Abstract
A fundamental requirement for a molecule to be considered a molecular wire (MW) is the ability to transport electrical charge with a reasonably low resistance. We have carried out two experiments that measure first, the charge transfer from an electrode to the molecule, and second, the dielectric response of the MW. The latter experiment requires no contacts to either end of the molecule. From our experiments we conclude that adsorbed individual DNA molecules have a resistivity similar to mica, glass, and silicon oxide substrates. Therefore adsorbed DNA is not a conductor, and it should not be considered as a viable candidate for MW applications. Parallel studies on other nanowires, including single-walled carbon nanotubes, showed conductivity as expected.
Electrical transport through molecular wires (MWs) has been the subject of intense interest the last few years (1–4). MWs are expected to present conductive properties considerably different from those of bulk conductors (5). In addition, conductive molecules could be used to interconnect different parts of a circuit in nanoscale devices, playing an important role in future electronics (6). Among the different molecule candidates for MWs, carbon nanotubes and DNA have been the subject of a number of studies in the last few years, which dealt with the transport properties of these two molecules (7, 8).
Carbon nanotubes (9) are derived from a curved graphene sheet and, depending on the rolling direction of this sheet, the current versus voltage, I/V characteristics evolve from linear (without gap) to a semiconductor type, with a clear gap near 0 V (7). Although the precise mechanism for electron transport in nanotubes remains unclear (3, 10–12), there is general agreement on the possibility of carbon nanotubes being MWs.
The current situation of the conducting properties of DNA is less clearly resolved. Depending on the study, DNA is a good conductor with linear I/V characteristics (13), a conductor with a gap that gets wider with temperature (14), a fairly good insulator (15–17), or even a superconductor with very low resistance at room temperature (18). In addition, it has even been proposed that DNA can be doped from a semiconducting molecule to a metallic conductor with linear I/Vs (19). In summary, published claims on DNA electrical conductivity span over 10 orders of magnitude!
The simplest circuit to measure the resistance of a given object is likely to require a battery, a current meter, and two contacts to the object. In the macroscopic world, the technology to perform electrical contacts is well established. In the nanoscopic world making reliable contacts is a nontrivial problem. In ref. 17 a circuit similar to the one described above was used in an attempt to measure the resistance of DNA molecules. In that experiment, the DNA molecule was partially covered by a macroscopic gold electrode while the second electrode was a metallic scanning force microscopy (SFM) tip. The large resistance between the two contacts measured in that work was attributed to the high resistance of the DNA molecule. Since that was a two-terminal measurement, it is possible to argue that the large resistance was caused by unreliable electrical contacts.
In the present work we perform a systematic study on the electrical properties of individual double-stranded DNA molecules. The basic idea of this study is to minimize the effects of the electrical contacts on the measurements, aiming at the intrinsic electronic properties of the DNA molecules. Accordingly, the experiments described below avoid mechanical contact of the SFM tip with the sample. The electrical properties of the molecules studied are inferred from the electrostatic force between tip and sample, which is a contactless measurement. We have performed three different experiments. In the first two experiments, one end of the DNA molecules is directly connected (i) to a gold electrode and (ii) to a single-walled carbon nanotube (SWNT) that itself is connected to a macroscopic gold electrode. In the third experiment, the DNA molecules are simply adsorbed on an insulating substrate with no contact to any conducting object. From the measured electrostatic forces induced between tip and sample in all three set-ups we conclude that the DNA molecules do not have any significant DC conductivity.
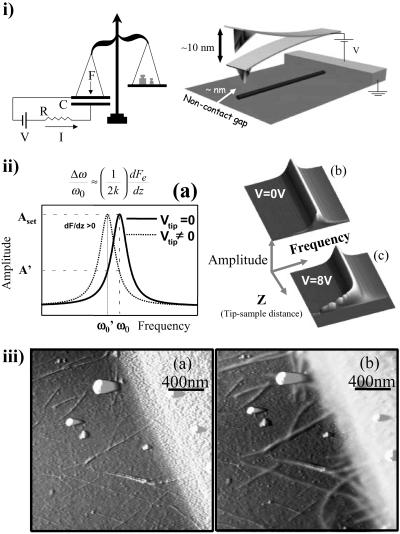
The physical principles involved in the experiments are related to the electrostatic forces between metallic and dielectric objects. These are determined by the geometry and the density of mobile and bound charges. Electrical transport properties are closely related to the density of mobile charges and the ability of these charges to move. The two first experiments are realized by applying a potential difference between a molecule and a SFM tip by using a battery (Fig. 1i). From an electrostatic point of view, any MW can be modeled as a resistance and a capacitance. In this situation, charge passes through the resistor, charging the capacitor. The amount of charge in the capacitor can be measured by attaching a force detector to the upper plate of the capacitor (Fig. 1i). On a nanoscopic scale an SFM can be used as a force detector (20–23). To measure this force, an SFM metallic tip is placed over the molecule. The tip is oscillated at its resonance frequency ω0, and the amplitude of the oscillation is monitored and kept constant. The electrostatic force gradient between the tip and the molecule induces a resonance frequency shift as is seen in the experimental data shown in Fig. 1ii. If no frequency shift is observed over the molecule we conclude that no charge has passed through the molecule and therefore it is an insulator.
Figure 1.
The nonintrusive method used in this work is illustrated. (i) The MW, characterized by a resistance (R) and a capacitance (C), is connected to a metal electrode. A SFM metallic tip of force constant k is placed over the molecule. The tip is oscillated at its resonance frequency ω0, and the amplitude of the oscillation is monitored and kept constant. When a voltage V is applied between the tip and the sample the molecule gets charged, and an attractive force appears between the tip and the sample. The RC factor of the MW determines the time required for the molecule to be charged. The experiment can be seen as a balance (force sensor), a battery, a resistor R, and a capacitance C. When the circuit is closed, electrical charges are accumulated at the plates, giving rise to an electrostatic force that is detected by the balance. The gradient of the electrostatic force introduces a shift of the resonance frequency to lower values. (iia) This frequency shift is shown. (iib) The oscillation amplitude A is measured as a function of the frequency ω and the tip-sample distance z A(ω,z), since no voltage is applied between the tip and metallic sample, no frequency shift caused by long-range interaction is observed. (iic) A bias voltage of 8 V is applied, producing a clear frequency shift. The total z displacement in these images is 200 nm. (iiia) SFM topography showing randomly dispersed SWNT on a silicon oxide substrate where some of the molecules appear partially covered by a gold electrode. (iiib) SFM topography showing the same area as in iiia but with a tip-sample bias voltage of +2 V. Notice that those SWNTs electrically connected to the metal electrode appear wider and higher because of the electrostatic interaction.
To understand the third experiment we should consider the case of an object placed in an external electric field. Charges will then be induced in proportion to the dielectric constant ɛ (in a metal ɛ can be considered to be very high) through polarization. The force produced near a polarized material, although smaller than in the case of a metal object, can also be measured with SFM (24). Bare molecules adsorbed on an insulating substrate will modify the electrostatic force between tip and substrate. In principle the electronic properties of the molecules can be determined from a precise measurement of this force. Since a quantitative determination of the electrostatic force is very difficult for a complex system such as SFM, in the present work we have performed a comparative measurement of DNA molecules with two other materials of known electronic properties: SWNTs and isolating substrates such as mica, glass, and silicon oxide.
Samples were prepared in a two-step process. The first step is the adsorption of a random population of molecules on an insulating substrate.‡ The sample is then inspected by SFM to check the density of adsorbed molecules. The second step, which is not always required, is the evaporation of a metal electrode. Along the electrode edge a number of molecules are partially covered by metal. In the present work we will focus on two different kinds of samples: SWNTs on SiO2 and SWNTs and DNA molecules coadsorbed on a variety of insulating substrates.
As a test, we measured the electrostatic response of a sample with SWNTs. Fig. 1iiia is an SFM topographic image showing the edge of a gold electrode and SWNTs randomly dispersed on the SiO2 substrate.§ A number of SWNTs are in direct contact with the gold electrode. When a biased metal tip is driven to contact with one of those molecules, an electric current passing between the SFM tip and the gold electrode through the SWNT is detected (25). Fig. 1iiib is an SFM topographic image of the same area shown in Fig. 1iiia but a bias voltage of 2 V is applied to the SFM tip.¶ We note that during acquisition of both images (Fig. 1 iiia and iiib) the tip is not driven to contact with the nanotube. The connected nanotubes appear wider and higher in Fig. 1iiib than in Fig. 1iiia. This is caused by the electrostatic force present between the tip and the conducting SWNTs. As the voltage is reduced the apparent width and height of the connected SWNTs decrease.
When the SFM cantilever, driven at its free resonance frequency, feels the electrostatic interaction of the molecule, the resonance frequency of the system shifts to smaller values, the oscillation amplitude decreases (see Fig. 1ii), and the SFM feedback withdraws the tip to recover the set point amplitude. For a more detailed discussion of this effect see ref. 26. Therefore, the biased SWNTs appear higher and wider in the topographic images. An important feature seen in Fig. 1iiib is that not only the SWNTs directly connected to the gold electrode show this effect, but those nanotubes connected via a second or even a third nanotube also present this electrostatic contrast. Thus, the experiment shown in Fig. 1iiib is able to detect a network of SWNTs electrically connected to the gold electrode (26).
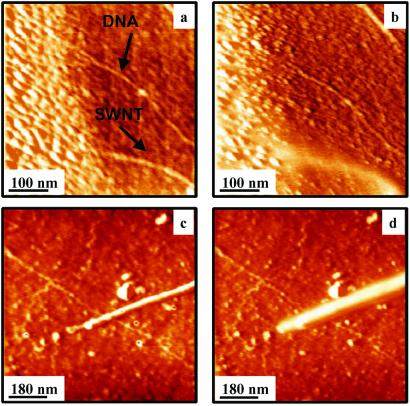
To compare the electrical properties of DNA and SWNTs, both molecules were coadsorbed on an isolating substrate.‡ Based on the above result, DNA molecules should also present electrostatic contrast if they are conducting. Fig. 2a shows an SFM topographic image of a mica substrate with a SWNT and a DNA molecule partially covered by the gold electrode. Fig. 2b is an image of the same area shown in Fig. 2a but with a bias voltage of +1.6 V applied between tip and electrode. Notice that while the SWNT appears higher and wider because of the presence of the electrostatic interaction, the DNA molecule remains unmodified. Therefore, no charges have passed from the gold electrode to the DNA, implying that DC conductivity in DNA is negligibly small. As a double-check experiment, the tip was driven into mechanical contact with the DNA. No current was detected above the noise level (1 pA) in good agreement with the results of ref. 17.
Figure 2.
(a) SFM topography showing a SWNT and a DNA molecule on a mica substrate. Both molecules are in clear contact with the gold electrode. (b) SFM topography showing the same area as in a but with a bias voltage of +1.6 V applied between the tip and the sample. While the effect of the bias voltage can be clearly seen on the gold electrode and the nanotube, the DNA molecule is not affected by the electrostatic interaction. (c) The SFM topographic image shows a DNA molecule on a glass substrate at zero bias voltage in contact with a SWNT. (d) Same as in c but with a voltage of +3 V between the tip and the sample. While the effect of the electrostatic interaction is clearly seen in the nanotubes, the contrast of DNA molecule does not change. To enhance the edges a high past filter has been performed on the images.
It could be argued that the connection to the DNA molecule by means of an evaporated macroscopic electrode could modify its properties. Therefore, in a further experiment, a more gentle electrical contact to the DNA was established through a SWNT. Fig. 2c shows a DNA molecule connected to a carbon nanotube in contact with a macroscopic gold electrode (not shown). Fig. 2d is a topographic image of the same area but with a tip-sample bias voltage of +3 V. As expected the SWNT is affected by the bias in the same way as in Fig. 1. On the contrary, the DNA molecule remains unmodified. The latter result can be contrasted with the experiments reported in Fig. 1iiib where several nanotubes, connected to the gold electrode via an intermediate SWNT, show electrostatic contrast.
Electrostatics deals with a system of charges in equilibrium. In a conductor electrostatic equilibrium is reached well below the typical time for SFM image acquisition (≈100 s). Hence in the SWNT case, we are measuring a system in electrostatic equilibrium since the charges have passed through SWNT molecules until their electrostatic potential has become equal to the one of the electrode. If a molecule with poor, but nonzero conductivity is adsorbed on a perfect insulator the time required to reach the electrostatic equilibrium with the electrode can be, in principle, very large. In this case, SFM images taken at a fixed bias would show molecules that begin to present electrostatic contrast as the charge slowly passes through them. Eventually, electrostatic equilibrium will be reached and the molecule will present an electrostatic contrast in the SFM image as observed for SWNTs. Therefore, electrostatic interaction can be used to measure extremely small currents since the time to reach electrostatic equilibrium can be very long. We have never observed this charging effect on DNA, even when a bias was applied to the electrode overnight. The interpretation of this result is that the insulating substrate has a high, but finite, resistance. Charges pass along the surface of the insulator into the volume and into the ground or surrounding atmosphere. Since we see no electrostatic contrast between the DNA and the insulating substrate, we conclude that the DNA molecules must have a resistance comparable to that of the substrate. In the case of glass (one of the substrates used) a lower bound for its resistivity is 1012 Ω⋅cm.
We have confirmed the above reported results in many additional experiments testing different substrates (mica, glass, silicon oxide) and metal electrodes (gold, silver, chromium). All of these experiments confirmed the results described above: electrostatic contrast in the case of SWNT but not in the case of DNA molecules. If poly(C)–poly(G) is used instead of λ DNA again a negative result is obtained. Therefore, this experiment is in disagreement with the one reported by Cai et al. (27). To test the influence of high-resistance nanowires, electrostatic images of vanadium pentoxide fibers (10 nm width, 1.5 nm height) were adsorbed on SiO2. V2O5 is a semiconductor MW with a high resistivity (0.5 Ω⋅cm, 500 times larger than the average values found in SWNTs) (28). When these molecules are used, a clear and marked contrast is obtained as the bias voltage is increased. This experiment suggests that the electrostatic method proposed in this work is a general tool, which can be applied to test nonzero conductivity of any MW.
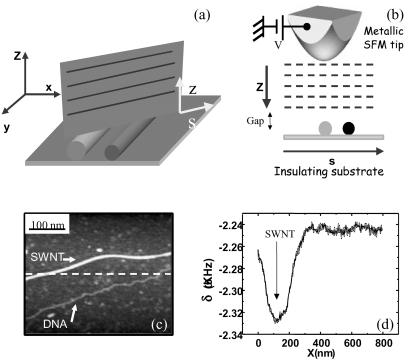
We have further confirmed our results by carrying out a final experiment without any electrodes, avoiding any problem caused by electrical contacts. This experiment is shown in Fig. 3. Fig. 3c shows an SFM topographic image of a DNA molecule parallel to a SWNT (upper molecule). Both molecules are not connected to any electrode. After this topographic image, the tip is lifted 100 nm above the mica substrate while oscillating at the free-resonance frequency (Fig. 3a). A bias voltage of +6 V is then applied to the metal-covered tip. As the tip approaches the surface while scanning over the SWNT and the DNA molecule (Fig. 3b) a monotonic decrease of the resonance frequency δω is observed because of the electrostatic force produced by the polarization of the insulating substrate (29). Fig. 3d shows a frequency-shift profile taken along the dashed line drawn in Fig. 3c, with the tip positioned at 10 nm above the sample. Whereas a remarkable decrement of the resonance frequency appears at the nanotube position, no signal, above our noise level, is detected when the tip is over the DNA molecule. Since the electrostatic force between tip and sample is basically produced by the effect of the polarization induced by the applied bias, the absence of a signal over the DNA molecule implies that the dielectric constant of the DNA and that of the substrate are similar. No significant contrast was detected over the DNA molecule at any bias voltage between ±10 V. However, the SWNT is a good conductor and hence its dielectric constant is very high. Thus, it presents a marked electrostatic signal with respect to the insulating substrate. If the experiment is repeated on a glass substrate a similar behavior is observed. This result is especially significant since it does not depend on any contact with the electrodes.
Figure 3.
(a and b) A scheme of the contactless experiment performed on two different molecules coadsorbed on an insulating substrate. Frequency-shift profiles are taken at different tip-sample distances. In the experiment, the SFM tip never establishes contact with the adsorbed molecules. (c) SFM topographic image showing a SWNT and a DNA molecule adsorbed on mica. (d) This graph shows a frequency-shift profile along the dashed line drawn in c. A clear frequency shift is observed above the SWNT, whereas no measurable change appears above the DNA molecule. This profile was taken with the tip placed at 10 nm above the surface. A phase-locked loop was used to keep the system at resonance. No feedback was applied on the oscillation amplitude.
Our experiments agree with a number of previous scanning tunneling microscopy (STM) experiments on DNA. Imaging DNA adsorbed on a surface has been always difficult because of the poor conductivity of the molecule (30–32). Guckenberger et al. (15) have obtained STM images of DNA adsorbed on mica. Their experiments were interpreted as resulting from the presence of a water layer adsorbed on the substrate, which carried H+ charges. Our experiments strongly disagree with the results of refs. 18 and 27 since our findings suggest a resistance similar to the resistance of the insulating substrates. DNA could still support AC conductivity as suggested in ref. 33, but according to our results DC conductivity in adsorbed DNA molecules must be ruled out. First, principle calculations performed in poly(C)–poly(G) DNA shows a band structure typical of a semiconductor (17), but in the λ DNA case the disorder introduced by the random base pair sequence entirely destroys the band structure. Electrical transport caused by polarons has been put forward as a second mechanism for DNA conductivity (34). Since polarons are associated with phonons, it is expected that adsorbed DNA molecules have a reduced degree of freedom, which significantly suppresses the phonon modes. Therefore, one would expect a decrease in the conductivity based on polaronic effects. Moreover, the structure of adsorbed DNA might also present distortions because of the molecule-substrate force that could modify its transport properties.
Our result is just another piece of evidence of an intriguing puzzle. To clarify the situation further, more theoretical and experimental work is required. In particular, precise calculations of the charge transfer rate caused by polarons for both free-standing and adsorbed DNA molecules would be particularly interesting. A recent theoretical work (35) shows that diffuse electrical transport at low rates only occurs in DNA in aqueous solution. This theoretical calculation together with our experimental results provide compelling evidence that shows that adsorbed DNA molecules do not exhibit MW behavior.
Acknowledgments
We thank S. Gómez-Moñivas, J. J. Sáenz, J. M. Soler, and R. Reifenberger for helpful discussions; M. T. Martinez, A. M. Benito, and W. K. Maser for kindly providing the solution with the SWNT; and M. Burghard for kindly providing the vanadium pentoxide fibers sample. We also acknowledge support from Ministerio de Educación y Cultura through Dirección General de Enseñanza Superior e Investigación Científica Projects Nos. BFM2001-0209 and MAT2001-0664. F.M.-H. is supported by a scholarship from Comunidad de Madrid.
Abbreviations
- MW
molecular wire
- SFM
scanning force microscopy
- SWNT
single-walled carbon nanotube
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Sample preparation. Clean substrates were pretreated with 3-aminopropyltriethoxysilane (APTES) by immersing them in a 0.1% volume of APTES for 15 min. Then, they were rinsed with 2-propanol and ultrapure water and dried with nitrogen. Substrates prepared this way are positively charged. A mixture of λ DNA and SWNT was dropped over the substrates and allowed to bind for 1 h. Then, it was washed with water and dried. Concentrations of DNA and SWNT were adjusted by SFM inspection until a final concentration of about one λ DNA molecule and 5–10 SWNT per micron square was obtained.
SFM imaging. The experiments were carried out by using a SFM working in noncontact dynamic mode. Olympus-type cantilevers with a resonance frequency of 80 kHz were consecutively covered with 20 nm of titanium and 20 nm of gold. Topography images were taken by using a dynamic force microscope with a free oscillating amplitude of 15 nm, and feedback was set at an amplitude slightly lower than this value (reduction of about 10%). The scan frequency was 1 Hz.
A similar effect was observed for negative voltages.
References
- 1.Datta S, Tian W, Hong S, Reifenberger R, Henderson J I, Kubiack C P. Phys Rev Lett. 1997;79:2530–2533. [Google Scholar]
- 2.Kondo Y, Takayanagi K. Phys Rev Lett. 1997;79:3455–3458. [Google Scholar]
- 3.Frank S, Poncharal P, Wang Z L, de Heer W A. Science. 1998;280:1744–1746. doi: 10.1126/science.280.5370.1744. [DOI] [PubMed] [Google Scholar]
- 4.Ohnishi H, Kondo Y, Takayanagi K. Nature (London) 1998;395:780–783. [Google Scholar]
- 5.Datta S. Electronic Transport in Mesoscopic Systems. Cambridge, U.K.: Cambridge Univ. Press; 1997. [Google Scholar]
- 6.Jortner J, Ratner M. Molecular Electronics. Oxford: Blackwell; 1997. [Google Scholar]
- 7.Saito R, Dresselhaus M S, Dresselhaus G. Physical Properties of Carbon Nanotubes. London: Imperial College Press; 1998. [Google Scholar]
- 8.Giese B, Amaudrut J, Kohler A K, Spormann M, Wessely S. Nature (London) 2001;412:318–320. doi: 10.1038/35085542. [DOI] [PubMed] [Google Scholar]
- 9.Iijima S. Nature (London) 1991;354:56–58. [Google Scholar]
- 10.Batchold A, Strunk C, Salvetat J-P, Bonard J-M, Forro L, Nussbaumer T, Schonenberger C. Nature (London) 1999;397:673–675. [Google Scholar]
- 11.Tans S J, Devoret M H, Dai H, Thess A, Smalley R E, Geerligs L J, Dekker C. Nature (London) 1997;386:474–477. [Google Scholar]
- 12.Kasumov A Y, Deblock R, Kociak M, Reulet B, Bouchiat H, Khodos I I, II, Gorbatov Y B, Volkov V T, Journet C, Burghard M. Science. 1999;284:1508–1511. doi: 10.1126/science.284.5419.1508. [DOI] [PubMed] [Google Scholar]
- 13.Fink H W, Schonenberger C. Nature (London) 1999;398:407–410. doi: 10.1038/18855. [DOI] [PubMed] [Google Scholar]
- 14.Porath D, Bezryadin A, de Vries S, Dekker C. Nature (London) 2000;403:635–638. doi: 10.1038/35001029. [DOI] [PubMed] [Google Scholar]
- 15.Guckenberger R, Heim M, Cevc G, Knapp H F, Wiegrabe W, Hillebrand A. Science. 1994;266:1538–1540. doi: 10.1126/science.7985024. [DOI] [PubMed] [Google Scholar]
- 16.Braun E, Eichen Y, Sivan U, Ben-Yoseph G. Nature (London) 1998;391:775–778. doi: 10.1038/35826. [DOI] [PubMed] [Google Scholar]
- 17.de Pablo P J, Moreno-Herrero F, Colchero J, Gómez-Herrero J, Herrero P, Baro A M, Ordejon P, Soler J M, Artacho E. Phys Rev Lett. 2000;85:4992–4995. doi: 10.1103/PhysRevLett.85.4992. [DOI] [PubMed] [Google Scholar]
- 18.Kasumov A Y, Kociak M, Gueron S, Reulet B, Volkov V T, Klinov D V, Bouchiat H. Science. 2001;291:280–282. doi: 10.1126/science.291.5502.280. [DOI] [PubMed] [Google Scholar]
- 19.Rakitin A, Aich P, Papadopoulos C, Kobzar Y, Vedeneev A S, Lee J S, Xu J M. Phys Rev Lett. 2001;86:3670–3673. doi: 10.1103/PhysRevLett.86.3670. [DOI] [PubMed] [Google Scholar]
- 20.Terris B D, Stern J E, Rugar D, Mamin H J. Phys Rev Lett. 1989;63:2662–2672. doi: 10.1103/PhysRevLett.63.2669. [DOI] [PubMed] [Google Scholar]
- 21.Schoenenberger C, Alvarado S F. Phys Rev Lett. 1990;65:3162–3164. doi: 10.1103/PhysRevLett.65.3162. [DOI] [PubMed] [Google Scholar]
- 22.Hao H W, Baro A M, Saenz J J. J Vac Sci Technol B. 1991;9:1323–1329. [Google Scholar]
- 23.Hu J, Xiao X D, Salmeron M. Appl Phys Lett. 1995;67:476–478. [Google Scholar]
- 24.Hu J, Xiao X D, Ogeltree D F, Salmeron M. Science. 1995;268:267–271. doi: 10.1126/science.268.5208.267. [DOI] [PubMed] [Google Scholar]
- 25.de Pablo P J, Martinez M T, Colchero J, Gómez-Herrero J, Maser W K, Benito A M, Muñoz E, Baro A M. Adv Materials. 2000;12:573–576. [Google Scholar]
- 26.de Pablo P J, Gómez-Navarro C, Gil A, Colchero J, Martinez M T, Benito A M, Maser W K, Gómez-Herrero J, Baró A M. Appl Phys Lett. 2001;79:2979–2981. [Google Scholar]
- 27.Cai L, Tabata H, Kaway T. Appl Phys Lett. 2000;77:3105–3106. [Google Scholar]
- 28.Muster J, Kim G T, Krstic V, Park J G, Roth S, Burghard M. Adv Materials. 2000;12:420–424. [Google Scholar]
- 29.Gómez-Navarro C, de Pablo P J, Moreno-Herrero F, Horcas I, Fernandez-Sanchez R, Colchero J, Gómez-Herrero J, Baro A M. Nanotechnology. 2002;13:314–317. [Google Scholar]
- 30.Salmeron M, Beebe T, Odriozola J, Wilson T, Ogletree D F, Siekhaus W. J Vac Sci Technol A. 1990;8:635–639. [Google Scholar]
- 31.Garcia R, Yuqiu J, Schabtach E, Bustamante C. Ultramicroscopy. 1992;42–44:1250–1254. doi: 10.1016/0304-3991(92)90431-i. [DOI] [PubMed] [Google Scholar]
- 32.Dunlap D D, Garcia R, Schabtach E, Bustamante C. Proc Natl Acad Sci USA. 1993;90:7652–7655. doi: 10.1073/pnas.90.16.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran P, Alavi B, Gruner G. Phys Rev Lett. 2000;85:1564–1567. doi: 10.1103/PhysRevLett.85.1564. [DOI] [PubMed] [Google Scholar]
- 34.Conwell E M, Rakhmanova S V. Proc Natl Acad Sci USA. 2000;97:4556–4560. doi: 10.1073/pnas.050074497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnett R, Cleveland C, Joy A, Landman U, Schuster G. Science. 2001;294:567–571. doi: 10.1126/science.1062864. [DOI] [PubMed] [Google Scholar]