Abstract
Mutations in mitochondrial DNA, affecting the activity of respiratory complexes, have been implicated in many chronic degenerative diseases. Mitochondrial proteins coded for by both the mitochondrial and nuclear genes are known to have important signaling roles in apoptosis. However, the impact of the inhibition of mitochondrial protein synthesis on apoptosis is largely unknown. This inhibition is particularly important in NO-dependent cytotoxicity, which is believed to have a significant mitochondrial component and depend on other factors such as glycolysis. In this study we have examined whether the inhibition of mitochondrial protein synthesis by chloramphenicol increases the susceptibility of endothelial cells to undergo NO-dependent apoptosis in glucose-free media. Bovine aortic endothelial cells were treated with chloramphenicol, which resulted in a decreased ratio of mitochondrial complex IV to cytochrome c and increased oxidant production in the cell. Inhibition of mitochondrial protein synthesis was associated with a greater susceptibility of the cells to apoptosis induced by NO in glucose-free medium.
Endothelial cell apoptosis is thought to play an important role in many chronic vascular diseases including atherosclerosis (1–3). Potential cytotoxic mediators include increased formation of reactive oxygen and nitrogen species (ROS/RNS) during the atherosclerotic process. Nitric oxide (NO) has been demonstrated to have a biphasic action on oxidative cell killing with low concentrations protecting against cell death, whereas higher concentrations are cytotoxic (4). High levels of NO can be produced by inducible nitric-oxide synthase in response to cytokine stimulation, primarily from macrophages (5), and elevated levels of NO can induce injury to the endothelium (6). NO induces cell death through mechanisms involving cytochrome c release and caspase activation (7, 8). Furthermore, ROS can induce mitochondrial DNA damage in endothelial cells, and this damage is accompanied by a decrease in mitochondrial RNA (mtRNA) transcripts, mitochondrial protein synthesis, and cellular ATP levels (9). Mitochondria have been recognized to play a pivotal role in the signaling cascade of apoptosis (10) and have been implicated in atherosclerosis-induced damage in endothelial cells (11, 12). Little is known of the effect of inhibition of mitochondrial protein synthesis on the sensitivity of endothelial cells to apoptosis; this effect is the focus of this study.
The processes involved in the signaling pathways leading to apoptosis are complex but have some degree of convergence between cell types including those in the vasculature. Release of cytochrome c from mitochondria is a proapoptotic signal, which activates several downstream signaling events including formation of the apoptosome and activation of caspases (13). The impact of the interaction of cytochrome c with its redox partners in the respiratory chain on apoptosis is less well understood. Ubiquinol cytochrome c reductase (complex III) is a site for ROS formation, and cytochrome c oxidase (complex IV) is a target for the interaction of NO in mitochondria (14–16). We have also shown that binding of NO to complex IV can inhibit cytochrome c release from mitochondria (17). Both these enzyme complexes contain subunits coded for by the mitochondrial genome and are thus susceptible to the consequences of mitochondrial DNA damage. A recent study suggested that the cytoprotective effects of NO in endothelial cells depended on the mitochondrial respiratory chain (18). An interplay between ROS or RNS derived from the mitochondrion and the availability of glucose for glycolysis has also been suggested, but how the synthesis of mitochondrial proteins would have an impact on apoptosis is not known. These data led us to hypothesize that inhibition of transcription of the mitochondrial genome would have an impact on the cytotoxicity of NO.
In this study selective inhibition of specific components of the mitochondrial respiratory chain was achieved by treatment of the cells with an inhibitor of mitochondrial protein synthesis. The susceptibility of these cells to apoptosis induced by the high fluxes of NO that can be generated by inducible nitric-oxide synthase was then examined.
Materials and Methods
Chloramphenicol, ATP, dichloroindophenol, ubiquinone, thenoyltrifluoroacetone, cytochrome c, Tris, acetyl-CoA, oxaloacetate, pyruvate, and 5,5′-dithiobis-(2,4-nitrobenzoic acid) were obtained from Sigma. DPTA-NONOATE was from Alexis Biochemicals, San Diego. All other reagents used were of analytical grade.
Cell Culture.
Bovine aortic endothelial cells (BAEC) harvested from descending thoracic aortas were maintained (37°C, 5% CO2) in DMEM growth medium (GIBCO) containing glutamine (4 mM), pyruvate (1 mM), sodium bicarbonate (3.7 g/liter), and 10% FCS (Atlanta Biologicals, Norcross, GA) with penicillin (100 units/ml) and streptomycin (100 ng/ml). Cells used in this study were between passages 5 and 11.
Treatment with Chloramphenicol and Nitric Oxide Donor.
Bovine aortic endothelial cells (BAECs) were grown to confluence and then treated with 20 μg/ml chloramphenicol throughout the entire experiment. Chloramphenicol was used in earlier models to inhibit mitochondrial protein synthesis selectively, as reported for BHK-21 cells (19). For treatment with the NO donor DPTA-NONOATE (400 μM), chloramphenicol-treated cells (48 h) were exposed to NO for the times indicated in either glucose-replete or glucose-free media.
Assay of Respiratory Complex Activities, Citrate Synthase, and ATP Levels.
Complex IV activity was measured by the oxidation of cytochrome c at 550 nm (20) and control activities were ≈9 ± 0.7 k (min−1⋅mg−1 protein). Data is represented as the peudo first order rate constant (k) divided by the protein concentration. Complex II activity was followed by measuring the reduction of dichloroindophenol at 600 nm with succinate as the substrate, and complex II/III was measured by monitoring the reduction of cytochrome c at 550 nm, also with succinate as the substrate (21). Replicate assays for complexes II and II/III within experiments were within 5%, but control activities ranged from 25 to 50 nmol⋅min−1⋅mg−1 protein with passage number. Complex I activity was measured by monitoring the oxidation of NADH to NAD+ at 340 nm (21) (control activity of approximately 140 ± 10 nmol⋅min−1⋅mg−1 protein), and citrate synthase was measured by using the coupled reaction with oxaloacetate, acetyl-CoA, and 5,5′-dithiobis-(2,4-nitrobenzoic acid) (22). ATP was measured by luminometry by using the Enliten luciferin-luciferase kit (Promega). Cell viability was measured as the proportion of lactate dehydrogenase released into the medium. Mitochondrial respiration and NO levels were determined as described (16).
Separation of Mitochondrial Fraction and Western Blot Analysis of Complex IV (Subunit I) and Cytochrome c.
BAECs were washed in an isotonic sucrose buffer composed of 10 mM Tris⋅HCl (pH 7.4), 1 mM EDTA, and 0.25 M sucrose (TES), and suspended in the same buffer. The cells were then homogenized in a Dounce homogenizer and centrifuged at 3,000 × g for 10 min to obtain the postnuclear supernatant. The supernatant was then centrifuged at 24,000 × g for 10 min to obtain the mitochondrial fraction. Aliquots (30 μg protein) of mitochondrial sample were then resolved on a SDS-15% polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Millipore). The polyvinylidene difluoride membrane was probed with complex IV (subunit I) (Molecular Probes) or cytochrome c (BD PharMingen) antibodies. Goat anti-mouse IgG conjugated to alkaline phosphatase was used as secondary antibody and developed by a chemiluminescence detection method.
Detection of Apoptosis.
Apoptosis in endothelial cells was assessed by examination of nuclear morphology and staining with the phosphatidylserine-specific ligand, annexin V. For nuclear staining, BAECs on glass slides were allowed to air dry after treatment. An ethanol/chloroform/acetic acid mixture (6:3:1 vol/vol) was used as fixative. After 10 min exposure, cells were stained with Hoechst 33258 (50 μg/ml) for 10 min. The cells were then examined under a Leitz Orthoplan fluorescent microscope with an UV filter. For the annexin-binding assay, cells were detached by using trypsin-EDTA, washed with PBS, and resuspended to 100 μl of annexin V-binding buffer, with 0.5 ng of Annexin V-FITC and 2.5 ng of propidium iodide (CLONTECH). The cells (105) were then analyzed on a FACScan (Becton Dickinson) by using WINMDI 2.8 software (The Scripps Research Institute Cytometry Software Page) within 30 min after staining.
Caspase Assay.
For detection of caspase 3 activity, BAECs were lysed in buffer (1% Triton X-100/10 mM Tris⋅HCl, pH 7.4/1 mM EDTA/0.25 M sucrose/100 μM phenylmethylsulfonyl fluoride, pH 7.4), followed by centrifugation (20,000 × g, 10 min). Caspase 3 activity was detected in the resulting supernatants by measuring the proteolytic cleavage of the colorimetric substrate acetyl-Asp-Glu-Val-Asp (DEVD)-pNA in assay buffer (100 mM Hepes/10% sucrose/0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), pH 7.5/1 mM phenylmethylsulfonyl fluoride/10 mM DTT) by using the absorbance of released para-nitroanilide at 405 nm.
Immunocytochemistry.
BAECs grown on glass slides were incubated with 500 nM Mitotracker Deep Red 633 (Molecular Probes) for staining of mitochondria. Cells were washed with PBS, fixed with 1% paraformaldehyde in PBS, and after permeabilization, incubated with a monoclonal cytochrome c antibody (1:200, BD PharMingen) for 60 min at 25°C. The secondary antibody was Oregon Green 488 conjugated goat anti-mouse (Molecular Probes). Nuclei were counterstained with Hoechst 33258 (20 μg/ml) (Sigma). Images were acquired through a Leitz Orthoplan microscope and analyzed with IP LAB SPECTRUM software (Scanalytics, Billerica, MA).
Measurement of Reactive Oxygen and Nitrogen Species.
For measurement of ROS/RNS coelenterazine (Molecular Probes, 20 μM) was added to 2–5 × 106 cells per ml resuspended in PBS + 1 mM Ca2+, in the absence of glucose. Chemiluminescence was then monitored for 2 min by using an Autolumat LB953 luminometer (23). The ROS/RNS production is expressed as relative light units per 106 cells.
Results
Effect of Chloramphenicol Treatment on Bioenergetics and Respiratory Chain Activity.
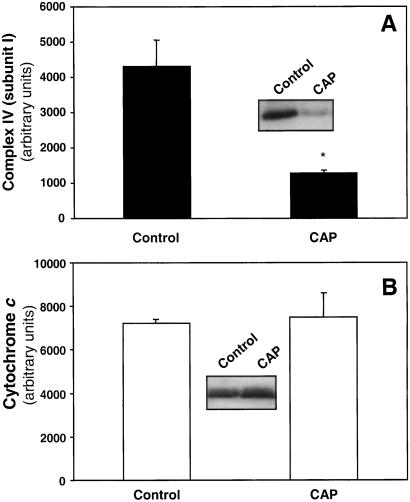
Treatment of BAEC with chloramphenicol (20 μg/ml) for 48 h inhibited complex IV activity by 70% and complex I activity by 40%, whereas complex II, complex II/III, and citrate synthase activity were unaffected, indicating the specificity of inhibition to mitochondrial-encoded proteins in endothelial cells (Fig. 1, Table 1). Chloramphenicol treatment for up to 55 h in glucose-replete media did not alter total cellular ATP levels (95.8 ± 6.26% of control, mean ± SEM, n = 3), or cell viability (90.3 ± 1.18%, mean ± SEM, n = 3). The inhibition of mitochondrial respiratory complex IV activity by chloramphenicol was reversed after withdrawal of the drug for 24 h from the medium (result not shown). To confirm that chloramphenicol decreased complex IV by inhibiting protein synthesis the levels of mitochondrially coded subunit I of the enzyme were determined and found to be decreased when compared with controls (Fig. 2A). However, the levels of the nuclear encoded cytochrome c in mitochondria were not affected (Fig. 2B).
Figure 1.
Activity of mitochondrial respiratory complexes after treatment with chloramphenicol. BAECs were treated with 20 μg/ml chloramphenicol for the times shown, and the activity of complex IV (A) and complex II (B) were measured. Specific activity is k (min−1⋅mg−1 protein) for complex IV and nmol⋅min−1⋅mg−1 protein for complex II. Values are mean ± SEM; n = 3.
Table 1.
Mitochondrial enzyme activities after treatment with chloramphenicol
| Mitochondrial enzymes | Specific activity, % control |
|---|---|
| Complex I | 61 ± 10.3* |
| Complex II | 89 ± 2.4 |
| Complex II/III | 84.3 ± 7.1 |
| Complex IV | 32 ± 4.0* |
| Citrate synthase | 105 ± 0.66 |
BAECs were treated with CAP (20 μg/ml) for 48 h, and the activity of the various respiratory complexes were measured. Values are mean ± SEM, n = 3.
, P < 0.05 compared with control.
Figure 2.
Complex IV (subunit I) and cytochrome c levels in mitochondria after treatment with chloramphenicol. BAECs were treated with 20 μg/ml chloramphenicol (CAP) for 48 h and the mitochondrial fraction separated and probed for complex IV (subunit I) (A) or cytochrome c (B). The intensity of the bands was determined and plotted as arbitrary units. (Inset) Representative blots showing levels of complex IV (subunit I) (A) and cytochrome c (B) in control and chloramphenicol-treated cells. (*, P < 0.05.)
Chloramphenicol Treatment Renders Endothelial Cells More Susceptible to NO-Induced Apoptosis.
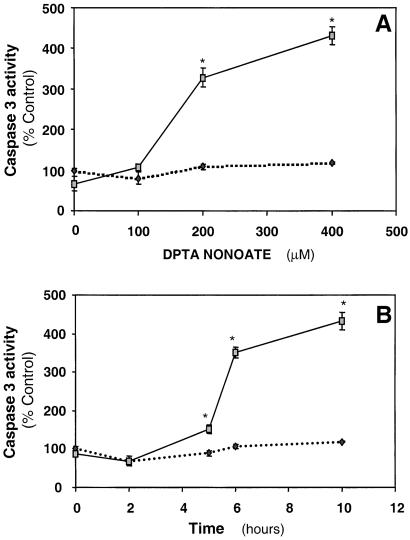
In agreement with the literature in which other cell types were used, NO (DTPA-NONOATE, 400 μM) did not cause cytotoxicity in endothelial cells, with or without chloramphenicol treatment, in glucose-replete media (24–27). However, chloramphenicol-treated endothelial cells exposed to the NO donor in glucose-free media (10 h) showed marked condensation of nuclei, consistent with apoptosis compared with control (Fig. 3A). The NO donor resulted in a steady state of 3.37 ± 0.28 μM NO (mean ± SEM, n = 3) in the media, which is comparable with the amounts produced in vivo after activation of inducible nitric-oxide synthase (28). Chloramphenicol-treated cells showed a higher number of annexin-positive cells when compared with NO treatment alone (Fig. 3B). The activity of caspase 3 was determined after treatment with a range of NO donor concentrations with or without chloramphenicol. It was found that caspase 3 increased as a function of the concentration of NO donor in chloramphenicol-pretreated cells with detectable activation within 5 h after NO exposure (Fig. 4). No significant induction of apoptotic markers in control or chloramphenicol-treated cells in glucose-replete media was observed in cells exposed to NO.
Figure 3.
Detection of apoptosis in BAECs after treatment with chloramphenicol and NO donor. (A) BAECs were treated with 20 μg/ml chloramphenicol for 48 h and then exposed to DPTA-NONOATE (400 μM) for 10 h. The cells were then stained with Hoechst 33258. Control cells (a), cells treated with chloramphenicol alone (b), or NO donor alone (c) show normal morphology. Apoptotic cells (arrowhead) are visible among cells treated with the NO donor after chloramphenicol treatment (d). (B) Fluorescence-activated cell sorter analysis for exposure of phosphatidylserine. BAECs were treated with 20 μg/ml chloramphenicol for 48 h and then exposed to DPTA-NONOATE (400 μM) for the various times indicated. The cells were then collected, labeled with annexin V antibody conjugated to FITC and propidium iodide and analyzed by fluorescence-activated cell sorter. Diagrams were obtained from bivariate annexin V/PI analysis. The percentage of apoptotic (annexin V-FITC positive) cells is indicated. - - -, NO alone; —, NO + chloramphenicol. The data are expressed as mean ± SEM; n = 3. (*, P < 0.05.)
Figure 4.
Caspase 3 activity in chloramphenicol-pretreated cells exposed to NO donor. BAECs were treated with 20 μg/ml chloramphenicol for 48 h and then exposed to varying concentrations of DPTA-NONOATE for 10 h (A) or exposed to DPTA-NONOATE (400 μM) for various times as indicated (B). The cells were then lysed and the caspase 3 activity determined. - - -, NO alone; —, NO + chloramphenicol. The data are expressed as mean ± SEM. (*, P < 0.05.)
Apoptosis After Chloramphenicol Pretreatment Involves the Mitochondrial Pathway.
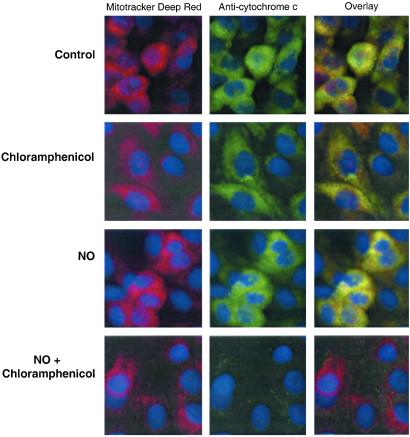
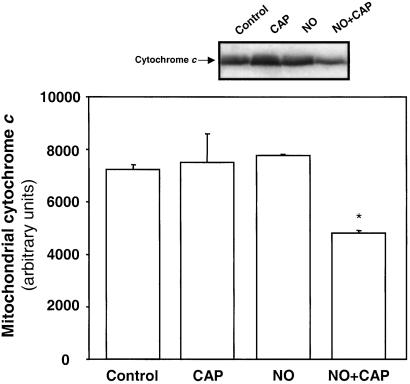
Treatment with the caspase 9 inhibitor (LEHD-fmk) resulted in a significant decrease in the activation of caspase 3 (caspase activation of 180.3 ± 17% of control with LEHD-fmk compared with 367 ± 36% without inhibitor, mean ± SEM, n = 3, P < 0.05), indicating that caspase 9 is upstream of caspase 3 and involved in NO-dependent apoptotic cell death. Because cytochrome c release from mitochondria is required for caspase 9 activation, localization of cytochrome c in BAECs after treatment with chloramphenicol and NO was examined by immunocytochemistry. As seen in Fig. 5, cytochrome c (green fluorescence) colocalized to mitochondria (red fluorescence) in the control and cells treated with chloramphenicol or NO alone. However, in cells treated with NO and chloramphenicol, a loss of cytochrome c fluorescence occurs, indicating release from the mitochondrion. This result was confirmed by Western blotting of mitochondrially enriched subfractions and showed a decrease in mitochondrially associated cytochrome c in cells treated with NO and chloramphenicol (Fig. 6).
Figure 5.
Depletion of cytochrome c from mitochondria in BAECs treated with NO and chloramphenicol. BAECs on glass slides were treated with 20 μg/ml chloramphenicol for 48 h and then exposed to DPTA-NONOATE (400 μM) for 10 h. Immunostaining for cytochrome c was then performed in permeabilized BAECs stained with Mitotracker Deep Red 633 to localize mitochondria. The panels on the right indicate the overlay of the first two micrographs illustrating colocalization of cytochrome c to mitochondria in all cells except those treated with NO and chloramphenicol, where cytochrome c is lost from mitochondria. The cell nuclei were counter-stained with Hoechst 33258.
Figure 6.
Western blot and quantitation of mitochondria for cytochrome c: BAECs were treated with 20 μg/ml chloramphenicol (CAP) for 48 h and then exposed to DPTA-NONOATE (400 μM) for 12 h. The mitochondrial fraction was then separated and probed for cytochrome c. The intensity of the bands was quantitated and expressed as arbitrary units. (*, P < 0.05.) A representative blot for cytochrome c in mitochondria after the various treatments is also shown.
Cellular ATP and ROS Production in Cells Treated with NO and Chloramphenicol.
As expected, a decrease in cellular ATP levels in control or chloramphenicol-treated cells is evident after withdrawal of glucose from the medium (Fig. 7A). NO treatment did not affect ATP levels in glucose-replete media in control cells but did show a significant decrease in the chloramphenicol-treated cells. Removal of glucose from the medium in cells exposed to NO results in a further suppression of ATP levels in both control and chloramphenicol-treated cells to approximately the same levels.
Figure 7.
ATP levels and free radical production after treatment of cells with CAP and NO. (A) BAECs were treated with 20 μg/ml chloramphenicol (CAP) for 48 h and then exposed to DPTA-NONOATE (400 μM) for 12 h in either glucose-replete (□) or glucose-free media (■). Total ATP levels were measured by luminometry. Values are mean ± SEM of three separate experiments. (*, P < 0.05 compared with control in glucose-replete media; #, P < 0.05 compared with control in glucose-free media.) (B) BAECs were initially pretreated with chloramphenicol for 72 h after confluence. The cells were then collected and resuspended in PBS containing 1 mM calcium for 30 min. Cells treated with NO donor were exposed to DPTA NONOATE (400 μM) at the time of resuspension for 30 min. ROS generation was measured for 2 min at 37°C by using the chemiluminescent probe, coelenterazine (20 μM). Results are means ± SEM for triplicate experiments, normalized to 106 cells (*, P < 0.05 relative to control).
The susceptibility of mitochondrial respiration to inhibition by NO (400 μM DPTA NONOATE) determined in cells suspended in an oxygen electrode was not altered after chloramphenicol treatment in BAECs (time for 100% inhibition of respiration for control being 107 ± 2 sec, compared with 108 ± 1 sec for chloramphenicol-treated cells). By using coelenterazine as a probe for ROS/RNS (23) the levels of chemiluminescence generated in the BAECs treated with chloramphenicol alone or NO alone were both significantly higher than in the control cells (Fig. 7B). However, no significant difference occurred in the ROS/RNS levels between these cells and those exposed to NO in the presence of chloramphenicol.
Discussion
Reactive oxygen and nitrogen species can inhibit mitochondrial protein synthesis, which suggests that during chronic exposure to inflammatory mediators differential inhibition of mitochondrially and nuclear coded proteins may occur. The importance of the respiratory chain in maintaining ATP levels is well recognized, but the effects of inhibiting mitochondrial protein synthesis on oxidant formation in cells and apoptosis are not understood in detail, which is potentially important because it has been proposed that the balance between ATP formation and cytochrome c release may regulate the pathway of cell death through necrosis or apoptosis (29). Nitric oxide, depending on concentration, cell type, and the absence or presence of glucose, has been shown to be either pro- or antiapoptotic through mechanisms thought to involve the mitochondria (7, 8, 30). Taken together these data led to the hypothesis that changes in the rate of mitochondrial protein synthesis and the resulting decrease in the activity of the mitochondrial respiratory chain would have an impact on apoptosis through several interrelated mechanisms: (i) through changing the relative proportion of cytochrome c to complex IV and complex III, (ii) by the decrease in the availability of complex IV as a site for NO to inhibit cytochrome c release, (iii) the ability of the respiratory chain to serve the cells energy requirements, and (iv) increasing the formation of ROS/RNS.
To test these ideas mitochondrial protein synthesis was inhibited with chloramphenicol and was clearly evident in a decrease of complex IV to approximately 30% of control levels. In contrast chloramphenicol had little or no effect on complex II, complex II/III or citrate synthase activity, consistent with restriction of the defect to the mitochondrially coded proteins. This reversible and partial inhibition of mitochondrial protein synthesis did not change ATP levels or cell viability in glucose-replete media supporting the use of this model of mitochondrial damage in endothelial cells.
The levels of cytochrome c, which is nuclear encoded, were not altered in these mitochondria. The diffusion rate, the concentration of cytochrome c, and concentration of complexes III and IV are rate limiting for maximal electron transport activity (31, 32). In addition, the cytochrome c potentially available for initiating the apoptotic cascade is not modified by chloramphenicol treatment. Mechanisms leading to cytochrome c release from the mitochondrion in apoptosis are controversial but it is likely a two-step process, involving its initial detachment from the inner membrane before exit through the outer membrane (33, 34). From these data we hypothesized that a potential consequence of inhibition of mitochondrial protein synthesis would be to increase cytochrome c available to initiate apoptosis on exposure to NO.
Chloramphenicol-treated cells, on exposure to NO, showed several changes characteristic of apoptotic cell death (35). For example, an increase in caspase 3 activation was observed with further involvement of caspase 9, indicating involvement of the mitochondrial pathway of apoptosis (13). Furthermore, exposure of chloramphenicol-treated cells to NO induced release of cytochrome c from the mitochondria.
However, NO-dependent apoptosis was only observed in glucose-free media. This dependence of NO cytotoxicity on glucose deprivation was also observed in several different studies (24, 25). For example, glycolytic metabolism of glucose was shown to affect IFNγ/lipopolysaccharide-induced cytotoxicity in mouse embryonic fibroblasts. Withdrawal of glucose from the media at later time points after treatment (30–48 h) was needed for cell death, whereas withdrawal of L-arginine from the culture medium prevented cell death, indicating that cytotoxicity in these cells was due to an NO- and glycolysis-dependent mechanism (36). Hypoxic HeLa cells were dramatically sensitive to heat under glucose deprivation (37), and this treatment also enhanced tumor necrosis factor-related apoptosis-inducing ligand–induced apoptosis in prostate adenocarcinoma DU-145 cells (38). Earlier studies showed that L1210 cells pretreated with cytotoxic-activated macrophages are susceptible to cell death on withdrawal of glucose from the medium, whereas control L1210 cells maintained viability in glucose-free medium (26). Taken together these data emphasize the critical interplay between cellular bioenergetics and the susceptibility of different cell types to NO-dependent apoptosis. Inhibition of glycolysis could enhance NO-dependent cytotoxicity through several mechanisms including decreased ATP formation and loss of the maintenance of NADPH for antioxidant defenses (25, 39). Also, NO, or other RNS, could further increase these effects by inhibition of glycolytic enzyme, notably glyceraldehyde-3-phosphate dehydrogenase (40).
The role of cellular bioenergetics and ROS production in modulating the increased susceptibility of chloramphenicol-treated cells to NO-induced apoptosis was then investigated. The finding that NO exposure of chloramphenicol-treated cells in glucose-replete media resulted in decreased ATP levels indicates that the bioenergetic status of these cells is compromised, as might be expected. In the absence of glucose, ATP levels are further decreased on NO exposure with chloramphenicol treatment, although this level is not significantly different from NO alone. Thus, the emergence of a threshold of cellular bioenergetics in the combined treatments of NO and inhibition of mitochondrial protein synthesis renders the cell susceptible to additional stressors which could include glucose deprivation and oxidants derived from the mitochondrion itself or other intracellular sources. Indeed, measurement of ROS/RNS reveals that chloramphenicol treatment also results in an increase in oxidant production as shown by others in RL 34 cells (41). NO also caused an increase in coelenterazine chemiluminescence in the control cells, but this chemiluminescence was not further increased by chloramphenicol treatment. The probe used here measures both superoxide and peroxynitrite and these cannot be distinguished using these protocols (23).
In conclusion, this study has shown NO-dependent apoptosis in endothelial cells is influenced by a complex interaction between several factors that have not been integrated in previous studies. These include (i) the relative ratio of cytochrome c to cytochrome c oxidase in the electron transport chain, (ii) the alteration in cellular bioenergetics due to inhibition of mitochondrial protein synthesis, (iii) increased ROS/RNS formation in cells in which mitochondrial protein synthesis is inhibited. The site of formation of the increased oxidant production in chloramphenicol-treated cells is not clear at present but these data suggest that mitochondrial function is more closely associated with the modulation of ROS/RNS in the cell than thought previously. Importantly, these data suggest that inhibition of mitochondrial protein synthesis contributes to the shift in the balance between the cytotoxic and cytoprotective properties of NO during chronic inflammation.
Acknowledgments
This study was supported by National Institutes of Health Grant RO1/HL58031. A.R. is supported by a fellowship from the American Heart Association, Southeast Affiliate. S.S. is a recipient of a National Institutes of Health training fellowship for cardiovascular research. E.C. is supported by a predoctoral National Science Foundation GK-12 fellowship.
Abbreviations
- BAEC
bovine aortic endothelial cell
- CAP
chloramphenicol
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Choy J C, Granville D J, Hunt D W, McManus B M. J Mol Cell Cardiol. 2001;33:1673–1690. doi: 10.1006/jmcc.2001.1419. [DOI] [PubMed] [Google Scholar]
- 2.Dimmeler S, Zeiher A M. Regul Pept. 2000;90:19–25. doi: 10.1016/s0167-0115(00)00105-1. [DOI] [PubMed] [Google Scholar]
- 3.Dimmeler S, Hermann C, Zeiher A M. Eur Cytokine Network. 1998;9:697–698. [PubMed] [Google Scholar]
- 4.Joshi M S, Ponthier J L, Lancaster J R., Jr Free Radical Biol Med. 1999;27:1357–1366. doi: 10.1016/s0891-5849(99)00179-3. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda U, Maeda Y, Shimada K. Clin Cardiol. 1998;21:473–476. doi: 10.1002/clc.4960210705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toborek M, Kaiser S. Basic Res Cardiol. 1999;94:295–314. doi: 10.1007/s003950050156. [DOI] [PubMed] [Google Scholar]
- 7.Taimor G, Hofstaetter B, Piper H M. Cardiovasc Res. 2000;45:588–594. doi: 10.1016/s0008-6363(99)00272-2. [DOI] [PubMed] [Google Scholar]
- 8.Yabuki M, Tsutsui K, Horton A A, Yoshioka T, Utsumi K. Free Radical Res. 2000;32:507–514. doi: 10.1080/10715760000300511. [DOI] [PubMed] [Google Scholar]
- 9.Ballinger S W, Patterson C, Yan C N, Doan R, Burow D L, Young C G, Yakes F M, Van Houten B, Ballinger C A, Freeman B A, et al. Circ Res. 2000;86:960–966. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- 10.Bratton S B, Cohen G M. Trends Pharmacol Sci. 2001;22:306–315. doi: 10.1016/s0165-6147(00)01718-1. [DOI] [PubMed] [Google Scholar]
- 11.Whereat A F. Ann Intern Med. 1970;73:125–127. doi: 10.7326/0003-4819-73-1-125. [DOI] [PubMed] [Google Scholar]
- 12.Kinscherf R, Deigner H P, Usinger C, Pill J, Wagner M, Kamencic H, Hou D, Chen M, Schmiedt W, Schrader M, et al. FASEB J. 1997;11:1317–1328. doi: 10.1096/fasebj.11.14.9409551. [DOI] [PubMed] [Google Scholar]
- 13.Hengartner M O. Nature (London) 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 14.Gille L, Nohl H. Arch Biochem Biophys. 2001;388:34–38. doi: 10.1006/abbi.2000.2257. [DOI] [PubMed] [Google Scholar]
- 15.Torres J, Darley-Usmar V, Wilson M T. Biochem J. 1995;312:169–173. doi: 10.1042/bj3120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiva S, Brookes P S, Patel R P, Anderson P G, Darley-Usmar V M. Proc Natl Acad Sci USA. 2001;98:7212–7217. doi: 10.1073/pnas.131128898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brookes P S, Salinas E P, Darley-Usmar K, Eiserich J P, Freeman B A, Darley-Usmar V M, Anderson P G. J Biol Chem. 2000;275:20474–20479. doi: 10.1074/jbc.M001077200. [DOI] [PubMed] [Google Scholar]
- 18.Paxinou E, Weisse M, Chen Q, Souza J M, Hertkorn C, Selak M, Daikhin E, Yudkoff M, Sowa G, Sessa W C, et al. Proc Natl Acad Sci USA. 2001;98:11575–11580. doi: 10.1073/pnas.201293198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipton J H, McMurray W C. Biochim Biophys Acta. 1977;477:264–272. doi: 10.1016/0005-2787(77)90051-x. [DOI] [PubMed] [Google Scholar]
- 20.Darley-Usmar V M, Capaldi R A, Takamiya S, Millett F, Wilson M T, Malatesta F, Sarti P. In: Mitochondria: A Practical Approach. Darley-Usmar V M, Rickwood D, Wilson M T, editors. Oxford: IRL; 1987. pp. 113–152. [Google Scholar]
- 21.Ragan C I, Wilson M T, Darley-Usmar V M, Lowe P N. In: Mitochondria: A Practical Approach. Darley-Usmar V M, Rickwood D, Wilson M T, editors. Oxford: IRL; 1987. pp. 79–112. [Google Scholar]
- 22.Shepherd D, Garland P B. Biochem J. 1969;114:597–610. doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarpey M M, White C R, Suarez E, Richardson G, Radi R, Freeman B A. Circ Res. 1999;84:1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- 24.Kim W K, Chung J H, Kim H C, Ko K H. Neurosci Res. 1999;33:281–289. doi: 10.1016/s0168-0102(99)00018-8. [DOI] [PubMed] [Google Scholar]
- 25.Le Goffe C, Vallette G, Jarry A, Bou-Hanna C, Laboisse C L. Biochem J. 1999;344:643–648. doi: 10.1042/0264-6021:3440643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granger D L, Taintor R R, Cook J L, Hibbs J B., Jr J Clin Invest. 1980;65:357–370. doi: 10.1172/JCI109679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beltran B, Mathur A, Duchen M R, Erusalimsky J D, Moncada S. Proc Natl Acad Sci USA. 2000;97:14602–14607. doi: 10.1073/pnas.97.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ameredes B T, Zamora R, Gibson K F, Billiar T R, Dixon-McCarthy B, Watkins S, Calhoun W J. J Leukocyte Biol. 2001;70:730–736. [PubMed] [Google Scholar]
- 29.Lemasters J J, Qian T, Trost L C, Herman B, Cascio W E, Bradham C A, Brenner D A, Nieminen A L. Biochem Soc Symp. 1999;66:205–222. doi: 10.1042/bss0660205. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Bombeck C A, Yang S, Kim Y M, Billiar T R. J Biol Chem. 1999;274:17325–17333. doi: 10.1074/jbc.274.24.17325. [DOI] [PubMed] [Google Scholar]
- 31.Gupte S S, Hackenbrock C R. J Biol Chem. 1988;263:5248–5253. [PubMed] [Google Scholar]
- 32.Gupte S S, Hackenbrock C R. J Biol Chem. 1988;263:5241–5247. [PubMed] [Google Scholar]
- 33.Krippner A, Matsuno-Yagi A, Gottlieb R A, Babior B M. J Biol Chem. 1996;271:21629–21636. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]
- 34.Ott M, Robertson J D, Gogvadze V, Zhivotovsky B, Orrenius S. Proc Natl Acad Sci USA. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hortelano S, Dallaporta B, Zamzami N, Hirsch T, Susin S A, Marzo I, Bosca L, Kroemer G. FEBS Lett. 1997;410:373–377. doi: 10.1016/s0014-5793(97)00623-6. [DOI] [PubMed] [Google Scholar]
- 36.Dijkmans R, Billiau A. Eur J Biochem. 1991;202:151–159. doi: 10.1111/j.1432-1033.1991.tb16356.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim J H, Kim S H, Alfieri A A. Radiat Res. 1988;116:337–342. [PubMed] [Google Scholar]
- 38.Nam S Y, Amoscato A A, Lee Y J. Oncogene. 2002;21:337–346. doi: 10.1038/sj.onc.1205068. [DOI] [PubMed] [Google Scholar]
- 39.Tatsumi T, Matoba S, Kawahara A, Keira N, Shiraishi J, Akashi K, Kobara M, Tanaka T, Katamura M, Nakagawa C, et al. J Am Coll Cardiol. 2000;35:1338–1346. doi: 10.1016/s0735-1097(00)00526-x. [DOI] [PubMed] [Google Scholar]
- 40.Hurst R D, Azam S, Hurst A, Clark J B. Brain Res. 2001;894:181–188. doi: 10.1016/s0006-8993(01)01992-8. [DOI] [PubMed] [Google Scholar]
- 41.Karbowski M, Kurono C, Wozniak M, Ostrowski M, Teranishi M, Soji T, Wakabayashi T. Biochim Biophys Acta. 1999;1449:25–40. doi: 10.1016/s0167-4889(98)00167-0. [DOI] [PubMed] [Google Scholar]