Abstract
The mechanism of pI258 arsenate reductase (ArsC) catalyzed arsenate reduction, involving its P-loop structural motif and three redox active cysteines, has been unraveled. All essential intermediates are visualized with x-ray crystallography, and NMR is used to map dynamic regions in a key disulfide intermediate. Steady-state kinetics of ArsC mutants gives a view of the crucial residues for catalysis. ArsC combines a phosphatase-like nucleophilic displacement reaction with a unique intramolecular disulfide bond cascade. Within this cascade, the formation of a disulfide bond triggers a reversible “conformational switch” that transfers the oxidative equivalents to the surface of the protein, while releasing the reduced substrate.
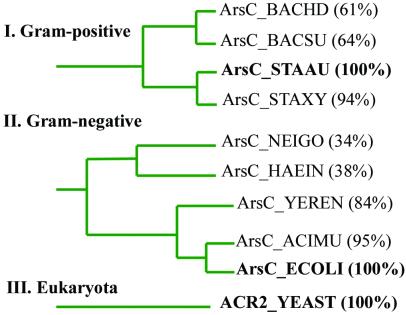
Arsenate reductases (ArsCs) catalyze the reduction of arsenate (HAsO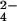 ) to arsenite (H3AsO3) and participate in the arsenic detoxification systems of prokaryotes and eukaryotes (1–5). ArsCs from different sources have unrelated sequences (Fig. 1) and folds. ArsC from Staphylococcus aureus plasmid pI258 (Gram-positive) has a tyrosine phosphatase (PTPase) I fold typical for low molecular weight (LMW) PTPases. It includes a P-loop with the characteristic CX5R sequence motif flanked by a β-strand and an α-helix (6). Acr2p from the yeast Saccharomyces cerevisiae also contains this motif but is homologous to the human cell cycle control phosphatase Cdc25a (7). ArsC from Escherichia coli plasmid R773 (Gram-negative) has a distinct HX3CX3R catalytic sequence motif and partially resembles crambin and partially glutaredoxin (8). Thus ArsCs form a very diverse family, in which ArsC from plasmid pI258 is representative for the Gram-positive group (Fig. 1).
) to arsenite (H3AsO3) and participate in the arsenic detoxification systems of prokaryotes and eukaryotes (1–5). ArsCs from different sources have unrelated sequences (Fig. 1) and folds. ArsC from Staphylococcus aureus plasmid pI258 (Gram-positive) has a tyrosine phosphatase (PTPase) I fold typical for low molecular weight (LMW) PTPases. It includes a P-loop with the characteristic CX5R sequence motif flanked by a β-strand and an α-helix (6). Acr2p from the yeast Saccharomyces cerevisiae also contains this motif but is homologous to the human cell cycle control phosphatase Cdc25a (7). ArsC from Escherichia coli plasmid R773 (Gram-negative) has a distinct HX3CX3R catalytic sequence motif and partially resembles crambin and partially glutaredoxin (8). Thus ArsCs form a very diverse family, in which ArsC from plasmid pI258 is representative for the Gram-positive group (Fig. 1).
Figure 1.
ArsC families from Gram-positive bacteria (I), Gram-negative bacteria (II), and eukaryota (III). Within each family, the sequence identity of ArsC proteins is shown behind the SWISS-PROT database name. ArsC_STAAU (S. aureus) (bold face) represents the Gram-positive family (Bacillus halodurans, B. subtilis, Staphylococcus xylosus), ArsC_ECOLI (E. coli) (bold face) represents the Gram-negative family (Neisseria gonorrhoeae, Haemophilus influenzae, Yersinia enterocolitica, Acidiphilium multivorum), and ACR 2 (S. cerevisiae) (bold face) represents the only known member of the eukaryotic family. The blast network service of the Swiss Institute of Bioinformatics was used to construct the phylogenetic tree from the percentage of sequence identity to the family representative. The interfamilial sequence identity is lower than 20%.
In pI258 ArsC, the flexible P-loop (residues 10–17) (9) and three cysteines (10, 82, and 89) are essential for catalysis. On arsenate reduction, a Cys-82–Cys-89 disulfide bond is formed (10), and this oxidized ArsC is regenerated via a coupled reaction with thioredoxin, thioredoxin reductase, and NADPH (11). pI258 ArsC also catalyzes the hydrolysis of p-nitrophenyl phosphate (6), albeit ≈1,000-fold slower than “acknowledged” LMW PTPases (12). The structurally similar Bacillus subtilis (64% sequence identity) is also capable of catalyzing the phosphatase reaction (13). This dual catalytic feature is limited to the Gram-positive ArsC family, because Acr2p and R773 ArsC have no measurable phosphatase activities (7, 8).
On the basis of the structures of the reduced and oxidized forms of pI258 ArsC, a reaction mechanism had been proposed, backed by kinetic arguments from some ArsC mutants (6). Here, we thoroughly document the mechanism. We provide the high-resolution structures of all critically important intermediates along the reaction pathway and corroborate the visual data of the x-ray electron densities with data from NMR and kinetic studies.
Methods
Site-Directed Mutagenesis, Purification, and Kinetic Analysis.
pET11a arsC wild-type plasmid (9) was used as DNA template in PCR amplification with primers designed to introduce the C89L, S17A, R16K, and D105A mutations. The resulting fragments were digested with NdeI and BamHI, cloned into the pET11a or pET14b (D105A) vector (Stratagene), transformed in E. coli JM109 (14), and expressed in E. coli strain BL21(DE3). Construction and expression of the C15A, C82S, and C10S ArsC mutants were previously reported (10). The D105A mutant was purified on Ni2+-NTA Superflow (Qiagen, Valencia, CA) in 20 mM Tris, pH 8.0/1 M NaCl/0.1 mM EDTA/1 mM DTT buffer solution and eluted with 1 M imidazole, followed by a gel filtration on Superdex75 HR (Amersham Pharmacia Biosciences). All other ArsC mutants were purified to homogeneity as described (9). The kinetic assays were performed as described in full in Messens et al. (9).
Single Catalytic Cycle Arsenite Analysis.
After the final Jupiter C18 reverse-phase purification step (9), the ArsC wild type and ArsC C89L were dialyzed against 20 mM Tris, pH 8.0/50 mM K2SO4. The concentration of active reduced ArsC in the solutions used for the assay was determined on an analytical Vydac (Hesperia, CA) C4 column (10). ArsC wild type and C89L were diluted to, respectively, 200, 400, and 800 nM and incubated overnight with 34 μM arsenate at 37°C. ArsC was removed on a solid-phase extraction cartridge (Waters Oasis HLB). After arsenate and arsenite separation on a Hamilton PRP-X100 anion exchange column (250 × 4.1 mm) operated in 20 mM KH2PO4/K2HPO4, pH 6.0 at 1 ml/min, the HPLC effluent was mixed with 1.5 M HCl and 2.5%/0.4% NaBH4/NaOH to form gaseous AsH3. The arsines were analyzed by using an atomic fluorescence spectrometer (Excalibur, PS Analytical, Orpington, U.K.) calibrated with a standard of arsenite.
Mass Spectrometry.
Reduced ArsC C89L and oxidized ArsC C89L obtained after 30-min incubation with arsenate at 37°C were purified with reverse-phase chromatography on a Vydac C4 column (4.6 × 250 mm, 214TP54) and concentrated by SpeedVac (Thermo Sarvant, Holbrook, NY). Their mass was determined with electrospray ionization–MS on a Quattro II quadrupole mass spectrometer (Micromass, Manchester, U.K.), as described previously (15). Detection of 18O isotopomers of arsenate was achieved in the negative ion mode with a capillary voltage of 2.75 kV and a cone voltage of 25 V. The scan time was 1 s in a window of 100–250 Da. Solutions of arsenate (500 μM) and phosphate (up to 200 mM) were incubated at 37°C in H O both with and without reduced ArsC C82S (10 μM) or reduced ArsC C10S (10 μM) and sampled at regular (10′) time intervals.
O both with and without reduced ArsC C82S (10 μM) or reduced ArsC C10S (10 μM) and sampled at regular (10′) time intervals.
NMR.
15N-enriched C82S ArsC and 13C,15N C89L ArsC were expressed and purified by using a previously published procedure (15). NMR samples contained either 1.5 mM C82S or 1.8 mM C89L in phosphate buffer pH 6.7, with 50 mM K2SO4, 1 mM DTT, and 0.1 mM EDTA, and were measured at 298 K. Studies involving the C82S ArsC were performed at 500 MHz on a Bruker (Billerica, MA) AMX (16). Before the binding study of arsenate with ArsC C82S, the 50 mM K2SO4 was removed by overnight dialysis. A series of 1H-15N heteronuclear sequential quantum correlation spectra (HSQC) were obtained at arsenate/C82S ArsC ratios of 0, 0.4, 0.9, 1.1, 2.0, 4.5, 7.0, and 10.0, by adding small aliquots of a concentrated arsenate solution to the NMR sample. 1H-15N HSQC total correlation spectroscopy and HSQC nuclear Overhauser effect spectroscopy spectra were recorded on the initial and final sample to aid the assignment of the 1H-15N spectra, by using the chemical shift values of ArsC wild type (16) as a guide. After titration, the NMR sample was subjected to overnight dialysis to remove any unbound arsenate and found to yield the original 1H-15N HSQC spectrum. Measurements involving ArsC C89L were performed on 13C,15N-labeled sample at 600 MHz on a Bruker DMX. HNCACB and CBCA(CO)NH spectra afforded backbone assignments, using acquisition parameters described previously (17). Titration of C89L with arsenate was performed in a fashion similar to C82S. 1H-15N spectra were obtained at arsenate/C89L ArsC ratios of 0, 0.75, 1.5, 2.7, 4.0, 5.0, 10.0, and 25.0. Dialysis to remove all arsenic species was performed overnight.
Crystallization.
ArsC wild type and C15A were purified as described (9). ArsC C89L was first oxidized with a 10 molar excess of arsenate at 37°C before reverse-phase chromatography Jupiter (Phenomenex, Torrance, CA) C18 purification (9) in 20 mM Tris, pH 8.0/2 mM 2-mercaptoethanol/15% acetonitrile. All were dialyzed against 20 mM Tris⋅HCl, pH 8.0/100 mM KCl and concentrated to obtain an OD280 nm between 25 and 30. Crystallization of ArsC C15A in the presence of 10 mM arsenite was performed as described for ArsC C10SC15A (6). ArsC wild type and C89L were crystallized at 20°C with the hanging drop vapor diffusion method with 100 mM Tris⋅HCl, pH 8.0/50 mM ammonium bicarbonate and 42.5% (wt/vol) polyethylene glycol 4000 (Hampton Research, Riverside, CA) in the bottom solution (1 ml). For ArsC wild type, 10 mM DTT was added to the bottom solution. The ArsC drops (3 μl) were left hanging in the sealed chamber for 20 h and then mixed with an equal volume of precipitant solution. Crystals grew within the next 16 h.
Data Collection and Structure Determination.
Data of ArsC wild type, C15A, and C89L were collected at 100 K at beamlines BW7A and BW7B of the Deutsches Elektronen Synchrotron in Hamburg, Germany. Diffraction images were processed by using the programs denzo, xdisplayf, and scalepack from the hkl package (18) and the ccp4 program truncate (19). The structures were determined by molecular replacement by using amore (20), with reduced C10SC15A ArsC as a model. Refinement was done by using the conjugate gradient least-square refinement protocols of the cns program (21), with the maximum likelihood target function based on structure factors. Residues 82–97 were built manually by using the program turbo. Bulk solvent and anisotropic B-factor corrections were used throughout the refinement. Figures were prepared by using the program molscript (22).
Results and Discussion
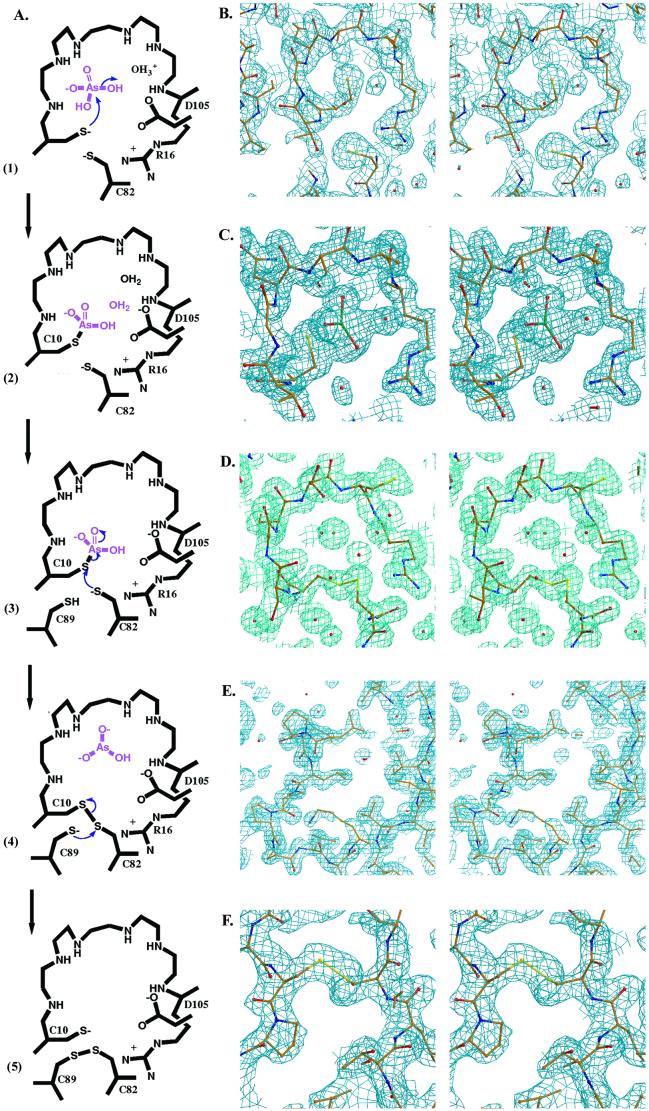
The Mechanism of Catalysis (Fig. 3A).
Figure 3.
(A) Scheme of the reaction mechanism of pI258 ArsC. (1) The nucleophilic attack of the thiol of Cys-10; (2) the formation of a covalent Cys-10-HAsO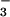 intermediate; (3) the nucleophilic attack of the thiol of Cys-82 with arsenite release; (4) the formation of a Cys-10–Cys-82 intermediate and the nucleophilic attack of the thiol of Cys-89; (5) the formation of a Cys-82–Cys-89 disulfide. (B–F) A stereo view of the 2Fo − Fc electron density maps contoured at 1.0 σ placed next to its corresponding reaction step in A. (B) The P-loop (residues 10–17) in the structure of reduced wild-type ArsC with Cys-10 in the center of the image. The P-loop is fully structured, despite the absence of bound oxyanion (2.0 Å). (C) In the structure of C15A ArsC-HAsO
intermediate; (3) the nucleophilic attack of the thiol of Cys-82 with arsenite release; (4) the formation of a Cys-10–Cys-82 intermediate and the nucleophilic attack of the thiol of Cys-89; (5) the formation of a Cys-82–Cys-89 disulfide. (B–F) A stereo view of the 2Fo − Fc electron density maps contoured at 1.0 σ placed next to its corresponding reaction step in A. (B) The P-loop (residues 10–17) in the structure of reduced wild-type ArsC with Cys-10 in the center of the image. The P-loop is fully structured, despite the absence of bound oxyanion (2.0 Å). (C) In the structure of C15A ArsC-HAsO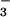 , an arsenic is covalently bound on Cys-10, surrounded by three oxygens in a plane and a water molecule opposite the sulfur of Cys-10 (1.4 Å). (D) Oxidized ArsC C89L with the intermediate Cys-10–Cys-82 disulfide bond (1.6 Å). (E) A view on the flexible looped-out region of oxidized ArsC C89L, where Cys-89 has left the hydrophobic core and is replaced by Leu-92 upon Cys-10–Cys-82 formation. The electron density in this highly flexible region is not so well defined. (F) A view on the surface of oxidized ArsC C10SC15A (6) with the Cys-82–Cys-89 disulfide bond.
, an arsenic is covalently bound on Cys-10, surrounded by three oxygens in a plane and a water molecule opposite the sulfur of Cys-10 (1.4 Å). (D) Oxidized ArsC C89L with the intermediate Cys-10–Cys-82 disulfide bond (1.6 Å). (E) A view on the flexible looped-out region of oxidized ArsC C89L, where Cys-89 has left the hydrophobic core and is replaced by Leu-92 upon Cys-10–Cys-82 formation. The electron density in this highly flexible region is not so well defined. (F) A view on the surface of oxidized ArsC C10SC15A (6) with the Cys-82–Cys-89 disulfide bond.
The reaction takes off with ArsC in the reduced state, featuring an empty P-loop. The first reaction step (step 1) is a nucleophilic attack of the thiol of Cys-10 on the arsenate substrate. A hydroxyl, to be protonated to a water molecule, then leaves the arsenic, leading to a covalent Cys-10-HAsO intermediate (step 2). In the next step, the first step of the disulfide cascade, Cys-82 attacks Cys-10 (step 3) with formation of a Cys-10–Cys-82 disulfide intermediate (step 4). In this process, the electrons from the S—As bond shuttle to arsenic, and arsenite is released. In the final step of the disulfide cascade, Cys-89 attacks Cys-82 (step 4), forming a Cys-82–Cys-89 disulfide (step 5) (6). In this step, the Cys-10 thiolate is regenerated, and the oxidative equivalents are exposed on the surface of ArsC. After the completion of the reaction, ArsC is regenerated by thioredoxin that reduces the formed Cys-82–Cys-89 disulfide (10). All steps are discussed in full.
intermediate (step 2). In the next step, the first step of the disulfide cascade, Cys-82 attacks Cys-10 (step 3) with formation of a Cys-10–Cys-82 disulfide intermediate (step 4). In this process, the electrons from the S—As bond shuttle to arsenic, and arsenite is released. In the final step of the disulfide cascade, Cys-89 attacks Cys-82 (step 4), forming a Cys-82–Cys-89 disulfide (step 5) (6). In this step, the Cys-10 thiolate is regenerated, and the oxidative equivalents are exposed on the surface of ArsC. After the completion of the reaction, ArsC is regenerated by thioredoxin that reduces the formed Cys-82–Cys-89 disulfide (10). All steps are discussed in full.
The Nucleophilic Attack of Cys-10 Thiolate on Arsenate.
The first reaction step takes place within the P-loop. In the structure of reduced ArsC (2.0 Å) (Fig. 2) (Table 1), we find an empty but structured P-loop with the Cys-10 sulfur in the center (Fig. 3B). The NH dipoles of the P-loop main chain nitrogens, the OH of Ser-17, as well as the macrodipole from the helix (residues 16–28) point toward the sulfur, all possibly stabilizing the nucleophilic thiolate form of Cys-10. The observation of an empty structured P-loop (Fig. 3B) was surprising. Previously, we showed by NMR that oxyanions are necessary to arrest the P-loop dynamics and to visualize all of the amide resonances of the P-loop residues (9). In the crystal, the P-loop is not directly involved in lattice contacts but might be stabilized by crystal contacts of neighboring residues 40–43 with two symmetry equivalent molecules. These residues are linked in their dynamics to the P-loop residues (6).
Figure 2.
Ribbon diagram of the overall structure of reduced ArsC wild type visualized from two different positions. The P-loop CX5R motif (red), the catalytic key residues in ball-and-stick representation, and the flexible short α-helix region (yellow) are shown.
Table 1.
Data collection and refinement statistics
| ArsC wild type | C15A ArsC-HAsO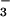
|
C89L ArsC | |
|---|---|---|---|
| Resolution limits, Å | 15–2.0 | 30–1.4 | 30–1.6 |
| Space group | P212121 | P212121 | P21 |
| Unit cell, Å; ° for β | a = 32.45, b = 32.77, c = 100.92 | a = 33.97, b = 37.83, c = 106.37 | a = 61.71, b = 35.48, c = 62.98 β = 118.01 |
| Wavelength, Å | 1.192 | 0.8453 | 0.8459 |
| Measured reflections | 203,478 | 188,689 | 214,661 |
| Unique reflections | 7,503 | 27,888 | 32,094 |
| Completeness/last shell, % | 96.5/74.8 | 94.8/99.6 | 97.3/97.6 |
| Rmerge/last shell, % | 7.0/45.1 | 5.2/23.9 | 3.3/6.5 |
| I/σ(I)/last shell | 22.5/3.1 | 34.0/6.77 | 23.7/14.4 |
| Refinement statistics | |||
| Rcryst/Rfree, % | 21.4/25.4 | 23.0/25.8 | 22.0/23.4 |
| rms deviation bonds, Å | 0.011 | 0.011 | 0.011 |
| rms angles, ° | 1.55 | 1.73 | 1.51 |
| Number of waters | 101 | 241 | 466 |
| Ligands | K+ | K+ | K+, Cl− |
The Cys-10 nucleophile can attack the arsenic atom in arsenate, concomitant with the expulsion of a hydroxyl from arsenate (step 1). We expected to trap the resulting covalent arsenate adduct by using the C82S mutant (10) but were not able to observe it by mass spectrometry. This observation indicates that if the adduct is formed, arsenate can also be released again by the inverse reaction, similar to phosphate hydrolysis in the second step of the well-documented phosphatase reaction (23). In agreement with this idea, 1H-15N HSQC analysis of the titration of ArsC C82S with arsenate shows that the formation of a 1:1 ArsC–arsenate complex is reversible. The reaction sequence, comprised of a single round of nucleophilic attack by Cys-10, the leaving of a hydroxyl group, the formation of an arsenic-bound intermediate, and its hydrolysis, is a futile cycle that results only in the exchange of an oxygen atom between arsenate and water. The occurrence of such a futile cycle catalyzed by the C82S mutant of ArsC could indeed be proven by detecting 18O incorporation in arsenate by mass spectrometry. The assignment of Cys-10 as the nucleophile in this reaction step was confirmed by the fact that the ArsC C10S mutant was not able to perform this futile cycle.
The Structure of the Arsenic-Bound Intermediate.
The structure of the arsenic-bound ArsC-HAsO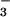 intermediate (step 2) was visualized from the 1.4-Å resolution structure of oxidized ArsC C15A (Fig. 3B). In this structure, Cys-82 and Cys-89 are engaged in a disulfide bond, whereas Cys-10, the proposed attacking nucleophile, is covalently bound to arsenic (S-As distance 2.4 Å) with three oxygens in a plane perpendicular to the As—S bond (As-O distances of 1.8 Å). These oxygens are well fixed in this position by interactions with backbone NHs of several residues of the P-loop (G12, N13, R16, S17) and with Nη2 of Arg-16. For comparison, an S-As distance of 2.18 Å was observed in R773 ArsC (8). A search in the Cambridge database of small-molecule structures (24) revealed that the S—As bond length of 132 entries varies between 1.5 and 3.0 Å. The in-plane As—O bond distances are in agreement with the value of 1.83 Å observed in a DTT–arsenite complex, where As-S distances of 2.24 and 2.25 Å are measured (25). A water molecule (As-O distance, 3.0 Å) is observed on the other side of the As-O plane opposite to the sulfur of Cys-10. Thus the sulfur and the water oxygen form the apices of a trigonal bipyramid with the arsenic at its center and three oxygens in equatorial positions. This geometry is reminiscent of that of the transition state of phosphoryl transfer reactions with the attacking and leaving groups at the apices (26). The “leaving” water ligated to the arsenic is cradled by the backbone NH and Nɛ of Arg-16 (Fig. 3C) and close (2.6 Å) to a water molecule that in turn is hydrogen bonded to Asp-105. The equivalent of Asp-105 in LMW PTPase protonates the leaving oxygen (27) in the dephosphorylation reaction. The position of the leaving water in ArsC, however, is quite different from that of the leaving oxygen in the LMW PTPase and much more buried in the active site. The overall geometry adopted by the substrate in the P-loop of ArsC in the first reaction step is different from that of PTPases, possibly because the smaller substrate of the reductase reaction permits a different orientation of the leaving group.
intermediate (step 2) was visualized from the 1.4-Å resolution structure of oxidized ArsC C15A (Fig. 3B). In this structure, Cys-82 and Cys-89 are engaged in a disulfide bond, whereas Cys-10, the proposed attacking nucleophile, is covalently bound to arsenic (S-As distance 2.4 Å) with three oxygens in a plane perpendicular to the As—S bond (As-O distances of 1.8 Å). These oxygens are well fixed in this position by interactions with backbone NHs of several residues of the P-loop (G12, N13, R16, S17) and with Nη2 of Arg-16. For comparison, an S-As distance of 2.18 Å was observed in R773 ArsC (8). A search in the Cambridge database of small-molecule structures (24) revealed that the S—As bond length of 132 entries varies between 1.5 and 3.0 Å. The in-plane As—O bond distances are in agreement with the value of 1.83 Å observed in a DTT–arsenite complex, where As-S distances of 2.24 and 2.25 Å are measured (25). A water molecule (As-O distance, 3.0 Å) is observed on the other side of the As-O plane opposite to the sulfur of Cys-10. Thus the sulfur and the water oxygen form the apices of a trigonal bipyramid with the arsenic at its center and three oxygens in equatorial positions. This geometry is reminiscent of that of the transition state of phosphoryl transfer reactions with the attacking and leaving groups at the apices (26). The “leaving” water ligated to the arsenic is cradled by the backbone NH and Nɛ of Arg-16 (Fig. 3C) and close (2.6 Å) to a water molecule that in turn is hydrogen bonded to Asp-105. The equivalent of Asp-105 in LMW PTPase protonates the leaving oxygen (27) in the dephosphorylation reaction. The position of the leaving water in ArsC, however, is quite different from that of the leaving oxygen in the LMW PTPase and much more buried in the active site. The overall geometry adopted by the substrate in the P-loop of ArsC in the first reaction step is different from that of PTPases, possibly because the smaller substrate of the reductase reaction permits a different orientation of the leaving group.
The Intramolecular Disulfide Bond Cascade.
The reaction sequence proceeds with the release of the reduced substrate via a nucleophilic attack of Cys-82 on Cys-10 (step 3). To provide direct evidence for this step in the reaction, we constructed a C89L mutant. The reduction of arsenate by ArsC C89L leads to an enzyme locked in the Cys-10–Cys-82 intermediate form (step 4) as observed in the electron density (Fig. 3 D and E). C89L ArsC crystallizes in a monoclinic space group with two molecules per asymmetric unit. The structure was determined at 1.6-Å resolution (Table 1). Both ArsC molecules unmistakably show the Cys-10–Cys-82 disulfide bond. As expected, ArsC C89L catalyzes a single catalytic cycle, producing arsenite with a turnover number of approximately 120 min−1 concomitant with the loss of 2 Da in the mass of the enzyme, indicative for disulfide bridge formation. Also, the kinetically inactive C82S mutant (10), where the disulfide bond cascade is blocked in an earlier stage of the reaction, is unable to produce arsenite through a single catalytic cycle. This observation proves the importance of Cys-82 for the transport of electrons toward the thiolate of Cys-10 and thus the release of arsenite.
The final step in the reaction results in the formation of the Cys-82–Cys-89 disulfide (step 5) and its exposure on the surface of ArsC (Fig. 3F) (6). For this reaction to take place, Cys-89 has to come out of its hydrophobic core and move over 10.2 Å to form the disulfide with Cys-82. Leu-92 replaces the cysteine in the hydrophobic core. The nucleophilic attack of Cys-89 requires a sizeable structural change, but it is not the first example of a functional “conformational switch.” A local protein refolding of the N-terminal loop in yeast glycogen phosphorylase regulates the accessibility of the phosphorylation site to protein kinases and phosphatases (28). In the regulatory protein OxyR, a distance of 17 Å separates the two redox-active cysteines, and a major structural change is also required (29).
The Essential Residues for Catalysis.
The catalytic roles of P-loop residues conserved among ArsCs from Gram-positive bacteria and LMW PTPase and of the structurally conserved aspartic acid (D105) were investigated in a thioredoxin, thioredoxin reductase, and NADPH-coupled steady-state kinetic assay (9). Next to Cys-10, -82, and -89 (10), Arg-16 is essential for catalysis as its replacement by a lysine kills the ArsC activity (kcat lower than 4.8 min−1, being the detection limit of the assay). This result stresses the structural importance of Arg-16 in the P-loop of ArsC (Fig. 3C). A catalytic essential Arg in the CX5R motif of the ArsC (Acr2p) from yeast was also reported (30). In ArsC, mutating residues N13 or S17 from the P-loop to alanine does not affect its Km but decreases the kcat with a factor more than 5. These muations result in a similar, although not so extreme, trend on the kcat as observed for LMW PTPases, where those mutations decrease the kcat with a factor of approximately 300 (31). In LMW PTPases, the Asn was proposed to be important to maintain the geometry of the P-loop and Ser to facilitate the correct ionization and orientation of the attacking Cys nucleophile (31).
The role of D105, the plausible analogue of the general acid of LMW PTPase, was also investigated. In ArsC, mutating D105 to alanine (Km = 3.8 mM, kcat = 58.5 min−1) increases the Km with a factor of 55 and decreases its kcat ≈4 times compared with wild-type ArsC. The respective Asp/Ala mutation in LMW PTPases, however, decreases the kcat with a factor of more than 1,000, while hardly affecting Km (27, 32). In ArsC, Asp-105 might have a somewhat different function, stabilizing the transition state via a bound (protonated) water molecule (Fig. 3C). It might be worth noting that the loop on which Asp-105 is located is not flexible, as it was observed for some PTPases (6, 33, 34).
Disulfide Bond Induced Flexibility.
The V77–K98 segment is seen to adopt a variety of conformations (Fig. 4), suggesting considerable flexibility in this segment. In the structure of oxidized C89L (Table 1), the two molecules in the asymmetric unit have different conformations for the stretch of residues from 82 to 97. In both molecules, Cys-10 and -82 form a disulfide bond, but in molecule A (Fig. 4, yellow), residues 82–97 still have a conformation similar to that of the reduced ArsC, with Cys-89 in the hydrophobic core In molecule B (Fig. 4, red), Leu-92 replaces Cys-89 in the hydrophobic core, residues 83–95 have moved, and the helix between Cys-82 and -89 partially loops out. Molecule B already resembles the oxidized form of ArsC.
Figure 4.

The movement of the “conformational switch” in the flexible segment (residues 80–98) trapped in four different ArsC crystals. Starting with a helix in the reduced ArsC wild type (blue), via oxidized ArsC C89L (Cys-10–Cys-82) in the first (yellow) and in the second (red) molecule in the asymmetric unit to finally looping out to form the C82-C89 disulfide (green). The two arrows indicate the movement of L92 and C89.
Treatment of C89L with arsenate induces conformational fluctuations on the microsecond-to-millisecond time scale, as evidenced by NMR (see supporting information on the PNAS web site, www.pnas.org). These fluctuations lead to exchange broadening of those segments of ArsC involved in arsenate catalysis: the P-loop from Cys-10 to Gln-18, the residues that are structurally organized around the P-loop and the complete V77–K98 segment. A highly dynamic P-loop was already documented for ArsC wild type, where oxyanions are necessary for the observation of all of the amide resonances (9, 16). Additional flexibility is induced in the V77–K98 segment on completion of the first two reaction steps. We propose that the simultaneously occurring reduced- and oxidized-like forms of ArsC in crystals of this mutant represent the structural extremes of a multistate conformational exchange process, responsible for the dynamic phenomena observed with NMR. The removal of the Cys-10–Cys-82 disulfide bond with DTT perfectly restores the original spectral appearance and stable dynamical state for this segment.
Thus, the formation of the Cys-10–Cys-82 disulfide bond triggers the structural change that allows Cys-89 to move out of its hydrophobic core, so that it can attack Cys-82.
Comparison to Other ArsCs and to Thiol-Disulfide Exchange Enzymes.
The reaction path of pI258 ArsC from S. aureus differs from that proposed in the other ArsC families. Acr2p of S. cerevisiae forms a mixed disulfide with glutathione before being reduced by glutaredoxin that regenerates the active Acr2p reductase (7). The reaction mechanism proposed for R773 ArsC from E. coli involves a peculiar thiarsahydroxyl derivative (Cys-S-As+-OH) of the active site cysteine, which is further processed by glutathione and glutaredoxin (8). Common among all ArsCs representing the three families (Fig. 1) is the sequential involvement of three different thiolate nucleophiles in the reaction mechanisms. In the case of R773 ArsC and Acr2p, the redox enzyme, glutathione and glutaredoxin, each contributes one thiolate to the intermolecular cascade to reduce arsenate (7, 8). All data we present prove that ArsC from pI258 is unique in that all three nucleophilic thiolates (Cys-10, -82, and -89) act intramolecularly through a reversible disulfide bond mediated “conformational switch.”
Intermolecular transport of electrons via thiol redox reactions is quite common (35), and reversible disulfide bond formation in oxidoreductases such as thioredoxin (36) and DsbA (37) introduces limited structural changes. Recently, a flexible intersubunit disulfide shuttle in thiooxidase Erv2p was reported (38). In that protein, a flexible C-terminal CX1C-segment swings into the vicinity of the CX2C segment in the opposite subunit to shuttle electrons.
Intramolecular transport of electrons via thiol redox reactions has been reported for the cytoplasmic membrane protein DsbD (39), for peptide methionine sulfoxide reductase MsrA (40), and for ribonucleotide reductase of class I and II (41). Each of these single domain thiol-disulfide mechanisms has distinct characteristics. The mechanism of pI258 ArsC can be catalogued within this group but has its own unique characteristics. For its reduction process, ArsC combines a phosphatase-like nucleophilic displacement reaction with a flexible intramolecular disulfide bond cascade.
Supplementary Material
Acknowledgments
We are grateful to Maya De Kerpel for sequencing the ArsC mutants, to Georges Laus for mass spectrometry analysis, to Stefan Loverix for quantum mechanic calculations, and to Remy Loris and Dominique Maes for help with data collection. We thank the European Community–Access to Research Infrastructure Action of the Improving Human Potential Program to the European Molecular Biology Laboratory (EMBL) Hamburg Outstation, contract no. HPRI-CT-1999-00017, and EMBL beamline ID14-3 at the European Synchrotron Radiation Facility, Grenoble, France. This work was funded in part by the Vlaams interuniversitair Instituut voor Biotechnologie, the European Space Agency Program Prodex, the Fund for Scientific Research Flanders (FWO) (Belgium), and the Research Council of the Vrije Universiteit Brussel. J.C.M. is a Postdoctoral Fellow of the FWO.
Abbreviations
- LMW PTPase
low molecular weight tyrosine phosphatase
- ArsC
arsenate reductase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The atomic coordinates reported in this paper have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID codes 1LJL, 1LJU, and 1LK0).
References
- 1.Broer S, Ji G, Broer A, Silver S. J Bacteriol. 1993;175:3480–3485. doi: 10.1128/jb.175.11.3480-3485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu C, Zhou T, Kuroda M, Rosen B P. J Biochem. 1998;123:16–23. doi: 10.1093/oxfordjournals.jbchem.a021904. [DOI] [PubMed] [Google Scholar]
- 3.Rosen B P, Dey S, Dou D, Ji G, Kaur P, Ksenzenko M Y, Silver S, Wu J. Ann NY Acad Sci. 1992;671:257–272. doi: 10.1111/j.1749-6632.1992.tb43801.x. [DOI] [PubMed] [Google Scholar]
- 4.Ji G, Silver S. Proc Natl Acad Sci USA. 1992;89:9474–9478. doi: 10.1073/pnas.89.20.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukhopadhyay R, Rosen B P. FEMS Microbiol Lett. 1998;168:127–136. doi: 10.1111/j.1574-6968.1998.tb13265.x. [DOI] [PubMed] [Google Scholar]
- 6.Zegers I, Martins J C, Willem R, Wyns L, Messens J. Nat Struct Biol. 2001;8:843–847. doi: 10.1038/nsb1001-843. [DOI] [PubMed] [Google Scholar]
- 7.Mukhopadhyay R, Shi J, Rosen B P. J Biol Chem. 2000;275:21149–21157. doi: 10.1074/jbc.M910401199. [DOI] [PubMed] [Google Scholar]
- 8.Martin P, DeMel S, Shi J, Gladysheva T, Gatti D L, Rosen B P, Edwards B F. Structure (London) 2001;9:1071–1081. doi: 10.1016/s0969-2126(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 9.Messens J, Martins J C, Brosens E, Van Belle K, Jacobs D M, Willem R, Wyns L. J Biol Inorg Chem. 2002;7:146–156. doi: 10.1007/s007750100282. [DOI] [PubMed] [Google Scholar]
- 10.Messens J, Hayburn G, Desmyter A, Laus G, Wyns L. Biochemistry. 1999;38:16857–16865. doi: 10.1021/bi9911841. [DOI] [PubMed] [Google Scholar]
- 11.Ji G, Garber E A, Armes L G, Chen C M, Fuchs J A, Silver S. Biochemistry. 1994;33:7294–7299. doi: 10.1021/bi00189a034. [DOI] [PubMed] [Google Scholar]
- 12.Ramponi G, Stefani M. Biochim Biophys Acta. 1997;1341:137–156. doi: 10.1016/s0167-4838(97)00087-3. [DOI] [PubMed] [Google Scholar]
- 13.Bennett M S, Guan Z, Laurberg M, Su X D. Proc Natl Acad Sci USA. 2001;98:13577–13582. doi: 10.1073/pnas.241397198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook J, Frietsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 15.Messens J, Hayburn G, Brosens E, Laus G, Wyns L. J Chromatogr B Biomed Sci Appl. 2000;737:167–178. doi: 10.1016/s0378-4347(99)00363-1. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs D M, Messens J, Wechselberger R W, Brosens E, Willem R, Wyns L, Martins J C. J Biomol NMR. 2001;20:95–96. doi: 10.1023/a:1011207007790. [DOI] [PubMed] [Google Scholar]
- 17.Landrieu I, Wieruszeski J M, Odaert B, Inze D, Grzesiek S, Lippen G. J Biomol NMR. 2000;17:271–272. doi: 10.1023/a:1008375707703. [DOI] [PubMed] [Google Scholar]
- 18.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 19.Collaborative Computational Project, N.4. Acta Crystallogr D. 1994;50:760–763. [Google Scholar]
- 20.Navaza J. Acta Crystallogr A. 1994;50:157–163. [Google Scholar]
- 21.Brunger A T, Adams P D, Clore G M, DeLano W L, Gros P, Grosse-Kunstleve R W, Jiang J S, Kuszewski J, Nilges M, Pannu N S, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 22.Esnouf R M. J Mol Graph Model. 1997;15:132–133. doi: 10.1016/S1093-3263(97)00021-1. [DOI] [PubMed] [Google Scholar]
- 23.Denu J M, Dixon J E. Curr Opin Chem Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 24.Allen F H, Kennard O. Chem Des Auto News. 1993;8:31–37. [Google Scholar]
- 25.Cruse W B T, James M N G. Acta Crystallogr B. 1972;28:1325–1331. [Google Scholar]
- 26.Denu J M, Lohse D L, Vijayalakshmi J, Saper M A, Dixon J E. Proc Natl Acad Sci USA. 1996;93:2493–2498. doi: 10.1073/pnas.93.6.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L, Zhang Z Y. Biochemistry. 1996;35:5426–5434. doi: 10.1021/bi952885a. [DOI] [PubMed] [Google Scholar]
- 28.Lin K, Rath V L, Dai S C, Fletterick R J, Hwang P K. Science. 1996;273:1539–1542. doi: 10.1126/science.273.5281.1539. [DOI] [PubMed] [Google Scholar]
- 29.Choi H, Kim S, Mukhopadhyay P, Cho S, Woo J, Storz G, Ryu S. Cell. 2001;105:103–113. doi: 10.1016/s0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay R, Rosen B P. J Biol Chem. 2001;276:34738–34742. doi: 10.1074/jbc.M103354200. [DOI] [PubMed] [Google Scholar]
- 31.Evans B, Tishmack P A, Pokalsky C, Zhang M, Van Etten R L. Biochemistry. 1996;35:13609–13617. doi: 10.1021/bi9605651. [DOI] [PubMed] [Google Scholar]
- 32.Taddei N, Chiarugi P, Cirri P, Fiaschi T, Stefani M, Camici G, Raugei G, Ramponi G. FEBS Lett. 1994;350:328–332. doi: 10.1016/0014-5793(94)00805-1. [DOI] [PubMed] [Google Scholar]
- 33.Stuckey J A, Schubert H L, Fauman E B, Zhang Z Y, Dixon J E, Saper M A. Nature (London) 1994;370:571–575. doi: 10.1038/370571a0. [DOI] [PubMed] [Google Scholar]
- 34.Schubert H L, Fauman E B, Stuckey J A, Dixon J E, Saper M A. Protein Sci. 1995;4:1904–1913. doi: 10.1002/pro.5560040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritz D, Beckwith J. Annu Rev Microbiol. 2001;55:21–48. doi: 10.1146/annurev.micro.55.1.21. [DOI] [PubMed] [Google Scholar]
- 36.Weichsel A, Gasdaska J R, Powis G, Montfort W R. Structure (London) 1996;4:735–751. doi: 10.1016/s0969-2126(96)00079-2. [DOI] [PubMed] [Google Scholar]
- 37.Guddat L W, Bardwell J C, Martin J L. Structure (London) 1998;6:757–767. doi: 10.1016/s0969-2126(98)00077-x. [DOI] [PubMed] [Google Scholar]
- 38.Gross E, Sevier C S, Vala A, Kaiser C A, Fass D. Nat Struct Biol. 2002;9:61–67. doi: 10.1038/nsb740. [DOI] [PubMed] [Google Scholar]
- 39.Katzen F, Beckwith J. Cell. 2000;103:769–779. doi: 10.1016/s0092-8674(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 40.Lowther W T, Brot N, Weissbach H, Honek J F, Matthews B W. Proc Natl Acad Sci USA. 2000;97:6463–6468. doi: 10.1073/pnas.97.12.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjoberg B M, Sahlin M. Methods Enzymol. 2002;348:1–21. doi: 10.1016/s0076-6879(02)48620-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.