Abstract
Background
Insulin resistance (IR) has been recognized as a critical factor in the progression of cardiovascular disease (CVD), yet its association with cardiovascular-kidney-metabolic (CKM) syndrome remains incompletely understood. This study aimed to evaluate the impact of IR, as measured by the estimated glucose disposal rate (eGDR), on the risk of future CVD events in individuals with CKM stages 0–3.
Methods
This study included 325,312 participants from the UK Biobank with CKM stages 0–3. IR was quantified using eGDR, a non-insulin-dependent metric, with lower values indicating greater IR. Participants were stratified into quartiles based on eGDR distribution. The primary outcome was incident CVD, including coronary heart disease, stroke, atrial fibrillation, heart failure, and peripheral artery disease.
Results
In the CKM 0–3 cohort, eGDR demonstrated the highest predictive value for future CVD events among non-insulin-dependent IR metrics. Incorporating eGDR significantly improved the predictive performance of the PREVENT Cardiovascular Disease Risk Equations (AUC: PREVENT Equations + eGDR 0.743 vs. PREVENT Equations 0.719, p < 0.001). Over a median follow-up of 13.57 years, 48,433 incident CVD cases were identified. The adjusted rates of CVD incidence (95% confidence interval [CI]) across eGDR quartiles (Q1–Q4) were 3.84 (3.62–4.07), 3.82 (3.66–3.98), 3.53 (3.41–3.65), and 3.37 (3.25–3.50) per 1000 person-years. RCS analysis revealed a significant nonlinear association between eGDR and CVD incidence (p for overall < 0.001; p for nonlinear = 0.020), with greater risk reduction at higher eGDR levels. A significant trend toward reduced CVD risk was observed across higher eGDR quartiles, with Q3 and Q4 demonstrating statistically significant reductions relative to Q1 (HR 0.920, 95% CI 0.871–0.971; and 0.883, 95% CI 0.827–0.942, respectively; p for trend < 0.001). Kaplan–Meier analysis further confirmed a graded decrease in CVD risk with increasing eGDR levels (log-rank p < 0.001).
Conclusion
This study establishes a strong association between IR severity and long-term CVD risk in individuals with CKM syndrome stages 0–3. The eGDR, a reliable surrogate marker of IR, independently predicts future CVD events and provides incremental predictive value beyond the PREVENT equations. These findings underscore the clinical utility of eGDR for risk stratification in CKM populations.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02860-z.
Keywords: Cardiovascular-kidney-metabolic syndrome, Insulin resistance, Estimated glucose disposal rate, Cardiovascular disease
Research insights
What is currently knownabout this topic?
IR drives CVD progression via inflammation and dysfunction.
eGDR correlates with insulin sensitivity and CVD risk.
CKM syndrome links metabolic, renal, and CVD risks.
What is the key researchquestion?
How does eGDR assessed IR predict CVD risk across CKM stages0–3?
What is new?
eGDR enhances the PREVENT model for CVD risk prediction.
eGDR-PAD L-shaped link suggests a 9.91 mg/kg/min threshold.
Women, younger individuals, and those with preserved renalfunction show stronger eGDR improvement.
eGDR’sprotective effect is strongest in early CKM stages (0–1).
RCS analysis reveals a nonlinear eGDR-CVD association with riskreduction as eGDR rises.
How might this studyinfluence clinical practice?
eGDR-guided precision interventions can optimize early CKM management and reduce CVD risk.
Introduction
The cardiovascular-kidney-metabolic (CKM) syndrome, a multisystem disorder stemming from the pathophysiological interplay of metabolic dysregulation, cardiovascular disease (CVD), and chronic kidney disease (CKD), represents an escalating global public health priority [1]. It reported that CVD accounted for 32% of worldwide mortality, with CKM syndrome affecting over 25% of U.S. adults and driving > 75% of national healthcare expenditures [2–4]. The American Heart Association (AHA) classifies CKM syndrome into five stages (0–4), advocating integrated cardiovascular-renal-metabolic management focused on primary prevention for stages 0–3, spanning risk-free individuals to those with subclinical CVD and high-risk metabolic profiles [5].
Insulin resistance (IR) drives CKM syndrome progression through multifaceted mechanisms, including chronic inflammation, endothelial dysfunction, and glomerular hyperfiltration, establishing itself as a central pathophysiological nexus linking metabolic dysregulation to end-organ damage [6, 7]. Emerging evidence indicates that systematic IR quantification and targeted intervention in CKM stages 0–3, encompassing individuals from risk-free status to those with subclinical cardiovascular pathology, may disrupt the pathophysiological cascade underlying cardiovascular events. While the hyperinsulinemic-euglycemic clamp (HEC) remains the gold standard for IR assessment, its procedural invasiveness and operational complexity limit clinical scalability [8]. Noninvasive IR surrogates, including the triglyceride-glucose index (TyG), metabolic score for insulin resistance (METS-IR), atherogenic index of plasma (AIP), and estimated glucose disposal rate (eGDR, where lower values indicate greater IR severity), are increasingly advocated for population studies due to their operational feasibility and validated associations with CVD risk [9–13].
Given the pivotal role of IR in CKM syndrome progression, this study conducted a comprehensive evaluation of quantitative associations between IR severity (assessed via eGDR, which calculated as 21.158 − [0.09 × Waist circumference] − [3.407 × Hypertension Status]– [0.551 × HbA1c]) and CVD risk among individuals with CKM stages 0–3. We innovatively evaluated the incremental predictive value of integrating this IR biomarker into the AHA PREVENT risk model—a validated multivariable-adjusted framework for cardiovascular risk stratification [14] (Supplementary Table S6). Our findings establish novel biomarker-driven strategies for early identification of high-risk populations while providing a methodological framework for precision interventions targeting CKM syndrome progression, with particular translational relevance for intercepting metabolic-cardiovascular-renal deterioration trajectories.
Methods
Data sources
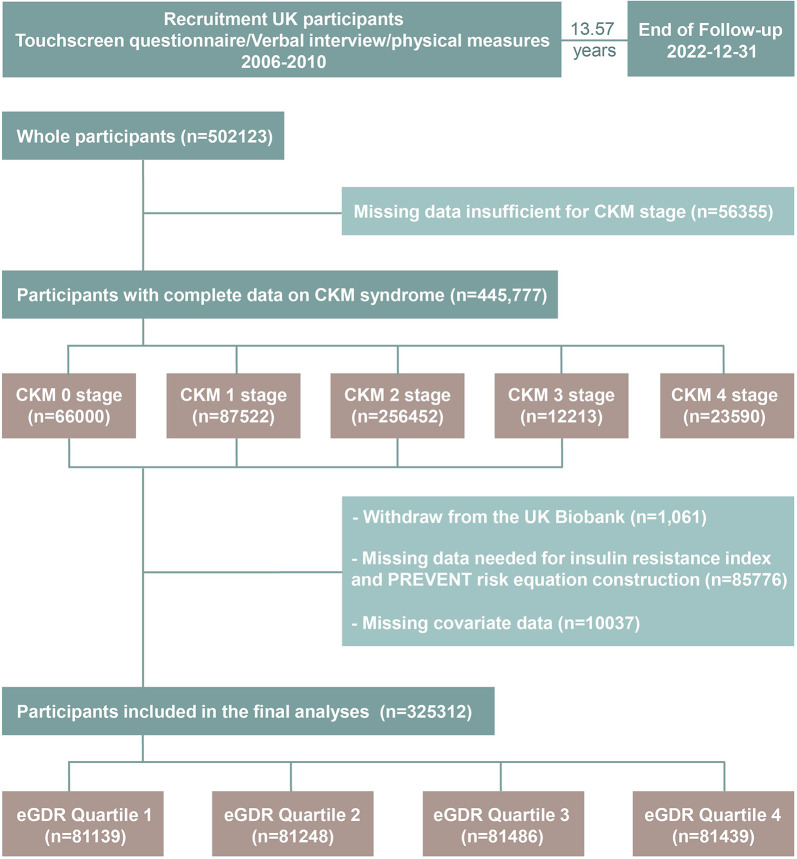
The UK Biobank (UKB) constitutes a large-scale retrospective cohort study that recruited over 502,123 participants aged 37–73 years across the United Kingdom between 2006 and 2010, achieving a response rate of 5.5%. The study collected extensive multimodal data through touchscreen questionnaires, verbal interviews, physical measurements, biological samples, and imaging modalities. Ethical approval for UK Biobank was granted by the Northwest Multi-Centre Research Ethics Committee (REC reference: 11/NW/0382), with all participants providing informed consent via electronic signatures post-enrollment. Detailed study protocols are publicly accessible (https://www.ukbiobank.ac.uk/). This analysis was conducted under UK Biobank application number 532333. Participants lacking data required for CKM staging (n = 56,355) were excluded from the analytical cohort. Additionally, individuals with baseline CVD diagnoses (CKM stage 4, n = 23,590) and those lost to follow-up during the median 13.57-year observation period (n = 1061) were systematically excluded. Furthermore, we excluded participants with incomplete covariate data essential for analysis (n = 95,814). After applying these exclusion criteria, the final analytical cohort comprised 325,312 individuals representative of the CKM stages 0–3 population (Fig. 1).
Fig. 1.
Flowchart of the study. eGDR, Estimated Glucose Disposal Rate; CKM, Cardiovascular-Kidney-Metabolic
Definition of CKM syndrome
CKM syndrome, as defined by the AHA, arises from the pathophysiological interplay among obesity, diabetes mellitus, CKD, and CVD, and is stratified into five progressive stages [1, 15]. In this study, stage 0 was defined as individuals with normal body mass index (BMI), waist circumference, normoglycemia, normotension, optimal lipid profiles, and absence of CKD or CVD. Stage 1 was characterized by excess adiposity, abdominal obesity, or adipose tissue dysfunction, without additional metabolic risk factors or CKD. Stage 2 encompassed individuals with confirmed metabolic risk factors—hypertriglyceridemia, hypertension, metabolic syndrome (MetS), or diabetes mellitus—and/or moderate-to-high risk CKD [16]. Stage 3 includes individuals with very-high-risk CKD (KDIGO G4-G5 or heat map 5-year progression risk ≥ 30%) or a 10-year PREVENT CVD risk ≥ 20%. Stage 4 referred to established CVD overlapping with features of stages 1–3, including heart failure (HF), atrial fibrillation (AF), coronary heart disease (CHD), stroke, or peripheral artery disease (PAD). Detailed definitions of CKM classification are provided in Supplementary Methods.
Assessment of CVD
In alignment with the AHA criteria for CKM stage 4, CVD diagnoses in this study were ascertained through hospital admission records and death registry data, encompassing HF, AF, CHD, stroke, or PAD. Hospitalization records were obtained from the Hospital Episode Statistics (HES) for England, Scottish Morbidity Record (SMR) for Scotland, and Patient Episode Database for Wales (PEDW). Mortality data for England and Wales were sourced from the National Health Service (NHS) Digital, while Scottish mortality records were acquired from the National Records of Scotland. Both primary and secondary hospital diagnoses, as well as underlying causes of death, were systematically coded using the International Classification of Diseases, Tenth Revision (ICD-10) system. CVD-specific ICD-10 codes were rigorously selected and validated by the UK Biobank Outcome Adjudication Group to ensure diagnostic accuracy and consistency (details see Supplementary Table S3).
Covariates
This study incorporated covariates encompassing sociodemographic characteristics, lifestyle factors, and comorbidities previously established as CVD risk determinants and potential confounders in exposure-outcome relationships. Self-reported data collected via touchscreen questionnaires included baseline age, sex, ethnicity, educational attainment, alcohol consumption status, smoking status, insomnia frequency, and dietary quality indices (see Supplementary Table S5). Socioeconomic deprivation was quantified using the Townsend Deprivation Index derived from residential postcodes. Physical activity levels were assessed according to International Physical Activity Questionnaire (IPAQ) guidelines, with compliance to 2017 UK physical activity recommendations defined as achieving ≥ 150 min of moderate-intensity activity/walking or ≥ 75 min of vigorous-intensity activity weekly [17]. Anthropometric measurements (height and waist circumference) were obtained by trained nurses, and BMI was calculated as weight (kg) / height (m2). Biochemical analyses of baseline blood samples quantified concentrations of high-density lipoprotein cholesterol (HDL-C), triglycerides, total cholesterol, fasting glucose, glycated hemoglobin (HbA1c), C-reactive protein (CRP), creatinine, and cystatin-C (formulae for eGFR [18] in Supplementary Table S7). Medical histories of hypertension, diabetes mellitus, and dyslipidemia, along with medication use (including lipid-lowering agents, antihypertensives, and glucose-lowering drugs) (see Supplementary Table S4), were ascertained through nurse-administered interviews. Details of definitions for these variables are detailed in Supplementary Table S2.
Statistical analysis
Complete-case analysis served as the primary analytical approach. Baseline demographic characteristics were stratified by eGDR quartiles [Q1 (≤ 6.62 mg/kg/min), Q2 (6.63–9.33 mg/kg/min), Q3 (9.34–10.70 mg/kg/min), and Q4 (≥ 10.71 mg/kg/min)], with continuous variables presented as mean [standard deviation (SD)] and categorical variables as frequencies (percentages). Poisson regression models with model-based standardization estimated crude and adjusted incidence rates of outcome events across eGDR quartiles, reported as events per 1000 person-years. Adjusted incidence rate differences and rate ratios (RRs) between quartiles were calculated. Dose–response relationships were evaluated using 4-knot restricted cubic splines (RCS) [19]. Cox proportional hazards models quantified associations between eGDR and CVD risk, expressed as hazard ratios (HRs) with 95% confidence interval (CIs). Survival curves derived from Cox models illustrated cumulative risk differences across eGDR quartile groups. Three sequential adjustment models were implemented: Model 1 (unadjusted) considered eGDR quartiles alone; Model 2 adjusted for demographic and lifestyle covariates (age, sex, physical activity, ethnicity, education, insomnia, Townsend deprivation index, alcohol consumption status, smoking status, dietary score); Model 3 further adjusted for clinical comorbidities (hypertension, type 2 diabetes, dyslipidemia), medications (lipid-lowering/antihypertensive/glucose-lowering agents), and BMI. Model 3 was selected as optimal based on Akaike and Bayesian Information Criterion (AIC/BIC) scores (Supplementary Table S8) for subsequent analyses.
Receiver operating characteristic (ROC) curves with area under the curve (AUC) evaluated the predictive performance of non-insulin-dependent IR surrogates for CVD. Optimal cut-off values were determined by maximizing Youden’s index. DeLong’s test compared AUCs between combined (eGDR + PREVENT risk) and single (PREVENT risk) prediction models. Time-dependent ROC curves assessed dynamic predictive performance, calculating AUCs at 1-, 3-, 5-, 10-, and 15-year intervals to quantify discriminative capacity between event-positive and event-negative cohorts. Bootstrap resampling (1000 iterations) generated 95% confidence interval (CI) for AUC estimates [20].
To validate the robustness of our findings, we conducted comprehensive sensitivity analyses. Firstly, we additionally adjusted for CKM stages to assess the result stability. Secondly, we excluded participants developing CVD within the first 3 years of follow-up to mitigate reverse causation bias. Thirdly, we employed the Fine and Gray competing risks regression model, treating all-cause death as competing risks. Fourthly, we conducted inverse probability weighting (IPW) and multiple imputation to evaluate potential selection bias from excluding participants with incomplete data. Furthermore, subgroup analyses were performed to evaluate potential effect modification by age, sex, ethnicity, education level, smoking status, alcohol consumption status, dietary patterns, physical activity, renal function, and inflammatory status. We compared Cox proportional hazards models with and without exposure-by-stratum interaction terms using Wald tests to evaluate interaction effects. All analyses were performed using R software version 4.2.2, with two-sided P values < 0.05 considered statistically significant. A Bonferroni-corrected significance level of P < 0.0015 (0.05/33) was used to adjust for multiple testing in subgroup analyses.
Results
The analytical cohort comprised 325,312 participants with complete data, including 180,833 males (55.6%) and 144,479 females (44.4%), with a mean age (SD) of 56.23 (8.09) years. During the follow-up period, 48,433 participants developed incident CVD, comprising 27,060 cases of CHD, 7430 strokes, 12,742 AF events, 9263 HF diagnoses, and 9739 PAD cases.
Through systematic screening of multiple non-insulin-based surrogate markers for IR from existing literature (formulae for IR indices provided in Supplementary Table S1), ROC curve analysis demonstrated eGDR's superior predictive performance for CVD events compared to alternative IR indicators, achieving an AUC of 0.716 (95% CI 0.714–0.719) (see Supplementary Figure S1).
Baseline characteristics of participants
Participants with lower eGDR quartiles which indicate greater IR, demonstrated distinct sociodemographic, lifestyle, and clinical profiles: they were predominantly female, older, and socioeconomically disadvantaged (lower income and educational attainment); exhibited higher rates of smoking, alcohol consumption, physical inactivity, and poor dietary quality; displayed adverse metabolic characteristics including elevated BMI, triglycerides, and cholesterol levels, accompanied by chronic inflammation, impaired glycemic control, and renal dysfunction; and manifested advanced clinical profiles with higher comorbidity burdens, complex medication regimens, and later-stage CKM classifications (Table 1). Supplementary Figure S2 illustrates the distribution trend of eGDR, showing a non-normal distribution, with a higher mean value in males compared to females. Further analysis across CKM stages revealed progressive phenotypic divergence: Stage 3 patients were characterized by older age, male predominance, lower educational attainment, and clustering of modifiable risk factors, including smoking, insomnia, alcohol misuse, poor diet, and physical inactivity. Notably, this advanced-stage subgroup exhibited the most pronounced IR signatures, underscoring the metabolic-renal-cardiovascular interplay in CKM progression (Supplementary Table S10).
Table 1.
Baseline characteristics stratified by eGDR quartiles among CKM stage 0–3 patients
| Total | Estimated glucose disposal rate (eGDR) (mg/kg/min) | ||||
|---|---|---|---|---|---|
| Quartile 1 (− 6.28–6.62) |
Quartile 2 (6.63–9.33) |
Quartile 3 (9.34–10.70) |
Quartile 4 (10.71–16.56) |
||
| n | 325,312 | 81,139 | 81,248 | 81,486 | 81,439 |
| Age, years, mean (SD) | 56.23 (8.09) | 59.07 (7.21) | 57.41 (7.84) | 54.95 (8.10) | 53.51 (8.01) |
| Sex, n (%) | |||||
| Female | 144,479 (44.4) | 28,915 (35.6) | 44,160 (54.4) | 37,593 (46.1) | 70,165 (86.2) |
| Male | 180,833 (55.6) | 52,224 (64.4) | 37,088 (45.6) | 43,893 (53.9) | 11,274 (13.8) |
| Ethnicity, n (%) | |||||
| British | 289,512 (89.0) | 72,192 (89.0) | 72,488 (89.2) | 72,423 (88.9) | 72,409 (88.9) |
| Others | 35,800 (11.0) | 8947 (11.0) | 8760 (10.8) | 9063 (11.1) | 9030 (11.1) |
| Townsend deprivation index, mean (SD) | − 1.40 (3.01) | − 1.09 (3.16) | − 1.41 (3.01) | − 1.51 (2.95) | − 1.60 (2.89) |
| PREVENT Risk, % | 6.83 (4.95) | 10.77 (5.56) | 7.46 (4.28) | 5.55 (3.58) | 3.55 (2.72) |
| Education, n (%) | |||||
| College or above | 117,152 (36.0) | 21,858 (26.9) | 27,402 (33.7) | 30,668 (37.6) | 37,224 (45.7) |
| High school or equivalent | 164,395 (50.5) | 40,887 (50.4) | 41,693 (51.3) | 41,373 (50.8) | 40,442 (49.7) |
| Less than high school | 43,765 (13.5) | 18,394 (22.7) | 12,153 (15.0) | 9445 (11.6) | 3773 (4.6) |
| Smoking status, n (%) | |||||
| Never | 180,875 (55.6) | 38,615 (47.6) | 44,144 (54.3) | 46,705 (57.3) | 51,411 (63.1) |
| Current | 32,530 (10.0) | 8545 (10.5) | 8538 (10.5) | 8488 (10.4) | 6959 (8.5) |
| Former | 111,907 (34.4) | 33,979 (41.9) | 28,566 (35.2) | 26,293 (32.3) | 23,069 (28.3) |
| Alcohol consumption status, n (%) | |||||
| Never | 13,384 (4.1) | 3754 (4.6) | 3534 (4.3) | 2899 (3.6) | 3197 (3.9) |
| Current | 301,143 (92.6) | 73,961 (91.2) | 74,887 (92.2) | 76,327 (93.7) | 75,968 (93.3) |
| Former | 10,785 (3.3) | 3424 (4.2) | 2827 (3.5) | 2260 (2.8) | 2274 (2.8) |
| Dietary score, n (%) | |||||
| 0 | 71,592 (22.0) | 21,637 (26.7) | 18,417 (22.7) | 19,402 (23.8) | 12,136 (14.9) |
| 1 | 173,368 (53.3) | 41,155 (50.7) | 42,586 (52.4) | 43,235 (53.1) | 46,392 (57.0) |
| 2 | 71,563 (22.0) | 16,326 (20.1) | 18,016 (22.2) | 16,955 (20.8) | 20,266 (24.9) |
| 3 | 8789 (2.7) | 2021 (2.5) | 2229 (2.7) | 1894 (2.3) | 2645 (3.2) |
| Physical activity, n (%)* | |||||
| Above recommendation | 226,324 (69.6) | 52,271 (64.4) | 55,870 (68.8) | 57,706 (70.8) | 60,477 (74.3) |
| Not meeting recommendation | 98,988 (30.4) | 28,868 (35.6) | 25,378 (31.2) | 23,780 (29.2) | 20,962 (25.7) |
| Insomnia, n (%) | |||||
| Never/rarely | 79,483 (24.4) | 18,362 (22.6) | 18,935 (23.3) | 22,208 (27.3) | 19,978 (24.5) |
| Sometimes | 155,824 (47.9) | 37,522 (46.2) | 38,858 (47.8) | 39,225 (48.1) | 40,219 (49.4) |
| Usually | 90,005 (27.7) | 25,255 (31.1) | 23,455 (28.9) | 20,053 (24.6) | 21,242 (26.1) |
| Physical measures and biomarkers, mean (SD) | |||||
| Body mass index, kg/m2 | 27.54 (4.71) | 31.33 (4.80) | 28.17 (4.53) | 27.15 (2.81) | 23.54 (2.57) |
| C reactive protein, mg/L | 2.58 (4.26) | 3.63 (4.97) | 2.80 (4.37) | 2.35 (3.93) | 1.55 (3.35) |
| eGFR, mL/min/1.73m2 | 95.41 (14.06) | 89.69 (15.08) | 93.89 (13.64) | 97.00 (12.75) | 101.03 (12.08) |
| Cholesterol, mmol/L | 5.73 (1.11) | 5.52 (1.19) | 5.83 (1.10) | 5.89 (1.07) | 5.70 (1.03) |
| HDL-C, mmol/L | 1.47 (0.38) | 1.28 (0.31) | 1.44 (0.38) | 1.43 (0.33) | 1.70 (0.38) |
| Triglycerides, mmol/L | 1.70 (1.01) | 2.14 (1.14) | 1.80 (1.05) | 1.72 (0.94) | 1.13 (0.52) |
| HbA1c, mmol/mol | 35.89 (6.27) | 39.15 (9.39) | 35.89 (5.18) | 34.87 (3.70) | 33.66 (3.45) |
| History of hypertension, n (%) | 124,969 (38.4) | 80,236 (98.9) | 44,582 (54.9) | 151 (0.2) | NA |
| History of diabetes, n (%) | 14,380 (4.4) | 11,021 (13.6) | 2411 (3.0) | 757 (0.9) | 191 (0.2) |
| History of Dyslipidemia, n (%) | 75,523 (23.2%) | 37,270 (45.9) | 21,196 (26.1) | 10,817 (13.3) | 6240 (7.7) |
| Medications and supplements, n (%) | |||||
| Lipid-lowering medication | 48,407 (14.9) | 28,372 (35.0) | 12,374 (15.2) | 5250 (6.4) | 2411 (3.0) |
| Antihypertensive medication | 64,266 (19.8) | 43,182 (53.2) | 19,148 (23.6) | 1181 (1.4) | 755 (0.9) |
| Anti-diabetic medication | 19,431 (6.0) | 13,271 (16.4) | 5540 (6.8) | 490 (0.6) | 130 (0.2) |
| IR measurement, mean (SD) | |||||
| METS-IR | 2.07 (0.18) | 2.20 (0.17) | 2.09 (0.18) | 2.08 (0.14) | 1.93 (0.12) |
| TyG | 8.68 (0.57) | 9.00 (0.56) | 8.75 (0.54) | 8.69 (0.50) | 8.30 (0.42) |
| TyG-BMI | 240.29 (49.67) | 282.15 (48.42) | 247.33 (46.75) | 236.21 (29.61) | 195.63 (25.64) |
| TyG-WC | 788.39 (148.33) | 928.12 (116.11) | 810.32 (142.43) | 788.31 (66.38) | 627.36 (62.80) |
| AIP | 0.02 (0.30) | 0.18 (0.27) | 0.05 (0.29) | 0.04 (0.27) | − 0.20 (0.22) |
| eGDR | 8.72 (2.42) | 5.35 (1.03) | 7.99 (0.85) | 10.05 (0.39) | 11.48 (0.52) |
| CKM classification, n (%) | |||||
| Stage 0 | 55,900 (17.2) | NA | 78 (0.1) | 9358 (11.5) | 46,464 (57.1) |
| Stage 1 | 52,717 (16.2) | 60 (0.1) | 6856 (8.4) | 26,065 (32.0) | 19,736 (24.2) |
| Stage 2 | 210,842 (64.8) | 76,067 (93.7) | 73,622 (90.6) | 45,929 (56.4) | 15,224 (18.7) |
| Stage 3 | 5853 (1.8) | 5012 (6.2) | 692 (0.9) | 134 (0.2) | 15 (0.0) |
*Physical activity that met the 2017 UK Physical activity guidelines (150 min of walking or moderate activity per week or 75 min of vigorous activity) was defined as above recommendation
eGDR, Estimated Glucose Disposal Rate; eGFR, Estimated Glomerular Filtration Rate; HbA1c, Glycated Hemoglobin A1c; HDL-C, High-Density Lipoprotein Cholesterol; IR, Insulin Resistance; METS-IR, Metabolic Score for Insulin Resistance; TyG, Triglyceride-Glucose Index; TyG-BMI, Triglyceride-Glucose-Body Mass Index; AIP, Atherogenic Index of Plasma; TyG-WC, Triglyceride-Glucose-Waist Circumference; BMI, Body Mass Index; WC, Waist Circumference; SD, Standard Deviation;; CKM, Cardiovascular-Kidney-Metabolic
The relationship between the eGDR and the incidence of CVD
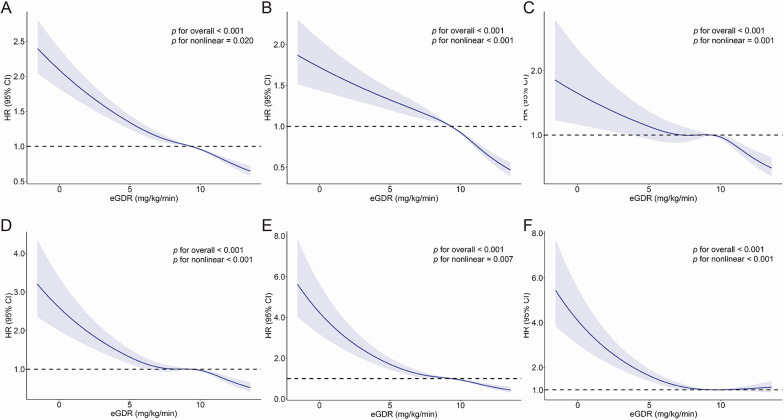
We employed RCS analysis to systematically evaluate the dose–response relationship between eGDR and CVD risk, including its component events, within the CKM syndrome stages 0–3 population (Fig. 2). The results revealed a significant inverse association between eGDR and overall CVD risk, as well as risks of composite events encompassing CHD, AF, HF, and stroke. A progressive decline in CVD risk was observed with increasing eGDR levels. Notably, the association between eGDR and PAD exhibited a distinct L-shaped curve pattern: PAD risk decreased markedly with rising eGDR levels below a threshold of 9.91 mg/kg/min, beyond which the risk reduction plateaued and demonstrated a non-significant upward trend at higher eGDR values.
Fig. 2.
Dose–response relationship between eGDR and the risk of cardiovascular disease and its component events in individuals with CKM syndrome stages 0–3. Adjustments were made for age, sex, physical activity level, ethnicity, education, insomnia, Townsend deprivation index, alcohol consumption status, smoking status, dietary score, diabetes, hypertension, dyslipidemia, body mass index (BMI), antihyperlipidemic medications, antihypertensive medications, and antidiabetic medications. The solid line represents the adjusted hazard ratio, and the shaded band indicates the 95% confidence interval. A Cardiovascular disease; B Coronary heart disease; C Stroke; D Atrial fibrillation; E Heart failure; F Peripheral artery disease. eGDR, Estimated Glucose Disposal Rate; CKM, Cardiovascular-Kidney-Metabolic
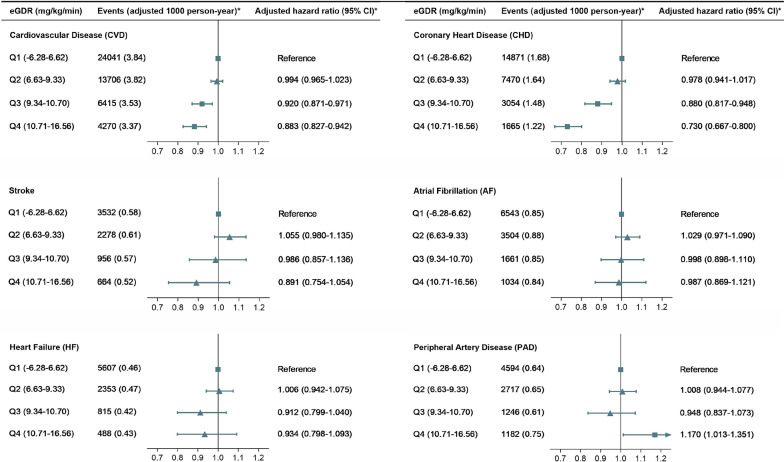
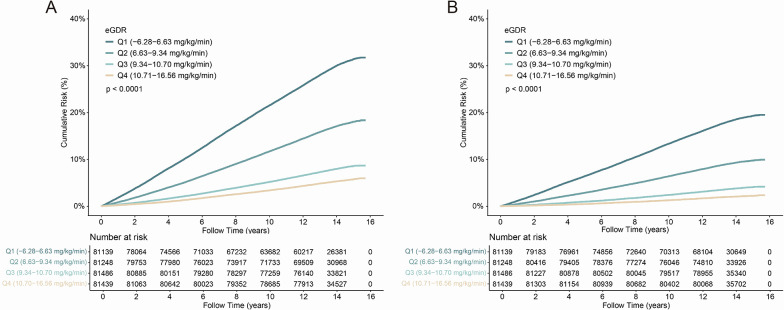
Multivariable-adjusted Cox regression analysis demonstrated a progressive reduction in CVD risk across increasing eGDR quartiles, the corresponding adjusted hazard compared to Q1 were 0.994 (95% CI 0.965–1.023) for Q2, 0.920 (95% CI 0.871–0.971) for Q3, and 0.883 (95% CI 0.827–0.942) for Q4. A similar graded inverse association was observed for CHD, with 0.880 (95% CI 0.817–0.948) for Q3 and 0.730 (95% CI 0.667–0.800) for Q4 showing statistically significant risk reductions (Fig. 3 and Supplementary Table S12). While analogous trends were noted for stroke, AF, HF, and PAD, statistical significance was inconsistent across these endpoints. Cumulative risk curves stratified by eGDR quartiles corroborated this dose-dependent protective relationship, with progressively lower CVD and CHD risks observed at higher eGDR levels (Fig. 4).
Fig. 3.
Adjusted hazard ratios for cardiovascular disease incidence in individuals with CKM syndrome stages 0–3, stratified by quartiles of eGDR. Adjustments were made for variables including age, sex, physical activity level, ethnicity, education, insomnia, Townsend deprivation index, alcohol consumption status, smoking status, dietary score, diabetes, hypertension, dyslipidemia, body mass index, antihyperlipidemic medications, antihypertensive medications, and antidiabetic medications. eGDR, Estimated Glucose Disposal Rate; CKM, Cardiovascular-Kidney-Metabolic
Fig. 4.
Cumulative risk curves for cardiovascular disease (CVD) (A) and coronary heart disease (CHD) (B) risk across different eGDR quartiles in individuals with CKM syndrome stages 0–3. eGDR, Estimated Glucose Disposal Rate; CKM, Cardiovascular-Kidney-Metabolic
To elucidate the stage-specific impact of eGDR on CVD risk, subgroup analyses stratified by CKM stages (0–3) demonstrated that higher eGDR quartiles were associated with reduced CVD risk across all stages, where the strongest protective effect occurred in stage 0 (HR 0.906, 95% CI 0.833–0.985), followed by stage 1 (HR 0.910, 95% CI 0.852–0.971), stage 2 (HR 0.931, 95% CI 0.916–0.946), and stage 3 (HR 0.907, 95% CI 0.862–0.954) (p for interaction < 0.001) (Supplementary Figure S3).
In the CKM stages 0–3 cohort, overall incidence rates per 1000 person-years were 11.55 (95% CI 11.44–11.65) for CVD, 6.24 (95% CI 6.17–6.32) for CHD, 1.66 (95% CI 1.62–1.70) for stroke, 2.88 (95% CI 2.83–2.93) for AF, 2.07 (95% CI 2.03–2.11) for HF, and 2.18 (95% CI 2.14–2.23) for PAD (Table 2). Moreover, with increasing CKM staging, the adjusted CVD incidence rates rose progressively: 3.02 (95% CI 2.89–3.15) for Q1, 3.10 (95% CI 2.97–3.23) for Q2, 8.33 (95% CI 8.12–8.54) for Q3, and 11.41 (95% CI 10.85–11.97) for Q4, per 1000 person-years (Supplementary Table S9). After stratification by eGDR quartiles, adjusted CVD incidence rates decreased sequentially: 3.84 (95% CI 3.62–4.07) for Q1, 3.82 (95% CI 3.66–3.98) for Q2, 3.53 (95% CI 3.41–3.65) for Q3, and 3.37 (95% CI 3.25–3.50) for Q4 per 1000 person-years. Similarly, adjusted CHD incidence declined from 1.68 (95% CI 1.55–1.81) in Q1 to 1.22 (95% CI 1.15–1.29) in Q4 per 1000 person-years. Compared to Q1, higher eGDR quartiles demonstrated progressive risk reductions, with absolute CVD risk differences of 0.31 (95% CI 0.06–0.57) for Q3 and 0.47 (95% CI 0.22–0.72) for Q4. Corresponding CHD risk differences were 0.20 (95% CI 0.05–0.35) for Q3 and 0.46 (95% CI 0.31–0.60) for Q4. Stroke, AF, and HF showed weaker inverse correlations, though consistent with the overall trend. Notably, PAD exhibited a distinct risk profile, with an elevated adjusted absolute risk of 0.11 (95% CI 0.01–0.21) observed in Q4. (Table 2).
Table 2.
Cardiovascular disease incidence rates, incidence rate differences, and incidence rate ratios by eGDR quartiles in individuals with CKM stages 0–3
| Estimated glucose disposal rate (eGDR) (mg/kg/min) | ||||||
|---|---|---|---|---|---|---|
| Total | Quartile 1 (− 6.28–6.62) |
Quartile 2 (6.63–9.33) |
Quartile 3 (9.34–10.70) |
Quartile 4 (10.71–16.56) |
||
| CVD | Event, n | 48,433 | 24,041 | 13,706 | 6415 | 4271 |
| Incidence event rate | 11.55 (11.44, 11.65) | 25.01 (24.69, 25.32) | 13.19 (12.97, 13.42) | 5.88 (5.74, 6.02) | 3.87 (3.75, 3.98) | |
| Adjusted incidence event rate | 3.84 (3.62, 4.07) | 3.82 (3.66, 3.98) | 3.53 (3.41, 3.65) | 3.37 (3.25, 3.50) | ||
| Adjusted incidence rate difference | Ref | − 0.02 (− 0.30, 0.25) | − 0.31 (− 0.57, − 0.06) | − 0.47 (− 0.72, − 0.22) | ||
| Adjusted incidence rate ratio | Ref | 0.99 (0.95, 1.04) | 0.92 (0.89, 0.95) | 0.88 (0.85, 0.91) | ||
| CHD | Event, n | 27,060 | 14,871 | 7470 | 3054 | 1665 |
| Incidence event rate | 6.24 (6.17, 6.32) | 14.51 (14.28, 14.75) | 6.9 (6.76, 7.08) | 2.75 (2.65, 2.84) | 1.49 (1.42, 1.56) | |
| Adjusted incidence event rate | 1.68 (1.55, 1.81) | 1.64 (1.55, 1.74) | 1.48 (1.41, 1.55) | 1.22 (1.15, 1.29) | ||
| Adjusted incidence rate difference | Ref | − 0.04 (− 0.20, 0.13) | − 0.20 (− 0.35, − 0.05) | − 0.46 (− 0.60, − 0.31) | ||
| Adjusted incidence rate ratio | Ref | 0.98 (0.92, 1.04) | 0.88 (0.84, 0.92) | 0.73 (0.69, 0.77) | ||
| Stroke | Event, n | 7430 | 3532 | 2278 | 956 | 664 |
| Incidence event rate | 1.66 (1.62, 1.70) | 3.19 (3.08, 3.29) | 2.04 (1.96, 2.13) | 0.85 (0.80, 0.90) | 0.59 (0.55, 0.63) | |
| Adjusted incidence event rate | 0.58 (0.50, 0.67) | 0.61 (0.55, 0.68) | 0.57 (0.53, 0.62) | 0.52 (0.47, 0.57) | ||
| Adjusted incidence rate difference | Ref | 0.03 (− 0.08, 0.14) | − 0.01 (− 0.11, 0.09) | − 0.06 (− 0.16, 0.04) | ||
| Adjusted incidence rate ratio | Ref | 1.06 (0.94, 1.17) | 0.99 (0.90, 1.07) | 0.89 (0.81, 0.97) | ||
| AF | Event, n | 12,743 | 6543 | 3504 | 1661 | 1035 |
| Incidence event rate | 2.88 (2.83, 2.93) | 6.08 (5.93, 6.22) | 3.18 (3.07, 3.28) | 1.49 (1.41, 1.56) | 0.92 (0.87, 0.98) | |
| Adjusted incidence event rate | 0.85 (0.76, 0.95) | 0.88 (0.81, 0.95) | 0.85 (0.80, 0.91) | 0.84 (0.78, 0.90) | ||
| Adjusted incidence rate difference | Ref | 0.02 (− 0.10, 0.15) | 0.00 (− 0.11, 0.11) | − 0.01 (− 0.13, 0.10) | ||
| Adjusted incidence rate ratio | Ref | 1.03 (0.94, 1.11) | 1.00 (0.93, 1.06) | 0.99 (0.91, 1.06) | ||
| HF | Event, n | 9263 | 5607 | 2353 | 815 | 488 |
| Incidence event rate | 2.07 (2.03–2.11) | 5.09 (4.96, 5.23) | 2.11 (2.02, 2.19) | 0.72 (0.67, 0.77) | 0.43 (0.39, 0.47) | |
| Adjusted incidence event rate | 0.46 (0.40, 0.53) | 0.47 (0.42, 0.51) | 0.42 (0.38, 0.46) | 0.43 (0.38, 0.47) | ||
| Adjusted incidence rate difference | Ref | 0.00 (− 0.08, 0.08) | − 0.04 (− 0.12, 0.03) | − 0.04 (− 0.11, 0.04) | ||
| Adjusted incidence rate ratio | Ref | 1.00 (0.90, 1.11) | 0.91 (0.83, 0.98) | 0.92 (0.83, 1.01) | ||
| PAD | Event, n | 9739 | 4594 | 2717 | 1246 | 1182 |
| Incidence event rate | 2.18 (2.14–2.23) | 4.17 (4.05, 4.29) | 2.44 (2.35, 2.53) | 1.11 (1.05, 1.17) | 1.05 (0.99, 1.11) | |
| Adjusted incidence event rate | 0.64 (0.56, 0.73) | 0.65 (0.58, 0.71) | 0.61 (0.56, 0.65) | 0.75 (0.69, 0.80) | ||
| Adjusted incidence rate difference | Ref | 0.01 (− 0.10, 0.11) | − 0.03 (− 0.13, 0.06) | 0.11 (0.01, 0.21) | ||
| Adjusted incidence rate ratio | Ref | 1.01 (0.91, 1.11) | 0.95 (0.88, 1.02) | 1.17 (1.08, 1.26) | ||
The bold text in Table 2 indicates statistical significance. eGDR, Estimated Glucose Disposal Rate; CVD, Cardiovascular Disease; CHD, coronary heart disease; AF, atrial fibrillation; HF, heart failure; PAD, peripheral artery disease; CKM, Cardiovascular-Kidney-Metabolic
Incremental predictive performance of eGDR in the risk assessment of CVD
ROC analysis revealed that integrating eGDR with the PREVENT risk model significantly enhanced CVD prediction (AUC 0.743 vs. 0.716 for PREVENT alone, P < 0.001), with stable discriminative capacity across time points (1-year AUC 0.724, 3-year 0.725, 5-year 0.721, 10-year 0.719, 15-year 0.718) (Supplementary Figure S4). Risk reclassification analysis demonstrated clinically meaningful improvements (NRI = 0.268, 95% CI 0.264–0.274; P < 0.001), with 8.9% of high-risk (PREVENT ≥ 20%) and 23.3% of intermediate-risk (PREVENT 5–20%) subjects appropriately reclassified to lower-risk categories. These results, supported by significant discrimination improvement (IDI = 0.015, P < 0.001), establish eGDR as providing incremental predictive value beyond conventional CVD risk assessment tools (Supplementary Figure S5).
Subgroup and sensitivity analyses
Subgroup analyses revealed significant heterogeneity in the eGDR-CVD associations in certain subgroups (Bonferroni correction P for interaction < 0.0015) (Supplementary Table S11). Protective effects were more pronounced in younger participants (< 65 years: Q4 HR 0.740; 95% CI 0.685–0.800 vs. ≥ 65 years: HR 0.843, 95% CI 0.748–0.950), female (Q4 HR 0.806; 95% CI 0.727–0.893), and those with preserved renal function (eGFR ≥ 90 mL/min/1.73m2: Q4 HR 0.842; 95% CI 0.770–0.920). Additionally, interaction effects were observed in subgroups defined by education level and ethnicity, whereas no interaction effects were detected in subgroups based on other covariates. The main results remained robust across various sensitivity analyses. Additional covariate adjustment (Supplementary Table S13), excluding participants who had a CVD event within 3 years of follow-up (see Supplementary Table S14), accounting for competing risks from all-cause death (see Supplementary Figures S6), and potential selection bias from excluding participants with incomplete data(see Supplementary Figures S7), resulted in slightly attenuated associations.
Discussion
This study identifies IR, indicated by lower eGDR, as a key predictor of increased CVD risk across CKM stages 0–3, with a notable impact on CHD. Adjusted CVD incidence rates show reduced differences across eGDR quartiles, suggesting it partly reflects metabolic and clinical burden, while multivariable Cox regression confirms its independent predictive value. Integrating eGDR with the PREVENT model boosts prognostic accuracy, maintains time-dependent discrimination, and enhances risk reclassification, affirming its dual role as a disease burden marker and prognostic tool in CKM populations.
Existing studies have established that the eGDR, a metabolic marker of tissue glucose utilization efficiency, is strongly associated with enhanced insulin sensitivity and holds significant promise for CVD prevention. Studies demonstrate that eGDR exhibits high concordance with the HEC, the gold standard for assessing IR, particularly in individuals with type 1 diabetes mellitus (T1DM) [8]. Its superior predictive accuracy compared to conventional metrics, such as Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), establishes its clinical utility in diabetes management [21, 22]. Clinical evidence indicates that elevated eGDR levels are associated with a reduced risk of CVD (HR 0.81, 95% CI 0.75–0.88), alongside decreased CVD incidence and all-cause mortality in both T1DM and type 2 diabetes mellitus (T2DM) populations [23–25]. The predictive value of eGDR extends beyond diabetic cohorts to broader metabolic dysregulation spectra, such as non-alcoholic fatty liver disease (NAFLD), where a dose–response relationship with arterial stiffness has been observed, suggesting its relevance in metabolic comorbidities [26].
Integrating eGDR into the CKM syndrome assessment framework highlights its role as a versatile predictor across metabolic syndromes and elucidates its capacity to capture heterogeneity within the CKM axis [27, 28]. In early CKM stages (0–1), IR originates from adipose tissue dysfunction, promoting lipid dysregulation characterized by elevated triglycerides, reduced HDL cholesterol, and increased small dense LDL particles. These factors synergize with proinflammatory cytokines (e.g., IL-6, TNF-α) to accelerate atherogenesis [29]. As CKM progresses to stages 2–3, IR amplifies risk through endothelial dysfunction and sympathetic overactivation, which diminishes nitric oxide bioavailability and promotes hypertension and ventricular remodeling [30, 31]. These pathological processes are further exacerbated by oxidative stress amplification and autophagy suppression, with mitochondrial dysfunction-mediated reactive oxygen species bursts perpetuating vascular injury [11, 27, 28]. The unique metabolic-renal crosstalk inherent to CKM syndrome may amplify cardiovascular risk through feedback loops. Experimental models demonstrate that IR-driven glomerular hyperfiltration and CKD-associated insulin clearance impairment reciprocally reinforce each other, creating a proinflammatory cycle that elevates CVD susceptibility [30]. Emerging human studies highlight novel mediators such as gut microbiota-derived trimethylamine N-oxide (TMAO) and epigenetic dysregulation (e.g., aberrant DNA methylation), which may sustain long-term cardiovascular damage via metabolic memory effects [32–35]. While these mechanisms await full validation in CKM populations, their convergence across studies provides critical insights into the multidimensional pathophysiology linking IR to cardiovascular outcomes. While these mechanisms await full validation in CKM populations, their convergence across studies provides critical insights into the multidimensional pathophysiology linking IR to cardiovascular outcomes.
Critically, such pathophysiological complexity manifests as clinically relevant heterogeneity in treatment responses. Subgroup analyses indicate that younger individuals, women, and those with preserved renal function might benefit more, possibly due to lower cumulative metabolic burden. This sexual dimorphism may be attributed to estrogen-mediated enhancement of endothelial function via PPARγ pathway regulation [36], compounded by sex hormone-driven differences in inflammatory responses and visceral adiposity patterns, which collectively exacerbate IR and CVD risk disparities [37]. In advanced CKM stages, particularly among those with renal impairment, activation of the NLRP3 inflammasome and vascular calcification amplify CVD risk, potentially attenuating eGDR’s protective effects [38, 39]. These findings advocate for personalized CVD prevention strategies informed by eGDR, tailored to gender, age, and renal function profiles, thereby providing a robust foundation for precision medicine.
This study establishes a strong association between IR severity, as measured by eGDR, and long-term CVD risk in individuals with CKM syndrome stages 0–3. While the overall inverse relationship between eGDR and CVD risk (including CHD, AF, HF, and stroke) aligns with the known protective effects of insulin sensitivity, the L-shaped relationship with PAD introduces a critical nuance. The RCS analysis identified a threshold at 9.91 mg/kg/min, below which increasing eGDR significantly reduced PAD risk, but beyond which the protective effect plateaued, with a non-significant upward trend at higher values. This pattern may reflect a complex interplay of metabolic and vascular factors. At lower eGDR levels, improved insulin sensitivity likely mitigates PAD risk by reducing endothelial dysfunction and inflammation, key drivers of peripheral atherosclerosis. However, at higher eGDR levels, other risk factors—such as advanced age, altered lipid profiles, or compensatory metabolic changes—might counteract these benefits, potentially explaining the plateau and slight increase in PAD risk. This threshold effect could also indicate a saturation point of insulin-mediated vascular protection, warranting further investigation into PAD-specific mechanisms, such as microvascular dysfunction or plaque stability, which may differ from macrovascular CVD endpoints.
Clinical implications
The integration of eGDR into the PREVENT risk stratification model significantly enhances cardiovascular risk prediction in patients with CKM syndrome, confirming its clinical utility for identifying high-risk populations. This enhancement is particularly significant for managing early-stage CKM, where preserved insulin sensitivity enables maximum preventive benefits through targeted interventions. Our stage-specific findings provide critical clinical insights for tailoring CKM interventions. In early stages (0–1), where eGDR’s protective effect is strongest, lifestyle changes (e.g., Mediterranean diet, exercise) or drugs may best reduce CVD risk [40–42]. In stage 0, lifestyle interventions can maintain high eGDR to prevent progression. In stage 1, adding risk factor management (e.g., statins) is needed. In stage 2, combining IR therapies (GLP-1 receptor agonists or SGLT2 inhibitors) enhances benefits [43]. In stage 3, multimodal treatments for advanced risks (e.g., antihypertensives) alongside IR interventions are essential. These insights support a precision medicine approach, leveraging eGDR and CKM stages to customize interventions effectively.
Notably, our study reveals a heightened responsiveness to eGDR improvement among women, younger individuals, and those with preserved renal function. Subgroup analyses in patients under 65 years demonstrate that higher eGDR levels provide enhanced protection against CVD in lower CKM stages (0–1), reflecting improved insulin sensitivity responses in younger patients (Supplementary Figure S8). These individuals typically exhibit a lower cumulative metabolic burden and are more frequently in early CKM stages (0 or 1), positioning them as priority targets for targeted interventions. Early interventions targeting IR, such as lifestyle modifications (e.g., Mediterranean diet, regular exercise) or pharmacological agents (e.g., metformin, SGLT2 inhibitors), show greater potential to reduce CVD risk in these individuals [44].
Limitations
This study has several limitations requiring cautious interpretation. First, the single-timepoint eGDR measurement restricts insight into temporal IR dynamics, potentially underestimating the long-term cardiovascular risk from metabolic fluctuations, while its components (e.g., HbA1c, blood pressure), influenced by renal function or medications, lack the precision of HEC methods. Second, the lack of specific data on SGLT2 inhibitors and GLP-1 receptor agonists—known to enhance insulin sensitivity and reduce CVD risk—limits stratified analyses, potentially underestimating IR in treated individuals and weakening the L-shaped CVD risk association. Thirdly, despite rigorous covariate adjustments and sensitivity analyses, residual confounding from unmeasured genetic or environmental factors persists, particularly in the Q1 group with higher comorbidities and socioeconomic challenges, which may bias risk estimates. Fourth, the retrospective UK Biobank design limits access to fasting insulin and subclinical atherosclerosis data (e.g., coronary artery calcium score, carotid intima-media thickness), hindering HOMA-IR comparisons and eGDR’s link to subclinical disease. Future imaging-based studies will address this gap, potentially enabling earlier and more targeted interventions in CKM populations.
Conclusion
IR exerts stage-specific effects on CVD risk across the CKM syndrome continuum (stages 0–3). The eGDR emerges as both a practical biomarker for cardiovascular risk stratification and a catalyst for precision prevention strategies, with validated incremental predictive value over existing models. Our findings underscore the therapeutic potential of early insulin sensitivity optimization to disrupt CKM progression trajectories. By identifying critical windows for intervention and delineating outcome-specific IR pathophysiology, this work advances personalized approaches for mitigating cardiometabolic risk in evolving CKM syndromes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The present study was conducted using the UK Biobank resource under the application number 532333. We are grateful to all the participants and researchers from the UK Biobank.
Author contributions
Hao Zhang: Writing–Original draft, Conceptualization. Sizhuang Huang: Project administration, Conceptualization. Yanwen Fang: Project administration, Methodology. Haihua Zhang: Literature search and analyses. Weixian Yang:, Review & editing, Supervision. Mengyue Yu: Writing—Review & editing, Formal analysis, Supervision, Conceptualization, Analyzed and interpreted the data.
Data availability
All data relevant to the study were acquired from the UK Biobank Resource under application number 532333. Data can be accessed through applications on UK Biobank website (https://www.ukbiobank.ac.uk/).
Declarations
Ethics approval and consent to participate
Ethical approval for the UK Biobank was granted by the North West Multicenter Research Ethics Committee (reference number: 11/NW/ 0382), and all participants provided written informed consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Zhang and Sizhuang Huang have contributed equally to this work.
Contributor Information
Weixian Yang, Email: fwywx66@fuwai.com.
Mengyue Yu, Email: yumengyue@fuwaihospital.org.
References
- 1.Ndumele CE, Rangaswami J, Chow SL, et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. 2023;148(20):1606–35. 10.1161/CIR.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 2.The Global Cardiovascular Risk Consortium. Global effect of cardiovascular risk factors on lifetime estimates. N Engl J Med. 2025. 10.1056/NEJMoa2415879 [DOI] [PubMed]
- 3.Ostrominski JW, Arnold SV, Butler J, et al. Prevalence and overlap of cardiac, renal, and metabolic conditions in US adults, 1999–2020. JAMA Cardiol. 2023;8(11):1050. 10.1001/jamacardio.2023.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogeli C, Shields AE, Lee TA, et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–5. 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SS, Coresh J, Pencina MJ, et al. Novel prediction equations for absolute risk assessment of total cardiovascular disease incorporating cardiovascular-kidney-metabolic health: a scientific statement from the American Heart Association. Circulation. 2023;148(24):1982–2004. 10.1161/CIR.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 6.Marassi M, Fadini GP. The cardio-renal-metabolic connection: a review of the evidence. Cardiovasc Diabetol. 2023;22(1):195. 10.1186/s12933-023-01937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–223. 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DSNT, Brookshire T, Krakoff J, Bunt JC. Repeatability and reproducibility of the hyperinsulinemic-euglycemic clamp and the tracer dilution technique in a controlled inpatient setting. Metabolism. 2009;58(3):304–10. 10.1016/j.metabol.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Y, Yin L, Huang H, Hu Y, Lin S. Assessing the impact of insulin resistance trajectories on cardiovascular disease risk using longitudinal targeted maximum likelihood estimation. Cardiovasc Diabetol. 2025;24(1):112. 10.1186/s12933-025-02651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan M, Zhao X, Li S, et al. Metabolic score for insulin resistance (METS-IR) predicts all-cause and cardiovascular mortality in the general population: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2024;23(1):243. 10.1186/s12933-024-02334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun R, Wang J, Li M, et al. Association of insulin resistance with cardiovascular disease and all-cause mortality in type 1 diabetes: systematic review and meta-analysis. Diabetes Care. 2024;47(12):2266–74. 10.2337/dc24-0475. [DOI] [PubMed] [Google Scholar]
- 12.Rabiee Rad M, Ghasempour Dabaghi G, Darouei B, Amani-Beni R. The association of atherogenic index of plasma with cardiovascular outcomes in patients with coronary artery disease: a systematic review and meta-analysis. Cardiovasc Diabetol. 2024;23(1):119. 10.1186/s12933-024-02198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang K, Wang X, Hu J, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol. 2024;23(1):8. 10.1186/s12933-023-02115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan SS, Matsushita K, Sang Y, et al. Development and validation of the American Heart Association’s PREVENT equations. Circulation. 2024;149(6):430–49. 10.1161/CIRCULATIONAHA.123.067626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minhas AMK, Mathew RO, Sperling LS, et al. Prevalence of the cardiovascular-kidney-metabolic syndrome in the United States. J Am Coll Cardiol. 2024;83(18):1824–6. 10.1016/j.jacc.2024.03.368. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes. Diabetes Care. 2008;31(9):1898–904. 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Wei L, He W, et al. Associations of severe liver diseases with cataract using data from UK Biobank: a prospective cohort study. eClinicalMedicine. 2024;68: 102424. 10.1016/j.eclinm.2024.102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–49. 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57. 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Zhang H, Li L, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun. 2020;40(7):301–12. 10.1002/cac2.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care. 2013;36(8):2280–5. 10.2337/dc12-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Zhao L, Lu Y, Xiao Y, Zhou X. Insulin resistance assessed by estimated glucose disposal rate and risk of incident cardiovascular diseases among individuals without diabetes: findings from a nationwide, population based, prospective cohort study. Cardiovasc Diabetol. 2024;23(1):194. 10.1186/s12933-024-02256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams KV, Erbey JR, Becker D, Orchard TJ. Improved glycemic control reduces the impact of weight gain on cardiovascular risk factors in type 1 diabetes. The epidemiology of diabetes complications study. Diabetes Care. 1999;22(7):1084–91. 10.2337/diacare.22.7.1084. [DOI] [PubMed] [Google Scholar]
- 25.Nyström T, Holzmann MJ, Eliasson B, Svensson A, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20(3):556–63. 10.1111/dom.13110. [DOI] [PubMed] [Google Scholar]
- 26.Song J, Ma R, Yin L. Associations between estimated glucose disposal rate and arterial stiffness and mortality among US adults with non-alcoholic fatty liver disease. Front Endocrinol. 2024;15:1398265. 10.3389/fendo.2024.1398265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T, Li M, Zeng T, et al. Association between insulin resistance and cardiovascular disease risk varies according to glucose tolerance status: a nationwide prospective cohort study. Diabetes Care. 2022;45(8):1863–72. 10.2337/dc22-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aroor AR, McKarns S, DeMarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62(11):1543–52. 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–67. 10.1210/er.2018-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nistala R, Whaley-Connell A. Resistance to insulin and kidney disease in the cardiorenal metabolic syndrome; role for angiotensin II. Mol Cell Endocrinol. 2013;378(1–2):53–8. 10.1016/j.mce.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng J, Zhang Y, Zhu Y, et al. Estimated glucose disposal rate for predicting cardiovascular events and mortality in patients with non-diabetic chronic kidney disease: a prospective cohort study. BMC Med. 2024;22(1):411. 10.1186/s12916-024-03582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S, Li X, Yang F, et al. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol. 2019;10:1360. 10.3389/fphar.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong H, Sun Y, Nie L, et al. Metabolic memory: mechanisms and diseases. Sig Transduct Target Ther. 2024;9(1):38. 10.1038/s41392-024-01755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bordoni L, Sawicka AK, Szarmach A, Winklewski PJ, Olek RA, Gabbianelli R. A pilot study on the effects of l-carnitine and trimethylamine-N-oxide on platelet mitochondrial DNA methylation and CVD biomarkers in aged women. IJMS. 2020;21(3):1047. 10.3390/ijms21031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valles-Colomer M, Menni C, Berry SE, Valdes AM, Spector TD, Segata N. Cardiometabolic health, diet and the gut microbiome: a meta-omics perspective. Nat Med. 2023;29(3):551–61. 10.1038/s41591-023-02260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Yang X, Wang Z, et al. Estrogen sulfotransferase (SULT1E1) regulates inflammatory response and lipid metabolism of human endothelial cells via PPARγ. Mol Cell Endocrinol. 2013;369(1–2):140–9. 10.1016/j.mce.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Gado M, Tsaousidou E, Bornstein SR, Perakakis N. Sex-based differences in insulin resistance. J Endocrinol. 2024;261(1): e230245. 10.1530/JOE-23-0245. [DOI] [PubMed] [Google Scholar]
- 38.Fang YP, Zhao Y, Huang JY, Yang X, Liu Y, Zhang XL. The functional role of cellular senescence during vascular calcification in chronic kidney disease. Front Endocrinol. 2024;15:1330942. 10.3389/fendo.2024.1330942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang Q, Wang P, Pan Y, et al. Irisin protects against vascular calcification by activating autophagy and inhibiting NLRP3-mediated vascular smooth muscle cell pyroptosis in chronic kidney disease. Cell Death Dis. 2022;13(3):283. 10.1038/s41419-022-04735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54. 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caturano A, Vetrano E, Galiero R, et al. Advances in the insulin-heart axis: current therapies and future directions. IJMS. 2024;25(18):10173. 10.3390/ijms251810173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd-Jones DM, Liu K, Colangelo LA, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation. 2007;115(8):1004–11. 10.1161/CIRCULATIONAHA.106.648642. [DOI] [PubMed] [Google Scholar]
- 43.Yamada T, Wakabayashi M, Bhalla A, et al. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol. 2021;20(1):14. 10.1186/s12933-020-01197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reduction in the incidence of Type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study were acquired from the UK Biobank Resource under application number 532333. Data can be accessed through applications on UK Biobank website (https://www.ukbiobank.ac.uk/).