Abstract
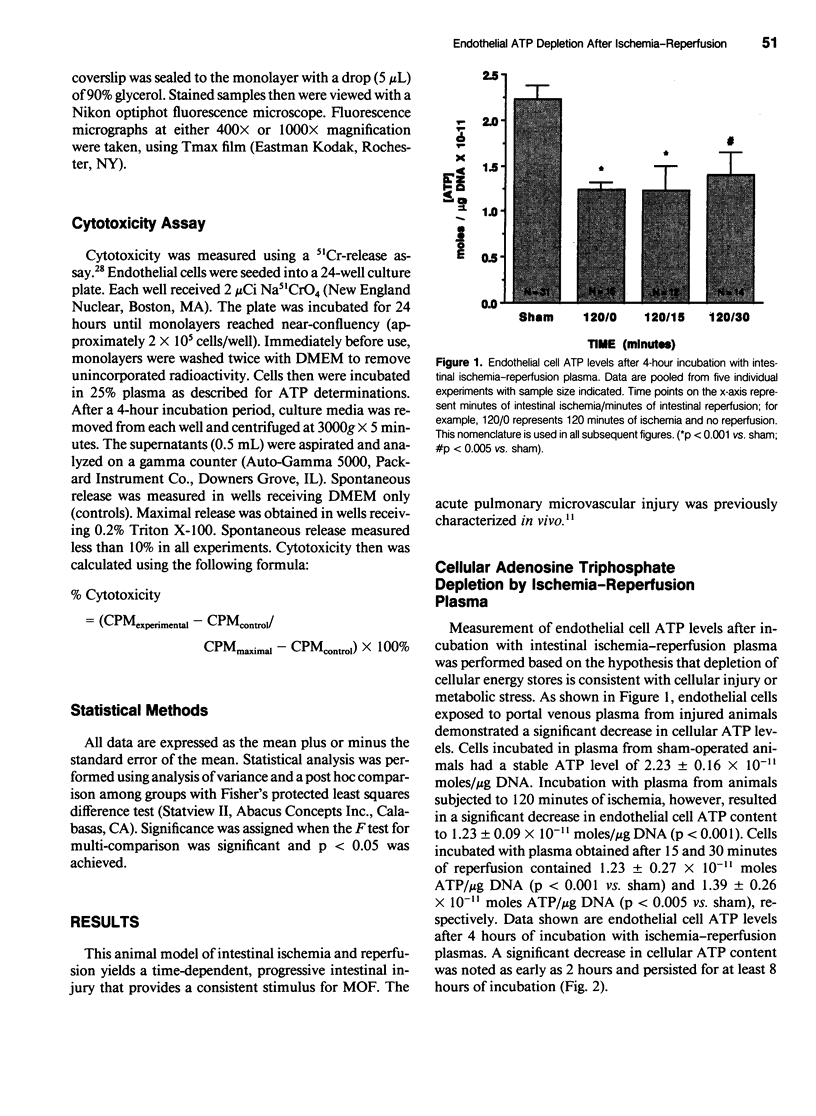
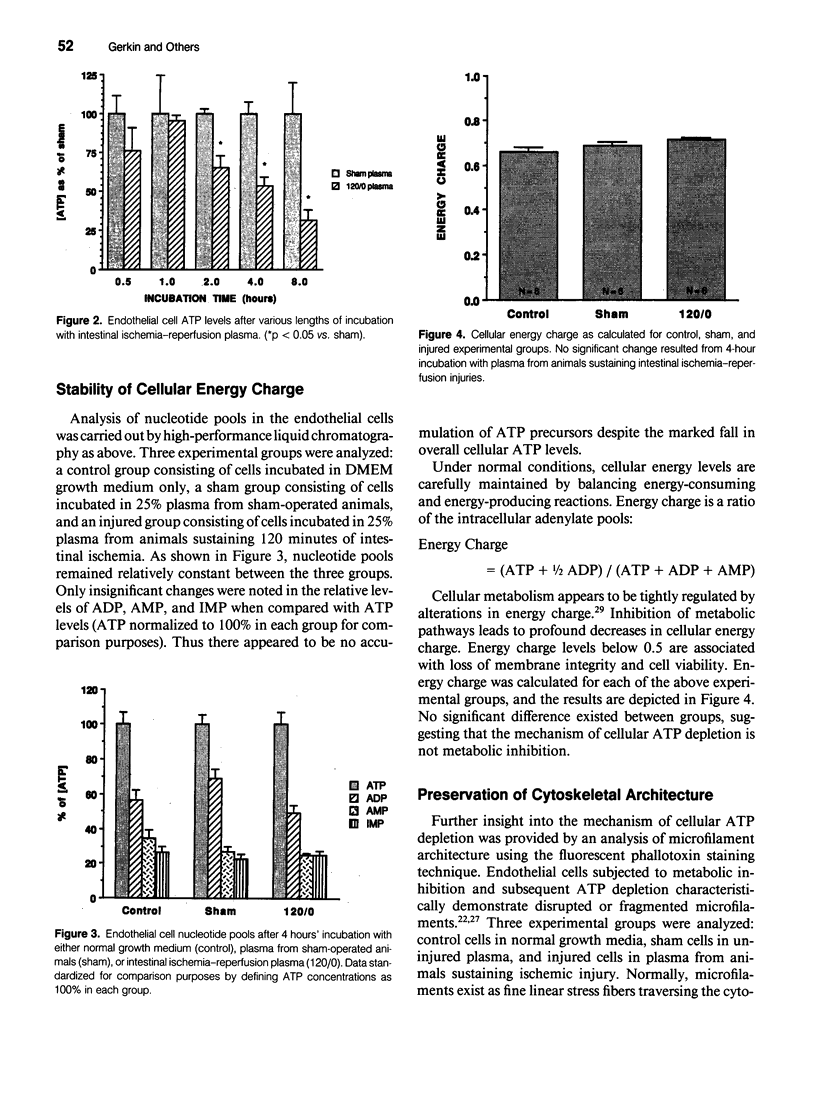
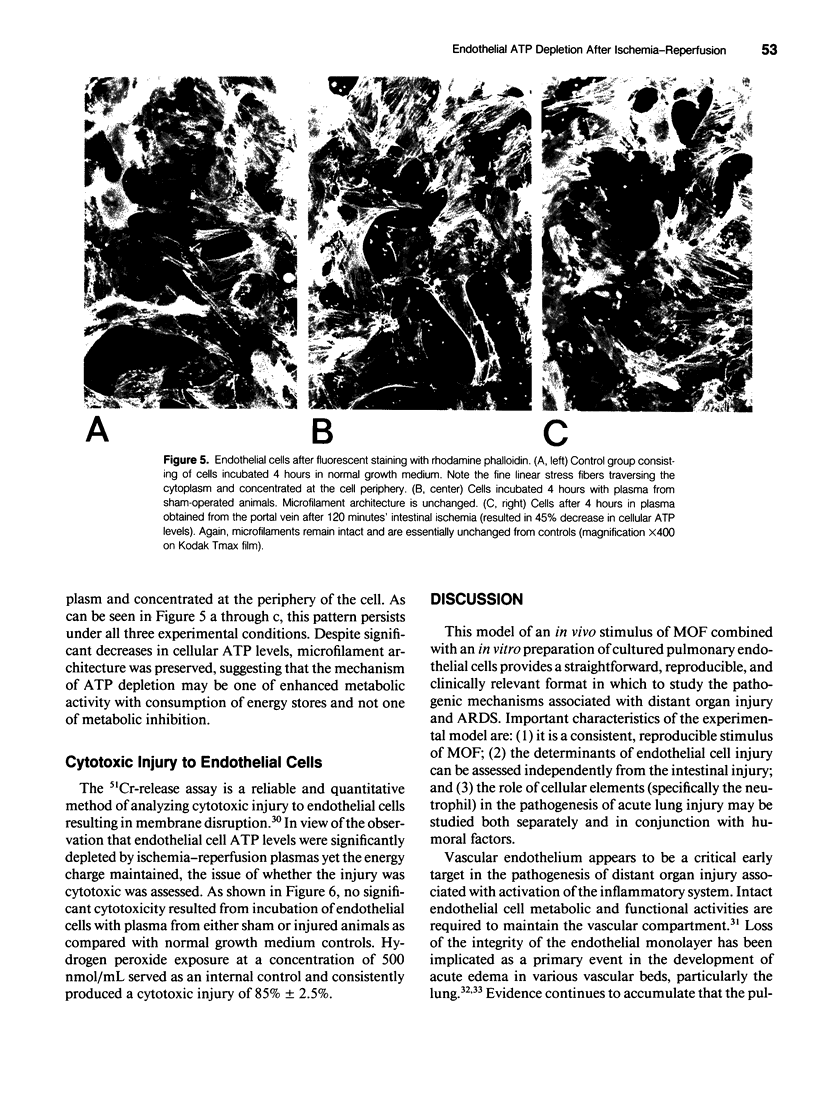
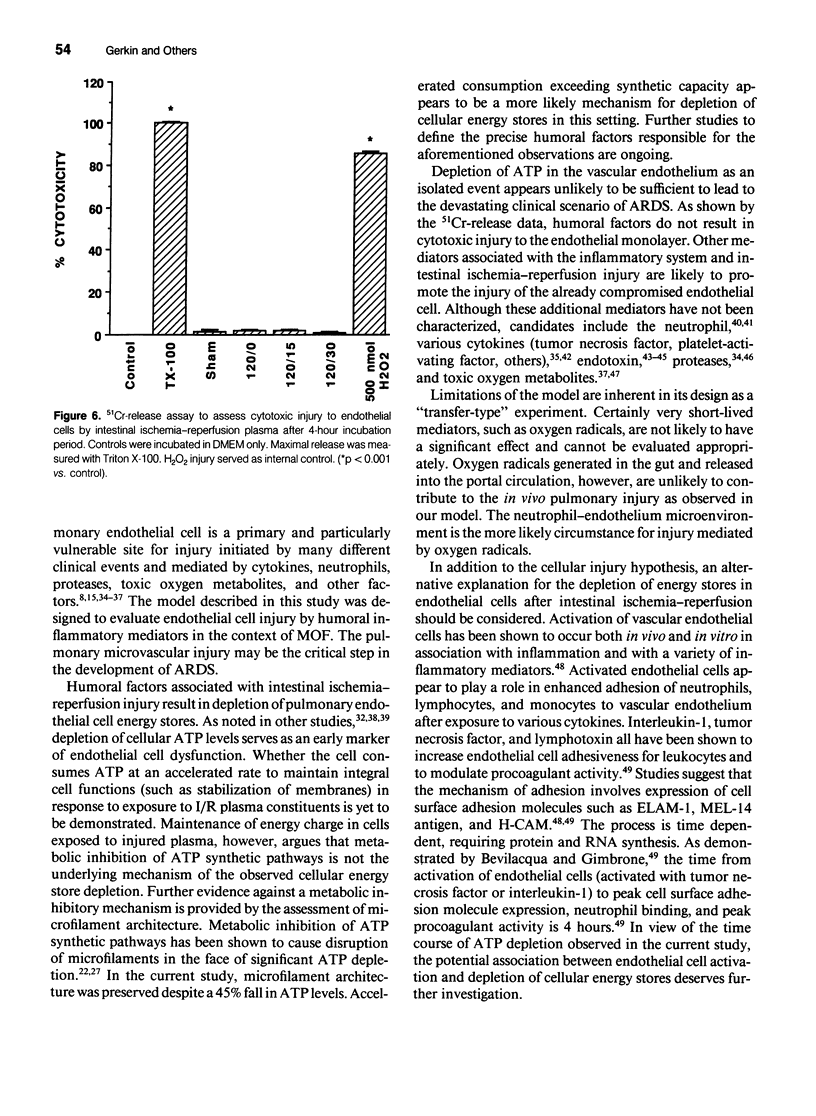
Intestinal ischemia-reperfusion is a common clinical event associated with both clinical and experimental distant organ injury. In particular, the pulmonary microvasculature appears to be susceptible to injury resulting from systemic inflammatory mediator activation. This study was designed to evaluate the hypothesis that noncellular humoral factors associated with intestinal ischemia-reperfusion result in pulmonary endothelial cell adenosine triphosphate (ATP) depletion. Male Sprague-Dawley rats had intestinal ischemia induced by microvascular clip occlusion of the superior mesenteric artery (SMA) for 120 minutes. Reperfusion resulted from superior mesenteric artery clip removal. After reperfusion for 0, 15, or 30 minutes, plasma samples were obtained from the portal vein. Monolayers of cultured rat pulmonary artery endothelial cells then were incubated with the plasma samples. Adenosine triphosphate levels were determined using a luciferin-luciferase assay. A 51Cr-release assay using labeled endothelial cells was performed under identical conditions to assess cytotoxicity. Potential mechanisms of ATP depletion were evaluated by analysis of cellular energy charge and assessment of microfilament architecture. Endothelial cell ATP levels decreased from 2.23 +/- 0.16 x 10(-11) moles/microgram DNA in sham preparations to 1.23 +/- 0.09 x 10(-11) moles/microgram DNA (p < 0.001) after 4 hours in plasma from animals undergoing 120 minutes of intestinal ischemia. For plasma obtained after 15 minutes of reperfusion, the decrease in cellular ATP concentration persisted (1.23 +/- 0.27 x 10(-11) moles/microgram DNA, p < 0.001 vs. sham). After 30 minutes' reperfusion, cellular ATP levels increased only slightly after the 4-hour incubation (1.39 +/- 0.26 x 10(-11) moles/microgram DNA, p < 0.005 vs. sham). No significant cytotoxic injury occurred in any group when compared with controls. Cellular energy charge was unchanged, and microfilament architecture was preserved. These data confirm the hypothesis that humoral factors, independent of the neutrophil, result in endothelial cell ATP depletion without metabolic inhibition or cell death. Depletion of energy stores by noncellular humoral factors may represent an early event that predisposes the cell to more severe injury by other mediators of the endogenous inflammatory response.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdalla E. K., Caty M. G., Guice K. S., Hinshaw D. B., Oldham K. T. Arterial levels of oxidized glutathione (GSSG) reflect oxidant stress in vivo. J Surg Res. 1990 Apr;48(4):291–296. doi: 10.1016/0022-4804(90)90061-6. [DOI] [PubMed] [Google Scholar]
- Andreadis N., Petty T. L. Adult respiratory distress syndrome: problems and progress. Am Rev Respir Dis. 1985 Dec;132(6):1344–1346. doi: 10.1164/arrd.1985.132.6.1344. [DOI] [PubMed] [Google Scholar]
- Bell R. C., Coalson J. J., Smith J. D., Johanson W. G., Jr Multiple organ system failure and infection in adult respiratory distress syndrome. Ann Intern Med. 1983 Sep;99(3):293–298. doi: 10.7326/0003-4819-99-3-293. [DOI] [PubMed] [Google Scholar]
- Berger N. A. Oxidant-induced cytotoxicity: a challenge for metabolic modulation. Am J Respir Cell Mol Biol. 1991 Jan;4(1):1–3. doi: 10.1165/ajrcmb/4.1.1. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Gimbrone M. A., Jr Inducible endothelial functions in inflammation and coagulation. Semin Thromb Hemost. 1987 Oct;13(4):425–433. doi: 10.1055/s-2007-1003519. [DOI] [PubMed] [Google Scholar]
- Demling R. H. Current concepts on the adult respiratory distress syndrome. Circ Shock. 1990 Apr;30(4):297–309. [PubMed] [Google Scholar]
- Demling R. H., Niehaus G., Will J. A. Pulmonary microvascular response to hemorrhagic shock, resuscitation, and recovery. J Appl Physiol Respir Environ Exerc Physiol. 1979 Mar;46(3):498–503. doi: 10.1152/jappl.1979.46.3.498. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Priest B. P., Condie R. M. Protective capacity of polyclonal and monoclonal antibodies directed against endotoxin during experimental sepsis. Arch Surg. 1988 Nov;123(11):1389–1393. doi: 10.1001/archsurg.1988.01400350103016. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Goris R. J., te Boekhorst T. P., Nuytinck J. K., Gimbrère J. S. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985 Oct;120(10):1109–1115. doi: 10.1001/archsurg.1985.01390340007001. [DOI] [PubMed] [Google Scholar]
- Grosso M. A., Brown J. M., Viders D. E., Mulvin D. W., Banerjee A., Velasco S. E., Repine J. E., Harken A. H. Xanthine oxidase-derived oxygen radicals induce pulmonary edema via direct endothelial cell injury. J Surg Res. 1989 Apr;46(4):355–360. doi: 10.1016/0022-4804(89)90201-1. [DOI] [PubMed] [Google Scholar]
- Guice K. S., Oldham K. T., Caty M. G., Johnson K. J., Ward P. A. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg. 1989 Dec;210(6):740–747. doi: 10.1097/00000658-198912000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guice K. S., Oldham K. T., Johnson K. J., Kunkel R. G., Morganroth M. L., Ward P. A. Pancreatitis-induced acute lung injury. An ARDS model. Ann Surg. 1988 Jul;208(1):71–77. doi: 10.1097/00000658-198807000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch M., Gnidec A. A., Bersten A. D., Troster M., Rutledge F. S., Sibbald W. J. Histologic and ultrastructural changes in nonpulmonary organs during early hyperdynamic sepsis. Surgery. 1990 Apr;107(4):397–410. [PubMed] [Google Scholar]
- Hinshaw D. B., Armstrong B. C., Burger J. M., Beals T. F., Hyslop P. A. ATP and microfilaments in cellular oxidant injury. Am J Pathol. 1988 Sep;132(3):479–488. [PMC free article] [PubMed] [Google Scholar]
- Hinshaw D. B., Burger J. M., Armstrong B. C., Hyslop P. A. Mechanism of endothelial cell shape change in oxidant injury. J Surg Res. 1989 Apr;46(4):339–349. doi: 10.1016/0022-4804(89)90199-6. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Sklar L. A., Bohl B., Schraufstatter I. U., Hyslop P. A., Rossi M. W., Spragg R. G., Cochrane C. G. Cytoskeletal and morphologic impact of cellular oxidant injury. Am J Pathol. 1986 Jun;123(3):454–464. [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Robkin L. Hydrogen peroxide lowers ATP levels in platelets without altering adenyalte energy charge and platelet function. J Biol Chem. 1977 Mar 10;252(5):1752–1757. [PubMed] [Google Scholar]
- Horgan M. J., Wright S. D., Malik A. B. Antibody against leukocyte integrin (CD18) prevents reperfusion-induced lung vascular injury. Am J Physiol. 1990 Oct;259(4 Pt 1):L315–L319. doi: 10.1152/ajplung.1990.259.4.L315. [DOI] [PubMed] [Google Scholar]
- Hsueh W., González-Crussi F., Arroyave J. L. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987 Nov;1(5):403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- Jutila M. A., Berg E. L., Kishimoto T. K., Picker L. J., Bargatze R. F., Bishop D. K., Orosz C. G., Wu N. W., Butcher E. C. Inflammation-induced endothelial cell adhesion to lymphocytes, neutrophils, and monocytes. Role of homing receptors and other adhesion molecules. Transplantation. 1989 Nov;48(5):727–731. doi: 10.1097/00007890-198911000-00001. [DOI] [PubMed] [Google Scholar]
- Klausner J. M., Paterson I. S., Kobzik L., Valeri C. R., Shepro D., Hechtman H. B. Leukotrienes but not complement mediate limb ischemia-induced lung injury. Ann Surg. 1989 Apr;209(4):462–470. doi: 10.1097/00000658-198904000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- LeRoy E. C., Ager A., Gordon J. L. Effects of neutrophil elastase and other proteases on porcine aortic endothelial prostaglandin I2 production, adenine nucleotide release, and responses to vasoactive agents. J Clin Invest. 1984 Sep;74(3):1003–1010. doi: 10.1172/JCI111467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral J. L., Dunn D. L. Cross-reactive murine monoclonal antibodies directed against the core/lipid A region of endotoxin inhibit production of tumor necrosis factor. J Surg Res. 1990 Oct;49(4):287–292. doi: 10.1016/0022-4804(90)90022-t. [DOI] [PubMed] [Google Scholar]
- Oldham K. T., Guice K. S., Till G. O., Ward P. A. Activation of complement by hydroxyl radical in thermal injury. Surgery. 1988 Aug;104(2):272–279. [PubMed] [Google Scholar]
- Otamiri T. Oxygen radicals, lipid peroxidation, and neutrophil infiltration after small-intestinal ischemia and reperfusion. Surgery. 1989 May;105(5):593–597. [PubMed] [Google Scholar]
- Rivkind A. I., Siegel J. H., Guadalupi P., Littleton M. Sequential patterns of eicosanoid, platelet, and neutrophil interactions in the evolution of the fulminant post-traumatic adult respiratory distress syndrome. Ann Surg. 1989 Sep;210(3):355–373. doi: 10.1097/00000658-198909000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Clements E., Habliston D., Ryan J. W. Isolation and culture of pulmonary artery endothelial cells. Tissue Cell. 1978;10(3):535–554. doi: 10.1016/s0040-8166(16)30347-0. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeling D. J., Caty M. G., Oldham K. T., Guice K. S., Hinshaw D. B. Evidence for neutrophil-related acute lung injury after intestinal ischemia-reperfusion. Surgery. 1989 Aug;106(2):195–202. [PubMed] [Google Scholar]
- Schraufstätter I. U., Revak S. D., Cochrane C. G. Proteases and oxidants in experimental pulmonary inflammatory injury. J Clin Invest. 1984 Apr;73(4):1175–1184. doi: 10.1172/JCI111303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffel U., Shiga J., Mittermayer C. The proliferation-inhibiting effect of endotoxin on human endothelial cells in culture and its possible implication in states of shock. Circ Shock. 1982;9(5):499–508. [PubMed] [Google Scholar]
- Shryock J. C., Rubio R., Berne R. M. Extraction of adenine nucleotides from cultured endothelial cells. Anal Biochem. 1986 Nov 15;159(1):73–81. doi: 10.1016/0003-2697(86)90309-x. [DOI] [PubMed] [Google Scholar]
- Spragg R. G., Hinshaw D. B., Hyslop P. A., Schraufstätter I. U., Cochrane C. G. Alterations in adenosine triphosphate and energy charge in cultured endothelial and P388D1 cells after oxidant injury. J Clin Invest. 1985 Oct;76(4):1471–1476. doi: 10.1172/JCI112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkanat K. K., Fox I. H. Isocratic separation of ATP and its degradation products from biological fluids by automated liquid chromatography. Clin Chem. 1988 May;34(5):925–932. [PubMed] [Google Scholar]
- Till G. O., Beauchamp C., Menapace D., Tourtellotte W., Jr, Kunkel R., Johnson K. J., Ward P. A. Oxygen radical dependent lung damage following thermal injury of rat skin. J Trauma. 1983 Apr;23(4):269–277. doi: 10.1097/00005373-198304000-00001. [DOI] [PubMed] [Google Scholar]
- Varani J., Bendelow M. J., Sealey D. E., Kunkel S. L., Gannon D. E., Ryan U. S., Ward P. A. Tumor necrosis factor enhances susceptibility of vascular endothelial cells to neutrophil-mediated killing. Lab Invest. 1988 Aug;59(2):292–295. [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Till G. O., Kunkel R. G., Ryan U. S., Ward P. A. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985 Dec;53(6):656–663. [PubMed] [Google Scholar]
- Vedder N. B., Fouty B. W., Winn R. K., Harlan J. M., Rice C. L. Role of neutrophils in generalized reperfusion injury associated with resuscitation from shock. Surgery. 1989 Sep;106(3):509–516. [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski R. B., Lagunoff D. Inhibition of endothelial cell retraction by ATP depletion. Am J Pathol. 1988 Jul;132(1):28–37. [PMC free article] [PubMed] [Google Scholar]



