Abstract
Vitamin A and its active metabolite, all-trans retinoic acid (RA), regulate the antibody response in vivo, although the underlying mechanisms are not well understood. We have investigated the regulation by RA of B cell population dynamics and Ig gene expression in purified splenic mouse B cells stimulated through the B cell antigen receptor (BCR) and/or CD38, a BCR coreceptor. After ligation of the BCR and/or CD38, B cells became more heterogeneous in size. RA substantially restrained this change, concomitant with inhibition of cell proliferation. To examine B cell heterogeneity more closely, we categorized stimulated B cells by size (forward angle light scatter) and determined cell division dynamics, germ-line Ig heavy chain gene transcription and surface IgG1 (sIgG1) expression. Flow cytometric analysis of carboxyfluorescein diacetate succinimidyl ester-labeled B cells costained for sIgG1 showed that the more proliferative groups of B cells were smaller, whereas cells expressing more sIgG1 were larger. RA enriched the latter population, whereas cell division frequency in general and the number of smaller B cells that had undergone division cycles were reduced. Although RA significantly inhibited Ig germ-line transcript levels in the total B cell population, CD19-IgG1+ B cells, which represent a more differentiated phenotype, were enriched. Furthermore, pax-5 mRNA was decreased and activation-induced cytidine deaminase mRNA was increased in RA-treated stimulated B cells. Thus, RA regulated factors known to be required for Ig class switch recombination and modulated the population dynamics of ligation-stimulated B cells, while promoting the progression of a fraction of B cells into differentiated sIgG-expressing cells.
Keywords: IgG1, pax-5, activation-induced cytidine deaminase, CD19, costimulation
Retinoic acid (RA), a natural bioactive metabolite of vitamin A (1), is well known as a hormone capable of promoting the differentiation of a wide variety of cell types and as a regulator of many physiological processes. Vitamin A deficiency in young children is associated with increased morbidity and mortality, which is widely attributed to an increased severity of infectious diseases (2, 3), and vitamin A supplementation has been shown to reduce mortality and disease severity (4–6). Vitamin A deficiency is also associated with exacerbation of immunodeficiency (7), reduced or unbalanced numbers of lymphocytes (8), and dysregulated production of antibodies (9). Animal experiments have demonstrated that an adequate level of vitamin A is necessary to mount an efficient antibody response (10, 11), whereas supplementation with vitamin A/RA can stimulate the antibody response to vaccination even in normal animals (10, 12, 13). Despite these interesting functional properties of vitamin A and RA on antibody production, little is known about the underlying mechanisms, especially related to B lymphocyte activation and differentiation.
Induction of an effective antibody response requires that immunocompetent naïve B cells be capable of responding to multiple signals delivered by antigens binding to the B cell antigen receptor (BCR) and by accessory molecules. Although the BCR is the central component mediating B cell activation (14–16) and antibody production, other B cell surface molecules, such as CD38, CD19, and CD40, are known to function as accessory receptors or costimulators in the activation of B cells (17–20). These surface molecules integrate the signals provided by antigen and activated T cells to induce a cascade of responses involving B cell proliferation, Ig heavy chain gene class switch recombination (CSR), and Ig gene expression, culminating in antibody production (19–21).
CD38 is variably expressed in hematopoietic cells and is tightly controlled during cell development and activation (17, 22, 23). CD38 is one of the earliest surface markers expressed on B cells. In murine B cells, CD38 is detectable at the pro-B cell stage, preceding the expression of surface IgM and IgD (17). CD38 is also a signaling molecule of the cell surface (24). Cross-linking of CD38 on mature B cells causes B cell proliferation and differentiation (25, 26). The extracellular domain of CD38, a type II membrane-associated glycoprotein, possesses intrinsic enzymatic activity in NAD+ metabolism, which, in ways not fully understood, is involved in intracellular calcium mobilization (27, 28). Although the short cytoplasmic domain of CD38 lacks autonomous signaling capability, CD38 may nevertheless promote cell activation through its interaction with other signal-transducing molecules, such as the Igα/Igβ components of the BCR (29). The activation of CD38 lowers the threshold of BCR activation, indicating a functional interaction of these two molecular clusters (29). It is interesting to note that mice lacking CD38 (30) and animals lacking adequate vitamin A (31, 32) have similar defects in antibody production, characterized by a significantly reduced antibody response to T cell-dependent and type 2 T cell-independent antigens, although the reasons are not understood at the present time.
Whereas previous studies have shown that B cell proliferation is reduced by RA (33, 34), RA significantly increased the antibody response in RA-treated rats and mice (10, 12, 13). To better understand the effects of RA on B cells, we have focused on the role of RA in the activation and differentiation of naïve mature splenic B cells stimulated through the BCR and coreceptor CD38. Whereas RA inhibited cell proliferation and germ-line (GL) IgG (γ1) and IgM (μ) gene transcription, inspection of flow cytometry data revealed that the B cell population became markedly heterogeneous in size after BCR/CD38 stimulation, which was substantially restrained in the presence of RA. Thus, we proceeded to characterize B cell division, the expression of IgG1 GL transcript, and B cell surface markers during cell activation and differentiation in relationship to B cell size. Our results showed that RA is a potent inhibitor of B cell proliferation and an inducer of changes in gene expression that favor the differentiation of a portion of BCR/CD38-stimulated B cells into surface IgG1 (sIgG1)-expressing cells, thus favoring B cell maturation.
Materials and Methods
Reagents. All-trans RA (Sigma) was prepared as a concentrated stock in ethanol and diluted right before applying to cells. Antibodies used for B cell stimulation were anti–mouse IgM F(ab′)2 fragment (115-006-075, Jackson ImmunoResearch) and anti-CD38 (clone NIMR-5, Southern Biotechnology Associates). Antibodies used for surface staining were anti-mouse B220-phycoerythrin (PE), CD19-PE, CD19-FITC, and IgG1-peridinin chlorophyll protein (PerCP) (BD Biosciences), anti-CD38-FITC (Santa Cruz Biotechnology), and Alexa Fluo 488-conjugated goat anti-IgG1 (Molecular Probes). Respective isotype controls (BD Biosciences) were also used. Carboxyfluorescein diacetate succinimidyl ester (CFSE) was from Molecular Probes.
B Cell Isolation. Naive splenic B cells from adult (>8 wk) C57BL/6 mice were purified by negative selection of total spleen cells by using density beads coupled with antibodies against non-B cells (Spin-Sep, StemCell Technologies, Vancouver). The purity of the recovered B cells (CD19+/B220+/CD38+) was ≈92%. B cells were also purified by sorting the splenocytes to collect CD19-positive cells; the splenocytes were first labeled with CD19-FITC antibody and then subjected to flow sorting (Coulter Elite ESP flow cytometer, Beckman Coulter). Isolated B cells were cultured in RPMI medium 1640 supplemented with 10% heat-inactivated FBS, 5 × 10-5 M 2-mercaptoethanol, 100 units/ml of penicillin, and 100 μg/ml streptomycin (Life Technologies, Carlsbad, CA), at 37°C in a 5% CO2 incubator. The RA was diluted with medium and added to cells at a concentration of 10 nM, similar to its physiological concentration in plasma (35).
Cell Proliferation Assay. B cells in 96-well plates (2 × 105 cells per well) were treated with and without stimuli (anti-μ and anti-CD38) and RA. Cells were cultured for a total of 72 h; [3H]thymidine (0.2 μCi per well, Amersham Pharmacia Biosciences) was added for the last 4 h after which the cells were harvested, and the incorporation of [3H]thymidine was determined by liquid scintillation spectrometry.
RNA Isolation and RT-PCR. B cells plated in 12-well plates (5 × 105 cells per ml) were treated with stimuli and RA under conditions as described in the figure legends. Total cellular RNA was isolated at the time of harvesting (RNeasy mini kit, Qiagen, Valencia, CA). One microgram of total RNA was subjected to reverse transcription and PCR analysis (Promega). Primers for GL Ig gene transcription were those reported by Muramatsu et al. (36). The pax-5 primers were: forward, 5-GGTCAGCCATGGTTGTGTCAG; reverse, 5-CGGCACTGGAGACTCCTGAAT. Activation-induced cytidine deaminase (AID) primers were: forward, 5-CCGCTCACCTGGTTCACCTCCT; reverse, 5-CCTTCCCAGGCTTTGAAAGTTCTTTCA. The mRNA for GAPH (37) and 18S ribosomal RNA (forward, 5-AATGGTGCTACCGGTCATTC; reverse, 5-ACCTCTCTTACCCGCTCTCC) were used as internal controls for RNA integrity and RT-PCR amplification. During PCR amplification, 0.1 μCi of [α-33P]dATP (Amersham Pharmacia) was added to the PCR mixture to label the PCR product. PCR conditions were optimized as denaturing at 94°C, 1 min, annealing at 62°C for 1 min, and extension at 72°C for 1 min, using 19 cycles for the housekeeping genes and 28 cycles for the other genes. After amplification, the PCR products were separated on polyacrylamide gel and quantified by a liquid scintillation counter to determine the relative gene expression level (37).
Flow Cytometric Analysis. To determine the level of cell surface markers B220, CD38, CD19, and IgG1, B cells were washed twice with PBS at the time of harvesting and stained with fluorochrome-conjugated antibodies. The proportion of positive-stained cells was measured by flow cytometry, using isotype-matched control antibody for background subtraction. Compensation was performed to ensure the positive staining in double-staining experiments, and data were analyzed by flowjo software (Tree Star, Ashland, OR).
B cells were also sorted by flow cytometry according to their size character. Stimulated B cells at day 5 were harvested and resuspended in medium at a concentration of 1 × 107 cells per ml. About 0.5–1 ml of the cells were sorted according to the forward angle light scatter (FALS); the cutoff was set at 300 units on the FALS scale. About 2 × 106 cells were collected in each group (upper and bottom), which were further subjected to RNA isolation and RT-PCR analysis.
Determination of Cell Division and sIgG1 Expression. Fluorescent labeling of B cells with CFSE was performed as described (38). Briefly, isolated splenic B cells were suspended at a density of 2 × 107 cells per ml in PBS, and CFSE was added to a final concentration of 10 nM. After 30-min incubation at 37°C, unbound CFSE was quenched by the addition of 10 ml of medium containing 10% FBS, and the cells were washed twice with medium to remove noninternalized dye. Cells were then plated at 2 × 105 cells per well in 96-well plates and treated with stimuli. At the time of harvesting, cells were stained with fluorochrome-conjugated antibody specific for IgG1. Flow cytometry analysis was performed to determine the cell distribution according to the side and forward scatter scale and the fluorescent labeling (CFSE and IgG1). The dilution of CSFE, indicative of cell division, was determined by using time-0 cells to set the gate for undivided cells.
Statistics. Student's t test was used for statistic analysis, and P < 0.05 was considered a significant difference.
Results
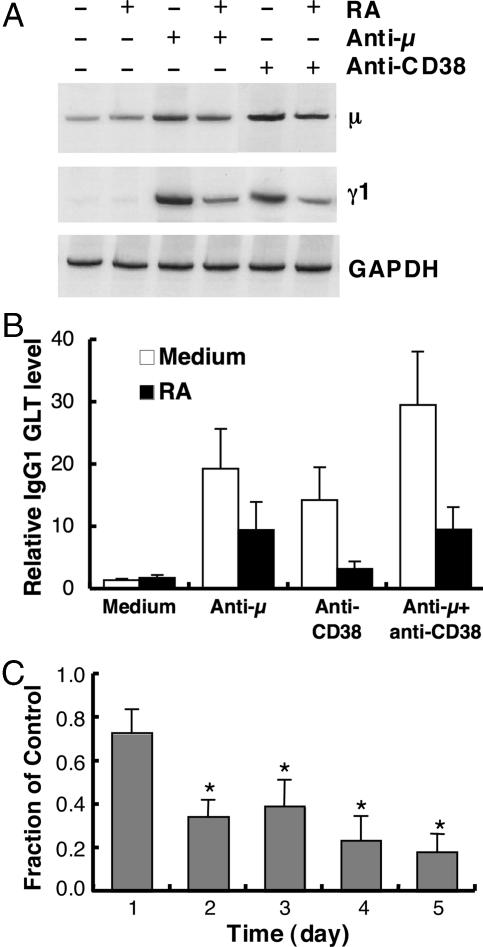
RA Inhibits BCR and CD38 Ligation-Induced Proliferation in Total B Cells. Initially, B cells were treated with increasing concentrations of agonistic antibodies to the BCR (anti-μ) or CD38 (anti-CD38) in the presence and absence of RA, and cell proliferative activity was measured. Ligation of the BCR or the costimulating receptor CD38 markedly induced B cell proliferation in a dose-dependent manner, and the presence of a physiological concentration of RA (10 nM) significantly inhibited stimulation-induced B cell proliferation (Fig. 1 A and B). The combination of anti-μ and anti-CD38 produced a more than additive increase in B cell proliferation, which again was greatly dampened by RA (Fig. 1C). These results suggest that although CD38 is known to share the BCR signaling pathway (17, 29), it may also have distinct pathways, all of which are affected by RA.
Fig. 1.
RA inhibits BCR and CD38 ligation-induced B cell proliferation. Primary murine B cells were cultured in the presence and absence of a physiological concentration of RA (10 nM) and the stimuli indicated for 72 h. [3H]thymidine was added to the cells in the last 4 h of culture to label the proliferating cells, and [3H]thymidine incorporation was determined. (A) Proliferation of B cells stimulated with low doses of anti-μ.(B) Proliferation of B cells stimulated with anti-CD38. (C) Proliferation of B cells stimulated with anti-CD38 and anti-μ (each 1 μg/ml) in the presence and absence of RA. The data shown represent the mean ± SE for five independent experiments conducted in triplicate. *, P < 0.01.
To confirm that the results presented above for density bead-purified B cells (≈92% CD19/B220/CD38-positive cells) are truly representative of B cells, the same experiments were performed after sorting spleen cells to obtain only the CD19+ (B cell) population. RA produced a comparable inhibition of proliferation in the sorted B cells (data not shown) as in bead-purified B cells. Thus, RA inhibited BCR/CD38-induced B cell proliferation, similar to the inhibition reported in other B cell models (33, 34).
RA Regulates the Level of GL mRNA Induced by BCR and CD38 Ligation. Ig gene transcription is an essential event before Ig class switching (39), and thus an early hallmark of B cell activation/differentiation. Because RA markedly reduced B cell proliferative activity, we performed the following experiment to examine whether RA also regulates the expression of GL Ig transcripts. Splenic B cells were cultured for up to 5 days in the presence and absence of RA and anti-μ or anti-CD38 antibodies. IgM (μ gene) and IgG (γ1) GL transcripts were chosen to evaluate the regulatory effect of stimuli and RA. B cell stimulation increased the levels of both μ and γ1 GL transcripts; however, the GL transcript levels were markedly reduced by RA as shown in Fig. 2 A and B for the 48-h data. RA inhibited stimulation-induced IgG1 GL transcript expression throughout the 5-day experiment; by day 5, the level in RA-treated cells was <20% of that in stimulated B cells cultured without RA (Fig. 2C).
Fig. 2.
RA regulates GL μ and γ1 mRNA levels induced by BCR and CD38 ligation. B cells were cultured as described in Fig. 1 for different times. Cellular total RNA was extracted and subjected to RT-PCR analysis. (A) Representative 33P-labeled PCR product showing the level of expression of μ and γ1 GL mRNA after 48 h of culture. GAPDH mRNA is shown as an internal control of the assay. (B) IgG1 GL transcript level in cells cultured for 48 h. Data were obtained by counting 33P-labeled PCR products and are presented as the mean ± SE from n = 4 independent experiments. (C) The ratio of GL transcript levels [cells cultured with RA (10 nM) compared with control without RA] in B cells stimulated with anti-μ plus anti-CD38 (1 μg/ml of each). *, P < 0.05, compared with day 1.
RA Reduces B Cell Division Rate as Indicated by CFSE Dilution. It has been reported that B cell antibody production and class switching depend on cell division (38, 40, 41). To examine whether RA, shown above to reduce DNA synthesis and GL Ig mRNA expression, affects the cell division cycle, CFSE staining was used to trace the activity of dividing cells. CFSE-labeled B cells were cultured for 5 days in the presence and absence of stimuli, with and without RA, and the cells were then costained with PerCP-conjugated anti-IgG1 upon harvesting and subjected to flow cytometric analysis.
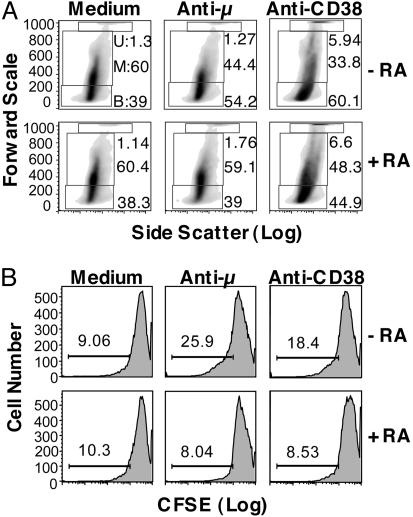
As analyzed by FALS and side scatter (Fig. 3A), B cells, which were relatively homogeneous before stimulation, became markedly heterogeneous after culture with stimuli. Some cells were small in size (low forward scatter), whereas another group of cells distributed in a relatively broad field along the middle part of the forward scatter–side scatter scales, and a cluster of cells formed at the top of the forward scatter scale. Hence, we initially divided the B cells into three putative populations, designated bottom, middle, and upper groups. Stimulation with anti-μ or anti-CD38 increased the percentage of B cells within the bottom group. RA had no effect on unstimulated cells; however, the shift in cell distribution that was induced by anti-μ or anti-CD38 was restrained in the presence of RA.
Fig. 3.
Regulation by RA of B cell distribution and cell division (CFSE dilution) elicited by BCR and CD38 ligation. Isolated B cells were labeled with CFSE and cultured for 5 days with and without stimuli (anti-μ or anti-CD38, each 1 μg/ml) and RA (10 nM). Cells were harvested on day 5 and analyzed for CFSE intensity by flow cytometry. (A) FALS versus side scatter plots were used to analyze the data. A representative panel of graphs of the distribution of B cells after treatment is shown. Three arbitrary gates (U, upper; M, middle; B, bottom) were made to divide the cell population according to the forward scatter. (B) Flow cytometric histograms of CFSE dilution in control and stimulated B cells with and without RA; the percentage of cells that had undergone dilution is indicated above the bars.
To assess the cell division status, cells that had undergone CFSE dilution, which represented the cells that had passed through at least one division cycle, were quantified. As shown in Fig. 3B, stimulation of the BCR or CD38 increased the population of B cells with CFSE dilution, indicative of having undergone cell division, as compared with the unstimulated control cells, in which there was very little CFSE dilution. RA significantly reduced the stimuli-induced CFSE dilution, indicating that RA inhibited the division of stimulated B cells. As we noticed a cell number reduction in RA-treated samples after the 5-day treatment, staining of annexin V was performed to assess potential apoptosis. However, no apparent apoptosis was found (data not shown).
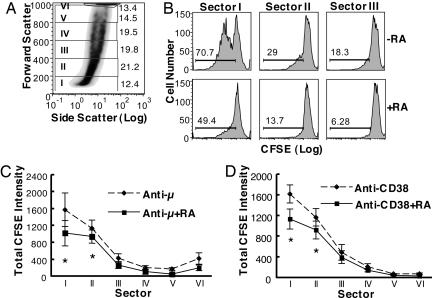
To analyze the data in a more detailed way, we further divided the cells into six sectors according to the forward scatter scale (increments of 200 scale units of forward scatter for sectors I to V, with the rest of the cells at the top of the scale designated as sector VI, illustrated in Fig. 4A). It was interesting to note that the cells having undergone the most division cycles were located mainly in sectors I and II (Fig. 4B, showing data for anti-μ-stimulated cells). The dividing B cells comprised ≈71% and 29% of the cells in those sectors, respectively. The larger B cells had undergone less CFSE dilution (sector III < II < I, as illustrated), indicative of less cell proliferation/division. In all sectors of cell size, RA markedly reduced the fraction of B cells that had divided. The data for all six sectors are summarized in Fig. 4 C and D, which combines the cell population with CFSE-diluted cells in each sector. The inhibitory effect of RA on cell division was observed in each sector, with the greatest inhibition on cells with the greatest CSFE dilution (cells in sectors I and II). When the B cells were categorized into quintiles of cell number along the forward scale, instead of by forward scale values, the results showed a similar trend with the greatest reduction in CFSE dilution in RA-treated cells in the lowest two quintiles of forward scatter. Thus, these analyses showed that the stimulation of B cells by ligation of the BCR and surface CD38 alters the cell size distribution and cell division activity in the B cell population. RA restrains the alterations induced by BCR/CD38 stimulation, modulating B cell dynamics.
Fig. 4.
B cell division (CFSE dilution) induced by BCR and CD38 ligation is reduced by RA. B cells were labeled with CFSE and cultured for 5 days with and without stimuli (anti-μ or anti-CD38, 1 μg/ml) and RA. Cells were harvested on day 5 and analyzed by flow cytometry for CFSE dilution. (A) A representative graph showing the way the B cell population was divided into six sectors according to forward scatter (every 200 scale units, with the cells clustered at the top as sector VI). (B) The inhibitory effect of RA on anti-μ-induced B cell CFSE dilution, illustrated for sectors I, II, and III as shown in A.(C and D). Charts summarizing studies in which CFSE dilution in each sector of A was determined for B cells stimulated by ligation of the BCR (C) and CD38 (D), with and without RA. *, P < 0.05.
RA Positively Regulates the Number of Activation-Induced sIgG1-Expressing Cells. To determine the cell differentiation status of the activated and RA-treated B cells, sIgG1 expression was assessed. Ligation of the BCR or CD38 increased the percentage of cells expressing sIgG1. Interestingly, RA further increased the proportion of sIgG1-positive cells (Fig. 5A). By analyzing the data for each of the six sectors of cell size described above, it was revealed that, in contrast to cells that had undergone CFSE dilution, the IgG1-positive cells were distributed mainly in the upper sectors, such as sectors III, IV, V, and VI (shown in Fig. 5B), and that RA enriched the number of sIgG1-positive cells in each sector (Fig. 5 B–D). In addition, RA increased the mean fluorescence intensity of sIgG1 by ≈20% (data not shown).
Fig. 5.
B cell sIgG1 expression induced by BCR and CD38 ligation is increased by RA. B cells were cultured for 5 days as described in Fig. 4. At the end of culture, cells were stained with a PerCP-labeled anti-IgG1 antibody and subjected to flow cytometric analysis. (A) Graphs showing the percentage of sIgG1-positive cells after BCR and CD38 ligation (1 μg/ml), with and without RA (10 nM). (B) Graphs showing that the majority of IgG1-positive cells were located in sectors III, IV, and V, and RA increased the percentage of IgG1-positive cells in each sector. Anti-μ was used as stimulus in the experiment shown. The dashed line illustrates the isotype control. (C and D) Charts summarizing the data for each sector and treatment. *, P < 0.05.
Combining the data on cell distribution, CFSE dilution, and sIgG1 expression shows that the smaller B cells located in sectors I and II were more active in proliferation, as CFSE was more diluted in those cells, whereas the larger B cells distributed in sectors III, IV, V, and VI had undergone less CFSE dilution and a higher proportion of these cells express sIgG1. Stimulation of B cells by ligation of BCR or CD38 resulted in enhancement of both cell proliferation/division rate and sIgG1 expression. However, the addition of RA to B cells reduced the cell proliferation induced by those stimuli while, concomitantly, it enhanced the expression of sIgG1. These data provided evidence that RA differentially regulates B cell functions, which may depend on the B cell activation stage. Although RA had a restraining effect on B cell proliferation, it favored the differentiation of B cells.
IgG1 GL Transcript Level Is Higher in the B Cell Population Expressing sIgG1. To determine whether IgG1 GL transcription also differs in the B cell subsets, a cell sorting experiment was performed. B cells stimulated with anti-μ plus anti-CD38 in the presence and absence of RA were cultured for 5 days and then sorted into two populations according to their position on the forward scatter scale: the bottom gate represented the smaller cells (mainly in the previous sectors I and II as described in Figs. 4 and 5), and the upper gate comprised the rest of the cells (sectors III, IV, V, and VI). After sorting, RNA was isolated, and the IgG1 GL transcript level was quantified in each group. Cells in the upper group (larger B cells previously shown to have gone through fewer division cycles) expressed more IgG1 GL transcripts compared with cells in the bottom group (Fig. 6), which was consistent with the sIgG1 staining showing that the upper sectors contained more of the sIgG1-positive cells. RA reduced IgG1 GL transcripts in both of these groups, in agreement with the data in Fig. 2 showing that RA inhibited the GL transcript expression in total B cell population.
Fig. 6.
RA regulates IgG1 GL mRNA expression in B cells sorted into smaller and larger-sized cell populations. B cells were cultured for 5 days with anti-μ plus anti-CD38 (1 μg/ml each) in the presence and absence of RA (10 nM). Cells were then sorted into two populations [B, bottom gate (similar to sectors I and II), and U, upper gate (similar to sectors III, IV, V, and VI in Figs. 4 and 5, based on FALS)]. Cellular RNA was isolated from these cells and RT-PCR was performed to determine the level of GL IgG1 mRNA (γ1 gene transcription). The expression pattern of the GL γ1 transcript in each treatment is shown by a representative gel of PCR products and 18S RNA as control. The chart summarizes the expression pattern of γ1 transcript in each gate for the treatments shown. **, the significant difference between groups with and without RA; *, the difference between bottom and upper groups, P < 0.05.
sIgG1 Expression on CD19-Negative B Cells Is Enriched by RA. CD19 is a B cell marker whose expression is reduced in the course of B cell differentiation (42); thus we reasoned that the level of CD19 and sIgG1 may be regulated differentially in BCR and CD38 ligation-activated B cells. We therefore performed a flow cytometric analysis on B cells with CD19 and sIgG1 costaining to determine the cell activation/differentiation status after BCR-CD38 stimulation in the presence and absence of RA. On the day of purification, ≈90% of the enriched B cell population was CD19-positive, and the percentage of CD19-positive cells declined steadily during in vitro culture and was further reduced in stimulated cells (Fig. 7A; see also Fig. 9, which is published as supporting information on the PNAS web site). Oppositely, only ≈20–30% of naïve B cells were positive for sIgG1 (most are CD19-positive), whereas the proportion of cells expressing sIgG1 started to increase 1 day after stimulation and reached a high level on day 3 (Figs. 7B and 9). On day 5, most of the IgG1-positive cells were CD19-negative. Although RA itself had no significant effect on CD19 expression (Fig. 7A) and did not immediately alter the proportion of sIgG1-positive B cells, significantly more B cells were sIgG1-positive after 3 days in the RA-treated cultures. Additionally, when RA was added to stimulated cells on day 3 of culture instead of day 0, it similarly enhanced the proportion of stimulation-induced sIgG1+CD19- cells on day 5 (data not shown).
Fig. 7.
CD19 and sIgG1 expression during BCR and CD38 ligation-induced B cell activation. B cells were cultured for 5 days in the presence and absence of anti-μ plus anti-CD38 (1 μg/ml each) and RA (10 nM). At the time of harvesting each day, surface CD19 and sIgG1 were detected by anti-CD19-PE and anti-IgG1-Alexa 488 antibodies, respectively, and analyzed by flow cytometry. (A) The percentage of CD19+ cells during culture and after stimulation. Con, control; S±RA, stimuli in the presence and absence of RA (which did not differ). (B) The percentage of sIgG1+/CD19- cells. *, the difference between S and S+RA; **, the difference between S and Con. P < 0.05.
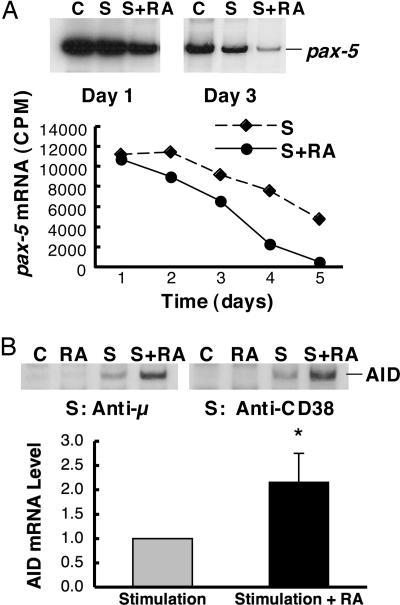
RA Regulates pax-5 and AID in BCR/CD38-Stimulated B Cells. B cell activation and differentiation, which is manifested by cell proliferation, expression of GL transcripts, CSR, and Ig production, is tightly regulated by the interaction of transcription factors (39, 43, 44). Pax-5 encodes the B cell-specific activator protein, BSAP, that plays an important role in B cell development and early activation (45); its expression is known to be extinguished during B cell differentiation, before plasma cell formation (44, 46). On the other hand, the expression of the AID is indispensable for CSR and somatic hypermutation (47). Because RA greatly decreased B cell proliferation and differentially regulated the levels of GL transcript and sIgG1 expression, we were interested in understanding whether RA also alters the expression of pax-5 and AID genes, which are associated with and/or determinants of B cell activation/differentiation. As shown in Fig. 8A, pax-5 was expressed constitutively in naïve B cells. Pax-5 mRNA gradually declined when cells were cultured in vitro with BCR/CD38 stimulation, and RA further reduced the level of pax-5 expression. In contrast, AID expression was low in naïve B cells, induced after BCR or CD38 ligation, and further up-regulated by RA (Fig. 8B). From these results we infer that the reduction of pax-5/BSAP expression, together with an increase in stimuli-induced AID, in BCR/CD38-stimulated RA-treated cells could be a major mechanism contributing to the changes in B cell dynamics and enrichment of sIgG1 induced by RA. These data suggest a unique molecular mechanism by which RA can favor B cell differentiation/maturation after BCR and CD38 ligation.
Fig. 8.
Regulation of pax-5 and AID gene expression during the activation of B cells in the presence of RA. B cells were cultured for different times, as shown, and then total RNA was subjected to RT-PCR analysis to assess pax-5 and AID mRNA levels. (A) Pax-5 expression in anti-μ and anti-CD38-stimulated B cells. (Inset) A gel showing the expression pattern of pax-5 on days 1 and 3 in the presence and absence of treatment. S represents stimulation with anti-μ and anti-CD38 (1 μg/ml for each). S+RA represents stimulation plus RA (10 nM). The chart illustrates pax-5 gene expression (cpm) in BCR and CD38 ligation-activated B cells during the 5-day experiment. (B) AID mRNA in B cells stimulated by anti-μ or anti-CD38, with and without RA, for 48 h. The chart summarizes n = 3 independent experiments. Stimulation represents anti-μ plus anti-CD38 (1 μg/ml for each). Data are presented as the ratio of AID mRNA in RA-treated cells compared with cells without RA. *, P < 0.05.
Discussion
The regulatory effect of RA on B cells is rather complex and closely depends on the manner of B cell stimulation and the cytokine milieu. RA inhibited anti-μ and IL-4-induced human peripheral blood B cell proliferation and cytokine (IL-6 and TNF-α) production (48) and was inhibitory for the growth of several tumorigenic B cells (33, 49). RA also inhibited IL-4-induced IgE and IgG1 production in LPS-stimulated murine B cells, whereas under the same conditions RA enhanced IL-5-induced IgA production (50). We have shown in our model of freshly isolated, normal naïve murine B cells that RA is inhibitory for B cell proliferation/division induced by surface BCR and CD38 ligation (Fig. 1). Although the stimulatory effect of anti-BCR and anti-CD38 on B cell proliferation is more than additive, suggesting that more than one pathway could be involved, RA markedly reduced the effect of either stimulus alone and both stimuli in combination. RA also significantly reduced stimulation-induced B cell division as demonstrated by monitoring the dilution of CFSE, indicative of cell division, over the 5 days of culture (Figs. 3 and 4). It was reported that RA could induce human B cell arrest in the late G1 phase of the cell cycle by repressing the expression of cyclin E and cyclin A (34), which may explain the reduction of B cell proliferative activity and CFSE dilution in the presence of RA. The results we and others have observed in B cells are also consistent with our previous observation in the monocytic cell line, THP-1, in which RA inhibited THP-1 cell proliferation by inducing cell cycle arrest and concurrently promoted the cell differentiation and functional maturation of the THP-1 monocytes to macrophage-like phagocytic cells (51).
It is well accepted that activated B cells undergo cell proliferation and Ig heavy chain rearrangement and that Ig production is related to B cell division cycles under the stimulation of T cell-dependent signals, such as those delivered by CD40 ligation and cytokines, especially IL-4 (38). Our isolated B cell model clearly demonstrates that activated B cells rapidly become heterogeneous during in vitro culture. Cells existed in two, and likely more, distinct activation/differentiation stages. Although some B cells were more proliferative, other subset(s) of B cells expressed higher levels of GL transcripts and sIgG1, suggesting that B cell proliferation and sIg expression are not necessarily always parallel to each other, and that the expression of sIgG1 in BCR- and CD38-ligated B cells does not require extensive cell proliferation. Whereas RA significantly inhibited the proliferation of the stimuli-activated B cells, it increased the number of sIgG1-positive cells, and the surface density of sIgG1, in our model. Furthermore, although RA reduced the level of stimulation-induced GL transcripts, it enriched the subset of B cells that had the higher level of γ1 GL transcript and greater sIgG1 intensity (Figs. 5 and 6). All of these lines of evidence support the hypothesis that RA favors the differentiation of B cells. It is also noteworthy that CD19, a B cell surface marker whose expression declines as B cells undergo activation and differentiation (42), was markedly decreased on B cells 1 day after BCR/CD38 stimulation. Although RA has no obvious effect on CD19 expression (Figs. 7A and 9), the expression of sIgG1 was increased in RA-treated stimulated cells by day 3 (Fig. 7B). A similar enhancement was observed whether RA was added on day 0 (with initial stimulation) or day 3 (data not shown), suggesting that the effect of RA on sIgG1 expression depends on the functional readiness of the B cells. The ability of RA to increase sIgG1 is superimposed only in stimulated, competent B cells at certain differentiation phase(s). Other investigators have provided evidence that the activation/differentiation of stimulated B and T cells is stochastically determined (40, 52). The cell fate and strength of the response solely depend on the type, concentration, and duration of the stimuli. RA does not appear to exert an “all or none” effect on B cell processes, but rather to exert multiple effects at different stages that influence cell dynamics, which result an increase in the number of B cells that reach a more differentiated state.
B cell proliferation and differentiation are regulated in concert by groups of transcription factors. For example, B cell-specific activator protein (BSAP), encoded by the pax-5 gene, is a B cell-specific transcription factor only expressed in pro-B, pre-B, and mature B cells, but not in plasma cells. It is a key factor in the control of B cell expansion (45), development, and proliferation (53), but down-regulation and extinction of pax-5/BSAP is necessary for activated B cells to differentiate into Ig-producing plasma cells (44, 46, 54, 55). Here, we have shown that whereas pax-5 was constitutively expressed in mature B cells, the level of pax-5 mRNA declined during culture and stimulation, similar to CD19 for which it is a regulatory factor (44, 46). Pax-5 mRNA was further reduced in the presence of RA (Fig. 8). The reduction in pax-5 expression could be a key event in the promotion of sIgG1 expression by RA, as it was observed early in the activation-differentiation process and led to nearly complete extinction of pax-5 gene expression by day 4. We also determined the expression level of AID because it is a required protein in CSR. AID mRNA was increased by RA in stimulated B cells, indicative of a positive effect of RA on the regulation of CSR and consistent with increased sIgG1 expression as demonstrated in Fig. 5.
Expression of GL Ig gene transcripts is known as a prerequisite signal for the Ig class switching that leads to antibody production (39). However, the function and regulation of this process is not well understood, and it is not yet clear whether the magnitude of GL transcription is of regulatory significance. In our study, GL Igγ1 mRNA and sIgG1 expression were differentially regulated by RA. Whereas the level of μ and γ1 GL transcripts was substantially reduced by RA at 24 h and later times (Fig. 2), sIgG1 expression was elevated by day 3 of stimulation, remained high during the detection time (to day 5), and was further increased in the presence of RA (Figs. 5 and 7). On the surface, these results seem disparate, and at present we cannot explain the opposing effects of RA on the levels of γ1 GL transcript level and sIgG1 expression. However, the results might be reconciled if the level of GL transcription in BCR/CD38-stimulated B cells is adequate to initiate DNA rearrangement and class switching, whether or not RA is added, and if GL transcription (once activated) must be down-regulated for B cell differentiation to proceed, which may be promoted by RA. Regardless of the mechanism, the data caution against using GL transcript levels as a surrogate for mature IgG gene expression, as they may be regulated differentially.
In summary, our experiments provide evidence that RA regulates the dynamics of BCR/CD38-stimulated B cell activation/differentiation in both early and later stages of cell activation. In the early phases of B cell activation RA functioned to restrain cell proliferation and reduce division frequency, GL transcript levels, and pax-5 gene expression. In the intermediate and later stages of differentiation, RA increased AID expression and enriched the population of B cells that express sIgG1. Overall, RA promoted stimulation-induced B cell progression to a more differentiated state, consistent with its ability to promote antibody production and isotype switching after immunization in vivo.
Supplementary Material
Acknowledgments
We thank Elaine Kunze for advice and assistance with cell sorting. This work was supported by National Institutes of Health Grant DK 41479 and funds from the Howard Heinz Endowment.
Author contributions: Q.C. and A.C.R. designed research; Q.C. performed research; Q.C. analyzed data; and Q.C. and A.C.R. wrote the paper.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 29, 2003.
Abbreviations: AID, activation-induced cytidine deaminase; BCR, B cell antigen receptor; CFSE, carboxyfluorescein diacetate succinimidyl ester; CSR, class switch recombination; FALS, forward angle light scatter; GL, germ line; PerCP, peridinin chlorophyll protein; RA, retinoic acid; sIgG1, surface IgG1.
See accompanying Profile on page 14139.
References
- 1.Ross, A. C., Zolfaghari, R. & Weisz, J. (2001) Curr. Opin. Gastroenterol. 17, 184-192. [DOI] [PubMed] [Google Scholar]
- 2.Reifen, R. (2002) Proc. Nutr. Soc. 61, 397-400. [DOI] [PubMed] [Google Scholar]
- 3.Semba, R. D. (1999) Proc. Nutr. Soc. 58, 719-727. [DOI] [PubMed] [Google Scholar]
- 4.Semba, R. D., Ndugwa, C., Perry, R. T., Clark, T. D., Jackson, J. B., Melikian, G., Tielsch, J. & Mmiro, F. (2005) Nutrition 21, 25-31. [DOI] [PubMed] [Google Scholar]
- 5.Villamor, E. & Fawzi, W. W. (2005) Clin. Microbiol. Rev. 18, 446-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grubesic, R. B. & Selwyn, B. J. (2003) J. Nurs. Scholarsh. 35, 15-20. [DOI] [PubMed] [Google Scholar]
- 7.Hanley, T. M., Kiefer, H. L., Schnitzler, A. C., Marcello, J. E. & Viglianti, G. A. (2004) J. Virol. 78, 2819-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjersing, J. L., Telemo, E., Dahlgren, U. & Hanson, L. A. (2002) Clin. Exp. Immunol. 130, 404-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephensen, C. B., Jiang, X. & Freytag, T. (2004) J. Nutr. 134, 2660-2666. [DOI] [PubMed] [Google Scholar]
- 10.DeCicco, K. L., Youngdahl, J. D. & Ross, A. C. (2001) Immunology 104, 341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeCicco, K. L., Zolfaghari, R., Li, N. & Ross, A. C. (2000) J. Infect. Dis. 182, Suppl. 1, S29-S36. [DOI] [PubMed] [Google Scholar]
- 12.Cui, D., Moldoveanu, Z. & Stephensen, C. B. (2000) J. Nutr. 130, 1132-1139. [DOI] [PubMed] [Google Scholar]
- 13.Ma, Y., Chen, Q. & Ross, A. C. (2005) J. Immunol. 174, 7961-7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poe, J. C., Hasegawa, M. & Tedder, T. F. (2001) Int. Rev. Immunol. 20, 739-762. [DOI] [PubMed] [Google Scholar]
- 15.Ruland, J. & Mak, T. W. (2003) Immunol. Rev. 193, 93-100. [DOI] [PubMed] [Google Scholar]
- 16.Dal Porto, J. M., Gauld, S. B., Merrell, K. T., Mills, D., Pugh-Bernard, A. E. & Cambier, J. (2004) Mol. Immunol. 41, 599-613. [DOI] [PubMed] [Google Scholar]
- 17.Donis-Hernandez, F. R., Parkhouse, R. M. & Santos-Argumedo, L. (2001) Eur. J. Immunol. 31, 1261-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFranco, A. L. (1996) Curr. Biol. 6, 548-550. [DOI] [PubMed] [Google Scholar]
- 19.Mills, D. M. & Cambier, J. C. (2003) Semin. Immunol. 15, 325-329. [DOI] [PubMed] [Google Scholar]
- 20.Baumgarth, N. (2000) Immunol. Rev. 176, 171-180. [DOI] [PubMed] [Google Scholar]
- 21.Tangye, S. G. & Hodgkin, P. D. (2004) Immunology 112, 509-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinis, M., Morra, M., Funaro, A., Di Primio, R. & Malavasi, F. (1997) J. Biol. Regul. Homeostasis Agents 11, 137-142. [PubMed] [Google Scholar]
- 23.Ridderstad, A. & Tarlinton, D. M. (1998) J. Immunol. 160, 4688-4695. [PubMed] [Google Scholar]
- 24.Shubinsky, G. & Schlesinger, M. (1997) Immunity 7, 315-324. [DOI] [PubMed] [Google Scholar]
- 25.Kaku, H., Horikawa, K., Obata, Y., Kato, I., Okamoto, H., Sakaguchi, N., Gerondakis, S. & Takatsu, K. (2002) Int. Immunol. 14, 1055-1064. [DOI] [PubMed] [Google Scholar]
- 26.Lund, F., Solvason, N., Grimaldi, J. C., Parkhouse, R. M. & Howard, M. (1995) Immunol. Today 16, 469-473. [DOI] [PubMed] [Google Scholar]
- 27.Flora, A. D. E., Zocchi, E., Guida, L., Franco, L. & Bruzzone, S. (2004) Ann. N.Y. Acad. Sci. 1028, 176-191. [DOI] [PubMed] [Google Scholar]
- 28.Franco, L., Guida, L., Bruzzone, S., Zocchi, E., Usai, C. & De Flora, A. (1998) FASEB J. 12, 1507-1520. [DOI] [PubMed] [Google Scholar]
- 29.Lund, F. E., Yu, N., Kim, K. M., Reth, M. & Howard, M. C. (1996) J. Immunol. 157, 1455-1467. [PubMed] [Google Scholar]
- 30.Cockayne, D. A., Muchamuel, T., Grimaldi, J. C., Muller-Steffner, H., Randall, T. D., Lund, F. E., Murray, R., Schuber, F. & Howard, M. C. (1998) Blood 92, 1324-1333. [PubMed] [Google Scholar]
- 31.Pasatiempo, A.M., Kinoshita, M., Taylor, C. E. & Ross, A. C. (1990) FASEB J. 4, 2518-2527. [DOI] [PubMed] [Google Scholar]
- 32.Wiedermann, U., Hanson, L.A., Kahu, H. & Dahlgren, U. I. (1993) Immunology 80, 581-586. [PMC free article] [PubMed] [Google Scholar]
- 33.Cariati, R., Zancai, P., Quaia, M., Cutrona, G., Giannini, F., Rizzo, S., Boiocchi, M. & Dolcetti, R. (2000) Int. J. Cancer 86, 375-384. [DOI] [PubMed] [Google Scholar]
- 34.Naderi, S. & Blomhoff, H. K. (1999) Blood 94, 1348-1358. [PubMed] [Google Scholar]
- 35.De Leenheer, A. P., Lambert, W. E. & Claeys, I. (1982) J. Lipid Res. 23, 1362-1367. [PubMed] [Google Scholar]
- 36.Muramatsu, M., Kinoshita, K., Fagarasan, S., Yamada, S., Shinkai, Y. & Honjo, T. (2000) Cell 102, 553-563. [DOI] [PubMed] [Google Scholar]
- 37.Chen, Q., Ma, Y. & Ross, A. C. (2002) Immunology 107, 199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasbold, J., Lyons, A. B., Kehry, M. R. & Hodgkin, P. D. (1998) Eur. J. Immunol. 28, 1040-1051. [DOI] [PubMed] [Google Scholar]
- 39.Nambu, Y., Sugai, M., Gonda, H., Lee, C. G., Katakai, T., Agata, Y., Yokota, Y. & Shimizu, A. (2003) Science 302, 2137-2140. [DOI] [PubMed] [Google Scholar]
- 40.Hasbold, J., Corcoran, L. M., Tarlinton, D. M., Tangye, S. G. & Hodgkin, P. D. (2004) Nat. Immunol. 5, 55-63. [DOI] [PubMed] [Google Scholar]
- 41.Tangye, S. G., Nichols, K. E., Hare, N. J. & van de Weerdt, B. C. (2003) J. Immunol. 171, 2485-2495. [DOI] [PubMed] [Google Scholar]
- 42.Nagy, M., Chapuis, B. & Matthes, T. (2002) Br. J. Haematol. 116, 429-435. [DOI] [PubMed] [Google Scholar]
- 43.Stavnezer, J. & Amemiya, C. T. (2004) Semin. Immunol. 16, 257-275. [DOI] [PubMed] [Google Scholar]
- 44.Lin, K. I., Angelin-Duclos, C., Kuo, T. C. & Calame, K. (2002) Mol. Cell. Biol. 22, 4771-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nutt, S. L., Eberhard, D., Horcher, M., Rolink, A. G. & Busslinger, M. (2001) Int. Rev. Immunol. 20, 65-82. [DOI] [PubMed] [Google Scholar]
- 46.Morrison, A. M., Nutt, S. L., Thevenin, C., Rolink, A. & Busslinger, M. (1998) Semin. Immunol. 10, 133-142. [DOI] [PubMed] [Google Scholar]
- 47.Sugai, M., Gonda, H., Nambu, Y., Yokota, Y. & Shimizu, A. (2004) J. Mol. Med. 82, 592-599. [DOI] [PubMed] [Google Scholar]
- 48.Blomhoff, H. K., Smeland, E. B., Erikstein, B., Rasmussen, A. M., Skrede, B., Skjonsberg, C. & Blomhoff, R. (1992) J. Biol. Chem. 267, 23988-23992. [PubMed] [Google Scholar]
- 49.Guidoboni, M., Zancai, P., Cariati, R., Rizzo, S., Dal Col, J., Pavan, A., Gloghini, A., Spina, M., Cuneo, A., Pomponi, F., et al. (2005) Cancer Res. 65, 587-595. [PubMed] [Google Scholar]
- 50.Tokuyama, Y. & Tokuyama, H. (1996) Cell Immunol. 170, 230-234. [DOI] [PubMed] [Google Scholar]
- 51.Chen, Q. & Catharine Ross, A. (2004) Exp. Cell Res. 297, 68-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanzavecchia, A. & Sallusto, F. (2002) Nat. Rev. Immunol. 2, 982-987. [DOI] [PubMed] [Google Scholar]
- 53.Wakatsuki, Y., Neurath, M. F., Max, E. E. & Strober, W. (1994) J. Exp. Med. 179, 1099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neurath, M. F., Max, E. E. & Strober, W. (1995) Proc. Natl. Acad. Sci. USA 92, 5336-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michaelson, J. S., Singh, M. & Birshtein, B. K. (1996) J. Immunol. 156, 2349-2351. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.