Abstract
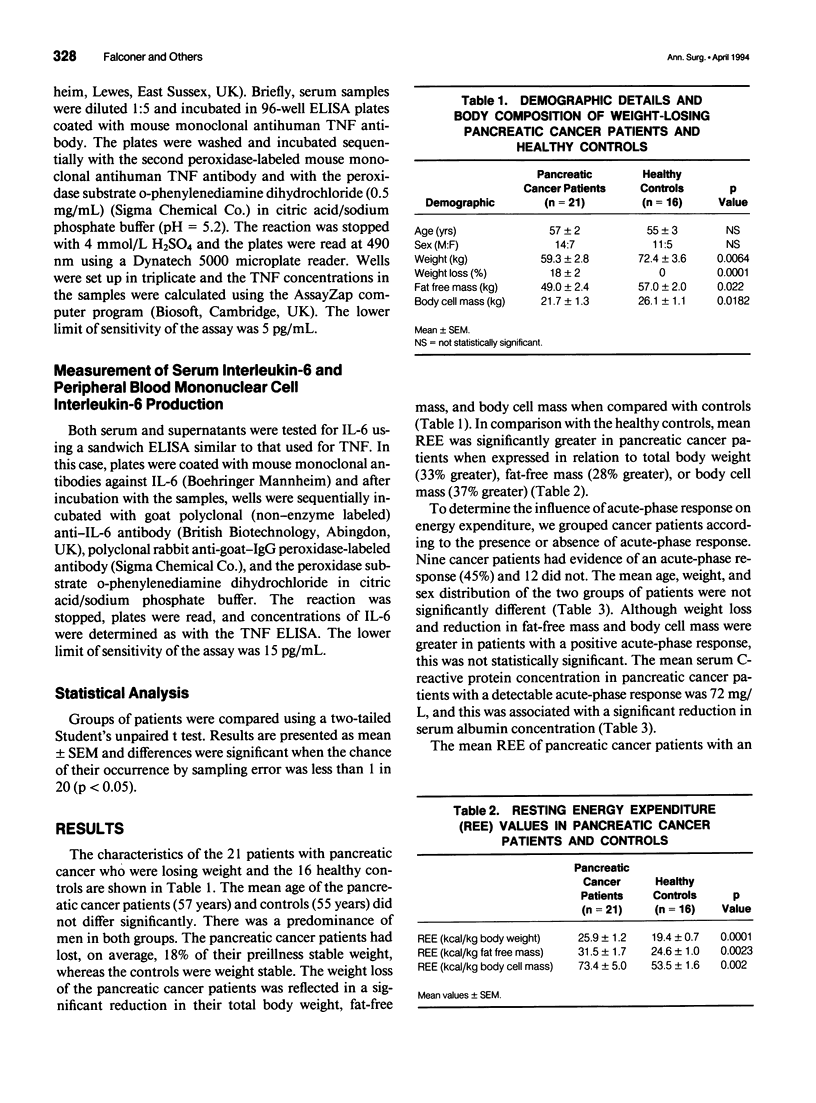
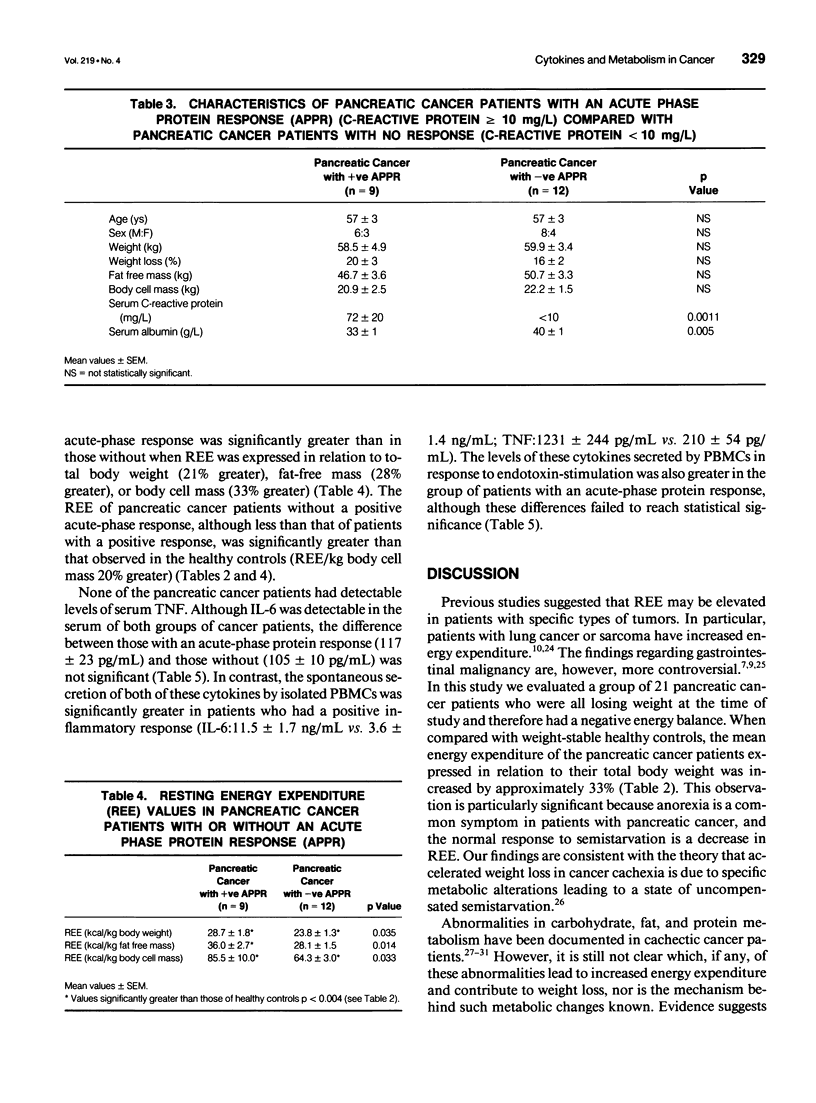
OBJECTIVE: To determine whether resting energy expenditure (REE) is increased in cachectic patients with pancreatic cancer and to define the relation of tumor necrosis factor (TNF) and interleukin-6 (IL-6) production to the acute-phase response and to REE. METHODS: Measurement of REE (indirect calorimetry) and assessment of body composition (bioelectrical impedance analysis) were done in 21 patients with unresectable pancreatic cancer and on 16 age-related controls. The systemic inflammatory response in peripheral blood of the cancer patients was assessed using the acute-phase protein, C-reactive protein, and the cytokines TNF and IL-6. Production of these cytokines by peripheral blood mononuclear cells in vitro was also measured. RESULTS: Patients with pancreatic cancer had an elevated REE when compared with controls (73.4 +/- 5.0 vs. 53.5 +/- 1.6 kcal/kg body cell mass; p < 0.003). Resting energy expenditure was significantly greater in cancer patients with an acute-phase response (C-reactive protein > 10 mg/L) than in those who did not have such a response (85.5 +/- 10.0 [n = 9] vs. 64.3 +/- 3.0 [n = 12] kcal/kg body cell mass; p < 0.04). Tumor necrosis factor was not detected in the serum of any of the cancer patients. Serum IL-6 was detected but levels were not significantly different among cancer patients with or without an acute-phase response. In contrast, spontaneous production of TNF and IL-6 by isolated peripheral blood mononuclear cells was significantly greater in cancer patients with an acute-phase response that in those without (TNF: 1231 +/- 244 vs. 210 +/- 54 pg/ml/10(5) cells; p < 0.001; IL-6: 11.5 +/- 1.7 vs. 3.6 +/- 1.4 ng/mL/10(5) cells; p < 0.003). CONCLUSIONS: In pancreatic cancer at least a component of weight loss is due to increased REE. Furthermore, the presence of an acute-phase response identifies a group of patients who are markedly hypermetabolic. The serum concentration of TNF of IL-6 does not correlate with the presence of an acute-phase response, whereas rates of cytokine production by peripheral blood mononuclear cells are significantly greater in patients with such a response. This suggests that local rather than systemic cytokine production may be important in regulating the acute-phase response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan M. F. Uncomplicated starvation versus cancer cachexia. Cancer Res. 1977 Jul;37(7 Pt 2):2359–2364. [PubMed] [Google Scholar]
- Cohn S. H., Vartsky D., Vaswani A. N., Sawitsky A., Rai K., Gartenhaus W., Yasumura S., Ellis K. J. Changes in body composition of cancer patients following combined nutritional support. Nutr Cancer. 1982;4(2):107–119. doi: 10.1080/01635588209513746. [DOI] [PubMed] [Google Scholar]
- Dempsey D. T., Feurer I. D., Knox L. S., Crosby L. O., Buzby G. P., Mullen J. L. Energy expenditure in malnourished gastrointestinal cancer patients. Cancer. 1984 Mar 15;53(6):1265–1273. doi: 10.1002/1097-0142(19840315)53:6<1265::aid-cncr2820530609>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Dewys W. D., Begg C., Lavin P. T., Band P. R., Bennett J. M., Bertino J. R., Cohen M. H., Douglass H. O., Jr, Engstrom P. F., Ezdinli E. Z. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980 Oct;69(4):491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- Edén E., Edström S., Bennegárd K., Lindmark L., Lundholm K. Glycerol dynamics in weight-losing cancer patients. Surgery. 1985 Feb;97(2):176–184. [PubMed] [Google Scholar]
- Fearon K. C., Hansell D. T., Preston T., Plumb J. A., Davies J., Shapiro D., Shenkin A., Calman K. C., Burns H. J. Influence of whole body protein turnover rate on resting energy expenditure in patients with cancer. Cancer Res. 1988 May 1;48(9):2590–2595. [PubMed] [Google Scholar]
- Fearon K. C., McMillan D. C., Preston T., Winstanley F. P., Cruickshank A. M., Shenkin A. Elevated circulating interleukin-6 is associated with an acute-phase response but reduced fixed hepatic protein synthesis in patients with cancer. Ann Surg. 1991 Jan;213(1):26–31. doi: 10.1097/00000658-199101000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon K. C., Richardson R. A., Hannan J., Cowan S., Watson W., Shenkin A., Garden O. J. Bioelectrical impedance analysis in the measurement of the body composition of surgical patients. Br J Surg. 1992 May;79(5):421–423. doi: 10.1002/bjs.1800790516. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Barber A., Manogue K., Tracey K. J., Kuo G., Fischman D. A., Cerami A. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am J Physiol. 1989 Mar;256(3 Pt 2):R659–R665. doi: 10.1152/ajpregu.1989.256.3.R659. [DOI] [PubMed] [Google Scholar]
- Fredrix E. W., Soeters P. B., Wouters E. F., Deerenberg I. M., von Meyenfeldt M. F., Saris W. H. Effect of different tumor types on resting energy expenditure. Cancer Res. 1991 Nov 15;51(22):6138–6141. [PubMed] [Google Scholar]
- Fredrix E. W., Wouters E. F., Soeters P. B., van der Aalst A. C., Kester A. D., von Meyenfeldt M. F., Saris W. H. Resting energy expenditure in patients with non-small cell lung cancer. Cancer. 1991 Oct 1;68(7):1616–1621. doi: 10.1002/1097-0142(19911001)68:7<1616::aid-cncr2820680725>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Geiger T., Andus T., Klapproth J., Hirano T., Kishimoto T., Heinrich P. C. Induction of rat acute-phase proteins by interleukin 6 in vivo. Eur J Immunol. 1988 May;18(5):717–721. doi: 10.1002/eji.1830180510. [DOI] [PubMed] [Google Scholar]
- Gelin J., Moldawer L. L., Lönnroth C., Sherry B., Chizzonite R., Lundholm K. Role of endogenous tumor necrosis factor alpha and interleukin 1 for experimental tumor growth and the development of cancer cachexia. Cancer Res. 1991 Jan 1;51(1):415–421. [PubMed] [Google Scholar]
- Hansell D. T., Davies J. W., Burns H. J. The relationship between resting energy expenditure and weight loss in benign and malignant disease. Ann Surg. 1986 Mar;203(3):240–245. doi: 10.1097/00000658-198603000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyltander A., Drott C., Körner U., Sandström R., Lundholm K. Elevated energy expenditure in cancer patients with solid tumours. Eur J Cancer. 1991;27(1):9–15. doi: 10.1016/0277-5379(91)90050-n. [DOI] [PubMed] [Google Scholar]
- Jeevanandam M., Horowitz G. D., Lowry S. F., Brennan M. F. Cancer cachexia and protein metabolism. Lancet. 1984 Jun 30;1(8392):1423–1426. doi: 10.1016/s0140-6736(84)91929-9. [DOI] [PubMed] [Google Scholar]
- Langstein H. N., Doherty G. M., Fraker D. L., Buresh C. M., Norton J. A. The roles of gamma-interferon and tumor necrosis factor alpha in an experimental rat model of cancer cachexia. Cancer Res. 1991 May 1;51(9):2302–2306. [PubMed] [Google Scholar]
- Macfie J., Burkinshaw L., Oxby C., Holmfield J. H., Hill G. L. The effect of gastrointestinal malignancy on resting metabolic expenditure. Br J Surg. 1982 Aug;69(8):443–446. doi: 10.1002/bjs.1800690803. [DOI] [PubMed] [Google Scholar]
- Makita K., Nunn J. F., Royston B. Evaluation of metabolic measuring instruments for use in critically ill patients. Crit Care Med. 1990 Jun;18(6):638–644. doi: 10.1097/00003246-199006000-00013. [DOI] [PubMed] [Google Scholar]
- Matthys P., Dijkmans R., Proost P., Van Damme J., Heremans H., Sobis H., Billiau A. Severe cachexia in mice inoculated with interferon-gamma-producing tumor cells. Int J Cancer. 1991 Aug 19;49(1):77–82. doi: 10.1002/ijc.2910490115. [DOI] [PubMed] [Google Scholar]
- McIntosh J. K., Jablons D. M., Mulé J. J., Nordan R. P., Rudikoff S., Lotze M. T., Rosenberg S. A. In vivo induction of IL-6 by administration of exogenous cytokines and detection of de novo serum levels of IL-6 in tumor-bearing mice. J Immunol. 1989 Jul 1;143(1):162–167. [PubMed] [Google Scholar]
- Moldawer L. L., Georgieff M., Lundholm K. Interleukin 1, tumour necrosis factor-alpha (cachectin) and the pathogenesis of cancer cachexia. Clin Physiol. 1987 Aug;7(4):263–274. doi: 10.1111/j.1475-097x.1987.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Nixon D. W., Lawson D. H., Kutner M., Ansley J., Schwarz M., Heymsfield S., Chawla R., Cartwright T. H., Rudman D. Hyperalimentation of the cancer patient with protein-calorie undernutrition. Cancer Res. 1981 Jun;41(6):2038–2045. [PubMed] [Google Scholar]
- Oliff A., Defeo-Jones D., Boyer M., Martinez D., Kiefer D., Vuocolo G., Wolfe A., Socher S. H. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Peacock J. L., Inculet R. I., Corsey R., Ford D. B., Rumble W. F., Lawson D., Norton J. A. Resting energy expenditure and body cell mass alterations in noncachectic patients with sarcomas. Surgery. 1987 Sep;102(3):465–472. [PubMed] [Google Scholar]
- Selby P., Hobbs S., Viner C., Jackson E., Jones A., Newell D., Calvert A. H., McElwain T., Fearon K., Humphreys J. Tumour necrosis factor in man: clinical and biological observations. Br J Cancer. 1987 Dec;56(6):803–808. doi: 10.1038/bjc.1987.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B. A., Gelin J., Fong Y., Marano M., Wei H., Cerami A., Lowry S. F., Lundholm K. G., Moldawer L. L. Anticachectin/tumor necrosis factor-alpha antibodies attenuate development of cachexia in tumor models. FASEB J. 1989 Jun;3(8):1956–1962. doi: 10.1096/fasebj.3.8.2721856. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Warren R. S., Jeevanandam M., Gabrilove J. L., Larchian W., Oettgen H. F., Brennan M. F. Tumor necrosis factor and the acute metabolic response to tissue injury in man. J Clin Invest. 1988 Oct;82(4):1321–1325. doi: 10.1172/JCI113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann G., Fong M., Kenney J. S., Jacob C. O. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992 May;89(5):1681–1684. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Wei H., Manogue K. R., Fong Y., Hesse D. G., Nguyen H. T., Kuo G. C., Beutler B., Cotran R. S., Cerami A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988 Mar 1;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIR J. B. DE B. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949 Aug;109(1-2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnold I., Lundholm K., Scherstén T. Energy balance and body composition in cancer patients. Cancer Res. 1978 Jun;38(6):1801–1807. [PubMed] [Google Scholar]
- Waterhouse C. Lactate metabolism in patients with cancer. Cancer. 1974 Jan;33(1):66–71. doi: 10.1002/1097-0142(197401)33:1<66::aid-cncr2820330113>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Alsina M. A., Chavez J. B., Bonewald L., Nishimura R., Mundy G. R. Evidence that tumor necrosis factor plays a pathogenetic role in the paraneoplastic syndromes of cachexia, hypercalcemia, and leukocytosis in a human tumor in nude mice. J Clin Invest. 1991 Mar;87(3):977–985. doi: 10.1172/JCI115106. [DOI] [PMC free article] [PubMed] [Google Scholar]


