Abstract
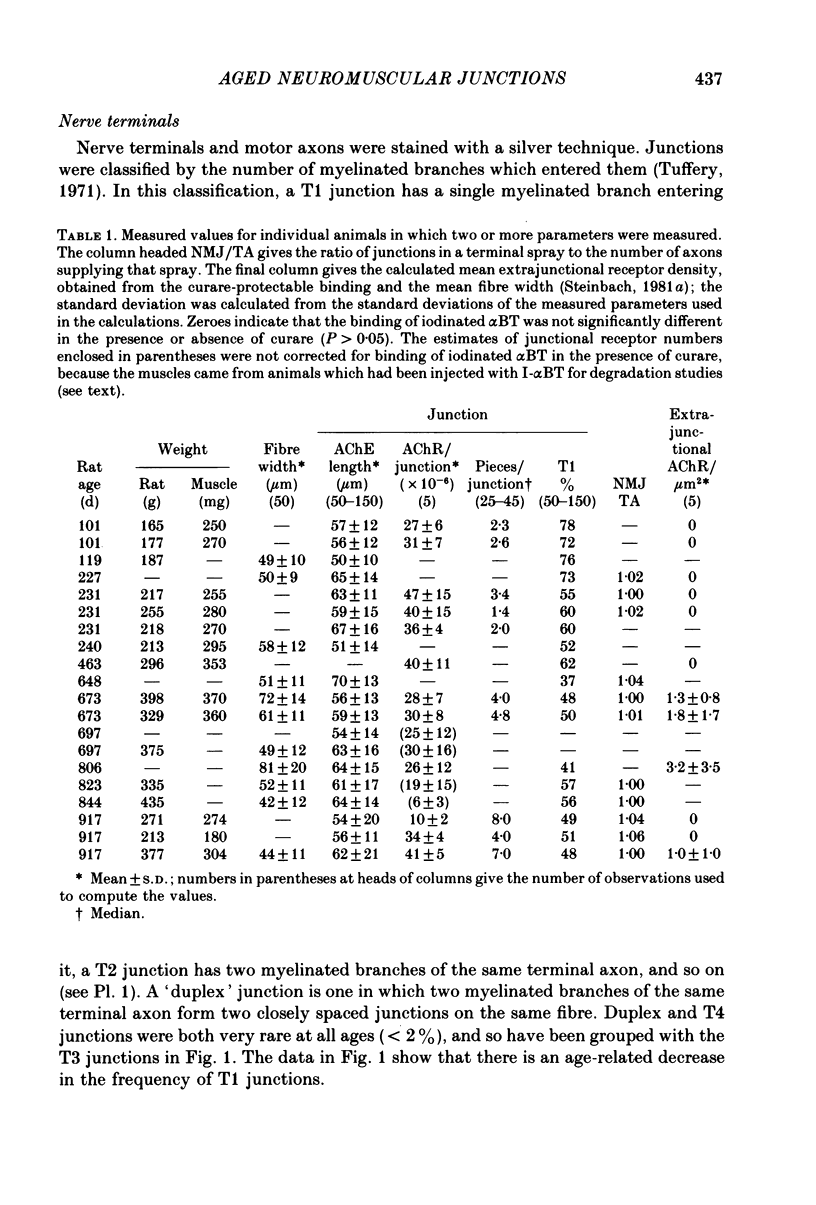
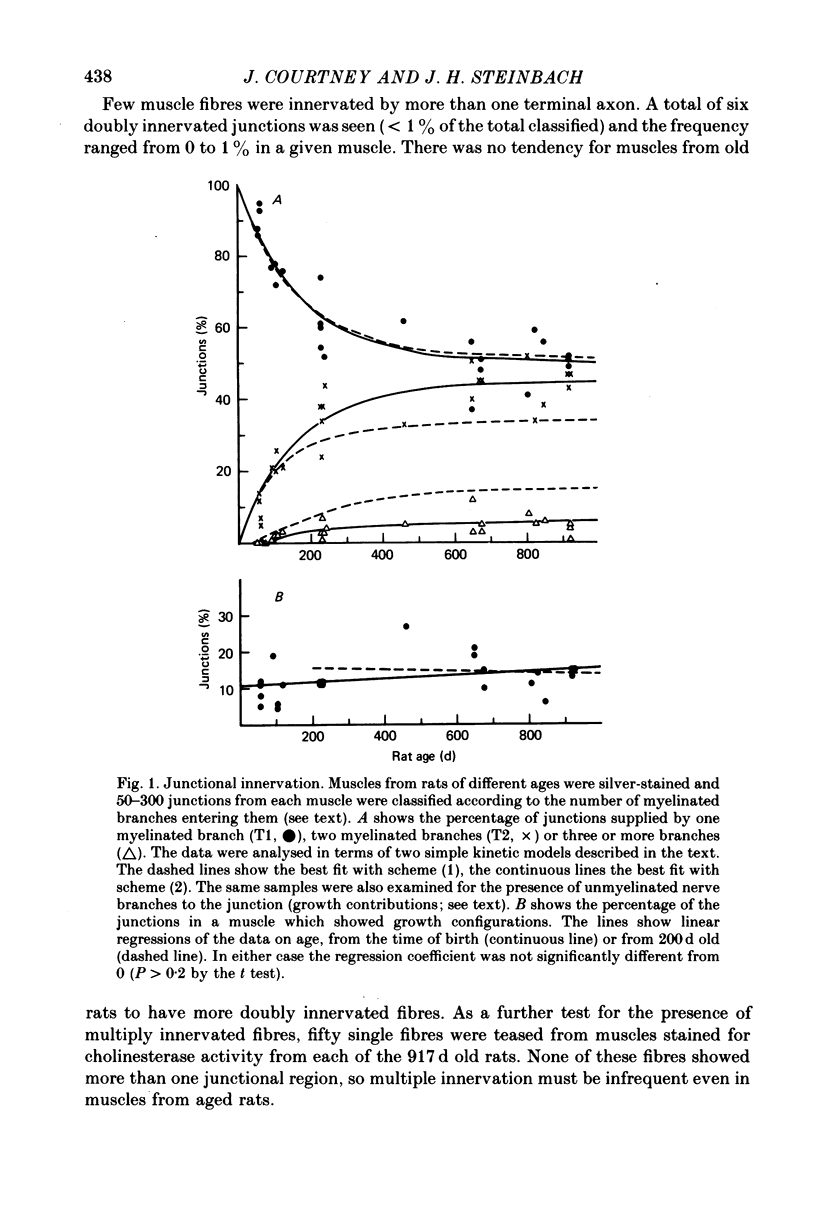
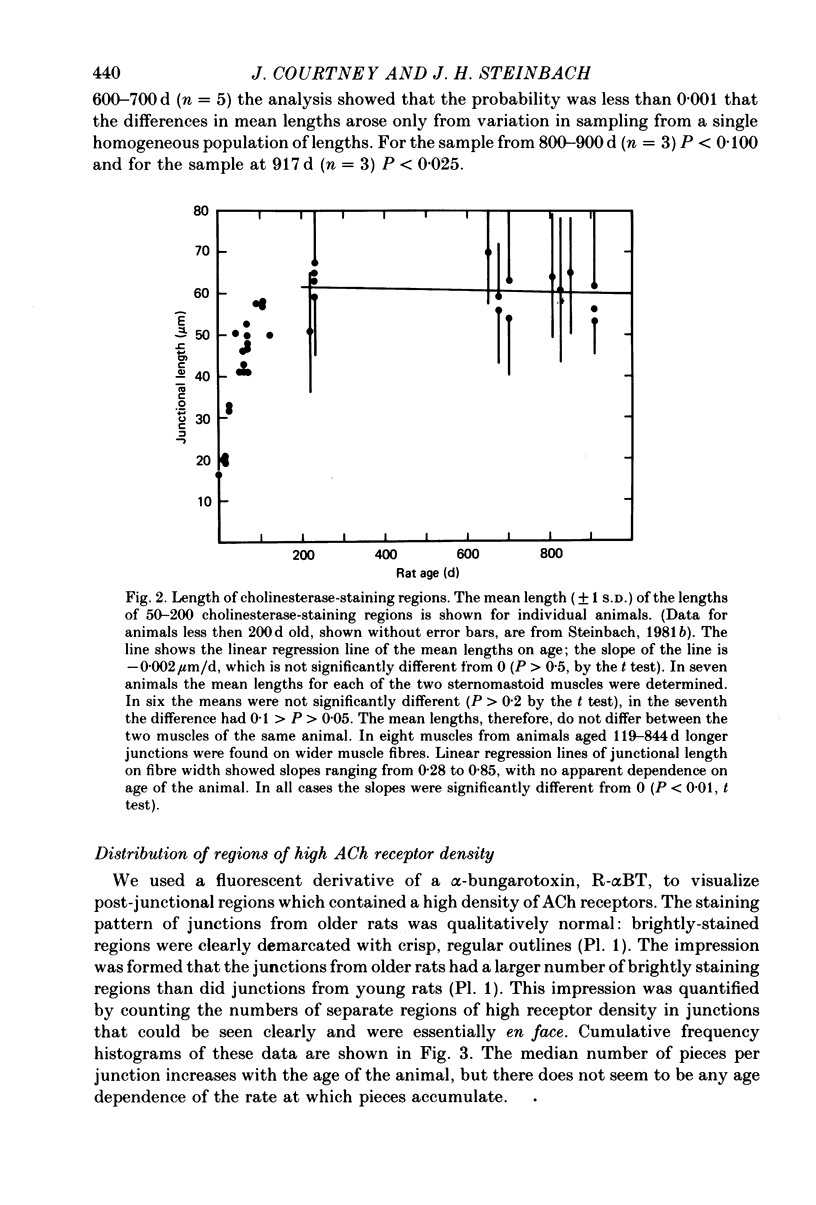
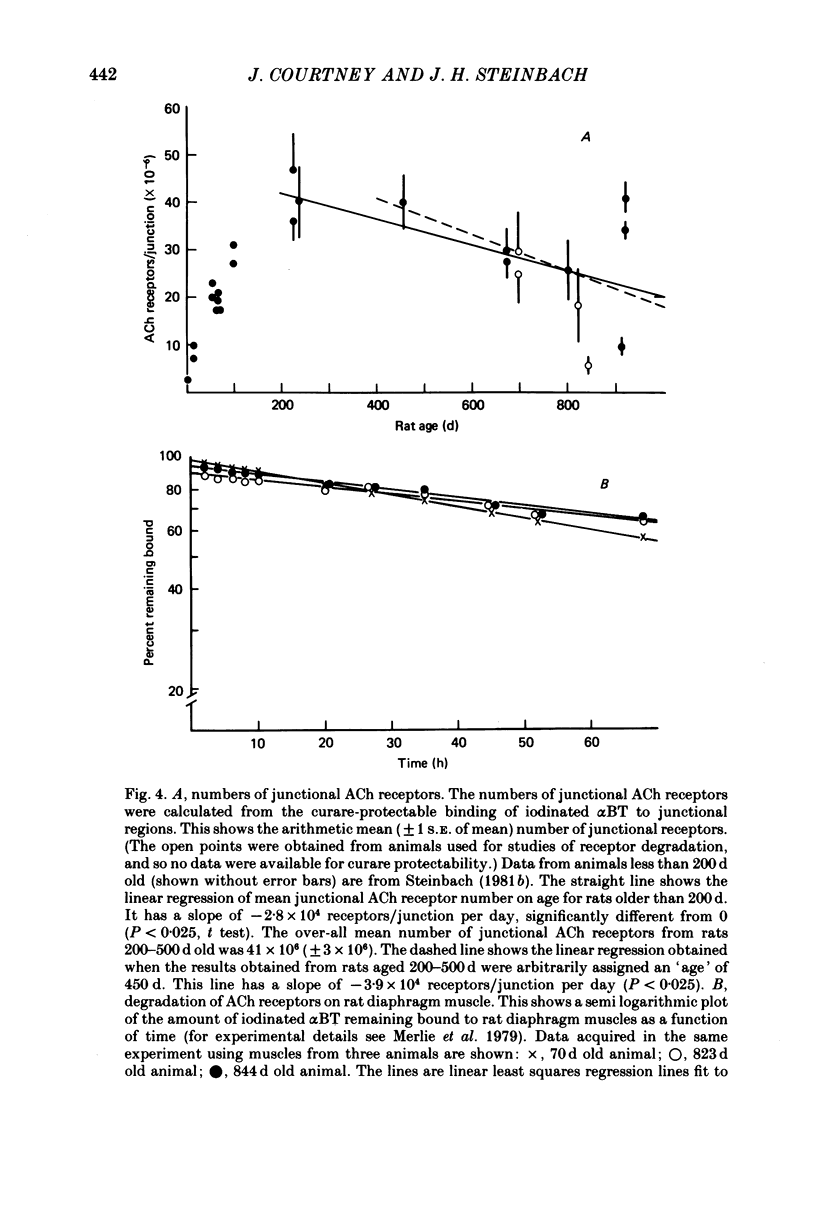
1. Neuromuscular junctions in the sternomastoid muscles of female Lewis rats were examined in animals up to 917 d old. 2. The average number of myelinated branches of terminal axons entering a junction increased with age of the animal, up to 400 d. This change could be described by a simple kinetic model which assumed that there was no influence of age on the ability of motoneurones to produce or maintain terminal branches, but that axons could produce or maintain only a limited number of branches. 3. There was a change in the over-all junctional length with age, but there was a significant increase in the number of discrete regions of high ACh receptor density in junctions from older animals. 4. There was a gradual decrease in the number of ACh receptors per junction with age after about 500 d, and muscles from some rats older than 500 d had detectable numbers of extrajunctional ACh receptors. 5. The changes in the neuromuscular junction with increased age occurred gradually over adult life.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W. Fluorescent staining of acetylcholine receptors in vertebrate skeletal muscle. J Physiol. 1974 Mar;237(2):385–400. doi: 10.1113/jphysiol.1974.sp010487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERG B. N. Muscular dystrophy in aging rats. J Gerontol. 1956 Apr;11(2):134–139. doi: 10.1093/geronj/11.2.134. [DOI] [PubMed] [Google Scholar]
- BERG B. N., WOLF A., SIMMSHS Degenerative lesions of spinal roots and peripheral nerves in aging rats. Gerontologia. 1962;6:72–80. doi: 10.1159/000211108. [DOI] [PubMed] [Google Scholar]
- Barker D., Ip M. C. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):538–554. doi: 10.1098/rspb.1966.0008. [DOI] [PubMed] [Google Scholar]
- Caccia M. R., Harris J. B., Johnson M. A. Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve. 1979 May-Jun;2(3):202–212. doi: 10.1002/mus.880020308. [DOI] [PubMed] [Google Scholar]
- Coërs C., Telerman-Toppet N., Gérard J. M. Terminal innervation ratio in neuromuscular disease. I. Methods and controls. Arch Neurol. 1973 Oct;29(4):210–214. doi: 10.1001/archneur.1973.00490280022002. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine receptors. Revised estimates of extrajunctional receptor density in denervated rat diaphragm. J Gen Physiol. 1974 Oct;64(4):468–472. doi: 10.1085/jgp.64.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore S. A. Spinal nerve root degeneration in aging laboratory rats: a light microscopic study. Anat Rec. 1972 Oct;174(2):251–257. doi: 10.1002/ar.1091740209. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Hanzlíková V. Fast and slow motor units in ageing. Gerontology. 1976;22(4):280–300. doi: 10.1159/000212144. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Hanzlíková V. Motor unit in old age. Nature. 1966 Feb 26;209(5026):921–922. doi: 10.1038/209921b0. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Hanzlíková V., Vysokocil F. Age changes in cross striated muscle of the rat. J Physiol. 1971 Jul;216(2):331–343. doi: 10.1113/jphysiol.1971.sp009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. S. The effect of age on neuromuscular transmission. J Physiol. 1978 Jan;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenenhouse A. Role of skeletal muscle activity in the control of muscle acetylcholine sensitivity. Exp Neurol. 1977 Jan;54(1):148–171. doi: 10.1016/0014-4886(77)90242-4. [DOI] [PubMed] [Google Scholar]
- Lewkowicz S. J. Motor innervation of the gastrocnemius muscle of wobbler and dystrophic mice. J Neurol Sci. 1979 Nov;43(3):405–419. doi: 10.1016/0022-510x(79)90019-4. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Heinemann S., Lindstrom J. M. Acetylcholine receptor degradation in adult rat diaphragms in organ culture and the effect of anti-acetylcholine receptor antibodies. J Biol Chem. 1979 Jul 25;254(14):6320–6327. [PubMed] [Google Scholar]
- Mills R. G., Bray J. J., Hubbard J. I. Effects of inactivity on membrane potentials in rat muscle. Brain Res. 1978 Jul 21;150(3):607–610. doi: 10.1016/0006-8993(78)90824-7. [DOI] [PubMed] [Google Scholar]
- Namba T., Nakamura T., Grob D. Staining for nerve fiber and cholinesterase activity in fresh frozen sections. Am J Clin Pathol. 1967 Jan;47(1):74–77. doi: 10.1093/ajcp/47.1.74. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B., Griffin J. W. Effect of muscle disuse on acetylcholine receptors. Nature. 1976 Mar 25;260(5549):352–353. doi: 10.1038/260352a0. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B., Griffin J. W. Effects of aging on nerve sprouting and regeneration. Exp Neurol. 1980 Oct;70(1):65–82. doi: 10.1016/0014-4886(80)90006-0. [DOI] [PubMed] [Google Scholar]
- Ravdin P., Axelrod D. Fluorescent tetramethyl rhodamine derivatives of alpha-bungarotoxin: preparation, separation, and characterization. Anal Biochem. 1977 Jun;80(2):585–592. doi: 10.1016/0003-2697(77)90682-0. [DOI] [PubMed] [Google Scholar]
- Rowe R. W. The effect of senility on skeletal muscles in the mouse. Exp Gerontol. 1969 Jul;4(2):119–126. doi: 10.1016/0531-5565(69)90034-5. [DOI] [PubMed] [Google Scholar]
- Stanmore A., Bradbury S., Weddell A. G. A quantitative study of peripheral nerve fibres in the mouse following the administration of drugs. 1. Age changes in untreated CBA mice from 3 to 21 months of age. J Anat. 1978 Sep;127(Pt 1):101–115. [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H., Merlie J., Heinemann S., Bloch R. Degradation of junctional and extrajunctional acetylcholine receptors by developing rat skeletal muscle. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3547–3551. doi: 10.1073/pnas.76.7.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H. Neuromuscular junctions and alpha-bungarotoxin-binding sites in denervated and contralateral cat skeletal muscles. J Physiol. 1981;313:513–528. doi: 10.1113/jphysiol.1981.sp013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi H., Yoshioka T., Kobayashi H. Age change of skeletal muscles of rats. Gerontologia. 1971;17(4):219–227. doi: 10.1159/000211826. [DOI] [PubMed] [Google Scholar]
- Tuffery A. R. Growth and degeneration of motor end-plates in normal cat hind limb muscles. J Anat. 1971 Nov;110(Pt 2):221–247. [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F., Gutmann E. Spontaneous transmitter release from nerve endings and contractile properties in the soleus and diaphragm muscles of senile rats. Experientia. 1972 Mar 15;28(3):280–281. doi: 10.1007/BF01928688. [DOI] [PubMed] [Google Scholar]



