Abstract
Larvae of the European cabbage butterfly, Pieris rapae (Pieridae), are beset with glandular hairs, bearing droplets of a clear oily secretion at their tip. The fluid consists primarily of a series of chemically labile, unsaturated lipids, the mayolenes, which are derived from 11-hydroxylinolenic acid. In bioassays with the ant Crematogaster lineolata, the secretion was shown to be potently deterrent, indicating that the fluid plays a defensive role in nature.
Keywords: chemical defense‖predation‖Lepidoptera‖Formicidae
The European cabbage butterfly, Pieris rapae, is a widely distributed insect. Native to Eurasia and North Africa, it was accidentally introduced into Canada in about 1860, from where it spread over most of North America. It also has become established in Bermuda, Australia, Hawaii, and other Pacific Islands (1). As a larva, it prefers plants of the crucifer and caper families (Brassicaceae, Capparidaceae), and it is a common pest on cabbage, Brussel sprouts, and cauliflower (1). Pursuant to a study of the defenses of a number of larval insects, we noted the caterpillars of P. rapae to be beset with glandular hairs, such as pierid larvae had been reported to possess (2), but had never been investigated. In P. rapae, these hairs are arranged in rows along the full length of the body. Their product is a clear oily fluid that collects as droplets at the tip of the hairs (Fig. 1). On the assumption that this secretion might be protective and responsible in part for P. rapae's extraordinary adaptive fitness, we looked into both the repellency and chemistry of the fluid. We found the secretion to be potently deterrent to ants, and to consist primarily of a series of unsaturated lipids, which we named the mayolenes. Here, we present these results.**
Figure 1.
P. rapae larva (bar = 2 mm).
Materials and Methods
P. rapae.
Secretion was obtained from larvae of a laboratory population of P. rapae maintained on cabbage plants (Brassica oleraceae). The larvae used in the ant bioassays stemmed either from laboratory-laid or field-collected eggs.
Crematogaster lineolata.
The ant of choice is one we maintain in the laboratory specifically for predation purposes. Colonies were collected in eastern Connecticut, including at the Trinity College Field Station at Church Farm, Ashford.
Ant Bioassay l (Acceptability of P. rapae Larvae and Tenebrio Molitor Larvae).
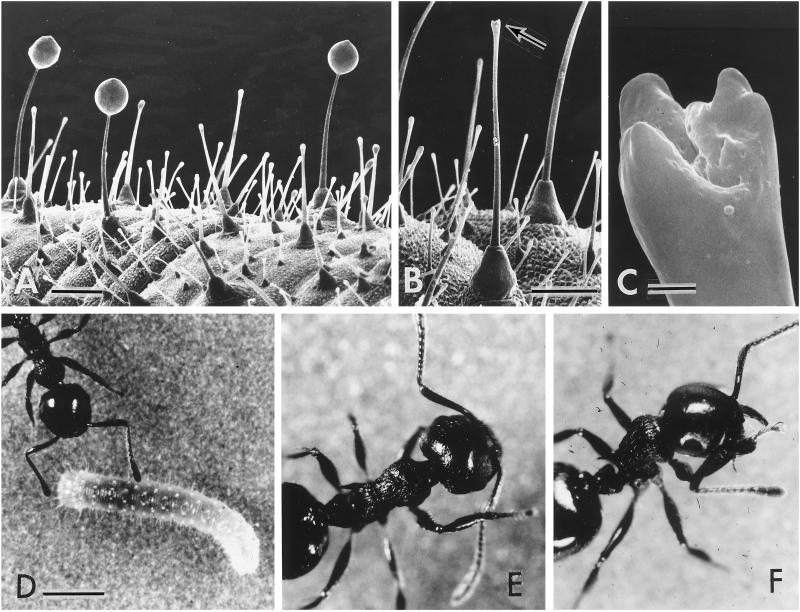
Trials were carried out in individual Petri dishes (5 cm diameter), the floor of which was roughened by sand-blasting to provide for an improved locomotory surface (the sides of the dish were Teflon-coated and thereby rendered escape-proof). An individual ant was first introduced by itself into the dish, and its behavior was videotaped (with time track) for 5 min (the control period), providing a record of its spontaneous cleaning activities [antennal wiping with forelegs (Fig. 2E); foreleg wiping with mouthparts (Fig. 2F); dragging of mouthparts against substrate; regurgitating]. A P. rapae larva (of measured length; second instar) was then introduced, having been transferred into the dish on a small piece of cabbage leaf, to avoid contacting its glandular hairs. Events were then again monitored, this time for 10 min, beginning from the time that the larva crawled off the leaf. The moment of first contact between ant and larva was noted, and the period from that moment to the end of the 10 min was designated as the experimental period. The videotape again provided a record of the behavioral events, including this time all ant-larva contacts (effected by the ant with an antenna, a leg, or the mouthparts), in addition to the ant's cleansing activities.
Figure 2.
(A) Detail of dorsum of a P. rapae larva, showing three droplet-bearing glandular hairs. (B) Same as preceding, but rinsed with dichloromethane; glandular hair (arrow) is seen to be secretion free. (C) Enlarged view of tip of hair denoted by arrow in B. (D) Crematogaster ant inspecting a P. rapae larva. (E) Ant, wiping right antenna with foreleg after contacting a P. rapae larva with that antenna. (F) Ant, cleansing its right foreleg with the mouthparts after using that leg to groom an antenna that had touched a P. rapae larva. Bars: A and B = 0.1 mm; C = 5 μm; D = 1 mm.
Events timed from the videotape were the following: the total time that the ant engaged in cleansing during the control period; the total time that it made contact with the larva during the experimental period (that is, the sum total of durations of the individual contact events); and the total time that it engaged in cleansing during the experimental period. These values provided a basis for calculating the fraction of a given period spent by the ant in contact with the larva or engaged in self-cleaning.
During the control and experimental periods, we sometimes lost sight of the ants when these moved momentarily out of the field of view of our video camera. Such times of ant invisibility (which amounted usually to less than 1% of the period) were subtracted from the values used to express the length of the period. In addition, because of inability to measure durations of under 1 s accurately from the videotapes, we arbitrarily scored ant-larval contacts that were shorter than 1 s to be of 1 s duration. Values given for total contact times are therefore slightly inflated.
An identical set of assays was carried out, by using mealworms (larvae of the tenebrionid beetle, Tenebrio molitor) instead of the P. rapae larvae. Mealworms lack glandular hairs, and they were chosen to be of a length (4.8 ± 0.2 mm) comparable to that of the P. rapae tested (4.5 ± 0.1 mm; t test, P = 0.36).
Trials were replicated 16 times with both the P. rapae larvae and the mealworms. Fresh individuals were used in all trials. After the experiments, the ants, larvae, and mealworms were confined individually and checked after 48 h for incidence of survival. A separate set of these insects (n = 16 of each) that had not been used in experiments were similarly set up and checked for mortality after 48 h.
Ant Bioassay 2 (Acceptability of P. rapae Larvae, With and Without Secretion).
To determine whether the secretion, independent of caterpillar behavior, accounted for the ant's response, dead larvae (freshly killed by freezing) were used. Two sets of tests, identical to the preceding, were carried out, respectively with a group of P. rapae larvae with secretion (n = 11), and with another set of larvae (n = 11) rinsed briefly with dichloromethane to wash away their secretion. Larvae were of the same size as those used in bioassay 1.
To establish whether or not dichloromethane itself serves as an attractant to the ants, two additional sets of trials were conducted. In both cases, the prey item was a mealworm killed by freezing just before the trial. One set of mealworms was rinsed with dichloromethane in a manner identical to that used to remove the secretion from the larval P. rapae, whereas the other set served as unrinsed controls. The mealworms were presented to ants, and the proportion of each ant's time spent in contact with the mealworm was then determined, following the protocol used for the experimental period described above.
Ant Bioassay 3 (Deterrency of Synthetic Mayolenes).
The two compounds (mayolene-16 and mayolene-18) were together applied topically to a food item readily acceptable to C. lineolata: an egg of the hemipteran Oncopeltus fasciatus (reared on sunflower seed, rather than the bug's cardenolide-laden natural diet of Asclepias seed). Experimental eggs received 20 μg of each of the two compounds, dissolved together in 2 μl of methanol. Control eggs received a volume of 2 μl of methanol. Eggs were presented as a pair, with the treated and control egg at diagonally opposite corners of a glass coverslip (22 × 22 mm). Trials were conducted with colonies (n = 4) of C. lineolata collected in eastern Connecticut and maintained in the laboratory in plastic boxes (56 × 41 × 22 cm). Within a colony's box, the coverslip was placed at the center of a circular platform (8.8 cm diameter) to which the ants had been previously trained to forage on chopped mealworm pieces. Several fresh mealworm pieces were added to the coverslip to maintain foraging activity at the site. During each trial, the feeding platform was examined at 1-min intervals for 1 h. Eggs were considered removed if the ants had carried them off the platform. Sixteen trials were performed, four with each ant colony.
Collection of Secretion.
Secretion was collected with fine glass micropipettes, which were touched to the hairs, causing the droplets to be taken up by capillarity. Hundreds of larvae were “milked” in this fashion. The micropipettes were stored at −20°C until rinsed with benzene-d6. The resulting extract was concentrated by evaporation in vacuo, yielding 2.4 mg of an oily, colorless residue, which was redissolved in 0.55 ml of benzene-d6 and directly analyzed by NMR spectroscopy. In addition, small samples of secretion were analyzed by using combinations of GC and electrospray ionization (ESI)-MS, as well as of HPLC and ESI-MS.
Analytical Procedures.
NMR-Spectra were recorded at 25°C by using a Varian UNITY+ (500 MHz proton, 126 MHz carbon) spectrometer with benzene-d6 as the solvent. Phase-sensitive double-quantum filtered correlation spectroscopy spectra and phase-sensitive nuclear Overhauser effect spectroscopy spectra were acquired by using the standard pulse sequences and phase cycling (3). For ESI-MS, a Hewlett–Packard 1090 II pump was linked to a Micromass (Manchester, U.K.) Quattro I mass spectrometer operated in positive ion electrospray mode. For GC-MS, a Hewlett–Packard HP5890A gas chromatograph was linked to a Hewlett–Packard mass-selective detector (MSD; 70 eV EI), using a 30 m DB5-MS coated column (J & W Scientific, Folsom, CA) with 0.25 mm i.d. and 0.25 μm film thickness.
NMR Spectroscopic Data for the Mixture of Mayolenes.
13C NMR (126 MHz, benzene-d6; ppm).
(i) Linolenic acid part: 14.2 (C-18), 20.7 (C-17), 132.9 (C-16), 126.7 (C-15), 26.4 (C-14), 131.9 (C-13), 128.05 (C-12), 66.3 (C-11), 127.8 (C-10), 133.9 (C-9), 28.23 (C-8), 29.4–29.6 (C-7, C-6, C-5), 29.2 (C-4), 24.9 (C-3), 33.9 (C-2), and 179.2 (C-1). (ii) Hexadecanoyl and octadecanoyl substituent: 14.4 (C-16′ or C-18′ for mayolene-16 or mayolene-18, respectively), 23.1 (C-15′ or C-17′), 32.2 (C-14′ or C-16′), 29.2–30.1 (C-5′, C-6′, C-13′ or C-15′), 29.34 (C-4′), 25.21 (C-3′), 34.53 (C-2′), and 172.3 (C-1′).
1H NMR (500 MHz, benzene-d6; ppm).
(i) Linolenic acid part: 2.07 (t, J2,3 = 7.3, 2 H, 2-H), 1.47 (m, J3,4 = 7.0, 2 H, 3-H), 1.11 (m, 2 H, 4-H), 1.14–1.22 (m, 4 H, 5-H and 6-H), 1.23–1.24 (m, J7,8a = J7,8b = 7.1 Hz, 2 H, 7-H), 2.205 (m, J8a,8b = 14 Hz, J8a,9 = 7.3 Hz, 1 H, 8-Ha), 2.32 (m, J8b,9 = 7.3 Hz, 1 H, 8-Hb), 5.49 (m, J9,10 = 10.9 Hz, 1 H, 9-H), 5.62 (m, J10,11 = 8.8 Hz, 1 H, 10-H), 6.78 (m, J11,12 = 8.8 Hz, 1 H, 11-H), 5.61 (m, J12,13 = 10.9 Hz, 1 H, 12-H), 5.50 (m, J13,14a = J13,14b = 7.6 Hz, 1 H, 13-H), 3.08 (m, J14a,14b = 15.7 Hz, J14a,15 = 5.4 Hz, 1 H, 14-Ha), 3.17 (m, J14b,15 = 5.4 Hz, 1 H, 14-Hb), 5.41–5.43 [m, J15,16 = 10.9 Hz, J16,17 = 5.7 Hz, 2 H, 15-H, and 16-H (coupling constants determined by using CD2Cl2 as the solvent where the signals of these two protons are well separated)], 2.07 (m, J17,18 = 7.6 Hz, 2 H, 17-H), 0.93 (t, 3 H, 18-H). (ii) Hexadecanoyl and octadecanoyl substituent: 2.19 (t, 2 H, J2′,3′ = 7.5 Hz, 2′-H), 1.601 (m, J3′,4′ = 6.8 Hz, 2H, 3′-H), 1.21 (m, 2 H, 4′-H), 1.15–1.37 (m, 5′-H, 6′-H, 7′-H, … 15′-H or 17′H, for mayolene-16 or mayolene-18, respectively), 0.92 (t, 3 H, J = 7.2 Hz, 16′-H or 18′-H for mayolene-16 or mayolene-18, respectively).
Absolute Configuration of the Mayolenes.
A sample of approximately 200 μg (≈0.4 μmol) of fresh secretion was dissolved in a methanolic solution of potassium hydroxide (300 μl, 2 M) and stirred for 23 h at 45°C. The reaction mixture was cooled to 25°C and concentrated in vacuo. The residue was dissolved in sodium acetate buffer (1.0 ml, 2 M, pH 5.5) and microextracted with pentane. The aqueous phase was saturated with sodium chloride and further extracted with pentane. Organic extracts were passed through a pipette column of anhydrous sodium sulfate and subsequently evaporated in vacuo. Analysis of the residue by 1H-NMR spectroscopy showed it to correspond to 11-hydroxyoctadeca-9(Z),12(Z),15(Z)-trienoic acid. The sample of 11-hydroxydecatrienoic acid thus obtained was dissolved in ether (50 μl) and pentane (100 μl) and treated with a fresh solution of diazomethane in ether/pentane (1:25). After stirring for 15 min at 25°C, the solution was concentrated in vacuo. Analysis of the residue by 1H-NMR spectroscopy showed it to correspond to 11-hydroxyoctadeca-9(Z),12(Z),15(Z)-trienoic acid methyl ester (4). Subsequently, the sample of trienoic acid methyl ester 4 was dissolved in methylene chloride (100 μl) and cooled to 0°C. The solution was treated with a solution of (−)-chloromenthoxydiphenylsilane (6.1 mg, 16.2 μmol) in methylene chloride (100 μl) followed by the addition of a solution of 4-dimethylaminopyridine (1.9 mg, 16.2 μmol) in methylene chloride (100 μl). After stirring the resulting mixture for 5 min at 0°C, the reaction was quenched by the drop-wise addition of methanol (750 μl). The solution was warmed to 25°C and concentrated in vacuo. Chromatography of the residue on a silica gel column (6 cm × 0.7 cm) eluting with 2.5% ethyl acetate in hexane, afforded methyl (11R)-11-[{(1′S,2′R,5′S)-(+)-menthoxydiphenylsilyl}oxy]octadeca-9(Z),12(Z),15(Z)-trienoate (5) as determined by comparison of its 1H-NMR spectrum with that of synthetic reference compounds. For experimental details for the synthesis of reference compounds see Weibel et al. (4).
Results
The Glandular Hairs.
As evidenced by Fig. 2A, the secretion withstands the high vacuum that is the concomitant of scanning electronmicroscopic viewing. The electronmicrographs also provide a possible explanation for why the droplets do not slide down the length of the hairs as they are being formed. The tip of each hair, where the secretion oozes forth, is not pointed, but is expanded and elaborately sculpted (Fig. 2B, arrow; Fig. 2C). The elaboration may allow the secretion to be held in place as the droplets build up.
Bioassay 1.
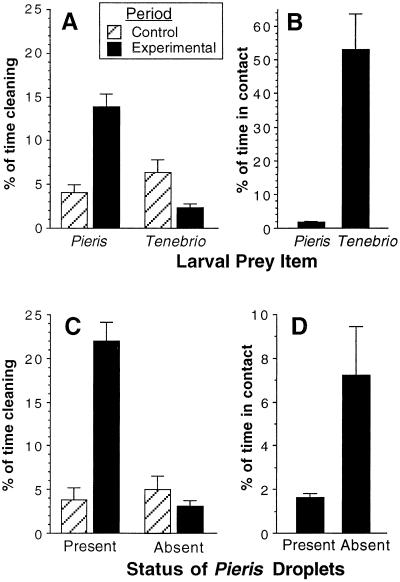
As is clear from Fig. 3A, exposure of the ant to the P. rapae larva resulted in a sharp increase in the time the ant spent cleansing, an effect that we attribute to the ant having made contact with the larva and therefore inevitably with the secretion. Total time of contact with the larva was short (Fig. 3B), indicating that the ant was promptly deterred on contact and never persisted in its assault.
Figure 3.
Bioassay 1: Predation behavior of Crematogaster ant vis à vis P. rapae larvae and mealworms (Tenebrio larvae). (A) Exposure to the P. rapae larva results in increased self-cleaning on the part of the ant (Wilcoxon signed rank, Z = −3.413, P = 0.0006*), whereas exposure to the mealworm results in decreased cleansing (Wilcoxon signed rank, Z = −2.844, P = 0.0045*). (B) The ant makes but brief contact with the P. rapae larva, but extensive contact with the mealworm (Mann–Whitney U, Z = −4.824, P < 0 0001*). For A and B, n =16 in all cases. Bioassay 2: Predation behavior of Crematogaster ant vis à vis P. rapae larvae with intact or experimentally removed secretory droplets. (C) Exposure to the former larvae results in increased cleansing (Wilcoxon signed rank, Z = −2.845, P = 0.0044*), whereas exposure to the latter has no such effect (Wilcoxon signed rank, Z = −0.968, P = 0.33). (D) The ant makes brief contact with the secretion-bearing larvae, but substantially more extensive contact with the secretion-free larvae (Mann–Whitney U, Z = −3.447, P = 0.0006*). For C and D, n = 11 in all cases. P values accompanied by an asterisk are significant at an experiment-wide α = 0.05, as determined by the sequential Bonferonni method. Data are presented as mean ± 1 SE.
In contrast, exposure to the mealworm resulted in a decrease in the amount of cleansing undertaken by the ant (Fig. 3A), indicating that the ant is not negatively affected by contact with the mealworm. Indeed, when confined with the mealworm, the ant actually spent upward of 50% of its time in contact with the mealworm (Fig. 3B).
All experimental P. rapae larvae and ants were live when examined after 48 h, as were their controls. However, whereas all control mealworms were live after 48 h, 10 of the 16 experimental mealworms did not survive (Gadj = 17.58, P < 0.0001); we presume the non-survivors to have been injured by the ants. Survival of ants at 48 h was unrelated to whether they had contacted a P. rapae larva (all 16 alive), a mealworm (15 of 16 alive), or were maintained as controls without contact (all 16 alive; Gadj = 1.34, P = 0.51).
Bioassay 2.
Whereas exposure to secretion-bearing larvae resulted in increased cleansing on the part of the ant, exposure to secretion-free larvae had no such effect (Fig. 3C). Moreover, whereas the ant made but brief contact with the secretion-bearing larvae, it made more persistent contact with the secretion-free larvae (Fig. 3D). All ants were live after 48 h.
The ants' increased contact with the secretion-free larvae does not appear to be a consequence of the dichloromethane itself rendering larvae more attractive, given that the percentage of ant's time spent in contact with dichloromethane-rinsed mealworms (18.7 ± 2.6%) and unrinsed mealworms (25.0 ± 4.0%) did not differ (Mann–Whitney U,2 = −1.103, P = 0.27, n = 14 in both cases).
Bioassay 3.
C. lineolata colonies removed at least one egg per pair in 15 of 16 trials. The ants removed control eggs much more frequently than eggs treated with the mixture of the two mayolenes (Gadj = 34.5, P < 0.001): in 14 trials the control egg alone was removed, whereas in one trial both eggs were removed. In all 15 trials, the treated egg persisted longer than the control egg (sign test, P < 0.001). Mean removal time was 44 ± 2 min (n = 15) and 57 min (n = 1) for the control and treated eggs, respectively.
Chemical Analyses.
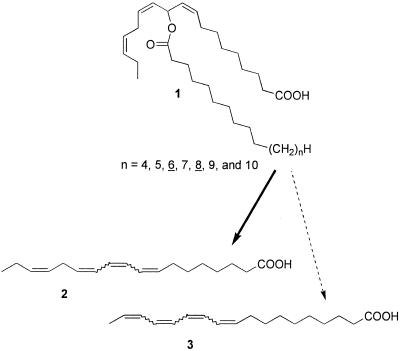
To obtain an overview of the secretion's composition, we started our investigations with direct NMR-spectroscopic analyses of the crude, unfractionated natural samples. A standard set of two-dimensional NMR experiments established that the secretion is composed chiefly of one or more O-acylated derivatives of 11-hydroxylinolenic acid, whereby the acyl groups appeared to be derived from simple straight-chained saturated fatty acids. To determine the chain length of these saturated fatty acids, and correspondingly, the molecular weight of the mayolenes, we proceeded to analyze the secretion by positive-ion electrospray mass spectrometry (ESI-MS). As can be expected from the doubly allylic nature of the ester moiety in these compounds, the mayolenes proved to be highly sensitive to acidic conditions as well as to elevated temperatures, which greatly complicated their mass-spectroscopic analyses. Only under carefully chosen conditions by using ammonia as the ionizing agent were we able to obtain spectra showing molecular ions or the corresponding ammonia adducts. The ESI-MS analyses indicated that the secretion contains a series of homologues (the “mayolenes”, 1) corresponding to 11-hydroxylinolenic acid esterified with straight chain saturated fatty acids of 14 to 20 carbon atoms, dominated by two compounds containing hexadecanoyl (“mayolene-16”) and octadecanoyl (“mayolene-18”) groups (Fig. 4). These two components made up about 80% of the total secretion. These results were corroborated by gas chromatographic analyses of secretion that had been subjected to alkaline hydrolysis and subsequent methylation.
Figure 4.
Structure of the mayolenes (1), and structures of tetra-unsaturated fatty acids 2 and 3 resulting from intramolecular elimination in the mayolenes.
Regarding the chemical stability of the mayolenes at room temperature, we found that, in the absence of acidic contamination, the mayolenes decompose slowly into a mixture of tetra-unsaturated fatty acids, which result from 1,4-elimination of the carboxylic acid ester. Under weakly acidic conditions, the elimination proceeds much more rapidly, and, below pH 4, the mayolenes (1) decompose almost instantaneously. Among the decomposition products, stereoisomers of 8,10,12,15-octadecatetraenoic acid (2) are most abundant. Interestingly, only traces of the entirely conjugated 9,11,13,15-octadecatetraenoic acid (3), which would result from the alternative direction of 1,4-elimination, are formed (Fig. 4). As could be expected considering the thermal sensitivity of these compounds, direct gas chromatographic analyses of the crude secretion failed, because of on-column decomposition. Accordingly, chiral gas chromatography could not be used to determine the absolute configuration of the mayolenes. We therefore pursued a different approach.
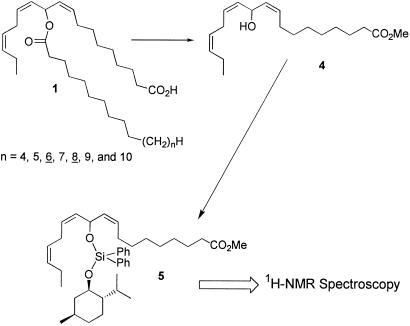
To determine the absolute configuration of the C-11 chiral center in the mayolenes (1), we carried out a hydrolysis of the ester moiety to give the corresponding linear secondary alcohol, with the intention of treating this alcohol with a chiral derivatizing agent such as MTPA chloride (5, 6), providing a product that could then be compared with synthetically accessible reference compounds. Hydrolysis of the ester in (1) with methanolic potassium hydroxide under carefully optimized conditions, followed by methylation of the resulting carboxylic acid, yielded 11-hydroxyoctadeca-9(Z),12(Z),15(Z)-trienoic acid methyl ester (4), containing a bis-allylic hydroxyl group (Fig. 5). Not surprisingly, the alcohol 4 also undergoes elimination easily, suggesting that ester-based chiral derivatizing reagents, such as MTPA chloride, cannot be used for the determination of its absolute stereochemistry. In fact, treatment of the alcohol 4 with MTPA chloride under various conditions consistently yielded a mixture of elimination products instead of the desired MTPA-derivative. To circumvent this problem, we developed a new group of chiral silylation reagents, which can be used for the determination of absolute configuration and enantiomeric excess of labile chiral alcohols such as 4 via NMR spectroscopy (7). Derivatization of 11-hydroxyoctadeca-9(Z),12(Z),15(Z)-trienoic acid methyl ester with (−)-chloromenthoxydiphenylsilane as described in (6) provided the corresponding (−)-menthoxydiphenylsilyl ether (5) (Fig. 5). Reference samples of optically pure (11R)-5 and (11S)-5 were synthesized from (2R)-glyceraldehyde acetonide (4).
Figure 5.
Derivatization of the natural mixture of mayolenes (1).
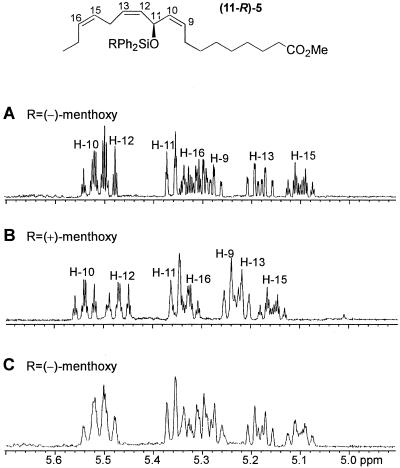
To determine the absolute configuration of the chiral center in the mayolenes, the 1H-NMR spectrum of the sample of 5 prepared from the natural material was compared with the spectra of the synthetic samples of (11R)-5 and (11S)-5 (Fig. 6). As shown in Fig. 6C, the sample of methyl 11-[{(1′S,2′R,5′S)-(−)-menthoxydiphenylsilyl}oxy]octadeca-9(Z),12(Z),15(Z)-trienoate (5) derived from the natural material comprises a single enantiomer, which displays a 1H-NMR spectrum identical to that obtained for (11R)-5 (Fig. 6A). Therefore, the configuration at C-11 in 1 is established as (11R).
Figure 6.
Assignment of absolute configuration for the chiral center at C-11 in the mayolenes. Characteristic region of the 1H-NMR spectra (500 MHz, C6D6) of (A) (11R)-methyl-11-[{(1′S,2′R,5′S)-(−)-menthoxydiphenylsilyl}oxy]octadeca-9(Z),12(Z),15(Z)-trienoate, (B) (11S)-methyl-11-[{(1′S,2′R,5′S)-(−)-menthoxydiphenylsilyl}oxy] octadeca-9(Z),12(Z),15(Z)-trienoate, and (C) methyl-11-[{(1′S,2′R,5′S)-(−)-menthoxydiphenylsilyl}oxy]octadeca-9(Z),12(Z),15(Z)-trienoate derived from hydrolysis and derivatization of natural material.
Discussion
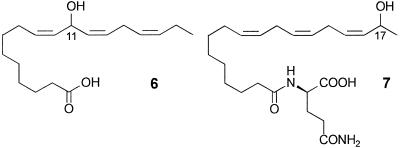
It seems established that the secretion of the glandular hairs of larval P. rapae is defensive and that its principal components are the mayolenes, i.e., acyl derivatives of 11-hydroxylinolenic acid (6) (Fig. 7). The secretion could clearly be effective against arthropods other than ants, including such enemies as wasps, bugs, beetles, spiders, harvestmen, and parasitoids.
Figure 7.
Structures of 11-hydroxylinolenic acid (6) and volicitin (7).
Other hydroxylated derivatives of linolenic acid play an important role in the regulation of chemical defense mechanisms in plants via the jasmonate pathway (8). It should be noted, however, that, in case of the mayolenes, the linolenic acid is hydroxylated at one of the bis-allylic methylene groups, without change in double bond position and geometry, which is different from the hydroxylation pattern generally associated with the jasmonate pathway. Surprisingly, hydroxylated derivatives of unsaturated fatty acids of this particular kind have only rarely been reported from animal sources. One prominent example is volicitin, 7, which has been identified from the regurgitate of Spodoptera exigua caterpillars. Volicitin, a derivative of 17-hydroxylinolenic acid, appears to act as an elicitor for the production of plant volatiles via the jasmonate pathway (8).
As in the case of volicitin, the determination of the absolute configuration at the allylic carbon in the mayolenes could not be achieved by using established methods such as chiral gas chromatography or comparison of suitable diastereomeric derivatives, because of the fact that 11- as well as 17-hydroxylinolenic acid derivatives are highly susceptible to elimination (9). The determination of the absolute configuration as described here represents the first application of a novel derivatization technique involving a chiral silylating agent, which we specifically developed for labile allylic alcohols such as 11-hydroxylinolenic acid, 2, or 17-hydroxylinolenic acid. The absolute configuration of volicitin, 7, has recently been determined by using a different approach based on HPLC separation of carbamoyl derivatives (9).
It should be noted that even very common fatty acids such as linolenic acid or oleic acid are quite effective as insect repellents (10). Therefore, the repellency of the mayolenes seems not surprising, given that these compounds simply represent O-acylated derivatives of 11-hydroxylinolenic acid. It is beyond the scope of this paper to compare quantitatively the repellency of the mayolenes with that of their building blocks, linolenic acid or stearic acid. It seems likely, however, that the high tendency to eliminate under formation of a highly conjugated tetra-unsaturated acid might play an important role in relation to the biological activity of the mayolenes.
Glandular hairs are a common defensive feature of plants (11–13), where they have been shown to be effectively protective against insects (14). They are of less frequent occurrence in insects, but the chemistry of the secretion of insectan hairs, to the extent that it is known, can be of interest. Thus, for instance, the pupal hairs of certain coccinellid beetles have been shown to produce, in one species, a series of azamacrolides, and in others a combinatorial mixture of macrocyclic polyamines (15–17). The present discovery of yet another novel group of secondary products from the glandular hairs of an insect suggests that the study of such defensive materials can be rewarding.
Glandular hairs, while present in some lepidopteran larvae (2), are not of general occurrence in caterpillars. Caterpillars are, however, variously protected chemically. Thus, many discharge enteric fluid, both fore and aft, when disturbed (18), whereas others have compressible glands from which they spray aimed jets of irritant fluid (containing, for instance, formic acid) when assaulted (19). Still others derive protection from eversible glandular structures (18), or from plant secondary metabolites such as cardenolides or pyrrolizidine alkaloids that they sequester from their food plants (18, 20). P. rapae might itself benefit from systemic sequestration of glucosinolate from is larval diet (21).
Acknowledgments
We named mayolenes in honor of May Berenbaum, whose visionary research, lectures, and writings on insects have helped spur entomophilia the world over. We thank Janice Schlesinger for rearing the insects, Maria Eisner for help with photography, Janis Strope for help with the manuscript, and Stefan Cover for identification of the ant. This study was supported by Grants AI02908 (T.E.) and GM 53850 (J.M.) from the National Institutes of Health, the Eppley Foundation for Research (S.R.S.), Trinity College Faculty Research Committee (S.R.S.), and a Pfizer Summer Undergraduate Research Fellowship (K.A.L.).
Abbreviation
- ESI
electrospray ionization
Footnotes
This is paper no. 183 from the series, Defense Mechanisms of Arthropods. Paper no. 182 is ref. 22.
References
- 1.Opler P A, Krizek G O. Butterflies East of the Great Plains. Baltimore: Johns Hopkins Univ. Press; 1984. [Google Scholar]
- 2.Newman E. An Illustrated Natural History of British Butterflies. London: W. Tweedie; 1871. [Google Scholar]
- 3.Griesinger C, Sorensen O W, Ernst R R. J Magn Reson. 1987;75:474–492. [Google Scholar]
- 4. Weibel, D. B., Shevy, L. E., Schroeder, F. C. & Meinwald, J. (2002) J. Org. Chem., in press. [DOI] [PubMed]
- 5.Dale J A, Dull D L, Mosher H S. J Org Chem. 1969;34:2543–2549. [Google Scholar]
- 6.Dale J A, Mosher H S. J Am Chem Soc. 1973;95:512–519. [Google Scholar]
- 7.Weibel D B, Walker T R, Schroeder F C, Meinwald J. Org Lett. 2000;2:2381–2383. doi: 10.1021/ol006162h. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder F. Angew Chem Int Ed. 1998;37:1213–1216. doi: 10.1002/(SICI)1521-3773(19980518)37:9<1213::AID-ANIE1213>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Spiteller D, Pohnert G, Boland W. Tetrahedron. 2001;42:1483–1485. [Google Scholar]
- 10.Eisner T, Attygalle A B, Conner W E, Eisner M, MacLeod E, Meinwald J. Proc Natl Acad Sci USA. 1996;93:3280–3283. doi: 10.1073/pnas.93.8.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahn A. Secretory Tissues in Plants. New York: Academic; 1979. [Google Scholar]
- 12.Rodriguez E, Healey P L, Mehta I. Biology and Chemistry of Plant Trichomes. New York: Plenum; 1984. [Google Scholar]
- 13.Levin D A. Q Rev Biol. 1973;48:3–15. [Google Scholar]
- 14.Goffreda J C, Mutscher M A, Tingey W M. Entomol Exp Appl. 1988;48:101–107. [Google Scholar]
- 15.Attygalle A B, McCormick K D, Blankespoor C L, Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1993;90:5204–5208. doi: 10.1073/pnas.90.11.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder F C, Farmer J J, Smedley S R, Attygalle A B, Eisner T, Meinwald J. J Am Chem Soc. 2000;122:3628–3634. [Google Scholar]
- 17.Schroeder F C, Smedley S R, Gibbons L K, Farmer J J, Attygalle A B, Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1998;95:13387–13391. doi: 10.1073/pnas.95.23.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brower L P. The Biology of Butterflies, Symposium of the Royal Entomological Society London No. 11. London: Academic; 1984. pp. 109–133. [Google Scholar]
- 19.Attygalle A B, Smedley S R, Meinwald J, Eisner T. J Chem Ecol. 1993;19:2089–2104. doi: 10.1007/BF00979649. [DOI] [PubMed] [Google Scholar]
- 20.Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1995;92:50–55. doi: 10.1073/pnas.92.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aplin R T, d'Arcy Ward R, Rothschild M. J Entomol. 1975;50:73–78. [Google Scholar]
- 22.Eisner T, Yack J, Aneshansley D J. Chemoecology. 2001;11:221–223. [Google Scholar]