Abstract
Introduction
Diabetic heart disease (DHD) is systolic and/or diastolic dysfunction of the heart muscle that occurs in patients with the presence of Type 2 diabetes mellitus. AMPKα1, a key regulator of glucose metabolism, has been shown to promote glucose uptake and catabolism. Alpha-linolenic acid (α-ALA) is an essential fatty acid that helps to prevent cardiovascular disease and is very important for human health. However, its role as a medical agent in preventing DHD by modulating AMPKα1is unknown.
Methods
An experimental type 2 diabetic mouse model was established by treating animals with a high-fat diet (HFD) for four weeks and intraperitoneal injection of streptozotocin (STZ) (50 mg/kg body weight). After induction of type 2 diabetes, the animals were treated orally with α-ALA (2 or 4 g/kg) for twelve weeks.
Results
The type 2 diabetic mice showed an increase in blood glucose levels, a decrease in body weight and cardiac dysfunction. Diabetic mice treated with α-ALA attenuated hyperglycaemia, dyslipidaemia, and cardiac dysfunction. In addition, α-ALA improved histological changes and fibrosis in HFD/STZ-induced mice. Type 2 diabetes in mice exacerbated the inflammatory status. α-ALA treatment significantly attenuated inflammation in diabetic hearts. The underlying mechanisms for this attenuation involved modulation of AMPKα1.
Conclusion
The results of this study provide evidence that α-ALA protects against HFD/STZ (T2DM)-induced cardiac injury by alleviating inflammation and upregulating AMPKα1.
Keywords: diabetic heart disease, α-ALA, AMPKα1, inflammation
Introduction
Diabetic heart disease (DHD) is defined as heart disease that is complicated or associated with diabetes mellitus. DHD is a leading cause of death in people with diabetes mellitus, particularly type 2 diabetes mellitus (T2DM).1,2 There is growing concern worldwide about T2DM-related illness and death, mainly due to physical inactivity and high intake of high-fat diets (HFD).3 Although intensive glucose lowering can help reduce the cardiovascular risk of T2DM, lifestyle management is just as crucial as using preventive medications, according to the Diabetes Clinical Guidelines.4 Early intervention reduces high individual risk and direct medical expenditure. Cardiac injury in patients with T2DM is characterised by alterations in cardiac function and structure triggered by high glucose and lipid levels, ultimately leading to adverse cardiac events. It has been previously reported that a combination of factors such as hyperglycaemia, dyslipidaemia, inflammation, and altered biomarkers of cardiac function contribute to the development of DHD.5 Inflammation plays a crucial role in the development and progression of cardiac damage in people with T2DM, as recent studies have shown.6 As a key sensor of cellular energy status, AMPK plays a crucial role in the regulation of cellular energy homeostasis and glycolipid metabolism. AMPK has been demonstrated to have a multifaceted role in T2DM and its complications, particularly with regard to aspects glucose uptake and metabolism, insulin resistance/sensitivity, and β-cell function.7 In recent years, the anti-inflammatory effects of AMPK and its signaling cascades have become of increasing interest, particularly in the context of metabolic diseases. Numerous studies have shown that anti-inflammatory drugs act through AMPK and related pathways.8,9
Replacing saturated and trans fats with polyunsaturated fats can help control blood lipids and is essential in the management of patients with T2DM, according to the American Diabetes Association.10 In the daily diet, alpha-linolenic acid (α-ALA) is the predominant polyunsaturated fatty acid and an essential fatty acid. It is an essential fatty acid of the omega-3 family with cholesterol-lowering properties. It is one of the alternative systems to pharmacological therapies for minimising the risk of cardiovascular disease. In terms of lipid-lowering prevention, α-ALA may have favourable effects on LDL cholesterol and triglyceride levels in adults and children alike. Furthermore, α-ALA has a protective effect against hypertension and helps balance blood pressure through dietary habits.11 One study reported a beneficial effect of α-ALA on non-alcoholic fatty liver disease, which was triggered by AMPK signaling.12 However, little is known about the effect of α-ALA on the prevention and treatment of DHD. Therefore, the present study investigated the mechanism of action of α-ALA based on the modulation of inflammation and regulation of cardiac injury by AMPK in HFD/streptozotocin (STZ)-induced DHD mice.
Chemicals and Reagents
α-ALA was acquired from NU-CHEK-PREP, INC (USA). Streptozotocin (STZ) was purchased from Sigma–Aldrich (USA). All other chemicals used in this study were purchased from local commercial suppliers.
Animal Experiments
Fifty adult (8 weeks old) C57BL/6J mice were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Animals were housed in a specific pathogen-free animal facility with temperature (22±2°C) and humidity (50±10%) regulated on a 12 h light/12 h dark cycle. Mice were acclimatized to laboratory conditions and underwent a week-long detailed health check. Mice were given free access to purified water and fed with standard rodent chow. All animal experiments were conducted in accordance with the Guidelines for Ethical Review of Animal Welfare in China, and animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Yi Shengyuan Gene Technology (Tianjin) Co., Ltd. (protocol number YSY-DWLL-2024472).
Diabetes Induction
After four weeks of a high-fat diet (Medicience, Jiangsu, China), the mice were administered a single low-dose STZ (50 mg/kg, ip) injection for 3d. Mice with fasting blood glucose levels above 11.1 mmol/L one week after STZ injection were considered to have T2DM and could be used in the study. Mice with substandard blood glucose levels were simply eliminated. T2DM mice were assigned at a ratio of 1:1:1 to continue receiving a high-fat diet. Randomisation was performed using SPSS 26.
Experimental Design
Standard rodent chow-fed mice were used as control (Control). HDF+STZ mice were randomly divided into 3 groups: the model group (DHD group), the 2 g/kg α-ALA group (α-ALA-L), and the 4 g/kg α-ALA group (α-ALA-H). The high-fat feeds supplemented with α-ALA were commissioned to Jiangsu Medison Biomedical Co. The doses of α-ALA used in this study were equivalent to population levels of 0.8 g/day and 1.6 g/day, respectively.13,14 After 12 weeks of α-ALA treatment, the mice were anaesthetised with isoflurane and then euthanised by taking a blood sample from their hearts.
Assessment of Weekly Body Weight, OGTT, and ITT
Weights were recorded weekly until the experiment ended. OGTT and ITT experiments were performed on mice 12 weeks after drug administration, and fasting blood glucose, 20% dextrose gavage, and 30 min, 60 min, 90 min, 120 min, and 180 min blood glucose (mmol/L) after intraperitoneal injection of 0.75 U/kg insulin were recorded, respectively.
Biochemical Analysis of Plasma
Before euthanasia, blood was collected in tubes with anticoagulants, coagulated at normal room temperature for no less than 30 minutes, and then centrifuged at 3000 rpm for 15 minutes to collect plasma. The collected plasma samples were tested for four lipids: low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C), triglycerides (TG), and total cholesterol (TC) (mmol/L). IL-6 (pg/mL), CRP (μg/L), and TNF-α (pg/mL) were measured using the ELISA kit.
Echocardiography
Mice were lightly anaesthetised with 3% isoflurane and maintained anaesthetised with 1.5% isoflurane during echocardiography. Transthoracic echocardiography was performed using ultrasound equipment (Vinno 6 Lab, China) to assess left ventricular function. Cardiac tissue was imaged in 2D mode in long-axis views at the level of the papillary muscles, and M-mode views were used to measure LV dimensions. Left ventricular ejection fraction (LVEF %) was used as an index of LV function.
Histopathological Analysis
Mouse hearts were harvested and placed in 4% paraformaldehyde solution for more than 48 h. Hearts were embedded in paraffin and sectioned at 4 μm thickness. Tissue sections were stained with haematoxylin and eosin (HE) to assess cellular and structural changes. Myocardial fibrosis was assessed by Masson’s trichrome. Three sections were analysed for each sample and each score was averaged over the three sections.
Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
When the mice were sacrificed, the hearts were quickly removed and snap frozen in a liquid nitrogen tank. Total RNA was isolated from heart tissue using TRIzol (Invitrogen, Life Technologies) and an equal amount of total RNA was reverse transcribed to cDNA using Transcriptor FirstStrand cDNA Synthesis (Roche, Switzerland). Real-time RT-PCR was performed on the StepOne RealTime PCR System (Bio-Rad, USA) using the Fast Start Universal SYBR Green Master Mix Kit (Roche, Switzerland) and specific primers to determine the mRNA expression of IL-6, IL-1β, TNF-α, and Prkaa1 in mouse heart samples. As an internal control, specific mRNA levels were normalised to β-actin (B662302-0001, Sangon Biotech, China) mRNA levels. The following primer sets were used (see Table 1).
Table 1.
Primers Used in This Study
| Gene | Forward Primer (5′-3′) | Reverse Primer (3′-5′) |
|---|---|---|
| IL-6 | GGACCAAGACCATCCAATTC | ACCACAGTGAGGAATGTCCA |
| IL-10 | TGAATTCCCTGGGTGAGAAG | CTCTTCACCTGCTCCACTGC |
| TNF-α | CTCTTCTGCCTGCTGCACTTTG | ATGGGCTACAGGCTTGTCACTC |
| Prkaa1 | GAGTGTTCGGAGGAGGAGGTC | CTGTTGTCTATGATGAGGTGGTAGG |
Western Blot Analysis
Cardiac tissues were lysed using RIPA and PMSF, and protein concentrations were determined using a BCA protein assay kit (Beyotime Biotechnology, China). Equal amounts of proteins were separated by SDS-PAGE, transferred to PVDF membranes and blocked with a blocking solution containing 5% skimmed milk. The membranes were incubated overnight with primary antibodies specific for AMPKα1 (1:2000, 10,929-2-AP, Proteintech, China), p-AMPKα1 (1:500, AF3423, Affinity, China), and β-actin (1:10,000, 81,115-1-RR, Proteintech, China), followed by horseradish peroxidase-conjugated secondary antibodies. Immunoreactive proteins were detected on a luminescence image analyser using the ECL chemiluminescence reagent system. The intensity of the bands was quantified using ImageJ software.
Statistical Analysis
The statistical analysis of the results was performed using GraphPad Prism 9.0 software. Unpaired t-test or one-way analysis of variance was used to calculate the differences in mean values. Differences with P values < 0.05 were considered significant.
Results
α-ALA Rescues High-Fat, HFD/STZ-Induced Abnormalities in Body Weight and Heart Weight in Type 2 Diabetic Mice
Figure 1 shows the weekly changes in body weight and heart weight in control and DHD mice. HFD-fed mice gained significantly more weight than normal chow-fed mice, whereas STZ injection resulted in a dramatic decrease in weight. However, treatment of type 2 diabetic mice with α-ALA resulted in a significant increase in body weight compared to diabetic mice (Figure 1A and B). Compared with control mice, diabetic mice showed a significant decrease in heart weight, whereas type 2 diabetic mice treated with α-ALA-H showed a significant improvement in heart weight compared with diabetic mice (Figure 1C). However, there was no significant change in heart weight/body weight in the experimental mice compared to the control group (Figure 1D).
Figure 1.
Effect of α-ALA on weekly body weight and heart weight in HDF +STZ induced diabetic mice. (A) Weekly Body; (B) Weight at the time of sacrifice; (C) Heart weight; (D) heart weight/body weight (HW/BW). Control: Normal control mice; DHD: HDF +STZ; α-ALA-L: HDF +STZ mice treated with 2 g/kg of α-ALA; α-ALA-H: HDF +STZ mice treated with 4 g/kg of α-ALA. Data are expressed as mean ± SD, n ≥5. #P < 0.05, ##P < 0.01 compared with control group. *P < 0.05, **P < 0.01 compared with DHD group.
α-ALA Affects Metabolic Parameters in HFD/STZ-Induced Type 2 Diabetic Mice
The Oral Glucose Tolerance Test (OGTT) is the classic test of the body’s ability to cope with a glucose challenge. The main purpose of the Insulin Tolerance Test (ITT) is to assess the degree of induction of exogenous insulin in experimental mice. The results of this study showed the effect of α-ALA on glucose metabolism in diabetic mice (Figure 2A–D). Before the end of the experiment, the fasting blood glucose level of DHD mice was higher than that of control mice, whereas after 12 weeks of α-ALA treatment, the fasting blood glucose level of DHD mice was significantly reduced (p < 0.05) and approached normal levels. Figure 2E shows that plasma TG and LDL levels were significantly higher and HDL levels were significantly lower in DHD mice compared with control mice (p < 0.05), suggesting altered lipid metabolism in DHD mice. In contrast, α-ALA treatment for 12 weeks reduced the levels of TG and LDL in DHD mice (p < 0.05) and at the same time increased the levels of HDL in DHD mice.
Figure 2.
Effect of α-ALA on glucolipid metabolic parameters in HDF +STZ induced diabetic mice. (A) Blood glucose levels at different time points of the OGTT experiment; (B) OGTT Folding Line; (C) Blood glucose levels at different time points of the ITT experiment; (D) ITT Folding Line; (E) Lipid metabolism. Control: Normal control mice; DHD: HDF +STZ; α-ALA-L: HDF +STZ mice treated with 2 g/kg of α-ALA; α-ALA-H: HDF +STZ mice treated with 4 g/kg of α-ALA. Data are expressed as mean ± SD, n ≥5. #P < 0.05, ##P < 0.01 is compared with the Control group. *P < 0.05, **P < 0.01 is compared with the DHD group.
Abbreviations: TG, Triglycerides; TC, Total Cholesterol; HDL, High-Density Lipoprotein; LDL, Low-Density lipoprotein.
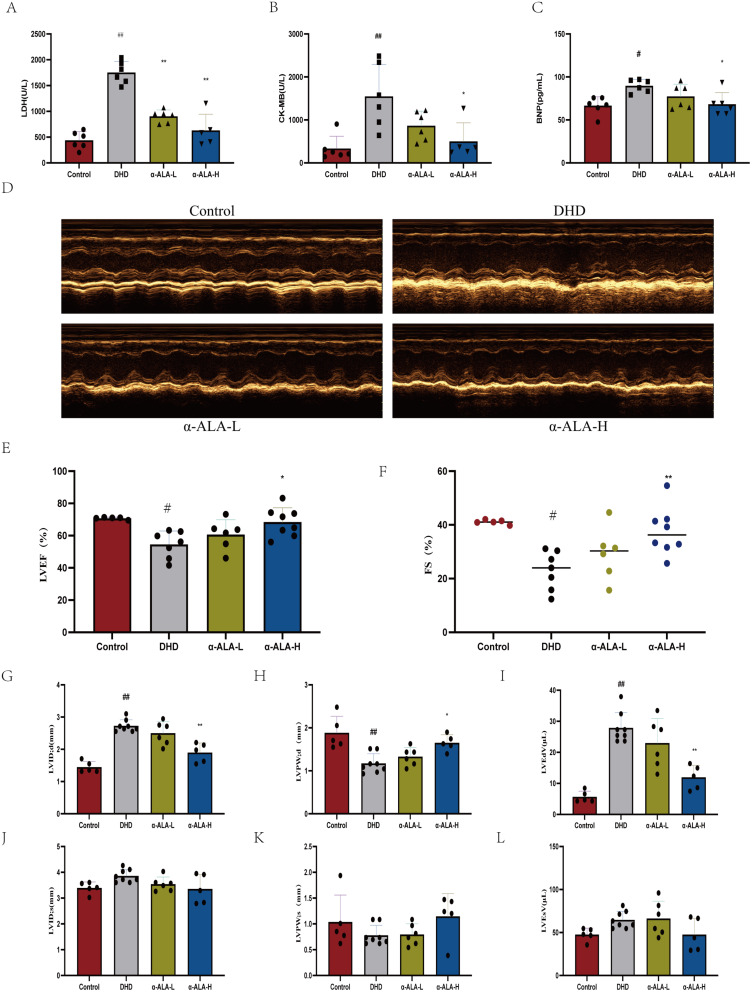
α-ALA Affected Plasma Cardiac Markers and Improved Cardiac Dysfunction in HFD/STZ-Induced Type 2 Diabetic Mice
Plasma levels of LDH, CK-MB, and BNP were significantly increased in diabetic mice compared to non-diabetic mice (p < 0.05). Treatment with α-ALA resulted in a significant reduction in LDH (Figure 3A). Treatment with α-ALA-H significantly reduced CK-MB (Figure 3B) and BNP (Figure 3C) levels compared to diabetic controls. Transthoracic echocardiography was used to measure cardiac function after 12 weeks of α-ALA treatment. Representative M-mode echocardiograms from each group are shown in Figure 3D. HDF+STZ significantly induced heart failure in mice, as evidenced by significant reductions in LV ejection fraction (Figure 3E) and fractional shortening (Figure 3F). α-ALA attenuated the diabetes-induced reductions in LV ejection fraction and fractional shortening. Importantly, in the early stages of DHD, abnormalities in LV diastolic function occur earlier and are more pronounced than those in systolic function.15 Our study showed that α-ALA significantly improved diabetes-induced diastolic dysfunction (Figure 3G–L).
Figure 3.
Effect of α-ALA on cardiac injury in HDF +STZ induced diabetic mice. (A) LDH levels in different groups; (B) CK-MB levels in different groups; (C) BNP levels in different groups; (D) Representative images from the parasternal long axis view of the heart acquired in M-mode. (E) Ejection fraction; (F) Fractional shortening; (G) LVID;d; (H) LVPW; d; (I) LVEdV; (J) LVID: s; (K) LVPW; s; (L) LVEsV. Control: Normal control mice; DHD: HDF +STZ; α-ALA-L: HDF +STZ mice treated with 2 g/kg of α-ALA; α-ALA-H: HDF +STZ mice treated with 4 g/kg of α-ALA. Data are expressed as mean ± SD, n ≥5. #P < 0.05, ##P < 0.01 is compared with the Control group. *P < 0.05, **P < 0.01 is compared with the DHD group.
α-ALA Affects Histopathological Changes and Myocardial Fibrosis in the Heart of HFD/STZ-Induced Type 2 Diabetic Mice
To clarify the effect of α-ALA treatment on the myocardial structure of DHD mouse hearts. Myocardial tissues from each group were fixed and sectioned for pathological staining. Histopathological analysis of the cardiac tissues by H&E and Masson staining are shown in Figure 4A and B, respectively. The DHD group showed mild inflammatory cell infiltration and diffuse fibrotic injury compared to the control group. In contrast, α-ALA ameliorated DHD-induced inflammatory cell infiltration and reduced the extent of diffuse fibrotic injury.
Figure 4.
Effects of α-ALA administration on histopathological changes and fibrotic markers in DHD mice. (A) Hematoxylin and eosin (H&E); (B) Masson.
α-ALA Reduces Inflammation in the Hearts of Type 2 Diabetic Mice Induced by HFD/STZ
Significant increases (p < 0.05) in the levels of IL-6 (Figure 5A), CRP (Figure 5B), and TNF-α (Figure 5C) were observed in the plasma of diabetic mice compared to control mice. In contrast, treatment of diabetic mice with α-ALA resulted in a significant improvement, as evidenced by reduced levels of IL-6, CRP, and TNF-α. RT-PCR gene expression studies revealed a significant (p < 0.05) upregulation of IL-6 (Figure 5D), IL-1β (Figure 5E), and TNF-α (Figure 5F) mRNA levels in cardiac tissue from diabetic mice compared to control mice. α-ALA showed a significant (p < 0.05) downregulation of IL-6, IL-1β, and TNF-α mRNA expression levels compared to untreated diabetic mice.
Figure 5.
Effect of α-ALA on inflammation status in HDF +STZ induced diabetic mice. (A) CRP levels in different groups; (B) IL-6 levels in different groups; (C) TNF-α levels in different groups; Relative mRNA expression of (D) IL-6; (E) IL-1β; (F) TNF-α. Control: Normal control mice; DHD: HDF +STZ; α-ALA-L: HDF +STZ mice treated with 2 g/kg of α-ALA; α-ALA-H: HDF +STZ mice treated with 4 g/kg of α-ALA. Data are expressed as mean ± SD, n ≥5. #P < 0.05, ##P < 0.01 is compared with the Control group. *P < 0.05, **P < 0.01 is compared with the DHD group.
α-ALA Influences AMPKα1 in the Cardiac Tissue of Type 2 Diabetic Mice Induced by HFD/STZ
RT-PCR evaluated the relative mRNA expression levels of AMPKα1. The mRNA expression level of AMPKα1 was significantly reduced in diabetic mice compared to control mice. In contrast, the expression level of AMPKα1 was significantly (p < 0.05) increased after treatment of diabetic animals with α-ALA (Figure 6A). Supporting these observations, the protein distribution levels of AMPKα1 and p-AMPKα1 were lower in diabetic hearts compared to control mice. Treating diabetic mice with α-ALA significantly increased the protein levels of AMPKα1 and p-AMPKα1 (Figure 6B–D).
Figure 6.
Effect of α-ALA on AMPKα1 in HDF +STZ induced diabetic mice. (A) Relative mRNA expression of AMPKα1; (B) Western blot analyses of AMPKα1 and p-AMPKα1; (C) AMPKα1 protein quantifications; (D) p-AMPKα1 protein quantifications. Control: Normal control mice; DHD: HDF +STZ; α-ALA-L: HDF +STZ mice treated with 2 g/kg of α-ALA; α-ALA-H: HDF +STZ mice treated with 4 g/kg of α-ALA. Data are expressed as mean ± SD, n ≥5. #P < 0.05, ##P < 0.01 is compared with the Control group. *P < 0.05, **P < 0.01 is compared with the DHD group.
Discussion
Type 2 diabetes mellitus (T2DM) is recognized as a global health concern and a significant risk factor for cardiovascular diseases. α-Linolenic acid (α-ALA), derived from Perilla frutescens, is an essential fatty acid that cannot be synthesized by humans. α-ALA can influence the thrombotic process by modulating the PI3K/Akt signaling pathway.16 It possesses anti-arrhythmic properties and is associated with cardiovascular disease and cancer.17,18 The dietary guidelines established by the World Health Organization recommend increasing the intake of polyunsaturated fatty acids, particularly α-ALA, with a suggested daily intake of about 1.3 g.19 However, the potential protective role and mechanisms of α-ALA against HFD/STZ-induced cardiotoxicity remain unclear. In this study, after establishing a T2DM mouse model, the animals were orally treated with 2 or 4 kg of α-ALA per kg of body weight for 12 weeks. For the treatment of T2DM, α-ALA mitigates HFD/STZ-induced weight loss and enhances glucose and insulin tolerance in mice. α-ALA also improves blood lipids, particularly TG, HDL, and LDL. Regarding the treatment of HFD/STZ-induced cardiac injury, α-ALA decreased the release of LDH, CK-MB, and BNP into the plasma in mice. Additionally, α-ALA improved cardiac function, particularly left ventricular ejection fraction, and corrected diastolic abnormalities. α-ALA reduced HFD/STZ-induced histopathological changes and fibrotic characteristics.
Dietary recommendations encourage the reduction of dietary saturated fatty acids and their replacement with polyunsaturated fatty acids to reduce the risk of metabolic disorders. The importance of dietary α-ALA in the prevention of chronic diseases is related to its anti-inflammatory function.20 Liquid chromatography-tandem mass spectrometry-mediated lipidomics reveals that α-ALA controls inflammation and tissue homeostasis through the enzymatic oxygenation pathway.21 Treatment of atherosclerotic cardiovascular disease (ASCVD) often requires a combination of lifestyle modifications and drug therapy to reduce the risk of cardiovascular disease. α-ALA and statins are commonly used in the treatment of cardiovascular disease to control cardiovascular risk factors. Statins are a crucial lipid-lowering therapy, primarily targeting low-density lipoprotein cholesterol (LDL-C) levels, whereas α-ALA targets triglyceride (TG) concentrations. Statins and ALA have several overlapping effects, including improving endothelial function, modulating inflammation, and stabilizing atherosclerotic plaque.22 In addition, α-ALA plays an active role in the treatment of diabetes mellitus. Supplementation with α-ALA for six months in diabetes mellitus reduced fasting blood glucose and serum triglyceride levels.23,24 Nevertheless, for DHD, most studies have utilized α-ALA in conjunction with other medications, instead of investigating the effectiveness of α-ALA alone against DHD.25,26 The aim of our study was to investigate the effectiveness of α-ALA as a stand-alone intervention for DHD. In this study, DHD mice showed a significant reduction in body weight compared to control mice, as supported by previous observations.27 Treating HFD/STZ-induced diabetic mice with α-ALA led to significant improvements in both body weight and heart weight. α-ALA helped reduce the heart weight deficit in diabetic animals, although relative heart weight remained unchanged. This may be because the reduction in heart weight was proportional to body weight, suggesting that α-ALA has a cardioprotective effect. T2DM is characterized by a constant increase in glucose levels and a decrease in insulin levels. These results were corroborated by metabolic alterations observed in HFD/STZ-induced diabetic mice.28 The other risk factor that is commonly associated with DHD is hyperlipidemia. α-ALA showed a significant reduction in triglyceride and LDL levels and a significant increase in HDL levels. This demonstrates the anti-hyperlipidemic effect of α-ALA.
Evaluating cardiac functional biomarkers provides crucial information about myocardial function, and biomarkers like CK-MB and BNP are regarded as gold-standard indicators for detecting myocardial damage. Treatment of α-ALA for 12 weeks in diabetic mice led to a significant improvement in cardiac function, evident through a drastic reduction in the levels of CK-MB, LDH, and BNP compared to their counterparts, showcasing its cardioprotective potential. Cardiac dysfunction in diabetic patients is often clinically silent and is often not recognised until the disease is advanced. It is widely recognised that one of the hallmarks of the diabetic heart is left ventricular diastolic dysfunction, which is one of the first signs of diabetic cardiomyopathy and is often detected earlier than clinically evident left ventricular systolic dysfunction.29 α-ALA improves HFD/STZ-induced diastolic dysfunction in the heart of diabetic mice. Inflammation exacerbates cardiac injury under type 2 diabetic conditions. Furthermore, chronic low-grade inflammation is thought to be another important factor in the pathogenesis of diabetic cardiomyopathy, with several inflammatory factors such as IL-6, CRP, and TNF-α playing a role in the pathogenesis of diabetic cardiomyopathy. The inflammatory response is a key factor in the development and progression of diabetic cardiomyopathy, involving immune cells, inflammatory factors, and inflammation-related pathways. Therefore, controlling the initiating factors of inflammation and modulating specific cellular subpopulations or related inflammatory pathways may prevent or ameliorate the course and pathological process of diabetic cardiomyopathy.30 Inflammatory factor levels in the circulating plasma and heart of mice were examined, and it was found that α-ALA reduced inflammatory factor levels. This suggests that α-ALA acts against HFD/STZ-induced cardiac injury by antagonizing the release of inflammatory factors.
AMPK (5’AMP-activated protein kinase) is an energy-sensing organelle in the cell.31 When activated, AMPK promotes the use of both glucose and fat for energy and inhibits the production of cholesterol and triglycerides. AMPK consists of one catalytic subunit, α, and two regulatory subunits, β and γ, of which PRKAA1 /AMPKα1 is the predominant isoform in angiocytes and macrophages. AMPKα1 accelerates glucose clearance, improves insulin sensitivity, and attenuates adipose inflammatory response.32 Inflammatory cytokines inhibit AMPKα1, whereas anti-inflammatory cytokines activate AMPKα1. Thus, in our HFD/STZ-induced mouse model, we found that CRP, TNFα, and IL-6 were upregulated in mice in response to diabetes. Therefore, we conclude that there is a pro-inflammatory shift in cytokine expression in diabetic mice. It has also been reported that elevated plasma levels of proinflammatory cytokines induce inflammation in cardiomyocytes and increase the risk of developing heart failure.33 Our results are consistent with these reports, as we found increased expression of inflammatory markers in a diabetic model. The expression of the inflammatory factors IL-6 and TNF-α can be attributed to the increased number of tissue-infiltrating inflammatory cells found in the myocardium of diabetic mice. High glucose had a dual inhibitory effect on AMPKα1 protein levels and kinase activity. The E3 ubiquitination ligase MG53 binds to AMPKα1, ubiquitinates AMPKα1 and leads to ubiquitination-dependent degradation of AMPKα1, resulting in a decrease in AMPKα protein levels. In addition, the high-sugar, high-fat environment leads to an increase in ROS, which causes dissociation of AMPKα1 from its upstream kinase LKB1, thereby inhibiting AMPKα1 activity.34 The activation of AMPKα1 can inhibit the NF-κB (p65) pathway to exert anti-inflammatory effects.35 Therefore, we hypothesized that α-ALA inhibits the release of inflammatory factors through the activation of AMPKα1, thereby suppressing the inflammatory response in cardiomyocytes. Our study showed that HFD/STZ significantly inactivated AMPKα1, whereas α-ALA upregulated AMPKα1 expression, consistent with our hypothesis.
Conclusion
In this study, we suggest that α-ALA, possibly through the activation of AMPKα1, may attenuate HFD/STZ-induced cardiac injury by suppressing the inflammatory response. Notably, we found that providing α-ALA for early intervention once T2DM has been diagnosed may slow the progression of DHD. The current study has a few limitations. Our study did not thoroughly investigate dose dependency, long-term efficacy, and synergistic effects. In the future, we will further explore the effects of different doses and their clinical translational potential. Overall, this study provides robust experimental evidence for the application of α-ALA in diabetic heart disease, demonstrating significant clinical promise.
Acknowledgments
The Animal Ethics Committee of Yi Shengyuan Gene Technology (Tianjin) Co., Ltd. conducted an ethical review of the study and has no conflicts of interest.
Funding Statement
This work was supported by the Tianjin Health Commission (2021140, TJWJ2022QN050) and Exceptional Young Talents Fostering Foundation 2021 of the Tianjin Fourth Central Hospital (tjdszxyy20210024).
Disclosure
The authors declare that this study was conducted without any commercial or financial relationship that could be perceived as a potential conflict of interest.
References
- 1.Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126:1501–1525. doi: 10.1161/CIRCRESAHA.120.315913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20:685–695. doi: 10.1038/s41569-023-00877-z [DOI] [PubMed] [Google Scholar]
- 3.Shriya ASK, Pawar VB, Paul AA. Diabetic heart disease: an intricate interplay of a widespread metabolic disorder with the cardiovascular system. Curr Diabetes Rev. 2025;21:93–101. doi: 10.2174/0115733998305019240702095537 [DOI] [PubMed] [Google Scholar]
- 4.Seferovic PM, Paulus WJ, Rosano G, et al. Diabetic myocardial disorder. A clinical consensus statement of the heart failure association of the ESC and the ESC working group on myocardial & pericardial diseases. Eur J Heart Fail. 2024;26:1893–1903. doi: 10.1002/ejhf.3347 [DOI] [PubMed] [Google Scholar]
- 5.Parim B, Sathibabu Uddandrao VV, Saravanan G. Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail Rev. 2019;24:279–299. doi: 10.1007/s10741-018-9749-1 [DOI] [PubMed] [Google Scholar]
- 6.Bellemare M, Bourcier L, Iglesies-Grau J, Boulet J, O’Meara E, Bouabdallaoui N. Mechanisms of diabetic cardiomyopathy: focus on inflammation. Diabetes Obes Metab. 2025;27:2326–2338. doi: 10.1111/dom.16242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Entezari M, Hashemi D, Taheriazam A, et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: a pre-clinical and clinical investigation. Biomed Pharmacother. 2022;146:112563. doi: 10.1016/j.biopha.2021.112563 [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Bai L, Yang X, et al. Recent advances in anti-inflammation via AMPK activation. Heliyon. 2024;10:e33670. doi: 10.1016/j.heliyon.2024.e33670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canbolat E, Cakiroglu FP. The importance of AMPK in obesity and chronic diseases and the relationship of AMPK with nutrition: a literature review. Crit Rev Food Sci Nutr. 2023;63:449–456. doi: 10.1080/10408398.2022.2087595 [DOI] [PubMed] [Google Scholar]
- 10.Joseph JJ, Deedwania P, Acharya T, et al; American Heart Association Diabetes Committee of the Council on, L. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American heart association. Circulation. 2022;145:e722–e759. doi: 10.1161/CIR.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 11.Bertoni C, Abodi M, D’Oria V, Milani GP, Agostoni C, Mazzocchi A. Alpha-linolenic acid and cardiovascular events: a narrative review. Int J Mol Sci. 2023;24:14319. doi: 10.3390/ijms241814319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han H, Xue T, Li J, et al. Plant sterol ester of alpha-linolenic acid improved non-alcoholic fatty liver disease by attenuating endoplasmic reticulum stress-triggered apoptosis via activation of the AMPK. J Nutr Biochem. 2022;107:109072. doi: 10.1016/j.jnutbio.2022.109072 [DOI] [PubMed] [Google Scholar]
- 13.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuang P, Shou Q, Lu Y, et al. Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2715–2726. doi: 10.1016/j.bbadis.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Miyoshi T, Yoshida M, et al. Pathophysiology and treatment of diabetic cardiomyopathy and heart failure in patients with diabetes mellitus. Int J Mol Sci. 2022;23(7):3587. doi: 10.3390/ijms23073587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonetto M, Infante M, Sacco RL, Rundek T, Della-Morte D. A novel anti-inflammatory role of omega-3 PUFAs in prevention and treatment of atherosclerosis and vascular cognitive impairment and dementia. Nutrients. 2019;11(10):2279. doi: 10.3390/nu11102279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalili Tilami S, Kourimska L. Assessment of the nutritional quality of plant lipids using atherogenicity and thrombogenicity indices. Nutrients. 2022;14:10.3390/nu14183795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimlou M, Jahromi NB, Hasanyani N, Ahmadi AR. Effects of flaxseed interventions on circulating inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:1108–1119. doi: 10.1093/advances/nmz048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shams-White MM, Pannucci TE, Lerman JL, et al. Healthy eating index-2020: review and update process to reflect the dietary guidelines for Americans,2020-2025. J Acad Nutr Diet. 2023;123:1280–1288. doi: 10.1016/j.jand.2023.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liput KP, Lepczynski A, Ogluszka M, et al. Effects of dietary n-3 and n-6 polyunsaturated fatty acids in inflammation and cancerogenesis. Int J Mol Sci. 2021;22:10.3390/ijms22136965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishihara T, Yoshida M, Arita M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int Immunol. 2019;31:559–567. doi: 10.1093/intimm/dxz001 [DOI] [PubMed] [Google Scholar]
- 22.Djuricic I, Calder PC. Omega-3 (n-3) fatty acid-statin interaction: evidence for a novel therapeutic strategy for atherosclerotic cardiovascular disease. Nutrients. 2024;16:962. doi: 10.3390/nu16070962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar M, Pal N, Sharma P, et al. Omega-3 fatty acids and their interaction with the gut microbiome in the prevention and amelioration of type-2 diabetes. Nutrients. 2022;14(9):1723. doi: 10.3390/nu14091723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mone P, Varzideh F, Kansakar U, et al. Omega-3 fatty acids coordinate glucose and lipid metabolism in diabetic patients. Lipids Health Dis. 2022;21:31. doi: 10.1186/s12944-022-01642-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y, Gao M, Xu H. Ginger extract and omega-3 fatty acids supplementation: a promising strategy to improve diabetic cardiomyopathy. Physiol Res. 2024;73:351–367. doi: 10.33549/physiolres.935266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eraky SM, Ramadan NM. Effects of omega-3 fatty acids and metformin combination on diabetic cardiomyopathy in rats through autophagic pathway. J Nutr Biochem. 2021;97:108798. doi: 10.1016/j.jnutbio.2021.108798 [DOI] [PubMed] [Google Scholar]
- 27.Guo J, Qiu X, Zhang L, Wei R. Smurf1 regulates macrophage proliferation, apoptosis and migration via JNK and p38 MAPK signaling pathways. Mol Immunol. 2018;97:20–26. doi: 10.1016/j.molimm.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Zhu C, Yang H, Deng J, Fan D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol Res. 2020;155:104746. doi: 10.1016/j.phrs.2020.104746 [DOI] [PubMed] [Google Scholar]
- 29.Daniels LJ, Macindoe C, Koutsifeli P, et al. Myocardial deformation imaging by 2D speckle tracking echocardiography for assessment of diastolic dysfunction in murine cardiopathology. Sci Rep. 2023;13:12344. doi: 10.1038/s41598-023-39499-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramesh P, Yeo JL, Brady EM, McCann GP. Role of inflammation in diabetic cardiomyopathy. Ther Adv Endocrinol Metab. 2022;13:20420188221083530. doi: 10.1177/20420188221083530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinberg GR, Hardie DG. New insights into activation and function of the AMPK. Nat Rev Mol Cell Biol. 2023;24:255–272. doi: 10.1038/s41580-022-00547-x [DOI] [PubMed] [Google Scholar]
- 33.Prandi FR, Evangelista I, Sergi D, Palazzuoli A, Romeo F. Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: molecular abnormalities and phenotypical variants. Heart Fail Rev. 2023;28:597–606. doi: 10.1007/s10741-021-10200-y [DOI] [PubMed] [Google Scholar]
- 34.Jiang P, Ren L, Zhi L, et al. Negative regulation of AMPK signaling by high glucose via E3 ubiquitin ligase MG53. Mol Cell. 2021;81:629–637e625. doi: 10.1016/j.molcel.2020.12.008 [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Lu J, Wang Y, et al. Canagliflozin attenuates lipotoxicity in cardiomyocytes by inhibiting inflammation and ferroptosis through activating AMPK pathway. Int J Mol Sci. 2023;24. doi: 10.3390/ijms24010858 [DOI] [PMC free article] [PubMed] [Google Scholar]