Abstract
Use of soy-based infant formulas and soy/isoflavone supplements has aroused concern because of potential estrogenic effects of the soy isoflavones genistein and daidzein. Here we show that s.c. genistein injections in ovariectomized adult mice produced dose-responsive decreases in thymic weight of up to 80%. Genistein's thymic effects occurred through both estrogen receptor (ER) and non-ER-mediated mechanisms, as the genistein effects on thymus were only partially blocked by the ER antagonist ICI 182,780. Genistein decreased thymocyte numbers up to 86% and doubled apoptosis, indicating that the mechanism of the genistein effect on loss of thymocytes is caused in part by increased apoptosis. Genistein injection caused decreases in relative percentages of thymic CD4+CD8− and double-positive CD4+CD8+ thymocytes, providing evidence that genistein may affect early thymocyte maturation and the maturation of the CD4+CD8− helper T cell lineage. Decreases in the relative percentages of CD4+CD8− thymocytes were accompanied by decreases in relative percentages of splenic CD4+CD8− cells and a systemic lymphocytopenia. In addition, genistein produced suppression of humoral immunity. Genistein injected at 8 mg/kg per day produced serum genistein levels comparable to those reported in soy-fed human infants, and this dose caused significant thymic and immune changes in mice. Critically, dietary genistein at concentrations that produced serum genistein levels substantially less than those in soy-fed infants produced marked thymic atrophy. These results raise the possibility that serum genistein concentrations found in soy-fed infants may be capable of producing thymic and immune abnormalities, as suggested by previous reports of immune impairments in soy-fed human infants.
Soy-based formula for human infant nutrition is widely used, with approximately 25% of formula-fed infants in the U.S. consuming soy-based formula (1). This number represents 15% of all infants in the U.S., or about 750,000 infants/year (1, 2). Infants consuming soy formula are exposed to high levels of genistein and daidzein, estrogenic isoflavones present in soybeans and soy products. On average, infants fed soy-based formula consume 6.0–11.9 mg of isoflavones/kg per day (3, 4), an order of magnitude greater than adults eating high-soy diets. Total plasma levels of isoflavones and genistein in soy-fed infants range from 2.0 to 6.6 and 1.5 to 4.4 μmol/liter, respectively (3), 10-fold greater than levels in Japanese adults whose diets have historically included soy, and 200-fold greater than plasma levels in infants fed cow's milk formula or human breast milk (3, 5). Levels of the free genistein aglycone as a percent of total genistein are higher in rat pups than in adults (6), but have not been measured in human infants. If a similar phenomenon occurs in humans, relative levels of the biologically active free aglycones may be even greater than the 10-fold difference documented in total (free + conjugated) serum isoflavone and genistein levels in soy-fed infants vs. adults eating high-soy diets.
Total plasma isoflavone levels in soy-fed infants are up to 22,000 times greater than 17β-estradiol (E2) levels (3). However, estrogenicity of genistein is only 1/1,000th to 1/10,000th that of E2 (7). In addition, only a small fraction of circulating genistein or daidzein is the active aglycone. Nonetheless, high genistein levels in infants could have effects despite limited estrogenic potency and the preponderance of conjugated forms in the circulation.
Work on estrogenic effects of phytoestrogens has focused on reproductive organs (7). However, thymus expresses both estrogen receptor (ER) α and ERβ, and estrogen treatment of developing rodents induces thymic atrophy and immune suppression (8, 9). Despite genistein's affinity for ERα and ERβ, thymic effects of genistein have not been studied. There are reports of genistein effects at high concentrations on immune cells in vitro (10), but it is unclear whether these effects occur at physiological concentrations or in vivo.
In the present report, we examined thymic and immune effects of genistein in mice. Our results indicate that genistein injections decreases thymic weight and thymic and splenic CD4+CD8− T cell numbers and result in lymphocytopenia and immune suppression. Of greatest concern, thymic atrophy is seen when mice are given dietary genistein levels that produce serum genistein concentrations less than those reported for soy-fed human infants.
Methods
Effect of Genistein on Thymic Weight, Apoptosis, and Thymocyte Subtypes in Adult Mice.
Female C57BL/6 mice (Harlan Breeders, Indianapolis) were ovariectomized 1 week before injection to mimic endocrine conditions in human infants, where circulating E2 levels are minimal in both males and females (3). They were fed ad libitum with a casein-based phytoestrogen-free diet (AIN-93G) starting 2 days before injections began. Mice (100 days old when injected) were given one s.c. injection/day for 7 or 21 days of either 0.02 ml DMSO (control), E2 (5 μg/kg per day), or genistein (Indofine Chemicals, Somerville, NJ) at 2–200 mg/kg per day.
We also determined the effects of genistein on thymic size in castrated males. Seventy-day-old males were castrated, and 5 days later they were placed on phytoestrogen-free diet. Beginning 1 week after castration, they were injected with DMSO, E2, or genistein (200 mg/kg body weight/day) for 21 days.
Direct immunofluorescence was used to analyze lymphocyte subpopulations in thymus of control and genistein-treated females. Thymic lymphocytes (1 × 106) were incubated with allophycocyanin-conjugated anti-mouse CD4 (L3T4, PharMingen) mAb and phycoerythrin-conjugated anti-mouse CD8 (LY-2, PharMingen) mAb. Cells were then fixed, and 10,000 cells were examined by flow cytometry (Coulter EPICS XL). Appropriate controls were run with each sample.
Thymocytes were stained for annexin V-FITC and propidium iodide (PI) by using the annexin-V-FLUOS staining kit (Roche Diagnostics), then apoptosis was quantitated by flow cytometry (11). Annexin staining measures cell membrane phophatidylserine externalization, which is a marker of the earlier stages of apoptosis. PI stains cells that have large plasma membrane ruptures characteristic of later stages of cell death. The use of annexin and PI staining allows simultaneous detection of independent apoptotic changes (12).
Effect of Genistein on Humoral Immunity and Blood Lymphocyte Percentages.
Juvenile (25–27 days old) mice were ovariectomized and placed on phytoestrogen-free feed 5 days later. Beginning 1 week after ovariectomy, they were given daily injections of DMSO, E2, or genistein (8–80 mg/kg per day) for 5 weeks. Mice were immunized with 100 μg of keyhole limpet haemocyanin (KLH) by using Bentonite as an adjuvant (13) 1 week after initiation of treatment. Three weeks later, a KLH booster dose was given, then animals were killed 1 week later, at the conclusion of the treatment period. Serum was obtained for subsequent measurement of anti-KLH antibody by ELISA in all groups, as described (13), and thymic weights were determined. Splenic lymphocyte subpopulations were determined as described above for thymic lymphocytes. A blood smear was made from animals used in the humoral immunity study. A differential count of 100 white blood cells was performed for each sample from the control and various treated groups (n = 5–8).
Measurement of Serum Genistein Levels in Mice Given Dietary or Injected Genistein.
Serum genistein levels were measured in mice that were ovariectomized at day 25–27 and placed on phytoestrogen-free feed as in the previous section. Beginning 1 week after ovariectomy, mice received two daily injections of one of the following: DMSO vehicle (n = 6) or genistein at 2, 8, 20, 80, and 200 mg/kg. Twenty-four hours after the second injection, blood was collected by decapitation (time 0; n = 6 from each group), while remaining mice were given a third injection and blood was collected at 0.5, 1, 2, and 6 h after dosing (n = 5–6 for each time point and treatment).
To test whether dietary genistein could cause thymic effects, we fed 32- to 34-day-old, ovariectomized mice either a phytoestrogen-free AIN-93G diet (control) or this diet supplemented with 1,000 or 1,500 ppm of genistein (Dyets, Bethlehem, PA). Mice were killed at lights on (8 a.m.) on day 12 of feeding; serum genistein levels at this time reflect levels seen during the night, when the mice are eating (14). Thymuses were weighed, and blood was collected for genistein measurement.
To determine genistein levels, blood was centrifuged and serum was removed. Amounts of total genistein (aglycone + conjugates) were determined by using duplicate 50-μl samples from each animal. Samples were mixed with equal volumes of acetonitrile, sonicated for 10 min, and centrifuged (15,000 rpm for 10 min). To measure total genistein, the supernatant was combined with 1 ml of sodium citrate buffer (25 mM, pH 5.0) containing β-glucuronidase and sulfatase to deconjugate the genistein. After incubation at 37°C for 4 h, the sample was loaded onto a C-18 SPE column (J & W Scientific, Folsom, CA) and washed with 30 ml of distilled water. The washed column was eluted with 1.5 ml of methanol, evaporated under vacuum with centrifugation, and resuspended in methanol for HPLC analysis. Prepared samples were separated by HPLC with a Nova Pak C-18 column (3.9 × 150 mm column, 4-μm particles; Waters) by using a gradient solvent system. The solvent system consisted of a sodium acetate buffer (50 mM, pH 4.8) and methanol (4:1) mixture and a sodium acetate buffer, methanol, and acetonitrile (2:2:1) mixture. The flow rate was 0.5 ml/min at a temperature of 30°C. For isoflavone detection, a Waters Photodiode Array (PDA) detector was used, with detection ranging from 210 to 400 nm. The assay had a minimum detection limit of 0.2 μmol/liter (15).
Statistical Analysis.
Results from all experiments were analyzed by one-way ANOVA followed by the Student–Newman–Keuls Multiple Comparisons test, and differences were considered significant at P < 0.05.
Results and Discussion
Genistein Decreases Thymic Size in Female and Male Mice Through ER and Non-ER-Mediated Mechanisms.
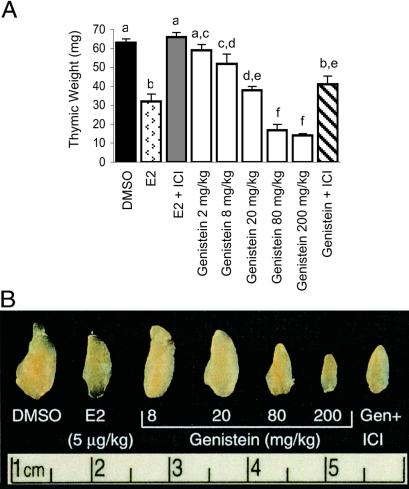
We examined thymic and immune effects of genistein treatment in ovariectomized mice and observed that genistein produced dose-responsive reductions in thymic weight and size (Fig. 1). Thymic weights in E2-treated (5 μg/kg per day) females were decreased 50% compared with controls; higher E2 doses (up to 12 μg/kg per day) did not produce greater decreases (not shown). Thymic weights in females given 200 mg/kg per day genistein were 78% less than controls and 56% less than E2-treated females. Thymic weights in the 8, 20, and 80 mg/kg per day genistein groups were 17%, 40% and 73% less than controls, respectively; genistein at 2 mg/kg per day did not produce significant decreases. Thus, genistein decreases thymic weight of female mice and does so to a greater degree than E2 at maximally effective doses.
Figure 1.
Thymic effects of genistein. (A) Thymic weights in 100-day-old ovariectomized mice given 21 daily s.c. injections with DMSO, E2 (5 μg/kg), or genistein (2–200 mg/kg). Some mice given E2 or 200 mg/kg genistein were also given weekly injections (5 mg/week) of the antiestrogen ICI 182,780. Genistein produced dose-dependent decreases in thymic weights, which exceeded those seen with E2 at higher genistein doses. ICI 182,780 only partially blocked genistein effects, indicating that genistein acts partly through a non-ER mechanism. Data are presented as mean ± SE; n ≥ 7 for all groups. Columns with different superscript letters are statistically different. (B) Whole-mount photographs of individual thymic lobes from mice treated as in A.
The observations that genistein suppressed thymic weight more profoundly than E2 indicated that genistein effects might not be mediated entirely through ER. We determined whether the anti-estrogen ICI 182,780 (Astra-Zeneca, Macclesfield, U.K.) blocked genistein effects on thymic weight (Fig. 1). Thymic weight was restored to control levels in ovariectomized mice given E2 (5 μg/kg per day) + ICI 182,780 (200 mg/kg per week). Conversely, ICI blocked only 50% of genistein's effect on thymic weight in the genistein (200 mg/kg per day) + ICI 182,780 (200 mg/kg per week) group, indicating that genistein's effects were partially non-ER mediated.
To ensure that ICI 182,780 treatment completely blocked ER-mediated genistein signaling, we measured uterine and vaginal weights, sensitive indicators of estrogenic effects. Genistein or E2 markedly increased uterine and vaginal weights. In both E2 + ICI and genistein + ICI groups, uterine and vaginal weights equaled those of ovariectomized controls (not shown), indicating that ICI 182,780 completely inhibited uterine and vaginal estrogenic responses to E2 and genistein. Thus, thymic effects of genistein seen after ICI 182,780 treatment occur through non-ER-mediated mechanisms. These could involve effects on protein tyrosine kinases and/or topoisomerase II, both of which have been shown to be inhibited in thymocytes and other cell types by high genistein concentrations in vitro (16, 17).
Thymic weight in genistein-treated castrated male mice was 42 ± 4 mg (n = 7), 49% less then the DMSO controls (82 ± 3 mg; n = 4) and 30% less than the E2-treated group (60 ± 2 mg; n = 7). All differences between groups were significant. Both genistein and E2 produced less pronounced thymic suppression in males than females, but genistein still caused greater thymic atrophy in males than a maximally effective E2 dose, indicating that genistein effects on thymic atrophy are similar in both sexes.
Genistein Increases Thymocyte Apoptosis and Preferentially Decreases CD4+CD8− and CD4+CD8+ Thymocytes.
Thymocyte numbers were measured with a hemocytometer to determine whether the effects of genistein involved reductions in these cells. Thymocyte numbers in DMSO, E2, and genistein (200 mg/kg per day) groups were 18.3 ± 1.6 × 106, 7.0 ± 1.3 × 106, and 2.6 ± 0.6 × 106, respectively (significant 62% and 86% reductions with E2 and genistein, respectively; all groups n ≥ 7). Genistein therefore decreases thymocyte numbers to an extent similar to its effect on thymic weight.
Reductions in thymocyte number in mice treated 21 days with 200 mg/kg per day of genistein were so severe that insufficient thymocytes were available for analysis of apoptosis. We therefore treated mice with DMSO, E2, or 80 mg/kg per day genistein for 7 days, and then quantitated apoptosis (11). The 7-day genistein treatment decreased thymic weight 62%, only slightly less than the 73% decrease after 21 days of treatment with this genistein dose, indicating genistein effects occur rapidly. The percentages of thymocytes in the relatively early stages of apoptosis, indicated by annexin staining, in the DMSO, E2, and genistein (80 mg/kg) groups were 0.8 ± 0.2%, 1.8 ± 0.2%, and 2.4 ± 0.4%, respectively (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). PI staining, which identifies cells in the late stages of cell death, was similarly increased by genistein (Fig. 6). PI staining was 2.6 ± 0.3%, 4.6 ± 0.3%, and 5.5 ± 0.8% in the DMSO, E2, and genistein (80 mg/kg per day) groups, respectively (n = 6 for each group and P < 0.05 for genistein and E2 vs. control for both annexin and PI staining).
These results demonstrate that decreases in thymic weight induced by genistein occur relatively quickly, accompanied by decreased thymocyte numbers and increased apoptosis. Thymocyte apoptosis would appear to be an important aspect of the mechanism of genistein's thymic effects, although genistein effects on other processes, such as cell proliferation, could also be contributory. The increased thymocyte apoptosis that accompanied the genistein-induced thymic atrophy is consistent with reports that estrogen-induced thymic atrophy also involves decreases in thymocyte numbers and increased apoptosis (18).
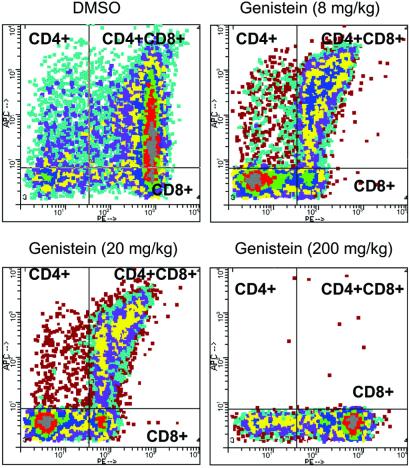
High doses of genistein (200 mg/kg per day) resulted in near-total elimination of CD4+CD8− thymocytes and similar striking inhibitions in double-positive CD4+CD8+ thymocytes (Fig. 2). These effects were dose-responsive, with the 8 mg/kg per day dose decreasing CD4+CD8− cells 41% relative to controls (P < 0.01). Relative percentages of double-positive thymocytes, an early stage in thymocyte maturation that develops from CD4−CD8− (double negative) thymocytes, also showed decreases (approximately 60%) at the 8 and 20 mg/kg doses (P < 0.01). These results indicate that genistein may affect early thymic thymocyte maturation and the maturation of the CD4+CD8− lineage. Decreases in double-positive, but not CD4+CD8−, thymocytes in response to estrogen have been reported previously (18). These previous findings indicate that although the decreases we observed in double-positive thymocytes may be mediated through genistein effects mediated through ER, reductions in CD4+CD8− thymocytes could reflect non-ER-mediated actions. This possibility is consistent with our preliminary observations that decreases in relative percentages of CD4+CD8− thymocytes are still observed after genistein + ICI 182,780 treatment (S.Y. and P.S.C., unpublished work).
Figure 2.
Flow cytometric analysis of thymocyte subtypes in 100-day-old ovariectomized mice treated for 21 days with DMSO or genistein (8, 20, or 200 mg/kg per day). Thymocytes were stained for CD4 (allophycocyanin) and CD8 (phycoerythrin) cell surface markers. (Upper Left) CD4+CD8−. (Upper Right) CD4+CD8+. (Lower Left) CD4−CD8− (not labeled). (Lower Right) CD4−CD8+ thymocytes. Data shown are from individual animals, but percentage decreases for the various cell types were based on n > 6 for all groups.
Genistein Decreases Humoral Immunity and Splenic CD4+CD8− Cells and Produces Lymphocytopenia.
Suppressive effects of genistein on thymic size and CD4+CD8− cell numbers raised the possibility that genistein could inhibit immune function. Because changes in immune function as a result of experimental treatments that affect the thymus are more pronounced early in life than in adulthood (19), we measured thymic weight, humoral immunity, splenic CD4+CD8− cell numbers, and blood lymphocytes in young ovariectomized females after treatment with DMSO or genistein. Genistein at 8, 20, and 80 mg/kg per day produced decreases in thymic weight of 30%, 35%, and 64%, respectively, compared with DMSO-treated control mice (n = 9–10, P < 0.05 for all groups vs. controls). Thus, the magnitude of the genistein response in terms of thymic weight reductions was comparable in young and 100-day-old animals.
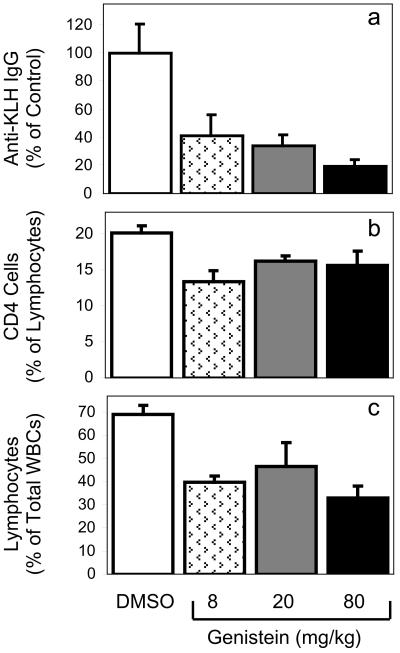
Genistein produced impairments in humoral immunity (Fig. 3a), with the 80 mg/kg per day genistein dose reducing KLH-specific antibody titers by over 80%, whereas doses as low as 8 mg/kg per day produced a greater than 50% decrease. Preliminary data indicates that genistein also decreases cell-mediated immunity (S.Y. and P.S.C., unpublished work), indicating that genistein may affect both the humoral and cell-mediated components of immunity. Although the immunosuppressive effects of high levels of exogenous estrogen have been established (20), the present report indicates that estrogenic dietary isoflavones such as genistein can produce immunosuppression in vivo.
Figure 3.
Anti-KLH IgG antibody titers (a), splenic CD4+CD8− T cell percentages (b), and relative blood lymphocyte percentages (c) in control and genistein-treated mice. (a) Juvenile (30 days old) ovariectomized mice were treated with DMSO or genistein for 5 weeks, and serum anti-KLH antibody was measured to determine whether genistein diminished antibody response. Results are expressed as a percentage of control. (b) Splenic CD4+CD8− T cell percentages in mice from a, measured by flow cytometry. (c) Relative blood lymphocyte percentages in mice shown in a and b. For all groups, n = 5–9. All genistein treatments were less than controls, but were not different from each other.
These mice also had decreases in CD4+CD8− T cells as a percentage of splenic lymphocytes at 8 mg/kg per day genistein or above (Fig. 3b). CD4−CD8+ T cell percentages were significantly lower at only the highest genistein 80 mg/kg per day dose (not shown). Thus, genistein decreases relative percentages of splenic T cells, and these are more severe in CD4+CD8− cells. These splenic effects are consistent with both thymic effects of genistein and its immunosuppressive effects discussed above.
Relative percentages of lymphocytes in blood of genistein-treated mice were reduced (Fig. 3c), consistent with genistein's effects on thymus, spleen, and immune function described above. This lymphocytopenia may reflect effects on T cell populations, but also inhibitory effects on B-lymphopoiesis (21).
Serum Levels of Genistein After Injection.
A critical question is whether these injected doses of genistein that produced thymic and immune effects produce physiological levels of genistein and are potentially relevant for soy-fed human infants. Ingestion of a compound normally leads to lower blood levels than injection, making it difficult to compare injected and ingested doses. Rodents, especially mice, metabolize genistein more quickly than humans do (14, 15). Therefore, it is problematic to compare effects of specific doses of genistein on mice and humans, and it is more physiologically relevant to examine the effects on mice of circulating genistein levels similar to those in soy-fed human infants.
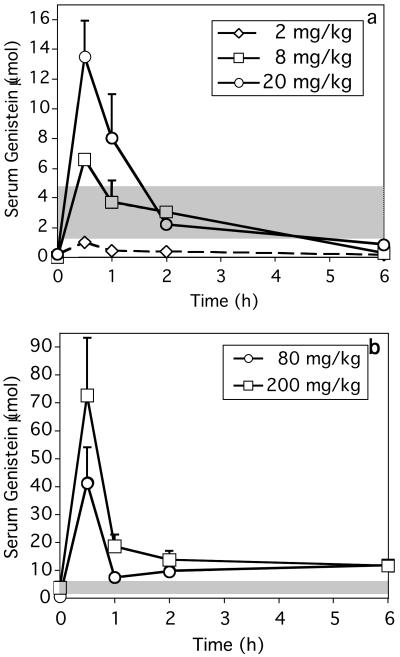
To compare serum genistein levels in our injected mice with those reported for soy-fed human infants, total genistein (aglycone + conjugates) was measured in young ovariectomized mice injected with 2–200 mg/kg per day of genistein, as described (15). Serum genistein levels in control samples were below the limit of detection. The 2 mg/kg dose produced transient increases in serum genistein, but peak levels were less than levels in soy-fed infants (Fig. 4a). Critically, serum genistein in mice dosed with 8 mg/kg were comparable to those in soy-fed infants at 0.5, 1, and 2 h (Fig. 4a), then levels declined to 0.4 ± 0.4 μmol/liter by 6 h and were not measurable after 24 h. At 0.5 and 1 h postinjection, the 20 mg/kg dose produced serum genistein levels 2- to 3-fold greater than maximal levels reported in soy-fed infants. Levels were comparable to those in soy-fed infants between 2 and 6 h. Genistein injections of 80 and 200 mg/kg produced peak serum levels 10- to 20-fold greater, respectively, than maximal levels in soy-fed infants (Fig. 4b). Levels in the 80 and 200 mg/kg groups remained greater than those seen in soy-fed infants at 6 and 24 h.
Figure 4.
Serum genistein levels in mice after injection of (a) 2–20 mg/kg or (b) 80–200 mg/kg of genistein. Data are mean ± SE and n = 5 for all points. Shaded bands represent the range of plasma genistein levels in 4-month-old soy-fed human infants (3). (a) Serum genistein after the 2 mg/kg dose was less than in soy-fed human infants. The 8 mg/kg dose produced genistein levels comparable to those in soy-fed human infants for the first 2 h postinjection; genistein declined to minimal levels by 6 h and was undetectable 24 h postinjection. The 20 mg/kg dose produced peak levels 3-fold greater than those in soy-fed human infants, then levels declined, equaling those in the 8 mg/kg dose by 2 h postinjection and becoming minimal by 6 h. (b) Genistein levels after injection of 80 or 200 mg/kg showed a temporal pattern similar to lower doses, although absolute levels from 0.5 to 6 h exceeded those in soy-fed human infants. Note different scales in a and b.
Plasma isoflavone levels of 2.0–6.6 μmol/liter (mean = 3.7 μmol/liter) and genistein levels of 1.5–4.4 μmol/liter (mean = 2.5 μmol/liter) have been reported in 4-month-old human infants (3). Maximal serum genistein in soy-fed infants are only modestly less than peak serum levels in mice after 8 mg/kg genistein injection, and this genistein persists less than 6 h, whereas soy-fed infants have relatively constant high levels caused by frequent nursing. Thus, genistein injected at an 8 mg/kg per day dose that produces peak blood levels comparable to those in soy-fed infants induces thymic and immune abnormalities.
Dietary Genistein Produces Thymic Atrophy.
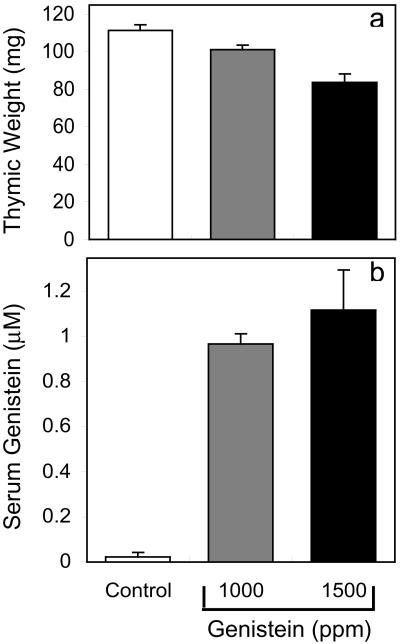
Injecting genistein produces different genistein pharmacokinetics and metabolism than administering this compound by the physiologically relevant route of dietary ingestion. It was therefore critical to determine whether dietary genistein could produce thymic effects comparable to those seen with genistein injection. Mice consuming feed supplemented with 1,000 or 1,500 ppm genistein had thymic weights approximately 10% and 25% less than those of controls (Fig. 5a) at the end of a 12-day treatment (n = 18, 20, and 10 for control, 1,000, and 1,500 ppm genistein groups, respectively). Body weight was not different in control and 1,000 ppm genistein groups and was slightly (<5%) lower in the 1,500 ppm group compared with controls. These results are consistent with previous reports that dietary genistein at 1,000 ppm did not affect body weight (22) and that genistein at 1,000 or 1,500 ppm did not affect food consumption (22, 23). Serum genistein levels in mice fed 1,000 and 1,500 ppm genistein were 0.97 ± 0.04 and 1.12 ± 0.18 μM, respectively, whereas genistein was not measurable in 17/18 control samples (Fig. 5b). These serum genistein levels are comparable to peak levels reported for mice after oral genistein administration (24), but are less than those in rats consuming similar genistein concentrations (14) because of the faster genistein metabolism in mice (14, 15, 24). Serum genistein levels in mice fed 1,500 ppm genistein, which produces a 25% decrease in thymic weight, are approximately 50% and 70% less than the average and maximal levels in soy-fed human infants of 2.5 and 4.4 μM, respectively (3). These results indicate that dietary genistein in mice decreases thymic weight at levels that produce serum genistein concentrations substantially below what human infants might be realistically exposed to.
Figure 5.
Thymic weights (a) and serum genistein levels (b) in ovariectomized, juvenile mice after 12 days of feeding with a phytoestrogen-free diet or this diet supplemented with 1,000 or 1,500 ppm genistein. (a) Control values were different from 1,000 or 1,500 ppm genistein groups at P < 0.05 and P < 0.001, respectively; the 1,000 or 1,500 ppm genistein groups were different at P < 0.01. (b) Control values were different from both genistein groups at P < 0.0001, but the genistein groups were not different from each other.
Our results indicating thymic effects in mice at serum genistein concentrations less than those of soy-fed human infants raise the critical question of whether there would be thymic and immune effects in infants consuming soy formula. A recent large-scale study of 811 adults fed soy formula as infants indicated that their growth was equal to a cow milk control group, and the soy-fed group had had no obvious alterations of reproductive development or adult function (2). However, the only parameter examined related to immune function, use of asthma or allergy medications, was different in the two groups. Women fed soy-formula as infants had an almost 90% increase in the regular use of allergy and asthma drugs, perhaps indicative of an increased incidence and/or severity of these conditions in adults fed soy formula as infants. Large-scale studies addressing immune function or morbidity in soy-fed infants are needed to conclusively determine whether there are immediate or long-term immune abnormalities associated with soy formula consumption, but data from this study, the largest to examine effects of soy formula consumption, are not inconsistent with this possibility. In addition, there are reports that gamma globulins and immunoglobulins are decreased in soy-fed infants compared with cow milk formula-fed controls (25, 26). T cell function appeared to be more impaired in soy-fed infants than B cell function (25). The decreased immunoglobulins and complement levels and impairments in T cell function suggested that soy-fed infants could have impaired humoral and cell-mediated immunity. Infants fed soy formula did indeed have reduced titers of antibodies against polio, tetanus, diptheria, and pertussis compared with infants that had been similarly vaccinated but fed cow milk formula (27). Morbidity, mainly upper respiratory infections and bronchitis, was increased in soy-fed vs. cow milk formula-fed infants (25, 27). Thus, the effects observed in this study with genistein effects on T cells and immune function in mice parallel some reports in human infants suggesting soy formula consumption impairs T cell and immune function while increasing morbidity.
T cell development is similar in mice and humans, so murine studies are potentially applicable to humans (28). However, extrapolation of results obtained with one species to another must be done with great caution, and clearly important questions still need to be addressed in the mouse model, such as the relative level of the aglycone and conjugated forms of genistein in mice consuming genistein relative to the soy-fed infant.
The recent popularity of dietary supplements containing either soy protein or isoflavones makes it possible for adults to ingest isoflavones doses severalfold greater than obtained with even a high-soy diet. Consumption of recommended doses of some of these products exposes adults to isoflavone levels similar to those in soy-fed infants on a per-weight basis, and plasma genistein levels in the soy-fed infant range were reported in adults ingesting commercial supplements (29). Thymic and immune effects of fetal and neonatal estrogen treatment are extensively documented, but estrogens administered to adult mice can cause thymic and immune impairments equaling those in younger animals (30). There have been no studies of thymic and immune function in adults consuming high supplement levels, but this question needs to be addressed. However, a recent study of the effects of a synthetic isoflavone derivative on osteoporosis in women produced the unexpected finding that some of these women had lymphocytopenia, which was consistent with our present findings and suggested that the immune effects of isoflavones documented here could account for these results (31).
In conclusion, in light of our present results and other work suggesting potential immune (25, 27), reproductive (32, 33), and endocrine (34, 35) effects in infants or adults as a result of high isoflavone consumption, the use of soy formula for infant nutrition and high soy/isoflavone intake by adults through the use of supplements needs to be approached with caution.
Supplementary Material
Acknowledgments
We thank D. Gross, D. Sherwood, D. Bunick, R. Hess, and R. Bigsby for critical discussions. This work was supported by grants from the United Soybean Board and Illinois Council on Food and Agricultural Research.
Abbreviations
- ER
estrogen receptor
- KLH
keyhole limpet hemocyanin
- E2
estradiol 17-β
- PI
propidium iodide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.American Academy of Pediatrics. Pediatrics. 1998;101:148–153. [Google Scholar]
- 2.Strom B L, Schinnar R, Ziegler E E, Barnhart K T, Sammel M D, Macones G A, Stallings V A, Drulis J M, Nelson S E, Hanson S A. J Am Med Assoc. 2001;286:807–814. doi: 10.1001/jama.286.7.807. [DOI] [PubMed] [Google Scholar]
- 3.Setchell K D, Zimmer-Nechemias L, Cai J, Heubi J E. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 4.Setchell K D, Zimmer-Nechemias L, Cai J, Heubi J E. Am J Clin Nutr. 1998;68:1453S–1461S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- 5.Adlercreutz H, Markkanen H, Watanabe S. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 6.Doerge D R, Churchwell M I, Chang H C, Newbold R R, Delclos K B. Reprod Toxicol. 2001;15:105–110. doi: 10.1016/s0890-6238(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 7.Whitten P L, Patisaul H B. Environ Health Perspect. 2001;109, Suppl. 1:5–20. doi: 10.1289/ehp.01109s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohen F, Abel L, Sharp A, Amir-Zaltsman Y, Somjen D, Luria S, Mor G, Knyszynski A, Thole H, Globerson A. Dev Immunol. 1998;5:277–285. doi: 10.1155/1998/62380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen N J, Kovacs W J. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 10.Atluru D, Gudapaty S. Vet Immunol Immunopathol. 1993;38:113–122. doi: 10.1016/0165-2427(93)90117-m. [DOI] [PubMed] [Google Scholar]
- 11.Hasper H J, Weghorst R M, Richel D J, Meerwaldt J H, Olthuis F M, Schenkeveld C E. Cytometry. 2000;40:167–171. [PubMed] [Google Scholar]
- 12.Rosal A, Geuna M. Cytometry. 2001;45:151–157. doi: 10.1002/1097-0320(20011001)45:2<151::aid-cyto1157>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Muhumuza L, Segre D, Segre M. Immunology. 1998;93:572–580. doi: 10.1046/j.1365-2567.1998.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang H C, Churchwell M I, Delclos K B, Newbold R R, Doerge D R. J Nutr. 2000;130:1963–1970. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- 15.Holder C L, Churchwell M I, Doerge D R. J Agric Food Chem. 1999;47:3764–3770. doi: 10.1021/jf9902651. [DOI] [PubMed] [Google Scholar]
- 16.Essex C. Br Med J. 1996;313:507–508. doi: 10.1136/bmj.313.7056.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustelin T. Science. 1990;247:1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- 18.Okasha S A, Ryu S, Do Y, McKallip R J, Nagarkatti M, Nagarkatti P S. Toxicology. 2001;163:49–62. doi: 10.1016/s0300-483x(01)00374-2. [DOI] [PubMed] [Google Scholar]
- 19.Holladay S D, Smialowicz R J. Environ Health Perspect. 2000;108:463–473. doi: 10.1289/ehp.00108s3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luster M I, Hayes H T, Korach K, Tucker A N, Dean J H, Greenlee W F, Boorman G A. J Immunol. 1984;133:110–116. [PubMed] [Google Scholar]
- 21.Ishimi Y, Miyaura C, Ohmura M, Onoe Y, Sato T, Uchiyama Y, Ito M, Wang X, Suda T, Ikegami S. Endocrinology. 1999;140:1893–1900. doi: 10.1210/endo.140.4.6663. [DOI] [PubMed] [Google Scholar]
- 22.Ju Y H, Allred C D, Allred K F, Karko K L, Doerge D R, Helferich W G. J Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- 23.Santell R C, Kieu N, Helferich W G. J Nutr. 2000;130:1665–1669. doi: 10.1093/jn/130.7.1665. [DOI] [PubMed] [Google Scholar]
- 24.Supko J G, Malspeis L. Int J Oncol. 1995;7:847–854. doi: 10.3892/ijo.7.4.847. [DOI] [PubMed] [Google Scholar]
- 25.Zoppi G, Gerosa F, Pezzini A, Bassani N, Rizzotti P, Bellini P, Todeschini G, Zamboni G, Vazzoler G, Tridente G. J Pediatr Gastroenterol Nutr. 1982;1:175–182. doi: 10.1097/00005176-198201020-00005. [DOI] [PubMed] [Google Scholar]
- 26.Zoppi G, Zamboni G, Bassani N, Vazzoler G. Eur J Pediatr. 1979;131:61–69. doi: 10.1007/BF00442786. [DOI] [PubMed] [Google Scholar]
- 27.Zoppi G, Gasparini R, Mantovanelli F, Gobio-Casali L, Astolfi R, Crovari P. Lancet. 1983;2:11–14. doi: 10.1016/s0140-6736(83)90004-1. [DOI] [PubMed] [Google Scholar]
- 28.Fadel S, Sarzotti M. Int Rev Immunol. 2000;19:173–193. doi: 10.3109/08830180009088504. [DOI] [PubMed] [Google Scholar]
- 29.Setchell K D R, Brown N M, Pankaj D, Zimmer-Nechemias L, Wolfe B E, Brashear W T, Kirschner A S, Cassidy A, Heubi J E. J Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 30.Smith B J, Holladay S D. J Appl Toxicol. 1997;17:265–271. doi: 10.1002/(sici)1099-1263(199709)17:5<265::aid-jat451>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Alexandersen P, Toussaint A, Christiansen C, Devogelaer J P, Roux C, Fechtenbaum J, Gennari C, Reginster J Y, Study I M E F. J Am Med Assoc. 2001;285:1482–1488. doi: 10.1001/jama.285.11.1482. [DOI] [PubMed] [Google Scholar]
- 32.Newbold R R, Banks E P, Bullock B, Jefferson W N. Cancer Res. 2001;61:4325–4328. [PubMed] [Google Scholar]
- 33.Hilakivi-Clarke L, Cho E, Onojafe I, Raygada M, Clarke R. Oncol Rep. 1999;6:1089–1095. doi: 10.3892/or.6.5.1089. [DOI] [PubMed] [Google Scholar]
- 34.Faber K A, Hughes C L., Jr Reprod Toxicol. 1993;7:35–39. doi: 10.1016/0890-6238(93)90007-t. [DOI] [PubMed] [Google Scholar]
- 35.Divi R L, Chang H C, Doerge D R. Biochem Pharmacol. 1997;54:1087–1096. doi: 10.1016/s0006-2952(97)00301-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.