Abstract
Cell-to-cell communication in plants involves the trafficking of macromolecules through specialized intercellular organelles, termed plasmodesmata. This exchange of proteins and RNA is likely regulated, and a role for protein phosphorylation has been implicated, but specific components remain to be identified. Here, we describe the molecular characterization of a plasmodesmal-associated protein kinase (PAPK). A 34-kD protein, isolated from a plasmodesmal preparation, exhibits calcium-independent kinase activity and displays substrate specificity in that it recognizes a subset of viral and endogenous non-cell-autonomous proteins. This PAPK specifically phosphorylates the C-terminal residues of tobacco mosaic virus movement protein (TMV MP); this posttranslational modification has been shown to affect MP function. Molecular analysis of purified protein established that tobacco (Nicotiana tabacum) PAPK is a member of the casein kinase I family. Subcellular localization studies identified a possible Arabidopsis thaliana PAPK homolog, PAPK1. TMV MP and PAPK1 are colocalized within cross-walls in a pattern consistent with targeting to plasmodesmata. Moreover, Arabidopsis PAPK1 also phosphorylates TMV MP in vitro at its C terminus. These results strongly suggest that Arabidopsis PAPK1 is a close homolog of tobacco PAPK. Thus, PAPK1 represents a novel plant protein kinase that is targeted to plasmodesmata and may play a regulatory role in macromolecular trafficking between plant cells.
INTRODUCTION
Plasmodesmata represent special intercellular organelles that establish cytoplasmic and endomembrane continuity between neighboring plant cells (Robards and Lucas, 1990; Lucas, 1995). This feature allows small molecules, such as ions, metabolites, and hormones, to diffuse cell to cell and thereby enhance the coordination of biochemical and physiological processes (Lucas et al., 1993). A fundamental difference between the intercellular communication channels found in animals, the gap junctions, and plasmodesmata is that the latter acquired an additional capacity to mediate the selective cell-to-cell trafficking of proteins and protein/RNA complexes (Gilbertson and Lucas, 1996; Zambryski and Crawford, 2000; Haywood et al., 2002; Heinlein, 2002; Ding et al., 2003; Wu et al., 2003; Lucas and Lee, 2004; Oparka, 2004).
A significant body of evidence now supports the concept that plasmodesmata establish a unique control system by allowing a special class of proteins, including transcription factors, to pass through fields of cells (Jackson, 2000; Hake, 2001; Wu et al., 2002; Cilia and Jackson, 2004). Examples of these transcription factors include KNOTTED1 (KN1), a homeobox protein in maize (Zea mays; Jackson et al., 1994; Lucas et al., 1995), LEAFY (LFY), the Arabidopsis thaliana homolog of the FLORICAULA that controls floral meristem identity (Weigel and Meyerowitz, 1993; Perbal et al., 1996; Sessions et al., 2000), SHORT-ROOT (SHR), a putative transcription factor required for endodermal specification (Helariutta et al., 2000; Nakajima et al., 2001; Gallagher et al., 2004), and CAPRICE, a protein central to hair cell formation (Schellmann et al., 2002; Wada et al., 2002; Pesch and Hulskamp, 2004). Many of these proteins appear to use the macromolecular trafficking capacity of plasmodesmata to act as non-cell-autonomous proteins (NCAPs), thereby regulating the biological events occurring outside the cells in which they are produced.
Given the roles played by these NCAPs, their cell-to-cell trafficking is likely to be a highly regulated process. Evidence consistent with this notion has been gained through genetic, molecular, and biochemical studies on the function of the 30-kD movement protein (MP) of Tobacco mosaic virus (TMV; Wolf et al., 1989; Citovsky, 1999) and other such viral MPs (Fujiwara et al., 1993; Noueiry et al., 1994; Rojas et al., 1998; Lazarowitz and Beachy, 1999; Heinlein, 2002; Waigmann et al., 2004). These viral-encoded proteins act as NCAPs, in that they mediate the cell-to-cell movement of infectious viral RNA (Lucas and Gilbertson, 1994; Carrington et al., 1996; Citovsky, 1999; Lazarowitz and Beachy, 1999; Tzfira et al., 2000; Zambryski and Crawford, 2000; Haywood et al., 2002). One form of regulation may well involve MP phosphorylation within the infected host cells (Watanabe et al., 1992; Citovsky et al., 1993; Haley et al., 1995; Kawakami et al., 1999). Direct support for the role of phosphorylation in regulating TMV MP movement was provided by Waigmann et al. (2000), who showed that mutant forms of TMV, carrying changes that mimicked phosphorylation of specific residues in the MP, could neither infect Nicotiana tabacum nor move cell to cell. Thus, MP phosphorylation, by a plant kinase, may well serve to block infection, perhaps by confining the MP within the plasmodesmata (Tomenius et al., 1987; Atkins et al., 1991; Ding et al., 1992; Moore et al., 1992), thereby preventing the spread of the infectious RNA.
Based on these findings, it would appear that plants likely evolved specific protein kinases that serve to regulate the cell-to-cell trafficking of both viral and endogenous NCAPs (Lee and Lucas, 2001; Lucas and Lee, 2004; Waigmann et al., 2004). Phosphorylation of such NCAPs, at or near plasmodesmata, could well play an important role in modulating the activity, extent of trafficking, and/or intracellular localization of these proteins within their target cells (Kawakami et al., 1999; Trutnyeva et al., 2005). Given that specific NCAPs are involved in the determination of cell fate and pattern formation in meristematic tissues (Zambryski and Crawford, 2000; Jackson, 2001; Haywood et al., 2002; Wu et al., 2002), one could argue that control over their spatial and temporal activation would be critical for precise developmental progression. A protein kinase having broad substrate specificity toward these NCAPs, that is itself positioned within or near plasmodesmata, might contribute to controlling the NCAP pathway (Lee and Lucas, 2001; Waigmann et al., 2004).
In this study, we provide the means to test this hypothesis through the development of a protocol that allowed us to isolate and characterize a plasmodesmal-associated protein kinase (PAPK). Molecular and bioinformatic analyses of an ∼34-kD PAPK, purified from tobacco, established that this protein belongs to the casein kinase I (CKI) family. Consistent with a role in macromolecular trafficking through plasmodesmata, this PAPK displayed substrate specificity toward a group of viral and plant NCAPs. Finally, colocalization of PAPK with the TMV MP, a protein well known to be targeted to and sequestered in plasmodesmata, confirmed that members of this plant PAPK family are similarly targeted to the same region of the cell periphery and presumably reside within plasmodesmata.
RESULTS
Purification of a Putative PAPK Using TMV MP as an in Vitro Substrate
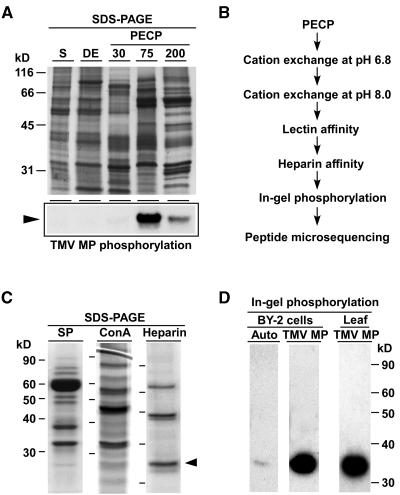
A biochemical approach was developed to identify the plasmodesmal-associated subcellular fraction that was enriched in kinase activity. For this purpose, we used a protocol recently developed for the isolation of a plasmodesmal-enriched cell wall protein (PECP) fraction from tobacco suspension-cultured cells (Lee et al., 2003). The TMV MP was used as substrate in phosphorylation assays performed on PECP subfractions (Figure 1A). This viral NCAP was selected as it was previously shown to be phosphorylated by a kinase present in cell wall extracts (Citovsky et al., 1993; Waigmann et al., 2004), and this modification was correlated with its altered movement function (Waigmann et al., 2000; Trutnyeva et al., 2005). Our experiments indicated that the highest kinase activity was present in the 75 mM CaCl2 (PECP-75) extracted proteins. It is important to note that NCAPP1 (Lee et al., 2003) was similarly highly enriched in this same PECP-75 subfraction (see Supplemental Figure 1 online). Importantly, the TMV MP was not phosphorylated by either the supernatant or the subcellular fraction containing detergent-extracted proteins from the cell wall pellet.
Figure 1.
Biochemical Isolation of a Tobacco PAPK.
(A) Enrichment of PAPK activity within the subfractions of a PECP preparation obtained using tobacco suspension cultured cells. Proteins from each fraction were resolved by electrophoresis in 10% SDS-PAGE gels and visualized by staining with GelCode Blue (top panel). The subcellular fractions include the supernatant (S), a 1% CHAPS extract of the cell wall pellets (DE), and PECP subfractions extracted with 30 mM (PECP30), 75 mM (PECP75), and 200 mM (PECP200) Ca2+ buffer. Each lane was loaded with 8 μg of protein. PAPK activity was determined by phosphorylation assays in which 1 μg of each fraction was added to assay mixtures containing TMV MP. Kinase assay reactions were resolved in 10% SDS-PAGE gels and autoradiography used to detected TMV MP phosphorylation (bottom panel, arrowhead).
(B) Biochemical protocol developed for PAPK purification. Phosphorylation assays were used to identify the chromatographic fractions containing peak enzyme activity for each step.
(C) Chromatographic purification of PAPK. Pooled PECP70 peak fractions were separated by cation exchange (SP; pH 6.8), lectin (Con A), and heparin columns. Proteins were resolved by electrophoresis in 10% SDS-PAGE gels and then stained with GelCode Blue. Protein loading volumes in each gel were 18, 25, and 35 μL for SP, Con A, and Heparin fractions, respectively. The arrowhead indicates ∼34-kD protein corresponding to the PAPK subsequently confirmed by in-gel phosphorylation assays.
(D) Purified heparin fraction, shown in (C), was used for in-gel autophosphorylation and TMV MP phosphorylation assays. For in-gel autophosphorylation, 20 μL of the heparin peak fraction was first resolved in 12% SDS-PAGE gels, then given denaturation/renaturation treatments, followed by incubation of the gel in complete assay buffer (2 mM DTT plus ATP mixture of 10 μCi/mL [γ32P]ATP) for 1 h. In-gel substrate phosphorylation assays were as above except that TMV MP was included at ∼300 μg/mL gel. PAPK was also purified from tobacco leaves using the same purification protocol as (B). Peak fractions were combined, concentrated to ∼80 μL, and 5-μL aliquots used for each in-gel TMV MP phosphorylation assay.
To identify the molecular nature of this putative PAPK, the enzyme was further purified by employing a combination of chromatographic protocols, including cation-exchange, lectin, and heparin columns (Figure 1B). The PECP-75 extracts from several preparations were pooled and proteins first fractionated, by cation-exchange chromatography, and their profile then resolved by SDS-PAGE. Fractions containing high levels of enzyme activity were again identified, based on our TMV MP phosphorylation assay. These peak fractions were again pooled, and a relatively high level of PAPK purification was achieved following lectin (concanavalin A [Con A]) and heparin chromatography. Coomassie blue–stained SDS-PAGE gels revealed the presence of several discrete bands within this purified and enriched PECP fraction (Figure 1C).
To determine the protein band corresponding to the putative PAPK, in-gel phosphorylation assays were next performed. These experiments established that the apparent molecular mass of the purified PAPK was ∼34 kD (Figure 1D). PAPK activity was also detected in PECP fractions prepared from tobacco leaf tissues (data not shown), and in-gel phosphorylation assays similarly revealed kinase activity associated with an ∼34-kD protein (Figure 1D).
Biochemical Characteristics of PAPK
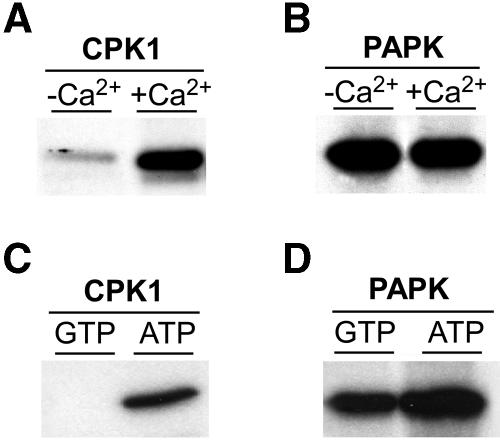
Biochemical assays were next performed to establish the functional characteristics of this ∼34-kD putative PAPK. Experiments were first conducted to ascertain whether PAPK exhibited calcium-dependent protein kinase activity. Pumpkin (Cucurbita maxima) CPK1 is a calcium-dependent protein kinase identified from pumpkin phloem sap (Yoo et al., 2002), and it was used as a control for calcium-dependent phosphorylation of the TMV MP in vitro (Figure 2A). In contrast with pumpkin CPK1, the phosphorylation of TMV MP, by PAPK, was calcium independent (Figure 2B). In general, ATP is preferred as a phosphate donor by most protein kinases (Edelman et al., 1987). Consistent with this notion, pumpkin CPK1 did not appear to use GTP as a phosphate donor (Figure 2C). Interestingly, both GTP and ATP could be used as good phosphate donors for the tobacco PAPK (Figure 2D).
Figure 2.
PAPK Exhibits Calcium-Independent Activity and Uses ATP or GTP as a Phosphate Donor.
(A) Control experiment employing pumpkin CPK1 demonstrated calcium-dependent phosphorylation of TMV MP. Kinase activity was assayed using 50 μL reaction buffer, plus 20 ng of purified recombinant enzyme and 1 μg of TMV MP. Reaction buffer contained 50 mM HEPES, pH 7.5, 10 mM MgCl2, 2 mM DTT, 2 mM EGTA, and 60 μM ATP mixed with 4 μCi of [γ32-P]ATP (3000 Ci/mmol), supplemented with 2.2 mM CaCl2.
(B) PAPK phosphorylated TMV MP in a calcium-independent manner. Kinase assays were performed in 50 μL of reaction buffer, plus 5 μL of heparin peak fraction and 1 μg of TMV MP ± 2.2 mM CaCl2.
(C) Pumpkin CPK1 uses ATP but not GTP as a phosphate donor. Reaction buffer was as above, except that it contained 4 μCi of [γ32P]GTP (3000 Ci/mmol) mixed with 60 μM ATP.
(D) PAPK can use GTP or ATP as the phosphate donor. Reaction buffer was as above, except that it contained 4 μCi of [γ32P]GTP (3000 Ci/mmol) mixed with 60 μM ATP.
PAPK Phosphorylates TMV MP at Its C Terminus
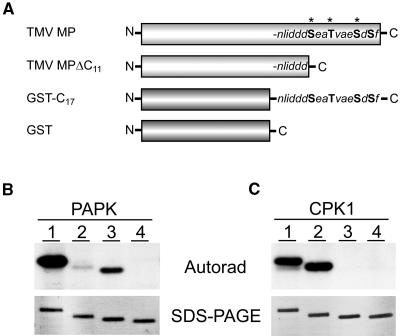
Recently, it was shown that a plant cell wall extract contained kinase activity that could phosphorylate TMV MP in a calcium-independent manner (Waigmann et al., 2000). The phosphorylation sites were mapped to the three residues, Ser258, Thr261, and Ser265, within the C-terminal end of the TMV MP. As PAPK is enriched in our PECP fraction and phosphorylates TMV MP, we next investigated whether this enzyme might be the corresponding endogenous kinase. For these studies, a deletion mutant that lacked the C-terminal 11 amino acid residues (TMV MPΔC11) and a recombinant glutathione S-transferase (GST) fusion protein containing the C-terminal 17 residues of TMV MP (GST-C17) were constructed (Figure 3A). In contrast with the full-length TMV-MP, the engineered TMV MPΔC11 served as a poor substrate for phosphorylation by the purified PAPK (Figure 3B). Densitometric analysis indicated that the level of phosphorylation of TMV MPΔC11 by PAPK was 10% of that observed for the TMV MP. This result indicated that the major phosphorylation site for PAPK is located within the deleted region of the TMV MP. As the expression of this mutant was as stable as that of full-length TMV MP, protein instability is unlikely to be responsible for this reduction in phosphorylation by the PAPK. Consistent with this notion, the TMV MPΔC11 served as a comparable substrate to the full-length TMV MP for phosphorylation by pumpkin CPK1 (Figure 3C).
Figure 3.
The TMV MP C-Terminal Domain Contains Major Phosphorylation Sites Recognized by PAPK.
(A) Schematic diagram illustrating the recombinant proteins used to identify the phosphorylation sites, present in TMV MP, recognized by PAPK. TMV MP: general structure indicating the positions of Ser/Thr residues in the C terminus (bold), including those previously identified as being in vivo phosphorylation sites (asterisks; Ser258, Thr261, and Ser265; Waigmann et al., 2000). TMV MPΔC11: deletion mutant lacking the 11 C-terminal residues. GST-C17:GST fusion protein containing the 17 C-terminal residues of the TMV MP. GST: control protein for our phosphorylation reactions.
(B) PAPK recognized, specifically, the Ser/Thr residues within the C terminus of TMV MP. Lane 1, TMV MP (30 kD); lane 2, TMV MPΔC11 (29 kD); lane 3, GST-C17 (28 kD); lane 4, GST (26 kD). Each phosphorylation assay contained 5 μL of heparin column-purified PAPK fraction and 1 μg of substrate protein in a 50-μL reaction volume.
(C) TMV MP C terminus lacked phosphorylation substrate sites for pumpkin CPK1. Parallel assays to those in (B) performed using 20 ng of purified recombinant pumpkin CPK1. Substrate phosphorylation visualized by autoradiography (top panels) and purified substrate proteins used in these assays (1 μg each) are shown in GelCode Blue–stained SDS-PAGE gels (bottom panels).
GST-C17 also served as a substrate for phosphorylation by PAPK; controls using GST alone demonstrated the specificity of this reaction (Figure 3B). However, in comparison with the full-length TMV MP, GST-C17 appeared to serve as a less favorable substrate. The level of phosphorylation of GST-C17 by PAPK was 30% of that observed for the TMV MP based on densitometric analysis. Specificity of the phosphorylation reaction, mediated by PAPK, toward the C terminus of TMV MP was supported by the finding that pumpkin CPK1 failed to phosphorylate the GST-C17. Taken together, our results indicate that the major PAPK phosphorylation site resides within the C-terminal 11 amino acid residues of TMV MP. These findings support the hypothesis that PAPK is a plant protein kinase that recognizes NCAPs, such as the TMV MP.
PAPK Phosphorylates a Subset of Endogenous and Viral NCAPs
It is possible that PAPK might be an endogenous kinase that modulates, specifically, TMV MP upon viral infection. If this were the case, PAPK would likely be expressed in TMV host plants, and its expression might well be affected by viral infection. Moreover, the substrate specificity of PAPK could be quite narrow, if not exclusive to TMV MP. Alternatively, PAPK could play a role in controlling NCAP trafficking during normal plant growth and development (i.e., viruses could have exploited this host mechanism) (Lucas and Wolf, 1993; Oparka, 2004; Waigmann et al., 2004). Under this scenario, PAPK would likely show broad substrate specificity toward both viral and endogenous NCAPs. To test between these two possibilities, we next examined the substrate specificity of PAPK.
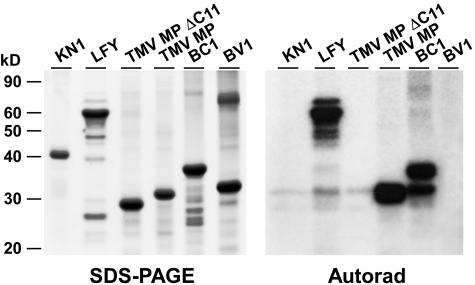
For this purpose, we selected four additional viral MPs and two well-characterized endogenous NCAPs. BC1 and BV1 represent MPs encoded by a DNA virus, Bean dwarf mosaic virus (Noueiry et al., 1994; Rojas et al., 1998), while the MPs of Cucumber mosaic virus (CMV) and Red clover mosaic virus (RCNMV) were selected as examples of RNA viruses (Fujiwara et al., 1993; Ding et al., 1995; Canto et al., 1997; Itaya et al., 1997; Wang et al., 1998). KN1 and LFY are plant transcription factors that are well characterized for their non-cell-autonomous activities (Lucas et al., 1995; Sessions et al., 2000; Kim et al., 2002, 2003; Lucas and Lee, 2004). Under the in vitro conditions used for our experiments, PAPK displayed substrate specificity, in that it phosphorylated LFY, BC1, and TMV MP, but not KN1 or BV1 (Figure 4). The MPs of CMV and RCNMV were also examined and found to serve as poor substrates for PAPK (see Supplemental Figure 2 online). These studies established that PAPK likely functions in the recognition and phosphorylation of a specific subset of NCAPs of both plant and viral origin.
Figure 4.
PAPK Displays Substrate Specificity for Viral and Endogenous Non-Cell-Autonomous Proteins.
Substrate specificity of PAPK tested using two plant NCAPs, KN1 and LFY, and two viral MPs, BC1 and BV1. TMV MP and TMV MPΔC11 were included as controls. Each in vitro phosphorylation assay contained 5 μL of heparin column-purified PAPK fraction and 1 μg of substrate protein in a 50-μL reaction volume. Note that LFY, TMV MP, and BC1 served as specific substrates for PAPK, whereas KN1 and BV1 were not phosphorylated.
PAPK Is a Member of the CKI Gene Family
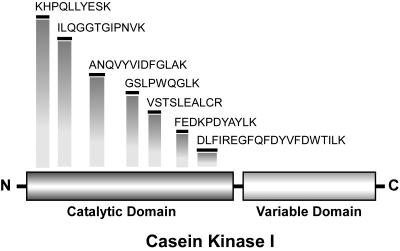
To identify the tobacco gene encoding PAPK, the ∼34-kD protein band (Figure 1C) was excised and subjected to conventional internal peptide microsequencing. A National Center for Biotechnology Information BLAST database search was conducted using the resultant peptide sequences, and this identified PAPK as a member of the CKI gene family (Figure 5). CKI is a Ser/Thr protein kinase family found in eukaryotes (Gross and Anderson, 1998). The kinase domains of plant CKI isoforms that were identified in the Arabidopsis and rice (Oryza sativa) genome databases shared 86% similarity at the amino acid sequence level (see Supplemental Figure 3 online). Interestingly, all PAPK-derived peptide microsequences were found to match this highly conserved kinase domain (Figure 5). This result prevented us from determining the actual tobacco CKI isoform corresponding to the biochemically purified PAPK. As both N- and C-terminal regions of purified PAPK were found to be blocked, we designed degenerate primers, based on the identified peptide sequences, to amplify PCR products that were then used to probe a tobacco cDNA library. A number of cDNA clones that contained regions closely matching the peptide microsequences were isolated. This indicated that the catalytic domain of CKI is highly conserved. However, we were unable to obtain any cDNA clones that completely matched all seven peptide microsequences and also encoded a 34-kD protein, the apparent molecular mass of purified PAPK.
Figure 5.
Peptide Microsequence Analysis Identifies PAPK as a Member of the CKI Gene Family.
All microsequences matched to the highly conserved catalytic domain of CKI; vertical bars indicate the relative locations within this domain.
To resolve this situation, we adopted an alternative approach to identify the PAPK gene, based on the expectation that PAPK would be localized to, or near, plasmodesmata. The Arabidopsis genome was found to contain some 14 Casein Kinase I-like (CKL) genes (Table 1), and within the conserved kinase domains, they shared 89% sequence similarity at the amino acid level. Of these genes, one (At4g08800) appeared to be a pseudogene, based on the fact that it contained an in-frame stop codon and/or short deletions within the conserved kinase subdomains; no reliable ESTs have been reported, and RT-PCR amplification of the putative transcript failed to yield any product. By contrast, all other members were successfully amplified by RT-PCR. As two isoforms, CKL9α and CKL9β, are apparently derived from alternative splicing, we concluded that 13 genes give rise to 14 CKL isoforms.
Table 1.
Molecular Information and Intracellular Localization of CKL Isoforms
| Isoform | AGI Locus Identifiera | GenBank Accession No. | No. of Amino Acid Residues | kDb | Intracellular Localization |
|---|---|---|---|---|---|
| CKL1 | At4g26100 | AY943842 | 450 | 51.1 | Punctate particles |
| CKL2 | At1g72710 | AY943843 | 465 | 52.0 | Cytoplasm/nucleus |
| CKL3 | At4g28880 | AY943852 | 415 | 47.0 | Cytoplasm/nucleus |
| CKL4 | At4g28860 | AY943853 | 414 | 46.8 | Cytoplasm/nucleus |
| CKL5 | At2g19470 | AY943844 | 433 | 49.0 | Strongly cytoplasm |
| CKL6 | At4g28540 | AY943845 | 479 | 54.1 | Punctate particles |
| CKL7 | At5g44100 | AY943846 | 476 | 53.7 | Strongly cytoplasm |
| CKL8 | At5g43320 | AY943854 | 480 | 54.5 | Punctate particles |
| CKL9α | At1g03930 | AY943847 | 319 | 36.8 | Cytoplasm/nucleus |
| CKL9β | At1g03930 | AY943848 | 471 | 53.1 | Strongly cytoplasm |
| CKL10 | At3g23340 | AY943849 | 442 | 50.5 | Punctate particles |
| CKL11 | At4g14340 | AY943850 | 457 | 52.0 | Cytoplasm/nucleus |
| CKL12 | At5g57015 | AY943855 | 435 | 49.5 | Strongly cytoplasm |
| CKL13 | At1g04440 | AY943851 | 468 | 53.3 | Punctate particles |
AGI, Arabidopsis Genome Initiative.
Calculated molecular mass.
Subcellular Localization Patterns of Arabidopsis CKL Isoforms
In yeast and humans, CKI isoforms contain variable domains, either at the N or C terminus (Gross and Anderson, 1998); in Arabidopsis, all CKL isoforms appeared to contain C-terminal extensions with variable lengths from ∼20 to 180 amino acid residues. Three out of four yeast isoforms, YCK1, 2, and 3, contain isoprenylation sites at their C termini and are associated with the plasma membrane (Wang et al., 1992; Vancura et al., 1994). However, no significant sequence conservation, predictable motifs, or domains for specific intracellular targeting/localization were detected within any of the Arabidopsis CKL extensions.
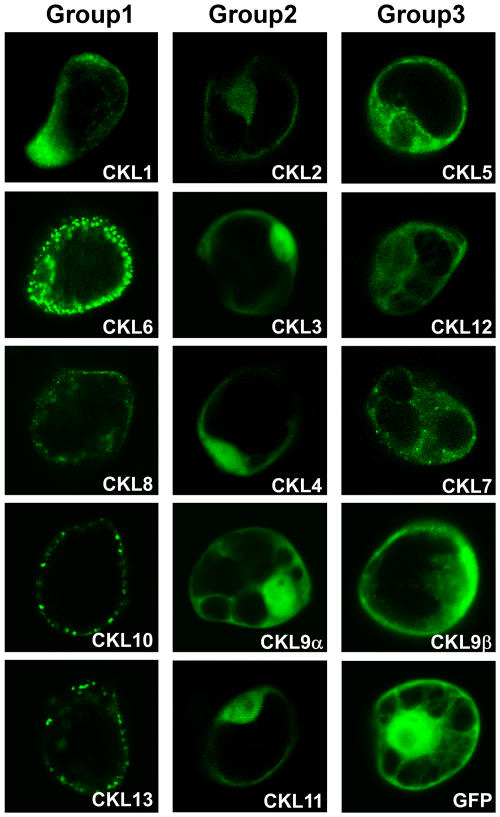
In view of this finding, the intracellular localization/targeting of each CKL isoform was determined by expressing N- or C-terminal green fluorescent protein (GFP) fusion proteins in plant cells. For transient expression of CKL-GFP fusion proteins, appropriate plasmid constructs were biolistically bombarded into tobacco suspension-cultured cells (Lee et al., 2003). We divided these CKL isoforms into three groups on the basis of localization patterns determined by fluorescence microscopy (Figure 6). Group 1 isoforms exhibited a predominantly punctate fluorescence pattern at the cell periphery; group 2 isoforms had strong fluorescent signals within the nucleus, but signal was also present in the cytoplasm; and for group 3 isoforms, signal was located predominantly in the cytoplasm, with only a low level detected in the nucleus. Parallel expression studies were also performed using tobacco leaf tissues. Here, similar results were obtained with these same CKL-GFP constructs when we analyzed epidermal cells of mature tobacco leaves (Figure 7).
Figure 6.
Subset of Arabidopsis CKL Isoforms Localizes to the Plasmodesmata/Cell Periphery.
The Arabidopsis genome contains 14 members of the CKI gene family (CKL1 to 14). Putative Arabidopsis PAPKs were identified on the basis of intracellular localization patterns observed for these CKL-GFP fusion proteins. Transient expression assays, using tobacco suspension culture cells, indicate the presence of three localization patterns, represented by genes in groups 1, 2, and 3. Fluorescent patterns were collected by laser scanning confocal microscopy 16 h after biolistic bombardment. Cytoplasmic and nuclear localization of GFP was used as a control. Based on the similarity in fluorescence patterns exhibited by group 1 isoforms and other plasmodesmal-targeted NCAPs, these are likely candidates for Arabidopsis PAPKs. Group 2 represents CKL isoforms that localize to the nucleus and cytoplasm. Group 3 CKLs accumulate mainly in the cytoplasm.
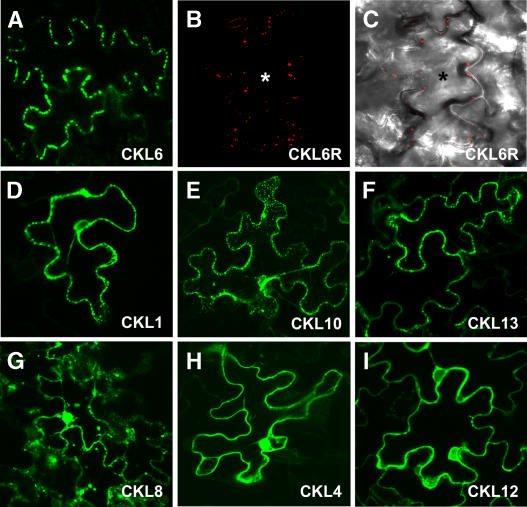
Figure 7.
Arabidopsis CKL6 Localizes to Discrete Regions at the Cell Periphery of Tobacco and Arabidopsis Epidermal Cells.
Tobacco leaf tissue transfected using Agrobacterium tumefaciens transformed with GFP-tagged CKL isoforms ([A] and [D] to [I]) was analyzed 48 h after agroinfiltration. Arabidopsis leaf tissue biolistically bombarded with plasmid DNA expressing RFP-tagged CKL6 ([B] and [C]) was analyzed 24 h after biolistic DNA delivery. Confocal microscopic images represent paradermal regions of epidermal cells expressing these fluorescently tagged constructs.
(A) CKL6-GFP develops a punctate fluorescence pattern reminiscent of plasmodesmal pit field distribution within epidermal cell walls.
(B) CLK6-RFP signal in an Arabidopsis epidermal cell shows punctate fluorescence signals similar to those observed in tobacco cells.
(C) Same image shown in (B) but combined with the transmitted light image to show the contour of the bombarded cell (indicated by an asterisk).
(D) to (F) CLK1-, CLK10-, and CLK13-GFP exhibit punctate fluorescence signals.
(G) CLK8-GFP signal appears to label cytoplasm and nucleus and to form punctate particles.
(H) CKL4-GFP signal accumulates in the nucleus and cytoplasm; note the absence of a punctate pattern over the cell wall.
(I) CKL12-GFP signal was strongest in the cytoplasm but was also present in the nucleus; note the absence of a punctate pattern over the cell wall.
Fusion of GFP either at the CKL N or C terminus did not appear to make a difference in terms of the observed localization patterns (data not shown). These results indicate that the specific localization of the CKL isoforms is not dependent on either terminus being exposed. Among the CKL genes, group 1 isoforms were considered most likely to contain the PAPK homolog(s), as they formed punctate fluorescent signals along the periphery of both suspension cultured and epidermal cells. Indeed, in epidermal cells, the fluorescence pattern associated with group 1 isoforms, including CKL1, CKL6, CKL10, and CKL13, was very similar to those earlier observed for viral MPs (Oparka et al., 1997; Itaya et al., 1998; Boyko et al., 2000; Crawford and Zambryski, 2001; Kotlizky et al., 2001; Lawrence and Jackson, 2001; Roberts et al., 2001; Gorshkova et al., 2003). The CKL8-associated fluorescence pattern appeared rather distinct, in that although the signal appeared punctate in nature the protein was also detected in both the cytoplasm and nucleus. Among the group 1 isoforms, CKL6 gave rise to the most stable expression and, therefore, was selected for further characterization as a putative Arabidopsis PAPK1. We first tested whether the localization pattern of CKL6 was consistent between Arabidopsis and tobacco. The experiments demonstrated that transient expression of CKL6 in Arabidopsis and tobacco leaves yielded similar punctate patterns (Figures 7A to 7C).
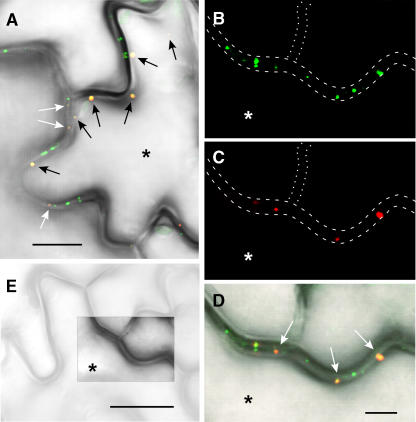
PAPK1 Colocalizes with TMV MP
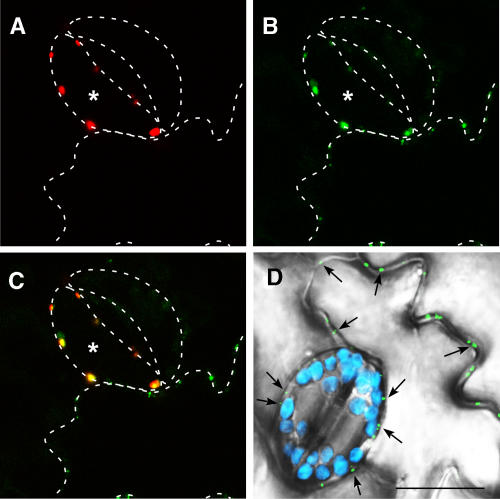
To address more directly the association of PAPK with plasmodesmata, we next conducted colocalization studies using PAPK1 and TMV MP. To this end, PAPK1 was transiently expressed, as a translational fusion to red fluorescent protein (RFP), in transgenic tobacco plants expressing TMV MP-GFP driven by the 35S constitutive promoter. To ascertain whether PAPK1 and TMV MP use the same intracellular delivery pathway (Ruiz-Medrano et al., 2004; Haupt et al., 2005), we first examined the fluorescence patterns present within specialized epidermal cells, the guard cells. These cells were chosen because, at maturity, they are symplasmically isolated as they do not have functional plasmodesmata (Wille and Lucas, 1984; Palevitz and Hepler, 1985; Ding et al., 1997). As such, these guard cells provide a single-cell-based assay within the context of an intact leaf. Expression of PAPK1-RFP, in individual guard cells, gave rise to strong punctate red fluorescent signals (Figure 8A). A similar green punctate pattern was detected for TMV MP-GFP within the same cell (Figure 8B). However, the strong accumulation of TMV MP-GFP signal within the target guard cell is apparently induced by the ectopic expression of PAPK1, as equivalent TMV MP-GFP signal is not detected within the member of the guard cell pair nor in other guard cells. Note the punctate green fluorescent signals within the epidermal cell walls (Figure 8D), previously shown to reflect the localization of TMV MP-GFP within plasmodesmal pit fields (i.e., the regions of high plasmodesma density; Robards and Lucas, 1990). Importantly, PAPK1-RFP and TMV MP-GFP signals were found to be colocalized (Figure 8C, yellow signals), consistent with the hypothesis that both proteins engage a similar intracellular delivery pathway.
Figure 8.
Colocalization of PAPK1 and TMV MP in Symplasmically Isolated Guard Cells.
PAPK1-RFP was transiently expressed, by particle bombardments, in transgenic tobacco leaves expressing TMV MP-GFP.
(A) Fluorescent signals, produced by PAPK1-RFP in a target guard cell (asterisk), detected in the red channel of the confocal microscope. Contours of the epidermal cells, drawn with white dashed lines, allow identification of the target cell in the context of the epidermal layer.
(B) Fluorescent signals, produced from TMV MP-GFP, were observed in the periphery of all epidermal cells, detected in the green channel on the confocal microscope. Note that the strong accumulation of TMV MP-GFP signal is only detected in one guard cell that ectopically expressed PAPK1-RFP, which is in contrast with the neighboring guard cell in which TMV MP-GFP was not detectable.
(C) Combined image of (A) and (B) showing overlapping signals between the red and green channels. Note the presence of strong yellow signals confined to the bombarded guard cell.
(D) Control image showing guard cells and surrounding epidermal cells in a TMV MP-GFP transgenic tobacco leaf. Picture represents a combined image of signals collected from three different channels. Punctate green signal, representing TMV MP-GFP, accumulates in plasmodesmata (arrows). Note that hardly any signals are detected within guard cells. Blue signal (false color): chloroplast autofluorescence restricted to the guard cell pair. Gray image: epidermal cells collected in the transmitted-light channel. Bar = 20 μm for (A) to (D).
Epidermal pavement cells exhibited a similar pattern of overlapping signals between PAPK1-RFP and TMV MP-GFP (Figure 9). In these experiments, the TMV MP-GFP was detected within the cross-walls as a green punctate signal. Again, it is important to note that, within individually bombarded target cells, punctate signals produced by PAPK1-RFP almost always overlapped with those of TMV MP-GFP (Figures 9A to 9D). These results provide strong support for the hypothesis that PAPK1 is targeted to plasmodesmata, or it is associated with novel plasmodesmal-targeting machinery. Moreover, these studies imply that the in vivo interaction between PAPK1 and TMV MP likely occurs either during targeting to and/or within plasmodesmata.
Figure 9.
PAPK1 and TMV MP Colocalize within the Cross-Walls of Neighboring Epidermal Cells.
Leaves of a TMV MP-GFP–expressing transgenic tobacco line were bombarded with PAPK1-RFP plasmid and observed by confocal microscopy 24 h after bombardment.
(A) Overlapping TMV MP-GFP and PAPK1-RFP signals (yellowish-orange; white arrows) detected within epidermal cross-walls; additional overlapping signals are also detected near the cell periphery (black arrows). Image represents the combination of green, red, and transmitted-light channels. Asterisk indicates the bombarded epidermal cell that is connected to its neighbors by plasmodesmata.
(B) to (D) High-resolution images show overlapping signals at the cross-wall of neighboring epidermal cells. Green channel, TMV MP-GFP (B); red channel, PAPK1-RFP (C); combined image demonstrates the overlapping nature of signals (D). Note the confinement of strong yellow-orange signals over the target cell wall (asterisk). Dashed lines in (B) and (C) illustrate the cell contour, whereas dotted lines indicate the position of a cross-wall slightly out of the focal plane.
(E) Epidermal cells imaged at lower magnification by transmitted light; boxed region represents the area shown in (B) to (D).
Bars = 10 μm in (A), 5 μm in (D), and 20 μm in (E). Bar in (D) is common to (B) and (C).
Arabidopsis PAPK1 Exhibits Similar Enzymatic Properties as Tobacco PAPK
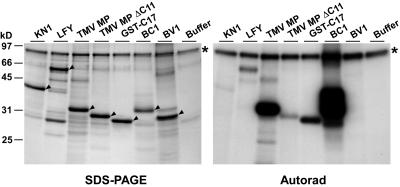
Colocalization data strongly suggested that Arabidopsis PAPK1 represents a homolog of the PAPK purified from tobacco. However, stronger evidence would be provided if these two isoforms shared similar enzymatic properties. To this end, full-length Arabidopsis PAPK1 was expressed as a GST fusion protein in Escherichia coli, affinity purified, and tested for phosphorylation activity. As shown in Figure 10, recombinant Arabidopsis PAPK1 phosphorylated TMV MP and its derivatives, BDMV BC1 and LFY, in a similar manner to that observed with the tobacco PAPK (see Figures 3B and 4). Interestingly, recombinant Arabidopsis PAPK1 appeared to have relatively stronger substrate preference toward BC1 than LFY. Densitometric analysis indicated that the levels of phosphorylation of TMV MPΔC11 and GST-C17 by Arabidopsis PAPK1 were 8 and 30% of that observed for the TMV MP, respectively, which is comparable to the tobacco PAPK result (see Figure 3). In 10 replicate experiments, KN1 and BDMV BV1 were not phosphorylated by recombinant Arabidopsis PAPK1, but all other proteins showed consistent patterns of phosphorylation. Taken together, the results for substrate specificity, as demonstrated by these experiments, provided strong support for the hypothesis that Arabidopsis PAPK1 is a close homolog of tobacco PAPK.
Figure 10.
PAPK1 Phosphorylates TMV MP in a Similar Manner to Tobacco PAPK.
Recombinant Arabidopsis PAPK1 phosphorylates full-length TMV MP and BDMV BC1 strongly and LFY less extensively. The C-terminal deletion mutant (TMV MPΔC11) and the C-terminal peptide (GST-C17) derivative of TMV MP showed reduced phosphorylation by Arabidopsis PAPK1 in a manner similar to that observed for tobacco PAPK. Among the tested proteins, KN1 and BDMV BV1 were not phosphorylated by Arabidopsis PAPK1. Negative control: phosphorylation reaction in the absence of substrate (buffer) shows GST-PAPK1 (indicated by asterisk) as an autophosphorylating band. Predicted molecular mass of GST-PAPK1 is ∼82 kD. Target substrate protein in each lane is marked by arrowheads. Phosphorylation assays were performed essentially as described for Figures 3 and 4, except that 0.4 μg of purified GST-PAPK1 was used in 50 μL of kinase reaction mixture. The assay was terminated by adding 10 μL of 6× SDS sample buffer, and 40 μL of each reaction was resolved on 12% SDS-PAGE. The gel was stained with GelCode Blue (SDS-PAGE), dried, and exposed to x-ray film (Autorad).
DISCUSSION
In this study, we describe the molecular characterization of a PAPK. This was achieved using a combination of biochemical enrichment of plasmodesmal proteins and purification methods using TMV MP as a substrate for this plant kinase (Figure 1). Tobacco PAPK was expressed in both suspension cells and leaf tissues. The purified PAPK exhibited calcium-independent kinase activity and could use both ATP and GTP as phosphate donors (Figure 2). PAPK appeared to display substrate specificity in terms of its recognition of viral and plant non-cell-autonomously acting proteins (Figures 3 and 4). Peptide microsequence analysis identified PAPK as a member of the CKI gene family (Figure 5). Punctate localization patterns of group 1 Arabidopsis CKL isoforms suggested that they might be closely related to the tobacco PAPK, as they both appear to be targeted to plasmodesmata (Figures 6 and 7). Colocalization studies using PAPK1-RFP and TMV MP-GFP provided support for the notion that these proteins might interact within the cytoplasm and are targeted to plasmodesmata (Figures 8 and 9). Phosphorylation of TMV MP by Arabidopsis PAPK1 in a similar manner to tobacco PAPK further strengthens our argument. Although a number of putative plasmodesmal proteins have been identified in recent studies (Escobara et al., 2003; Lucas and Lee, 2004; Oparka, 2004), PAPK1 is a novel gene to be cloned and characterized. Furthermore, our studies allowed us to assign a novel function for one member of the plant CKI gene family.
PAPK Phosphorylates TMV MP
Phosphorylation of TMV MP, at multiple Ser/Thr residues, has been observed in both in vitro and in vivo studies (Watanabe et al., 1992; Citovsky et al., 1993; Haley et al., 1995; Karpova et al., 1999; Waigmann et al., 2000; Lee and Lucas, 2001; Karger et al., 2003). Among these various TMV MP phosphorylation sites, the C-terminal three Ser/Thr residues were previously shown to be phosphorylated in vivo, and this posttranslational modification was important for movement function in a host-dependent manner (Waigmann et al., 2000; Trutnyeva et al., 2005). The translatability of a TMV MP-viral RNA complex also appears to be strongly affected by phosphorylation, and this posttranslational modification is correlated with trafficking through plasmodesmata (Karpova et al., 1997, 1999). These attributes made this viral NCAP an ideal substrate for our study. Based on our experiments, the major phosphorylation sites for this PAPK reside within the 11 C-terminal residues of the TMV MP (Figure 3), including the three earlier established in vivo phosphorylation sites (Waigmann et al., 2000). Importantly, this region contains the consensus phosphorylation sites of CKI (i.e., Ser/Thr immediately following a cluster of negatively charged residues; Gross and Anderson, 1998). Previous studies have demonstrated that substrate peptides of 10 to 18 amino acid residues are sufficient for recognition and phosphorylation by CKI (Agostinis et al., 1989; Flotow and Roach, 1991). Consistent with this observation, the GST fusion peptide containing the TMV MP C-terminal 17 residues served as a specific substrate for PAPK (Figure 3).
In our experiments, both PAPK and pumpkin CPK1 could phosphorylate the TMV MP in vitro. However, these kinases clearly recognize distinct sites, as PAPK phosphorylated the C-terminal residues, whereas pumpkin CPK1 did not. These findings are consistent with the presence of multiple phosphorylation sites on TMV MP (Watanabe et al., 1992; Citovsky et al., 1993; Haley et al., 1995; Karpova et al., 1999; Waigmann et al., 2000; Lee and Lucas, 2001; Karger et al., 2003). The role of additional protein kinases, in terms of modulating the functions performed by TMV MP, will require identification and characterization of such entities. In this regard, it is of interest to note that a plant kinase associated with the endoplasmic reticulum was recently shown to phosphorylate TMV MP at Thr104 (Karger et al., 2003).
PAPK Substrate Specificity for Viral and Endogenous NCAPs
It is well established that viral MPs interact with components of the plant NCAP machinery to traffic the infectious material into neighboring cells (Lucas and Gilbertson, 1994; Carrington et al., 1996; Gilbertson and Lucas, 1996; Lazarowitz and Beachy, 1999; Waigmann et al., 2004). Furthermore, cell biological studies have revealed the operation of distinct pathways engaged by various viral systems (Kragler et al., 1998, 2000; Haywood et al., 2002). Thus, an alteration in a particular pathway would affect a subset of viral MPs as well as any NCAPs using this same cell-to-cell trafficking pathway (Lee et al., 2003). Consistent with this notion, our phosphorylation experiments demonstrated that LFY, TMV MP, and BV1 acted as good in vitro substrates of PAPK, whereas KN1 and BV1 (Figure 4), as well as CMV MP and RCNMV MP, were either not recognized or served as poor substrates for this kinase.
This level of substrate specificity, as a means of regulating a particular intercellular communication pathway, was earlier demonstrated by our study of NCAPP1 (Lee et al., 2003). Here, a mutant form of NCAPP1 was shown to block the trafficking of the TMV MP and LFY, but not KN1 nor the CMV MP. Identification of PAPK now provides us with an important tool for the further molecular dissection of the components that operate in the LFY/TMV MP trafficking pathway. Importantly, as LFY has been suggested to move cell to cell via the nontargeted plasmodesmata pathway (Crawford and Zambryski, 2001; Wu et al., 2003; Lucas and Lee, 2004), it will be of considerable interest to ascertain whether trafficking of this NCAP is regulated by phosphorylation. Finally, the TMV MP–based assay used to purify PAPK can now be expanded to screen for kinases that recognize other NCAPs, such as KN1, SHR, CMV MP, and so forth.
Colocalization of PAPK1 and TMV MP
The assignment of CKL6 as PAPK1 was based on a number of features. First, it accumulated within the periphery of the cytoplasm, as distinct fluorescent foci (punctuate pattern) (Figures 6 and 7). This pattern is identical to that observed for TMV MP, for which accumulation within plasmodesmata has long been known (Waigmann et al., 2004). Second, in regions where plasmodesmata are grouped into pit fields, TMV MP-GFP fluorescence has been shown to colocalize with a callose-based immunofluorescence signal (Oparka et al., 1997). Callose is a wall material that forms an extracellular collar around plasmodesmata (Northcote et al., 1989; Trethewey and Harris, 2002). Our finding that PAPK1-RFP becomes colocalized with TMV MP-GFP, in connecting cell walls (Figure 9), provides evidence consistent with the hypothesis that it is a plasmodesmal-targeted/localized protein. Third, Arabidopsis PAPK1 phosphorylates TMV MP at its C terminus (Figure 10), which suggests that PAPK1 has similar enzymatic properties to that of tobacco PAPK. The fact that PAPK1 can phosphorylate TMV MP in vitro and that they colocalize within cells strongly suggest that they also interact in vivo. Direct evidence for an in vivo interaction between PAPK1 and TMV MP remains to be obtained.
Colocalization of PAPK1-RFP and TMV MP-GFP, within the periphery of mature guard cells that lack functional plasmodesmata, suggests they use a common pathway for delivery to and accumulation at a region near the plasma membrane (Figure 8). Additionally, the fact that TMV MP-GFP accumulates to higher levels in PAPK1-RFP expressing guard cells (Figure 8) supports the notion that PAPK1 in some way acts to stabilize the TMV MP. This is a stark contrast because normally the TMV MP-GFP signals within the guard cells of transgenic tobacco plants are under detection limit (see Figure 8 and legend). For colocalization studies, we used DsRed2 (Clontech) to label PAPK1 because this version of RFP is modified to reduce the aggregation problem of the original DsRed. However, it still forms tetramers that might prevent PAPK1 from associating properly within plasmodesmata. In this regard, the use of monomeric RFP might be helpful. In any event, localization of PAPK1, at the transmission electron microscopic level, would provide additional information on PAPK1 localization within or near plasmodesmal channels. Generation of PAPK1-specific antibodies would provide the tool for these studies. Finally, based on the similarity in localization patterns observed among group 1 CKL isoforms, these proteins might well be homologs with similar function. The possibility exists, however, that these isoforms exhibit distinct substrate and kinetic characteristics and/or that temporal/spatial expression patterns govern substrate specificity in planta.
CKI Attributes Are Consistent with PAPK Function
CKI is a unique Ser/Thr protein kinase family highly conserved in all eukaryotes from yeast to humans and plants. All CKI isoforms contain a highly conserved kinase domain, flanked by a variable domain. The kinase domain is basic in nature and distinct in sequence when compared with other Ser/Thr protein kinase gene families (Gross and Anderson, 1998; Vielhaber and Virshup, 2001). In addition, members of the CKI family display broad substrate specificities and are targeted to a range of subcellular domains. These characteristics contribute to the involvement of CKI isoforms in a wide array of regulatory events; recent advances in diverse fields strongly suggested that this kinase family is important in regulating critical signaling processes (Peters et al., 1999; Vielhaber and Virshup, 2001; Price and Kalderon, 2002; Eichwald et al., 2004; Jia et al., 2004; Preuss et al., 2004). In yeast, CKI isoforms are genetically linked to vesicular trafficking, DNA repair, cell cycle progression, and cytokinesis (Vancura et al., 1994; Gross and Anderson, 1998; Kafadar et al., 2003; Moriya and Johnston, 2004; Sun et al., 2004).
Although relatively little information is available as to the cellular function of CKI isoforms in metazoans and plants, they have been implicated in regulating Wnt signaling, circadian rhythms, and nuclear import (Peters et al., 1999; Vielhaber and Virshup, 2001; Price and Kalderon, 2002; Eichwald et al., 2004; Jia et al., 2004; Preuss et al., 2004). Here, it is noteworthy that a rice CKI isoform was recently linked to control over root development (Liu et al., 2003). In any event, the two most important properties of CKI family members are that subcellular localization is pivotal to their function, and they appear to be constitutively active and not subject to regulation by classical second messengers (Edelman et al., 1987; Gross and Anderson, 1998). These operational features make CKI family members ideal candidates for conscription into plasmodesmata to exert control over cell-to-cell trafficking of specific macromolecules.
Finally, such PAPKs could well provide an additional level of regulation, as CKI can phosphorylate substrates that have already been modified by other kinases (Flotow et al., 1990; Meggio et al., 1991; Xu et al., 1995). This form of control, in which multiple levels of protein phosphorylation are required for both targeting and modulation of protein function, is emerging as a paradigm for NCAP trafficking in plants (Ivanov et al., 2003; Lucas and Lee, 2004; Waigmann et al., 2004; Trutnyeva et al., 2005). Our observation that both the tobacco and Arabidopsis PAPK1 phosphorylate TMV MP suggests that phosphorylation is a common mechanism involved in TMV infection in both species. Previous studies demonstrated that TMV infects Arabidopsis and that the ability of TMV to move cell to cell is important for the spread of TMV infection (Dardick et al., 2000; Golem and Culver, 2003). Further investigation of the functional relationship between PAPK1 phosphorylation and TMV infection will provide important insights into the role of PAPK1 in NCAP trafficking.
METHODS
PECP Preparations
Maintenance of BY-2 tobacco (Nicotiana tabacum) suspension culture cells and preparation of PECP proteins were performed essentially as previously described (Lee et al., 2003). Briefly, cells were harvested 5 d after transfer by filtering 4 liters of culture through Miracloth (Calbiochem). Cells were then given a nondisrupting wash in 75 mM calcium solution to remove noncovalently bound proteins. PECP preparations were also obtained using young leaves of 3-week-old tobacco plants; this tissue was first chopped into small pieces using a BioHomogenizer (BioSpec Products) and then homogenized (BeadBeater; BioSpec Products) using 2.5-mm beads. BY-2 cells or leaf tissue were homogenized, at 4°C, in buffer H (40 mM HEPES, pH 6.8, 20 mM KCl, 5 mM KH2PO4, 1 mM EDTA, and 10% glycerol) containing a mixture of protease inhibitors (10 μg/mL each of leupeptin and aprotinin and 10 mM phenylmethylsulfonyl fluoride). Cell wall pellets were collected by low-speed (5 min at 300g) centrifugation of cell homogenates and were repeatedly subjected to resuspension in buffer H and low-speed centrifugation until a white-colored pellet was obtained. This pellet was then washed with 1% CHAPS followed by extraction of PECP proteins; subfractions were extracted using a step gradient of 30, 75, and 200 mM calcium.
Proteins contained in each subcellular fraction were resolved by electroporesis in SDS-PAGE gels and stained with GelCode Blue (Pierce) to reveal their protein profiles. Protein levels were determined using the Bradford assay (Bio-Rad).
PAPK Purification
All subsequent purification steps were performed at 4°C. Proteins were concentrated using Centricon Plus-80 (Millipore) and dialyzed against buffer A (40 mM HEPES, pH 6.8, 2 mM EDTA, and 10% glycerol). Samples were then loaded onto a HiTrap-SP column (Amersham Biosciences) equilibrated with buffer A, and 1 mL fractions were collected chromatographically in an increasing salt gradient with 1 M NaCl. Peak fractions with TMV MP–phosphorylating activity were pooled, dialyzed, and then loaded onto a new cation exchange column equilibrated with buffer A at pH 8.0. Next, a Con A Sepharose affinity column, equilibrated with buffer C (20 mM Tris-HCl, pH 7.2, 1 mM MnCl2, 1 mM CaCl2, and 0.5 M NaCl), was employed. Finally, pooled proteins were purified using a HiTrap Heparin column (Amersham Biosciences) equilibrated with 40 mM HEPES, pH 6.8, and 2 mM EDTA. Bound proteins were eluted with 1 M NaCl in a gradient and collected in 0.5-mL fractions.
Phosphorylation Assays
Kinase activity assays contained 5 μL of a chromatographic fraction plus 1 μg of TMV MP (or other purified proteins) in a 50-μL reaction volume: assay buffer contained 50 mM HEPES, pH 7.5, 10 mM MgCl2, 2 mM DTT, 2 mM EGTA, and 60 to 100 mM ATP mixed with 2 μCi of [γ32-P]ATP (3000 Ci/mmol; Amersham Biosciences). For calcium-dependent phosphorylation assays, the reaction buffer was supplemented with 2.2 mM CaCl2 (Lee et al., 1998). In assays employing Cucurbita maxima CPK1, 20 ng of purified recombinant enzyme was used. For kinase assays involving recombinant Arabidopsis thaliana PAPK1, 0.4 μg of purified GST-PAPK1 was added to the kinase assay mixture. In some assays, [γ -32P]GTP (3000 Ci/mmol) was used as the phosphate donor. Kinase assay components were assembled in each tube, on ice, and following transfer to 30°C, the ATP mixture was added and incubated for 10 min. Reactions were stopped by adding 10 μL of SDS sample loading buffer. Aliquots (15 to 30 μL) of each reaction mixture were resolved in 10 to 12% SDS-PAGE gel and autoradiographed to identify kinase activity. Densitometry analysis of autoradiograph was performed using NIH ImageJ software (http://rsb.info.nih.gov/ij/).
In-Gel Phosphorylation
In-gel autophosphorylation assays were performed as previously described (Yoo et al., 2002). Briefly, following electrophoresis, the SDS-PAGE gels were washed (2 × 1 h) in buffer D (20% 2-propanol and 40 mM HEPES, pH 7.5). Gels were then incubated for 1 h in buffer D containing 5 mM 2-mercaptoethanol before protein denaturation (1 h) in the same buffer containing 6 M guanidine-HCl. Proteins were renatured by incubation in buffer D containing 3 and 1 M guanidine-HCl (3 h each step). Gels were then incubated (3 h) in buffer D containing 0.04% Tween 20, followed by equilibration (2 × 15 min) in phosphorylation assay buffer containing 50 mM HEPES, pH 7.5, 10 mM MgCl2, and 2 mM EGTA.
In-gel phosphorylation was performed by incubating the conditioned gel in a complete assay buffer including 2 mM DTT and ATP mixture (10 μCi/mL [γ32P]ATP) for 1 h. Phosphorylation reactions were stopped by rinsing the gels with water and then incubating (4 × 1 h) in 5% trichloroacetic acid and 1% sodium pyrophosphate. In-gel substrate phosphorylation, in which TMV MP was polymerized to a final concentration of ∼300 μg/mL gel, was performed as for in-gel autophosphorylation assays.
Expression and Purification of Recombinant Proteins
LFY was expressed in and purified from Escherichia coli. pLG2-8 containing LFY cDNA in frame to GST was digested with HindIII and EcoRI, blunt-ended, and self-ligated to produce a C-terminal deletion mutant whose expression in E. coli yields functional LFY (Parcy et al., 2002). Soluble GST fusion protein was purified using glutathione-agarose (Sigma-Aldrich) following a standard protocol. Other proteins used in phosphorylation assays were prepared by renaturing inclusion bodies. Briefly, protein expression in actively growing BL21(DE3, pLys) cells (Invitrogen) was induced by treatment with 1 mM isopropyl-β-d-thiogalactopyranoside for 3 h at 37°C. Cells were then lysed in B-Per bacterial lysis buffer (Pierce) by sonication, and inclusion bodies were pelleted by centrifugation (5000g, 15 min). Pellets were then washed several times and resuspended in denaturation buffer (2% SDS and 0.1 M Na HCO3, pH 9.0), boiled for 4 min, and cooled on ice for 2 min. Renaturation of proteins was performed by a stepwise dialysis in which the ratio between renaturation (10 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, 10% glycerol, and 2 mM DTT) and denaturation buffers was gradually increased from 20:80 to 100:0.
PAPK1 was recalcitrant to expression in E. coli, possibly due to its toxic effect to the bacteria. However, the following protocol allowed the production of active full-length enzyme. Briefly, the Arabidopsis PAPK1 open reading frame was fused to GST, in frame, using pGEX-KG plasmid (Guan and Dixon, 1991) by PCR cloning. Following DNA sequence confirmation, pGST-PAPK1 was transformed into an E. coli host, Rosetta (DE3) pLys (Novagen). Transformed cells were streaked onto Luria-Bertani plates containing chloramphenicol and carbenicillin and grown overnight at 37°C. Cells were then transferred to 750 mL Luria-Bertani media containing antibiotics and grown at 25°C by shaking at 250 rpm until an OD of ∼0.8 was attained. Protein expression was induced by adding 1 mM isopropyl-β-d-thiogalactopyranoside to the culture and incubating for 2 h at 18°C with gentle shaking. Cells were harvested and lysed with B-PER E. coli extraction buffer (Pierce) according to the manufacturer's protocol. Cell extracts were briefly sonicated and centrifuged for 15 min at 4°C. The supernatant was then added to 1 mL of glutathione-agarose beads pre-equilibrated with 50 mM Tris (pH 7.5) and 150 mM NaCl and incubated for 30 min at 4°C while rotating the beads end over end. Following four times of wash in equilibration buffer, GST-PAPK1 was eluted from beads with 10 mM glutathione buffer, pH 8.0, quantified, and used in phosphorylation assays.
Arabidopsis CKL cDNA Cloning and Plasmid Fusion Construction
Plasmid vectors, pdGN, pdGC, and pdR2N, were constructed for the expression of GFP or RFP fusion proteins in plants. The multicloning site (MCS) of pdGN was constructed by transferring the enhanced GFP (EGFP) from pEGFP-PL (Lee et al., 2003) to the expression cassette of a plant expression vector, pN6. First, pN6 was modified to remove the SacI site by blunt-ending the site and self-ligation. The vector was then digested with BamHI followed by blunt-ending and XhoI digestion to remove an intron sequence between the 35S promoter and Ocs terminator. The MCS-EGFP fragment, released from the pEGFP-PL by restriction enzyme digestions with NotI followed by blunt-ending and XhoI, was inserted into the expression cassette by ligation. pdGC was constructed in a similar manner such that it contained the same MCS as that of pdGN but allowed for the formation of EGFP fusion protein in the reverse orientation (i.e., C-terminal GFP-fusion). In pdR2N, EGFP was replaced with DsRed2 (Clontech) to allow the expression of N-terminal RFP fusion proteins.
CKL open reading frames, or cDNAs, were cloned by RT-PCR. For this purpose, total RNA and mRNA were prepared from Arabidopsis root tissue using Trizol (Gibco BRL) and the RNeasy (Qiagen) mRNA purification kit, following the protocols provided by the suppliers. Following the RT reactions, employing PowerScript reverse transcriptase (Clontech), PCR amplification was performed with gene-specific primers and a high-fidelity Taq-polymerase (Stratagene). PCR products were then cloned into pdGN, pdGC, or pdR2C, via TOPO cloning kits (Invitrogen). The sequence of all CKL inserts in these TOPO clones was confirmed before subcloning into the respective expression vectors.
Transient Expression in BY-2 Cells and Plants
For localization of GFP and RFP fusion constructs, transient assays were performed by employing both biolistic DNA delivery and Agrobacterium tumefaciens–mediated transfection (agroinfiltration). Transient expression in BY-2 cells was as described (Lee et al., 2003), and bombardment of N. tabacum or Arabidopsis leaf tissues was performed basically as for BY-2 cells, except for the use of rupture discs with lower strength (960 p.s.i.). Agroinfiltration in N. benthamiana was performed as described (Liu et al., 2002). After 16 to 48 h of incubation, at 25°C, cells expressing GFP or RFP fusion protein were observed by laser scanning confocal microscopy, as described below.
Microscopic Imaging
Tobacco suspension cultured cells (BY-2) were imaged using a laser scanning confocal microscope (Leica LSM) as described (Lee et al., 2003). Confocal images of leaf cells were acquired on an Axiovert 200M inverted microscope equipped with a Zeiss LSM 510 NLO (Carl Zeiss) using a Zeiss 20× Plan-Apochromat lens (numerical aperture 0.75) or ×40 C-Apochromat (numerical aperture 1.2) objective lens. Data acquisition of EGFP was obtained using the 488-nm laser line of a 25-mW argon laser (LASOS) with a 505LP emission filter. Multichannel images of EGFP and DsRed2 were acquired in fastline-switch mode using the 488- and 543-nm helium neon laser lines (LASOS) with the 500–550 band-pass and 560 long-pass emission filters, respectively. Images were captured as single optical sections (two dimensional) or as a z-series of optical sections (three dimensional). For renderings, three-dimensional data sets were displayed as single maximum intensity projections generated using Zeiss LSM software v3.2.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY943842 to AY943855.
Supplementary Material
Acknowledgments
We thank D. Weigel, P. Waterhouse, and M. Rojas for kindly providing LFY, pN6, and BC1 and BV1 DNA plasmids, respectively. We also thank A. Omid and S. Wolf for their generous sharing of TMV MP-GFP transgenic tobacco and K. Czymmek (Delaware Biotechnology Institute Bioimaging Center) for technical support. This work was supported by grants from the National Science Foundation (IBN 03151174; to W.J.L.), the Department of Energy, Division of Energy Biosciences (DOE-FG03-94ER 20134; to W.J.L.), the National Institutes of Health (P20 RR-15588; to J.-Y.L. and B.-C.Y.), the National Science Foundation (MCB 0445626; to J.Y.L.), and by a University of Delaware Research Foundation award (to J.Y.L.).
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) are: Jung-Youn Lee (lee@dbi.udel.edu) and William J. Lucas (wjlucas@ucdavis.edu).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.034330.
References
- Agostinis, P., Pinna, L.A., Meggio, F., Marin, O., Goris, J., Vandenheede, J.R., and Merlevede, W. (1989). A synthetic peptide substrate specific for casein kinase I. FEBS Lett. 259, 75–78. [DOI] [PubMed] [Google Scholar]
- Atkins, D., Hull, R., Wells, B., Roberts, K., Moore, P., and Beachy, R.N. (1991). The tobacco mosaic virus-30k movement protein in transgenic tobacco plants is localized to plasmodesmata. J. Gen. Virol. 72, 209–211. [DOI] [PubMed] [Google Scholar]
- Boyko, V., van der Laak, J., Ferralli, J., Suslova, E., Kwon, M.O., and Heinlein, M. (2000). Cellular targets of functional and dysfunctional mutants of tobacco mosaic virus movement protein fused to green fluorescent protein. J. Virol. 74, 11339–11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto, T., Prior, D.A.M., Hellwald, K.H., Oparka, K.J., and Palukaitis, P. (1997). Characterization of cucumber mosaic virus. 4. Movement protein and coat protein are both essential for cell-to-cell movement of cucumber mosaic virus. Virology 237, 237–248. [DOI] [PubMed] [Google Scholar]
- Carrington, J.C., Kasschau, K.D., Mahajan, S.K., and Schaad, M.C. (1996). Cell-to-cell and long-distance transport of viruses in plants. Plant Cell 8, 1669–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia, M.L., and Jackson, D. (2004). Plasmodesmata form and function. Curr. Opin. Cell Biol. 16, 500–506. [DOI] [PubMed] [Google Scholar]
- Citovsky, V. (1999). Tobacco mosaic virus: A pioneer of cell-to-cell movement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354, 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky, V., McLean, B.G., Zupan, J.R., and Zambryski, P. (1993). Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase. Genes Dev. 7, 904–910. [DOI] [PubMed] [Google Scholar]
- Crawford, K.M., and Zambryski, P.C. (2001). Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 125, 1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardick, C.D., Golem, S., and Culver, J.N. (2000). Susceptibility and symptom development in Arabidopsis thaliana to Tobacco mosaic virus is influenced by virus cell-to-cell movement. Mol. Plant Microbe Interact. 13, 1139–1144. [DOI] [PubMed] [Google Scholar]
- Ding, B., Haudenshield, J.S., Hull, R.J., Wolf, S., Beachy, R.N., and Lucas, W.J. (1992). Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell 4, 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, B., Itaya, A., and Qi, Y.J. (2003). Symplasmic protein and RNA traffic: Regulatory points and regulatory factors. Curr. Opin. Plant Biol. 6, 596–602. [DOI] [PubMed] [Google Scholar]
- Ding, B., Kwon, M.O., Hammond, R., and Owens, R. (1997). Cell-to-cell movement of potato spindle tuber viroid. Plant J. 12, 931–936. [DOI] [PubMed] [Google Scholar]
- Ding, B.A., Li, Q.B., Nguyen, L., Palukaitis, P., and Lucas, W.J. (1995). Cucumber mosaic virus 3a protein potentiates cell-to-cell trafficking of Cmv RNA in tobacco plants. Virology 207, 345–353. [DOI] [PubMed] [Google Scholar]
- Edelman, A.M., Blumenthal, D.K., and Krebs, E.G. (1987). Protein serine/threonine kinases. Annu. Rev. Biochem. 56, 567–613. [DOI] [PubMed] [Google Scholar]
- Eichwald, C., Jacob, G., Muszynski, B., Allende, J.E., and Burrone, O.R. (2004). Uncoupling substrate and activation functions of rotavirus NSP5: Phosphorylation of Ser-67 by casein kinase 1 is essential for hyperphosphorylation. Proc. Natl. Acad. Sci. USA 101, 16304–16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobara, N.M., Haupt, S., Thow, G., Boevink, P., Chapmana, S., and Oparka, K. (2003). High-throughput viral expression of cDNA-green fluorescent protein fusions reveals novel subcellular addresses and identifies unique proteins that interact with plasmodesmata. Plant Cell 15, 1507–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotow, H., Graves, P.R., Wang, A.Q., Fiol, C.J., Roeske, R.W., and Roach, P.J. (1990). Phosphate groups as substrate determinants for casein kinase I action. J. Biol. Chem. 265, 14264–14269. [PubMed] [Google Scholar]
- Flotow, H., and Roach, P.J. (1991). Role of acidic residues as substrate determinants for casein kinase I. J. Biol. Chem. 266, 3724–3727. [PubMed] [Google Scholar]
- Fujiwara, T., Giesmancookmeyer, D., Ding, B., Lommel, S.A., and Lucas, W.J. (1993). Cell-to-cell trafficking of macromolecules through plasmodesmata potentiated by the red clover necrotic mosaic virus movement protein. Plant Cell 5, 1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, K.L., Paquette, A.J., Nakajima, K., and Benfey, P.N. (2004). Mechanisms regulating SHORT-ROOT intercellular movement. Curr. Biol. 14, 1847–1851. [DOI] [PubMed] [Google Scholar]
- Gilbertson, R.L., and Lucas, W.J. (1996). How do viruses traffic on the ‘vascular highway’? Trends Plant. Sci. 1, 260–268. [Google Scholar]
- Golem, S., and Culver, J.N. (2003). Tobacco mosaic virus induced alterations in the gene expression profile of Arabidopsis thaliana. Mol. Plant Microbe Interact. 16, 681–688. [DOI] [PubMed] [Google Scholar]
- Gorshkova, E.N., Erokhina, T.N., Stroganova, T.A., Yelina, N.E., Zamyatnin, A.A., Kalinina, N.O., Schiemann, J., Solovyev, A.G., and Morozov, S.Y. (2003). Immunodetection and fluorescent microscopy of transgenically expressed hordeivirus TGBp3 movement protein reveals its association with endoplasmic reticulum elements in close proximity to plasmodesmata. J. Gen. Virol. 84, 985–994. [DOI] [PubMed] [Google Scholar]
- Gross, S.D., and Anderson, R.A. (1998). Casein kinase I: Spatial organization and positioning of a multifunctional protein kinase family. Cell. Signal. 10, 699–711. [DOI] [PubMed] [Google Scholar]
- Guan, K.L., and Dixon, J.E. (1991). Eukaryotic proteins expressed in Escherichia coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192, 262–267. [DOI] [PubMed] [Google Scholar]
- Hake, S. (2001). Transcription factors on the move. Trends Genet. 17, 2–3. [DOI] [PubMed] [Google Scholar]
- Haley, A., Hunter, T., Kiberstis, P., and Zimmern, D. (1995). Multiple serine phosphorylation sites on the 30 kDa TMV cell-to-cell movement protein synthesized in tobacco protoplasts. Plant J. 8, 715–724. [DOI] [PubMed] [Google Scholar]
- Haupt, S., Cowan, G.H., Ziegler, A., Roberts, A.G., Oparka, K.J., and Torrance, L. (2005). Two plant-viral movement proteins traffic in the endocytic recycling pathway. Plant Cell 17, 164–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood, V., Kragler, F., and Lucas, W.J. (2002). Plasmodesmata: Pathways for protein and ribonucleoprotein signaling. Plant Cell 14, S303–S325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein, M. (2002). Plasmodesmata: Dynamic regulation and role in macromolecular cell-to-cell signaling. Curr. Opin. Plant Biol. 5, 543–552. [DOI] [PubMed] [Google Scholar]
- Helariutta, Y., Fukaki, H., Wysocka-Diller, J., Nakajima, K., Jung, J., Sena, G., Hauser, M.T., and Benfey, P.N. (2000). The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101, 555–567. [DOI] [PubMed] [Google Scholar]
- Itaya, A., Hickman, H., Bao, Y.M., Nelson, R., and Ding, B. (1997). Cell-to-cell trafficking of cucumber mosaic virus movement protein green fluorescent protein fusion produced by biolistic gene bombardment in tobacco. Plant J. 12, 1223–1230. [Google Scholar]
- Itaya, A., Woo, Y.M., Masuta, C., Bao, Y.M., Nelson, R.S., and Ding, B. (1998). Developmental regulation of intercellular protein trafficking through plasmodesmata in tobacco leaf epidermis. Plant Physiol. 118, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, K.I., Puustinen, P., Gabrenaite, R., Vihinen, H., Ronnstrand, L., Valmu, L., Kalkkinen, N., and Makinen, K. (2003). Phosphorylation of the potyvirus capsid protein by protein kinase CK2 and its relevance for virus infection. Plant Cell 15, 2124–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, D. (2000). Opening up the communication channels: Recent insights into plasmodesmal function. Curr. Opin. Plant Biol. 3, 394–399. [DOI] [PubMed] [Google Scholar]
- Jackson, D. (2001). The long and the short of it: Signaling development through plasmodesmata. Plant Cell 13, 2569–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, D., Veit, B., and Hake, S. (1994). Expression of maize Knotted1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120, 405–413. [Google Scholar]
- Jia, J., Tong, C., Wang, B., Luo, L., and Jiang, J. (2004). Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature 432, 1045–1050. [DOI] [PubMed] [Google Scholar]
- Kafadar, K.A., Zhu, H., Snyder, M., and Cyert, M.S. (2003). Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes Dev. 17, 2698–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karger, E.M., Frolova, O.Y., Fedorova, N.V., Baratova, L.A., Ovchinnikova, T.V., Susi, P., Makinen, K., Ronnstrand, L., Dorokhov, Y.L., and Atabekov, J.G. (2003). Dysfunctionality of a tobacco mosaic virus movement protein mutant mimicking threonine 104 phosphorylation. J. Gen. Virol. 84, 727–732. [DOI] [PubMed] [Google Scholar]
- Karpova, O.V., Ivanov, K.I., Rodionova, N.P., Dorokhov, Y.L., and Atabekov, J.G. (1997). Nontranslatability and dissimilar behavior in plants and protoplasts of viral RNA and movement protein complexes formed in vitro. Virology 230, 11–21. [DOI] [PubMed] [Google Scholar]
- Karpova, O.V., Rodionova, N.P., Ivanov, K.I., Kozlovsky, S.V., Dorokhov, Y.L., and Atabekov, J.G. (1999). Phosphorylation of tobacco mosaic virus movement protein abolishes its translation repressing ability. Virology 261, 20–24. [DOI] [PubMed] [Google Scholar]
- Kawakami, S., Padgett, H.S., Hosokawa, D., Okada, Y., Beachy, R.N., and Watanabe, Y. (1999). Phosphorylation and/or presence of serine 37 in the movement protein of tomato mosaic tobamovirus is essential for intracellular localization and stability in vivo. J. Virol. 73, 6831–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.Y., Yuan, Z., and Jackson, D. (2003). Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development 130, 4351–4362. [DOI] [PubMed] [Google Scholar]
- Kim, J.Y., Yuan, Z.A., Cilia, M., Khalfan-Jagani, Z., and Jackson, D. (2002). Intercellular trafficking of a KNOTTED1 green fluorescent protein fusion in the leaf and shoot meristem of Arabidopsis. Proc. Natl. Acad. Sci. USA 99, 4103–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlizky, G., Katz, A., van der Laak, J., Boyko, V., Lapidot, M., Beachy, R.N., Heinlein, M., and Epel, B.L. (2001). A dysfunctional movement protein of Tobacco mosaic virus interferes with targeting of wild-type movement protein to microtubules. Mol. Plant Microbe Interact. 14, 895–904. [DOI] [PubMed] [Google Scholar]
- Kragler, F., Monzer, J., Shash, K., Xoconostle-Cazares, B., and Lucas, W.J. (1998). Cell-to-cell transport of proteins: Requirement for unfolding and characterization of binding to a putative plasmodesmal receptor. Plant J. 15, 367–381. [Google Scholar]
- Kragler, F., Monzer, J., Xoconostle-Cazares, B., and Lucas, W.J. (2000). Peptide antagonists of the plasmodesmal macromolecular trafficking pathway. EMBO J. 19, 2856–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, D.M., and Jackson, A.O. (2001). Interactions of the TGB1 protein during cell-to-cell movement of Barley stripe mosaic virus. J. Virol. 75, 8712–8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz, S.G., and Beachy, R.N. (1999). Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell 11, 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.Y., and Lucas, W.J. (2001). Phosphorylation of viral movement proteins—Regulation of cell-to-cell trafficking. Trends Microbiol. 9, 5–8. [DOI] [PubMed] [Google Scholar]
- Lee, J.Y., Yoo, B.C., and Harmon, A.C. (1998). Kinetic and calcium-binding properties of three calcium-dependent protein kinase isoenzymes from soybean. Biochemistry 37, 6801–6809. [DOI] [PubMed] [Google Scholar]
- Lee, J.Y., Yoo, B.C., Rojas, M.R., Gomez-Ospina, N., Staehelin, L.A., and Lucas, W.J. (2003). Selective trafficking of non-cell-autonomous proteins mediated by NtNCAPP1. Science 299, 392–396. [DOI] [PubMed] [Google Scholar]
- Liu, W., Xu, Z.H., Luo, D., and Xue, H.W. (2003). Roles of OsCKI1, a rice casein kinase I, in root development and plant hormone sensitivity. Plant J. 36, 189–202. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Schiff, M., Marathe, R., and Dinesh-Kumar, S.P. (2002). Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 30, 415–429. [DOI] [PubMed] [Google Scholar]
- Lucas, W.J. (1995). Plasmodesmata: Intercellular channels for macromolecular transport in plants. Curr. Opin. Cell Biol. 7, 673–680. [DOI] [PubMed] [Google Scholar]
- Lucas, W.J., Bouchepillon, S., Jackson, D.P., Nguyen, L., Baker, L., Ding, B., and Hake, S. (1995). Selective trafficking of Knotted1 homeodomain protein and its messenger RNA through plasmodesmata. Science 270, 1980–1983. [DOI] [PubMed] [Google Scholar]
- Lucas, W.J., Ding, B., and Vanderschoot, C. (1993). Plasmodesmata and the supracellular nature of plants. New Phytol. 125, 435–476. [DOI] [PubMed] [Google Scholar]
- Lucas, W.J., and Gilbertson, R.L. (1994). Plasmodesmata in relation to viral movement within leaf tissue. Annu. Rev. Phytopathol. 32, 387–411. [Google Scholar]
- Lucas, W.J., and Lee, J.Y. (2004). Plasmodesmata as a supracellular control network in plants. Nat. Rev. Mol. Cell Biol. 5, 712–726. [DOI] [PubMed] [Google Scholar]
- Lucas, W.J., and Wolf, S. (1993). Plasmodesmata: The intercellular organelles of green plants. Trends Cell Biol. 3, 308–315. [DOI] [PubMed] [Google Scholar]
- Meggio, F., Perich, J.W., Reynolds, E.C., and Pinna, L.A. (1991). A synthetic beta-casein phosphopeptide and analogues as model substrates for casein kinase-1, a ubiquitous, phosphate directed protein kinase. FEBS Lett. 283, 303–306. [DOI] [PubMed] [Google Scholar]
- Moore, P.J., Fenczik, C.A., Deom, C.M., and Beachy, R.N. (1992). Developmental changes in plasmodesmata in transgenic tobacco expressing the movement protein of tobacco mosaic virus. Protoplasma 170, 115–127. [Google Scholar]
- Moriya, H., and Johnston, M. (2004). Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc. Natl. Acad. Sci. USA 101, 1572–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, K., Sena, G., Nawy, T., and Benfey, P.N. (2001). Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311. [DOI] [PubMed] [Google Scholar]
- Northcote, D.H., Davey, R., and Lay, J. (1989). Use of antisera to localize callose, xylan and arabinogalactan in the cell plate, primary and secondary walls of plant-cells. Planta 178, 353–366. [DOI] [PubMed] [Google Scholar]
- Noueiry, A.O., Lucas, W.J., and Gilbertson, R.L. (1994). Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 76, 925–932. [DOI] [PubMed] [Google Scholar]
- Oparka, K.J. (2004). Getting the message across: How do plant cells exchange macromolecular complexes? Trends Plant Sci. 9, 33–41. [DOI] [PubMed] [Google Scholar]
- Oparka, K.J., Prior, D.A.M., SantaCruz, S., Padgett, H.S., and Beachy, R.N. (1997). Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus (TMV). Plant J. 12, 781–789. [DOI] [PubMed] [Google Scholar]
- Palevitz, B.A., and Hepler, P.K. (1985). Changes in dye coupling of stomatal cells of Allium and Commelina by microinjection of Lucifer yellow. Planta 164, 473–479. [DOI] [PubMed] [Google Scholar]
- Parcy, F., Bomblies, K., and Weigel, D. (2002). Interaction of LEAFY, AGAMOUS and TERMINAL FLOWER1 in maintaining floral meristem identity in Arabidopsis. Development 129, 2519–2527. [DOI] [PubMed] [Google Scholar]
- Perbal, M.C., Haughn, G., Saedler, H., and Schwarz-Sommer, Z. (1996). Non-cell-autonomous function of the Antirrhinum floral homeotic proteins DEFICIENS and GLOBOSA is exerted by their polar cell-to-cell trafficking. Development 122, 3433–3441. [DOI] [PubMed] [Google Scholar]
- Pesch, M., and Hulskamp, M. (2004). Creating a two-dimensional pattern de novo during Arabidopsis trichome and root hair initiation. Curr. Opin. Genet. Dev. 14, 422–427. [DOI] [PubMed] [Google Scholar]
- Peters, J.M., McKay, R.M., McKay, J.P., and Graff, J.M. (1999). Casein kinase I transduces Wnt signals. Nature 401, 345–350. [DOI] [PubMed] [Google Scholar]
- Preuss, F., Fan, J.Y., Kalive, M., Bao, S., Schuenemann, E., Bjes, E.S., and Price, J.L. (2004). Drosophila doubletime mutations which either shorten or lengthen the period of circadian rhythms decrease the protein kinase activity of casein kinase I. Mol. Cell. Biol. 24, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, M.A., and Kalderon, D. (2002). Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell 108, 823–835. [DOI] [PubMed] [Google Scholar]
- Robards, A.W., and Lucas, W.J. (1990). Plasmodesmata. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41, 369–419. [Google Scholar]
- Roberts, I.M., Boevink, P., Roberts, A.G., Sauer, N., Reichel, C., and Oparka, K.J. (2001). Dynamic changes in the frequency and architecture of plasmodesmata during the sink-source transition in tobacco leaves. Protoplasma 218, 31–44. [DOI] [PubMed] [Google Scholar]
- Rojas, M.R., Noueiry, A.O., Lucas, W.J., and Gilbertson, R.L. (1998). Bean dwarf mosaic geminivirus movement proteins recognize DNA in a form- and size-specific manner. Cell 95, 105–113. [DOI] [PubMed] [Google Scholar]
- Ruiz-Medrano, R., Xoconostle-Cazares, B., and Kragler, F. (2004). The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Curr. Opin. Plant Biol. 7, 641–650. [DOI] [PubMed] [Google Scholar]
- Schellmann, S., Schnittger, A., Kirik, V., Wada, T., Okada, K., Beermann, A., Thumfahrt, J., Jurgens, G., and Hulskamp, M. (2002). TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J. 21, 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions, A., Yanofsky, M.F., and Weigel, D. (2000). Cell-cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science 289, 779–781. [DOI] [PubMed] [Google Scholar]
- Sun, B., Chen, L., Cao, W., Roth, A.F., and Davis, N.G. (2004). The yeast casein kinase Yck3p is palmitoylated, then sorted to the vacuolar membrane with AP-3-dependent recognition of a YXXPhi adaptin sorting signal. Mol. Biol. Cell 15, 1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomenius, K., Clapham, D., and Meshi, T. (1987). Localization by immunogold cytochemistry of the virus-coded 30k protein in plasmodesmata of leaves infected with tobacco mosaic virus. Virology 160, 363–371. [DOI] [PubMed] [Google Scholar]
- Trethewey, J.A.K., and Harris, P.J. (2002). Location of (1→3)- and (1→3),(1→4)-beta-D-glucans in vegetative cell walls of barley (Hordeum vulgare) using immunogold labelling. New Phytol. 154, 347–358. [DOI] [PubMed] [Google Scholar]
- Trutnyeva, K., Bachmaier, R., and Waigmann, E. (2005). Mimicking carboxyterminal phosphorylation differentially effects subcellular distribution and cell-to-cell movement of Tobacco mosaic virus movement protein. Virology 332, 563–577. [DOI] [PubMed] [Google Scholar]
- Tzfira, T., Rhee, Y., Chen, M.H., Kunik, T., and Citovsky, V. (2000). Nucleic acid transport in plant-microbe interactions: The molecules that walk through the walls. Annu. Rev. Microbiol. 54, 187–219. [DOI] [PubMed] [Google Scholar]
- Vancura, A., Sessler, A., Leichus, B., and Kuret, J. (1994). A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J. Biol. Chem. 269, 19271–19278. [PubMed] [Google Scholar]
- Vielhaber, E., and Virshup, D.M. (2001). Casein kinase I: From obscurity to center stage. IUBMB Life 51, 73–78. [DOI] [PubMed] [Google Scholar]
- Wada, T., Kurata, T., Tominaga, R., Koshino-Kimura, Y., Tachibana, T., Goto, K., Marks, M.D., Shimura, Y., and Okada, K. (2002). Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129, 5409–5419. [DOI] [PubMed] [Google Scholar]
- Waigmann, E., Chen, M.H., Bachmaier, R., Ghoshroy, S., and Citovsky, V. (2000). Regulation of plasmodesmal transport by phosphorylation of tobacco mosaic virus cell-to-cell movement protein. EMBO J. 19, 4875–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waigmann, E., Ueki, S., Trutnyeva, K., and Citovsky, V. (2004). The ins and outs of nondestructive cell-to-cell and systemic movement of plant viruses. CRC Crit. Rev. Plant Sci. 23, 195–250. [Google Scholar]
- Wang, H.L., Wang, Y., Giesman-Cookmeyer, D., Lommel, S.A., and Lucas, W.J. (1998). Mutations in viral movement protein alter systemic infection and identify an intercellular barrier to entry into the phloem long-distance transport system. Virology 245, 75–89. [DOI] [PubMed] [Google Scholar]
- Wang, P.C., Vancura, A., Mitcheson, T.G., and Kuret, J. (1992). Two genes in Saccharomyces cerevisiae encode a membrane-bound form of casein kinase-1. Mol. Biol. Cell 3, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, Y., Ogawa, T., and Okada, Y. (1992). In vivo phosphorylation of the 30-kDa protein of tobacco mosaic virus. FEBS Lett. 313, 181–184. [DOI] [PubMed] [Google Scholar]
- Weigel, D., and Meyerowitz, E.M. (1993). Activation of floral homeotic genes in Arabidopsis. Science 261, 1723–1726. [DOI] [PubMed] [Google Scholar]
- Wille, A.C., and Lucas, W.J. (1984). Ultrastructural and histochemical-studies on guard-cells. Planta 160, 129–142. [DOI] [PubMed] [Google Scholar]
- Wolf, S., Deom, C.M., Beachy, R.N., and Lucas, W.J. (1989). Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science 246, 377–379. [DOI] [PubMed] [Google Scholar]
- Wu, X., Dinneny, J.R., Crawford, K.M., Rhee, Y., Citovsky, V., Zambryski, P.C., and Weigel, D. (2003). Modes of intercellular transcription factor movement in the Arabidopsis apex. Development 130, 3735–3745. [DOI] [PubMed] [Google Scholar]
- Wu, X.L., Weigel, D., and Wigge, P.A. (2002). Signaling in plants by intercellular RNA and protein movement. Genes Dev. 16, 151–158. [DOI] [PubMed] [Google Scholar]
- Xu, R.M., Carmel, G., Sweet, R.M., Kuret, J., and Cheng, X. (1995). Crystal structure of casein kinase-1, a phosphate-directed protein kinase. EMBO J. 14, 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, B.C., Lee, J.Y., and Lucas, W.J. (2002). Analysis of the complexity of protein kinases within the phloem sieve tube system: Characterization of Cucurbita maxima calmodulin-like domain protein kinase 1. J. Biol. Chem. 277, 15325–15332. [DOI] [PubMed] [Google Scholar]
- Zambryski, P., and Crawford, K. (2000). Plasmodesmata: Gatekeepers for cell-to-cell transport of developmental signals in plants. Annu. Rev. Cell Dev. Biol. 16, 393–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.