Abstract
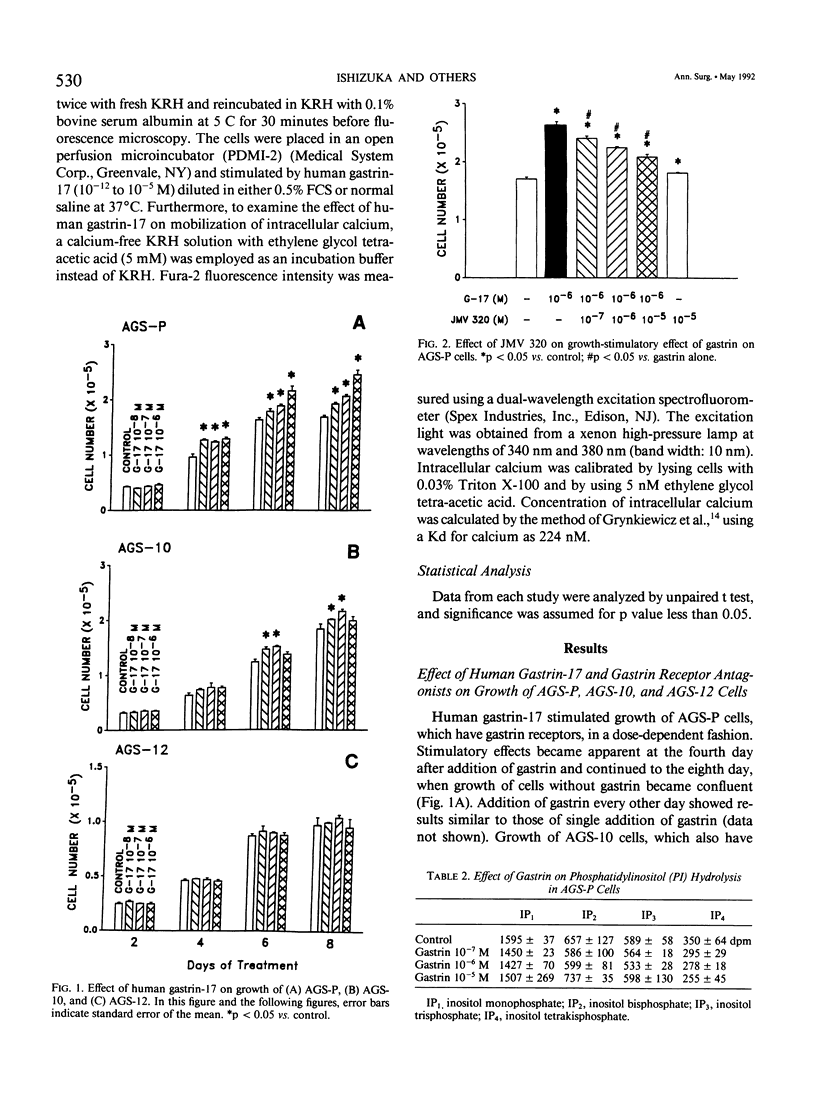
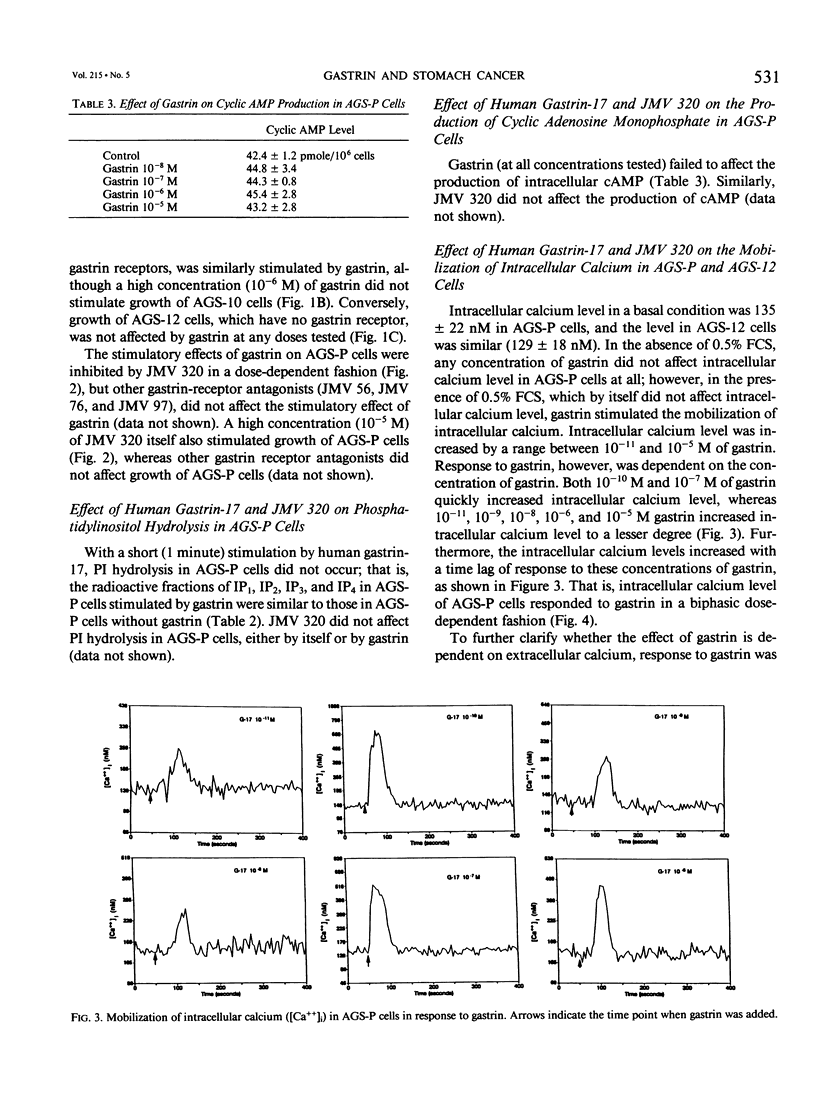
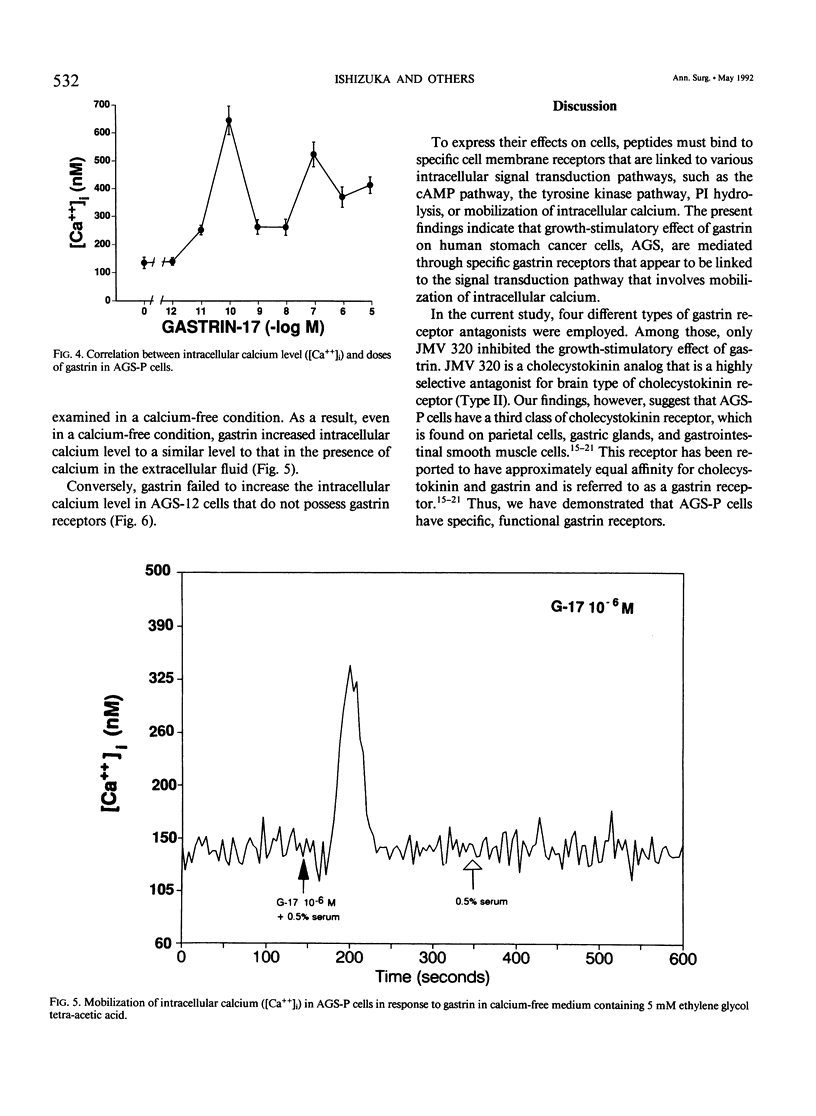
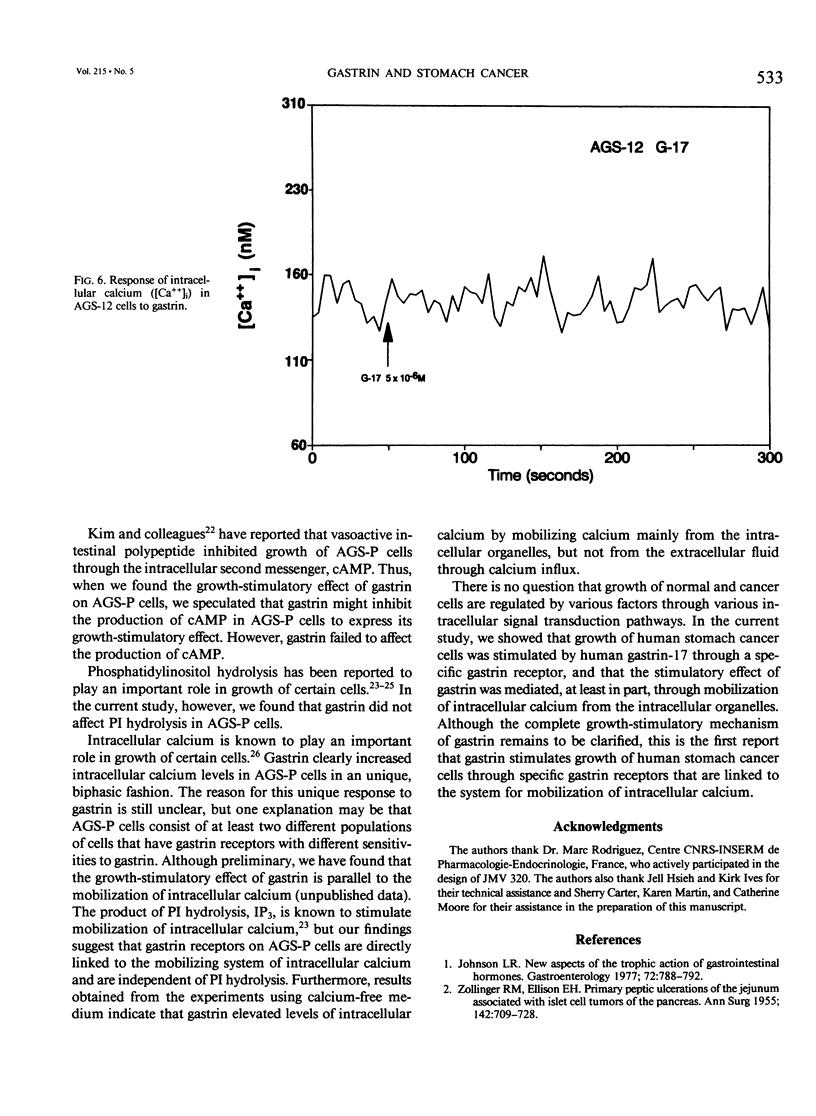
Gastrin is known as a trophic factor for some stomach and colorectal cancer cells; however, the roles of gastrin receptors and the intracellular signal transduction pathways by which gastrin regulates cell growth are still unknown. The authors examined the effect of synthetic human gastrin-17 on growth of human stomach cancer cells (the parent line, AGS-P, and two different clones, AGS-10 and AGS-12), which were established (and have been maintained) in our laboratory. Gastrin stimulated growth of AGS-P and AGS-10 cells, which have gastrin receptors, in a dose-dependent fashion. A highly selective gastrin receptor antagonist, JMV 320, inhibited the growth-stimulatory effect of gastrin on AGS-P cells in a dose-dependent fashion. Concentrations of gastrin (10(-8) to 10(-6) M), which stimulated growth of AGS-P cells, did not affect either cyclic adenosine monophosphate production or phosphatidylinositol hydrolysis. Gastrin (10(-11) to 10(-5) M) mobilized calcium from the intracellular organelles to increases intracellular calcium level in AGS-P cells. The AGS-12 clone has no gastrin receptors, and gastrin did not affect growth or mobilization of intracellular calcium in these cells. Our findings indicate that gastrin stimulates growth of AGS cells through a mechanism that involves binding to specific gastrin receptors that are linked to the system for mobilization of intracellular calcium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bitar K. N., Makhlouf G. M. Receptors on smooth muscle cells: characterization by contraction and specific antagonists. Am J Physiol. 1982 Apr;242(4):G400–G407. doi: 10.1152/ajpgi.1982.242.4.G400. [DOI] [PubMed] [Google Scholar]
- Cherner J. A., Sutliff V. E., Grybowski D. M., Jensen R. T., Gardner J. D. Functionally distinct receptors for cholecystokinin and gastrin on dispersed chief cells from guinea pig stomach. Am J Physiol. 1988 Feb;254(2 Pt 1):G151–G155. doi: 10.1152/ajpgi.1988.254.2.G151. [DOI] [PubMed] [Google Scholar]
- Durrant L. G., Watson S. A., Hall A., Morris D. L. Co-stimulation of gastrointestinal tumour cell growth by gastrin, transforming growth factor alpha and insulin like growth factor-I. Br J Cancer. 1991 Jan;63(1):67–70. doi: 10.1038/bjc.1991.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourmy D., Zahidi A., Fabre R., Guidet M., Pradayrol L., Ribet A. Receptors for cholecystokinin and gastrin peptides display specific binding properties and are structurally different in guinea-pig and dog pancreas. Eur J Biochem. 1987 Jun 15;165(3):683–692. doi: 10.1111/j.1432-1033.1987.tb11495.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hawkins D., Enyedi P., Brown E. The effects of high extracellular Ca2+ and Mg2+ concentrations on the levels of inositol 1,3,4,5-tetrakisphosphate in bovine parathyroid cells. Endocrinology. 1989 Feb;124(2):838–844. doi: 10.1210/endo-124-2-838. [DOI] [PubMed] [Google Scholar]
- Johnson L. R. New aspects of the trophic action of gastrointestinal hormones. Gastroenterology. 1977 Apr;72(4 PT2):788–792. [PubMed] [Google Scholar]
- Kim S. W., Beauchamp R. D., Townsend C. M., Jr, Thompson J. C. Vasoactive intestinal polypeptide inhibits c-myc expression and growth of human gastric carcinoma cells. Surgery. 1991 Aug;110(2):270–276. [PubMed] [Google Scholar]
- Martinez J., Bali J. P., Rodriguez M., Castro B., Magous R., Laur J., Lignon M. F. Synthesis and biological activities of some pseudo-peptide analogues of tetragastrin: the importance of the peptide backbone. J Med Chem. 1985 Dec;28(12):1874–1879. doi: 10.1021/jm00150a020. [DOI] [PubMed] [Google Scholar]
- Martinez J., Rodriguez M., Bali J. P., Laur J. Phenethyl ester derivative analogues of the C-terminal tetrapeptide of gastrin as potent gastrin antagonists. J Med Chem. 1986 Nov;29(11):2201–2206. doi: 10.1021/jm00161a012. [DOI] [PubMed] [Google Scholar]
- Menozzi D., Gardner J. D., Jensen R. T., Maton P. N. Properties of receptors for gastrin and CCK on gastric smooth muscle cells. Am J Physiol. 1989 Jul;257(1 Pt 1):G73–G79. doi: 10.1152/ajpgi.1989.257.1.G73. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ochiai A., Yasui W., Tahara E. Growth-promoting effect of gastrin on human gastric carcinoma cell line TMK-1. Jpn J Cancer Res. 1985 Nov;76(11):1064–1071. [PubMed] [Google Scholar]
- Rodriguez M., Amblard M., Galas M. C., Lignon M. F., Aumelas A., Martinez J. Synthesis of cyclic analogues of cholecystokinin highly selective for central receptors. Int J Pept Protein Res. 1990 May;35(5):441–451. doi: 10.1111/j.1399-3011.1990.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Dubreuil P., Bali J. P., Martinez J. Synthesis and biological activity of partially modified retro-inverso pseudopeptide derivatives of the C-terminal tetrapeptide of gastrin. J Med Chem. 1987 May;30(5):758–763. doi: 10.1021/jm00388a002. [DOI] [PubMed] [Google Scholar]
- Singh P., Rae-Venter B., Townsend C. M., Jr, Khalil T., Thompson J. C. Gastrin receptors in normal and malignant gastrointestinal mucosa: age-associated changes. Am J Physiol. 1985 Dec;249(6 Pt 1):G761–G769. doi: 10.1152/ajpgi.1985.249.6.G761. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Amirian D. A., Thomas L. P., Reedy T. J., Elashoff J. D. Gastrin receptors on isolated canine parietal cells. J Clin Invest. 1984 May;73(5):1434–1447. doi: 10.1172/JCI111348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tihon C., Goren M. B., Spitz E., Rickenberg H. V. Convenient elimination of trichloroacetic acid prior to radioimmunoassay of cyclic nucleotides. Anal Biochem. 1977 Jun;80(2):652–653. doi: 10.1016/0003-2697(77)90693-5. [DOI] [PubMed] [Google Scholar]
- Townsend C. M., Jr, Beauchamp R. D., Singh P., Thompson J. C. Growth factors and intestinal neoplasms. Am J Surg. 1988 Mar;155(3):526–536. doi: 10.1016/s0002-9610(88)80128-4. [DOI] [PubMed] [Google Scholar]
- Townsend C. M., Jr, Singh P., Thompson J. C. Gastrointestinal hormones and gastrointestinal and pancreatic carcinomas. Gastroenterology. 1986 Oct;91(4):1002–1006. doi: 10.1016/0016-5085(86)90707-9. [DOI] [PubMed] [Google Scholar]
- Yoder D. G., Moody T. W. High affinity binding of cholecystokinin to small cell lung cancer cells. Peptides. 1987 Jan-Feb;8(1):103–107. doi: 10.1016/0196-9781(87)90171-9. [DOI] [PubMed] [Google Scholar]
- Yu D. H., Noguchi M., Zhou Z. C., Villanueva M. L., Gardner J. D., Jensen R. T. Characterization of gastrin receptors on guinea pig pancreatic acini. Am J Physiol. 1987 Dec;253(6 Pt 1):G793–G801. doi: 10.1152/ajpgi.1987.253.6.G793. [DOI] [PubMed] [Google Scholar]
- ZOLLINGER R. M., ELLISON E. H. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955 Oct;142(4):709-23; discussion, 724-8. [PMC free article] [PubMed] [Google Scholar]


