Cytosolic calcium (Ca2+) is a focal point of many signal transduction pathways and modulates a diverse array of cellular activities ranging from fertilization to cell death (1). Cells generate Ca2+ signals through both internal and external Ca2+ sources. In most cell types, the major internal Ca2+ stores are the endoplasmic reticulum/sarcoplasmic reticulum (ER/SR). One mechanism for mobilizing such stores involves the classical phosphoinositide pathway. Essentially, the binding of many hormones to specific receptors on the plasma membrane leads to the activation of an enzyme (phosphoinositidase C) that catalyses the hydrolysis of phospholipids to produce the intracellular messenger inositol 1,4,5-trisphosphate (InsP3). Although derived from a lipid, InsP3 is water soluble and diffuses into the cell interior where it encounters InsP3 receptors (InsP3Rs) on the ER/SR. The binding of InsP3 changes the conformation of InsP3Rs such that an integral channel is opened, thus allowing the Ca2+ stored at high concentrations in the ER/SR to enter the cytoplasm. A critical feature of InsP3Rs is that their opening is regulated by the cytosolic Ca2+ concentration. This sensitivity to cytosolic Ca2+ allows them to act as Ca2+-induced Ca2+ release (CICR) channels that promote the rapid amplification of smaller trigger events (1).
It was thought that the binding of InsP3 was obligatory for channel opening. However, in an elegant study in this issue of PNAS, Foskett and colleagues (2) demonstrate that a protein could supplant the need for InsP3. Using a yeast two-hybrid screen, those authors demonstrate a high-affinity interaction between the NH2-terminal 600 aa of an InsP3R and a member of a previously cloned group of proteins called Ca2+ binding proteins (CaBPs; ref. 3). These proteins belong to a superfamily of proteins that includes the well-known protein calmodulin, which bind Ca2+ by EF-hand motifs. CaBPs belong to a subfamily of EF-hand-containing proteins known as neuronal calcium sensors (NCS) (4). Although members of this group of proteins show great structural similarities, they have very diverse functional properties. The NCS subfamily consists of small proteins (molecular mass ≈20 kDa) that are exclusively expressed in neurons or retinal photoreceptors. They have a high degree of sequence similarity to calmodulin, but differ with respect to the number of functional Ca2+-binding EF hands. The proposed physiological roles of NCS proteins include modulation of neurotransmitter release and regulation of gene transcription (4).
The study by Foskett and colleagues (2) proposes a new function for the CaBP members of the NCS family—regulation of InsP3Rs and Ca2+ release from intracellular stores. CaBPs interacted with InsP3Rs only in the amino-terminal region that was used for bait, which also contains the domain where InsP3 itself binds to the channel. Patch-clamp recording of single InsP3Rs in the outer nuclear membrane of Xenopus oocytes indicated that CaBPs could activate the channels in the presence or absence of InsP3. In fact, CaBP alone gave rise to substantial opening of the InsP3Rs (Po ≈0.8 in optimal conditions), with significant gating observed at 10 nM CaBP. The interaction between CaBPs and InsP3Rs was greatly potentiated by increasing cytosolic Ca2+ over its physiological range (0.1–1 μM). This process critically depended on the EF hands, because mutations that inhibited Ca2+ binding to these domains abrogated the ability of CaBP to bind and activate InsP3Rs.
CaBPs can act as endogenous high-affinity ligands of InsP3Rs.
The picture that emerges is that CaBPs can act as endogenous high-affinity ligands of InsP3Rs, which can activate Ca2+ release without the need for InsP3 production via the phosphoinositide pathway (see Fig. 1). Because the action of CaBPs depends on Ca2+ binding, InsP3R signaling would still operate in a CICR mode; CaBPs can act on InsP3Rs to potentiate trigger Ca2+ signals arising from other sources, but presumably cannot initiate Ca2+ transients. At least five different CaBP isoforms with multiple splice variants exist, and they appear to have a clear tissue-specific expression profile (3). Some of the isoforms are abundantly expressed in the cerebral cortex and hippocampus, suggesting that CaBPs may be key mediators of Ca2+ signaling in the brain.
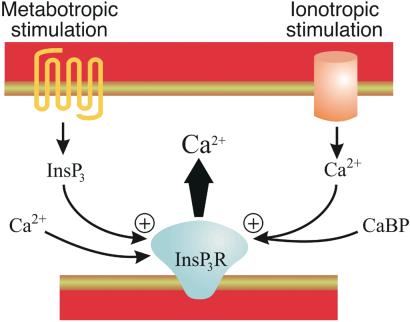
Figure 1.
Dual pathways for activation of InsP3Rs. Shown are the alternative pathways leading to InsP3R activation in neurons expressing CaBPs. Metabotropic receptors lead to InsP3 production via phospholipase C, whereas ionotropic stimulation produces an influx of Ca2+ that engages CaBP-bound InsP3Rs or recruits CaBP to the InsP3R. Both metabotropic and ionotropic pathways lead to the activation of InsP3Rs. InsP3 requires the concurrent binding of Ca2+ to an activatory site on the InsP3R. It is presently unclear whether the Ca2+-bound form CaBP is sufficient for InsP3R opening, or if Ca2+ is also required to bind to InsP3Rs independently.
Although we can presently only speculate on the functions of CaBPs in neuronal Ca2+ signaling, their subcellular localization suggests some intriguing possibilities. Cellular fractionation and immunohistochemistry of CaBP-expressing neurons suggests that these proteins are present in synaptic and dendritic compartments, where InsP3Rs are also located. These are strategic locations where information processing occurs. If CaBPs can participate in Ca2+ signals occurring during synaptic signaling they may have an important role in memory formation. Ca2+ signaling within spines underlie changes in synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD), which are models for learning and memory. Ca2+ signals underlie synaptic plasticity, and it is well established that InsP3Rs are involved in some of these paradigms (5, 6). It has been proposed that InsP3Rs act as “coincidence detectors” to interpret simultaneous signals arising from metabotropic receptors (producing InsP3) and the Ca2+ entry through voltage-operated Ca2+ channel and receptor-operated Ca2+ channels (such as N-methyl-d-aspartate receptors) (7). CaBPs could obviate the need for metabotropic stimulation during LTD/LTP, thus enabling InsP3Rs to participate in information processing in the absence of metabotropic receptor activation. The versatility of InsP3Rs would be enhanced through this responsiveness to InsP3 or CaBP, thus enabling them to operate after activation of metabotropic or ionotropic mechanisms.
Unless signals become amplified by CICR, buffering and sequestration usually determines that Ca2+ has a very limited diffusion within the cytosol of most cells. InsP3 is more freely diffusible, although it is typically metabolized to the non-Ca2+ releasing messengers inositol 1,4-bisphosphate (InsP2) and inositol 1,3,4,5-tetrakisphosphate (InsP4) within seconds. The enzyme that catalyses the latter reaction to produce InsP4, InsP33-kinase, has been proposed to act as a “firewall” within spines to stop the spread of InsP3 into dendritic shafts and neighboring spines (8). If Ca2+-bound CaBP can act as a mobile signal, it may smuggle an activatory signal beyond the barrier that prevents InsP3 diffusion, to allow InsP3R-dependent Ca2+ signaling over significant distances. An analogous situation occurs with calmodulin, which can diffuse over tens of micrometers within cells to allow local Ca2+ signals to activate gene transcription (9, 10).
Just how mobile CaBPs are is not fully resolved. One feature of the NCS family is that its members contain consensus sequences for myristoylation (4). This posttranslational modification results in Ca2+-dependent translocation of these proteins, although along distinct routes (11). Expression of isoform variants of a CaBP in Chinese hamster ovary cells revealed that it could have distinct cellular localizations—a short form was found at or near the plasma membrane, whereas a longer homologue was associated with the cytoskeleton (3). The ability of CaBPs to bind and activate InsP3Rs would clearly be altered if they were tethered by lipid groups or structural proteins distant from InsP3Rs.
Although the study from Foskett and colleagues (2) clearly demonstrates that CaBPs can regulate the activation of InsP3Rs, there are clearly many outstanding questions concerning their mode of action. InsP3 can have complicated effects on InsP3Rs; it has been demonstrated to cause intrinsic inactivation of InsP3Rs and at high concentrations to protect them from Ca2+-dependent inhibition (12). It will be interesting to see whether CaBPs can replicate all of the effects of InsP3 on channel gating. Furthermore, it is possible that activation of InsP3Rs is not the sole function of CaBPs. In common with other members of the NCS subfamily, they appear to interact with several targets (4). CaBPs have been demonstrated to activate calmodulin-dependent kinase II and G protein-coupled receptor kinases in vitro (3). Whether such interactions occur in vivo is not yet known, however, it points to a potential pleiotropic action of the CaBPs after a Ca2+ signal. In addition, the CaBPs have consensus sequences for phosphorylation and have been shown to be phosphorylated in hippocampal slices in situ (13). Their functions could therefore be modulated by upstream kinases. Another possibility is that the expression of CaBPs could be modulated in a developmental- or activity-dependent manner, in which case their effects on the InsP3Rs could vary substantially.
For the activation of calmodulin-dependent kinase II and G protein-coupled receptor kinases, CaBPs essentially mimicked the role of calmodulin (3). Interestingly, InsP3Rs also have calmodulin binding regions, which can modulate the activity of the channels (14–16). One of the calmodulin binding sites has been mapped to the NH2-terminal 159 aa of the InsP3R (17), which is within the “bait” region used by Yang et al. (2) in their yeast two-hybrid screen. However, in their study, calmodulin had little effect on the interaction of CaBPs with InsP3Rs, suggesting that they have distinct binding sites. In addition, calmodulin did not activate InsP3R channel activity in membrane patches. Therefore, the effects of CaBP and calmodulin on InsP3Rs appear to occur through distinct binding sites. It is plausible that these two proteins may have opposite effects on the opening of InsP3Rs, with CaBPs causing Ca2+-dependent activation and calmodulin evoking Ca2+-dependent inhibition.
CaBPs are not the only members of the NCS family that regulate intracellular Ca2+ signals. Neuronal Ca2+ sensor 1/frequenin has been shown to activate phosphatidylinositol 4-kinase (18) and facilitate the activation of P/Q-type voltage-operated Ca2+ channels (19). Therefore, several of the NCS proteins have the capacity to both interpret and modulate neuronal Ca2+ signals.
The demonstration that CaBPs can activate InsP3Rs in the absence of InsP3 raises many important questions for future work. CaBPs essentially endow InsP3Rs with the ability to display CICR without InsP3 binding. This would make them functionally equivalent to ryandodine receptors (RyRs), which are intracellular Ca2+ release channels directly gated by Ca2+. Neurons expressing CaBPs, InsP3Rs, and RyRs would therefore possess two channels capable of autonomous CICR and potentially causing steeply regenerative Ca2+ signals. It will be important to find out what decelerates CaBP-induced Ca2+ release to prevent it from simply running away with itself. Additional questions include the localization and translocation of CaBPs. Does this limit or enhance their effects on InsP3Rs? The observation that CaBPs can bind to InsP3Rs at low Ca2+ concentration (2) suggests that the Ca2+-free forms of these proteins can be prebound to InsP3Rs. This may be a way of speeding up the responsiveness of InsP3Rs, in a similar way that complexing apo-calmodulin does for P/Q-type voltage-operated Ca2+ channels (20). Finally, are InsP3Rs engaged with CaBP still responsive to InsP3, or do CaBPs completely decouple the phosphoinositide signaling pathway from Ca2+ release? Clearly, the novel function for CaBPs proposed by Foskett and colleagues (2) opens up a new concept in the regulation of Ca2+ release.
Footnotes
See companion article on page 7711.
References
- 1.Berridge M J, Lipp P, Bootman M D. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, McBride S, Mak D-OD, Vardi N, Palczewski K, Haeseleer F, Foskett J K. Proc Natl Acad Sci USA. 2002;99:7711–7716. doi: 10.1073/pnas.102006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haeseleer F, Sokal I, Verlinde C L M J, Erdjument-Bromage H, Tempst P, Pronin A N, Benovic J L, Fariss R N, Palczewski K. J Biol Chem. 2000;275:1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgoyne R D, Weiss J L. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue T, Kato K, Kohda K, Mikoshiba K. J Neurosci. 1998;18:5366–5373. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyata M, Finch E A, Khiroug L, Hashimoto K, Hayasaka S, Oda S-I, Inouye M, Takagishi Y, Augustine G J, Kano M. Neuron. 2000;28:233–244. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 7.Berridge M J. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 8.Schell M J, Erneux C, Irvine R F. J Biol Chem. 2001;276:37537–37546. doi: 10.1074/jbc.M104101200. [DOI] [PubMed] [Google Scholar]
- 9.Deisseroth K, Heist E K, Tsien R W. Nature (London) 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 10.Craske M, Takeo T, Gerasimenko O, Vaillant C, Török K, Petersen O H, Tepikin A V. Proc Natl Acad Sci USA. 1999;96:4426–4431. doi: 10.1073/pnas.96.8.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Callaghan D W, Ivings L, Weiss J L, Ashby M C, Tepikin A V, Burgoyne R D. J Biol Chem. 2002;277:14227–14237. doi: 10.1074/jbc.M111750200. [DOI] [PubMed] [Google Scholar]
- 12.Bootman M D, Lipp P. Curr Biol. 2000;9:R876–R878. doi: 10.1016/s0960-9822(00)80072-x. [DOI] [PubMed] [Google Scholar]
- 13.Seidenbecher C I, Langnaese K, Sanmart'- Vila L, Boeckers T M, Smalla K-H, Sabel B A, Garner C C, Gundelfinger E D, Kreutz M R. J Biol Chem. 1998;273:21324–21331. doi: 10.1074/jbc.273.33.21324. [DOI] [PubMed] [Google Scholar]
- 14.Missiaen L, Parys J B, Weidema A F, Sipma H, Vanlingen S, De Smet P, Callewaert G, De Smedt H. J Biol Chem. 1999;274:13748–13751. doi: 10.1074/jbc.274.20.13748. [DOI] [PubMed] [Google Scholar]
- 15.Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, Mikoshiba K. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- 16.Adkins C E, Morris S A, De Smedt H, Sienaert I, Torok K, Taylor C W. Biochem J. 2000;345:357–363. [PMC free article] [PubMed] [Google Scholar]
- 17. Sienaert, I., Nadif Kasri, N., Vanlingen, S., Parys, J. B., Callewaert, G., Missiaen, L. & De Smedt, H. (2002) Biochem. J., 10.1042/BJ20020144. [DOI] [PMC free article] [PubMed]
- 18.Hendricks K B, Wang B Q, Schnieders E A, Thorner J. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- 19.Tsujimoto T, Jeromin A, Saitoh N, Roder J C, Takahashi T. Science. 2002;295:2276–2279. doi: 10.1126/science.1068278. [DOI] [PubMed] [Google Scholar]
- 20.DeMaria C D, Soong T W, Alseikhan B A, Alvania R A, Yue D T. Nature (London) 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]