of H1N1 A/Solomon Islands/03/2006 (H1/SI06) HA in complex with AG11–2F01 and 16.ND.92 to resolutions of 2.89 Å and 2.82 Å, respectively (Fig. 5A to E and table S2). Contrary to our hypothesis, the cryo-EM structures revealed very different binding modes between the two antibodies. Whereas AG11-2F01 bound to HA stem horizontally, 16.ND.92 had a downward approaching angle towards HA (Fig. 5A). Relatedly, the epitope of 16.ND.92 shifted slightly upward compared with that of AG11–2F01 (Fig. 5B).
Figure 5. Structural analysis of AG11–2F01 and 16.ND.92 reveals distinct binding mode of IGHD3–3 HA stem antibodies.

(A) Cryo-EM structures of AG11–2F01 and 16.ND.92 in complex with H1/SI06 HA. HA1 is in light gray. HA2 is in dark gray. Heavy chain variable domain (VH) is in orange. Light chain variable domain (VL) is in pink. (B) Epitopes of AG11–2F01 and 16.ND.92 are shown. VH contacts are in orange. VL contacts are in pink. Contacts shared by both VH and VL are in purple. (C) Interactions between light chain and HA are shown. H-bonds are represented by black dashed lines. (D) Interactions between CDR H3 and HA are shown. (E) Overlay of the CDR H3 loops from IGHD3–3 HA stem-reactive antibodies. HA is in surface representation. (F) Contributions of CDR H3 (orange), non-CDR H3 VH (yellow), and VL (pink) to the paratope buried surface areas (BSA) of the indicated antibodies. (G) Contributions of IGHD3–3-encoded residues to VH paratope BSA of the indicated antibodies.
Although both 16.ND.92 and AG11–2F01 were encoded by IGKV1–5, their light chains interacted with the HA stem differently. For example, VL S30 of AG11–2F01 H-bonded with HA2 Q38, whereas that of 16.ND.92 H-bonded with HA1 K32 (Fig. 5C). Similarly, despite sharing an FG[V/L] motif in their IGHD3–3-encoded regions, 16.ND.92 and AG11–2F01 used this motif to interact with different parts of the HA stem (Fig. 5D). For the FGL motif in AG11–2F01, VH F100 inserted into a hydrophobic pocket in the HA stem centering at HA2 I48, whereas VH L100b inserted into a lower pocket centering at HA2 W21. By contrast, this lower pocket was occupied by the VH F100a of the FGV motif in 16.ND.92. The paratope of 16.ND.92 also involved IGHD3–3-encoded VH V100, I100e, and I100f, allowing its CDR H3 to bind to upper pockets in the HA stem that were not engaged by AG11–2F01 (Fig. 5D). Together, our structural analyses showed that AG11–2F01 and 16.ND.92 formed distinct molecular interactions with HA stem.
16.ND.92 utilizes IGHD3-3 in a unique manner for binding to the HA stem
Previous studies have determined the structures of several HA stem antibodies with an FG[V/L/I] motif in the CDR H3 that was encoded by reading frame +3 of IGHD3–3, including MEDI8852, 56.a.09, 54–1G05, 39.29, PN-SIA28, and 429 B01 (8, 31–35). These six HA stem antibodies used either IGHV6–1 or IGHV3–30, unlike AG11–2F01 and 16.ND.92, which used IGHV4-38-2 and IGHV3–74, respectively. Nevertheless, the CDR H3 conformation of AG11–2F01 resembled that of MEDI8852, 56.a.09, 54–1G05, 39.29, PN-SIA28, and 429 B01 (Fig. 5E). Moreover, the IGHD3–3-encoded FG[V/L/I] motifs of these seven antibodies bound to the HA stem in a similar fashion (Fig. 5E). In comparison, the CDR H3 conformation of 16.ND.92 was different due to more extensive involvement of IGHD3–3 in binding (Fig. 5D and E).
The unique usage of IGHD3–3 for binding enables 16.ND.92 VH to interact with the HA stem exclusively through CDR H3, whereas the VH paratopes of other IGHD3–3 HA stem antibodies involved non-CDR H3 regions (Fig. 5F and table S3). Similarly, IGHD3–3 accounted for 98.6% of the buried surface area of the 16.ND.92 VH paratope, but 38% to 63% of the VH paratopes of other IGHD3–3 HA stem antibodies (Fig. 5G and table S3). These observations not only substantiated that reading frame +3 of IGHD3–3 was a recurring sequence feature of HA stem antibodies, but also demonstrated that it could pair with diverse IGHV genes and interact with the HA stem through different binding modes.
AG11-2F01 and 16.ND.92 are neutralizing antibodies with in vivo protection activity
Given the different binding modes of AG11–2F01 and 16.ND.92, we further aimed to compare their binding breath, in vitro neutralization, and in vivo protection activity. ELISA showed that both AG11–2F01 and 16.ND.92 bound to all H1 and H5 HAs tested (Fig. 6A and fig. S14). Microneutralization assay against six H1N1 strains as well as one H5N1 strain further revealed their neutralizing activity (Fig. 6B). AG11–2F01 showed the strongest binding and neutralization against H5N1 A/bald eagle/Florida/W22–134-OP/2022 (H5/FL22) among all strains tested. H5/FL22 belongs to the 2.3.4.4b clade that is currently circulating in dairy cattle and has led to human infection, including one recent fatality in the US (36–40). Of note, our H5/FL22 was a recombinant virus with the multibasic cleavage site removed and was rescued using the six internal segments from H1N1 A/Puerto Rico/8/1934 (PR8) to minimize biosafety concerns. We then evaluated the in vivo protection activity of AG11–2F01 and 16.ND.92. In brief, mice were given the indicated antibody at 5 mg/kg intraperitoneally at 4 hours pre-infection (prophylaxis) or 4 hours post-infection (therapeutics). AG11–2F01 and 16.ND.92 protected mice against a lethal challenge of H1N1 PR8, based on the weight loss profiles (Fig. 6C and D) and survival analyses (Fig. 6E and F). We also measured lung viral titers at day 3 post-infection (Fig. 6G and H). Whereas 20% (1/5) and 80% (4/5) of the mice therapeutically treated with AG11–2F01 and 16.ND.92 survived (Fig. 6E), 100% (5/5 and 5/5) of the mice prophylactically treated with AG11–2F01 and 16.ND.92 survived (Fig. 6F). None of the control-treated mice survived (Fig. 6E and F). These results demonstrated the protective ability of the IGHD3–3 HA stem antibodies.
Figure 6. AG11–2F01 and 16.ND.92 confer in vitro neutralization and in vivo protection.
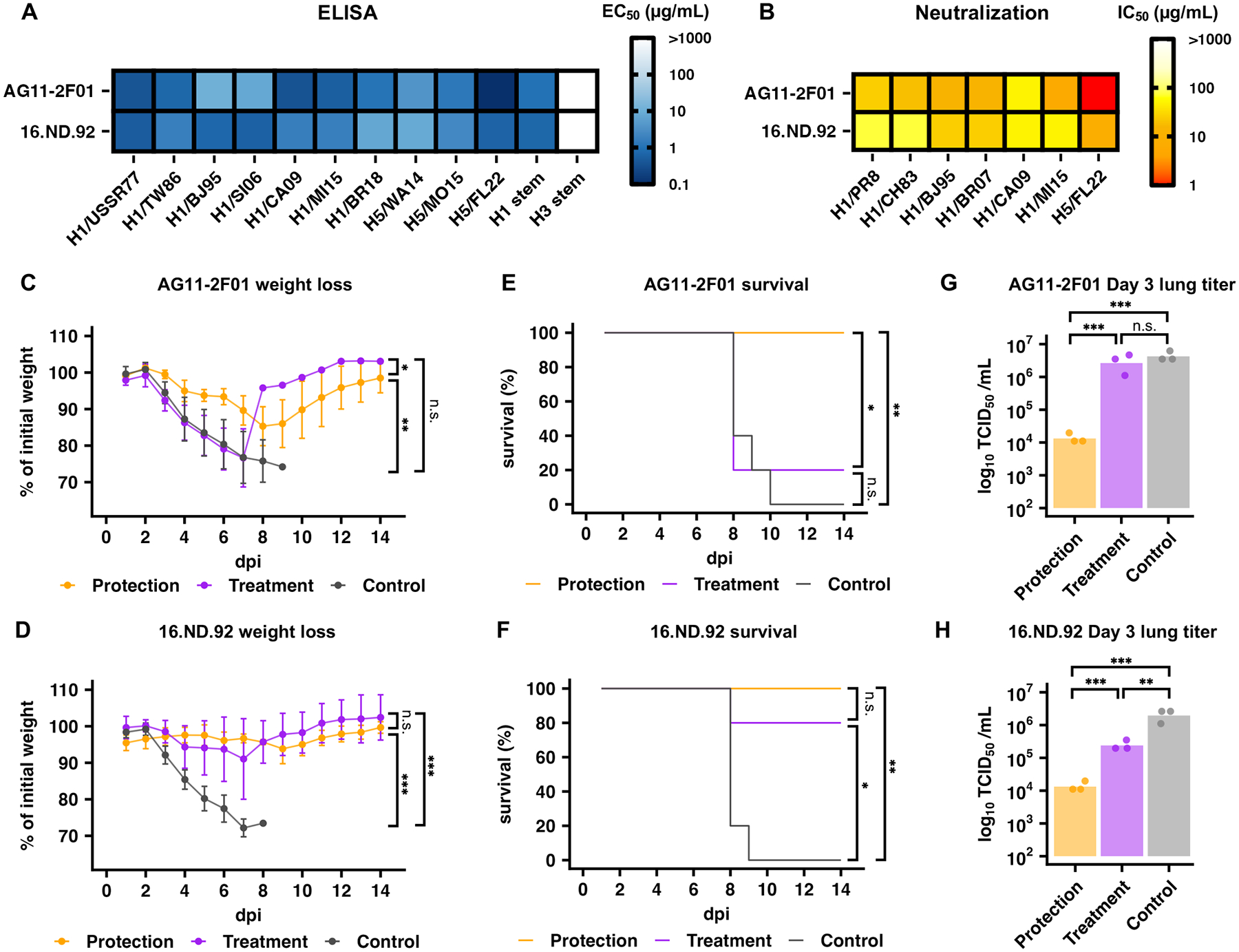
(A) The binding activities of AG11–2F01 and 16.ND.92 against recombinant HA proteins from the indicated H1 and H5 strains were measured by ELISA. The half-maximal effective concentration (EC50) values are shown as a heatmap. Technical duplicates were performed. (B) The neutralization activity of AG11–2F01 and 16.ND.92 against different recombinant H1N1 viruses was measured by a microneutralization assay. The half-maximal inhibitory concentration (IC50) values are shown as a heatmap. For (A) and (B) Strain names are abbreviated as follows: H1N1 A/Puerto Rico/8/1934 (H1/PR8), H1N1 A/USSR/90/1977 (H1/USSR77), H1N1 A/Chile/1/1983 (H1/CH83), H1N1 A/Taiwan/01/1986 (H1/TW86), H1N1 A/Beijing/262/1995 (H1/BJ95), H1N1 A/Solomon Island/3/2006 (H1/SI06), H1N1 A/Brisbane/59/2007 (H1/BR07), H1N1 A/California/04/2009 (H1/CA09), H1N1 A/Michigan/45/2015 (H1/MI15), H1N1 A/Brisbane/02/2018 (H1/BR18), H5N8 A/northern pintail/WA/40964/2014 (H5/WA14), H5N2 A/snow goose/Missouri/CC15–84A/2015 (H5/MO15), and H5N1 A/bald eagle/Florida/W22–134-OP/2022 (H5/FL22). H1 HA stem and H3 HA stem represent the HA stem constructs designed based on H1N1 A/Brisbane/59/2007 HA and H3N2 A/Finland/486/2004 HA, respectively (28, 29). Technical duplicates were performed. (C to H) The in vivo protective activity of AG11–2F01 (C, E, and G) and 16.ND.92 (D, F, and H) given 4 hours before (Protection) or after (Therapeutic) infection was assessed using lethal challenge of PR8 virus (n= 5 for all groups in C to F; n=3 for all groups in G and H; separate groups of mice were followed for weight loss and survival versus lung viral titers). Shown are weight loss profiles (C and D), Kaplan-Meier survival curves (E and F), and lung viral titers at day 3 post-infection (G and H). dpi, days post infection. Data in (C and D) represent mean ± standard deviation. Bars in (G) and (H) represent mean values. Two-way ANOVA followed by Tukey’s post hoc tests were performed in (C), (D), (G), and (H). Mantel-Cox log rank tests were performed in (E) and (F). n.s.: not significant; *:P < 0.05; **:P < 0.01; ***:P < 0.001.
DISCUSSION
Antibody discovery has led to major advancements on many fronts, including antibody-based therapeutics as well as vaccine design (9, 41–44). Discovery of natively paired antibody sequences has been hugely accelerated by scBCR-seq in the past few years (1). However, going from sequence information to specificity characterization remains a major bottleneck in antibody discovery. In this study, we presented oPool+ display, an experimental platform that allows specificity characterization of antibodies with defined sequences in a highly parallel fashion. Importantly, oPool+ display was more cost-efficient (approximately $30 per antibody) and faster (approximately 3–5 days hands-on time and approximately 5 days hands-off time, from oligo design to screening results) than the conventional methods that require cloning and recombinant expression of individual antibodies (approximately $200–350 per antibody, weeks to months), as oPool+ display leveraged the cost-efficiency of the oligo pool synthesis and multiplexed screening methods (table S4). As a proof-of-concept, we applied oPool+ display to delineate the binding specificity of hundreds of HA antibodies that were left uncharacterized in the literature. Follow-up analysis of AG11–2F01 and 16.ND.92 further revealed the versatility of IGHD3–3 in targeting the HA stem.
A key feature of oPool+ display is its relatively simple protocol. A previous study has shown that the throughput for screening antibodies with defined sequences can be increased by using liquid handlers to express individual antibodies one by one (45). In comparison, oPool+ display uses a near one-pot strategy for antibody library synthesis and screening. As suggested by our results, one 96-well plate PCR can allow rapid assembly of library up to 20,000 natively paired scFvs. With current display technologies, such as mRNA display, screening of the library against 10 to 20 antigens can be done by one person in hours. Moreover, it only requires standard benchtop equipment commonly found in a regular molecular biology lab. In addition, the constructed library can be stored long-term as DNA for future use when new antigens of interest emerge, such as novel HA variants or subtypes. The library can also be expanded by merging with additional oligo pools when new panels of antibodies are discovered. Therefore, oPool+ display not only bridges the gap between scBCR-seq and protein display technologies through massively parallel reconstruction of sequenced antibodies, but also provide more flexibility of the current antibody discovery pipelines. After the natively paired antibody sequences are obtained from scBCR-seq, oPool+ display can be applied to validate and characterize the specificity of a large panel of antibody candidates at any time. The synergy between oPool+ display and scBCR-seq can streamline the transition from antibody discovery to antibody characterization.
Previous studies have shown that IGHV6–1 and IGHV3–30 HA stem antibodies often utilize IGHD3–3-encoded FG[V/L/I] motif for binding to HA stem (8, 31–35). As demonstrated by our work here, IGHD3–3-encoded FG[V/L/I] motif can also pair with other IGHV genes to target HA stem, substantiating that it is an IGHV-independent recurring sequence feature of HA stem antibodies. Our results further revealed that IGHD3–3 can engage the HA stem through different binding modes. These observations are comparable to those of IGHD3–9, which is utilized by HA stem antibodies with various IGHV genes and can bind to HA stem in two different reading frames (12). Similarly, recent studies have identified IGHD3–22 as an IGHV-independent recurring sequence feature of antibodies that target a conserved site on the SARS-CoV-2 spike protein (46, 47). Although antibody sequence analysis typically focuses on IGHV genes, the contribution of IGHD genes to antibody responses should not be overlooked, since emerging evidence suggests that it might be more important than previously thought.
Although this study focused on influenza HA stem as a proof-of-concept, oPool+ display can be generalized to any antigens of interest as long as they can be recombinantly purified. Importantly, oPool+ display can be leveraged for epitope mapping, given that it enables competition screening. Such application will be particularly valuable for antigens that have largely unknown antigenicity but have several antibodies with known epitopes, such as those from emerging pathogens (48, 49). Furthermore, the capability of constructing custom-made antibody libraries means that oPool+ display has the potential to benefit the development of machine learning models for antibody engineering, specificity prediction, and de novo design, as a major throughput bottleneck still exists in experimental validation (14, 50–52). We envision that prediction results from these models can be rapidly validated by oPool+ display, which will in turn facilitate iterative refinement of the models for more advanced applications.
We acknowledge that oPool+ display has some limitations. First, oPool+ display requires antibodies to be presented as scFvs, which may lose functionality compared to its Fab counterpart (53–55). Second, antibodies with a fast off-rate may result in false negatives in oPool+ display, since it depends on monovalent binding. Inadequate wash during selection could also partially explain the false positive hits in our results. Nonetheless, a few solutions can be adopted in future studies. Performing multiple rounds of mRNA display selection, or replacing it with other protein display technologies that support multivalent binding, such as yeast display and phage display, can further reduce false negative rate, though throughput needs to be taken into consideration to accommodate the diversity of assembled libraries (56, 57). In addition, a more stringent wash protocol can potentially reduce false positive rate. Nevertheless, antibodies with weak affinity, which may exhibit similar neutralization activity as those with stronger affinity (58), would be missed under a highly stringent wash protocol. Therefore, the optimum wash protocol depends on the purpose of the application. As the length of oligo pool synthesis continues to improve, the cost and complexity of oPool+ display will further decrease. Overall, we believe that oPool+ display represents a starting point for the future advancement of high-throughput approaches to characterize antibodies.
MATERIALS AND METHODS
Study design
The objective of this study was to develop a strategy that can streamline antibody binding characterization. The HA antibodies in this study were chosen from a paired chain HA antibody sequence dataset we previously curated. The selection criteria is described below and in fig. S1. The library assembly design was first tested in low complexity, where oligos consisting of 10 antibodies were synthesized and assembled to optimize conditions. The screening method was first tested with positive and negative control antibodies in single-construct screens. Washing and elution conditions were optimized to reduce potential false positive and false negative results. We opted to validate the screening results using two different antibody formats (IgG and Fab) and two orthogonal methods (BLI and ELISA) to ensure rigor and reproducibility. All mouse experiments were performed in compliance with best practice, and sample sizes were determined based on previous literature. No data was excluded, and no outliers were observed in the study.
Selection of HA antibodies for paired antibody library synthesis
Members of the natively paired antibody library were selected from a previously curated dataset containing 5,561 human monoclonal antibodies to influenza HA from 60 research publications and three patents (14). Filters were applied to exclude antibodies that 1) had incomplete sequence information, 2) utilized germline genes that were regarded as recurring sequence features of HA stem antibodies, namely IGHV1–69, IGHV6–1, IGHV1–18 and IGHD3-9 (8, 10–12), and 3) were members of known HA stem antibody clonotypes. This resulted in 292 antibody sequences from 7 publications. Three HA stem antibodies, 31.a.55, AG11-2F01, and 042-100809-2F04, as well as 30 HA head antibodies were randomly selected as positive and negative controls (7, 8, 13, 15, 17–24). Of note, both AG11-2F01 and 042-100809-2F04 were not previously labeled as an HA stem antibody in the curated dataset (14). Through literature search, AG11-2F01 was found to compete with CR9114 (30), which is an HA stem antibody, for binding to H1 (15), whereas 042-100809-2F04 was found to bind to group 2 HA stem domain.
Overlap PCR assembly of the natively paired antibody library
A total of 13 oligo pools were synthesized (Integrated DNA Technologies). The lengths of oligos ranged from around 180 to 330 nucleotides. Each oligo pool contained 100 oligos resuspended in 200 μL water. An assembly PCR was set up for each oligo pool using 1,600 ng of oligos as input. The assembly was performed using KAPA HiFi HotStart ReadyMix (Roche) and a Mastercycler nexus GX2 (Eppendorf). PCR was set up in the absence of primers. From cycles 1 to 40, PCR was performed with minimal ramp rate (0.1°C/s) in between the denaturing (98°C, 20 s) and annealing steps (62°C, 15 s) to reduce erroneous annealing events. After cycle 40, a universal forward primer 5’-TTC TAA TAC GAC TCA CTA TAG GGA CAA TTA CTA AAG GAG TAT CC-3’ and a universal reverse primer 5’-GGA GCC GCT ACC CTT ATC GTC GTC ATC CTT GTA ATC GGA TCC T-3’ were added to the PCR. The italicized region in the forward primer sequence is the T7 promoter, whereas the italicized region in the reverse primer sequence encodes a FLAG tag. Subsequently, another 15 cycles of PCR were performed to amplify the assembled product. The final PCR product was purified using a Monarch Gel Extraction Kit (New England Biolabs). Two technical replicates of the assembly were performed.
Antibody screening using mRNA display
The mRNA display was performed based on the protocols provided by previous studies (26, 27, 59) with slight modifications. Two biological replicates were performed for all screens. The DNA library was first transcribed by a MEGAscript T7 Transcription Kit (Thermo Fisher Scientific) and purified by a MEGAclear Transcription Clean-Up Kit (Thermo Fisher Scientific) according to manufacturer’s instructions. Ligation was performed using 1 nmol of the mRNA product, 1.1 nmol of the splint oligo (5’-TTT TTT TTT TTT GGA GCC GCT ACC-3’), and 1.2 nmol of the puromycin linker (5’-/5Phos/-d(A)21-(C9)3-d(ACC)-puromycin-3’) by the T4 DNA ligase (New England Biolabs) in a 100 μL reaction for 1 hour at room temperature, followed by Lambda exonuclease (New England Biolabs) digestion for 30 mins at 37°C. The puromycin-conjugated mRNA product was purified using a Dynabeads mRNA DIRECT Purification Kit (Thermo Fisher Scientific), aliquoted, and stored at −20°C until used.
The puromycin-conjugated mRNA templates were translated using a PURExpress In Vitro Protein Synthesis Kit (New England Biolabs) with the addition of PURExpress Disulfide Bond Enhancer (New England Biolabs) for 1 hour at 37°C. The reaction was then incubated with 500 mM KCl and 60 mM MgCl2 for at least 30 mins at room temperature to promote fusion between the translated scFv and puromycin. EDTA was then added to dissociate ribosomes. The full-length mRNA-scFv product was then purified using Anti-FLAG M2 Magnetic Beads (Millipore Sigma) followed by elution using 3×FLAG peptides (GlpBio). Subsequently, the purified mRNA-scFv product was reverse transcribed using SuperScript IV reverse transcriptase (Thermo Fisher Scientific). The cDNA/mRNA-scFv product was referred as the “pre-selection library”.
Biotinylated HA constructs were coated onto the Dynabeads M280-straptavidin (Thermo Fisher Scientific) according to the manufacturer’s instruction. Briefly, 150 pmol of biotinylated proteins were incubated with 50 μL of the beads for 30 mins to 1 hour at room temperature with gentle rotation. The beads were then washed with TBST (20 mM Tris-HCl at pH 7.5, 100 mM NaCl, and 0.025% Tween-20) five times using the DynaMag-2 magnetic holder (Thermo Fisher Scientific) and then resuspended to the original volume.
Selection of antibodies against HA constructs were carried out in parallel. Briefly, the pre-selection library was mixed with 25 μL of beads coated with each HAs and incubated for 1 hour at room temperature with gentle rotation. After incubation, the beads were washed thrice with 400 L TBST. The beads were then resuspended in water. These samples were referred as the “post-selection libraries”.
The CR9114 competition screen was performed as describe above with the addition of a CR9114 blocking step prior to the selection. In brief, 25 μL beads coated with HAs were blocked with 2 μM CR9114 IgG (30), followed by the addition of 7.5 L of input library and incubation for 1 hour at room temperature with gentle rotation. After incubation, the beads were washed thrice with 400 μL TBST. The beads were then resuspended in water. These samples were referred as the “post-competition libraries”.
Analysis of next-generation sequencing data
Circular consensus sequences (CCSs) were generated from the raw subreads using SMRTLink v13.0, setting the parameters to require 99.9% accuracy and a minimum of 3 passes. CCSs in FASTQ format were parsed using the SeqIO module in BioPython (60) and filtered based on the base calling quality score, where any read with more than five nucleotides of phred quality score below 40 were removed. The adapter sequences were then identified on each read and trimmed from the scFv sequences. Reads that did not have the complete adapter sequences were also removed. The filtered reads were then aligned to the reference scFv sequences and classified into three categories: 1) natively paired scFvs with no mutation, 2) natively paired scFvs with mutation, 3) others (non-natively paired scFvs and incomplete assemblies). In our assembly coverage analysis, if any given scFv had correct assemblies yet only in one replicate, it would not be considered as successful. Additionally, only reads encoding natively paired scFvs with no mutation were used for downstream analysis, including assembly reproducibility, assembly coverage, as well as frequency/score calculation. Frequency (F) of a scFv i a given replicate k of a given antigen s was computed for each replicate as follows:
| (1) |
A pseudocount of 1 was added to each mutant to avoid division by zero in subsequent steps. We then calculated the enrichment (E) of a scFv i of a given replicate k of a given antigen s after the mRNA display selection as follows:
| (2) |
We calculated the mean enrichment of a scFv i of a given antigen s over two replicates, then inferred the binding score (BS) of a scFv i of a given antigen s using robust scaling:
| (3) |
Where median(E) represents the median of a given dataset, IQR(E) representing the interquartile range of a given dataset. Custom cutoffs were set for each screen to determine hits (table S5). For a given antigen s, the CR9114 competition index (CI) of a scFv i was computed as below:
| (4) |
Cryo-EM sample preparation, data collection, and data processing
The AG11–2F01 Fab was incubated with H1/SI06 HA on ice overnight followed by size exclusion chromatography. The peak fraction of the Fab-HA complex was concentrated to around 1 mg/mL for cryo-EM sample preparation. Cryo-EM grids were prepared using a Vitrobot Mark IV (Thermo Fisher Scientific). 3.5 μL of the sample was applied to a 300-mesh Quantifoil R1.2/1.3 Cu grid pretreated with glow-discharge. Excess liquid was blotted away using filter paper with blotting force −5 and blotting time 3 s. The grid was then flash frozen in liquid ethane. Movies were then collected on a Titan Krios microscope equipped with Gatan BioQuantum K3 imaging filter and camera (Thermo Fisher Scientific). Images were recorded at 130,000× magnification, corresponding to a pixel size of 0.33 Å/pixel at super-resolution mode of the camera. A defocus range of −0.8 μm to −1.5 μm was set. A total dose of 50 e−/Å2 of each exposure was fractionated into 50 frames. Both untilted and 30-degree-tilted data were collected and combined to alleviate the preferred orientation problem of the sample.
CryoSPARC (61) was used to process the cryo-EM data. For model building, ABodyBuilder (62) was used to generate an initial model for AG11–2F01 Fab. This model, together with the model of H1/SI06 HA (PDB 6FYT) (63), was fitted into the cryo-EM density map using UCSF Chimera (64). The model was manually adjusted in Coot (65) and refined with Phenix real-space refinement program (66). This process was iterated for several cycles until no substantial improvement of the model was observed.
The 16.ND.92 Fab was incubated with H1/SI06 HA and FISW84 Fab, a known HA anchor antibody (67), on ice overnight followed by size exclusion chromatography. The peak fraction of the Fab-HA complex was concentrated to around 3 mg/mL for cryo-EM sample preparation. 0.1% (w/v) of n-octyl-ß-D-glucoside was added to reduce orientation bias. Cryo-EM grids were prepared using a Vitrobot Mark IV (Thermo Fisher Scientific). 3 μL of the sample was applied to a 400-mesh Quantifoil R1.2/1.3 Cu grid pretreated with glow-discharge. Excess liquid was blotted away using filter paper with blotting force 0 and blotting time 3 s. The grid was then flash frozen in liquid ethane. Movies were then collected on a Titan Krios microscope equipped with Gatan BioQuantum K3 imaging filter and camera (Thermo Fisher Scientific). Images were recorded at 81,000 × magnification, corresponding to a pixel size of 0.53 Å/pixel at super-resolution mode of the camera. A defocus range of −0.5 μm to −5 μm was set. A total dose of 57.35 e−/Å2 of each exposure was fractionated into 40 frames.
CryoSPARC (61) was used to process the cryo-EM data. DeepEMhancer (68) was used to generate the sharpened density map for downstream model building. For model building, IgFold (69) was used to generate an initial model for 16.ND.92 Fab. This model, together with the model of H1/SI06 HA (PDB 6FYT) (63), was fitted into the cryo-EM density map using Phenix DockinMap module (70). The models were manually adjusted in Coot (65) and refined with Phenix real-space refinement program (66). This process was iterated for several cycles until no substantial improvement of the model was observed.
Microneutralization assay
For the microneutralization assay involving H1N1 viruses, MDCK-SIAT1 cells were seeded in 96-well plates. After reaching 100% confluency, MDCK-SIAT1 cells were washed once with 1 × PBS. Minimal essential media (Gibco) containing 25 mM HEPES (Gibco) was then added to the cells. Monoclonal antibodies were serially diluted 10-fold starting from 100 μg/ml and mixed with 100 TCID50 (median tissue culture infectious dose) of viruses at equal volume and incubated at 37°C for 1 hour. Subsequently, the mixture was inoculated into cells and incubated at 37°C for another hour. Cell supernatants were discarded and replaced with minimal essential media containing 25 mM HEPES, and 1 μg/mL TPCK-trypsin (Sigma). Plates were incubated at 37°C for 72 hours, and virus presence was detected by hemagglutination assay to determine the MN50 titers.
For the microneutralization assay involving H5N1 A/bald eagle/Florida/W22–134-OP/2022 virus on a PR8 backbone (6:2 reassortant) with the multibasic cleavage site removed, protocols were followed as previously described (71). Briefly, MDCK cells were seeded in 96-well plates at 2.5 × 10⁴ cells per well and incubated overnight at 37°C. Monoclonal antibodies were serially 2-fold diluted in MEM containing 1 μg/mL TPCK-treated trypsin and mixed with 100 TCID₅₀ of virus. After one hour of incubation at 37°C, antibody-virus mixtures were added to pre-washed MDCK cells and incubated for an additional hour. The inoculum was then removed, and cells were overlaid with corresponding antibody dilutions and incubated for 20 hours. Cells were fixed with 80% acetone, stained with anti-NP primary (Sigma) at 1:1000 filution, followed by HRP-conjugated secondary antibodies (Southern Biotech) at 1:1000 dilution, and developed using an ELISA substrate. Optical density was measured at 405 nm. All assays were performed in technical duplicate and independently repeated twice.
Mice and influenza infection
The animal experiments were performed in accordance with protocols approved by UIUC Institutional Animal Care and Use Committee (IACUC). Six-week-old female BALB/c mice (Jackson Laboratory) were used for all animal experiments. Female BALB/c mice at 6 weeks old (n = 5 per group) were anesthetized with isoflurane and intranasally infected with 5 × lethal dose (LD50) of recombinant PR8 virus. Mice were given the indicated antibody at a dose of 5 mg/kg intraperitoneally at 4 hours before infection (prophylaxis) or 4 hours after infection (therapeutics). Weight loss was monitored daily for 14 days. The humane endpoint was defined as a weight loss of 25% from initial weight at day 0. Of note, although the BALB/c mice used here were not modified to facilitate interaction with human IgG1, human IgG1 could interact with mouse Fc gamma receptors (72–74).To determine the lung viral titers at day 3 post-infection, lungs of infected mice were harvested and homogenized in 1 mL of minimal essential media with 10% bovine serum albumin using a gentleMACS C Tube (Miltenyi Biotec). Subsequently, virus titers were measured by TCID50 assay as described previously (75).
Statistical analysis
All data used for statistical analysis are provided in data file S5. Pearson correlation calculations, two-way ANOVA followed by Tukey’s post hoc test, and Mantel-Cox log rank tests were performed using R (76). The alpha level of 0.05 was used for all tests to determine significance. Four Parameter Logistic (4PL) Regression were performed using GraphPad Prism v.10.2.3 (GraphPad Software). Means and standard deviation were presented for weight loss. Means with individual level data were presented for the day 3 lung titers.
Supplementary Material
LIST OF SUPPLEMENTARY MATERIALS
Figures S1 to S14
Data file S1 to S5
ACKNOWLEDGEMENTS
We thank A. Olson, C. Brooke, D. Kranz, and S. Sligar for helpful discussion and the Roy J. Carver Biotechnology Center at the University of Illinois at Urbana-Champaign for assistance with PacBio sequencing. We thank K. Flatt and the Materials Research Laboratory Central Research Facilities at the University of Illinois at Urbana-Champaign for access to cryo-EM instrumentation during the screening of the Fab-HA complexes. We are grateful to the Cryo-EM Core and the Core Facility for Advanced Research Computing at Case Western Reserve University for the access of cryo-EM instrumentation and data processing. We also thank F. Vago and the Cryo-EM Facility at Purdue University for the access of cryo-EM instrumentation and data collection. We thank R. Webby for providing the H5N1/PR8 reassortant virus used in this study.
FUNDING
This work is supported by National Institutes of Health DP2 AI177692 (to J.J.G.), R01 AI167910 (to N.C.W.), DP2 AT011966 (to N.C.W.), the Searle Scholars Program (to N.C.W.), Howard Hughes Medical Institute Emerging Pathogens Initiative (to J.J.G. and N.C.W.), UIUC William T. and Lynn Jackson Graduate Student Fellowship (to W.O.O.), UIUC Gregorio Weber Graduate Student Fellowship (to W.O.O.), and Carl R. Woese Institute for Genomic Biology Postdoctoral Fellowship (to H.L.).
Footnotes
DECLARATION OF INTERESTS
N.C.W. consults for HeliXon. The authors declare no other competing interests.
DATA AVAILABILITY
All data associated with this study are in the paper or supplementary materials. Raw sequencing data have been submitted to the NIH Short Read Archive under accession number: BioProject PRJNA1150188. Cryo-EM density maps and coordinates have been deposited to EMDB and PDB with accession numbers: EMD-46727 and EMD-45930; PDB 9DBX and 9CU7. Biological materials including oligo pools and the assembled scFv library are available by contacting the corresponding author (N.C.W.). Custom scripts as well as raw data for experimental validations have been deposited to DOI: 10.5281/zenodo.15550057.
REFERENCES AND NOTES
- 1.Pedrioli A, Oxenius A, Single B cell technologies for monoclonal antibody discovery. Trends Immunol. 42, 1143–1158 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Valldorf B, Hinz SC, Russo G, Pekar L, Mohr L, Klemm J, Doerner A, Krah S, Hust M, Zielonka S, Antibody display technologies: selecting the cream of the crop. Biol. Chem 403, 455–477 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Persson MA, Caothien RH, Burton DR, Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc. Natl. Acad. Sci. U. S. A 88, 2432–2436 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheets MD, Amersdorfer P, Finnern R, Sargent P, Lindquist E, Schier R, Hemingsen G, Wong C, Gerhart JC, Marks JD, Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc. Natl. Acad. Sci. U. S. A 95, 6157–6162 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, García-Sastre A, Influenza. Nat. Rev. Dis. Primer 4, 1–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick E, Qiu X, Wilson PC, Bahl J, Krammer F, The influenza virus hemagglutinin head evolves faster than the stalk domain. Sci. Rep 8, 10432 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zost SJ, Wu NC, Hensley SE, Wilson IA, Immunodominance and antigenic variation of Influenza virus hemagglutinin: implications for design of universal vaccine immunogens. J. Infect. Dis 219, S38–S45 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joyce MG, Wheatley AK, Thomas PV, Chuang G-Y, Soto C, Bailer RT, Druz A, Georgiev IS, Gillespie RA, Kanekiyo M, Kong W-P, Leung K, Narpala SN, Prabhakaran MS, Yang ES, Zhang B, Zhang Y, Asokan M, Boyington JC, Bylund T, Darko S, Lees CR, Ransier A, Shen C-H, Wang L, Whittle JR, Wu X, Yassine HM, Santos C, Matsuoka Y, Tsybovsky Y, Baxa U, NISC Comparative Sequencing Program, Mullikin JC, Subbarao K, Douek DC, Graham BS, Koup RA, Ledgerwood JE, Roederer M, Shapiro L, Kwong PD, Mascola JR, McDermott AB, Vaccine-induced antibodies that neutralize group 1 and group 2 influenza A viruses. Cell 166, 609–623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu NC, Wilson IA, Influenza hemagglutinin structures and antibody recognition. Cold Spring Harb. Perspect. Med 10, a038778 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews SF, Graham BS, Mascola JR, McDermott AB, Is it possible to develop a “universal” influenza virus vaccine? Immunogenetic considerations underlying B-cell biology in the development of a pan-subtype influenza A vaccine targeting the hemagglutinin stem. Cold Spring Harb. Perspect. Biol 10, a029413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas L, Foglierini M, Piccoli L, Kallewaard NL, Turrini F, Silacci C, Fernandez-Rodriguez B, Agatic G, Giacchetto-Sasselli I, Pellicciotta G, Sallusto F, Zhu Q, Vicenzi E, Corti D, Lanzavecchia A, Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature 516, 418–422 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Wu NC, Yamayoshi S, Ito M, Uraki R, Kawaoka Y, Wilson IA, Recurring and adaptable binding motifs in broadly neutralizing antibodies to influenza virus are encoded on the D3–9 segment of the Ig gene. Cell Host Microbe 24, 569–578.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung CS-F, Fruehwirth A, Paparoditis PCG, Shen C-H, Foglierini M, Joyce MG, Leung K, Piccoli L, Rawi R, Silacci-Fregni C, Tsybovsky Y, Verardi R, Wang L, Wang S, Yang ES, Zhang B, Zhang Y, Chuang G-Y, Corti D, Mascola JR, Shapiro L, Kwong PD, Lanzavecchia A, Zhou T, Identification and structure of a multidonor class of head-directed influenza-neutralizing antibodies reveal the mechanism for its recurrent elicitation. Cell Rep. 32, 108088 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Lv H, Teo QW, Lei R, Gopal AB, Ouyang WO, Yeung Y-H, Tan TJC, Choi D, Shen IR, Chen X, Graham CS, Wu NC, An explainable language model for antibody specificity prediction using curated influenza hemagglutinin antibodies. Immunity, doi: 10.1016/j.immuni.2024.07.022 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry C, Zheng N-Y, Huang M, Cabanov A, Rojas KT, Kaur K, Andrews SF, Palm A-KE, Chen Y-Q, Li Y, Hoskova K, Utset HA, Vieira MC, Wrammert J, Ahmed R, Holden-Wiltse J, Topham DJ, Treanor JJ, Ertl HC, Schmader KE, Cobey S, Krammer F, Hensley SE, Greenberg H, He X-S, Wilson PC, Influenza virus vaccination elicits poorly adapted B cell responses in elderly individuals. Cell Host Microbe 25, 357–366.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry Dunand CJ, Leon PE, Kaur K, Tan GS, Zheng N-Y, Andrews S, Huang M, Qu X, Huang Y, Salgado-Ferrer M, Ho IY, Taylor W, Hai R, Wrammert J, Ahmed R, García-Sastre A, Palese P, Krammer F, Wilson PC, Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J. Clin. Invest 125, 1255–1268 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Henry Dunand CJ, Taylor WM, Lim S, Huang M, Qu X, Lee J-H, Salgado-Ferrer M, Krammer F, Palese P, Wrammert J, Ahmed R, Wilson PC, Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med 7, 316ra192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dugan HL, Guthmiller JJ, Arevalo P, Huang M, Chen Y-Q, Neu KE, Henry C, Zheng N-Y, Lan LY-L, Tepora ME, Stovicek O, Bitar D, Palm A-KE, Stamper CT, Changrob S, Utset HA, Coughlan L, Krammer F, Cobey S, Wilson PC, Preexisting immunity shapes distinct antibody landscapes after influenza virus infection and vaccination in humans. Sci. Transl. Med 12, eabd3601 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekiert DC, Kashyap AK, Steel J, Rubrum A, Bhabha G, Khayat R, Lee JH, Dillon MA, O’Neil RE, Faynboym AM, Horowitz M, Horowitz L, Ward AB, Palese P, Webby R, Lerner RA, Bhatt RR, Wilson IA, Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489, 526–532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry C, Palm A-KE, Utset HA, Huang M, Ho IY, Zheng N-Y, Fitzgerald T, Neu KE, Chen Y-Q, Krammer F, Treanor JJ, Sant AJ, Topham DJ, Wilson PC, Monoclonal antibody responses after recombinant hemagglutinin vaccine versus subunit inactivated influenza virus vaccine: a comparative study. J. Virol 93, e01150–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H, Voss J, Zhang G, Buchy P, Zuo T, Wang L, Wang F, Zhou F, Wang G, Tsai C, Calder L, Gamblin SJ, Zhang L, Deubel V, Zhou B, Skehel JJ, Zhou P, A human antibody recognizing a conserved epitope of H5 hemagglutinin broadly neutralizes highly pathogenic avian influenza H5N1 viruses. J. Virol 86, 2978–2989 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Tan H-X, Koutsakos M, Jegaskanda S, Esterbauer R, Tilmanis D, Aban M, Kedzierska K, Hurt AC, Kent SJ, Wheatley AK, Cross-lineage protection by human antibodies binding the influenza B hemagglutinin. Nat. Commun 10, 324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe A, McCarthy KR, Kuraoka M, Schmidt AG, Adachi Y, Onodera T, Tonouchi K, Caradonna TM, Bajic G, Song S, McGee CE, Sempowski GD, Feng F, Urick P, Kepler TB, Takahashi Y, Harrison SC, Kelsoe G, Antibodies to a conserved Influenza head interface epitope protect by an IgG subtype-dependent mechanism. Cell 177, 1124–1135.e16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Boutz DR, Chromikova V, Joyce MG, Vollmers C, Leung K, Horton AP, DeKosky BJ, Lee C-H, Lavinder JJ, Murrin EM, Chrysostomou C, Hoi KH, Tsybovsky Y, Thomas PV, Druz A, Zhang B, Zhang Y, Wang L, Kong W-P, Park D, Popova LI, Dekker CL, Davis MM, Carter CE, Ross TM, Ellington AD, Wilson PC, Marcotte EM, Mascola JR, Ippolito GC, Krammer F, Quake SR, Kwong PD, Georgiou G, Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat. Med 22, 1456–1464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts RW, Szostak JW, RNA-peptide fusions for the in vitro selection of peptides and proteins. Proc. Natl. Acad. Sci. U. S. A 94, 12297–12302 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu NC, Dai L, Olson CA, Lloyd-Smith JO, Sun R, Adaptation in protein fitness landscapes is facilitated by indirect paths. eLife 5, e16965 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka S, Olson CA, Barnes CO, Higashide W, Gonzalez M, Taft J, Richardson A, Martin-Fernandez M, Bogunovic D, Gnanapragasam PNP, Bjorkman PJ, Spilman P, Niazi K, Rabizadeh S, Soon-Shiong P, Rapid identification of neutralizing antibodies against SARS-CoV-2 variants by mRNA display. Cell Rep. 38, 110348 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RMB, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, de Man M, Ding Z, Apetri A, Kükrer B, Sneekes-Vriese E, Tomkiewicz D, Laursen NS, Lee PS, Zakrzewska A, Dekking L, Tolboom J, Tettero L, van Meerten S, Yu W, Koudstaal W, Goudsmit J, Ward AB, Meijberg W, Wilson IA, Radošević K, A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349, 1301–1306 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Corbett KS, Moin SM, Yassine HM, Cagigi A, Kanekiyo M, Boyoglu-Barnum S, Myers SI, Tsybovsky Y, Wheatley AK, Schramm CA, Gillespie RA, Shi W, Wang L, Zhang Y, Andrews SF, Joyce MG, Crank MC, Douek DC, McDermott AB, Mascola JR, Graham BS, Boyington JC, Design of nanoparticulate group 2 influenza virus hemagglutinin stem antigens that activate unmutated ancestor B cell receptors of broadly neutralizing antibody lineages. mBio 10, e02810–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJWM, Geelen E, Sahin Ö, Sieuwerts M, Brakenhoff JPJ, Vogels R, Li OTW, Poon LLM, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RHE, Highly conserved protective epitopes on influenza B viruses. Science 337, 1343–1348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kallewaard NL, Corti D, Collins PJ, Neu U, McAuliffe JM, Benjamin E, Wachter-Rosati L, Palmer-Hill FJ, Yuan AQ, Walker PA, Vorlaender MK, Bianchi S, Guarino B, Marco AD, Vanzetta F, Agatic G, Foglierini M, Pinna D, Fernandez-Rodriguez B, Fruehwirth A, Silacci C, Ogrodowicz RW, Martin SR, Sallusto F, Suzich JA, Lanzavecchia A, Zhu Q, Gamblin SJ, Skehel JJ, Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166, 596–608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu NC, Andrews SF, Raab JE, O’Connell S, Schramm CA, Ding X, Chambers MJ, Leung K, Wang L, Zhang Y, Mascola JR, Douek DC, Ledgerwood JE, McDermott AB, Wilson IA, Convergent evolution in breadth of two VH6–1-encoded Influenza antibody clonotypes from a single donor. Cell Host Microbe 28, 434–444.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda K, Huang J, Zhou T, Sheng Z, Kang BH, Ishida E, Griesman T, Stuccio S, Bolkhovitinov L, Wohlbold TJ, Chromikova V, Cagigi A, Leung K, Andrews S, Cheung CSF, Pullano AA, Plyler J, Soto C, Zhang B, Yang Y, Joyce MG, Tsybovsky Y, Wheatley A, Narpala SR, Guo Y, Darko S, Bailer RT, Poole A, Liang CJ, Smith J, Alexander J, Gurwith M, Migueles SA, Koup RA, Golding H, Khurana S, McDermott AB, Shapiro L, Krammer F, Kwong PD, Connors M, Prolonged evolution of the memory B cell response induced by a replicating adenovirus-influenza H5 vaccine. Sci. Immunol 4, eaau2710 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Wang F, Yin L, Jiang H, Lu X, Bi Y, Zhang W, Shi Y, Burioni R, Tong Z, Song H, Qi J, Gao GF, Structural basis for a human broadly neutralizing influenza A hemagglutinin stem-specific antibody including H17/18 subtypes. Nat. Commun 13, 7603 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura G, Chai N, Park S, Chiang N, Lin Z, Chiu H, Fong R, Yan D, Kim J, Zhang J, Lee WP, Estevez A, Coons M, Xu M, Lupardus P, Balazs M, Swem LR, An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe 14, 93–103 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Confirmations of highly pathogenic avian Influenza in commercial and backyard flocks, APHIS. https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/commercial-backyard-flocks.
- 37.Caserta LC, Frye EA, Butt SL, Laverack M, Nooruzzaman M, Covaleda LM, Thompson AC, Koscielny MP, Cronk B, Johnson A, Kleinhenz K, Edwards EE, Gomez G, Hitchener G, Martins M, Kapczynski DR, Suarez DL, Alexander Morris ER, Hensley T, Beeby JS, Lejeune M, Swinford AK, Elvinger F, Dimitrov KM, Diel DG, Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 634, 669–676 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen T-Q, Hutter CR, Markin A, Thomas M, Lantz K, Killian ML, Janzen GM, Vijendran S, Wagle S, Inderski B, Magstadt DR, Li G, Diel DG, Frye EA, Dimitrov KM, Swinford AK, Thompson AC, Snekvik KR, Suarez DL, Lakin SM, Schwabenlander S, Ahola SC, Johnson KR, Baker AL, Robbe-Austerman S, Torchetti MK, Anderson TK, Emergence and interstate spread of highly pathogenic avian influenza A(H5N1) in dairy cattle in the United States. Science 388, eadq0900 (2025). [DOI] [PubMed] [Google Scholar]
- 39.Garg S, Reinhart K, Couture A, Kniss K, Davis CT, Kirby MK, Murray EL, Zhu S, Kraushaar V, Wadford DA, Drehoff C, Kohnen A, Owen M, Morse J, Eckel S, Goswitz J, Turabelidze G, Krager S, Unutzer A, Gonzales ER, Abdul Hamid C, Ellington S, Mellis AM, Budd A, Barnes JR, Biggerstaff M, Jhung MA, Richmond-Crum M, Burns E, Shimabukuro TT, Uyeki TM, Dugan VG, Reed C, Olsen SJ, Highly Pathogenic Avian Influenza A(H5N1) Virus Infections in Humans. N. Engl. J. Med 392, 843–854 (2025). [DOI] [PubMed] [Google Scholar]
- 40.Mahase E, Bird flu: US reports first human death in person infected with H5N1. BMJ 388, r28 (2025). [DOI] [PubMed] [Google Scholar]
- 41.Lanzavecchia A, Frühwirth A, Perez L, Corti D, Antibody-guided vaccine design: identification of protective epitopes. Curr. Opin. Immunol 41, 62–67 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Haynes BF, Wiehe K, Borrow P, Saunders KO, Korber B, Wagh K, McMichael AJ, Kelsoe G, Hahn BH, Alt F, Shaw GM, Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat. Rev. Immunol 23, 142–158 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toussi SS, Hammond JL, Gerstenberger BS, Anderson AS, Therapeutics for COVID-19. Nat. Microbiol 8, 771–786 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Schoeder CT, Gilchuk P, Sangha AK, Ledwitch KV, Malherbe DC, Zhang X, Binshtein E, Williamson LE, Martina CE, Dong J, Armstrong E, Sutton R, Nargi R, Rodriguez J, Kuzmina N, Fiala B, King NP, Bukreyev A, Jr JEC, Meiler J, Epitope-focused immunogen design based on the ebolavirus glycoprotein HR2-MPER region. PLOS Pathog. 18, e1010518 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunt AC, Vögeli B, Hassan AO, Guerrero L, Kightlinger W, Yoesep DJ, Krüger A, DeWinter M, Diamond MS, Karim AS, Jewett MC, A rapid cell-free expression and screening platform for antibody discovery. Nat. Commun 14, 3897 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Iketani S, Guo Y, Reddem ER, Casner RG, Nair MS, Yu J, Chan JF-W, Wang M, Cerutti G, Li Z, Morano NC, Castagna CD, Corredor L, Chu H, Yuan S, Poon VK-M, Chan CC-S, Chen Z, Luo Y, Cunningham M, Chavez A, Yin MT, Perlin DS, Tsuji M, Yuen K-Y, Kwong PD, Sheng Z, Huang Y, Shapiro L, Ho DD, An antibody class with a common CDRH3 motif broadly neutralizes sarbecoviruses. Sci. Transl. Med 14, eabn6859 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H, Kaku CI, Song G, Yuan M, Andrabi R, Burton DR, Walker LM, Wilson IA, Human antibodies to SARS-CoV-2 with a recurring YYDRxG motif retain binding and neutralization to variants of concern including Omicron. Commun. Biol 5, 766 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson JE, Hastie KM, Cross RW, Yenni RE, Elliott DH, Rouelle JA, Kannadka CB, Smira AA, Garry CE, Bradley BT, Yu H, Shaffer JG, Boisen ML, Hartnett JN, Zandonatti MA, Rowland MM, Heinrich ML, Martínez-Sobrido L, Cheng B, de la Torre JC, Andersen KG, Goba A, Momoh M, Fullah M, Gbakie M, Kanneh L, Koroma VJ, Fonnie R, Jalloh SC, Kargbo B, Vandi MA, Gbetuwa M, Ikponmwosa O, Asogun DA, Okokhere PO, Follarin OA, Schieffelin JS, Pitts KR, Geisbert JB, Kulakoski PC, Wilson RB, Happi CT, Sabeti PC, Gevao SM, Khan SH, Grant DS, Geisbert TW, Saphire EO, Branco LM, Garry RF, Most neutralizing human monoclonal antibodies target novel epitopes requiring both Lassa virus glycoprotein subunits. Nat. Commun 7, 11544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Sun M, Zhang H, Zhang X, Yao Y, Li M, Li K, Fan P, Zhang H, Qin Y, Zhang Z, Li E, Chen Z, Guan W, Li S, Yu C, Zhang K, Gong R, Chiu S, Potent human neutralizing antibodies against Nipah virus derived from two ancestral antibody heavy chains. Nat. Commun 15, 2987 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joubbi S, Micheli A, Milazzo P, Maccari G, Ciano G, Cardamone D, Medini D, Antibody design using deep learning: from sequence and structure design to affinity maturation. Brief. Bioinform 25, bbae307 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett NR, Watson JL, Ragotte RJ, Borst AJ, See DL, Weidle C, Biswas R, Shrock EL, Leung PJY, Huang B, Goreshnik I, Ault R, Carr KD, Singer B, Criswell C, Vafeados D, Sanchez MG, Kim HM, Torres SV, Chan S, Baker D, Atomically accurate de novo design of single-domain antibodies. BioRxiv Prepr. Serv. Biol, 2024.03.14.585103 (2024). [Google Scholar]
- 52.Frey NC, Hotzel I, Stanton SD, Kelly RL, Alberstein RG, Makowski EK, Martinkus K, Berenberg D, Bevers J, Bryson T, Chan P, Czubaty A, D’Souza TA, Dwyer H, Dziewulska A, Fairman JW, Goodman A, Hofmann JL, Isaacson HH, Ismail AA, James S, Joren T, Kelow SP, Kiefer JR, Kirchmeyer M, Kleinhenz J, Koerber JT, Lafrance-Vanasse J, Leaver-Fay A, Lee JH, Lee E, Lee DW, Liang W-C, Lin JY-Y, Lisanza S, Loukas A, Ludwiczak J, Mahajan SP, Mahmood O, MohammadiPeyhani H, Nerli S, Park JW, Park J, Ra S, Robinson SA, Saremi S, Seeger F, Sinha I, Sokol AM, Spiess C, Tagasovska N, To HV, Wagstaff E, Wang A, Watkins AM, Wilson B, Wu S, Zadorozhny K, Marioni JC, Regev A, Wu Y, Cho K, Bonneau R, Gligorijevic V, Lab-in-the-loop therapeutic antibody design with deep learning. bioRxiv [Preprint] (2025). 10.1101/2025.02.19.639050. [DOI] [Google Scholar]
- 53.Wu NC, Grande G, Turner HL, Ward AB, Xie J, Lerner RA, Wilson IA, In vitro evolution of an influenza broadly neutralizing antibody is modulated by hemagglutinin receptor specificity. Nat. Commun 8, 15371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quintero-Hernández V, Juárez-González VR, Ortíz-León M, Sánchez R, Possani LD, Becerril B, The change of the scFv into the Fab format improves the stability and in vivo toxin neutralization capacity of recombinant antibodies. Mol. Immunol 44, 1307–1315 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Kang TH, Seong BL, Solubility, stability, and avidity of recombinant antibody fragments expressed in microorganisms. Front. Microbiol 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rondot S, Koch J, Breitling F, Dübel S, A helper phage to improve single-chain antibody presentation in phage display. Nat. Biotechnol 19, 75–78 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Chao G, Lau WL, Hackel BJ, Sazinsky SL, Lippow SM, Wittrup KD, Isolating and engineering human antibodies using yeast surface display. Nat. Protoc 1, 755–768 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He W-T, Limbo O, Smith C, Song G, Woehl J, Yang L, Abbott RK, Callaghan S, Garcia E, Hurtado J, Parren M, Peng L, Ramirez S, Ricketts J, Ricciardi MJ, Rawlings SA, Wu NC, Yuan M, Smith DM, Nemazee D, Teijaro JR, Voss JE, Wilson IA, Andrabi R, Briney B, Landais E, Sok D, Jardine JG, Burton DR, Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 369, 956–963 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olson CA, Wu NC, Sun R, A comprehensive biophysical description of pairwise epistasis throughout an entire protein domain. Curr. Biol 24, 2643–2651 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cock PJA, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, Friedberg I, Hamelryck T, Kauff F, Wilczynski B, de Hoon MJL, Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA, cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Leem J, Dunbar J, Georges G, Shi J, Deane CM, ABodyBuilder: automated antibody structure prediction with data–driven accuracy estimation. mAbs 8, 1259–1268 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laursen NS, Friesen RHE, Zhu X, Jongeneelen M, Blokland S, Vermond J, van Eijgen A, Tang C, van Diepen H, Obmolova G, van der Neut Kolfschoten M, Zuijdgeest D, Straetemans R, Hoffman RMB, Nieusma T, Pallesen J, Turner HL, Bernard SM, Ward AB, Luo J, Poon LLM, Tretiakova AP, Wilson JM, Limberis MP, Vogels R, Brandenburg B, Kolkman JA, Wilson IA, Universal protection against influenza infection by a multidomain antibody to influenza hemagglutinin. Science 362, 598–602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE, UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Emsley P, Cowtan K, Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Afonine PV, Poon BK, Read RJ, Sobolev OV, Terwilliger TC, Urzhumtsev A, Adams PD, Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. Sect. Struct. Biol 74, 531–544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benton DJ, Nans A, Calder LJ, Turner J, Neu U, Lin YP, Ketelaars E, Kallewaard NL, Corti D, Lanzavecchia A, Gamblin SJ, Rosenthal PB, Skehel JJ, Influenza hemagglutinin membrane anchor. Proc. Natl. Acad. Sci. U. S. A 115, 10112–10117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez-Garcia R, Gomez-Blanco J, Cuervo A, Carazo JM, Sorzano COS, Vargas J, DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol 4, 874 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruffolo JA, Chu L-S, Mahajan SP, Gray JJ, Fast, accurate antibody structure prediction from deep learning on massive set of natural antibodies. Nat. Commun 14, 2389 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Millán C, McCoy AJ, Terwilliger TC, Read RJ, Likelihood-based docking of models into cryo-EM maps. Acta Crystallogr. Sect. Struct. Biol 79, 281–289 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guthmiller JJ, Han J, Utset HA, Li L, Lan LY-L, Henry C, Stamper CT, McMahon M, O’Dell G, Fernández-Quintero ML, Freyn AW, Amanat F, Stovicek O, Gentles L, Richey ST, de la Peña AT, Rosado V, Dugan HL, Zheng N-Y, Tepora ME, Bitar DJ, Changrob S, Strohmeier S, Huang M, García-Sastre A, Liedl KR, Bloom JD, Nachbagauer R, Palese P, Krammer F, Coughlan L, Ward AB, Wilson PC, Broadly neutralizing antibodies target a haemagglutinin anchor epitope. Nature 602, 314–320 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lei R, Kim W, Lv H, Mou Z, Scherm MJ, Schmitz AJ, Turner JS, Tan TJC, Wang Y, Ouyang WO, Liang W, Rivera-Cardona J, Teo C, Graham CS, Brooke CB, Presti RM, Mok CKP, Krammer F, Dai X, Ellebedy AH, Wu NC, Leveraging vaccination-induced protective antibodies to define conserved epitopes on influenza N2 neuraminidase. Immunity 56, 2621–2634.e6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JHW, Bleeker WK, Parren PWHI, Crosstalk between human IgG isotypes and murine effector cells. J. Immunol. Baltim. Md 1950 189, 3430–3438 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Dekkers G, Bentlage AEH, Stegmann TC, Howie HL, Lissenberg-Thunnissen S, Zimring J, Rispens T, Vidarsson G, Affinity of human IgG subclasses to mouse Fc gamma receptors. mAbs 9, 767–773 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheung PPH, Watson SJ, Choy K-T, Fun Sia S, Wong DDY, Poon LLM, Kellam P, Guan Y, Malik Peiris JS, Yen H-L, Generation and characterization of influenza A viruses with altered polymerase fidelity. Nat. Commun 5, 4794 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.R Core Team, R: A language and environment for statistical computing. https://www.R-project.org/.
- 77.Swindells MB, Porter CT, Couch M, Hurst J, Abhinandan KR, Nielsen JH, Macindoe G, Hetherington J, Martin ACR, abYsis: Integrated antibody sequence and structure-management, analysis, and prediction. J. Mol. Biol 429, 356–364 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Fu L, Niu B, Zhu Z, Wu S, Li W, CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL, BLAST+: architecture and applications. BMC Bioinformatics 10, 421 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krissinel E, Henrick K, Inference of macromolecular assemblies from crystalline state. J. Mol. Biol 372, 774–797 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Ye J, Ma N, Madden TL, Ostell JM, IgBLAST: an immunoglobulin variable domain sequence analysis tool. Nucleic Acids Res. 41, W34–40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neumann G, Fujii K, Kino Y, Kawaoka Y, An improved reverse genetics system for influenza A virus generation and its implications for vaccine production. Proc. Natl. Acad. Sci. U. S. A 102, 16825–16829 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are in the paper or supplementary materials. Raw sequencing data have been submitted to the NIH Short Read Archive under accession number: BioProject PRJNA1150188. Cryo-EM density maps and coordinates have been deposited to EMDB and PDB with accession numbers: EMD-46727 and EMD-45930; PDB 9DBX and 9CU7. Biological materials including oligo pools and the assembled scFv library are available by contacting the corresponding author (N.C.W.). Custom scripts as well as raw data for experimental validations have been deposited to DOI: 10.5281/zenodo.15550057.


