Abstract
Missense mutations in Cu,Zn-superoxide dismutase (SOD1) account for ≈20% of familial amyotrophic lateral sclerosis (FALS) through some, as yet undefined, toxic gain of function that leads to gradual death of motor neurons. Mitochondrial swelling and vacuolization are early signs of incipient motor neuron death in FALS. We previously reported that SOD1 exists in the intermembrane space of mitochondria. Herein, we demonstrate that the entry of SOD1 into mitochondria depends on demetallation and that heat shock proteins (Hsp70, Hsp27, or Hsp25) block the uptake of the FALS-associated mutant SOD1 (G37R, G41D, or G93A), while having no effect on wild-type SOD1. The binding of mutant SOD1 to Hsps in the extract of neuroblastoma cells leads to formation of sedimentable aggregates. Many antiapoptotic effects of Hsps have been reported. We now propose that this binding of Hsps to mutant forms of a protein abundant in motor neurons, such as SOD1, makes Hsps unavailable for their antiapoptotic functions and leads ultimately to motor neuron death. It also appears that the Hsp–SOD1 complex recruits other proteins present in the neuroblastoma cell and presumably in motor neurons to form sedimentable aggregates.
Amyotrophic lateral sclerosis (ALS) occurs in familial (FALS) and sporadic (SALS) forms and is caused by a late-onset, progressive loss of motor neurons, leading to paralysis and death. FALS and SALS are distinguishable genetically, but not clinically. Approximately 20% of cases of FALS have been associated with more than 90 different mutations in the Cu,Zn-superoxide dismutase (SOD1). These mutations are predominantly single amino acid replacements seemingly randomly scattered throughout the structure of this homodimeric 32,000-Da metalloprotein. Most of these mutant SODs retain essentially full activity, as measured in vitro, and there is overwhelming evidence for a toxic gain of function as the cause of the disease (1, 2).
What common gain of function could possibly be caused by many different point mutations in SOD1 and how can the similarities in FALS and SALS be explained? In what follows we build on reports to the effect that: FALS may be caused by protein binding to mutant forms of SOD1 (3); mutant SOD1 is proapoptotic in a neuroblastoma cell line (4); the proapoptotic effect of mutant SOD1 in cultured fibroblasts was opposed by heat shock protein (Hsp) 70 (5); and mutant SOD1 binds Hsp70, Hsp40, and αB-crystalline (6).
Because mitochondrial swelling and vacuolization are early signs of incipient motor neuron death (1, 2) and because SOD1 is found in the intermembrane space of rat (7) and yeast (8), mitochondria and SOD1 accumulate in neuronal mitochondria of transgenic mice expressing either wild type (WT) or mutant SOD1 (9); we began by examining the uptake of SOD1 into these organelles. We now demonstrate that whereas partially or wholly demetallated SOD1 is taken into mitochondria, the holo enzyme is not. This was true of both the WT and FALS-associated mutant SODs. Strikingly, a cytosolic fraction from heat-shocked mouse neuroblastoma N2A cells inhibited this uptake, whereas cytosol from N2A cells, not so treated, did not. Furthermore, whereas the uptake of mutant SOD1s was blocked by the cytosol from heat-shocked cells, the uptake of WT SOD1 was much less affected. This finding indicated that some heat shock-inducible proteins preferentially bind to mutant SOD1s and thus prevent mitochondrial uptake of the mutant SOD1s.
Materials and Methods
Mitochondrial Uptake of SOD1.
WT and G93A SOD1s were prepared (10) and then demetallated as described (11, 12). Mice (C57BL6) were killed by CO2 inhalation, and the livers were rapidly removed and used. Mouse neuroblastoma N2A cells (Duke University Cell Culture Facility) were grown as described (4). Mitochondria were isolated from the mouse liver or nontransfected N2A cells as described (13) and suspended in MRM medium (250 mM sucrose/10 mM Hepes-KOH/1 mM ATP/5 mM Na-succinate/0.08 mM ADP/2 mM K2HPO4, pH 7.5) (13). These mitochondria were incubated with 20 μg/ml human WT or G93A SOD1 ±5 μM bongkrekic acid (Sigma) or ±40 μg/ml alamethicin (Sigma) for 1 h at 25°C. The mitochondria were then sedimented and thrice washed in the cold MRM medium, before being lysed in lysis buffer [50 mM Hepes/150 mM NaCl/0.5% NP-40 (igepal CA-630, Sigma)/0.2% digitonin (Sigma)/0.23 mM PMSF] at pH 7.6 for 1 h at 0°C, followed by 10 min at 25°C. When the effect of cytosol was examined, the mitochondria were suspended in the 35,000 × g supernatant fraction of the heat shock-treated or untreated nontransfected N2A cells homogenized in the MRM medium. When the effect of Hsp was examined, 1 μg Hsp70 (StressGen Biotechnologies, Victoria, Canada) was added to the mitochondrial suspension with MRM medium instead of cytosolic fraction. The mitochondrial lysates were clarified by centrifugation at 15,000 × g for 10 min and subjected to SDS/PAGE at 10 μg protein/lane.
Immunoblotting.
Human WT, G37R, or G41D SOD1 permanently transfected N2A cells from D. R. Borchelt (The Johns Hopkins School of Medicine, Baltimore) (4) and M. Patel (National Jewish Medical and Research Center, Denver) were fractionated for mitochondria (13) and cytosol (35,000 × g surpernatant), and then electrophoresed (5 μg/lane). The resultant electropherograms were blotted onto nitrocellulose membrane (Amersham Pharmacia). Immunoblot analysis used anti-human SOD1 antibody that cross reacts with mouse or rat SOD1 (1:1,000, Santa Cruz Biotechnology) and the enhanced chemiluminescence reagent (ECL-plus, Amersham Pharmacia). Swelling of mitochondria suspended in MRM medium was followed as the decrease in absorbance at 540 nm (14). SOD1-positive bands, after immunoblotting, were quantitated by densitometry with National Institutes of Health IMAGE 1.62 software. We very carefully examined the densitometry of ECL blots to make certain that quantitation was based on the linear portion of calibration curves.
Immunoprecipitation.
Human SOD1-transfected N2A cells were lysed in lysis buffer at pH 7.6 for 1 h at 0°C, followed by 10 min at 25°C. Immunoblots were examined by immunoprecipitation both before and after centrifugal clarification at 15,000 × g for 10 min. For immunoprecipitation, rabbit polyclonal anti-Hsp25 (1:100, StressGen) or mouse monoclonal anti-Hsp70 [1:100, W27 that reacts with both Hsp70 (inducible Hsp70; Hsp72) and Hsc70 (constitutive Hsp70; Hsp73), Santa Cruz Biotechnology] was added to 800 μg (see Fig. 4A) or 3 mg (see Fig. 4B) of protein in 1 ml and incubated at 4°C overnight with constant agitation. The immunocomplexes were precipitated with protein-G-Sepharose (Amersham Pharmacia) by incubation at 4°C for 3 h. The beads were washed four times with lysis buffer and extracted with SDS/PAGE sample buffer at 100°C for 5 min, and then subjected to SDS/PAGE. Proteins were separated on 9% (Hsps 25 and 70) or 12% (SOD) SDS/PAGE, blotted, and enhanced by ECL-plus as described above for immunoblot analysis. Immunoblot analysis used Hsp25 antibody (1:1,000), Hsp70 antibody (1:1,000), or SOD1 antibody (1:1,000).
Figure 4.
Preferential binding of mutant SOD1 by Hsp70 and Hsp25. (A) Induction of Hsp25 and Hsp70. (B) Mutant SODs bind Hsps. Binding of mutant SOD1 and Hsps was examined by immunoprecipitation (IP) and immunoblotting (IB). Total cell lysate from normal or heat-shocked mouse N2A cells that permanently transfected human WT, G37R, or G41D SOD1 was examined before (total) and after (sup) centrifugal clarification by immunoprecipitation. (A) Immunoprecipitation and immunoblotting used with anti-Hsp25 or anti-Hsp70 antibody. (B) Immunoprecipitation used with anti-Hsp25 or anti-Hsp70 antibody, and immunoblotting used with anti-SOD1 antibody.
Results
Effect of Swelling on Mouse Liver Mitochondrial Uptake of Apo-SOD1.
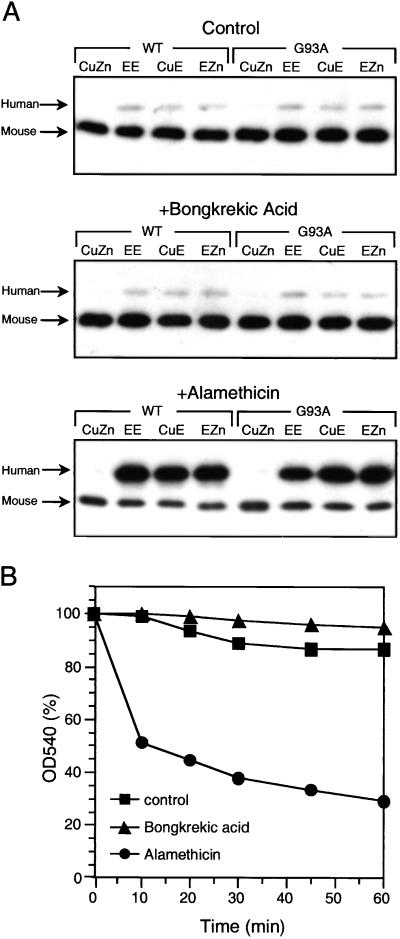
When human SOD1 was incubated with isolated mouse mitochondria, which were subsequently thrice washed, the holo enzyme was not taken up, but the enzymes lacking Zn, Cu, or both, were taken into and retained by these organelles. Bongkrekic acid, which inhibits mitochondrial swelling, did not influence uptake of the demetallated SOD1, but alamethicin, which causes mitochondrial swelling, greatly enhanced this uptake (Fig. 1A). The influence of bongkrekic acid and alamethicin on mitochondrial swelling, under the conditions imposed, are shown in Fig. 1B. Clearly, there was little mitochondrial swelling during the 60-min duration of these incubations, and that minor swelling was inhibited by bongkrekic acid and greatly augmented by alamethicin. The results in Fig. 1 are fully in accord with studies demonstrating the presence of SOD1 in the intermembrane space of mitochondria (7, 8) and the proposed uptake of the apo, but not the holo, SOD1 into this organelle (8).
Figure 1.
Uptake of SOD1 and swelling of mouse mitochondria. (A) Uptake of SOD1 by mouse liver mitochondria. Isolated mitochondria were incubated with holo or demetallated SOD1 and then analyzed for content of SOD1 by immunoblotting as described in Materials and Methods. CuZn, holo enzyme; EE, apo; CuE, SOD1 lacking Zn; EZn, SOD1 lacking Cu. Each lane was loaded with 10 μg of protein. (B) Mitochondrial swelling. Suspensions of mitochondria in the MRM medium at 25°C were incubated ± bongkrekic acid at 5 μM or alamethicin at 40 μg/ml and swelling was followed at 540 nm.
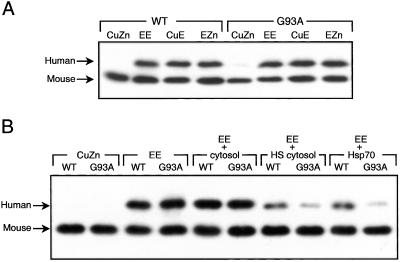
Effect of Cytosol on Mitochondrial Uptake of Apo-SOD1.
Mitochondria, isolated from N2A cells, like those isolated from mouse liver, took up the demetallated, but not the holo, SOD1, as shown in Fig. 2A. Cytosol from heat-shocked, but not from normal, N2A cells, blocked uptake of demetallated SOD1 and blocked G93A SOD1 more than WT SOD1 (Fig. 2B). These data indicate that some heat shock-inducible proteins can selectively bind to and thus prevent mitochondrial uptake of mutant SOD1.
Figure 2.
Effect of cytosol and Hsp70 on uptake of SOD1 into N2A mitochondria. (A) Mitochondrial uptake of SOD1. (B) Effect of Hsps. The uptake of SOD1 by mitochondria isolated from N2A cells was examined as in Fig. 1 in the absence and presence of cytosolic fractions. (A) In the absence of cytosol fraction. (B) In the presence of cytosol, cytosol from heat-shocked cells, or 1 μg Hsp70 (StressGen). CuZn, holo enzyme; EE, apo; CuE, SOD1 lacking Zn; EZn, SOD1 lacking Cu.
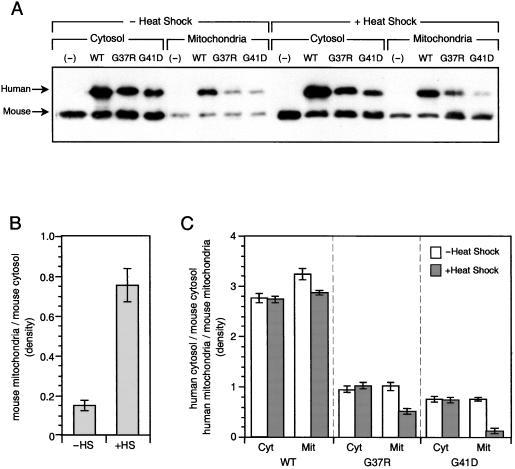
Effect of Heat Shock on Levels of SOD1 in N2A Cells.
Stably transfected N2A cells that expressed human WT, G37R, or G41D SOD1s were use for the next studies. Fig. 3A shows that human WT, G37R, and G41D were expressed in these cells and found both in cytosol and mitochondria, as was the endogenous mouse SOD1. Fig. 3B demonstrates that heat shock increased the proportion of mouse SOD1 found in mitochondria, probably because heat shock causes swelling of mitochondria (15). Fig. 3C shows that while heat shock similarly affected the amount of the mouse and human WT SOD1s in the cytosol of N2A cells, it specifically decreased the amount of the mutant G37R and G41D in the mitochondria. This finding indicates that mutant SOD1, after synthesis in the cytosol, and before metallation, can be intercepted by some heat-inducible protein and prevented from entering the mitochondria.
Figure 3.
Effect of heat shock on levels of SOD1 in N2A cells. (A) Immunoblots of SOD1, human WT, G37R, or G41D-SOD1 permanently transfected mouse N2A cells were fractionated, electrophoresed (5 μg/lane), and examined as Fig. 1. (B) Mouse endogenous cytosolic and mitochondrial SOD1. Bands were quantitated by densitometry with National Institutes of Health IMAGE 1.62 software. The effect of heat shock (HS) on the ratios of mitochondrial/cytosolic mouse SOD1 is shown. (C) Human cytosolic (Cyt) and mitochondrial (Mit) SOD1 normalized to mouse endogenous cytosolic/mitochondrial SOD1. The effect of heat shock on the ratio of cytosolic/mitochondrial human WT, G37R, and G41D SOD1s is normalized to the corresponding ratios of mouse SOD1.
Hsp25 and Hsp70 Preferentially Bind Mutant SOD1.
We next turned to immunoprecipitation, using antibodies to Hsp25, Hsp27, Hsp70, and SOD1, to directly demonstrate what could be inferred from Fig. 3. Thus the N2A cells, both normal and heat shocked, were lysed, and one portion of each lysate was clarified by 10 min of centrifugation at 15,000 × g; whereas the other portion was used without clarification. Fig. 4A shows that heat shock of these cells increased expression of Hsp25 in the cytosol (16), in the case of the cells expressing the WT SOD1, but had a much smaller effect on cells expressing the mutant SOD1s. We could detect Hsp25 or Hsp70 in mutant SOD1-expressing cells that had not been subjected to heat shock when we used 3 mg protein per lane (data not shown). But 3 mg protein was too much in the case of heat shock-treated cell extracts. Consequently we show only the data that was obtained by using 800 μg protein per lane. It should be noted that centrifugation of the lysates before immunoprecipitation decreased the detectable Hsps and mutant SOD1s. It follows that the mutant SOD1s made much of the Hsp25 insoluble, most probably by binding to it. Similar results were obtained for inducible and constitutive Hsp70 (Fig. 4A). When the blots were probed with anti-SOD (Fig. 4B) the mutant SOD1s, but not the endogenous mouse and human WT SOD1s, were seen to bind Hsp25 and Hsp70. This effect was greater with lysate from heat-shocked cells, as expected because of induction of these Hsps by heat shock. In accord with these results, isolated Hsp27, Hsp25, and Hsp70 (StressGen) were seen to bind to G93A SOD1 but not to WT SOD1 (not shown).
Discussion
Neurons are subjected to chronic oxidative stress caused by low-level excitotoxicity. It is noteworthy in this context that glutamate antagonists delay death in a mouse model of FALS (17). The relationship between excitotoxicity and oxidative stress has been reviewed (18). Motor neurons are particularly prone to the results of this stress because their very long axons and slow axonal transport make turnover of damaged macromolecules a slow process. This proapoptotic condition is ordinarily opposed by the antiapoptotic effect of the Hsps; however, the presence of any abundant mutated protein, which because of slightly aberrant folding binds the Hsps, will have the effect of decreasing their availability for other antiapoptotic functions. SOD1 is an abundant protein whose mutant forms bind Hsps and thus leads to eventual apoptosis.
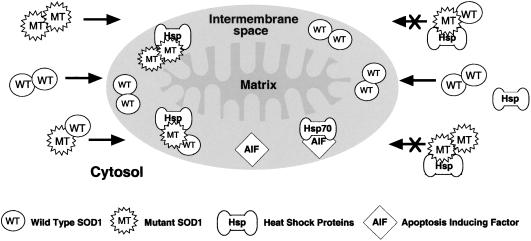
In humans, with FALS caused by mutations in SOD1, we could expect the intermembrane space of mitochondria in such neurons to be deficient in SOD1, because of the blocking of uptake into these organelles consequent upon the binding to Hsps in the cytosol. The mitochondria in the neurons would thus be denied the full protective effect of SOD1 and thus be selectively prone to oxidative damage. This proposal is illustrated in Fig. 5, which shows to the left of the mitochondrion that demetallated SOD1 dimers whether WT or mutant are taken up by this organelle. To the right of the mitochondrion we see that WT SOD1, which does not bind Hsp, is taken up; whereas homodimer of the mutant SOD1 or heterodimer containing one subunit of mutant and one of WT bind to the Hsp and are denied entry. This scheme predicts that the level of SOD1 in the intermembrane space of mitochondria would be decreased by the presence of the mutant SOD1.
Figure 5.
Proposed effect of mutant (MT) SOD1 on uptake of WT SOD1 into mitochondria.
Recent reviews (1, 2) present evidence supporting the roles of toxic protein aggregates, mitochondrial defects, apoptosis, excitotoxicity, and oxidative stress in the etiology of ALS. These can now be assembled into a coherent whole. Thus, excitotoxicity is known to impose oxidative stress (1, 19), which is known to be proapoptotic. The antiapoptotic effects of Hsp27, Hsp70, and Hsp90 are established (20). Apoptosis-inducing factor (AIF) exists in the intermembrane space of mitochondria, as does Hsp70 (21), and the binding of one to the other would be antiapoptotic (22). Mutant SOD1 in this space thus be in competition with AIF for binding to Hsp70 thus increasing the level of free AIF (Fig. 5). Proteasome expression and activity have been reported to decrease with age in spinal cord (23). Mutant SOD1 turns over more rapidly than WT SOD1, and an inhibitor of proteasome action inhibits this turnover and thus selectively increases the steady-state level of mutant SOD1 (24). Thus, the age-dependent decline in proteasome expression and activity would in this way account for the typically late onset of ALS symptoms in affected humans.
There are other abundant proteins in neurons, and mutant forms of these could have a similar effect and could thus be responsible for the 80% of cases of FALS that are not associated with mutations in SOD1. In a similar vein SALS could be caused by de novo mutations in any protein that is abundant in motor neurons. It should be noted here that neuronal protein aggregates are seen in both FALS and SALS and that they contain SOD1 in the former case but not in the latter (25). Other neurological diseases could likewise be caused by binding of Hsps to aberrant proteins. Thus in the polyglutamine expansion diseases, such as Huntington's disease, Hsp40 and Hsp70 binding to the aberrant huntingtin has been reported (26).
Hence motor neurons survive by virtue of the balance between the proapoptotic effect of oxidative stress and the antiapoptotic effect of Hsps. Mutations in any protein that is abundant in these neurons have the probability of imposing binding of Hsps and formation of protein aggregates and depletion of free Hsps, which will ultimately tip the balance in favor of apoptosis. In addition to the reasons already discussed, motor neurons may be selectively at risk because of their high level of expression of SOD1 (27).
Acknowledgments
We are indebted to Drs. Manisha Patel and David R. Borchelt, who supplied the SOD-transfected N2A cells; Drs. Jonathan S. Stamler and Paul Blackwelder, who supplied the mouse livers; and Dr. Paul Modrich, who kindly provided cell culture facilities. This work was supported by grants from the National Institutes of Health (1RO1DK59868-01), the Amyotrophic Lateral Sclerosis Association, and Incara Pharmaceuticals Inc.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- FALS
familial ALS
- SALS
sporadic ALS
- SOD1
Cu,Zn-superoxide dismutase
- Hsp
heat shock protein
- WT
wild type
- AIF
apoptosis-inducing factor
References
- 1.Cluskey S, Ramsden D B. J Clin Pathol Mol Pathol. 1991;54:386–392. [PMC free article] [PubMed] [Google Scholar]
- 2.Julien J P. Cell. 2001;104:581–591. doi: 10.1016/s0092-8674(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 3.Kunst C B, Mezey E M, Brownstein J, Patterson D. Nat Genet. 1997;15:91–94. doi: 10.1038/ng0197-91. [DOI] [PubMed] [Google Scholar]
- 4.Pasinelli P, Borchelt D R, Houseweart M K, Cleveland D W, Brown R H., Jr Proc Natl Acad Sci USA. 1998;95:15763–15768. doi: 10.1073/pnas.95.26.15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruening W, Roy J, Giasson B, Figlewicz D A, Mushynski W E, Durham H D. J Neurochem. 1999;72:693–699. doi: 10.1046/j.1471-4159.1999.0720693.x. [DOI] [PubMed] [Google Scholar]
- 6.Shinder G A, Lacourse M C, Minotti S, Durham H D. J Biol Chem. 2001;276:12791–12796. doi: 10.1074/jbc.M010759200. [DOI] [PubMed] [Google Scholar]
- 7.Okado-Matsumoto A, Fridovich I. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 8.Sturts L A, Diekert K, Jensen L T, Lill R, Culotta V C. J Biol Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 9.Jaarsma D, Rognoni F, Duijn W V, Verspaget H W, Haasdijk E D, Holstege J C. Acta Neuropathol. 2001;102:293–305. doi: 10.1007/s004010100399. [DOI] [PubMed] [Google Scholar]
- 10.Liochev S I, Chen L L, Hallewell R A, Fridovich I. Arch Biochem Biophys. 1997;346:263–268. doi: 10.1006/abbi.1997.0298. [DOI] [PubMed] [Google Scholar]
- 11.Sutter B, Bounds P L, Koppenol W H. Protein Expression Purif. 2000;19:53–56. doi: 10.1006/prep.2000.1241. [DOI] [PubMed] [Google Scholar]
- 12.Valentine J S, Pantoliano M W. In: Metal Ions in Biology. Spiro T G, editor. Vol. 3. New York: Wiley; 1981. pp. 291–358. [Google Scholar]
- 13.Goping I S, Gross A, Lavoie J N, Nguyen M, Jemmerson R, Roth K, Korsmeyer S J, Shore G C. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang D, Sullivan P G, Sensi S L, Steward O, Weiss J H. J Biol Chem. 2001;276:47524–47529. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- 15.Welch W J, Suhan J P. J Cell Biol. 1985;101:1198–1211. doi: 10.1083/jcb.101.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrido C, Bruey J M, Fromentin A, Hammann A, Arrigo A P, Solary E. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- 17.Gurney M E, Cutting F B, Zhai P, Doble A, Taylor C P, Andrus P K, Hall E D. Ann Neurol. 1996;39:147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- 18.Bondy S C, LeBel C P. Free Radical Biol Med. 1993;14:633–642. doi: 10.1016/0891-5849(93)90144-j. [DOI] [PubMed] [Google Scholar]
- 19.Li Q Y, Pedersen C, Day B J, Patel M. J Neurochem. 2001;78:746–755. doi: 10.1046/j.1471-4159.2001.00457.x. [DOI] [PubMed] [Google Scholar]
- 20.Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Biochem Biophys Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- 21.Bruey J M, Ducasse C, Bonniaud P, Ravagnan L, Susin S A, DiazLatoud C, Gurbuxani S, Arrigo A P, Kroemer G, Solary E, Garrido C. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 22.Ravagnan L, Surbuxani S, Susin S A, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger J M, Garrido C, Kroemer G. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 23.Keller J N, Huang F F, Markesbery W R. Neuroscience. 2000;98:149–156. doi: 10.1016/s0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman E K, Wilcox H M, Scott R W, Siman R. J Neurol Sci. 1996;139:15–20. [PubMed] [Google Scholar]
- 25.Fukuda K, Nagano S, Satoh M, Tohyama C, Nakanishi T, Shimizu A, Yanagihara T, Sakoda S. Eur J Neurosci. 2001;14:2032–2036. doi: 10.1046/j.0953-816x.2001.01828.x. [DOI] [PubMed] [Google Scholar]
- 26.Wyttenbach A, Carmichael J, Swartz J, Furlong R A, Narain Y, Rankin J, Rubinsztein D C. Proc Natl Acad Sci USA. 2000;97:2898–2903. doi: 10.1073/pnas.97.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardo C A, Xu Z, Borchelt D R, Price D L, Sisodia S S, Cleveland D W. Proc Natl Acad Sci USA. 1995;92:954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]