Abstract
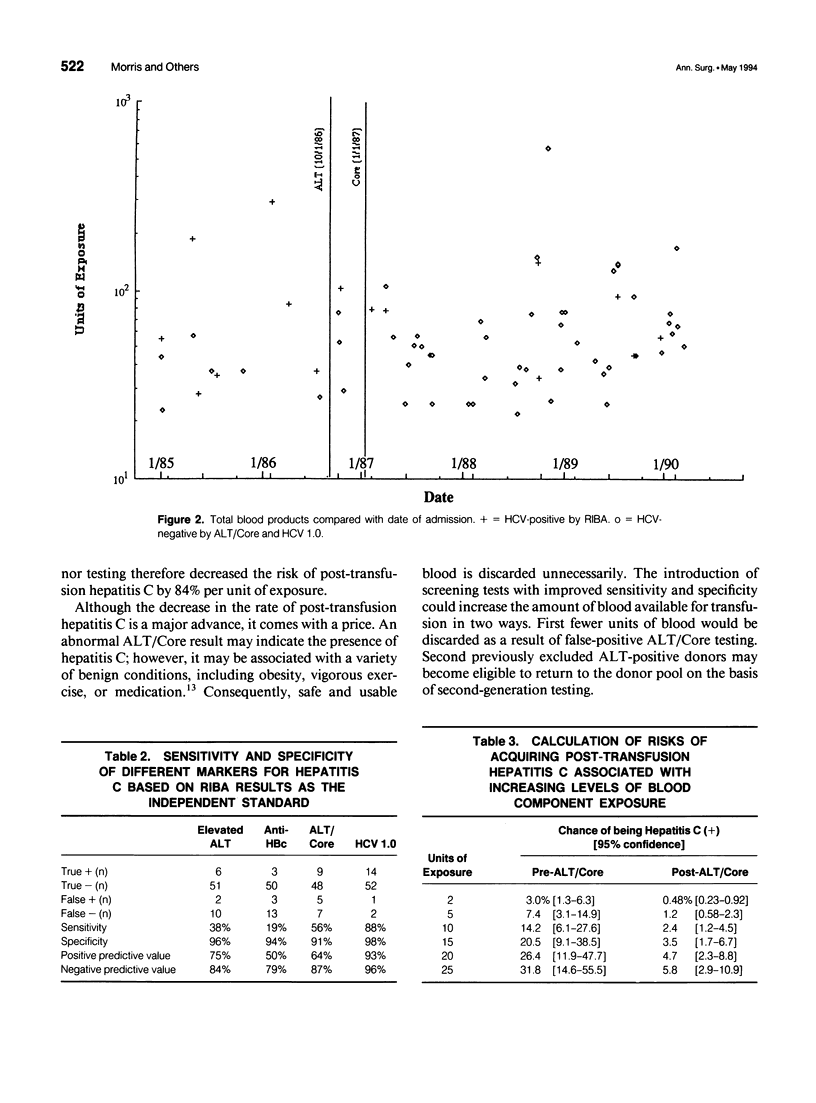
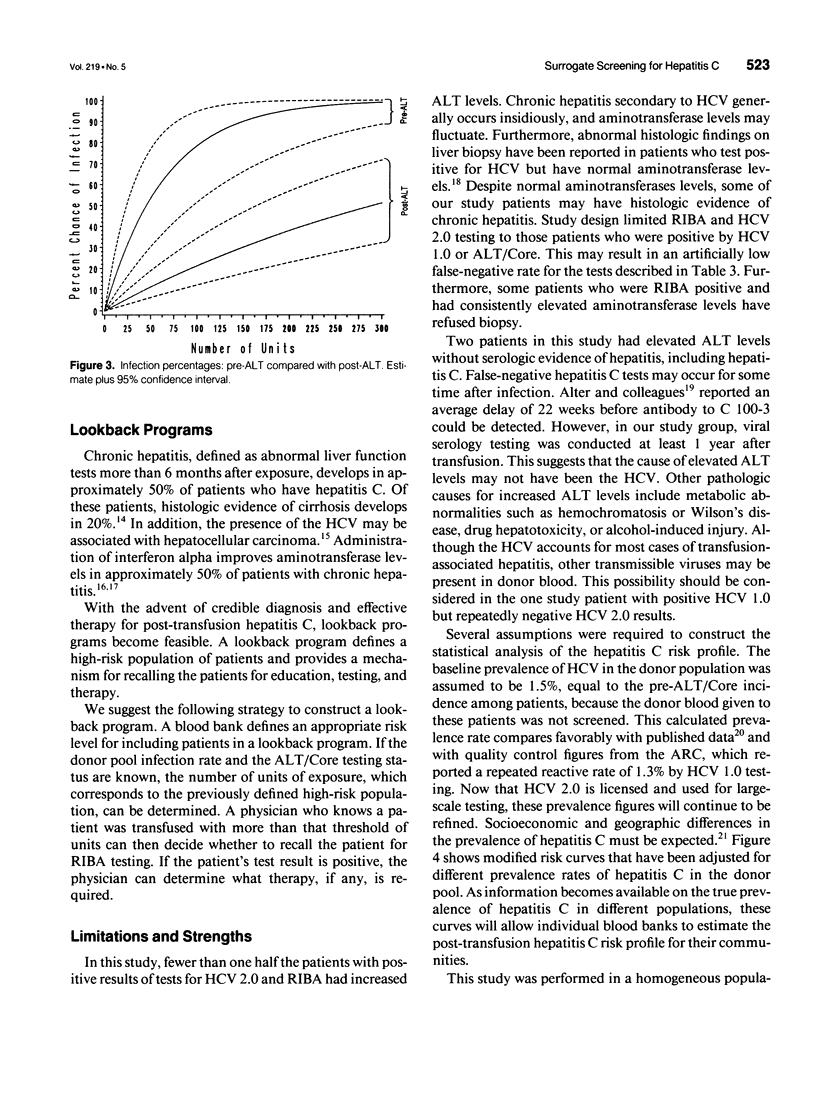
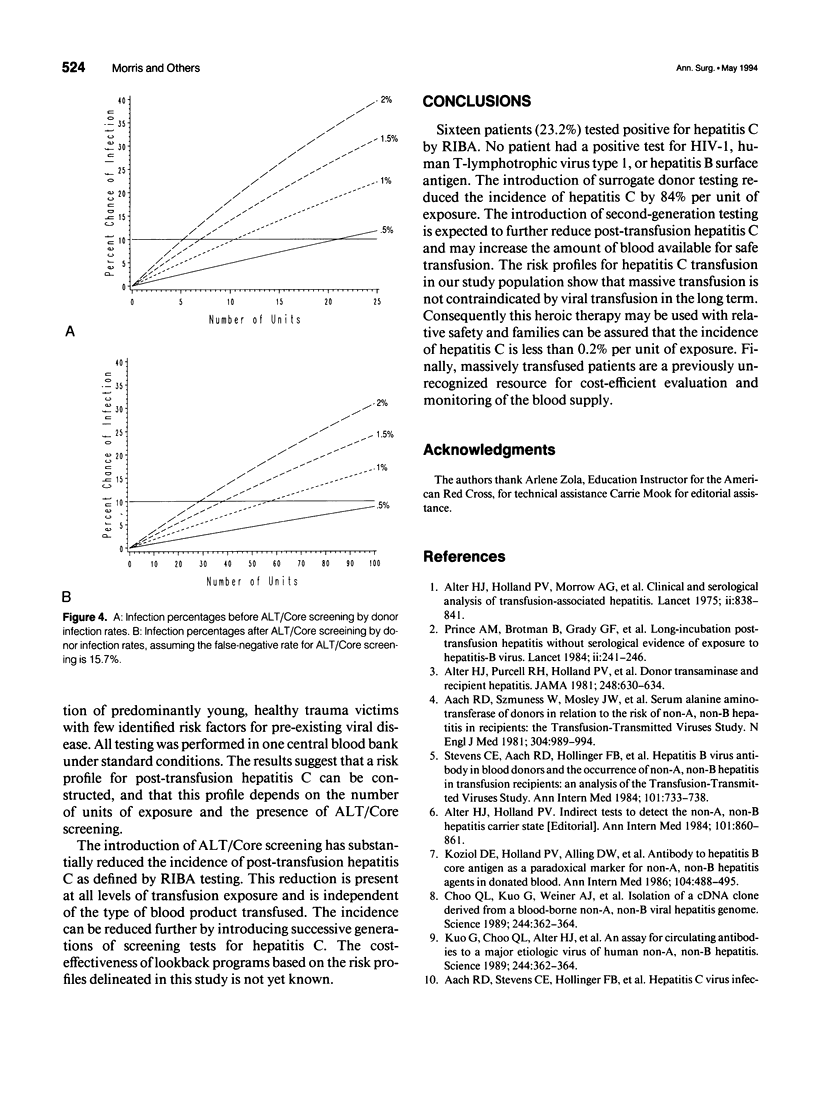
OBJECTIVE: To define a risk profile for post-transfusion hepatitis C in patients receiving massive transfusion. SUMMARY BACKGROUND DATA: Hepatitis C accounts for more than 90% of post-transfusion hepatitis. METHODS: Two-hundred twenty-one of 8,765 consecutive trauma admissions to a Level I trauma center received more than 20 units of erythrocytes. Sixty-nine survivors had positive viral serologic tests at least 1 year after transfusion. Surrogate testing for hepatitis C using alanine aminotransferase (ALT) levels and antibodies to hepatitis B core antigen (Core) began in October 1986 and January 1987, respectively. Donor blood for group 1 (pre-ALT/Core) was transfused before surrogate screening was introduced. Donor blood for group 2 (post-ALT/Core) was transfused after surrogate screening. RESULTS: Sixty-nine patients received blood products from 4,987 donors (mean, 72.3 units of exposure). No patient tested positive for antibodies to hepatitis B surface antigen, human immunodeficiency virus, or human T-lymphotrophic virus type 1. However 23.2% tested positive for hepatitis C virus (HCV) as measured by a second-generation enzyme immunoassay (HCV 2.0) and a recombinant immunoblot assay (RIBA), and 21.7% tested positive by HCV 1.0. Antibodies to Core were found in 8.7% of patients. The risk for post-transfusion hepatitis C per unit of exposure is estimated to be 1.52% group 1 (pre-ALT/Core) and 0.239% for group 2 (post-ALT/Core). CONCLUSIONS: The introduction of ALT/Core donor screening by a blood bank reduced the incidence of post-transfusion hepatitis C by 84%. The risk for post-transfusion hepatitis C depends on units of exposure, screening techniques, and prevalence of hepatitis C in the donor population. In our community, the risk for post-transfusion hepatitis C is less than 0.2% per unit of exposure. The population of massively transfused patients may serve as our effective resource for monitoring the safety of the blood supply.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aach R. D., Szmuness W., Mosley J. W., Hollinger F. B., Kahn R. A., Stevens C. E., Edwards V. M., Werch J. Serum alanine aminotransferase of donors in relation to the risk of non-A,non-B hepatitis in recipients: the transfusion-transmitted viruses study. N Engl J Med. 1981 Apr 23;304(17):989–994. doi: 10.1056/NEJM198104233041701. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Holland P. V. Indirect tests to detect the non-A, non-B hepatitis carrier state. Ann Intern Med. 1984 Dec;101(6):859–861. doi: 10.7326/0003-4819-101-6-859. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Holland P. V., Morrow A. G., Purcell R. H., Feinstone S. M., Moritsugu Y. Clinical and serological analysis of transfusion-associated hepatitis. Lancet. 1975 Nov 1;2(7940):838–841. doi: 10.1016/s0140-6736(75)90234-2. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Purcell R. H., Holland P. V., Alling D. W., Koziol D. E. Donor transaminase and recipient hepatitis. Impact on blood transfusion services. JAMA. 1981 Aug 7;246(6):630–634. [PubMed] [Google Scholar]
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Davis G. L., Balart L. A., Schiff E. R., Lindsay K., Bodenheimer H. C., Jr, Perrillo R. P., Carey W., Jacobson I. M., Payne J., Dienstag J. L. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989 Nov 30;321(22):1501–1506. doi: 10.1056/NEJM198911303212203. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L. Non-A, non-B hepatitis. I. Recognition, epidemiology, and clinical features. Gastroenterology. 1983 Aug;85(2):439–462. [PubMed] [Google Scholar]
- Hasan F., Jeffers L. J., De Medina M., Reddy K. R., Parker T., Schiff E. R., Houghton M., Choo Q. L., Kuo G. Hepatitis C-associated hepatocellular carcinoma. Hepatology. 1990 Sep;12(3 Pt 1):589–591. doi: 10.1002/hep.1840120323. [DOI] [PubMed] [Google Scholar]
- Hoofnagle J. H., Mullen K. D., Jones D. B., Rustgi V., Di Bisceglie A., Peters M., Waggoner J. G., Park Y., Jones E. A. Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986 Dec 18;315(25):1575–1578. doi: 10.1056/NEJM198612183152503. [DOI] [PubMed] [Google Scholar]
- Kelen G. D., Green G. B., Purcell R. H., Chan D. W., Qaqish B. F., Sivertson K. T., Quinn T. C. Hepatitis B and hepatitis C in emergency department patients. N Engl J Med. 1992 May 21;326(21):1399–1404. doi: 10.1056/NEJM199205213262105. [DOI] [PubMed] [Google Scholar]
- Klein H. G. Controversies in transfusion medicine. Alanine aminotransferase screening of blood donors: pro. Transfusion. 1990 May;30(4):363–367. doi: 10.1046/j.1537-2995.1990.30490273446.x. [DOI] [PubMed] [Google Scholar]
- Koziol D. E., Holland P. V., Alling D. W., Melpolder J. C., Solomon R. E., Purcell R. H., Hudson L. M., Shoup F. J., Krakauer H., Alter H. J. Antibody to hepatitis B core antigen as a paradoxical marker for non-A, non-B hepatitis agents in donated blood. Ann Intern Med. 1986 Apr;104(4):488–495. doi: 10.7326/0003-4819-104-4-488. [DOI] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Sherman K. E., Dodd R. Y. Alanine aminotransferase levels among volunteer blood donors: geographic variation and risk factors. J Infect Dis. 1982 Mar;145(3):383–386. doi: 10.1093/infdis/145.3.383. [DOI] [PubMed] [Google Scholar]
- Stevens C. E., Aach R. D., Hollinger F. B., Mosley J. W., Szmuness W., Kahn R., Werch J., Edwards V. Hepatitis B virus antibody in blood donors and the occurrence of non-A, non-B hepatitis in transfusion recipients. An analysis of the Transfusion-Transmitted Viruses Study. Ann Intern Med. 1984 Dec;101(6):733–738. doi: 10.7326/0003-4819-101-6-733. [DOI] [PubMed] [Google Scholar]
- Sørensen T. I., Orholm M., Bentsen K. D., Høybye G., Eghøje K., Christoffersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet. 1984 Aug 4;2(8397):241–244. doi: 10.1016/s0140-6736(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Van der Poel C. L., Cuypers H. T., Reesink H. W., Weiner A. J., Quan S., Di Nello R., Van Boven J. J., Winkel I., Mulder-Folkerts D., Exel-Oehlers P. J. Confirmation of hepatitis C virus infection by new four-antigen recombinant immunoblot assay. Lancet. 1991 Feb 9;337(8737):317–319. doi: 10.1016/0140-6736(91)90942-i. [DOI] [PubMed] [Google Scholar]


