Abstract
The liver possesses the remarkable ability to regenerate to its original size after a 70% partial hepatectomy. There has been little effort to characterize the Kupffer cells' role in this unique mammalian reparative physiologic phenomenon. The capacity of rat Kupffer cells (KC) isolated at specific intervals after partial hepatectomy to produce interleukin-6 (IL-6) and prostaglandin E2 (PGE2) in response to endotoxin was evaluated in standard RPMI-1640 (1200 microM L-arginine) and arginine-depleted RPMI-1640 (10 microM L-arginine) media. Regenerating liver KC 48 to 120 hours after partial hepatectomy responded to endotoxin stimulation with a significantly greater (p less than 0.05) production of IL-6 in standard RPMI-1640. Because Kupffer cells function in an environment where high arginase activity results in negligible L-arginine levels, the 10 microM L-arginine RPMI-1640 was used to simulate the true hepatic microenvironment. Production of IL-6 by regenerating liver KC was further increased (p less than 0.05) by placing these same KC in 10 microM L-arginine RPMI-1640 tissue culture media. During the same period, regenerating liver KC produced significantly (p less than 0.01) more PGE2 than sham-operated KC in both standard and low-arginine media. When the cyclo-oxygenase inhibitor indomethacin (1 x 10(-5) M) was added to cultures, the PGE2 production was inhibited, and IL-6 production was upregulated (p less than 0.05) in arginine-depleted cultures. The authors conclude that during hepatic regeneration KC IL-6 production is elevated but controlled in an autoregulatory fashion by KC PGE2 production.
Full text
PDF

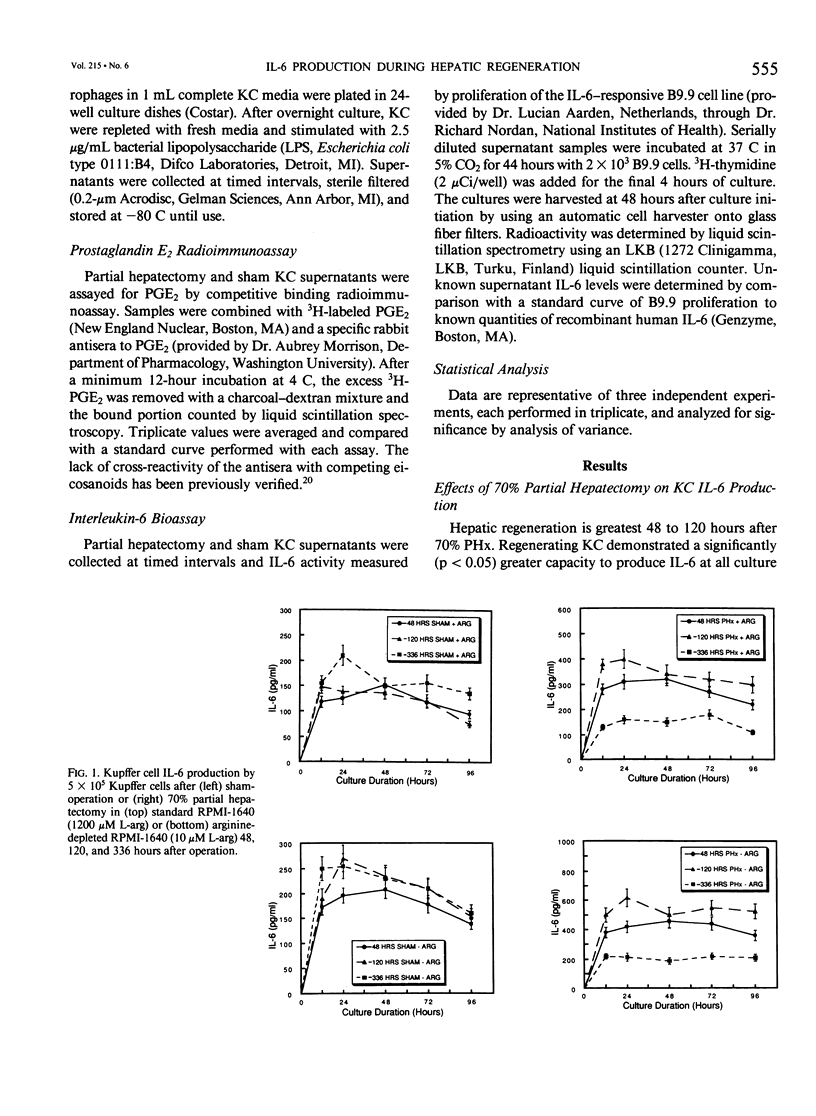
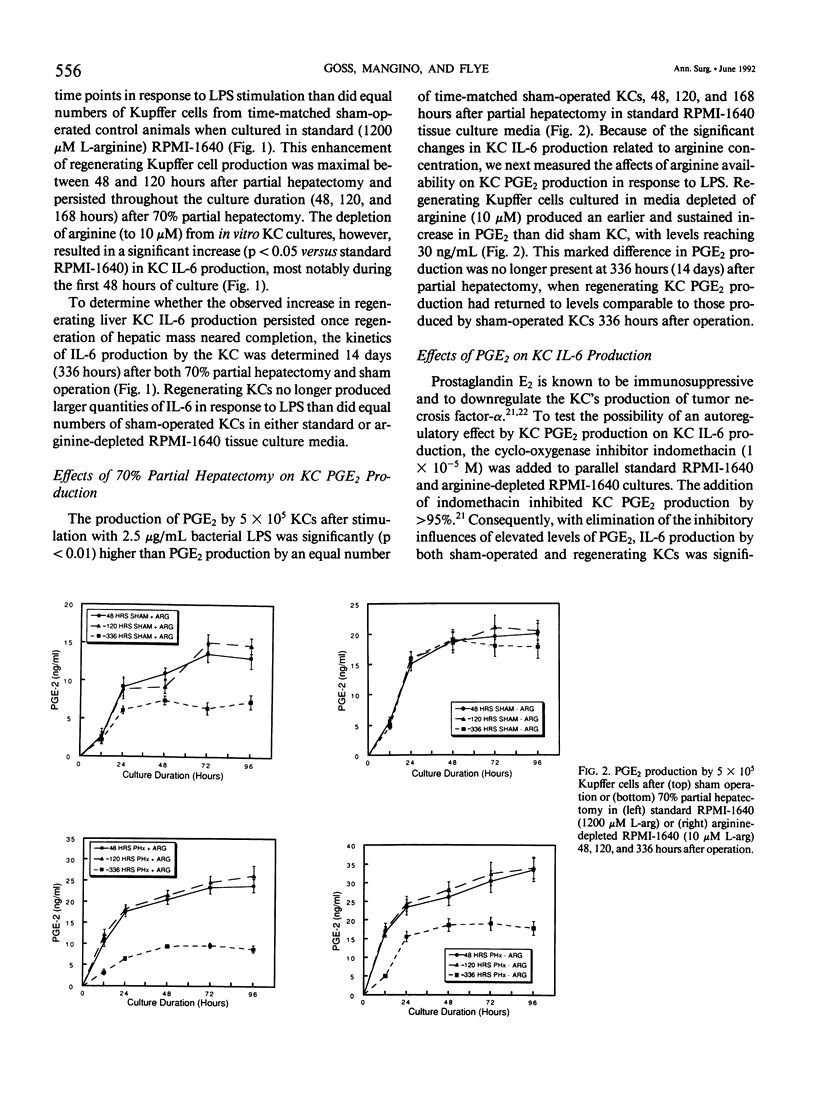
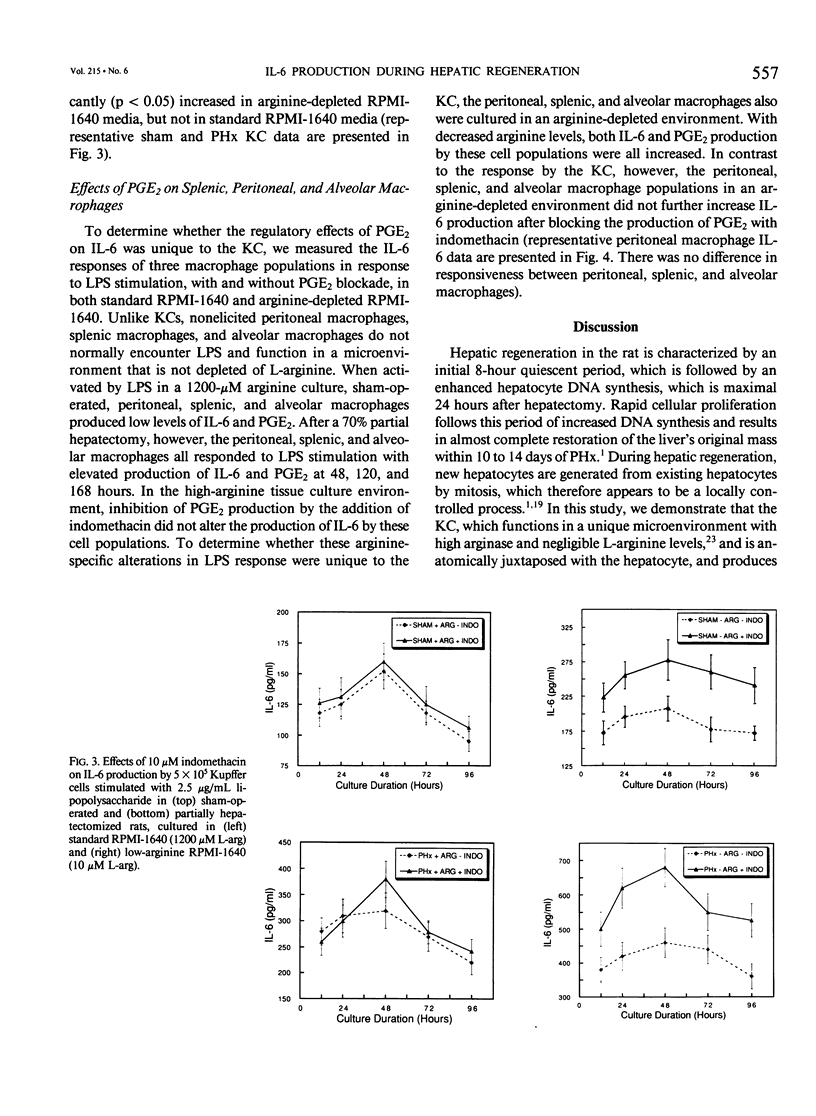
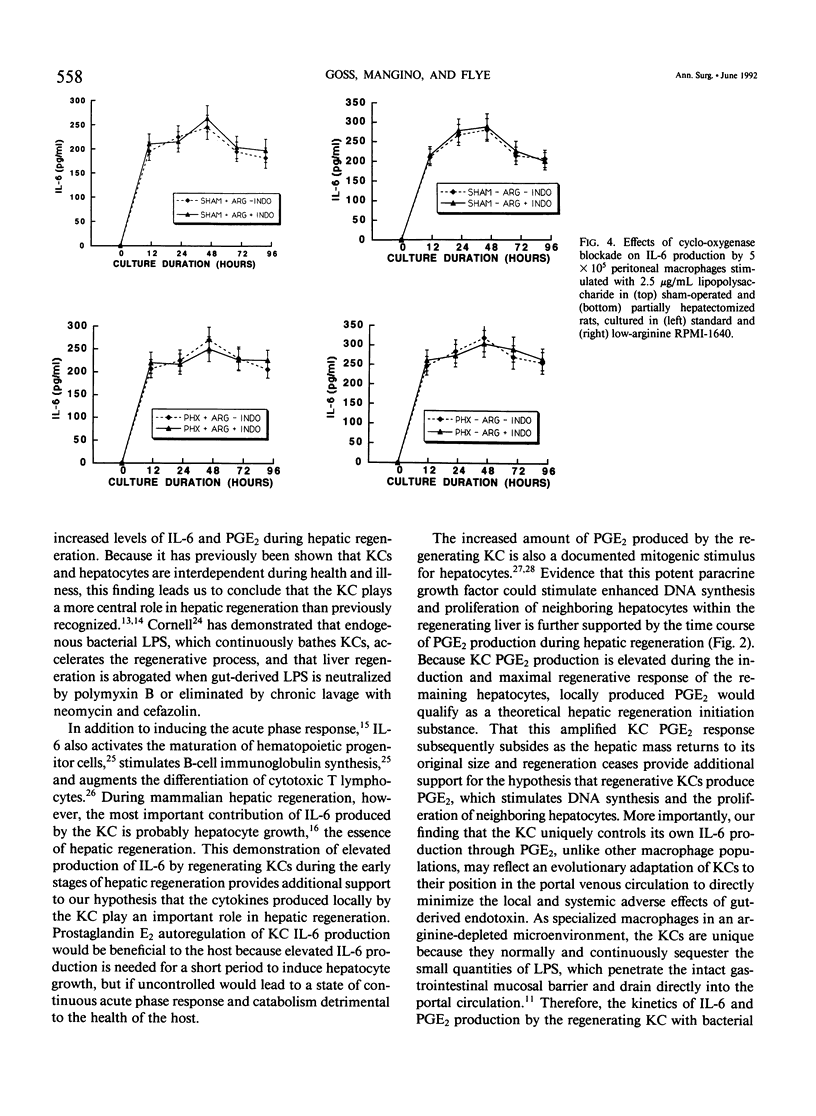

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreis P. G., Whitfield J. F., Armato U. Stimulation of DNA synthesis and mitosis of hepatocytes in primary cultures of neonatal rat liver by arachidonic acid and prostaglandins. Exp Cell Res. 1981 Aug;134(2):265–272. doi: 10.1016/0014-4827(81)90425-0. [DOI] [PubMed] [Google Scholar]
- Andus T., Bauer J., Gerok W. Effects of cytokines on the liver. Hepatology. 1991 Feb;13(2):364–375. [PubMed] [Google Scholar]
- Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr. 1986 Mar-Apr;10(2):227–238. doi: 10.1177/0148607186010002227. [DOI] [PubMed] [Google Scholar]
- Bucher M. L., Swaffield M. N. Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1157–1160. doi: 10.1073/pnas.72.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callery M. P., Kamei T., Flye M. W. Kupffer cell tumor necrosis factor-alpha production is suppressed during liver regeneration. J Surg Res. 1991 May;50(5):515–519. doi: 10.1016/0022-4804(91)90034-j. [DOI] [PubMed] [Google Scholar]
- Callery M. P., Mangino M. J., Flye M. W. A biologic basis for limited Kupffer cell reactivity to portal-derived endotoxin. Surgery. 1991 Aug;110(2):221–230. [PubMed] [Google Scholar]
- Callery M. P., Mangino M. J., Flye M. W. Kupffer cell prostaglandin-E2 production is amplified during hepatic regeneration. Hepatology. 1991 Aug;14(2):368–372. [PubMed] [Google Scholar]
- Cornell R. P. Restriction of gut-derived endotoxin impairs DNA synthesis for liver regeneration. Am J Physiol. 1985 Nov;249(5 Pt 2):R563–R569. doi: 10.1152/ajpregu.1985.249.5.R563. [DOI] [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Karck U., Peters T., Decker K. Comparative study of cytotoxicity, tumor necrosis factor, and prostaglandin release after stimulation of rat Kupffer cells, murine Kupffer cells, and murine inflammatory liver macrophages. J Leukoc Biol. 1989 Feb;45(2):139–146. doi: 10.1002/jlb.45.2.139. [DOI] [PubMed] [Google Scholar]
- Fausto N. New perspectives on liver regeneration. Hepatology. 1986 Mar-Apr;6(2):326–327. doi: 10.1002/hep.1840060229. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBrecque D. R., Pesch L. A. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975 Jun;248(2):273–284. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino M. J., Brunt E. M., Von Doersten P., Anderson C. B. Effects of the thromboxane synthesis inhibitor CGS-12970 on experimental acute renal allograft rejection. J Pharmacol Exp Ther. 1989 Jan;248(1):23–28. [PubMed] [Google Scholar]
- Mayanskii D. N., Scherbakoff V. I., Mayanskaya N. N., Panin L. E. Lysosomal enzyme activity in hepatocytes and Kupffer cells from intact and partially hepatectomized rats. Biochem Exp Biol. 1980;16(3):309–314. [PubMed] [Google Scholar]
- Miura Y., Fukui N. Prostaglandins as possible triggers for liver regeneration after partial hepatectomy. A review. Cell Mol Biol Incl Cyto Enzymol. 1979;25(3):179–184. [PubMed] [Google Scholar]
- Moolten F. L., Bucher N. L. Regeneration of rat liver: transfer of humoral agent by cross circulation. Science. 1967 Oct 13;158(3798):272–274. doi: 10.1126/science.158.3798.272. [DOI] [PubMed] [Google Scholar]
- Morley C. G., Kingdon H. S. The regulation of cell growth. I. Identification and partial characterization of a DNA synthesis stimulating factor from the serum of partially hepatectomized rats. Biochim Biophys Acta. 1973 May 10;308(2):260–275. doi: 10.1016/0005-2787(73)90156-1. [DOI] [PubMed] [Google Scholar]
- Namasivayam A., Padmanabhan N. The possible role of reticulo endothelial system in hepatic regeneration. Indian J Physiol Pharmacol. 1982 Apr-Jun;26(2):105–112. [PubMed] [Google Scholar]
- Price J. B., Jr Insulin and glucagon as modifiers of DNA synthesis in the regenerating rat liver. Metabolism. 1976 Nov;25(11 Suppl 1):1427–1428. doi: 10.1016/s0026-0495(76)80157-6. [DOI] [PubMed] [Google Scholar]
- SIGEL B., ACEVEDO F. J., DUNN M. R. The effect of patial hepatectomy on autotransplanted liver tissue. Surg Gynecol Obstet. 1963 Jul;117:29–36. [PubMed] [Google Scholar]
- Saba T. M. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970 Dec;126(6):1031–1052. [PubMed] [Google Scholar]
- Starzl T. E., Jones A. F., Terblanche J., Usui S., Porter K. A., Mazzoni G. Growth-stimulating factor in regenerating canine liver. Lancet. 1979 Jan 20;1(8108):127–130. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Wong G. G., Clark S. C., Burakoff S. J., Herrmann S. H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):508–512. [PubMed] [Google Scholar]
- West M. A., Billiar T. R., Curran R. D., Hyland B. J., Simmons R. L. Evidence that rat Kupffer cells stimulate and inhibit hepatocyte protein synthesis in vitro by different mechanisms. Gastroenterology. 1989 Jun;96(6):1572–1582. doi: 10.1016/0016-5085(89)90529-5. [DOI] [PubMed] [Google Scholar]
- West M. A., Billiar T. R., Mazuski J. E., Curran R. J., Cerra F. B., Simmons R. L. Endotoxin modulation of hepatocyte secretory and cellular protein synthesis is mediated by Kupffer cells. Arch Surg. 1988 Nov;123(11):1400–1405. doi: 10.1001/archsurg.1988.01400350114018. [DOI] [PubMed] [Google Scholar]


