Abstract
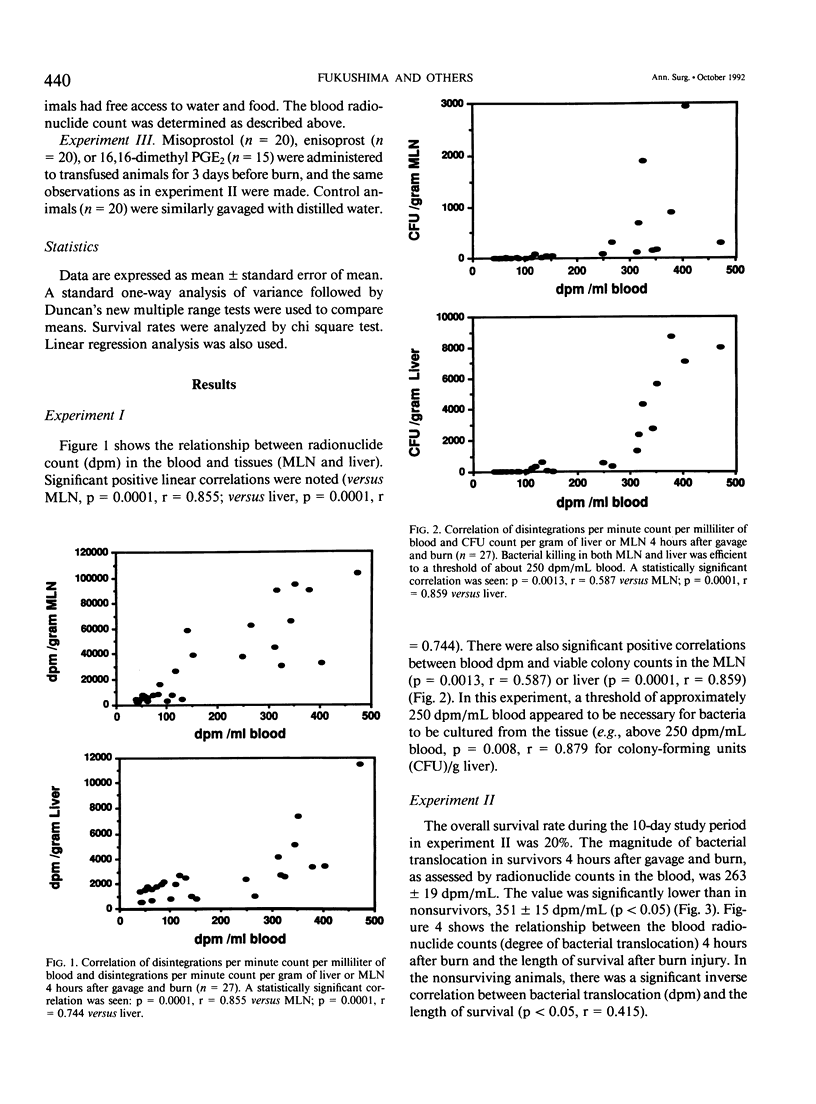
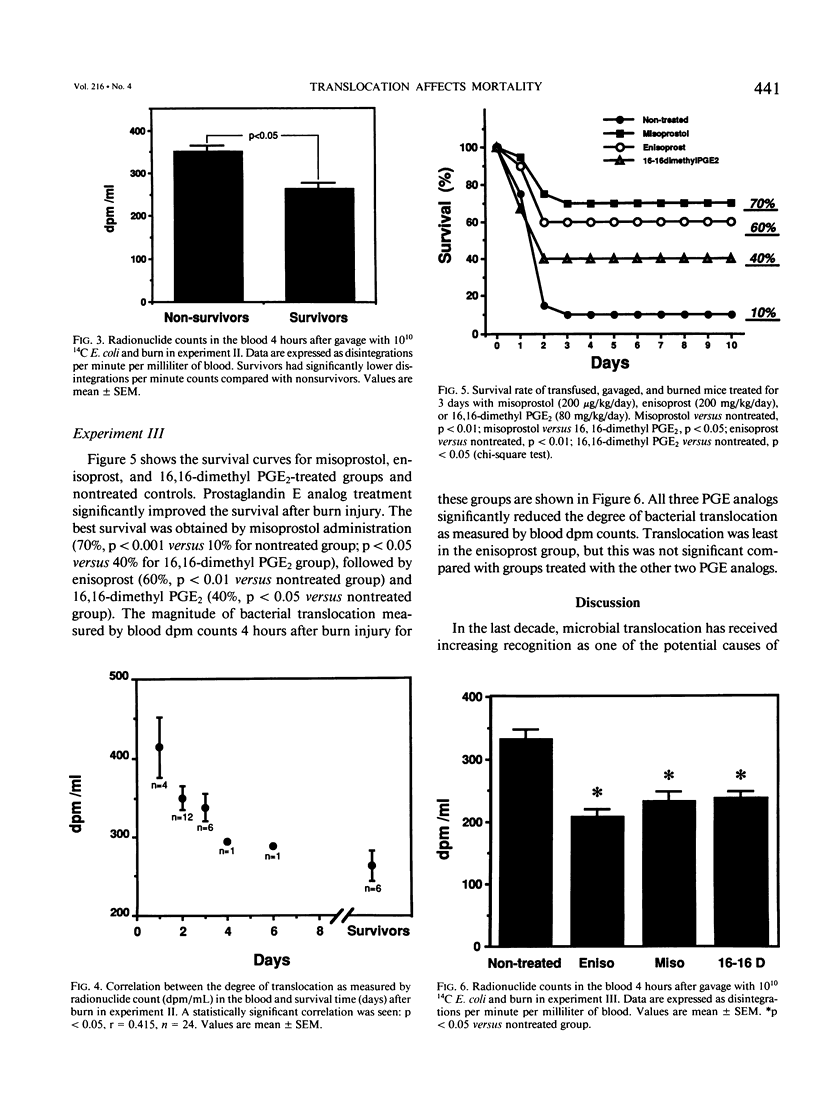
Bacterial translocation and related mortality rates were examined in previously transfused BALB/c mice that were gavaged with 14C radioisotope-labeled Escherichia coli before inflicting a 20% full-thickness flame burn. Radionuclide counts were measured in blood obtained by retro-orbital puncture 4 hours postburn, and survival was recorded for 10 days. Radionuclide counts in the blood correlated well with both radionuclide counts and numbers of viable bacterial in the tissues. Survivors had significantly less bacterial translocation as evidenced by blood radionuclide counts compared with nonsurvivors, and there was a significant inverse correlation between the degree of translocation and the length of survival. In the next experiment, the prostaglandin E (PGE) analogs misoprostol, enisoprost, or 16,16-dimethyl PGE2 were administered to transfused animals for 3 days before burn. Prostaglandin E analogs significantly reduced bacterial translocation as measured by blood radionuclide counts 4 hours postburn and improved survival. The data demonstrate that the intensity of bacterial translocation after burn injury is significantly associated with subsequent death. Improvement of survival by PGE analogs is associated with decreased bacterial translocation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Boyce S. T., Babcock G. F., Gianotti L., Peck M. D., Dunn D. L., Pyles T., Childress C. P., Ash S. K. The process of microbial translocation. Ann Surg. 1990 Oct;212(4):496–512. doi: 10.1097/00000658-199010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. W., Gianotti L., Pyles T., Carey M. A., Babcock G. F. Distribution and survival of Escherichia coli translocating from the intestine after thermal injury. Ann Surg. 1991 Jun;213(6):558–567. doi: 10.1097/00000658-199106000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alverdy J. C., Aoys E., Moss G. S. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988 Aug;104(2):185–190. [PubMed] [Google Scholar]
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Promotion of the translocation of enteric bacteria from the gastrointestinal tracts of mice by oral treatment with penicillin, clindamycin, or metronidazole. Infect Immun. 1981 Sep;33(3):854–861. doi: 10.1128/iai.33.3.854-861.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman H., Stahl G. L., Terashita Z., Lefer A. M. Mechanisms of action of PGE1 in hemorrhagic shock in rats. Ann Emerg Med. 1988 May;17(5):457–462. doi: 10.1016/s0196-0644(88)80236-1. [DOI] [PubMed] [Google Scholar]
- Border J. R., Hassett J., LaDuca J., Seibel R., Steinberg S., Mills B., Losi P., Border D. The gut origin septic states in blunt multiple trauma (ISS = 40) in the ICU. Ann Surg. 1987 Oct;206(4):427–448. doi: 10.1097/00000658-198710000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C. J., Meakins J. L., Marshall J. C., Fry D., Maier R. V. Multiple-organ-failure syndrome. Arch Surg. 1986 Feb;121(2):196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Chatenoud L., Klatzmann D., Fradelizi D. The mechanisms of inhibition of human IL 2 production. II. PGE2 induction of suppressor T lymphocytes. J Immunol. 1984 Apr;132(4):1851–1857. [PubMed] [Google Scholar]
- Deitch E. A. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg. 1989 Jun;124(6):699–701. doi: 10.1001/archsurg.1989.01410060065013. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Taylor M., Grisham M., Ma L., Bridges W., Berg R. Endotoxin induces bacterial translocation and increases xanthine oxidase activity. J Trauma. 1989 Dec;29(12):1679–1683. doi: 10.1097/00005373-198912000-00017. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Winterton J., Berg R. Thermal injury promotes bacterial translocation from the gastrointestinal tract in mice with impaired T-cell-mediated immunity. Arch Surg. 1986 Jan;121(1):97–101. doi: 10.1001/archsurg.1986.01400010111015. [DOI] [PubMed] [Google Scholar]
- Gianotti L., Pyles T., Alexander J. W., Babcock G. F., Carey M. A. Impact of blood transfusion and burn injury on microbial translocation and bacterial survival. Transfusion. 1992 May;32(4):312–317. doi: 10.1046/j.1537-2995.1992.32492263443.x. [DOI] [PubMed] [Google Scholar]
- Grbic J. T., Mannick J. A., Gough D. B., Rodrick M. L. The role of prostaglandin E2 in immune suppression following injury. Ann Surg. 1991 Sep;214(3):253–263. doi: 10.1097/00000658-199109000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcroft J. W., Vassar M. J., Weber C. J. Prostaglandin E1 and survival in patients with the adult respiratory distress syndrome. A prospective trial. Ann Surg. 1986 Apr;203(4):371–378. doi: 10.1097/00000658-198604000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakami Y., Nakao Y., Koizumi T., Katakami N., Ogawa R., Fujita T. Regulation of tumour necrosis factor production by mouse peritoneal macrophages: the role of cellular cyclic AMP. Immunology. 1988 Aug;64(4):719–724. [PMC free article] [PubMed] [Google Scholar]
- Krause W., Matheis H., Wulf K. Fungaemia and funguria after oral administration of Candida albicans. Lancet. 1969 Mar 22;1(7595):598–599. doi: 10.1016/s0140-6736(69)91534-7. [DOI] [PubMed] [Google Scholar]
- Lehmmann V., Benninghoff B., Dröge W. Tumor necrosis factor-induced activation of peritoneal macrophages is regulated by prostaglandin E2 and cAMP. J Immunol. 1988 Jul 15;141(2):587–591. [PubMed] [Google Scholar]
- Levitt M. A., Lefer A. M. Beneficial effects of prostaglandin E1 infusion in experimental traumatic shock. Crit Care Med. 1987 Aug;15(8):769–773. doi: 10.1097/00003246-198708000-00012. [DOI] [PubMed] [Google Scholar]
- Maejima K., Deitch E., Berg R. Promotion by burn stress of the translocation of bacteria from the gastrointestinal tracts of mice. Arch Surg. 1984 Feb;119(2):166–172. doi: 10.1001/archsurg.1984.01390140032006. [DOI] [PubMed] [Google Scholar]
- Mahatma M., Agrawal N., Dajani E. Z., Nelson S., Nakamura C., Sitton J. Misoprostol but not antacid prevents endotoxin-induced gastric mucosal injury: role of tumor necrosis factor-alpha. Dig Dis Sci. 1991 Nov;36(11):1562–1568. doi: 10.1007/BF01296398. [DOI] [PubMed] [Google Scholar]
- Miller T. A. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol. 1983 Nov;245(5 Pt 1):G601–G623. doi: 10.1152/ajpgi.1983.245.5.G601. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Trocki O., Dominioni L., Brackett K. A., Joffe S. N., Alexander J. W. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg. 1984 Sep;200(3):297–310. doi: 10.1097/00000658-198409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M., Mozes M. F., Maddux M. S., Veremis S., Bartkus C., Ketel B., Pollak R., Wallemark C., Jonasson O. Prevention of acute graft rejection by the prostaglandin E1 analogue misoprostol in renal-transplant recipients treated with cyclosporine and prednisone. N Engl J Med. 1990 Apr 26;322(17):1183–1188. doi: 10.1056/NEJM199004263221703. [DOI] [PubMed] [Google Scholar]
- Offenbartl K., Bengmark S. Intraabdominal infections and gut origin sepsis. World J Surg. 1990 Mar-Apr;14(2):191–195. doi: 10.1007/BF01664872. [DOI] [PubMed] [Google Scholar]
- Renz H., Gong J. H., Schmidt A., Nain M., Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988 Oct 1;141(7):2388–2393. [PubMed] [Google Scholar]
- Robert A., Nezamis J. E., Lancaster C., Hanchar A. J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979 Sep;77(3):433–443. [PubMed] [Google Scholar]
- Rush B. F., Jr, Redan J. A., Flanagan J. J., Jr, Heneghan J. B., Hsieh J., Murphy T. F., Smith S., Machiedo G. W. Does the bacteremia observed in hemorrhagic shock have clinical significance? A study in germ-free animals. Ann Surg. 1989 Sep;210(3):342–347. doi: 10.1097/00000658-198909000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush B. F., Jr, Sori A. J., Murphy T. F., Smith S., Flanagan J. J., Jr, Machiedo G. W. Endotoxemia and bacteremia during hemorrhagic shock. The link between trauma and sepsis? Ann Surg. 1988 May;207(5):549–554. doi: 10.1097/00000658-198805000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydjari R., Beerthuizen G. I., Townsend C. M., Jr, Herndon D. N., Thompson J. C. Bacterial translocation and its relationship to visceral blood flow, gut mucosal ornithine decarboxylase activity, and DNA in pigs. J Trauma. 1991 May;31(5):639–644. doi: 10.1097/00005373-199105000-00007. [DOI] [PubMed] [Google Scholar]
- Sori A. J., Rush B. F., Jr, Lysz T. W., Smith S., Machiedo G. W. The gut as source of sepsis after hemorrhagic shock. Am J Surg. 1988 Feb;155(2):187–192. doi: 10.1016/s0002-9610(88)80691-3. [DOI] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Carpenter C. B. Prostaglandin as an effective antirejection therapy in rat renal allograft recipients. Transplantation. 1983 Apr;35(4):279–281. doi: 10.1097/00007890-198304000-00002. [DOI] [PubMed] [Google Scholar]
- Tartter P. I. Blood transfusion and postoperative infections. Transfusion. 1989 Jun;29(5):456–459. doi: 10.1046/j.1537-2995.1989.29589284149.x. [DOI] [PubMed] [Google Scholar]
- Tartter P. I., Quintero S., Barron D. M. Perioperative blood transfusion associated with infectious complications after colorectal cancer operations. Am J Surg. 1986 Nov;152(5):479–482. doi: 10.1016/0002-9610(86)90207-2. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Volkheimer G., Schulz F. H. The phenomenon of persorption. Digestion. 1968;1(4):213–218. doi: 10.1159/000196856. [DOI] [PubMed] [Google Scholar]
- Waymack J. P., Robb E., Alexander J. W. Effect of transfusion on immune function in a traumatized animal model. II. Effect on mortality rate following septic challenge. Arch Surg. 1987 Aug;122(8):935–939. doi: 10.1001/archsurg.1987.01400200085016. [DOI] [PubMed] [Google Scholar]
- Waymack J. P., Yurt R. W. The effect of blood transfusions on immune function. V. The effect on the inflammatory response to bacterial infections. J Surg Res. 1990 Feb;48(2):147–153. doi: 10.1016/0022-4804(90)90207-i. [DOI] [PubMed] [Google Scholar]
- Wiederkehr J. C., Dumble L., Pollak R., Moran M. Immunosuppressive effect of misoprostol: a new synthetic prostaglandin E1 analogue. Aust N Z J Surg. 1990 Feb;60(2):121–124. [PubMed] [Google Scholar]


