Abstract
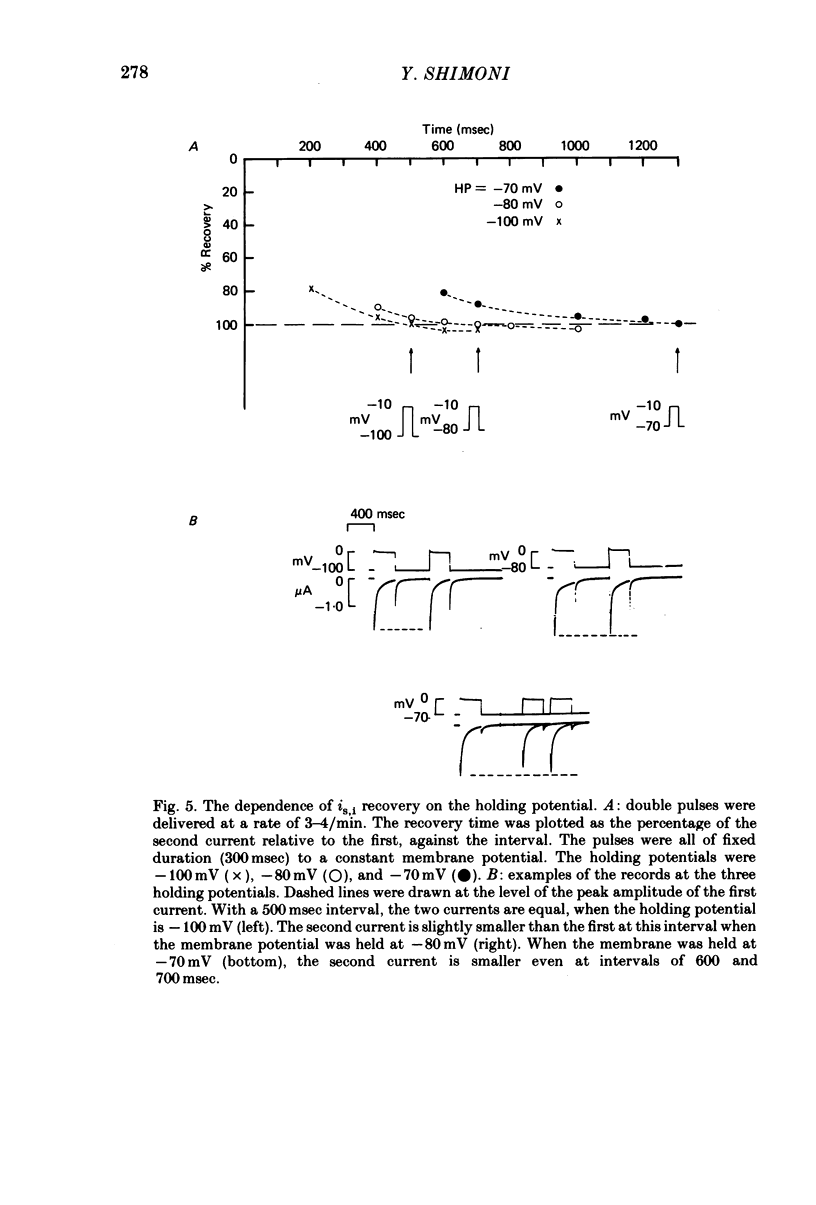
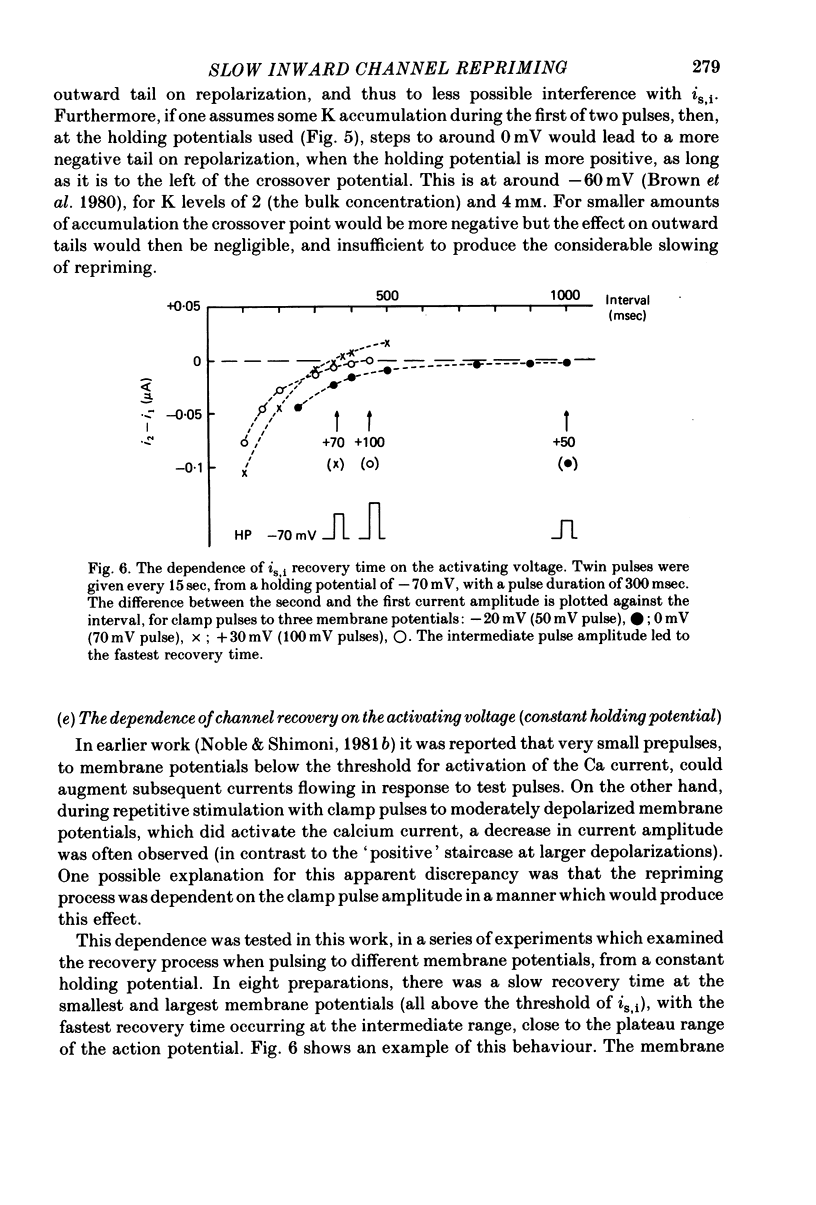
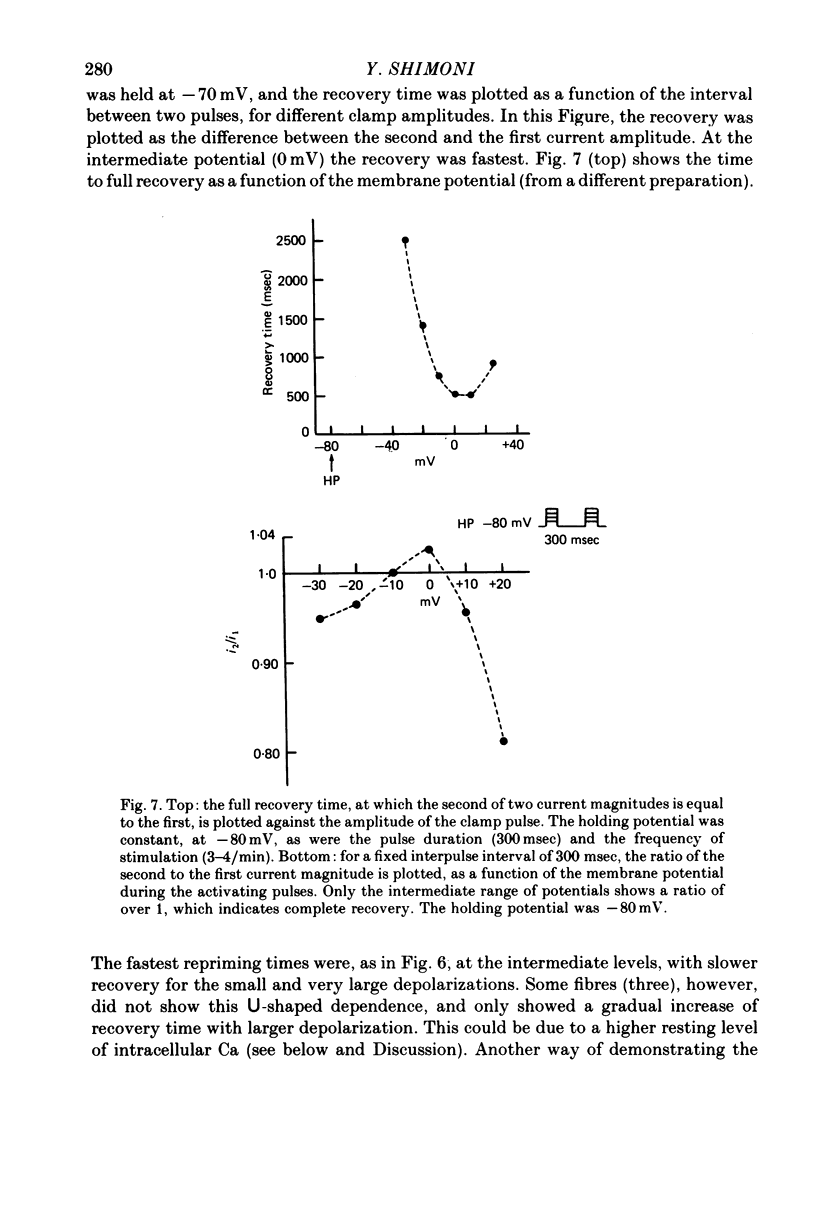
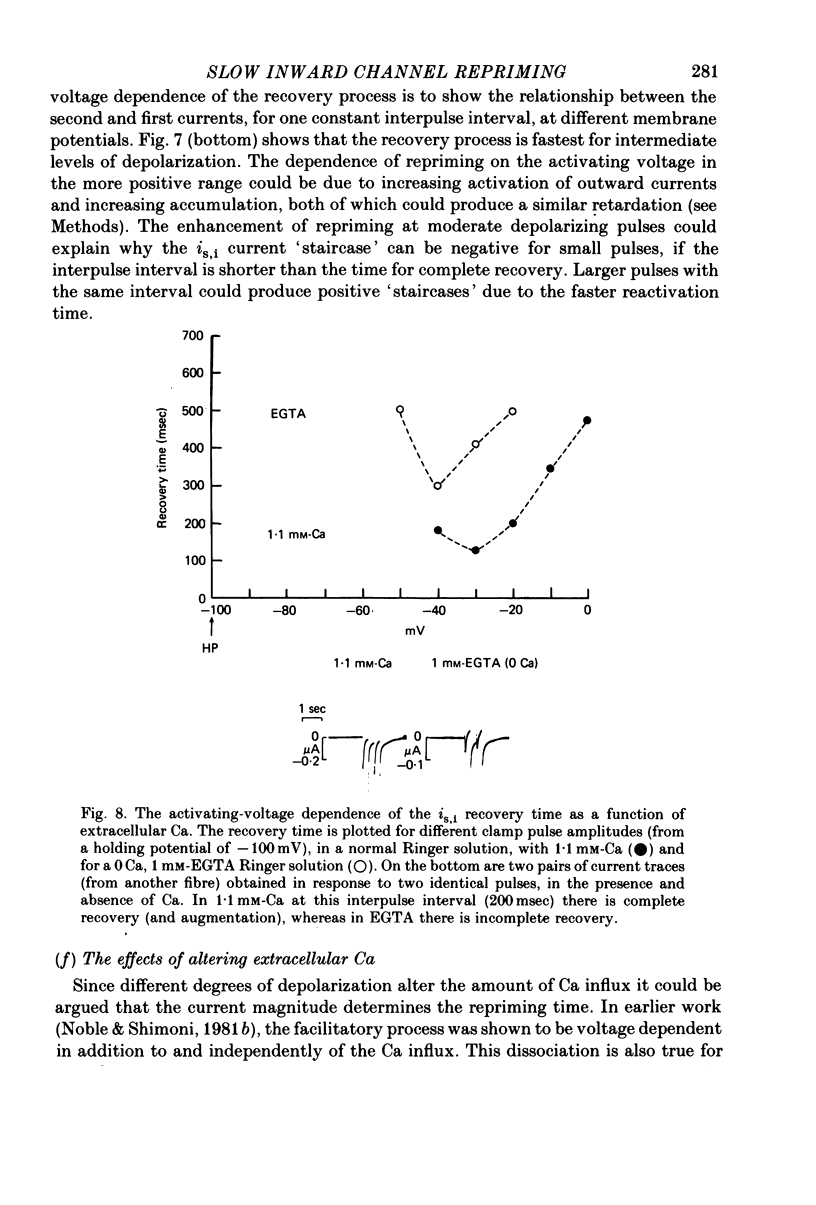
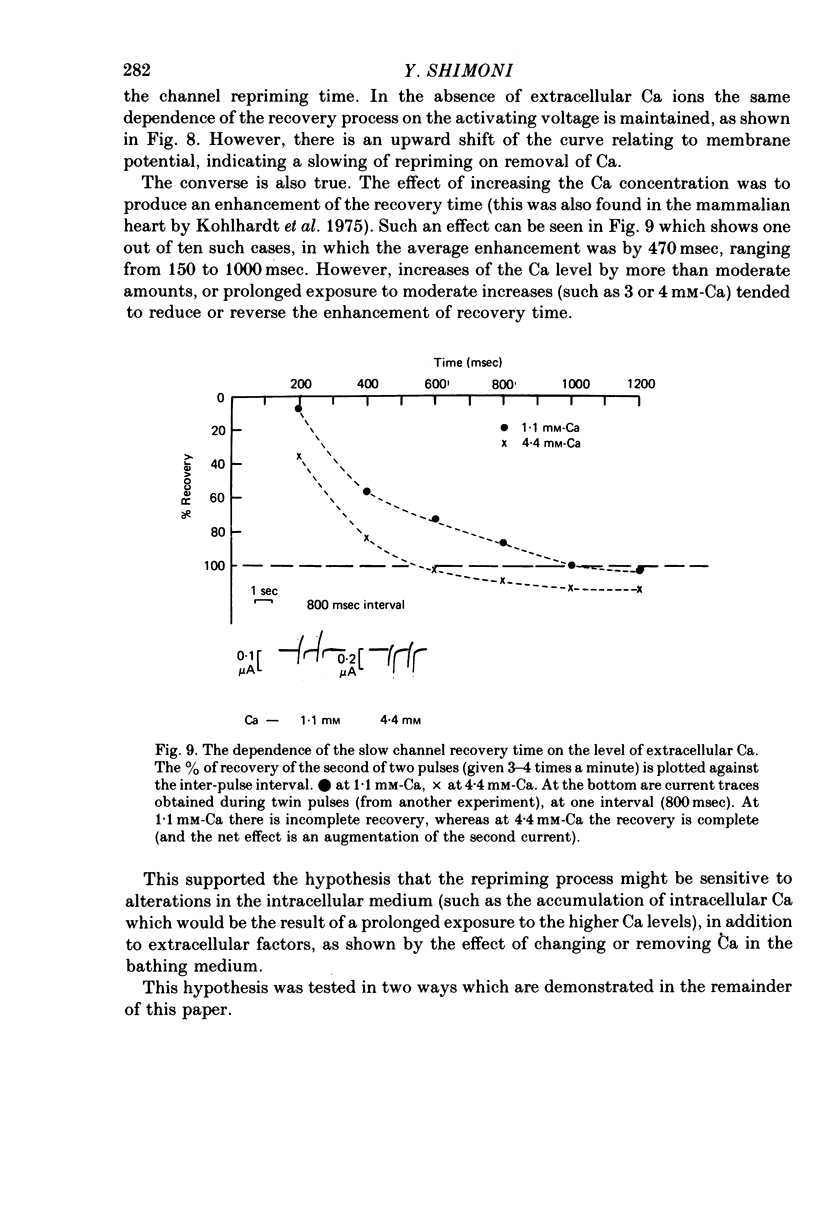
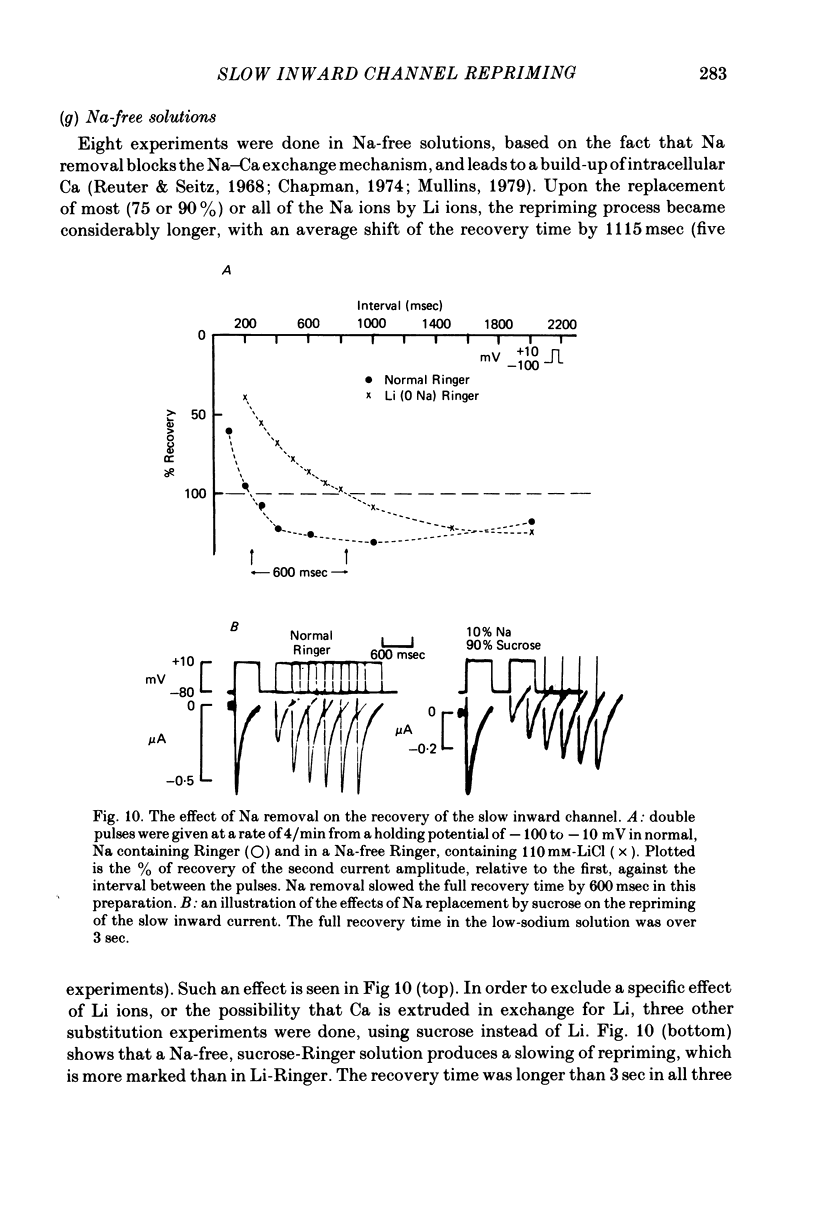
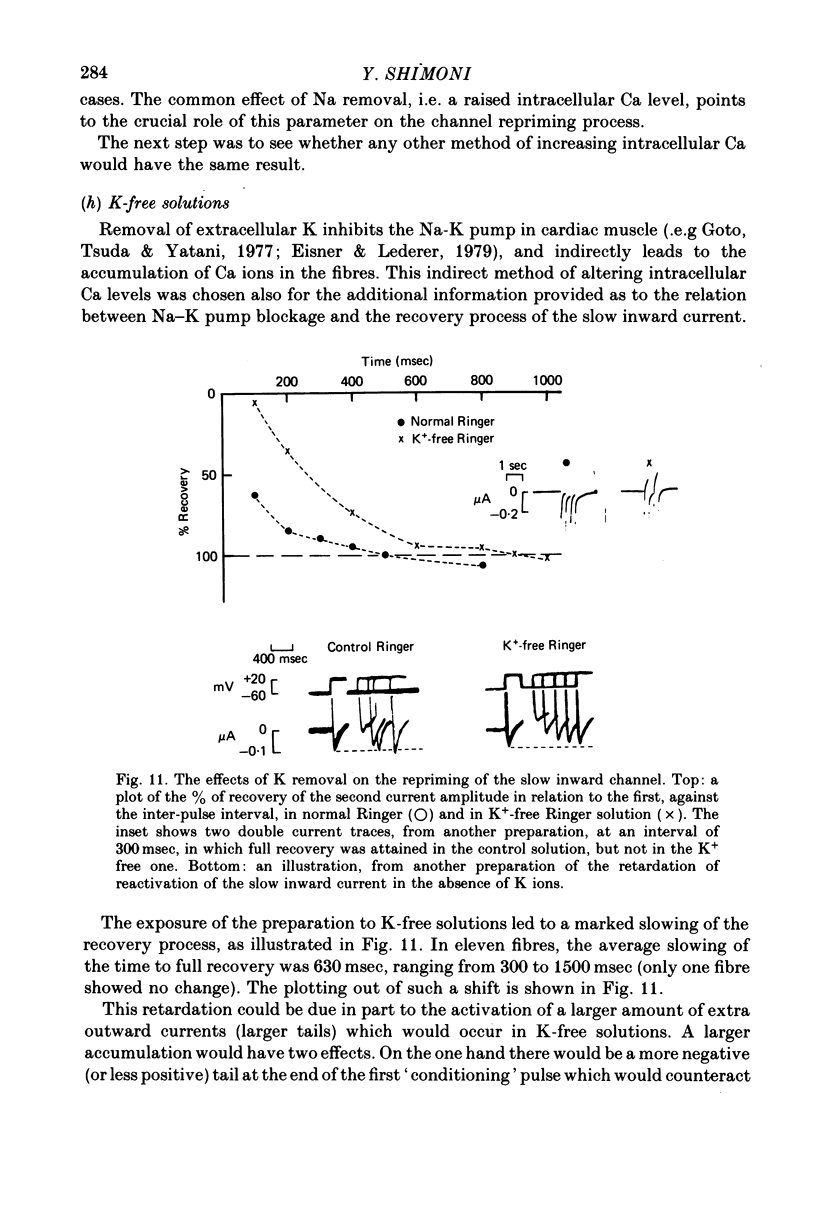
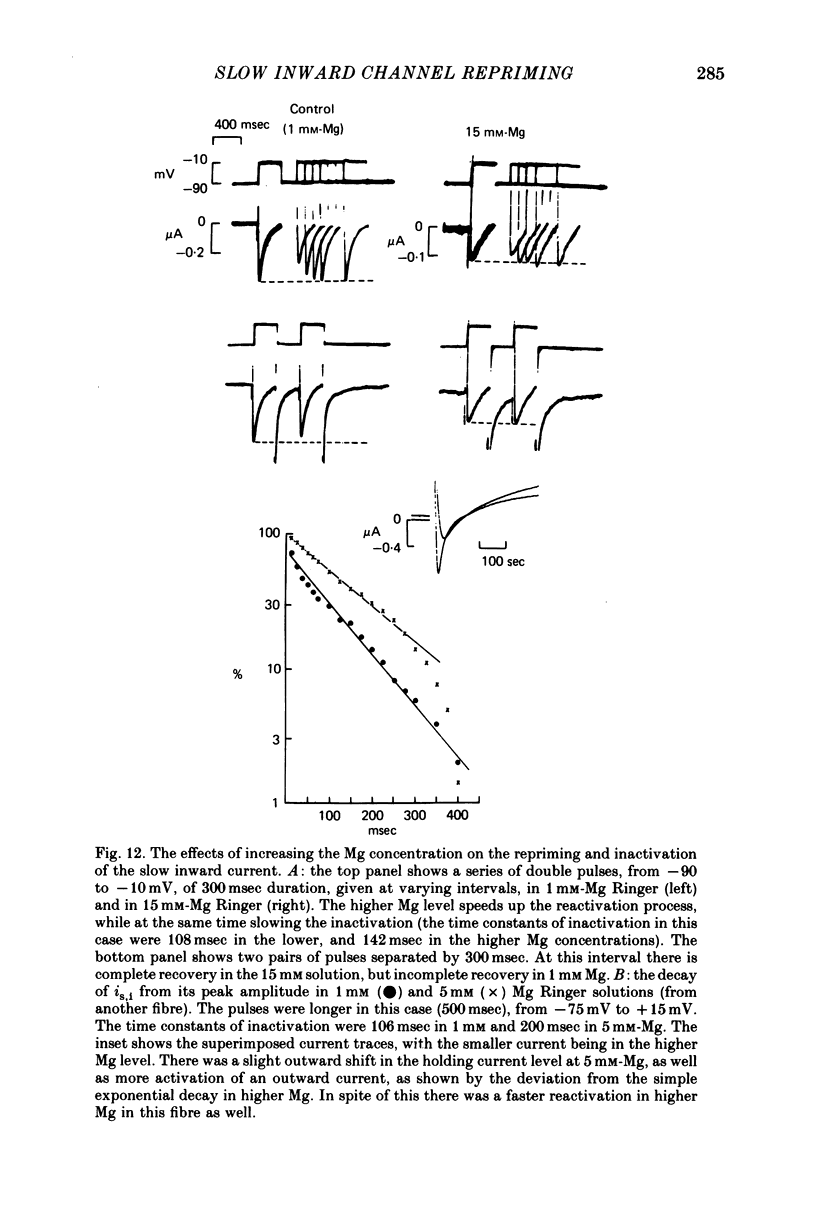
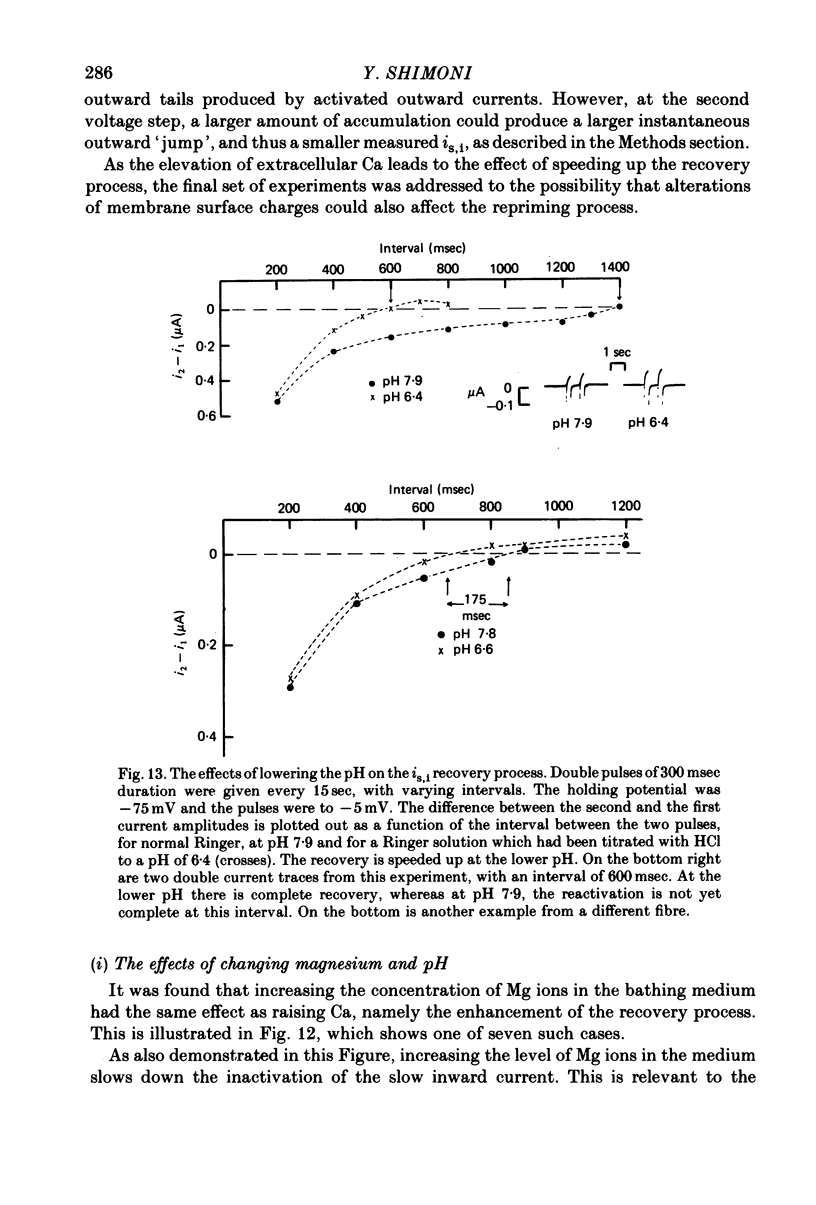
1. The time of recovery (from the inactivation) of the slow inward current was studied in the frog atrium, using the double sucrose gap voltage clamp technique. 2. The 'repriming' process was found to be distinct from the current inactivation, and to depend on experimental protocol: double pulses given at low frequencies (at 'rest') gave a faster recovery time when compared to recovery during constant stimulation, with interposed stimuli monitoring the recovery. Longer durations of the clamp pulses led to a faster recovery process. 3. Changing the holding potential of the membrane (with double pulses to the same absolute membrane potential monitoring the recovery process) greatly affect the repriming with depolarized levels slowing down the process. 4. The recovery time was fastest following clamp pulses to intermediate membrane potentials (in the plateau range). This was determined by double pulses, from a constant hold potentials, to different levels. 5. Decreasing extracellular Ca prolonged, and increasing Ca enhanced the recovery process. 6. The recovery process was markedly slowed down in Na or in K-free solutions. 7. The recovery process was enhanced in solutions with a raised concentration of Mg or H ions (lower pH). In higher Mg solutions, the inactivation of the slow inward current was slower. 8. It is proposed that the recovery process is sensitive to alterations in intracellular Ca ions and to variations in extracellular surface charges. The possible implications are discussed.
Full text
PDF


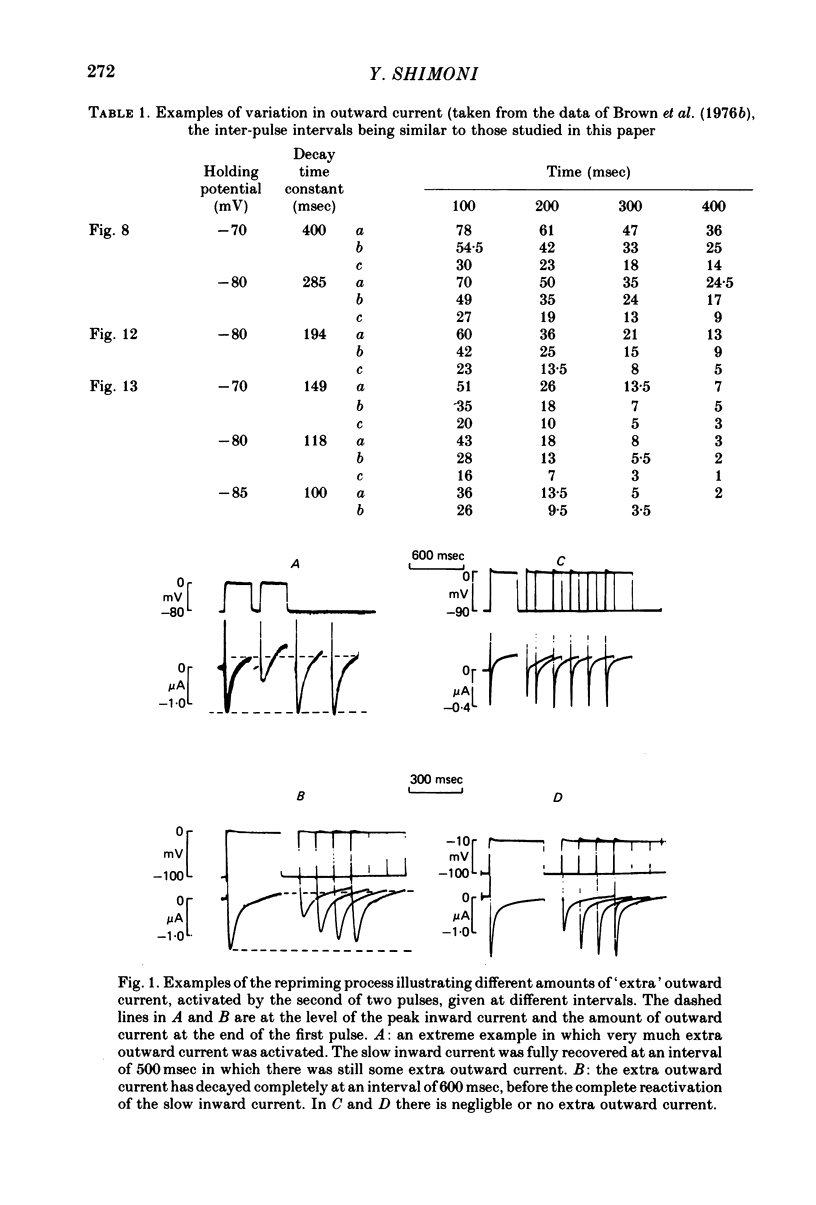


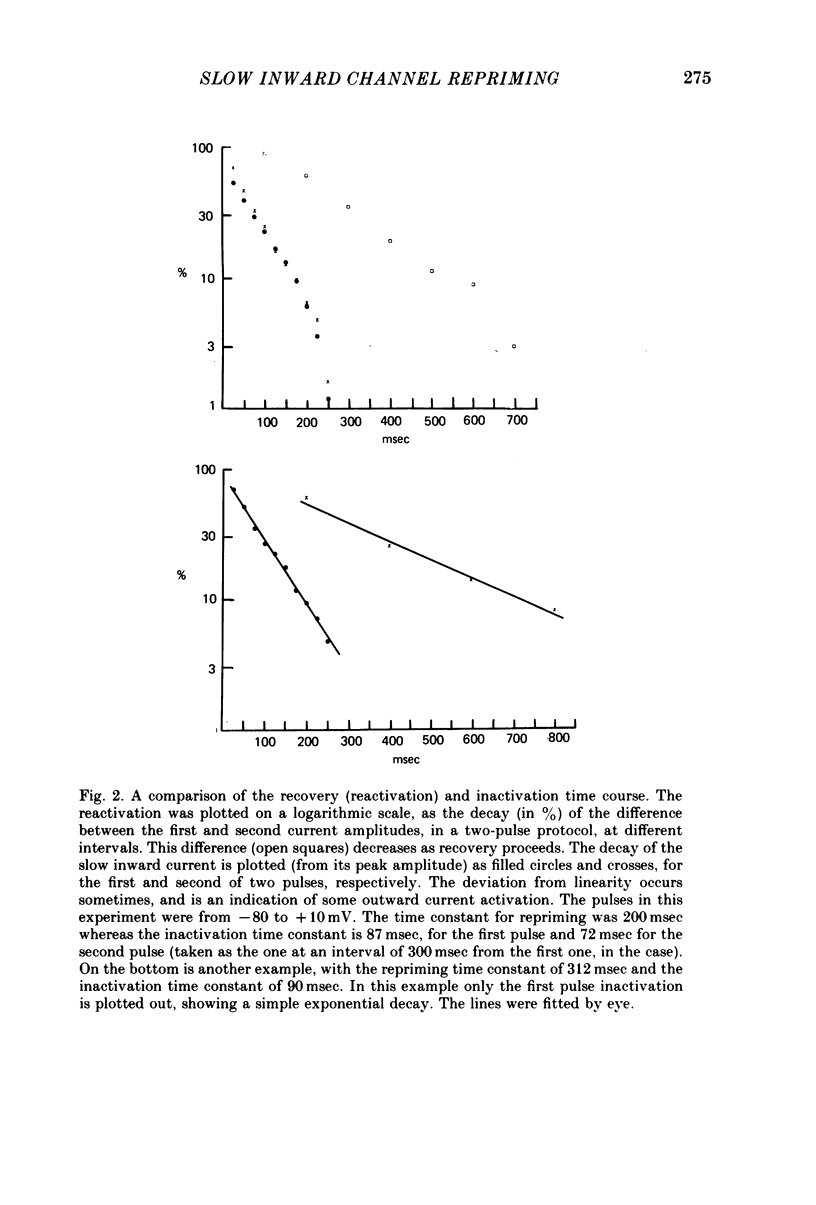
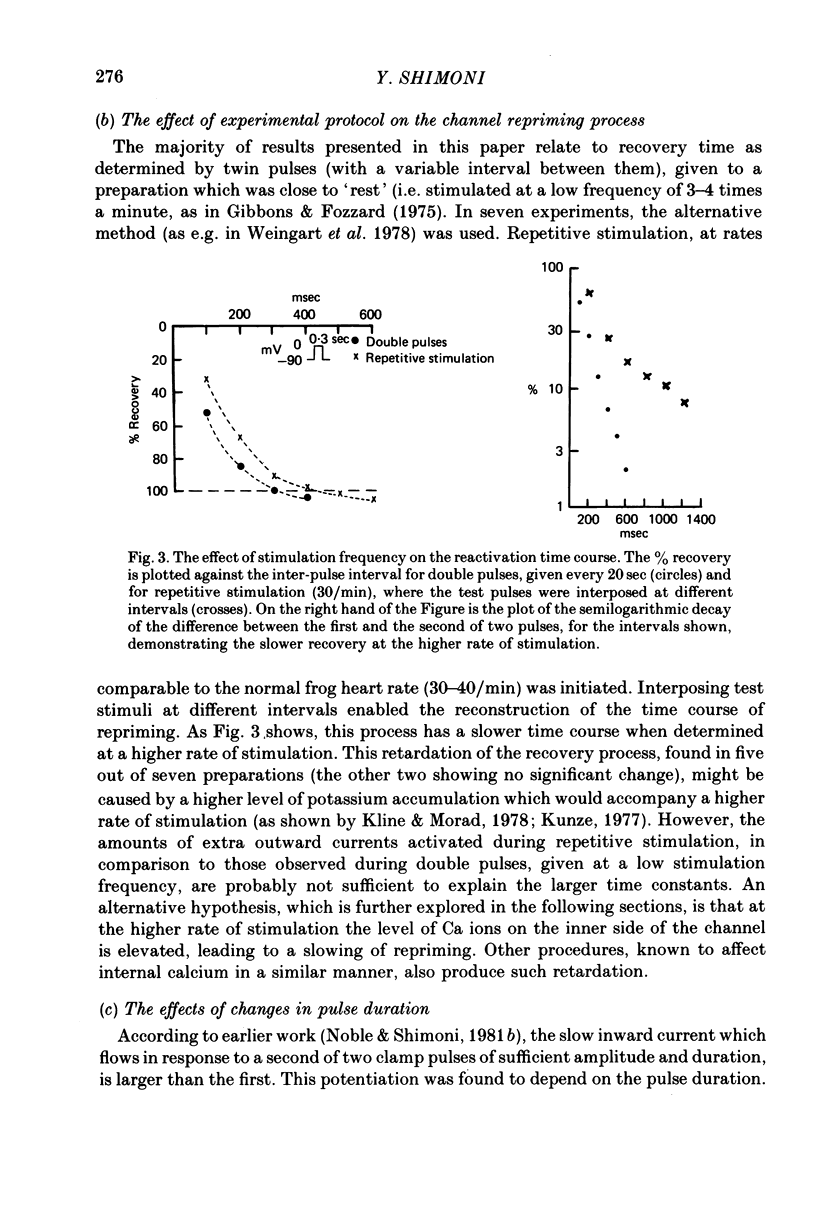
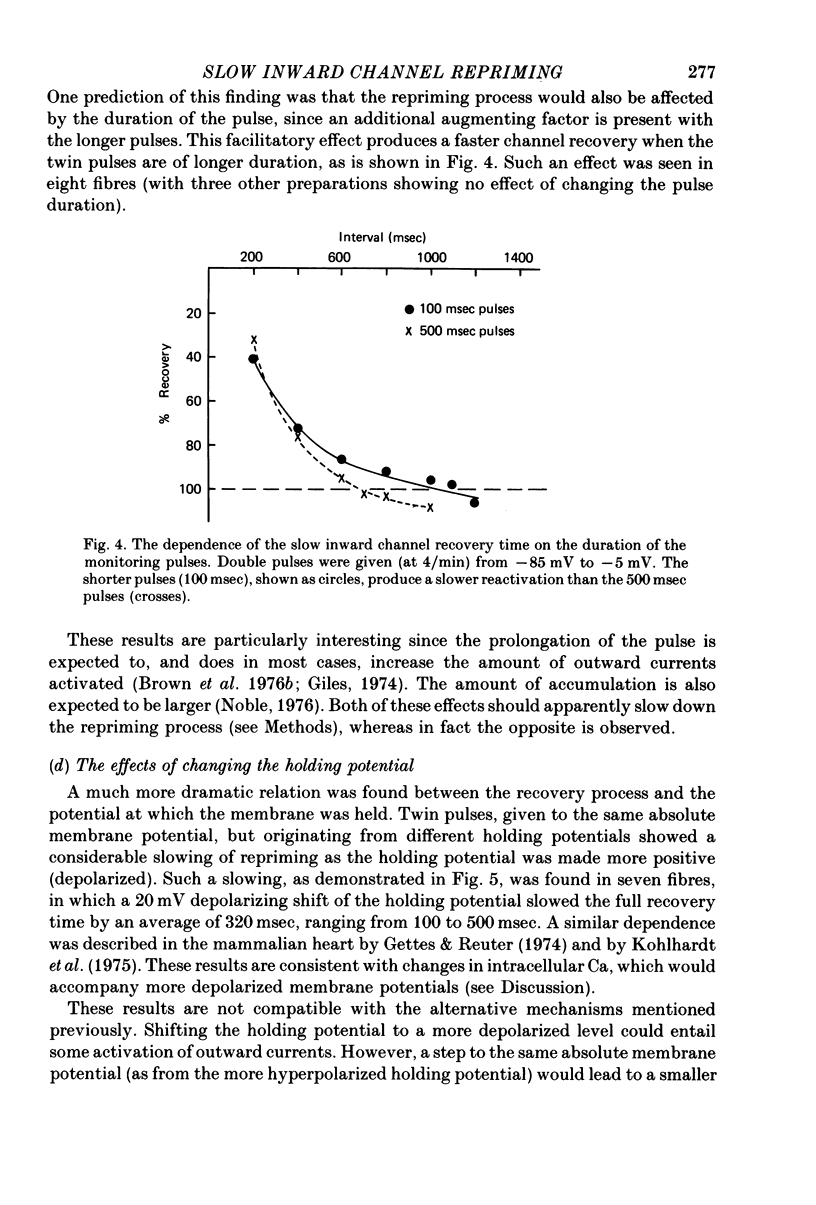





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseau A. Analyse, selon le modèle de Hodgkin-Huxley, des conductances membranaires du myocarde de grenouille (Rana esculenta. J Physiol (Paris) 1972;64(6):647–670. [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Clark A., Noble S. J. Analysis of pace-maker and repolarization currents in frog atrial muscle. J Physiol. 1976 Jul;258(3):547–577. doi: 10.1113/jphysiol.1976.sp011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Clark A., Noble S. J. Identification of the pace-maker current in frog atrium. J Physiol. 1976 Jul;258(3):521–545. doi: 10.1113/jphysiol.1976.sp011434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H., DiFrancesco D., Noble D., Noble S. The contribution of potassium accumulation to outward currents in frog atrium. J Physiol. 1980 Sep;306:127–149. doi: 10.1113/jphysiol.1980.sp013388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. H., Jr, Noble D. Displacement of activator thresholds in cardiac muscle by protons and calcium ions. J Physiol. 1978 Sep;282:333–343. doi: 10.1113/jphysiol.1978.sp012466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. A study of the contractures induced in frog atrial trabeculae by a reduction of the bathing sodium concentration. J Physiol. 1974 Mar;237(2):295–313. doi: 10.1113/jphysiol.1974.sp010483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The interaction of sodium and calcium ions at the cell membrane and the control of contractile strength in frog atrial muscle. J Physiol. 1980 Aug;305:109–123. doi: 10.1113/jphysiol.1980.sp013353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Noble D., Ohba M., Ojeda C. Action of salicylate ions on the electrical properties of sheep cardiac Purkinje fibres. J Physiol. 1979 Dec;297(0):163–185. doi: 10.1113/jphysiol.1979.sp013033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J., Barr L., Jakobsson E. Electrical characteristics of frog atrial trabeculae in the double sucrose gap. Biophys J. 1975 Oct;15(10):1047–1067. doi: 10.1016/S0006-3495(75)85882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T., Daufmann R. The voltage- and time-dependent effects of (-)-verapamil on the slow inward current in isolated cat ventricular myocardium. J Pharmacol Exp Ther. 1978 Oct;207(1):49–55. [PubMed] [Google Scholar]
- Einwächter H. M., Haas H. G., Kern R. Membrane current and contraction in frog atrial fibres. J Physiol. 1972 Dec;227(1):141–171. doi: 10.1113/jphysiol.1972.sp010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Inotropic and arrhythmogenic effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:255–277. doi: 10.1113/jphysiol.1979.sp012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettes L. S., Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974 Aug;240(3):703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Slow inward current and contraction of sheep cardiac Purkinje fibers. J Gen Physiol. 1975 Mar;65(3):367–384. doi: 10.1085/jgp.65.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Noble S. J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J Physiol. 1976 Sep;261(1):103–123. doi: 10.1113/jphysiol.1976.sp011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Tsuda Y., Yatani A. Two mechanisms for positive inotropism of low-K Ringer solution in bullfrog atrium. Nature. 1977 Aug 25;268(5622):755–757. doi: 10.1038/268755a0. [DOI] [PubMed] [Google Scholar]
- Hausworth O., Noble D., Tsien R. W. The dependence of plateau currents in cardiac Purkinje fibres on the interval between action potentials. J Physiol. 1972 Apr;222(1):27–49. doi: 10.1113/jphysiol.1972.sp009786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Calcium conductance in relation to contractility in frog myocardium. J Physiol. 1976 Aug;259(3):597–616. doi: 10.1113/jphysiol.1976.sp011485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Sodium-calcium exchange in regulation of cardiac contractility. Evidence for an electrogenic, voltage-dependent mechanism. J Gen Physiol. 1979 Apr;73(4):403–424. doi: 10.1085/jgp.73.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. P., Morad M. Potassium efflux in heart muscle during activity: extracellular accumulation and its implications. J Physiol. 1978 Jul;280:537–558. doi: 10.1113/jphysiol.1978.sp012400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Krause H., Kübler M., Herdey A. Kinetics of inactivation and recovery of the slow inward current in the mammalian ventricular myocardium. Pflugers Arch. 1975 Mar 22;355(1):1–17. doi: 10.1007/BF00584795. [DOI] [PubMed] [Google Scholar]
- Kunze D. L. Rate-dependent changes in extracellular potassium in the rabbit atrium. Circ Res. 1977 Jul;41(1):122–127. doi: 10.1161/01.res.41.1.122. [DOI] [PubMed] [Google Scholar]
- Maughan D. W. Some effects of prolonged polarization on membrane currents in bullfrog atrial muscle. J Membr Biol. 1973;11(4):331–352. doi: 10.1007/BF01869829. [DOI] [PubMed] [Google Scholar]
- Morad M., Trautwein W. The effect of the duration of the action potential on contraction in the mammalian heart muscle. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299(1):66–82. doi: 10.1007/BF00362542. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. The ionic nature of slow inward current and its relation to contraction. Pflugers Arch. 1972;334(1):24–38. doi: 10.1007/BF00585998. [DOI] [PubMed] [Google Scholar]
- Noble S. J. Potassium accumulation and depletion in frog atrial muscle. J Physiol. 1976 Jul;258(3):579–613. doi: 10.1113/jphysiol.1976.sp011436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S., Shimoni Y. The calcium and frequency dependence of the slow inward current 'staircase' in frog atrium. J Physiol. 1981 Jan;310:57–75. doi: 10.1113/jphysiol.1981.sp013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S., Shimoni Y. Voltage-dependent potentiation of the slow inward current in frog atrium. J Physiol. 1981 Jan;310:77–95. doi: 10.1113/jphysiol.1981.sp013538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Irisawa H., Kokobun S., Kotake H., Nishimura M., Watanabe Y. Slow current systems in the A-V node of the rabbit heart. Nature. 1980 May 22;285(5762):228–229. doi: 10.1038/285228a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Yanagihara K., Irisawa H. Inward current of the rabbit sinoatrial node cell. Pflugers Arch. 1977 Nov 25;372(1):43–51. doi: 10.1007/BF00582205. [DOI] [PubMed] [Google Scholar]
- Ojeda C., Rougier O. Kinetic analysis of the delayed outward currents in frog atrium. Existence of two types of preparation. J Physiol. 1974 May;239(1):51–73. doi: 10.1113/jphysiol.1974.sp010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol. 1967 Sep;192(2):479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F., Tripathi O. Calcium conductance and tension in mammalian ventricular muscle. Pflugers Arch. 1975;354(1):55–74. doi: 10.1007/BF00584503. [DOI] [PubMed] [Google Scholar]
- Weingart R., Kass R. S., Tsien R. W. Is digitalis inotropy associated with enhanced slow inward calcium current? Nature. 1978 Jun 1;273(5661):389–392. doi: 10.1038/273389a0. [DOI] [PubMed] [Google Scholar]


