Abstract
The serine protease, tissue-type plasminogen activator (tPA) is a key regulator of extracellular proteolytic cascades. We demonstrate a requirement for tPA signaling in the experience-dependent plasticity of mouse visual cortex during the developmental critical period. Proteolytic activity by tPA in the binocular zone was typically increased within 2 days of monocular deprivation (MD). This regulation failed to occur in glutamic acid decarboxylase (GAD) 65 knockout mice, an animal model of impaired ocular dominance plasticity because of reduced γ-aminobutyric acid (GABA)-mediated transmission described previously. Loss of responsiveness to the deprived eye consequent to MD was conversely suppressed in mice lacking tPA despite normal levels of neuronal activity. Plasticity was restored in a gene dose-dependent manner, or by direct tPA infusion. Permissive amounts of tPA may, thus, couple functional to structural changes downstream of the excitatory-inhibitory balance that triggers visual cortical plasticity. Our results not only support a molecular cascade leading to neurite outgrowth after sensory deprivation, but also identify a valuable tool for further proteomic and genomic dissection of experience-dependent plasticity downstream of electrical activity.
Loss of responsiveness to an eye deprived of vision is mediated by rapid functional disconnection followed by anatomical rearrangement within visual cortex (1, 2). Extracellular proteolysis is one mechanism that can remodel neuronal circuits by degradation of extracellular matrix proteins or cell recognition molecules. The serine protease, tissue-type plasminogen activator (tPA) controls a cascade of extracellular proteolytic activities involved in neurite outgrowth (3), cell migration (4, 5), long-lasting potentiation (6–8), learning and memory (8–10), excitotoxic cell death (11–13), and regeneration or recovery from injury in the nervous system (14).
In kitten visual cortex, blocking tPA function with either an endogenous inhibitor of plasminogen activator (PAI-I; ref. 15), or tPA-stop (16), suppresses ocular dominance plasticity. However, pharmacological studies yield conflicting results. It remains unclear whether tPA contributes selectively to recovery of lost input after reverse suture (16) or more generally to critical period plasticity (15). An obvious source of discrepancy lies in the different potencies of tPA inhibitors used, underscoring the drawbacks of this approach. Moreover, the previous work in cat visual cortex did not make clear where tPA stood in relation to other molecules thought to be permissive or essential for cortical plasticity.
Here, we define an essential role for tPA in visual cortical development by taking advantage of a mouse model (17) to combine biochemical, genetic, and electrophysiological analyses in vivo. Gene-targeted reduction of stimulated γ-aminobutyric acid (GABA) release previously demonstrated that inhibitory-excitatory balance within visual cortex initiates the plastic process (18, 19). Our present results establish tPA knockout (KO) mice as an animal model with reversible impairment of ocular dominance plasticity downstream of neuronal activity per se.
Methods
Mice bearing functional disruption of GAD65 (18, 20) or tPA (17) were generated as described.
Proteolytic Assay.
Mice (n = 110) were anesthetized with halothane and killed by cervical dislocation, and each visual cortex was removed. In Fig. 1 B and D, tPA activity was determined by chromogenic assay kit (Spectrolyse/fibrin, Biopool, Umeå, Sweden). Activity was measured by adding Glu-plasminogen, chromogenic plasmin substrate, and fibrin at neutral pH. In the presence of fibrin, tPA converts plasminogen to plasmin, which subsequently cleaves the chromogen substrate. The amount of color (yellow) developed during 3 h was proportional to the amount of tPA activity in the homogenate. In Fig. 1C, fibrinolytic activity was measured as fibrin clot lysis time, as described previously (21).
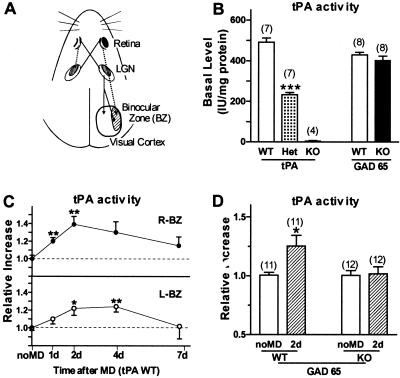
Figure 1.
(A) Central visual pathways in mice. Contralateral eye input projects to all of primary visual cortex (V1) via the lateral geniculate nucleus (LGN). Ipsilateral eye afferents innervate only the lateral one third of V1 [binocular zone (BZ)], where most cells respond to both eyes viewing the central 30° of visual space. (B) Basal tPA activity levels in binocular zone of WT, tPA Het, tPA KO, and GAD65 KO (18, 19). Note normal basal tPA levels in GAD65 KO mice. ***, P < 0.005 vs. WT. (C) tPA proteolytic activity in WT mouse V1 after monocular deprivation (MD) during critical period. Significant elevation in both hemispheres after left eye occlusion for 1, 2, 4, or 7 days (at P25–26). Six mice per group. *, P < 0.05, **, P < 0.01, Student's t test vs. no MD (13 ± 0.4 ng/mg protein). (D) GAD65 KO mice fail to regulate tPA activity after brief MD (2 days), reflecting impaired plasticity in visual cortex (18). *, P < 0.05 vs. no MD WT. Number of mice per group is indicated above each bar in B and D.
Ocular Dominance Experiments.
Extracellular single-unit recording from primary visual cortex (V1) in vivo was performed according to standard techniques (18, 22). Briefly, mice prepared blind to genotype were anesthetized with Nembutal (50 mg/kg, Abbott) and fixed to a stereotaxic apparatus after tracheotomy. Resin-coated tungsten microelectrodes (3–5 MΩ) were used to record visually active cells in vertical penetrations from the monocular zone to the V1/V2 boundary, including at least three sites (200 μm apart) within the binocular zone (20–30 cells). The ocular dominance of each neuron was classified on a seven-point scale, and cumulative histograms were constructed for each group (4–11 mice each). A contralateral bias index (CBI) was calculated as CBI = [(n1 − n7) + (2/3)(n2 − n6) + (1/3)(n3 − n5) + N]/2N, where N is the total number of cells and nx is the number of cells with ocular dominance score x on the seven-point scale (22). For monocular deprivation (MD), eyelid margins were trimmed, and lids were sutured shut under halothane anesthesia at postnatal days 22–26 (P22–26) for short-term MD (STMD; 4–5 days) or at P18–19 for long-term MD (LTMD) spanning the critical period (>2 wk). Rescue was attempted by daily injection (intracerebroventricularly 1.5 μl per side) of diazepam (2 mg/ml, Wako Biochemicals, Osaka) or vehicle (50% propylene glycol), or recombinant tPA (160 units/μl E6010, a gift from Eisai Co. Ltd., Ibaraki, Japan) or vehicle (in mg/ml: 7.6 arginine, 7.0 aspartate, 30.8 mannitol).
Results
In brain, tPA as an immediate early gene induced by seizures, kindling, and long-term potentiation was first identified in rat hippocampus (23). We, therefore, determined the level of tPA available to elicit structural changes in visual cortex by measuring tPA proteolytic activity directly in the binocular zone during the critical period for plasticity (Fig. 1A). Basal levels were negligible in mice carrying a targeted disruption of the tPA gene (tPA KO, ref. 17) and half that of wild-type (WT) mice in tPA heterozygotes (Het, Fig. 1B), confirming the sensitivity of our bioassay (21). Interestingly, tPA activity was significantly increased in both hemispheres within 2 days after MD in WT mice and was maintained at these levels for at least 4 days (Fig. 1C), consistent with the saturating plastic effects of brief MD (18, 19, 22).
Elevated tPA activity, thus, reflected imbalanced input from the two eyes, similar to the disinhibition of cAMP response element (CRE) activation (24), and was initiated before morphological changes in thalamo-cortical axonal arbors (2). Significantly, a mouse model with impaired visual cortical plasticity (18, 19) because of targeted deletion of glutamic acid decarboxylase (GAD) 65 exhibited normal constitutive levels (Fig. 1B), but failed to regulate tPA activity in response to brief MD (Fig. 1D), implicating this molecule as a downstream effector in the plasticity process.
To directly assess whether tPA couples physiological perturbation of sensory input to synaptic rearrangement, we examined ocular dominance plasticity in tPA KO mice (17). Response qualities to a moving light-slit (retinotopy, response strength, habituation, receptive field size, and spontaneous activity) were normal when recorded from nondeprived WT and tPA KO mouse visual cortex. Some enhanced activation in response to stationary stimuli was noted in the KO (30 ± 7% of 186 cells, 7 mice). The binocular zone showed no gross morphological abnormality (data not shown), was similarly localized in WT and KO, and exhibited equivalent ocular dominance histograms across genotypes (Fig. 2 A and B).
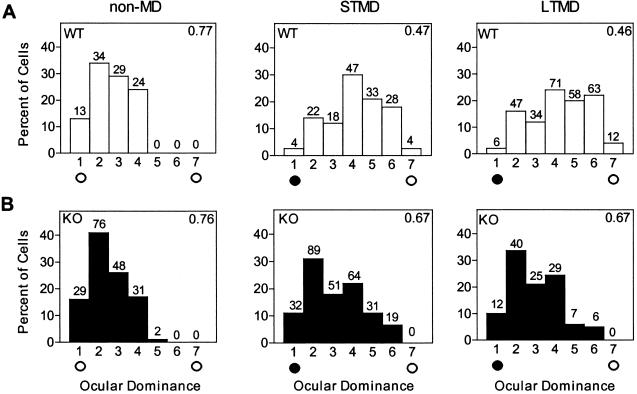
Figure 2.
(A) Ocular dominance histograms for WT mice (non-MD, four mice) normally shift toward ipsilateral, open eye (open circle) after short-term MD of contralateral input (filled circle, 4 days, STMD; 10 mice. P < 0.0001, χ2-test vs. non-MD) or long-term MD spanning the critical period (2 wk, LTMD; 10 mice, P < 0.0001, χ2-test vs. non-MD). (B) Distribution of non-MD tPA KO mice is similar to WT (seven mice, P = 0.51, χ2-test vs. non-MD WT). However, MD effects are weaker in tPA KO mice after both STMD (11 mice, P < 0.001, χ2-test vs. STMD in WT) and LTMD (10 mice, P < 0.0001, χ2-test vs. LTMD in WT). Contralateral bias index (CBI) in upper right corner ranges from 0 to 1 and decreases when plasticity occurs (18, 22). Number of cells per ocular dominance group is indicated above each bar.
As expected (19, 22), these distributions shifted robustly in WT mice after STMD as well as LTMD spanning the entire critical period (Fig. 2A). In contrast, plasticity was strongly suppressed in tPA KO mice (Fig. 2B). Both STMD and LTMD yielded little or no reduction in contralateral bias compared with WT (Fig. 3A). The critical period was not simply delayed in tPA KO mice, because extra-long MD for 6 weeks from P18 produced no additional effects compared with MD during the critical period (CBI ± SD = 0.67 ± 0.04, five mice).
Figure 3.
(A) Summary of MD effect in WT and tPA KO mice. Each symbol represents CBI value of individual animals (open circles, WT; filled circles, KO). Plasticity is consistently and significantly suppressed in all tPA KO mice after both STMD (CBI ± SD = 0.66 ± 0.06 vs. 0.49 ± 0.05, KO and WT) and LTMD (0.69 ± 0.06 vs. 0.44 ± 0.04). Shaded region indicates range of nondeprived CBI for both WT (0.73 ± 0.02) and KO mice (0.76 ± 0.04). ***, P < 0.001, Student's t test. (B) Diazepam (DZ, 2 mg/ml) fails to restore plasticity to tPA KO mice. Neither drug nor vehicle (Veh, 50% propylene glycol) infusion daily throughout brief MD (P26–30) enables the expected decrease in CBI. (C) No gross disruption of neuronal activity in tPA KO mice is observed by egr-1 mRNA expression with respect to WT under three conditions: normal light/dark cycle (L), 5 days complete darkness (D), and 30 min photo-stimulation (1 Hz, 20 J) after dark-adaptation (P). Expression was normalized by housekeeping gene G3PDH. *, P < 0.05, **, P < 0.01, ***, P < 0.001, Student's t test vs. D value.
We previously reported disruption of experience-dependent plasticity in GAD65 KO mice because of inadequate inhibition (18, 19). Enhancing GABAergic transmission with diazepam during MD rescues ocular dominance plasticity in GAD65 KO mice (18) but was ineffective in tPA KO mice (Fig. 3B). Contralateral bias remained high in tPA KO mice treated during MD, indicating that the impairment lies downstream of inhibitory-excitatory balance. Consistent with this view, prolonged neuronal discharge in response to a moving light-slit was prevalent in GAD65 KO mice (18, 19) but rarely observed in tPA KO mice (13 ± 5% of 186 cells, seven mice).
As a further measure of neuronal activation, we quantified expression of the sensory marker egr-1/zif268 (25), an immediate early gene whose response is up-regulated in GAD65 KO mouse visual cortex (18). In WT animals (Fig. 3C), egr-1 mRNA was expressed constitutively in the light (L) and decreased after dark-adaptation (D). A 2-fold increase was induced by photo-stimulation (30 min, 1 Hz, 20 J) after dark-adaptation (P). Expression of egr-1 in frontal cortex was not significantly altered by photo-stimulation (data not shown). No differences from WT were observed in egr-1 gene regulation in the visual cortex of tPA KO mice across these three conditions (L, D, P; Fig. 3C).
To estimate the amount of tPA that might enable ocular dominance plasticity, we recorded from tPA Het mice. The amounts of tPA mRNA (6478 ± 540 vs. 2951 ± 620 arbitrary units, four mice each, P < 0.05; ref. 15), protein (17.4 ± 0.8 vs. 8.6 ± 1.3 ng/mg, four mice each, P < 0.05; ref. 21), and proteolytic activity (Fig. 1B) were essentially half that of WT. Normal ocular dominance distributions and CBIs were observed in nondeprived mutants. After brief MD (4 days) during the critical period, CBI values in tPA Het were slightly reduced, but there was no obvious rescue from KO levels (Fig. 4A, STMD).
Figure 4.
(A) Permissive levels of tPA restore ocular dominance plasticity. Short-term MD during the critical period slightly reduces CBI values in tPA Het from non-MD levels (0.64 ± 0.02 and 0.77 ± 0.02, respectively) but does not reach WT levels (shaded zone; P < 0.001, Student's t test vs. STMD for WT in Fig. 3A) and is no better than tPA KO levels (P = 0.5 vs. STMD for KO in Fig. 3A). With long-term MD, CBI values in tPA Het (0.53 ± 0.07) are significantly reduced from brief MD and tPA KO (P < 0.001 vs. LTMD for KO in Fig. 3A). Late MD effects in tPA Het (late-LTMD at P60; 0.66 ± 0.05) are negligible in postcritical period adult animals similar to WT (CBI = 0.65 ± 0.07, n = 4; P = 0.8). (B) Monocular TTX injections (4 days) during the critical period significantly reduce CBIs in tPA Het compared with brief MD (P < 0.01 vs. STMD for Het in A), but remain weaker than in WT mice (CBI = 0.54 ± 0.05 and 0.39 ± 0.10, Het vs. WT). Note monocular TTX produces more powerful plasticity than eyelid suture (shaded zone) in WT animals (18). (C) Direct intracranial infusion of recombinant tPA during 1-wk MD (7 days from P25–26) restores plasticity to tPA KO mice. No gross abnormalities were observed after tPA injections (not shown). (CBI = 0.44 ± 0.02, 0.69 ± 0.01, and 0.54 ± 0.01 for WT, vehicle KO, and tPA KO, respectively). *, P < 0.05; **, P < 0.01; ***, P < 0.005, Student's t test.
In contrast, robust ocular dominance shifts were observed after long-term MD (Fig. 4A, LTMD) or monocular tetrodotoxin (TTX) injections (18), which completely silence retinal activity to enhance the contrast with the open input (Fig. 4B). Rescue was restricted to tPA Het mice deprived during the typical critical period for WT animals (19, 22), because the effect of late MD in tPA Het adults (2 wk from P60; L-LTMD) was significantly weaker than in young Het animals (Fig. 4A; P < 0.001). Plasticity was also restored by direct infusion of human recombinant tPA throughout 1 week of MD not only to tPA KO (Fig. 4C), but also to GAD65 KO mice (CBI ± SD = 0.50 ± 0.03, three animals). These CBI values were all comparable to WT mice after brief eyelid suture (Fig. 3A, STMD; Fig. 4, shaded region).
Discussion
Extracellular proteolytic events are a likely cellular mechanism that can couple physiological changes to structural rearrangement during experience-dependent plasticity. During MD, two processes may contribute to the ocular dominance shift: a loss of input from the deprived eye, and a strengthening of input from the open eye. The relative contribution of these two processes depends on the initial state of the binocular visual cortex before MD. In the biased mouse visual cortex (Fig. 1A), a major structural effect of MD is to expand the open eye's geniculocortical connections without causing the closed contralateral eye projection to shrink (2). Given the brief duration of MD used here, rapid remodeling of intracortical connections is likely to lead any afferent rewiring, as described in the cat (26).
We propose that tPA specifically promotes growth of connections serving the open eye. Similarly, the effect of tPA inhibitors in kittens is more pronounced in situations such as reverse suture, where growth demands on inputs serving the reopened eye are greatest (16, 27). Muller and Griesinger (16) may have failed to detect an effect of tPA inhibitors on the initial MD because they recorded from cortex ipsilateral to the deprived eye, where open, contralateral input is already strong at the outset. When ocular dominance plasticity is measured in cat cortex contralateral to the deprived eye (15), strengthening of initially weak ipsilateral input is more sensitive to tPA manipulation as in mice. Notably, tPA disruption never fully blocks the effect of MD (Figs. 2 and 3A; ref. 15), suggesting that alternative mechanisms for disconnecting deprived eye input may be involved in a modest role, if at all (28).
Our results indicate that a certain amount of tPA is necessary for full expression of synaptic plasticity in mouse visual cortex. Disparity of visual inputs during MD is first detected as a perturbation of excitatory-inhibitory balance (18, 19). Several intervening messengers may then lie upstream of tPA that have been implicated in visual cortical plasticity as well (Fig. 5). Neuronal depolarization (13, 16) or direct activation of adenylate cyclase by forskolin induces tPA release (7), whereas neuromodulators coupled to cAMP (e.g., norepinephrine) and protein kinase A (PKA) itself are required for ocular dominance plasticity (15, 29, 30).
Figure 5.
Proposed molecular cascade of visual cortical plasticity. Inhibitory-excitatory balance detects perturbed sensory input (18)(a). Neuromodulators (b) regulate coupling (29) to protein kinase A (30)(c); phosphorylation of MAP kinase (35) and CREB (24) (d) reciprocally induces and is stimulated by BDNF (34)(e), which is known to release tPA from cortical neurons (33).
Increasing evidence suggests a role for brain-derived neurotrophic factor (BDNF) in visual cortical plasticity, through multiple potential pathways (31, 32). Interestingly, BDNF stimulates tPA release and proteolytic activity in primary culture of cortical neurons (33). Both protein kinase A and BDNF induce phosphorylation of mitogen-activated protein (MAP) kinase and cAMP response element binding protein (CREB; ref. 34). Active MAP kinase is required for ocular dominance plasticity (35) and CRE activation is up-regulated in visual cortex after brief MD similar to and in advance of tPA activity (24). Promoter elements in the tPA gene contain binding sites for these regulatory factors (36).
Endogenous tPA could act, like BDNF (32), to establish an initial balance between excitation and inhibition necessary for ocular dominance plasticity, by modulating GABAergic transmission (6) or cleaving the amino terminus of N-methyl-d-aspartate (NMDA) receptor subunits to enhance their function (13). However, this result seems unlikely for three reasons. First, we found no gross hyperexcitability typical of GAD65 KO mice (18) in the absence of tPA (Fig. 3C). Second, prolonged neuronal discharge was unaltered by tPA rescue of GAD65 KO mice (70 ± 5 vs. 63 ± 4% of 76 and 91 cells, vehicle vs. drug, respectively; P = 0.36, Student's t test). This result is consistent with the view that tPA is a permissive factor for plasticity far downstream of the initial excitatory-inhibitory detector (Fig. 5), which is modestly engaged even in GAD65 KO mice when MD exceeds 1 wk (18). Finally, we failed to rescue tPA KO mice by enhancing inhibition (Fig. 3B).
Instead, plasticity was only gradually restored by prolonged tPA exposure (Fig. 4), suggesting a cumulative effect during MD. Neurons contain tPA (9, 13, 16) and secrete it from the growth cone of neurites (3). We thus propose that degradation of extracellular matrix or cell recognition molecules by a tPA proteolytic cascade creates a permissive environment for structural reorganization of circuits within visual cortex (1, 2, 26). Neural cell-adhesion molecule (NCAM) and laminin are well-known extracellular substrates for the tPA-plasmin system (37, 38) and they are implicated in the maintenance of long-lasting forms of synaptic potentiation (39–41). Although these mechanisms remain to be tested in visual cortex, our findings firmly establish a general role for tPA in the experience-dependent remodeling of circuits.
Acknowledgments
We thank S. Fujishima-Ohashi for technical assistance, P. Carmeliet and D. Collen for providing tPA KO mice, K. Obata for GAD65 KO mice, Eisai Co. Ltd. for E6010, and J. J. Emies for rabbit anti-rat tPA anti-serum. This work received additional support by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science & Technology (to T.K.H. and N.M.) and Special Coordination Funds for Promoting Science & Technology from Japan Science & Technology Corp (to T.K.H.).
Abbreviations
- tPA
tissue-type plasminogen activator
- GAD65
glutamic acid decarboxylase 65 kDa isoform
- BDNF
brain-derived neurotrophic factor
- KO
knockout
- WT
wild type
- Het
heterozygote
- MD
monocular deprivation
- CBI
contralateral bias index
- STMD
short-term MD
- LTMD
long-term MD
- V1
primary visual cortex
- GABA
γ-aminobutyric acid
- TTX
tetrodotoxin
- P
postnatal day
- CREB
cAMP-response element binding protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Antonini A, Stryker M P. J Comp Neurol. 1996;369:64–82. doi: 10.1002/(SICI)1096-9861(19960520)369:1<64::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Antonini A, Fagiolini M, Stryker M P. J Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krystosek A, Seeds N W. Science. 1981;213:1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- 4.Seeds N W, Basham M E, Haffke S P. Proc Natl Acad Sci USA. 1999;96:14118–14123. doi: 10.1073/pnas.96.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moonen G, Grau-Wagemans M P, Selak I. Nature (London) 1982;298:753–755. doi: 10.1038/298753a0. [DOI] [PubMed] [Google Scholar]
- 6.Frey U, Muller M, Kuhl D. J Neurosci. 1996;16:2057–2063. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baranes D, Lederfein D, Huang Y-Y, Chen M, Bailey C H, Kandel E R. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 8.Madani R, Hulo S, Toni N, Madani H, Steimer T, Muller D, Vassalli J-D. EMBO J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeds N W, Williams B L, Bickford P C. Science. 1995;270:1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi P, Napolitano M, Centonze D, Marfia G A, Gubellini P, Teule M A, Berretta N, Bernardi G, Frati L, Tolu M, Gulino A. Eur J Neurosci. 2000;12:1002–1012. doi: 10.1046/j.1460-9568.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsirka S E, Gualandris A, Amaral D G, Strickland S. Nature (London) 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 12.Nagai N, De Mol M, Lijnen H R, Carmeliet P, Collen D. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 13.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie E T, Vivien D, Buisson A. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 14.Siconolfi L B, Seeds N W. J Neurosci. 2001;21:4336–4347. doi: 10.1523/JNEUROSCI.21-12-04336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mataga N, Imamura K, Shiomitsu T, Yoshimura Y, Fukamauchi F, Watanabe Y. Neurosci Lett. 1996;218:149–152. doi: 10.1016/s0304-3940(96)13139-6. [DOI] [PubMed] [Google Scholar]
- 16.Muller C M, Griesinger C B. Nat Neurosci. 1998;1:47–53. doi: 10.1038/248. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord J J, Collen D, Mulligan R C. Nature (London) 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 18.Hensch T K, Fagiolini M, Mataga N, Stryker M P, Baekkeskov S, Kash S F. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagiolini M, Hensch T K. Nature (London) 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 20.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji F Y, Kanbara N, Kuzume H, Sanbo M, Yagi T, et al. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 21.Nagai N, Urano T, Endo A, Takahashi H, Takada Y, Takada A. Neurosci Res. 1999;33:147–154. doi: 10.1016/s0168-0102(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 22.Gordon J A, Stryker M P. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Z, Gilbert M E, Colicos M A, Kandel E R, Kuhl D. Nature (London) 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 24.Pham T A, Impey S, Storm D R, Stryker M P. Neuron. 1999;22:63–72. doi: 10.1016/s0896-6273(00)80679-0. [DOI] [PubMed] [Google Scholar]
- 25.Mataga N, Fujishima S, Condie B G, Hensch T K. J Neurosci. 2001;21:9724–9732. doi: 10.1523/JNEUROSCI.21-24-09724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trachtenberg J T, Stryker M P. J Neurosci. 2001;21:3476–3482. doi: 10.1523/JNEUROSCI.21-10-03476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonini A, Gillespie D C, Crair M C, Stryker M P. J Neurosci. 1998;18:9896–9909. doi: 10.1523/JNEUROSCI.18-23-09896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renger J J, Hartman K N, Tsuchimoto Y, Yokoi M, Nakanishi S, Hensch T K. Proc Natl Acad Sci USA. 2002;99:1041–1046. doi: 10.1073/pnas.022618799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imamura K, Kasamatsu T, Shirokawa T, Ohashi T. Proc R Soc London Ser B Biol Sci. 1999;266:1507–1516. doi: 10.1098/rspb.1999.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaver C J, Ji Q, Fischer Q S, Daw N W. Nat Neurosci. 2001;4:159–163. doi: 10.1038/83985. [DOI] [PubMed] [Google Scholar]
- 31.McAllister A K, Katz L C, Lo D C. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 32.Huang Z J, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear M F, Maffei L, Tonegawa S. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 33.Fiumelli H, Jabaudon D, Magistretti R J, Martin J-L. Eur J Neurosci. 1999;11:1639–1646. doi: 10.1046/j.1460-9568.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- 34.Pizzorusso T, Ratto G M, Putignano E, Maffei L. J Neurosci. 2000;20:2809–2816. doi: 10.1523/JNEUROSCI.20-08-02809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Cristo G, Berardi N, Cancedda L, Pizzorusso T, Putignano E, Ratto G M, Maffei L. Science. 2001;292:2337–2340. doi: 10.1126/science.1059075. [DOI] [PubMed] [Google Scholar]
- 36.Leonardsson G, Ny T. Eur J Biochem. 1997;248:676–683. doi: 10.1111/j.1432-1033.1997.t01-1-00676.x. [DOI] [PubMed] [Google Scholar]
- 37.Endo A, Nagai N, Urano T, Takada Y, Hashimoto K, Takada A. Neurosci Res. 1999;33:1–8. doi: 10.1016/s0168-0102(98)00105-9. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z-L, Strickland S. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman K B, Martinez J, Lynch G. Brain Res. 1998;811:29–33. doi: 10.1016/s0006-8993(98)00906-8. [DOI] [PubMed] [Google Scholar]
- 40.Cremer H, Chazal G, Carleton A, Goridis C, Vincent J-D, Lledo P-M. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagami Y, Abe K, Nishiyama N, Matsuki N. J Neurosci. 2000;20:2003–2010. doi: 10.1523/JNEUROSCI.20-05-02003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]