Abstract
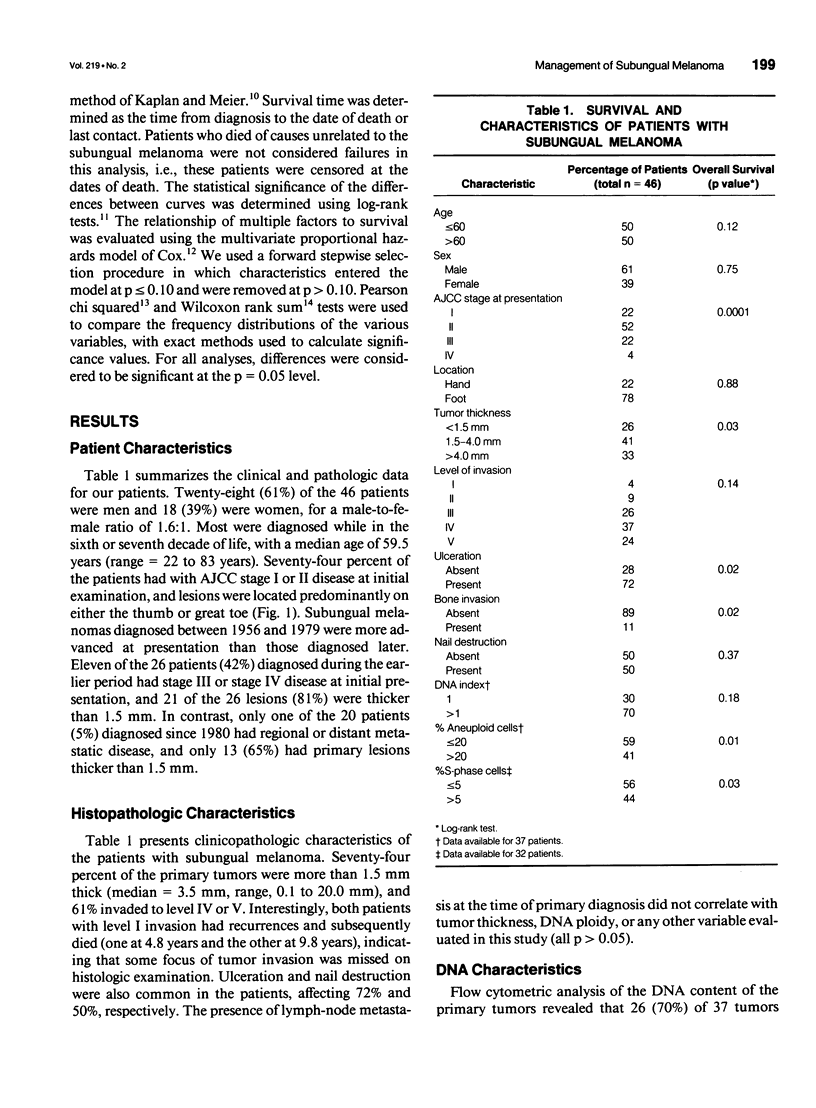
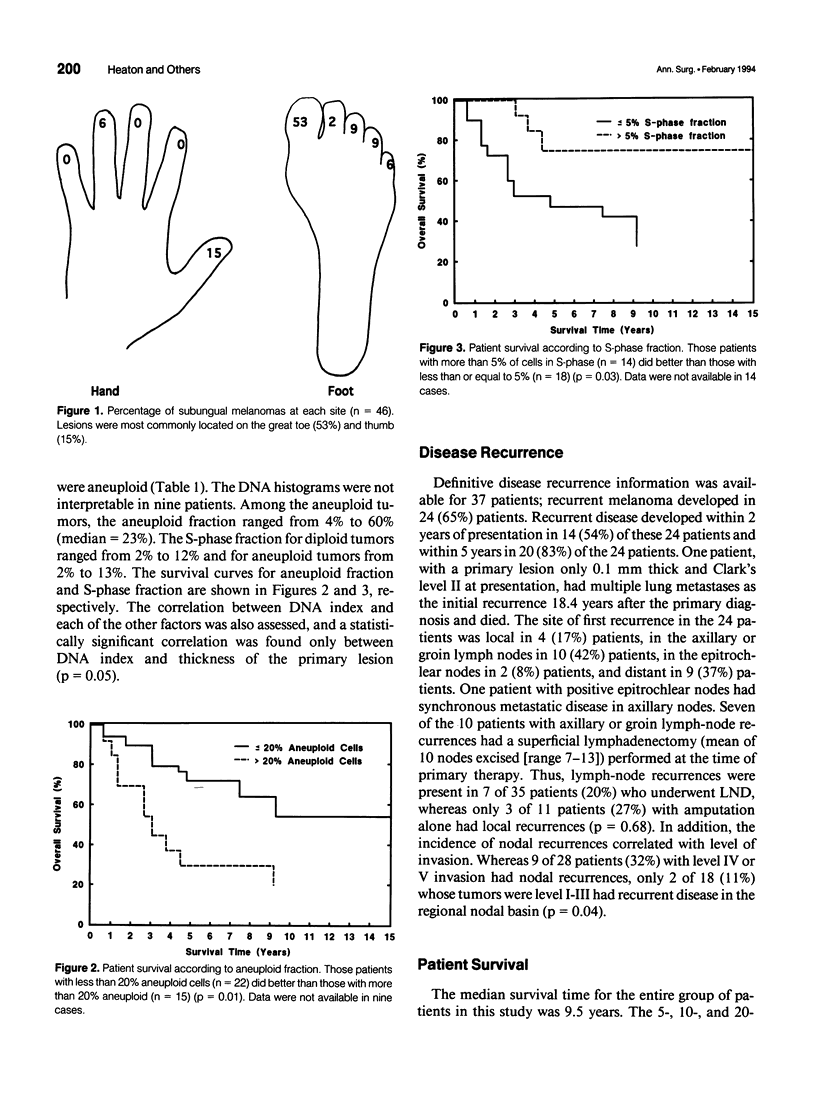
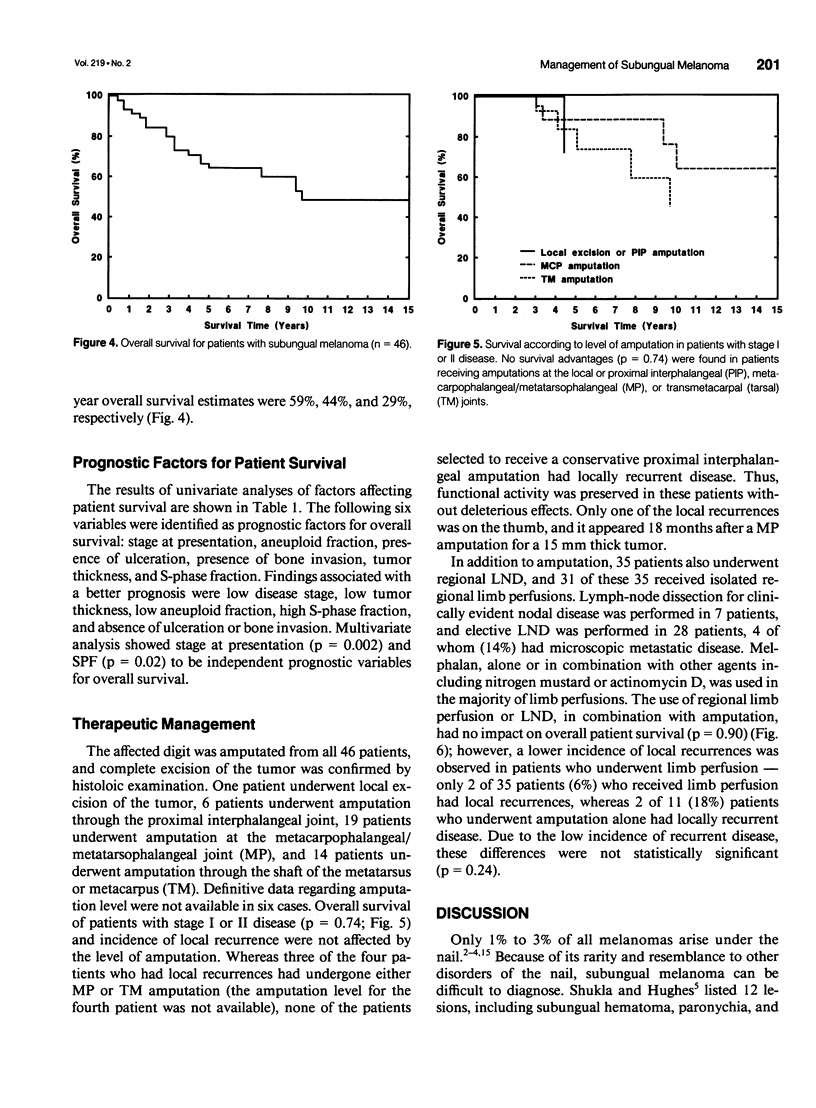
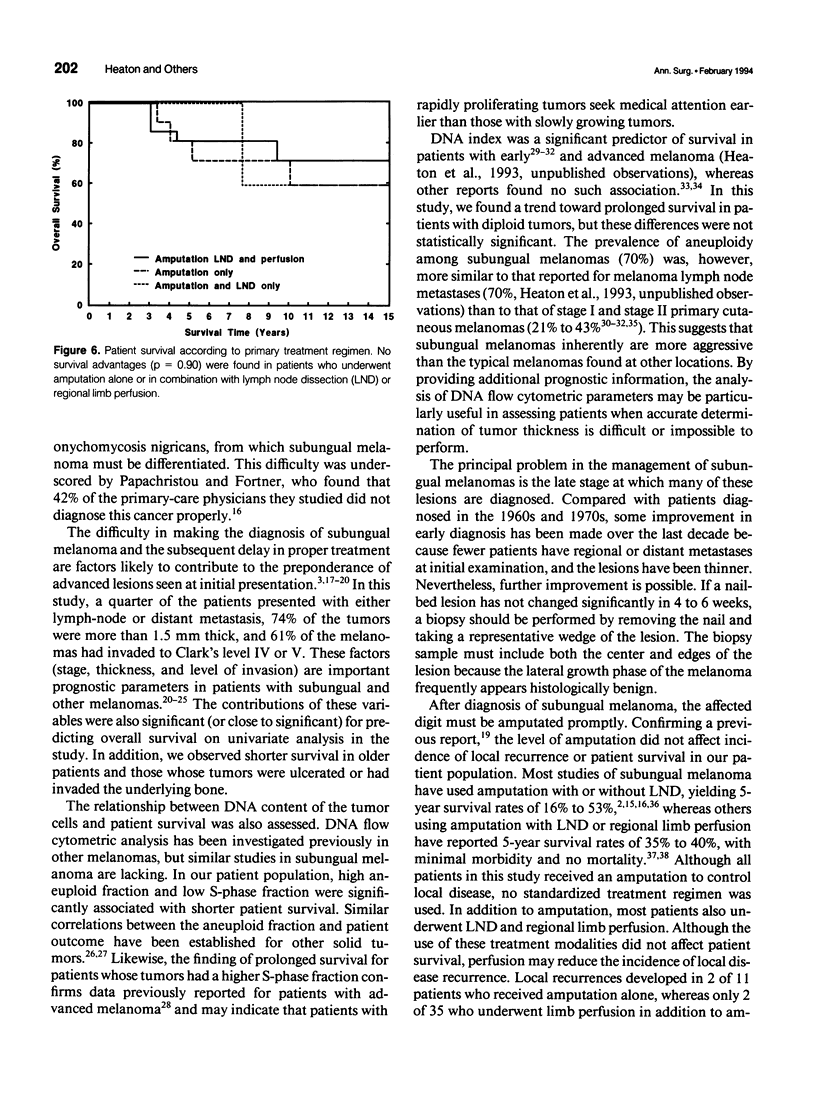
OBJECTIVE: Forty-six cases of subungual melanoma were reviewed to identify significant clinicopathologic prognostic factors, determine the role of DNA content analysis in the biologic assessment of these tumors, and evaluate the effectiveness of amputation level, lymph node dissection (LND), and regional limb perfusion on the survival of these patients. BACKGROUND: Subungual melanoma is a unique and rare subtype of melanoma, constituting only 1% to 3% of cases. Thus, little is known about prognostic factors and optimal management of patients with this disease. Moreover, the appropriate level of amputation and LND and limb perfusion in the management of subungual melanoma remain controversial. METHODS: Forty-six patients underwent amputation alone or in combination with LND and/or regional limb perfusion for primary subungual melanoma. The effects of these treatment modalities and the prognostic significance of patient and tumor-related variables, including DNA flow cytometric data, on overall survival were assessed. RESULTS: Univariate statistical analysis identified six factors that significantly affected patient survival. They were stage at diagnosis (p = 0.0001), percentage of aneuploid cells (p = 0.01), presence of ulceration (p = 0.02) or bone invasion (p = 0.02), thickness of the primary lesion (p = 0.03), and percentage of cells in S-phase (p = 0.03). Multivariate analyses identified tumor stage and S-phase fraction as independent prognostic factors in these patients. Survival did not differ among patients who received amputation alone or those who underwent amputation in combination with LND or perfusion (p = 0.90); however, the use of limb perfusion reduced the incidence of locally recurrent disease. The level of amputation did not affect patient survival (p = 0.74) or the incidence of local recurrence. CONCLUSIONS: The study identified several significant prognostic factors, including DNA flow cytometric parameters, in patients with subungual melanoma. In addition, it showed that conservative amputation of the affected digit at the level of the proximal interphalangeal or metacarpophalangeal/metatarsophalangeal joint appears to be safe, provided that clear margins are obtained. Although isolated limb perfusion may reduce the incidence of local recurrence, LND, or limb perfusion in the routine management of subungual melanoma remains controversial.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P. C., Hoekstra H. J., Schraffordt Koops H., Oosterhuis J. W., Oldhoff J. Isolated regional perfusion in the treatment of subungual melanoma. Arch Surg. 1989 Mar;124(3):373–376. doi: 10.1001/archsurg.1989.01410030123020. [DOI] [PubMed] [Google Scholar]
- Baisch H., Göhde W., Linden W. A. Analysis of PCP-data to determine the fraction of cells in the various phases of cell cycle. Radiat Environ Biophys. 1975 Jun 13;12(1):31–39. doi: 10.1007/BF02339807. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Murad T. M., Soong S. J., Ingalls A. L., Richards P. C., Maddox W. A. Tumor thickness as a guide to surgical management of clinical stage I melanoma patients. Cancer. 1979 Mar;43(3):883–888. doi: 10.1002/1097-0142(197903)43:3<883::aid-cncr2820430316>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Soong S. J., Milton G. W., Shaw H. M., McGovern V. J., Murad T. M., McCarthy W. H., Maddox W. A. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982 Dec;196(6):677–684. doi: 10.1097/00000658-198212001-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlogie B., Göhde W., Johnston D. A., Smallwood L., Schumann J., Drewinko B., Freireich E. J. Determination of ploidy and proliferative characteristics of human solid tumors by pulse cytophotometry. Cancer Res. 1978 Oct;38(10):3333–3339. [PubMed] [Google Scholar]
- Bartkowiak D., Schumann J., Otto F. J., Lippold A., Drepper H. DNA flow cytometry in the prognosis of primary malignant melanoma. Oncology. 1991;48(1):39–43. doi: 10.1159/000226892. [DOI] [PubMed] [Google Scholar]
- Blessing K., Kernohan N. M., Park K. G. Subungual malignant melanoma: clinicopathological features of 100 cases. Histopathology. 1991 Nov;19(5):425–429. doi: 10.1111/j.1365-2559.1991.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Collins R. J. Melanoma in the Chinese of Hong Kong. Emphasis on volar and subungual sites. Cancer. 1984 Oct 1;54(7):1482–1488. doi: 10.1002/1097-0142(19841001)54:7<1482::aid-cncr2820540745>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- DASGUPTA T., BRASFIELD R. SUBUNGUAL MELANOMA: 25-YEAR REVIEW OF CASES. Ann Surg. 1965 Apr;161:545–552. doi: 10.1097/00000658-196504000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. M., Berlin R., Urmacher C. Subungual melanoma: a 25-year review of cases. J Surg Oncol. 1987 Jun;35(2):107–112. doi: 10.1002/jso.2930350209. [DOI] [PubMed] [Google Scholar]
- Feibleman C. E., Stoll H., Maize J. C. Melanomas of the palm, sole, and nailbed: a clinicopathologic study. Cancer. 1980 Dec 1;46(11):2492–2504. doi: 10.1002/1097-0142(19801201)46:11<2492::aid-cncr2820461130>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gattuso P., Reddy V., Solans E., Kathuria S., Aranha G. V., Jacobs H. K., Walloch J. Is DNA ploidy of prognostic significance in stage I cutaneous melanoma? Surgery. 1990 Oct;108(4):702–709. [PubMed] [Google Scholar]
- Hansson J., Tribukait B., Lewensohn R., Ringborg U. Flow cytofluorometric DNA analyses of metastases of human malignant melanomas. Anal Quant Cytol. 1982 Jun;4(2):99–104. [PubMed] [Google Scholar]
- Jones D. J., Moore M., Schofield P. F. Refining the prognostic significance of DNA ploidy status in colorectal cancer: a prospective flow cytometric study. Int J Cancer. 1988 Feb 15;41(2):206–210. doi: 10.1002/ijc.2910410208. [DOI] [PubMed] [Google Scholar]
- Kheir S. M., Bines S. D., Vonroenn J. H., Soong S. J., Urist M. M., Coon J. S. Prognostic significance of DNA aneuploidy in stage I cutaneous melanoma. Ann Surg. 1988 Apr;207(4):455–461. doi: 10.1097/00000658-198804000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krementz E. T., Feed R. J., Coleman W. P., 3rd, Sutherland C. M., Carter R. D., Campbell M. Acral lentiginous melanoma. A clinicopathologic entity. Ann Surg. 1982 May;195(5):632–645. doi: 10.1097/00000658-198205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppard B., Sanderson K. V., Behan F. Subungual malignant melanoma: difficulty in diagnosis. Br Med J. 1974 Feb 23;1(5903):310–312. doi: 10.1136/bmj.1.5903.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- McLemore D. D., el Naggar A., Stephens L. C., Jardine J. H. Modified methodology to improve flow cytometric DNA histograms from paraffin-embedded material. Stain Technol. 1990;65(6):279–291. doi: 10.3109/10520299009105619. [DOI] [PubMed] [Google Scholar]
- Muchmore J. H., Krementz E. T., Carter R. D., Sutherland C. M., Godfrey R. S. Regional perfusion for the treatment of subungual melanoma. Am Surg. 1990 Feb;56(2):114–118. [PubMed] [Google Scholar]
- Muhonen T., Pyrhönen S., Laasonen A., Asko-Seljavaara S., Franssila K. DNA ploidy pattern and S-phase characteristics of metastatic melanoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(4):253–257. doi: 10.1007/BF02899554. [DOI] [PubMed] [Google Scholar]
- Pack G. T., Oropeza R. Subungual melanoma. Surg Gynecol Obstet. 1967 Mar;124(3):571–582. [PubMed] [Google Scholar]
- Papachristou D. N., Fortner J. G. Melanoma arising under the nail. J Surg Oncol. 1982 Dec;21(4):219–222. doi: 10.1002/jso.2930210405. [DOI] [PubMed] [Google Scholar]
- Park K. G., Blessing K., Kernohan N. M. Surgical aspects of subungual malignant melanomas. The Scottish Melanoma Group. Ann Surg. 1992 Dec;216(6):692–695. doi: 10.1097/00000658-199212000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. H., Helwig E. B. Subungual malignant melanoma: a clinical-pathologic study. Cancer. 1980 Nov 1;46(9):2074–2087. doi: 10.1002/1097-0142(19801101)46:9<2074::aid-cncr2820460928>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Scott N. A., Rainwater L. M., Wieand H. S., Weiland L. H., Pemberton J. H., Beart R. W., Jr, Lieber M. M. The relative prognostic value of flow cytometric DNA analysis and conventional clinicopathologic criteria in patients with operable rectal carcinoma. Dis Colon Rectum. 1987 Jul;30(7):513–520. doi: 10.1007/BF02554780. [DOI] [PubMed] [Google Scholar]
- Shukla V. K., Hughes L. E. Differential diagnosis of subungual melanoma from a surgical point of view. Br J Surg. 1989 Nov;76(11):1156–1160. doi: 10.1002/bjs.1800761115. [DOI] [PubMed] [Google Scholar]
- Slingluff C. L., Jr, Vollmer R., Seigler H. F. Acral melanoma: a review of 185 patients with identification of prognostic variables. J Surg Oncol. 1990 Oct;45(2):91–98. doi: 10.1002/jso.2930450207. [DOI] [PubMed] [Google Scholar]
- Søndergaard K., Larsen J. K., Møller U., Christensen I. J., Hou-Jensen K. DNA ploidy-characteristics of human malignant melanoma analysed by flow cytometry and compared with histology and clinical course. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;42(1):43–52. doi: 10.1007/BF02890369. [DOI] [PubMed] [Google Scholar]
- Takematsu H., Obata M., Tomita Y., Kato T., Takahashi M., Abe R. Subungual melanoma. A clinicopathologic study of 16 Japanese cases. Cancer. 1985 Jun 1;55(11):2725–2731. doi: 10.1002/1097-0142(19850601)55:11<2725::aid-cncr2820551134>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Zaloudik J., Moore M., Ghosh A. K., Mechl Z., Rejthar A. DNA content and MHC class II antigen expression in malignant melanoma: clinical course. J Clin Pathol. 1988 Oct;41(10):1078–1084. doi: 10.1136/jcp.41.10.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Roenn J. H., Kheir S. M., Wolter J. M., Coon J. S. Significance of DNA abnormalities in primary malignant melanoma and nevi, a retrospective flow cytometric study. Cancer Res. 1986 Jun;46(6):3192–3195. [PubMed] [Google Scholar]


