Abstract
Although direct cytotoxicity is a well-established phenomenon of tumor necrosis factor alpha (TNF alpha)-induced tissue damage, the intracellular events leading to cell death are still poorly understood. To study the cytotoxic effects of TNF alpha on normal parenchymal cells, rat hepatocytes were purified and incubated with various concentrations of TNF alpha. Mitochondrial respiration, total protein synthesis, and enzyme release were measured to assess metabolic performance and cell integrity. Treatment with TNF alpha suppressed mitochondrial respiration in a concentration-dependent fashion, resulting in a reduction of the activity of complex I of the respiratory chain to 67.0 +/- 3.5% of that of untreated hepatocytes by 2000 U/mL TNF alpha. Under these conditions protein synthesis and the release of intracellular enzymes were significantly increased. Both hepatocellular enzyme release and inhibition of mitochondrial respiration appear to be associated with the generation of reactive oxygen intermediates by the hepatocyte itself, because oxygen radical scavengers prevented these adverse effects of TNF alpha. Inhibition of protein synthesis by cycloheximide as well as addition of cyclic adenosine monophosphate synergistically enhanced the suppression of mitochondrial respiration by TNF alpha, resulting in complex I activity of 6.9 +/- 1.6% and 24.9 +/- 2.9% of that of untreated cells. These data indicate that inhibition of mitochondrial respiration is one of the mechanisms by which TNF alpha induces tissue injury.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S., Drysdale B. E., Shin H. S. Tumor necrosis factor-mediated cytotoxicity involves ADP-ribosylation. J Immunol. 1988 Jun 15;140(12):4187–4192. [PubMed] [Google Scholar]
- Aggarwal B. B., Eessalu T. E., Hass P. E. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Bankey P. E., Mazuski J. E., Ortiz M., Fulco J. M., Cerra F. B. Hepatic acute phase protein synthesis is indirectly regulated by tumor necrosis factor. J Trauma. 1990 Oct;30(10):1181–1188. doi: 10.1097/00005373-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Bankey P., Carlson A., Ortiz M., Singh R., Cerra F. Tumor necrosis factor production by Kupffer cells requires protein kinase C activation. J Surg Res. 1990 Sep;49(3):256–261. doi: 10.1016/0022-4804(90)90130-t. [DOI] [PubMed] [Google Scholar]
- Beutler B. A. Orchestration of septic shock by cytokines: the role of cachectin (tumor necrosis factor). Prog Clin Biol Res. 1989;286:219–235. [PubMed] [Google Scholar]
- Brett J., Gerlach H., Nawroth P., Steinberg S., Godman G., Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989 Jun 1;169(6):1977–1991. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
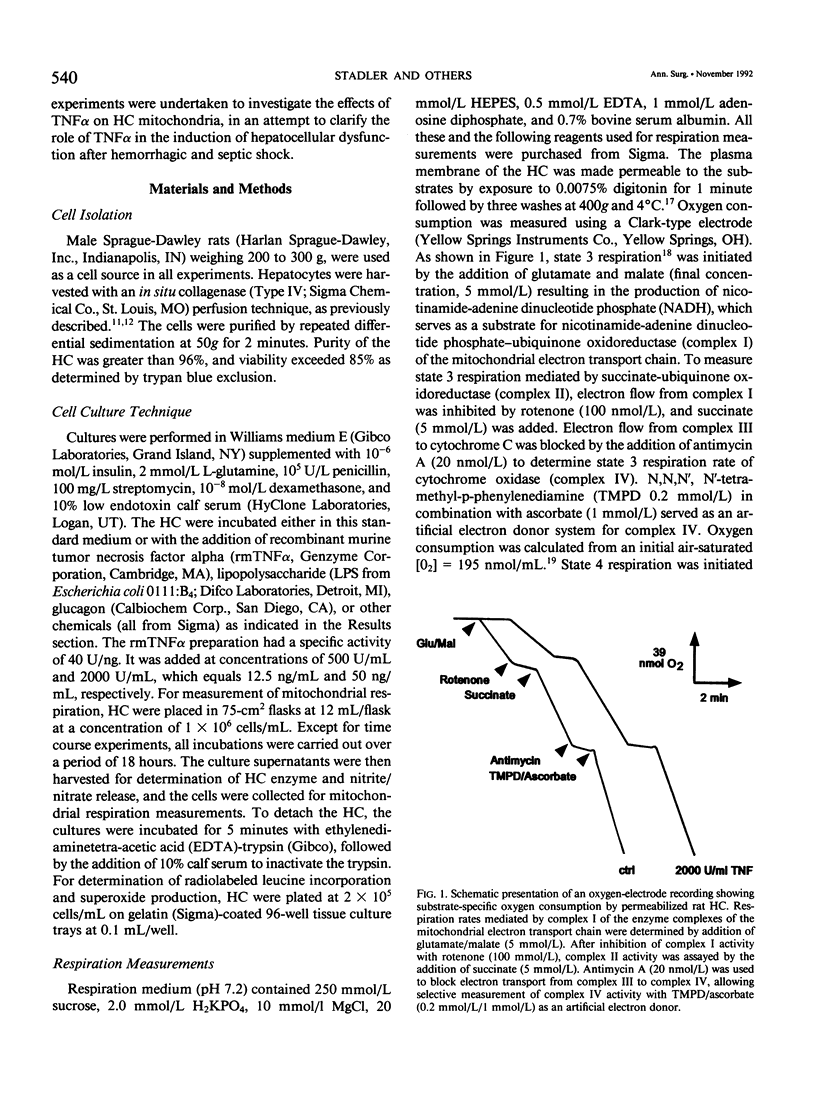
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerra F. B. Hypermetabolism, organ failure, and metabolic support. Surgery. 1987 Jan;101(1):1–14. [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Ochoa J. B., Harbrecht B. G., Flint S. G., Simmons R. L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990 Oct;212(4):462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas P., Reuter A., Gysen P., Demonty J., Lamy M., Franchimont P. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit Care Med. 1989 Oct;17(10):975–978. doi: 10.1097/00003246-198910000-00001. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Girardin E., Grau G. E., Dayer J. M., Roux-Lombard P., Lambert P. H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988 Aug 18;319(7):397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
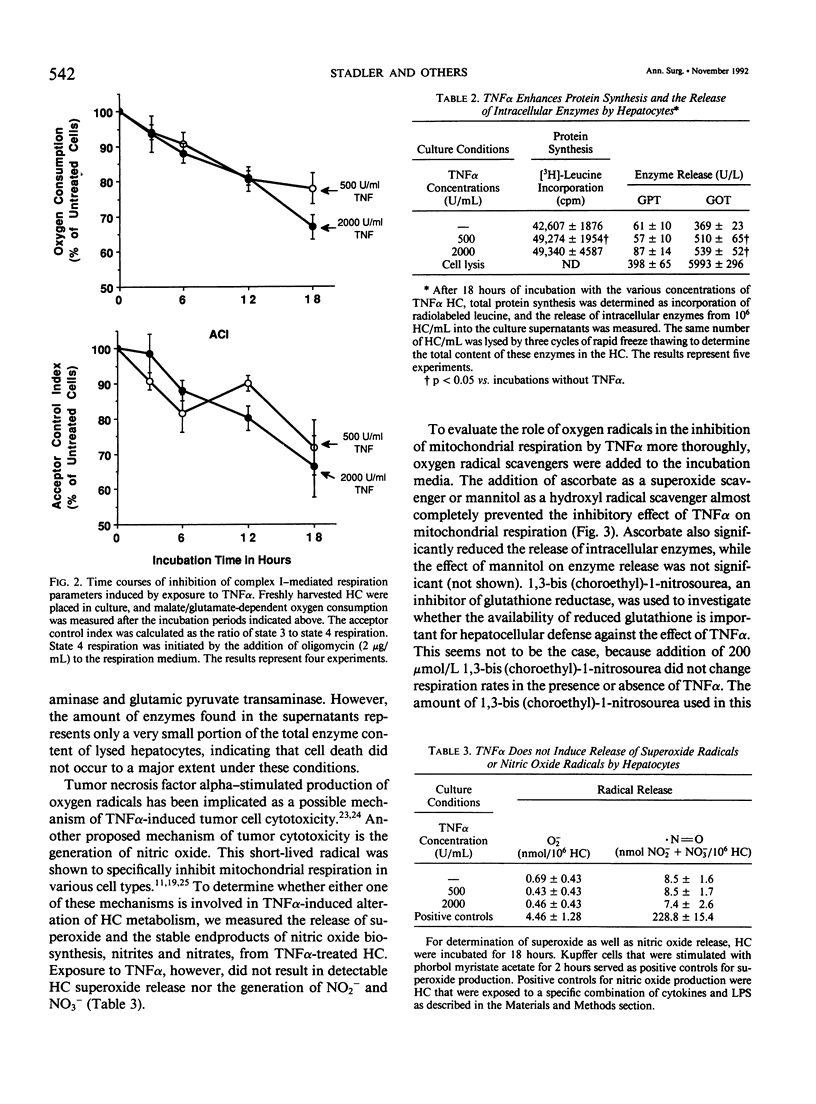
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Keller G. A., West M. A., Cerra F. B., Simmons R. L. Multiple systems organ failure. Modulation of hepatocyte protein synthesis by endotoxin activated Kupffer cells. Ann Surg. 1985 Jan;201(1):87–95. [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., West M. A., Harty J. T., Wilkes L. A., Cerra F. B., Simmons R. L. Modulation of hepatocyte protein synthesis by endotoxin-activated Kupffer cells. III. Evidence for the role of a monokine similar to but not identical with interleukin-1. Ann Surg. 1985 Apr;201(4):436–443. doi: 10.1097/00000658-198504000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., West M. A., Wilkes L. A., Cerra F. B., Simmons R. L. Modulation of hepatocyte protein synthesis by endotoxin-activated Kupffer cells. II. Mediation by soluble transferrable factors. Ann Surg. 1985 Apr;201(4):429–435. doi: 10.1097/00000658-198504000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull F. C., Jr, Cuatrecasas P. Possible requirement of internalization in the mechanism of in vitro cytotoxicity in tumor necrosis serum. Cancer Res. 1981 Dec;41(12 Pt 1):4885–4890. [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Cuatrecasas P. Cellular receptor for 125I-labeled tumor necrosis factor: specific binding, affinity labeling, and relationship to sensitivity. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5756–5760. doi: 10.1073/pnas.82.17.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
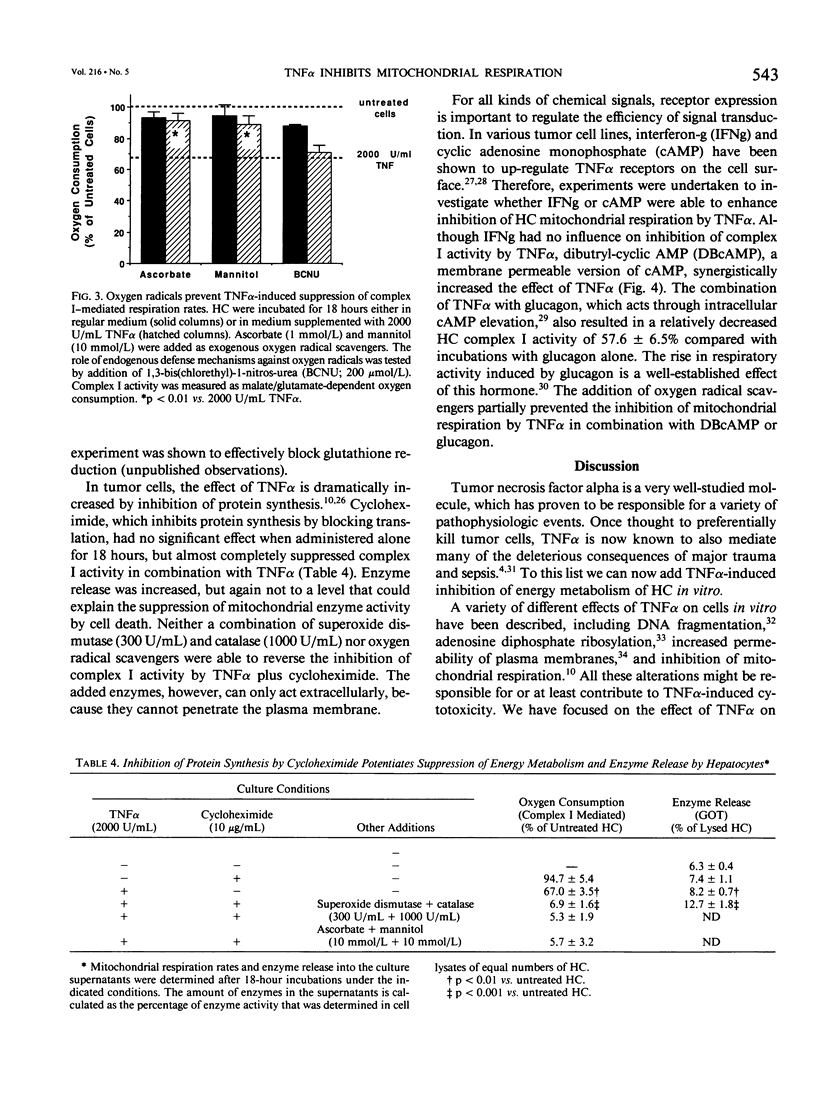
- Lancaster J. R., Jr, Laster S. M., Gooding L. R. Inhibition of target cell mitochondrial electron transfer by tumor necrosis factor. FEBS Lett. 1989 May 8;248(1-2):169–174. doi: 10.1016/0014-5793(89)80454-5. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Wright S. C. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J. 1990 Nov;4(14):3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
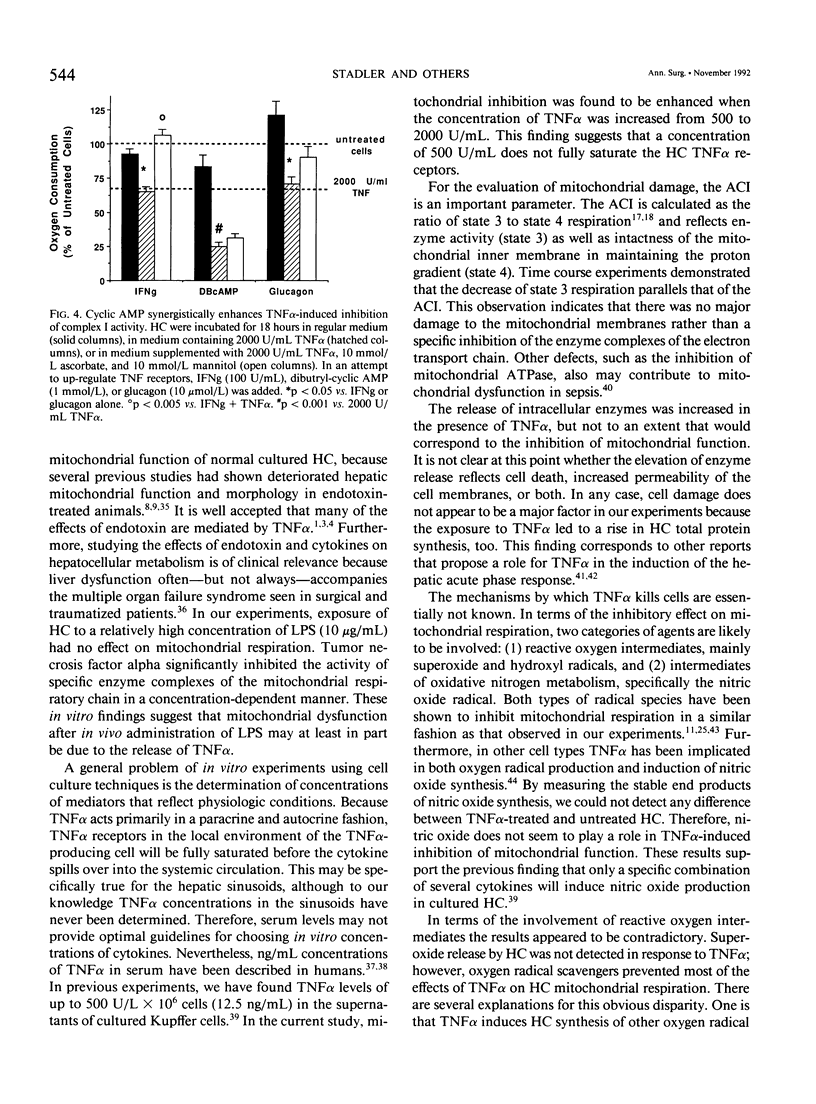
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Levy E., Slusser R. J., Ruebner B. H. Hepatic changes produced by a single dose of endotoxin in the mouse. Electron microscopy. Am J Pathol. 1968 Feb;52(2):477–502. [PMC free article] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Matthews N. Anti-tumour cytotoxin produced by human monocytes: studies on its mode of action. Br J Cancer. 1983 Sep;48(3):405–410. doi: 10.1038/bjc.1983.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Neale M. L., Jackson S. K., Stark J. M. Tumour cell killing by tumour necrosis factor: inhibition by anaerobic conditions, free-radical scavengers and inhibitors of arachidonate metabolism. Immunology. 1987 Sep;62(1):153–155. [PMC free article] [PubMed] [Google Scholar]
- Mela L., Bacalzo L. V., Jr, Miller L. D. Defective oxidative metabolism of rat liver mitochondria in hemorrhagic and endotoxin shock. Am J Physiol. 1971 Feb;220(2):571–577. doi: 10.1152/ajplegacy.1971.220.2.571. [DOI] [PubMed] [Google Scholar]
- Mela L., Miller L. D., Bacalzo L. V., Jr, Olofsson K., White R. R., 4th Role of intracellular variations of lysosomal enzyme activity and oxygen tension in mitochondrial impairment in endotoxemia and hemorrhage in the rat. Ann Surg. 1973 Dec;178(6):727–735. doi: 10.1097/00000658-197312000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreadith R. W., Fiskum G. Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal Biochem. 1984 Mar;137(2):360–367. doi: 10.1016/0003-2697(84)90098-8. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Rubin B. Y., Smith L. J., Hellermann G. R., Lunn R. M., Richardson N. K., Anderson S. L. Correlation between the anticellular and DNA fragmenting activities of tumor necrosis factor. Cancer Res. 1988 Nov 1;48(21):6006–6010. [PubMed] [Google Scholar]
- Scheurich P., Köbrich G., Pfizenmaier K. Antagonistic control of tumor necrosis factor receptors by protein kinases A and C. Enhancement of TNF receptor synthesis by protein kinase A and transmodulation of receptors by protein kinase C. J Exp Med. 1989 Sep 1;170(3):947–958. doi: 10.1084/jem.170.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumer W., Das Gupta T. K., Moss G. S., Nyhus L. M. Effect of endotoxemia on liver cell mitochondria in man. Ann Surg. 1970 Jun;171(6):875–882. doi: 10.1097/00000658-197006010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Billiar T. R., Curran R. D., Stuehr D. J., Ochoa J. B., Simmons R. L. Effect of exogenous and endogenous nitric oxide on mitochondrial respiration of rat hepatocytes. Am J Physiol. 1991 May;260(5 Pt 1):C910–C916. doi: 10.1152/ajpcell.1991.260.5.C910. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti S. V., Kwak E. L., Miller S. C., Miller L. L., Ringold G. M., Myambo K. B., Young A. P., Torti F. M. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988 Sep 5;263(25):12638–12644. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Videla L. A. Hepatic antioxidant-sensitive respiration. Effect of ethanol, iron and mitochondrial uncoupling. Biochem J. 1984 Nov 1;223(3):885–891. doi: 10.1042/bj2230885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K. Glucagon stimulation of mitochondrial respiration. J Biol Chem. 1975 Oct 10;250(19):7924–7930. [PubMed] [Google Scholar]
- Yasmineh W. G., Parkin J. L., Caspers J. I., Theologides A. Tumor necrosis factor/cachectin decreases catalase activity of rat liver. Cancer Res. 1991 Aug 1;51(15):3990–3995. [PubMed] [Google Scholar]
- Zhang Y., Marcillat O., Giulivi C., Ernster L., Davies K. J. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 1990 Sep 25;265(27):16330–16336. [PubMed] [Google Scholar]
- Zimmerman R. J., Chan A., Leadon S. A. Oxidative damage in murine tumor cells treated in vitro by recombinant human tumor necrosis factor. Cancer Res. 1989 Apr 1;49(7):1644–1648. [PubMed] [Google Scholar]
- Zimmerman R. J., Marafino B. J., Jr, Chan A., Landre P., Winkelhake J. L. The role of oxidant injury in tumor cell sensitivity to recombinant human tumor necrosis factor in vivo. Implications for mechanisms of action. J Immunol. 1989 Feb 15;142(4):1405–1409. [PubMed] [Google Scholar]


