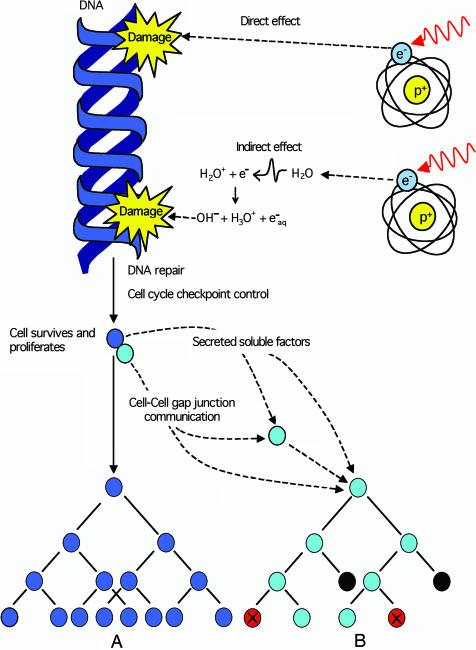
Implicit in understanding the biological effects of ionizing radiation and subsequent risks associated with such exposure is that only cells “hit” by the radiation are likely to carry the legacy of radiation damage. When a cell is hit, the deposition of energy can result in direct damage to the genetic material or indirect damage to critical nuclear targets through the radiolysis of water (Fig. 1). The subsequent action of DNA repair processes either removes the lesion(s) or misrepairs the induced damage such that all surviving progeny of an irradiated cell carry the burden of radiation exposure, e.g., a gene mutation and/or a chromosomal rearrangement (Fig. 1, diagram A). This central tenet in our understanding of the biological effects of ionizing radiation has now been called into question by the description of a number of nontargeted effects associated with radiation exposure. These effects can occur in the progeny of irradiated cells generations after the initial exposure, and/or in cells that were not directly traversed by ionizing radiation but were some distance from the hit cells (reviewed in refs. 1 and 2) (Fig. 1, diagram B).
Fig. 1.
Ionizing radiation induces direct DNA damage and indirect damage through the radiolysis of water. This damage is either eliminated or fixed in the cell as a mutation or chromosomal rearrangement by DNA repair processes. After release by cell cycle checkpoint control mechanisms, an irradiated surviving cell (blue) may proliferate, passing on the legacy of radiation to its progeny (diagram A), potentially initiating the carcinogenic process. The irradiated cell (blue) can also communicate with nonirradiated neighboring cells (cyan) by cell-to-cell gap junction communication and/or secretion of soluble factors eliciting nontargeted apoptosis (black) or micronucleation (red) in cells that have never been exposed to radiation (diagram B).
In this issue of PNAS, the work of Belyakov et al. (3) demonstrates nontargeted bystander responses in a three-dimensional human tissue model system. The investigators use a microbeam to deliver defined numbers of charged particles, in this case α-particles (the type of radiation associated with radon decay) with high accuracy to precise locations (4, 5). A defined area of cells was irradiated in a thin vertical plane, approximately two cell diameters, to bisect the tissue sample. Because of the inherent nature of the α-particles, there is very little radiation scatter. Consequently, cells more than a few micrometers away from the plane of irradiated cells receive zero radiation dose. At 72 h postirradiation, the tissues were formalin-fixed, paraffin-embedded, and sectioned in strips at increasing distances parallel to the plane of irradiated cells. This strategy allowed analysis of tissue slices containing only nonirradiated cells at known distances from the plane of irradiated cells. Nonirradiated cells up to 1 mm away from the irradiated cells, ≈50–75 cell diameters distant, showed a significant enhancement in the measured fractions of apoptotic and micronucleated cells.
These results indicate that, in a normal three-dimensional human tissue system, unirradiated cells can respond to radiation-induced damage occurring a large distance away. This so-called “bystander response” occurs in nonirradiated cells that were “bystanders” at the time of irradiation. Although the magnitude of the response is less than for directly hit cells, these results could have significant implications for determining risk associated with low-dose radiation situations. Due to the particulate nature of high linear energy radiations, exposure to low doses of α-particles would result in far more potential bystander cells than directly hit cells. This in turn would effectively increase the number of cells, or volume of tissue, affected by a low-radiation dose.
There is an expanding body of literature on nontargeted effects of ionizing radiation. These effects include radiation-induced genomic instability (6, 7), bystander effects (8), clastogenic factors (9), the death-inducing effect (10), and heritable effects occurring in the offspring after parental irradiation before conception (11, 12). Nontargeted effects rely on cell-to-cell communication to convey the response from the irradiated cell to its non-hit neighbor(s). This signaling occurs via cellular gap junctions (13) and/or through soluble factors secreted by irradiated cells (14). To date, the majority of these nontargeted effects have been described in cell culture systems in vitro. Such experimental models generally lack three-dimensional structure and, therefore, the full range of intercellular signaling characteristic of true tissue responses. The demonstration of bystander responses in a model tissue system by Belyakov et al. (3) therefore represents a significant advance.
The target for radiation damage is greater than the initial tissue volume irradiated.
In addition to the effects occurring in hit cells, now potential nontargeted effects in nonirradiated cells must also be considered when evaluation exposure to ionizing radiation. In terms of consequences to the irradiated tissue or organism, nontargeted bystander responses may be considered both detrimental and beneficial (15). Elevated fractions of micronuclei, a manifestation of cytogenetic damage or mitotic failure, might be associated with detrimental consequences. However, elimination of damaged cells by apoptosis might well protect the tissue by removing potentially damaged cells that may contribute to long-term deleterious effects associated with radiation exposure. Perhaps it is not surprising that damaged cells can communicate responses to nonirradiated neighboring cells after exposure to a DNA-damaging agent (16). An induced stress response may ultimately benefit the exposed tissue or organism in terms of overall survival. Nevertheless, the data of Belyakov et al. (3) clearly indicate that the target for radiation damage is greater than the initial tissue volume irradiated. It remains to be seen whether such responses are limited to the specific organ irradiated (17) or a limited region of the body (18), or whether the whole body is the ultimate target.
Humankind lives in a radiation environment. Ionizing radiation is in the air we breathe, the food we consume, the buildings we live in, and the earth we live on. As our society continues to evolve, human-made radiation adds to this natural background, and the potential for human exposure increases. Ionizing radiation is an intriguing contradiction for the public, regulators, and society in general. On one hand, it is a well documented mutagen and carcinogen, instilling fear that exposure will result in cancer and congenital abnormalities and invoking the menace of environmental contamination, radiological terrorism, and nuclear disaster. On the other, it is an efficient energy source and has widespread applications industrially and clinically, with an estimated two billion procedures involving ionizing radiation in diagnostic and radiotherapy-related medicine worldwide each year (http://lowdose.tricity.wsu.edu). A recent report from the National Academies' National Research Councils Board on the Effects of Ionizing Radiation (19) concluded that the preponderance of scientific evidence shows that there is no threshold of exposure below which low levels of ionizing radiation can be demonstrated to be harmless or beneficial, and that all exposures are likely to pose some risk of adverse health effects. Whatever the case, the communication of the radiation response outside the volume irradiated has potential implications for all types of occupational, environmental, and medical exposures to ionizing radiation and our future assessment of radiation risk.
Author contributions: W.F.M. and M.B.S. wrote the paper.
See companion article on page 14203.
References
- 1.Morgan, W. F. (2003) Radiat. Res. 159, 567-580. [DOI] [PubMed] [Google Scholar]
- 2.Morgan, W. F. (2003) Radiat. Res. 159, 581-596. [DOI] [PubMed] [Google Scholar]
- 3.Belyakov, O. V., Mitchell, S. A., Parikh, D., Randers-Pehrson, G., Marino, S. A., Amundson, S. A., Geard, C. R. & Brenner, D. J. (2005) Proc. Natl. Acad. Sci. USA 102, 14203-14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randers-Pehrson, G., Geard, C. R., Johnson, G., Elliston, C. D. & Brenner, D. J. (2001) Radiat. Res. 156, 210-214. [DOI] [PubMed] [Google Scholar]
- 5.Wu, L. J., Randers-Pehrson, G., Xu, A., Waldren, C. A., Geard, C. R., Yu, Z. & Hei, T. K. (1999) Proc. Natl. Acad. Sci. USA 96, 4959-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadhim, M. A., MacDonald, D. A., Goodhead, D. T., Lorimore, S. A., Marsden, S. J. & Wright, E. G. (1992) Nature 355, 738-740. [DOI] [PubMed] [Google Scholar]
- 7.Marder, B. A. & Morgan, W. F. (1993) Mol. Cell. Biol. 13, 6667-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mothersill, C. & Seymour, C. B. (2004) Nat. Rev. Cancer 4, 158-164. [DOI] [PubMed] [Google Scholar]
- 9.Emerit, I., Levy, A., Cernjavski, L., Arutyunyan, R., Oganesyan, N., Pogosian, A., Mejlumian, H., Sarkisian, T., Gulkandanian, M., Quastel, M., et al. (1994) J. Cancer Res. Clin. Oncol. 120, 558-561. [DOI] [PubMed] [Google Scholar]
- 10.Nagar, S., Smith, L. E. & Morgan, W. F. (2003) Cancer Res. 63, 324-328. [PubMed] [Google Scholar]
- 11.Dubrova, Y. E., Plumb, M., Brown, J., Fennelly, J., Bois, P., Goodhead, D. & Jeffreys, A. J. (1998) Proc. Natl. Acad. Sci. USA 95, 6251-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niwa, O. & Kominami, R. (2001) Proc. Natl. Acad. Sci. USA 98, 1705-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azzam, E. I., de Toledo, S. M. & Little, J. B. (2001) Proc. Natl. Acad. Sci. USA 98, 473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowa Resat, M. B. & Morgan, W. F. (2004) J. Cell Biochem. 92, 1013-1019. [DOI] [PubMed] [Google Scholar]
- 15.Lorimore, S. A., Coates, P. J., Scobie, G. E., Milne, G. & Wright, E. G. (2001) Oncogene 20, 7085-7095. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg, Z. & Lehnert, B. E. (2002) Int. J. Oncol. 21, 337-349. [PubMed] [Google Scholar]
- 17.Brooks, A. L., Benjamin, S. A., Hahn, F. F., Brownstein, D. G., Griffith, W. C. & McClellan, R. O. (1983) Radiat. Res. 96, 135-151. [PubMed] [Google Scholar]
- 18.Camphausen, K., Moses, M. A., Menard, C., Sproull, M., Beecken, W. D., Folkman, J. & O'Reilly, M. S. (2003) Cancer Res. 63, 1990-1993. [PubMed] [Google Scholar]
- 19.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council (2005) Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII-Phase 2 (The National Academies Press, Washington, DC). [PubMed]