Abstract
The inositol trisphosphate (InsP3) receptor (InsP3R) is a ubiquitously expressed intracellular Ca2+ channel that mediates complex cytoplasmic Ca2+ signals, regulating diverse cellular processes, including synaptic plasticity. Activation of the InsP3R channel is normally thought to require binding of InsP3 derived from receptor-mediated activation of phosphatidylinositol lipid hydrolysis. Here we identify a family of neuronal Ca2+-binding proteins as high-affinity protein agonists of the InsP3R, which bind to the channel and activate gating in the absence of InsP3. CaBP/caldendrin, a subfamily of the EF-hand-containing neuronal calcium sensor family of calmodulin-related proteins, bind specifically to the InsP3-binding region of all three InsP3R channel isoforms with high affinity (Ka ≈ 25 nM) in a Ca2+-dependent manner (Ka ≈ 1 μM). Binding activates single-channel gating as efficaciously as InsP3, dependent on functional EF-hands in CaBP. In contrast, calmodulin neither bound with high affinity nor activated channel gating. CaBP1 and the type 1 InsP3R associate in rat whole brain and cerebellum lysates, and colocalize extensively in subcellular regions in cerebellar Purkinje neurons. Thus, InsP3R-mediated Ca2+ signaling in cells is possible even in the absence of InsP3 generation, a process that may be particularly important in responding to and shaping changes in intracellular Ca2+ concentration by InsP3-independent pathways and for localizing InsP3-mediated Ca2+ signals to individual synapses.
The inositol trisphosphate signaling pathway is present in nearly all cells. Activation of phospholipase C by G-protein-coupled receptors and receptor tyrosine kinases results in the hydrolysis of the membrane lipid phosphatidylinositol 4,5-bisphosphate to two products, diacylglycerol and the water-soluble inositol 1,4,5-trisphosphate (InsP3) (1). InsP3 diffuses in the cytoplasm and binds to a receptor, the InsP3R, an integral membrane protein in the endoplasmic reticulum (ER), activating it as a Ca2+ channel to liberate stored Ca2+ from the ER lumen into the cytoplasm. This rapid release of Ca2+ modulates the cytoplasmic free Ca2+ concentration ([Ca2+]i), providing a ubiquitous intracellular signal with highly complex features that endow it with high temporal and spatial specificity (1, 2). The InsP3-mediated [Ca2+]i-signaling system regulates a diversity of cellular processes, including secretion, contraction, gene transcription, intercellular communication, membrane transport, and synaptic plasticity (1, 3).
The ability of the InsP3 signaling pathway to be at once ubiquitously expressed but nevertheless provide highly specific spatiotemporal [Ca2+]i signals has been attributed to the diversity of InsP3R isoform expression (4, 5), subcellular distributions of the InsP3R channels (6–10), and complex regulation of the channel by both InsP3 and [Ca2+]i (1, 11, 12). Gating of the InsP3-liganded InsP3R channel is modulated with a biphasic dependence on [Ca2+]i (13–15). Importantly, Ca2+ binding to specific sites associated with the channel is necessary for InsP3 to activate the channel. The requirement for Ca2+ binding enables the InsP3R to participate in Ca2+-induced Ca2+ release (CICR), believed to be the fundamental feature that determines the spatial extent and magnitude of InsP3-induced [Ca2+]i signals (2, 16). Furthermore, the requirement for both InsP3 and Ca2+ binding enables the channel to function as a coincidence detector, which is believed to be important in determining the fidelity of signaling and in physiological processes, including synaptic plasticity (17). Here, we show that a family of Ca2+ sensor proteins (CaBPs) can relieve the requirement for InsP3 in InsP3R channel activation by acting as direct ligands of the channel. CaBPs bind to the InsP3-binding domain of the channel with high affinity in a Ca2+-sensitive manner and activate channel gating in the absence of InsP3.
Materials and Methods
Yeast Two-Hybrid.
The cDNA encoding the NH2-terminal 600 aa of the rat InsP3R-3 was cloned into pLexA and used as a bait to screen a human brain cDNA library (Invitrogen). Identification of positive colonies from ≈6 × 106 primary transformants, recovery of library plasmids, and identification of prey sequences were performed according to the manufacturer's instructions (CLONTECH) and as described (18). Positive plasmids were confirmed by retransformation. Nonspecific interactions were detected by cotransformation with library plasmids and the pLexA vector.
Cell Culture, Molecular Biology, and Biochemistry.
COS-7 (Cercopithecus aethiops kidney) cells, grown in DMEM/high-glucose medium containing 10% FBS (GIBCO/BLR), were transfected with Lipofectamine (GIBCO/BLR) according to manufacturer's instructions. After 60 h, the cells were washed twice with 1× PBS and harvested in 1 ml of lysis buffer (10 mM Hepes, 250 mM NaCl, pH 7.1, 1% Triton X-100, and protease inhibitors). Coimmunoprecipitation and Western blotting were performed according to standard protocols. Unless specified, the Ca2+ concentration was estimated as ≈80 μM in lysates used in coimmunoprecipitation experiments. pGST-CaBP1 was constructed by cloning the cDNA corresponding to residues 19 to the COOH terminus (numbering based on s-CaBP1) from a positive clone in pYESTrp into the pGEX-6P-1 vector (Amersham Pharmacia). Glutathione S-transferase (GST)-CaBP1 was expressed in BL-21 (Stratagene) and purified on glutathione-Sepharose 4B (Amersham Pharmacia). Lysates, prepared from Xenopus oocytes as described (14, 19) and supplemented with an additional 100 mM NaCl and 500 μM free Ca2+ (4.5 mM EGTA, 5 mM Ca2+), were incubated with the GST-CaBP1 for 1 h at 4°C. The beads were centrifuged to remove the supernatant, washed three times with lysis buffer, and prepared for Western blot analysis. Δ1–600-InsP3R-3 was constructed in a modified pSP64 vector (pSP-InsP3R-3-Δ1–600) by PCR-amplification from pSP-InsP3R-3 (19). Production of mRNA and its injection into oocytes were performed as described (14, 19). CaBP1 (residues 19 to the COOH terminus) was removed from GST-CaBP1 by digestion with PreScission protease (Amersham Pharmacia), and after elimination of the protease by using GSH-Sepharose 4B, it was dialyzed with Path buffer (10 mM Hepes/140 mM KCl, pH 7.2) and concentrated by using a 5 kDa concentrator (Millipore). Purified s-CaBP1 protein was prepared as described (20).
Electrophysiology.
Patch-clamp experiments were performed by using isolated Xenopus oocyte nuclei as previously described (7, 13, 21, 22). Single-channel currents were amplified with antialiasing filtering at 1 kHz and digitized at 5 kHz. Pipette solutions contained 140 mM KCl and 10 mM Hepes with pH adjusted to 7.1 by using KOH. By using K+ as the current carrier and appropriate quantities of the high-affinity Ca2+ chelator BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) (100–1000 μM), or the low-affinity Ca2+ chelator 5,5′-dibromo-BAPTA (100–400 μM), or ATP (0.5 mM) alone to buffer Ca2+ in the experimental solutions, free Ca2+ concentrations were tightly controlled. Bath solutions had 140 mM KCl, 10 mM Hepes, 380 μM CaCl2, 500 μM BAPTA ([Ca2+] = 500 nM), and pH 7.1. Only single-channel records of sufficient duration were used in analysis of open probability (Po).
Immunofluorescence Microscopy.
Because antibodies to both InsP3R and CaBP were raised in rabbits, rat brain sections were incubated sequentially as follows: CaBP1 antibody, excess goat anti-rabbit Fab′ fragments conjugated to FITC (to cover all rabbit epitopes), InsP3R-1 antiserum, and goat anti-rabbit IgG conjugated to rhodamine. Control experiments were similar except that antiserum against InsP3R-1 was omitted. CaBP1-staining was specific because it was blocked by preabsorbing the antibody with CaBP1 peptide. Binding of the rhodamine-conjugated anti-rabbit antibody to the anti-CaBP1 antibody was insignificant, as demonstrated by the absence of labeling of stellate cells (see Results) with the rhodamine dye. Images were obtained with a Confocal Microscope (Leica).
Results
Identification of CaBPs as InsP3R-Interacting Proteins.
To explore the possibility that InsP3 and Ca2+-coincidence detection by the InsP3R channel could be regulated in cells through protein interactions with the channel, we used the yeast two-hybrid system to identify proteins that interact with the NH2-terminal 600 aa of the rat type 3 InsP3R (r-InsP3R-3). This region is present on the cytoplasmic portion of the channel and contains the InsP3-binding domain, believed to lie within a region extending approximately from residue 225 to residue 580 (23–25). We screened a human brain cDNA library and identified all 14 positive clones as COOH-terminal fragments of a previously described gene family termed CaBP (20). CaBPs, originally cloned from retinal cDNA libraries (20), belong to the neuronal Ca2+-binding protein (NCBP) subset of EF-hand-containing proteins. NCBP family members include recoverin, hippocalcin, neuronal calcium sensor-1 (frequenin), visinin, VILIPs, GCAPs, and KChips (calsenilin) (20, 26). NCBPs are similar to calmodulin family members in having four EF-hand motifs, but they are distinguished in that one or two of the motifs may be nonfunctional in Ca2+ binding, and they frequently are myristoylated at the NH2 terminus (20, 26). The CaBP subfamily is distinguished by its unique combination of functional EF-hand motifs, with the second EF-hand disabled, and by the presence of an extra turn in the central α-helix. Five members have been identified: CaBP1–5 (20) (Fig. 1A). Alternative splicing of the NH2-terminal regions also generates long and short forms of CaBP1 and CaBP2 (20). Another identified protein from this family termed caldendrin may represent a third splice variant of CaBP1 (20, 27). A protein containing the distal two EF-hands has been termed calbrain (28) but it is probably a partial clone of CaBP1 (20). Our screen identified caldendrin and CaBP1, which share identical COOH termini containing the EF-hand motifs. The longest clone identified in our two hybrid screen encompassed the COOH-terminal 256 aa containing all four EF-hands, whereas the shortest clone represented the COOH-terminal 103 aa containing three EF-hands, suggesting that CaBP1 and caldendrin interacted with the InsP3-binding region of the r-InsP3R-3 through their COOH-terminal EF-hand-containing region.
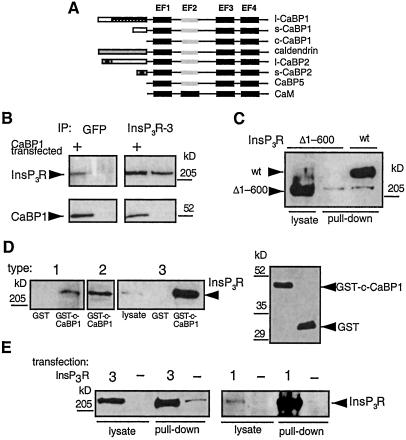
Figure 1.
Interaction of the InsP3R with CaBP1. (A) Domain structures of CaBPs and calmodulin. (B) Coimmunoprecipitation of CaBP1 and InsP3R-3 from control COS-7 cells (lanes 2 and 4) and COS-7 cells transiently transfected with s-CaBP1-GFP (lanes 1 and 3). Immunoprecipitates were probed with an InsP3R type 3-specific antibody (Transduction Laboratories, Lexington, KY), (Upper) or anti-CaBP1 antibody (Lower). COS-7 cells do not express endogenous CaBP1 (lane 4). The InsP3R-3 antibody does not interact with CaBP1 or GFP (not shown). (C) In vitro binding of InsP3R-3 to CaBP1 requires the NH2-terminal 600 residues of the InsP3R. Lysates from Xenopus oocytes expressing full-length r-InsP3R-3 (lane 3, lysate from 50 oocytes) or type 3 InsP3R lacking the first 600 residues (Δ1–600-InsP3R-3) (lane 2, lysate from 50 oocytes) were incubated with GST-CaBP1, and bound InsP3R was detected with a COOH-terminal InsP3R-3 antibody (49). Expression of Δ1–600-InsP3R-3 was verified by immunoprecipitation and Western blotting (lane 1, lysate from 14 oocytes). (D) All three mammalian InsP3R isoforms interact with CaBP1 in vitro. COS-7 cell lysates were incubated with GST-c-CaBP1, and bound InsP3R was detected with isoform-specific antibodies. Type 1 was pulled down with GST only (in 5 mg of lysate, lane 1) or with GST-c-CaBP1 (from 5 mg of lysate, lane 2), type 2 in GST-c-CaBP1 pull-down from 1.25 mg of lysate (lane 3), and type 3 present in 50 μg of lysate (lane 4), in pull-down with GST only (from 1.25 mg of lysate) and in pull-down with GST-c-CaBP1 (from 1.25 of mg lysate). Equivalent GST-fusion protein concentrations were present in in vitro binding reactions as shown in Right a Western blot with anti-GST antibody (Amersham Pharmacia). Intensities are within the linear range. Inspection of intensities and normalization of lysates used indicate stoichiometric interaction of InsP3R and CaBP1. (E) Homotetrameric rat types 1 and 3 InsP3R isoforms interact with CaBP1. Lysates from control COS-7 cells (−) or COS-7 cells transfected with types 3 (3, Left) or 1 (1, Right) InsP3R was incubated with GST-CaBP1, and bound InsP3R was detected with isoform-specific antibodies. Type 3 (Left): 5 μg or 250 μg of cell lysate used in first and second pairs of lanes, respectively. Type 1 (Right): 25 μg or 250 μg of cell lysate used in third and fourth pairs of lanes, respectively. Because of high level overexpression of the InsP3R, pull-down intensity is not proportional to the amount of InsP3R input in this experiment.
To confirm in mammalian cells the protein interactions detected in yeast, a green fluorescent protein (GFP)-tagged short splice variant of human CaBP1 (s-CaBP1-GFP) (20) (Fig. 1B) was expressed in COS-7 cells, which endogenously express the InsP3R-3 (29). Immunoprecipitation of the InsP3R-3 with an isoform-specific monoclonal antibody efficiently coprecipitated CaBP1, detected by using a CaBP1 polyclonal antibody (Fig. 1B, lane 3). In the reciprocal experiment, immunoprecipitation of CaBP1 with an anti-GFP antibody (Chemicon) coprecipitated InsP3R-3 (Fig. 1B, lane 1). These results confirm and extend the observations in yeast by demonstrating that the full-length proteins can interact.
To determine whether the NH2-terminal 600 residues used in the two-hybrid screen represented the only region of the InsP3R involved in the binding to CaBP1, in vitro “pull-down” assays were used. The conserved COOH terminus of CaBP1/caldendrin (c-CaBP1) was fused to GST to generate a fusion protein (GST-c-CaBP1) that was coupled to glutathione-Sepharose beads. Full-length r-InsP3R-3 or a mutant type 3 InsP3R lacking the first 600 residues (Δ1–600-InsP3R-3) was expressed in Xenopus oocytes (Fig. 1C, lanes 3 and 1, respectively), which lack an endogenous type 3 receptor (19), and cellular lysates were passed over the GST-c-CaBP1 column. The full-length InsP3R-3 was efficiently pulled down by GST-c-CaBP1 (Fig. 1C, lane 3), whereas the Δ1–600-InsP3R-3 was not detected in the pull-down (Fig. 1C, lane 2). Therefore, amino acids contained within the NH2-terminal 600 residues of the InsP3R-3 are both necessary and sufficient for the interaction with CaBP1. These results also demonstrate that the conserved COOH-terminal region of CaBP1/caldendrin containing the four EF-hands mediates the binding, as inferred from the two-hybrid results.
The NH2-terminal ligand-binding region used in the two-hybrid screen is conserved among the three different InsP3R isoforms (30), suggesting that in addition to the type 3 receptor, CaBP1/caldendrin might bind to the corresponding regions in the other two isoforms as well. To determine whether CaBP1 also binds to other InsP3R isoforms, GST-CaBP1 was used in pull-down experiments by using lysates from COS-7 cells, which express predominantly the types 2 and 3 InsP3R isoforms as well as low levels of InsP3R-1 (29). All three channel isoforms bound to CaBP1 (Fig. 1D). Given its low level of expression (29), the efficient pull-down of the endogenous type 1 isoform from COS-7 cell lysates (Fig. 1D, lane 2) suggests that the CaBP1–InsP3R interaction is a high-affinity one. However, because InsP3R isoforms may exist in hetero-oligomeric complexes, it was possible that the efficient pull-down of the type 1 isoform was indirect, due to its association with the endogenous type 3 isoform bound to CaBP1. Because transiently expressed recombinant types 1 and 3 InsP3Rs do not associate in hetero-oligomeric complexes with the endogenous InsP3Rs in COS-7 cells (29), we transfected COS-7 cells with r-InsP3R-1 and determined whether the expressed protein bound to CaBP1. As a control, r-InsP3R-3 was transiently expressed in parallel experiments. Both recombinant-channel isoforms efficiently bound to CaBP1 (Fig. 1E), demonstrating that CaBP1 can interact with homotetrameric types 1 and 3 InsP3R channels. Similar experiments were not performed with the type 2 isoform, but its high sequence homology with the other two isoforms suggests that it too likely binds directly to CaBP1/caldendrin.
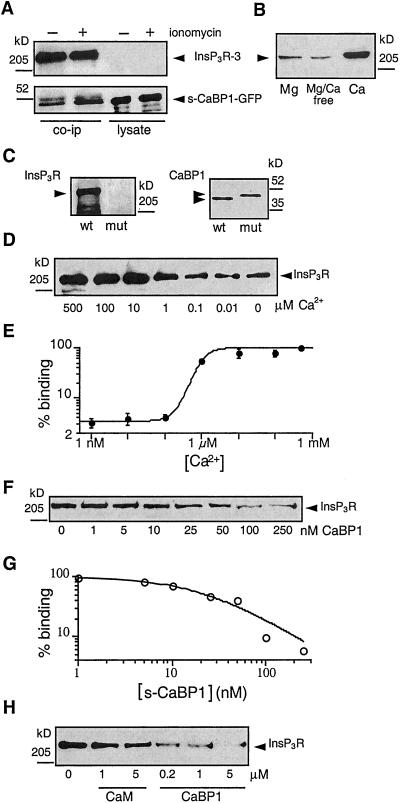
The presence of EF-hand motifs suggests that CaBPs bind Ca2+. Ca2+-binding to NCBPs induces structural alterations that affect their binding to target proteins (26, 31). To investigate the functional significance of Ca2+ binding to CaBP1 on the interaction with the InsP3R, we treated s-CaBP1-GFP-expressing COS-7 cells with the Ca2+ ionophore ionomycin (2 μM) for 2 min to raise [Ca2+]i. Treatment with ionomycin enhanced the amount of s-CaBP1-GFP detected in InsP3R-3 immunoprecipitates (Fig. 2A), suggesting that Ca2+ enhances the interaction between the two proteins. The divalent cation dependencies of binding were investigated in more detail by fixing the concentrations of free Ca2+ and Mg2+ in COS-7 cell lysates, by using EGTA or EDTA buffers. In the absence of Ca2+, raising Mg2+ had little effect on binding (Fig. 2B, lane 1 vs. 2). In contrast, raising [Ca2+] to 500 μM enhanced the binding of CaBP1 to the InsP3R (Fig. 2B, lane 3) by over 20-fold compared with that observed in the absence of Ca2+, although binding was still observed in the absence (≈2–5 nM) of Ca2+ (Fig. 2B, lane 2). To explore the requirement of Ca2+-binding to CaBP1 for optimal interaction of CaBP1 with the InsP3R, we mutated to alanine 6 conserved residues involved in Ca2+-coordination in functional EF-hands 1 (D96A, D98A), 3 (D173A, N175A), and 4 (D210A, N212A) [numbering based on the l-CaBP1 sequence (20)]. This CaBP1 triple-EF-hand mutant failed to bind to InsP3R-3, even in the presence of 500 μM Ca2+ (Fig. 2C). Thus, Ca2+ binding to the functional EF-hands of CaBP1 appears to be required for optimal interaction with the InsP3R, although it is possible that Ca2+ binding to the InsP3R might affect the interaction of the two proteins. Wild-type CaBP1 binding to the InsP3R was strongly enhanced when [Ca2+] was raised from 100 nM to ≈5 μM (Fig. 2D), with an apparent Ca2+-affinity of ≈1 μM (Fig. 2E). Ca2+-induced CaBP1 binding to the InsP3R therefore occurs over a physiologically relevant range of [Ca2+]i, suggesting that in vivo changes in [Ca2+]i may dynamically regulate the interaction between the two proteins.
Figure 2.
Ca2+ dependence of CaBP1-InsP3R interaction. (A) Elevation of [Ca2+]i enhances the interaction of the InsP3R with CaBP1. Coimmunoprecipitation, using type 3 InsP3R antibody, of CaBP1 with InsP3R-3 from lysates of CaBP1-GFP-transfected COS-7 cells (Left) exposed (+) or not (−) for 2 min to the Ca2+ ionophore ionomycin (2 μM). Immunoprecipitates (Left) or cell lysates (Right; 5 μg each) were probed for InsP3R-3 (Upper) or CaBP1 (Lower). Ionomycin enhanced the amount of CaBP1 detected in immunoprecipitates, which contained equal amounts of InsP3R (lanes 1 and 2, Top) and s-CaBP1-GFP (lanes 3 and 4, Bottom). (B) In vitro binding of InsP3R-3 to CaBP1 is specifically enhanced by Ca2+. COS-7 cell lysates, with free [Mg2+] and [Ca2+] fixed to 500 μM Mg2+/0 Ca2+ (left lane), 0 Mg2+/0 Ca2+ (center lane) or 0 Mg2+/500 μM Ca2+ (right lane) were incubated with GST-c-CaBP1, and bound InsP3R was detected with type-3-specific antibody. (C) Functional Ca2+-binding EF-hands are required for CaBP1 to interact with the InsP3R. Endogenous InsP3R-3 from COS-7 cell lysate was pulled down with GST-CaBP1 (wt) but not with GST-CaBP1 triple-EF-hand mutant (mut). Equivalent GST-fusion protein concentrations were present in in vitro binding reactions (Right, Coomassie stain). (D) [Ca2+]-dependence of in vitro interaction of CaBP1 and InsP3R. Endogenous InsP3R-3 in COS-7 cell lysate (1.25 mg) with [Ca2+] fixed as indicated was pulled down with GST-c-CaBP1 and probed with InsP3R-3 antibody. (E) [Ca2+]-dependence of InsP3R-3 interaction with CaBP1 by quantitative densitometry of gels similar to that shown in D (n = 3) with data normalized to binding observed in 500 μM
CaBPs Are Protein Ligands of the InsP3R Channel.
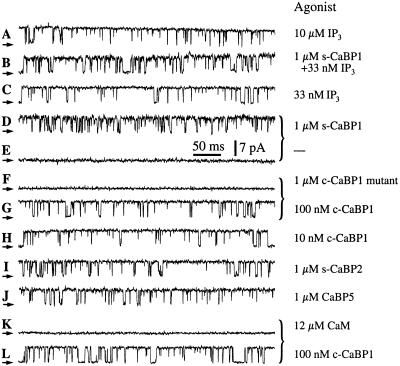
We explored the functional consequences of the interaction of CaBP1 with the InsP3R channel, by patch clamp recording of single-channel currents of endogenous Xenopus type 1 InsP3R channels in their native membrane environment in the outer membrane of isolated oocyte nuclei (7, 21). Robust channel activity of the InsP3R (Fig. 3A) is observed only when the solutions in the patch electrodes contain InsP3 and an appropriate [Ca2+] (7, 13), because the cytoplasmic side of the channel, which contains the InsP3- and Ca2+-binding regions, faces into the pipette (cf. Fig. 3E). To observe effects of CaBP1 on channel gating, purified s-CaBP1 or its COOH-terminal binding region (c-CaBP1) (1 μM) together with InsP3 (33 nM) was included in the pipette solution at an optimal [Ca2+] (13). However, robust channel activity was similarly observed in the presence or absence of CaBP1 (Fig. 3 B and C). Because CaBP1 interacts with the channel in a region that contains the InsP3-binding domain, we also examined the effects of CaBP1 in the absence of InsP3. Surprisingly, channel gating with high (Po) (≈0.8) was observed when 1 μM s-CaBP1 was included in the pipette solution (Fig. 3D, in 15 of 17 patches). The identification of the activated channels as InsP3Rs was based on the distinct conductance and gating properties of the channel in the oocyte nuclear membrane. Activation of gating was caused by CaBP1, because patches obtained from the same regions of the nucleus using pipettes lacking CaBP1 did not display channel activities (Fig. 3E, in 33 of 36 patches). The CaBP1 triple-EF-hand mutant protein (1 μM) failed to activate the channel (Fig. 3F, in 10 of 11 patches). In contrast, channel gating was activated in the same membrane areas when the pipette solution contained CaBP1 in lieu of the CaBP1 triple-EF-hand mutant protein (Fig. 3G, in 12 of 12 patches), which demonstrated that the failure to observe channels by using pipettes lacking either InsP3 or CaBP1 or containing the EF-hand mutant CaBP1 was not due to the absence of InsP3R channels in the membrane patches. Thus, Ca2+-dependent binding of CaBP1 to the InsP3R (Fig. 2) mediates the activation of channel gating. CaBP1-dependent activation of InsP3R channels with reasonably high Po (≈0.7) was observed even with CaBP1 concentrations reduced to 10 nM (Fig. 3H). These electrophysiological results indicate that CaBP1 is a high affinity activator of the InsP3R. Taken together, our functional and biochemical results demonstrate that CaBP1 is a high-affinity, specific protein ligand of the InsP3R, the Ca2+-dependent binding of which activates channel gating in the absence of InsP3 with features (Po, gating kinetics) remarkably similar to those activated by InsP3 (7, 32).
Figure 3.
Typical patch-clamp current records from outer membrane patches obtained from isolated Xenopus oocyte nuclei. Applied potential = 20 mV. The arrows indicate the closed-channel current level. The pipette solutions contained: 10 μM InsP3 (A), 33 nM InsP3 and 1 μM s-CaBP1(B), 33 nM InsP3 (C), 1 μM s-CaBP1 (D), no agonist (E), 1 μM CaBP1 triple-EF-hand mutant (F), 100 nM c-CaBP1 (G), 10 nM c-CaBP1 (H), 1 μM s-CaBP2 (I), 1 μM CaBP5 (J), 12 μM calmodulin (K), or 100 nM c-CaBP1 (L). Current traces D and E, F and G, and K and L (those indicated with braces) were recorded with membrane patches obtained from the same region of the same oocyte nuclei. Free Ca2+ concentrations used in all pipette solutions were optimal for achieving maximum channel Po (1.5–21 μM; ref. 13). n = number of patches used to determine Po. Po = 0.78 ± 0.03, n = 12 (A); Po = 0.65 ± 0.06, n = 4 (B); Po = 0.77 ± 0.08, n = 5 (C); Po = 0.78 ± 0.03, n = 10 (D); Po = 0.71 ± 0.03, n = 9 (G, L); Po = 0.70 ± 0.06, n = 6 (H); Po = 0.87 ± 0.02, n = 7 (I); Po = 0.87 ± 0.02, n = 5 (J). Ca2+. (F) CaBP1-binding affinity for the InsP3R-3. Endogenous InsP3R-3 was pulled down with GST-CaBP1 from COS-7 cell lysates (1.25 mg) containing defined concentrations of s-CaBP1. (G) Quantitative analysis of competition for CaBP1 binding to InsP3R-3 by s-CaBP1 with data normalized to binding in the absence of added s-CaBP1. (H) Specificity of the interaction with the InsP3R of CaBP1 vs. calmodulin (CaM). Endogenous COS-7 cell InsP3R-3 was pulled down with GST-c-CaBP1 from lysates (1.25 mg) supplemented with various concentrations of CaM or s-CaBP1.
We examined the specificity of the effects of CaBP1 by performing electrophysiological studies using other CaBP family members. Purified bovine s-CaBP2 and mouse CaBP5 (1 μM each) (20), with COOH-terminal sequences that are ≈85% similar to human CABP1/caldendrin, each stimulated InsP3R channel gating with high Po in optimal [Ca2+]i (Fig. 3 I and J). These results therefore identify the CaBP Ca2+ sensors as a family of protein ligands of the InsP3R channel.
CaBP1/caldendrin as well as other NCBPs belong to a superfamily of EF-hand-containing proteins, of which calmodulin is the prototypical member. Calmodulin has been implicated in the regulation of the InsP3R (33, 34) and some studies have suggested that calmodulin may affect InsP3 binding to the channel, possibly by interacting with residues near or within the InsP3-binding region (34, 35). Calmodulin has ≈60% sequence similarity over the CaBP1 region used in our binding and electrophysiology studies (20). To determine whether calmodulin could also bind to the CaBP1-interacting region of the InsP3R, we performed in vitro binding competition experiments. Purified calmodulin or s-CaBP1 protein was added to COS-7 cell lysates, from which the InsP3R-3 was pulled down by passage over a GST-c-CaBP1 column. s-CaBP1 competitively inhibited the binding of the InsP3R-3 channel to GST-c-CaBP1 with an apparent affinity (half-maximal inhibition) of ≈25 nM (Fig. 2 F and G). In contrast, calmodulin, even at high concentrations (5 μM), had relatively little effect on the binding (Fig. 2H), indicating that it does not interact well with the CaBP-binding site of the InsP3R. Electrophysiological experiments were conducted with calmodulin (12 μM) included in the pipette solution. However, calmodulin never activated channel gating (Fig. 3K, in 17 of 17 patches) in membrane regions where c-CaBP1 did (Fig. 3L, in 13 of 14 patches), in accord with the protein-binding data. Thus, the interactions of CaBPs with the InsP3R channel are highly specific ones that are not recapitulated by calmodulin.
CaBP1 and InsP3R Interact and Colocalize in Brain.
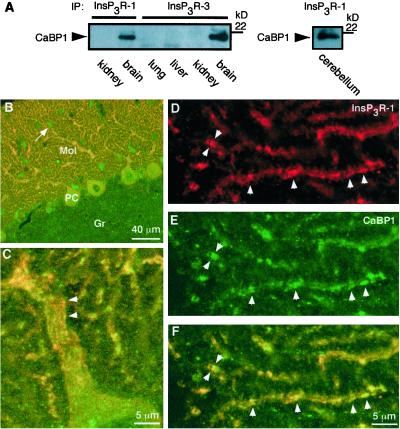
CaBP1 and caldendrin are expressed in specific cell types in the retina and throughout the brain, including cortex, cerebellum, and hippocampus (20, 27, 36, 37), whereas CaBP2, -3, and -5 may be retina-specific (20). Caldendrin expression in the brain appears to be restricted to neurons, where it has been localized to the somatodendritic compartment, with particular enrichment at dendritic postsynaptic densities (27, 36). The InsP3R is widely distributed throughout the brain, with the type 1 isoform (InsP3R-1) most highly expressed, and it is also localized in neuronal somatic and dendritic compartments (38–40). To verify the interaction of endogenous InsP3R and CaBP1 in brain, we performed immunoprecipitation and colocalization experiments. CaBP1 was present in immunoprecipitates of InsP3R-1 or InsP3R-3 from whole rat brain (Fig. 4A). Immunoprecipitation of InsP3R-1 from cerebellum, where it is expressed at very high levels in Purkinje cells (41, 42) coimmunoprecipitated CaBP1. Like InsP3R-1, staining for CaBP1 in cerebellum was strong in Purkinje cell somas and in their dendrites in the molecular layer (Fig. 4B), with staining also present in small somas of stellate cells in the molecular layer, and in fine puncta in the granular cell layer. CaBP1 and InsP3R-1 co-localized (within the ≈0.4-μm optical resolution of the microscope) extensively in Purkinje cell somas and dendrites (Fig. 4 B and F). Colocalization was nearly complete in dendrites, and in striated structures (Fig. 4 C and F) that are most likely the smooth ER that runs parallel to the dendritic axis immediately adjacent to the plasma membrane (subsurface or hypolemmal cisternae) (41, 43). These results demonstrate that CaBP1 is membrane-localized, perhaps through its myristoylated N terminus, in close proximity to the InsP3R in neurons.
Figure 4.
Interaction of InsP3R and CaBP1 in brain. (A) Coimmunoprecipitation of CaBP1 with InsP3R-1 (Left and Right) or InsP3R-3 (Left) from whole mouse brain (Left) and cerebellum (Right), but not from nonneural tissues (Left). Immunoprecipitates were probed with anti-CaBP1 antibody. The reciprocal experiment could not be performed because the CaBP antibody is directed against the same region to which the InsP3R binds. (B–F) Confocal immunolocalization of InsP3R-1 and CaBP1 in rat cerebellum sagittal sections. (B) Low magnification. CaBP1 (green) and InsP3R-1 (red) are localized to Purkinje cell somas (PC) and their dendrites in the molecular layer (Mol) (colocalization indicated by yellow). CaBP1 (but not InsP3R-1) is also localized to unidentified fine structures within the granular cell layer (Gr) and to stellate cells (arrow) in the molecular layer. (C–F) Higher magnification demonstrating subcellular colocalization on endoplasmic reticulum. (C) Striated colocalization (yellow) in Purkinje cell primary dendrite. (D–F) Dendritic tips of Purkinje cells. CaBP1 (E; green) and InsP3R-1 (D; red) are colocalized (F; yellow) to linear subregions within thin dendrites (arrowheads).
Discussion
We have identified members of a subfamily of the neuronal Ca2+-sensor protein family as high-affinity ligands of all of the mammalian isoforms of the InsP3R Ca2+ release channel. Elevation of cytoplasmic [Ca2+] promotes binding of CaBPs to the region of the InsP3R-channel that contains the InsP3-binding domain and activates channel gating in the absence of InsP3.
The existence of protein ligands of the InsP3R may suggest that InsP3R-mediated Ca2+ signaling could be recruited under conditions in which the phosphoinositide signaling system remains unengaged. The InsP3R, as well as the other major Ca2+-release channel in cells, the ryanodine receptor, is activated by Ca2+ binding, enabling both to participate in Ca2+-induced Ca2+ release (CICR), a process critical for their signaling functions. However, the InsP3R has been distinguished from the ryanodine receptor by its requirement for ligand binding. Identification of CaBPs as ligands whose binding to the InsP3R is promoted by Ca2+ may enable the channel to become activated by a rise of Ca2+ concentration without the necessity for InsP3 and Ca2+ coincidence detection, thereby enabling it to be involved in regenerative Ca2+ release in a pure, albeit distinct, CICR process. Physiologically, such a mechanism may be important in amplifying Ca2+ signals generated by other mechanisms, including Ca2+ influx through the plasma membrane or release from other intracellular stores. For example, Ca2+ stores in excitable cells function as integrators and amplifiers of Ca2+ influx, roles that are particularly important in dendritic spines and in synaptic plasticity (3, 17). Induction of binding of CaBP to the InsP3R by elevations of [Ca2+]i due to Ca2+ influx through voltage-gated Ca2+ channels could provide Ca2+-store-mediated amplification of the Ca2+ signal, thereby inducing synaptic plasticity without the requirement for parallel activation of InsP3-generating receptor pathways.
Cerebellar Purkinje cells express very high levels of the InsP3R-1, where it is involved in long-term depression (39, 44). Paradoxically, the channel is remarkably insensitive to InsP3 in this cell type, so that Ca2+ release requires InsP3 concentrations that are over two orders of magnitude higher than those required to gate the channel in electrophysiological studies (45). The molecular basis for the in vivo insensitivity of the channel to InsP3 is unknown. The identification of CaBP proteins as ligands that interact with the InsP3-binding site of the channel may suggest a possible mechanism. The apparent close physical proximity of the InsP3R and CaBP1 in Purkinje cells (Fig. 4) may enable CaBP to bind to the channel with high avidity even when cytoplasmic Ca2+ concentration is low under resting conditions. The channel in this state may be less sensitive to InsP3. Titration by CaBP of the effective affinity of InsP3 could provide a mechanism to ensure that InsP3-induced Ca2+ signals are generated and restricted to highly localized regions immediately adjacent to the sites of InsP3 production, as observed at synaptic inputs in Purkinje cells (39).
Because InsP3R-mediated Ca2+ signals are shaped by messenger diffusion, degradation, and removal, processes that will have distinct kinetics for InsP3 compared with CaBPs, the identification of protein ligands for the InsP3R provides insights into the dimensions and versatility of this ubiquitous signaling pathway.
Acknowledgments
We thank A. Tanimura and S. Joseph for antibodies, and M. Wallenstein for technical support. This work was supported by research grants from the National Institutes of Health (GM56328 and MH59937 to J.K.F.), American Heart Association (9906220U to D.-O.D.M.), Research to Prevent Blindness, Inc. (EY08061 to the Department of Ophthalmology, University of Washington), and the E.K. Bishop Foundation. K.P. is recipient of a Research to Prevent Blindness Senior Investigator Award.
Abbreviations
- InsP3, inositol trisphosphate
InsP3R, inositol trisphosphate receptor
- [Ca2+]i
cytoplasmic free Ca2+ concentration
- Po
open probability
- GFP
green fluorescent protein
- ER
endoplasmic reticulum
- GST
glutathione S-transferase
- NCBP
neuronal Ca2+-binding protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 7320.
References
- 1.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M J. J Exp Biol. 1997;200:315–319. doi: 10.1242/jeb.200.2.315. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M J, Bootman M D, Lipp P. Nature (London) 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 4.Taylor C W, Genazzani A A, Morris S A. Cell Calcium. 1999;26:237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- 5.Patel S, Joseph S K, Thomas A P. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021. [DOI] [PubMed] [Google Scholar]
- 6.Hirose K, Iino M. Nature (London) 1994;372:791–794. doi: 10.1038/372791a0. [DOI] [PubMed] [Google Scholar]
- 7.Mak D-O D, Foskett J K. J Gen Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barritt G J. Biochem J. 1999;337:153–169. [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M G, Xu X, Zeng W Z, Diaz J, Wojcikiewicz R J H, Kuo T H, Wuytack F, Racymaekers L, Muallem S. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto-Hino M, Miyawaki A, Segawa A, Adachi E, Yamashina S, Fujimoto T, Sugiyama T, Furuichi T, Hasegawa M, Mikoshiba K. J Cell Biol. 1998;141:135–142. doi: 10.1083/jcb.141.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorn P. Cell Calcium. 1996;20:203–214. doi: 10.1016/s0143-4160(96)90107-4. [DOI] [PubMed] [Google Scholar]
- 12.Bootman M D, Berridge M J. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 13.Mak D-O D, McBride S, Foskett J K. Proc Natl Acad Sci USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mak D-O D, McBride S, Foskett J K. J Gen Physiol. 2001;117:435–446. doi: 10.1085/jgp.117.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bezprozvanny I, Watras J, Ehrlich B E. Nature (London) 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 16.Marchant J S, Parker I. J Gen Physiol. 2000;116:691–695. doi: 10.1085/jgp.116.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berridge M J. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 18.Hallows K R, Raghuram V, Kemp B E, Witters L A, Foskett J K. J Clin Invest. 2000;105:1711–1721. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mak D-O D, McBride S, Raghuram V, Yue Y, Joseph S K, Foskett J K. J Gen Physiol. 2000;115:241–255. doi: 10.1085/jgp.115.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haeseleer F, Sokal I, Verlinde C L M J, Erdjument-Bromage H, Tempst P, Pronin A N, Benovic J L, Fariss R N, Palczewski K. J Biol Chem. 2000;275:1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak D-O D, Foskett J K. J Biol Chem. 1994;269:29375–29378. [PubMed] [Google Scholar]
- 22.Mak D-O D, Foskett J K. Am J Physiol. 1998;275:C179–C188. doi: 10.1152/ajpcell.1998.275.1.C179. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa F, Morita M, Monkawa T, Michikawa T, Furuichi T, Mikoshiba K. J Biol Chem. 1996;271:18277–18284. doi: 10.1074/jbc.271.30.18277. [DOI] [PubMed] [Google Scholar]
- 24.Mignery G A, Sudhof T C. EMBO J. 1990;9:3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyawaki A, Furuichi T, Ryou Y, Yoshikawa S, Nakagawa T, Saitoh T, Mikoshiba K. Proc Natl Acad Sci USA. 1991;88:4911–4915. doi: 10.1073/pnas.88.11.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgoyne R D, Weiss J L. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- 27.Seidenbecher C I, Langnaese K, Sanmarti-Vila L, Boeckers T M, Smalla K-H, Sabel B A, Garner C C, Gundelfinger E D, Kreutz M R. J Biol Chem. 1998;273:21324–21331. doi: 10.1074/jbc.273.33.21324. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Yamaguchi F, Miyamoto O, Sugimoto K, Konishi R, Hatase O, Tokuda M. J Biol Chem. 1999;274:3610–3616. doi: 10.1074/jbc.274.6.3610. [DOI] [PubMed] [Google Scholar]
- 29.Joseph S K, Bokkala S, Boehning D, Zeigler S. J Biol Chem. 2000;275:16084–16090. doi: 10.1074/jbc.M000506200. [DOI] [PubMed] [Google Scholar]
- 30.Mikoshiba K. Trends Pharmacol Sci. 1993;14:86–89. doi: 10.1016/0165-6147(93)90069-v. [DOI] [PubMed] [Google Scholar]
- 31.Sokal I, Li N, Verlinde C L M J, Haeseleer F, Baehr W, Palczewski K. Biochim Biophys Acta. 2000;1498:233–251. doi: 10.1016/s0167-4889(00)00099-9. [DOI] [PubMed] [Google Scholar]
- 32.Mak D-O D, McBride S, Foskett J K. J Gen Physiol. 2001;117:299–314. doi: 10.1085/jgp.117.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, Mikoshiba K. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- 34.Adkins C E, Morris S A, De Smedt H, Sienaert I, Török K, Taylor C W. Biochem J. 2000;345:357–363. [PMC free article] [PubMed] [Google Scholar]
- 35.Sipma H, De Smet P, Sienaert I, Vanlingen S, Missiaen L, Parys J B, De Smedt H. J Biol Chem. 1999;274:12157–12162. doi: 10.1074/jbc.274.17.12157. [DOI] [PubMed] [Google Scholar]
- 36.Menger N, Seidenbecher C I, Gundelfinger E D, Kreutz M R. Cell Tissue Res. 1999;298:21–32. doi: 10.1007/s004419900060. [DOI] [PubMed] [Google Scholar]
- 37.Haverkamp S, Wassle H. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- 38.Sharp A H, Dawson T M, Ross C A, Fotuhi M, Mourey R J, Snyder S H. Neuroscience. 1993;53:927–942. doi: 10.1016/0306-4522(93)90478-x. [DOI] [PubMed] [Google Scholar]
- 39.Finch E A, Augustine G J. Nature (London) 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 40.Furuichi T, Mikoshiba K. J Neurochem. 1995;64:953–960. doi: 10.1046/j.1471-4159.1995.64030953.x. [DOI] [PubMed] [Google Scholar]
- 41.Ross C A, Meldolesi J, Milner T A, Satoh T, Supattapone S, Snyder S H. Nature (London) 1989;339:468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- 42.Otsu H, Yamamoto A, Maeda N, Mikoshiba K, Tashiro Y. Cell Struct Funct. 1990;15:163–173. doi: 10.1247/csf.15.163. [DOI] [PubMed] [Google Scholar]
- 43.Peters A, Palay L S, Webster H. The Fine Structure of the Nervous System. Neurons and Their Supporting Cells. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- 44.Khodakhah K, Armstrong C M. Proc Natl Acad Sci USA. 1997;94:14009–14014. doi: 10.1073/pnas.94.25.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khodakhah K, Ogden D. Proc Natl Acad Sci USA. 1993;90:4976–4980. doi: 10.1073/pnas.90.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]