Full text
PDF


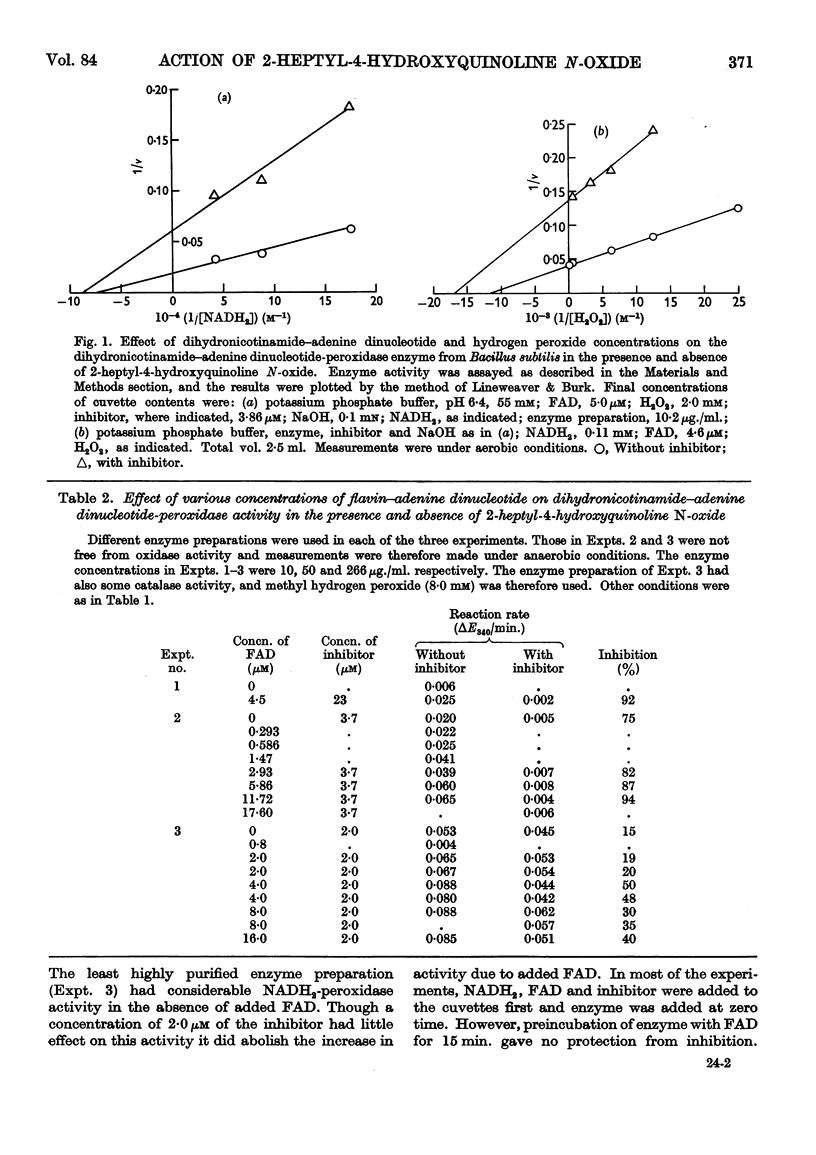
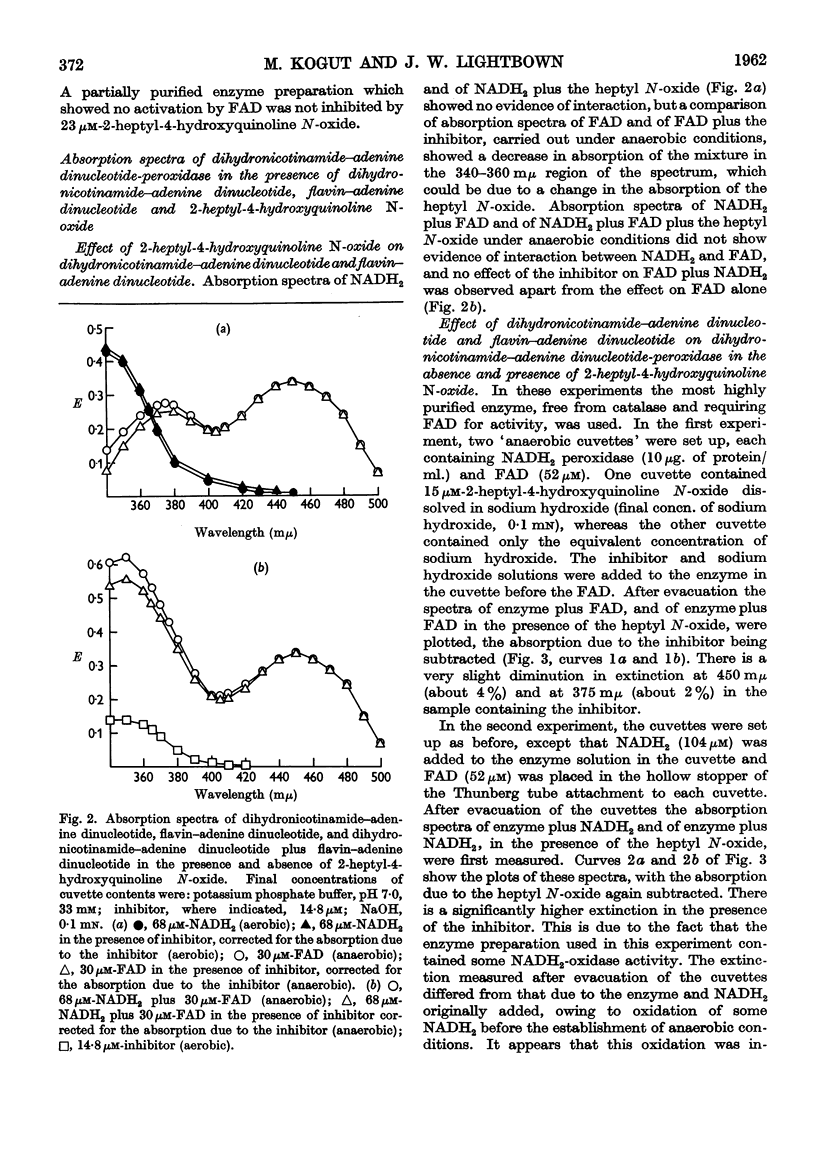
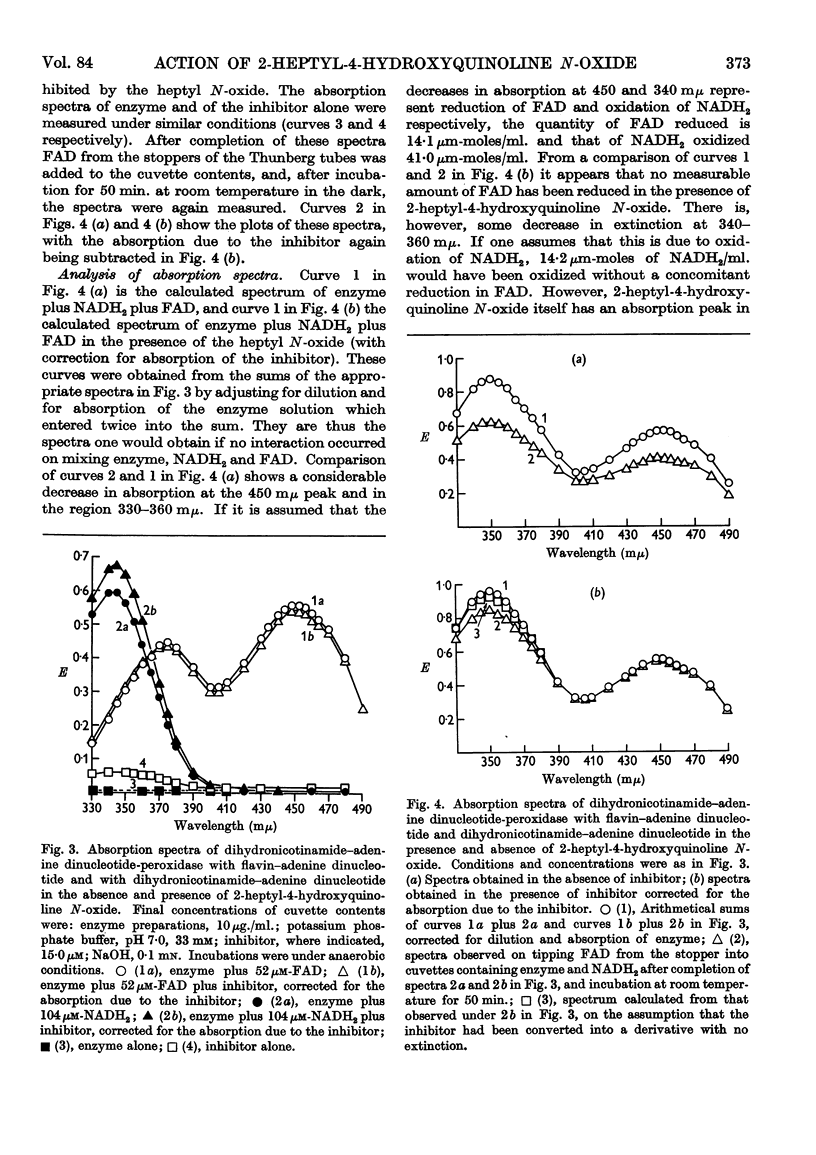

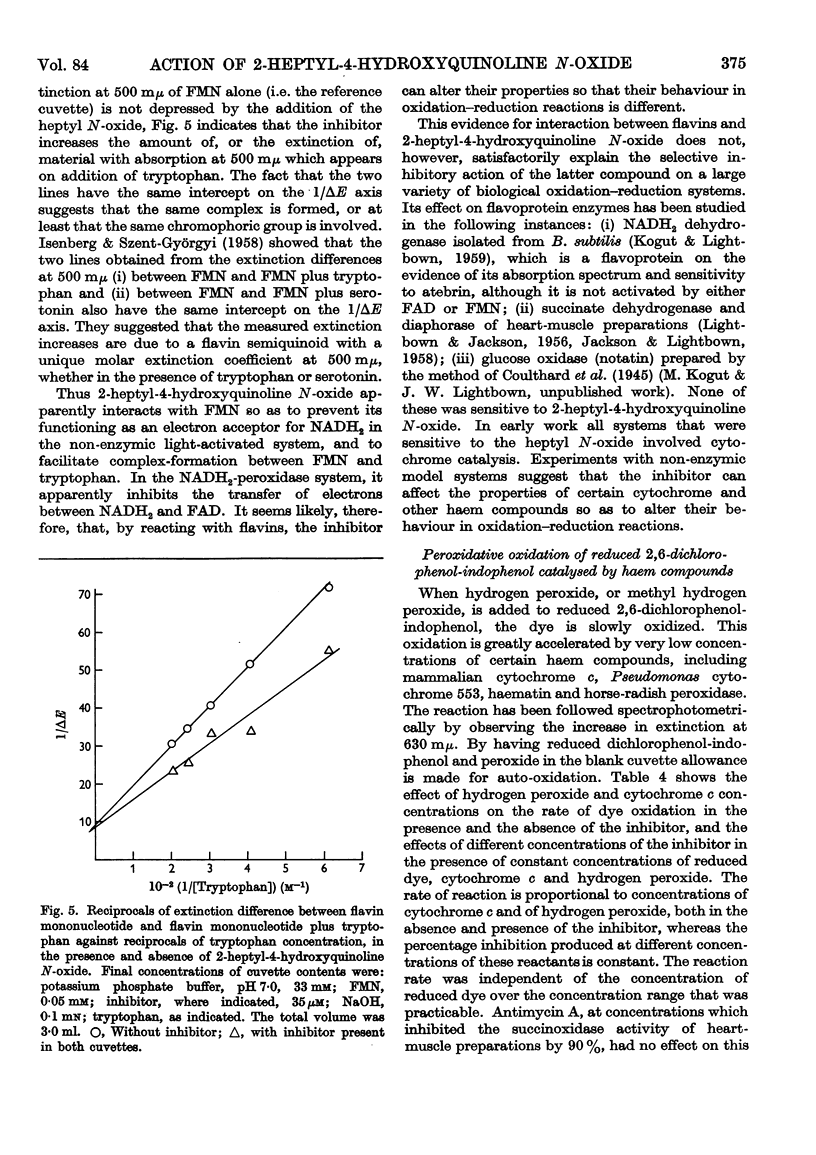




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. The inhibition of photoreactions of chloroplasts by 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1961 Apr;78:735–739. doi: 10.1042/bj0780735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth S., Rabinowitch E. Electron Transfer and Absorption Spectra of Complexes. Science. 1960 Jan 29;131(3396):303–303. doi: 10.1126/science.131.3396.303. [DOI] [PubMed] [Google Scholar]
- BRAY R. C., MALMSTROM B. G., VANNGARD T. The chemistry of xanthine oxidase. Electron-spin resonance of xanthine oxidase solutions. Biochem J. 1959 Sep;73:193–197. doi: 10.1042/bj0730193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMONER B., HEISE J. J., LIPPINCOTT B. B., NORBERG R. E., PASSONNEAU J. V., TOWNSEND J. Biological activity of free radicals. Science. 1957 Jul 12;126(3263):57–63. doi: 10.1126/science.126.3263.57. [DOI] [PubMed] [Google Scholar]
- CORNFORTH J. W., JAMES A. T. Structure of a naturally occurring antagonist of dihydrostreptomycin. Biochem J. 1956 May;63(1):124–130. doi: 10.1042/bj0630124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulthard C. E., Michaelis R., Short W. F., Sykes G. Notatin: an anti-bacterial glucose-aerodehydrogenase from Penicillium notatum Westling and Penicillium resticulosum sp. nov. Biochem J. 1945;39(1):24–36. doi: 10.1042/bj0390024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLIN M. I. The Streptococcus faecalis oxidases for reduced diphosphopyridine nucleotide. III. Isolation and properties of a flavin peroxidase for reduced diphosphopyridine nucleotide. J Biol Chem. 1957 Mar;225(1):557–573. [PubMed] [Google Scholar]
- DOLIN M. I. The Streptococus faecalis oxidases for reduced diphosphopyridine nucleotide. IV. Properties of the enzyme-substrate complex formed between reduced diphosphopyridine nucleotide peroxidase and pyridine nucleotides. J Biol Chem. 1960 Feb;235:544–550. [PubMed] [Google Scholar]
- EHRENBERG A., LUDWIG G. D. Free radical formation in reaction between old yellow enzyme and reduced triphosphopyridine nucleotide. Science. 1958 May 16;127(3307):1177–1178. doi: 10.1126/science.127.3307.1177. [DOI] [PubMed] [Google Scholar]
- Frisell W. R., Mackenzie C. G. THE PHOTOCHEMICAL OXIDATION OF DPNH WITH RIBOFLAVIN PHOSPHATE. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1568–1572. doi: 10.1073/pnas.45.11.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg I., Szent-Györgyi A. FREE RADICAL FORMATION IN RIBOFLAVIN COMPLEXES. Proc Natl Acad Sci U S A. 1958 Sep 15;44(9):857–862. doi: 10.1073/pnas.44.9.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON F. L., LIGHTBOWN J. W. Inhibition of dihydrocozymase-oxidase activity of heart-muscle preparations and of certain cell-free bacterial preparations by 2-heptyl-4-hydroxyquinoline N-oxide. Biochem J. 1958 May;69(1):63–67. doi: 10.1042/bj0690063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOGUT M., PODOSKI E. P. Oxidative pathways in a fluorescent Pseudomonas. Biochem J. 1953 Dec;55(5):800–811. doi: 10.1042/bj0550800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHTBOWN J. W. An antagonist of streptomycin and dihydrostreptomycin produced by Pseudomonas aeruginosa. J Gen Microbiol. 1954 Dec;11(3):477–492. doi: 10.1099/00221287-11-3-477. [DOI] [PubMed] [Google Scholar]
- LIGHTBOWN J. W., JACKSON F. L. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1956 May;63(1):130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E. The chromatographic behaviour of cytochrome c on cation exchangers. Biochem J. 1954 Apr;56(4):535–543. doi: 10.1042/bj0560535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E. The use of ion exchangers in the preparation and purification of cytochrome c. Biochem J. 1954 Apr;56(4):529–535. doi: 10.1042/bj0560529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULLMAN M. E., COLOWICK S. P., KAPLAN N. O. Comparison of diphosphopyridine nucleotide with its deaminated derivative in various enzyme systems. J Biol Chem. 1952 Feb;194(2):593–602. [PubMed] [Google Scholar]
- SMITH L., BALTSCHEFFSKY M. Respiration and light-induced phosphorylation in extracts of Rhodospirillum rubrum. J Biol Chem. 1959 Jun;234(6):1575–1579. [PubMed] [Google Scholar]
- TANIGUCHI S., ITAGAKI E. Nitrate reductase of nitrate respiration type from E. coli. I. Solubilization and purification from the particulate system with molecular characterization as a metalloprotein. Biochim Biophys Acta. 1960 Nov 4;44:263–279. doi: 10.1016/0006-3002(60)91562-6. [DOI] [PubMed] [Google Scholar]
- VERNON L. P., ASH O. K. The photo-reduction of pyridine nucleotides by illuminated chromatophores of Rhodospirillum rubrum in the presence of succinate. J Biol Chem. 1959 Jul;234(7):1878–1882. [PubMed] [Google Scholar]
- VERNON L. P. Photo-oxidations catalyzed by chromatophores of Rhodospirillum rubrum under anaerobic conditions. J Biol Chem. 1959 Jul;234(7):1883–1888. [PubMed] [Google Scholar]
- WHITBY L. G. A new method for preparing flavin-adenine dinucleotide. Biochem J. 1953 Jun;54(3):437–442. doi: 10.1042/bj0540437. [DOI] [PMC free article] [PubMed] [Google Scholar]


