Abstract
The aerobic respiratory chain is vital to bacterial and eukaryotic cell energy transformation. Embedded in the mitochondrial inner membrane and the bacterial plasma membrane, the respiratory chain couples sequential redox reactions with ion pumping, thereby generating the motive force that is used to drive ATP synthesis. Due to the essential role of oxidative phosphorylation in cellular life, the electron transport chain proteins, their cofactors, and ATP synthase components serve as a target for antibacterial, antifungal, and antiparasitic drugs. Whether by (1) inhibition of electron flow through transport chain complexes, (2) collapsing of the motive force, (3) competitive inhibition, or (4) blocking proton flow through the catalytic subunits of ATP synthase, small molecules can selectively inhibit bacterial, fungal, and parasitic life while not showing high toxicity in mammalian systems. Because of robust antimicrobial resistance against the traditional mechanisms of microbial control (cell wall integrity, protein synthesis, nucleotide and nucleic acid synthesis, etc.), the study of alternative targets, such as the respiratory chain, is prudent and timely. This review summarizes the current research on small molecule and peptide inhibition of the aerobic respiratory chain complexes, electron flow, and ion translocation in a series of human and plant pathogens.
Keywords: Drug development, bedaquiline, respiratory chain, atovaquone, pathogen metabolism
Introduction
The mitochondrial respiratory chain and its role in cell energetics
The respiratory chain, also known as the electron transport chain, plays a crucial role in aerobic organisms by serving as the final stage of cellular respiration, supporting the majority of ATP production. This metabolic pathway is ubiquitous, consisting of a series of protein complexes and electron carriers that transfer electrons supplied by NADH, succinate, UQH2, and other sources that reduce flavoenzymes (FADH2/FMNH2), which are derived from nutrients like glucose, amino acids, and lipids (Lehninger 1971). As electrons move through the chain and are delivered to the final electron acceptor(s), energy is released in the form of Δp, which is used to synthesize ATP. In eukaryotes, the respiratory chain is located in the inner mitochondrial membrane and consists of four multi-subunit redox protein complexes (I-IV) and two mobile electron carriers: ubiquinone and cytochrome c (Nicholls and Ferguson 2002; Wikström 2005) (Figure 1A). These components work concertedly to oxidize NADH and other electron carriers generated during glycolytic, tricarboxylic acid cycle and fatty acid metabolism, via flavoenzymes, and reduce molecular oxygen. This reaction represents a physiological step that distinguishes aerobic respiration from anaerobic counterparts. As electrons flow through the transport chain, protons are pumped from the mitochondrial matrix into the intermembrane space (Nicholls and Ferguson 2002; Wikström 2005). The coupling of these reactions thereby creates an electrochemical gradient that drives ATP synthesis via complex V, completing the process of oxidative phosphorylation (Mitchell 2011).
Figure 1.

Human (A), bacterial (B) and parasitic/fungal (C) respiratory chains.
The mitochondrial respiratory complexes play distinct roles in electron transport chain physiology. Complex I (NADH:ubiquinone oxidoreductase) transfers electrons from NADH to ubiquinone, and pumps protons from the matrix to the intermembrane space, with a H+/e− stoichiometry of 2:1. Complex II (succinate: ubiquinone oxidoreductase) or succinate dehydrogenase (SDH), forms part of the Krebs cycle and the respiratory chain, and oxidizes succinate to fumarate while delivering electrons to ubiquinone. Complex III (ubiquinol:cytochrome c oxidoreductase) or cytochrome bc1 complex, oxidizes ubiquinol to ubiquinone and transfers electrons to cytochrome c, translocating protons from the matrix to the intermembrane space, with a H+/e− of 2:1. Complex IV (cytochrome c oxidase) oxidizes cytochrome c, reduces molecular oxygen to water, and pumps protons from the matrix to the intermembrane space, with a H+/e− ratio of 1:1. Complex V (ATP synthase) facilitates the movement of protons along their concentration gradient back into the matrix through its two ion hemichannels, utilizing the energy released by the process to synthesize ATP from ADP and inorganic phosphate (Roberts GCK 2013; Cook et al. 2014). These five complexes work together to establish the proton motive force across the inner mitochondrial membrane, whose energy is harnessed and transduced to more usable cellular energy currency in the form of ATP.
The bacterial respiratory chain
The bacterial respiratory chain is located in the cell membrane and differs from the relatively simple mammalian respiratory chain, as it is able to obtain electrons through various routes due to its modular and branched architecture (Richardson 2000; Cook et al. 2014; Kaila and Wikström 2021) (Figure 1B). Branched respiratory chains containing “alternative” complexes provide flexibility to use respiratory components in different patterns, allowing them to thrive in diverse niches and under adverse conditions (Roberts GCK 2013; Cook et al. 2014; Kaila and Wikström 2021). For instance, many types of microorganisms can readily switch from aerobic to anaerobic environments by expressing terminal oxidases which have adapted to use electron acceptors other than oxygen, like nitrate or fumarate (Unden and Bongaerts 1997; Richardson 2000). Bacteria share the same, albeit structurally different, respiratory enzymes with mitochondria, including complexes I-IV. Bacterial complex I comprises 13–14 subunits, compared to >45 in mitochondria (Yagi 1991; Schägger 2002; Nolfi-Donegan et al. 2020). On the other hand, SDH is highly similar to mitochondrial complex II (Hägerhäll 1997; Schägger 2002; Nolfi-Donegan et al. 2020). Complex III is composed of only 3–4 subunits in comparison with its eukaryotic counterpart, which comprises 8–11 subunits (Schäfer and Penefsky 2008; Xia et al. 2013; Nolfi-Donegan et al. 2020). Aa3-type cytochrome c oxidases are found in the mitochondrial (Complex IV) and bacterial respiratory chains (Schägger 2002; Nolfi-Donegan et al. 2020; Borisov et al. 2021). The respiratory chain is coupled to ATP production via F-ATPases (Complex V), found in all domains of life (Schäfer and Penefsky 2008; Cook et al. 2014; Ahmad M et al. 2023) and conserved between aerobic and anaerobic organisms. The mitochondrial complex V contains non-catalytic supernumerary subunits, absent in bacterial counterparts (Vaillier et al. 1999; Lee J et al. 2015).
In addition to these “core” enzymes, bacterial respiratory chains contain accessory enzymes, which at first sight appear redundant, but play essential roles in different conditions. In addition to complex I, bacteria contain two other NADH dehydrogenases that transfer electrons from NADH to ubiquinone, including NDH-2 and NQR (Yagi 1991). NDH-2 is a ubiquitous single subunit enzyme that lacks proton pumping activity and contains FAD as the sole cofactor (Grandier-Vazeille et al. 2001; Melo et al. 2004; Saleh et al. 2007; Marreiros et al. 2016). NQR is a six subunit respiratory complex found mostly in proteobacteria, Chlamydiae, Chlorobi and Bacteroides (Reyes-Prieto et al. 2014). This is the only respiratory complex that is able to pump sodium, using a unique ion transport mechanism, which drives processes related to homeostasis and is also involved in pathogenesis (Juárez et al. 2010). In addition to several NADH dehydrogenases, the bacterial respiratory chains use a wide variety of other dehydrogenases that directly feed the respiratory chain, allowing the operation of a flexible metabolism. These dehydrogenases include malate, ethanol, glucose, and lactate, among others (Liang P et al. 2020; González-Montalvo et al. 2024). In contrast with eukaryotes, which have Complex IV as the only terminal oxidase, bacteria have a wider array of terminal oxidases that support aerobic metabolism (Richardson 2000,Nolfi-Donegan et al. 2020; Roberts GCK 2013; Kaila and Wikström 2021). Bacteria carry several types of heme-copper oxidases that differ from the eukaryotic Complex IV in structure, subunit composition, electron donors, and stoichiometric reactions. These oxidases include aa3 and ccb3 cytochrome c oxidases, as well as bo3 ubiquinol oxidases (Roberts GCK 2013; Borisov et al. 2021; Kaila and Wikström 2021; Siletsky and Borisov 2021). Furthermore, bacteria also carry non-copper bd-type ubiquinol oxidases (Roberts GCK 2013; Borisov et al. 2021; Kaila and Wikström 2021; Siletsky and Borisov 2021). In addition to the respiratory enzymes, the quinone carrier preference in bacteria differs from eukaryotes. While mitochondria exclusively use ubiquinone, anaerobic and microaerophilic bacteria use menaquinone (Søballe and Poole 1999; Franza and Gaudu 2022). The great flexibility in the components of bacterial respiratory chains allows quick adaptation and metabolic switching depending on the growth conditions or environmental cues, making bacteria highly adaptable. Due to this complexity, the study of the respiratory chain is critical, as several of its components are emerging as potential targets for drug development.
Fungal and parasitic respiratory chains
Fungal and several types of eukaryotes, including parasites, carry the core components described in the mammalian respiratory chain, complexes I-V. In addition, these eukaryotes contain accessory (alternative) respiratory complexes (Figure 1C), including NDH-2, which can be internal (NDH-2i), facing the mitochondrial matrix, or external (NDH-2e), facing the periplasmic space (Kerscher et al. 2008). Moreover, these organisms also carry an alternative oxidase, AOX, which bypasses complexes III and IV, and transfers electrons directly from ubiquinol to oxygen (Elthon and McIntosh 1987; Juárez et al. 2006), similar to bo3 and bd oxidases, but without pumping protons in the process. These complexes appear to be important for pathogenicity in some species, offering flexibility to the respiratory chain that allows for redox pool regulation and operation of the respiratory chain under stress conditions (Kerscher et al. 2008; Moore et al. 2013; Shiba et al. 2013).
The respiratory chain as a target for antibiotic development
Antimicrobial resistance (AMR) is among the most pressing public health challenges of our time. It causes approximately 1.7 million deaths annually and is a contributing factor in nearly 5 million deaths worldwide (Murray et al. 2022; Naghavi et al. 2024), with associated economic costs of billions of dollars per year. It is projected that by 2050, AMR will produce 10 million deaths yearly (Naghavi et al. 2024; O’Neill 2016), with one trillion dollars in additional healthcare costs in the US alone (Jonas et al. 2017; Dadgostar 2019). The extensive use of antibiotics has produced the emergence of multidrug-resistant (MDR) strains for most human pathogens (Putman et al. 2000; Alanis 2005; Davies and Davies 2010; Andersson and Hughes 2011), which greatly complicates the treatment of infectious diseases. AMR resistance now includes all classes of commercially available antibiotics (Alanis 2005; Hurdle et al. 2011; Avner et al. 2012), and will likely expand to newer generations of antibiotics, since in most cases they are directed against the same molecular targets and are based on the same general structures (Shahid et al. 2009; Sykes 2010; Bassetti et al. 2013; Bassetti and Righi 2015). The overexploitation of certain antibiotic scaffolds and lack of new suitable targets pose a major challenge in drug discovery (Overington et al. 2006; Hurdle et al. 2011; Lewis 2013; Santos and Torres 2013). Current classes of antibiotics, to which pathogens have already acquired resistance, all target the same biological processes: DNA replication, transcription, protein biosynthesis, and cell wall synthesis. Therefore, it is crucial to identify novel molecular targets for the development of new classes of antibiotics against essential cellular processes for innovative antimicrobial treatments.
Bacterial energy metabolism, and in particular pathogenic respiratory chains, represent interesting molecular targets for the development of new antibiotics. The respiratory chains of pathogenic organisms play essential roles in cell physiology, sustaining the entire metabolic network and all transport activities throughout the membrane, which are crucial to maintaining homeostatic processes and growth. Notably, pathogenic respiratory chains are critical in disease pathogenesis, making them highly attractive pharmacologic targets. Recently, the respiratory chain has been the target for novel antimicrobials against different bacteria. For instance, targeting the respiratory chain has been successful for developing new antibiotics against Mycobacterium tuberculosis, which produces one of the deadliest infections in the world. Tuberculosis treatment is complex, spanning 6–9 months with multiple antibiotics, and can exceed two years in MDR cases (CDC 2024). M. tuberculosis is notorious for producing dormant forms that cause persistent infections and treatment failure. However, recent developments show that inhibition of the M. tuberculosis respiratory chain represents a successful pathway for infection elimination (Sindhu and Debnath 2022). M. tuberculosis ATP synthase (Mahajan 2013), NDH-2, and Complex III are predicted to be essential for optimal growth in this pathogen, and are being pursued as drug targets (Pethe et al. 2013; Sellamuthu et al. 2017; Beites et al. 2019; Kim J et al. 2022; Capela et al. 2023). Thus, understanding the operation of bacterial, fungal, and parasitic respiratory chains, and how to disrupt them, can provide critical insights for the development of novel and more effective antimicrobials. By targeting specific components and mechanisms within these respiratory chains, compounds that are more selective, stable, and less prone to resistance can be selected or synthesized, ultimately enhancing their therapeutic potential. This work describes recent advances in the development of new antibiotics, antifungals and antiparasitics that target the respiratory chain and ATP synthase, as well as membrane-based bioenergetics.
Respiratory enzymes as metabolic targets for antibiotic, antifungal, and antiparasitic development
NDH-2
The non-proton pumping Type II NADH: ubiquinone oxidoreductases (NDH-2) comprise a group of five different isotypes (A-D) (Marreiros et al. 2016) that are found in all domains of life, but are absent in mammals (Melo et al. 2004). NDH-2s are widely distributed among bacteria and serve as the primary NADH dehydrogenase in several pathogens including Staphylococcus aureus, Streptococcus agalactiae, M. tuberculosis, M. leprae, Klebsiella aerogenes, Escherichia coli, and Listeria monocytogenes (Schurig-Briccio et al. 2014; Yano et al. 2014; Lencina et al. 2018; Beites et al. 2019; Smith et al. 2023; González-Montalvo et al. 2024). NDH-2 regulates the NADH/NAD+ ratio by uncoupling NADH oxidation from the generation of the ion gradient, as this enzyme lacks H+ pumping activity (Gyan et al. 2006; Vamshi Krishna and Venkata Mohan 2019). Since NDH-2 is not inhibited by ionic gradients, it allows for an increase in biosynthetic pathway involvement and ATP synthesis while simultaneously reducing ROS formation (Cook et al. 2014). NDH-2 is a 40–70 kDa single subunit (Heikal et al. 2014; Nakatani et al. 2017) homodimeric protein (Heikal et al. 2014; Blaza et al. 2017; Nakatani et al. 2017) that belongs to the two-dinucleotide binding domains flavoprotein superfamily (Ojha et al. 2007; Marreiros et al. 2017), which has two Rossmann folds (Blaza et al. 2017; Marreiros et al. 2017; Nakatani et al. 2017) forming the non-covalently bound FAD cofactor site and the NADH/NADPH binding site (Blaza et al. 2017; Marreiros et al. 2017) (Figure 2A,B). In bacteria, NDH-2 is a monotopic enzyme bound to the cytoplasmic side of the innermost membrane by two amphipathic helices (Marreiros et al. 2017; Nakatani et al. 2017). In fungi and parasites, NDH-2 can be found on the external or internal sides of the mitochondrial inner membrane (Feng Y et al. 2012; Heikal et al. 2014).
Figure 2.

Structure of NDH-2 accessed from PDB ID: 6BDO (Berman et al. 2000; Petri et al. 2018). (A) Tri-dimensional structure with inhibitor HQNO and cofactor FAD shown as spheres, light gray: FAD, dark gray: HQNO. (B) Close view of FAD binding. (C) Close view of HQNO binding. (D) Two-dimensional structure of NDH-2 inhibitors.
NDH-2 dehydrogenases are suitable drug targets due to their absence in human mitochondria. Indeed, several compounds have been identified as NDH-2 inhibitors (Cook et al. 2014; Heikal et al. 2014), such as phenothiazines, quinolinyl pyrimidines (Shirude et al. 2012), and quinoline quinones (Heikal et al. 2016) (Figure 2D; Table 1). M. tuberculosis studies show that NDH-2 is essential for growth in laboratory media (Sassetti et al. 2003; Griffin et al. 2011; Nakatani et al. 2020), and cells lacking this protein exhibit reduced virulence in vivo (Beites et al. 2019). Mycobacterial NDH-2 can be effectively inhibited using phenothiazines such as chlorpromazine and trifluoperazine (Amaral et al. 2001; Weinstein et al. 2005; Yano et al. 2006) (Figure 2D). Phenothiazine analogues have shown promising results in a mouse infection model, where it was demonstrated to reduce bacterial load by 90% over the course of an 11-day treatment (Weinstein et al. 2005). Moreover, these molecules are effective in the treatment of MDR-tuberculosis (Sellamuthu et al. 2017). In addition to phenothiazines, phenazines such as clofazimine have also shown efficacy as antibiotics against mycobacteria. Clofazimine, an FDA-approved drug against leprosy, was recently repurposed to treat MDR-tuberculosis, targeting NDH-2 (Lechartier and Cole 2015). The action mechanism of clofazimine is interesting as it is reduced by NDH-2, producing ROS, which dissipate the membrane potential (Feng X et al. 2015) and are bactericidal (Yano et al. 2011).
Table 1.
Reported NDH-2 inhibitors.
| Compound | Microorganism targeted | IC50 | MIC50 | Toxicity |
|---|---|---|---|---|
|
| ||||
| Trifluoperazine | M. tuberculosis | 12 μM (Yano et al. 2006) | MIC 9 μg/mL (Yano et al. 2006) | Hepatotoxic (Long term therapy) (National Institute of Diabetes and Digestive and Kidney Diseases 2012a) |
| Chlorpromazine | M. tuberculosis | 10 μM (Weinstein et al. 2005) | MIC 19 μg/mL (Weinstein et al. 2005) | Hepatotoxic (Long term therapy) (National Institute of Diabetes and Digestive and Kidney Diseases 2012b) |
| Trifluoperazine | S. aureus | 19 μM (Schurig-Briccio et al. 2014) | 70 μM (Schurig-Briccio et al. 2014) | Hepatotoxic (Long term therapy) (National Institute of Diabetes and Digestive and Kidney Diseases 2012a) |
| Thioridazine | S. aureus | 7 μM (Schurig-Briccio et al. 2014) | 70 μM (Schurig-Briccio et al. 2014) | Retinal (Rare), Hepatotoxic (Feinberg et al. 2025) |
| Triclabendazole | S. agalactiae | 0.14 μM (Lencina et al. 2018) | EC50: 15 μM (Lencina et al. 2018) | Rarely shown liver injury, associated with fluke clearing (National Institute of Diabetes and Digestive and Kidney Diseases 2012c) |
| Zafirlukast | S. agalactiae | 0.83 μM (Lencina et al. 2018) | EC50: 6 μM (Lencina et al. 2018) | Hepatotoxic (High dose) (Dhaliwal and Bajaj 2025) |
| Quinlinyl pyrimidine Compound-1 | M. tuberculosis | 0.03 μM (Lu L et al. 2022) | MIC 3 μg/mL (Lu L et al. 2022) | HEPG2 TD50 0.2 μM (Lu L et al. 2022) MRC-5 IC50 1.3 μM (Lu L et al. 2022) |
| Quinlinyl pyrimidine Compound-1 | M. smegmatis | 2 μM (Lu L et al. 2022) | ||
| Quinlinyl pyrimidine Compound-6 | M. tuberculosis | 1 μM (Lu L et al. 2022) | MIC 13 μg/mL (Lu L et al. 2022) | TD50 17 μM (Lu L et al. 2022) |
| Compound 13a | M. tuberculosis | 0.04 μM (Shirude et al. 2012) | MIC 2 μg/mL (Shirude et al. 2012) | |
| Diphenylene iodonium chloride (DPI) | P. falciparum | 16 μM (Biagini et al. 2006) (DB) 25 (CoQ) |
EC50 0.24 μM (Biagini et al. 2006) | Non-specific toxic effects. Acute gastrointestinal |
| Phenyl iodonium chloride (IDP) | P. falciparum | 66 μM (Biagini et al. 2006) (DB) | EC50 6 μM (Biagini et al. 2006) | Mitotoxic via ROS production and apoptosis induction (Li N et al. 2003) |
| HDQ (1-hydroxy-2-alkyl-4(1) quinolone) | T. gondii | EC50 4 nM (Saleh et al. 2007) | ||
| HDQ | P. falciparum | EC50 15 nM (Saleh et al. 2007) | ||
In S. aureus, NDH-2 is the sole NADH dehydrogenase of the respiratory chain (Schurig-Briccio et al. 2014) and a suitable drug target (Zhou J-L et al. 2024). Phenothiazines have been found to be effective inhibitors of S. aureus NDH-2 with strong antibiotic properties (Schurig-Briccio et al. 2014). In S. agalactiae, the elimination of NDH-2 does not affect growth in culture media but it attenuates organ colonization in a mouse systemic model (Lencina et al. 2018). FDA-approved drugs, such as zafirlukast (Figure 2D) and triclabendazole have been shown to inhibit NDH-2 activity and bacterial growth in the micromolar range (Lencina et al. 2018) (Table 1). Additionally, quinolinyl pyrimidines have been proposed as alternative options for phenothiazines, which have significant effects on the central nervous system (Shirude et al. 2012). This family of compounds inhibits NDH-2 and bacterial growth in the sub μM range (Shirude et al. 2012; Lu L et al. 2022) (Table 1). Interestingly, these molecules increase NADH oxidation and ROS overproduction, which is both toxic and starves the respiratory chain of redox equivalents, decreasing ATP synthesis (Heikal et al. 2016). In addition to its essential role in bacterial metabolism, NDH-2 also plays a relevant role in eukaryotic parasites like Plasmodium falciparum (Biagini et al. 2006; Saleh et al. 2007) and Toxoplasma gondii (Saleh et al. 2007), in which this enzyme is the only NADH dehydrogenase. Growth of T. gondii and P. falciparum is inhibited by 1-hydroxy-2-dodecyl-4(1H)quinolone (HDQ), which appears to inhibit NDH-2 (Table 1) (Saleh et al. 2007).
NQR
The proton (Raba et al. 2018)/sodium (Juárez et al. 2009) pumping NADH:ubiquinone oxidoreductase (NQR complex) is an essential membrane-associated protein found in numerous bacterial species (Häse and Mekalanos 1998; Stephens et al. 1998; Verkhovsky and Bogachev 2010; Reyes-Prieto et al. 2014; Tuz et al. 2015; Kamath et al. 2016; Liang P et al. 2018; González-Montalvo et al. 2024). NQR plays a critical role in the bacterial respiratory chain, contributing to energy conservation by generating either a proton or sodium motive force across the membrane (Dimroth 1994; Barquera et al. 2002; Raba et al. 2018). The resulting ion gradient supplies energy for essential cellular processes such as ATP synthesis, flagellar rotation, drug extrusion, and nutrient transport (Kojima et al. 1999; Häse and Barquera 2001; Juárez and Barquera 2012; Reyes-Prieto et al. 2014).
NQR is a transmembrane protein complex composed of six subunits, NqrA-F, and a unique collection of cofactors that facilitate the transfer of electrons through the enzyme (Verkhovsky and Bogachev 2010; Juárez and Barquera 2012; Steuber et al. 2015). NQR contains six redox cofactors: two [2Fe-2S] centers and four flavins (Juárez et al. 2009; 2010) (Figure 3). One of the hallmark features of the NQR family is the use of riboflavin as a redox-active molecule (Barquera et al. 2002; Juárez et al. 2008). NQR, and its close relative ferredoxin: NAD+ oxidoreductase (RNF) (Vitt et al. 2022), are the only enzymes reported that directly use this molecule as a cofactor (Barquera et al. 2002; Juárez et al. 2008). NQR is widely distributed among pathogenic bacteria and parasites, prominently in Gram-negative bacteria like Vibrio cholerae (Häse and Mekalanos 1998), Pseudomonas aeruginosa (Liang P et al. 2020), and Haemophilus influenzae (Hayashi et al. 1996). In V. cholerae, the NQR complex is crucial not only for energy production but also for the regulation of virulence. It influences the transcription of toxT, a key regulator that activates the expression of cholera toxin (ctxAB), toxin-coregulated pilus (tcpA), and accessory colonization factors (Häse and Mekalanos 1999). Elimination of NQR disrupts this regulatory pathway, impairing the pathogen’s ability to express virulence genes and effectively colonize the host. NQR plays a central role in the aerobic respiratory chain of P. aeruginosa, where it serves as the main NADH dehydrogenase. Over 70% of the oxygen consumption linked to NADH dehydrogenase activity in this organism is sensitive to the NQR inhibitor HQNO, demonstrating the dominant role of this enzyme in energy metabolism (Raba et al. 2018). Additionally, for the obligate intracellular parasite Chlamydia trachomatis, NQR plays a vital role during infection and intracellular multiplication (Liang P et al. 2018), underscoring the enzyme’s evolutionary conservation and essentiality across diverse pathogenic organisms (Reyes-Prieto et al. 2014). Given its crucial metabolic role, NQR serves as an attractive target for developing antimicrobial strategies (Dibrov et al. 2017).
Figure 3.
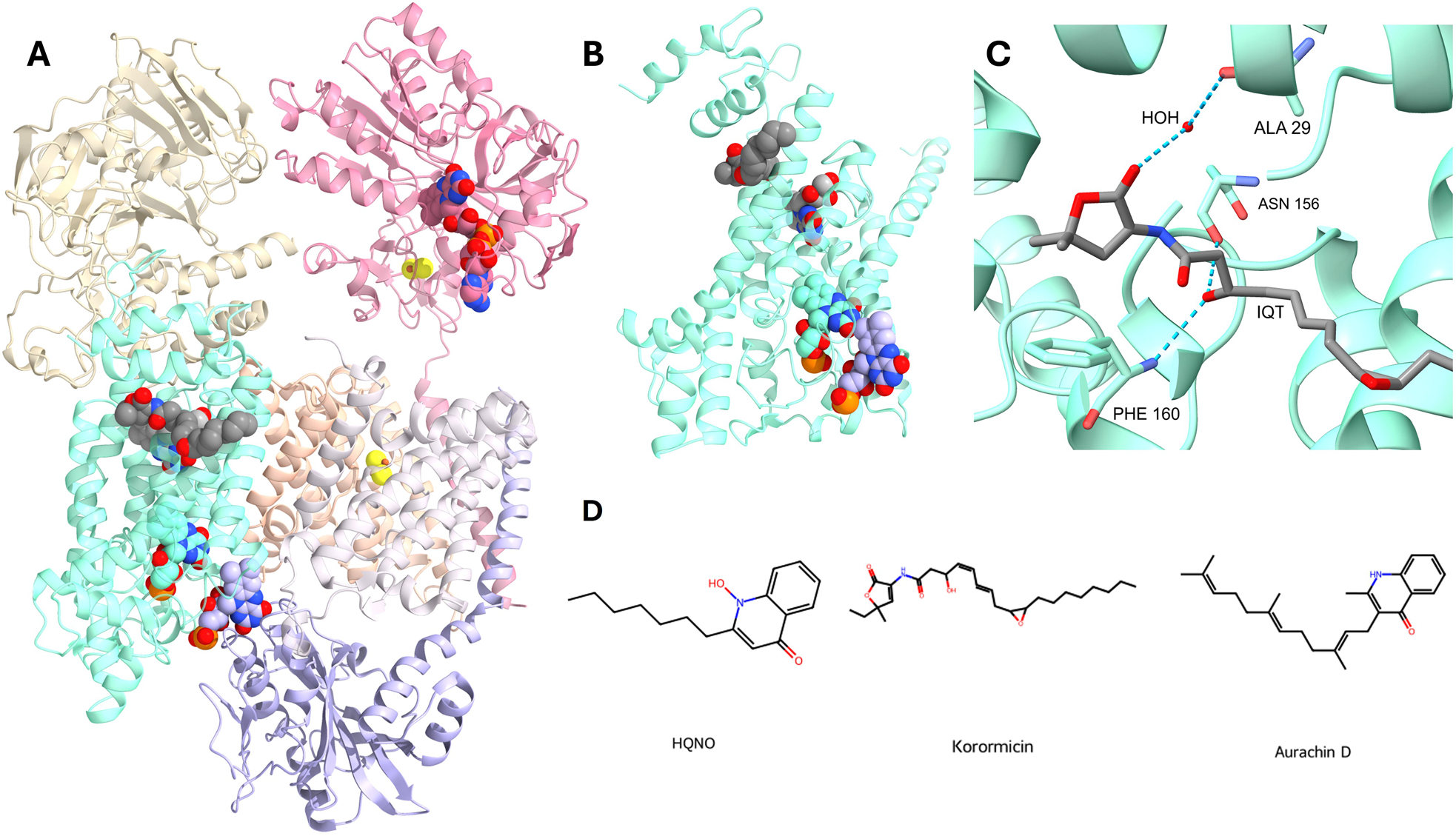
Structure of NQR accessed from PDB ID: 7XK7 (Berman et al. 2000; Kishikawa et al. 2022). (A) Tri-dimensional structure with cofactors/ligands show as spheres. Dark gray: Korormicin, yellow: 2Fe-2S clusters, teal: FMNB, lavender: FMNC, pink: FAD. (B) Close view of tri-dimensional structure of NQR chain B. Dark gray: Korormicin, light gray: Riboflavin, teal: FMNB, lavender: FMNC. (C) Close view of the Koror.
Recent studies highlight NQR as a critical target in pathogens, exemplified by the promising repurposing of FDA-approved drugs. For instance, the antibiotic clofazimine, a phenothiazine derivative, demonstrates potent inhibitory effects against NQR in V. cholerae. Clofazimine targets the catalytic ubiquinone-binding site, inhibiting bacterial growth with an IC50 of approximately 3 μM and significantly reducing production of the cholera toxin virulence factor. Its effective therapeutic concentration lies within the clinical dosage range, suggesting minimal toxicity risks for human hosts (Table 2). This dual antimicrobial and antivirulence activity makes clofazimine an attractive candidate for treating cholera and potentially other bacterial infections reliant on NQR-mediated metabolism (Yuan et al. 2025).
Table 2.
Reported NQR inhibitors.
| Compound | Microorganism | IC50 | MIC |
|---|---|---|---|
|
| |||
| PEG-2S | C. trachomatis | 2 nM (Dibrov et al. 2017) | 0.7 μM (Dibrov et al. 2017) |
| Korormicin A | V. cholerae | 5 nM (Ito et al. 2017) | 20 μM (Ito et al. 2017) |
| Aurachin D-42 | V. cholerae | 2 nM (Ito et al. 2017) | – |
| Clofazimine | V. cholerae | 3 μM (Yuan et al. 2025) | 5 μM (Yuan et al. 2025) |
| Thioridazine | V. cholerae | 27 μM (Yuan et al. 2025) | – |
Additionally, quinone-like inhibitors, including korormicin and aurachin, effectively target bacterial NQR (Figure 3). Aurachin D-42 and korormicin A are strong inhibitors, with IC50s of 2 and 5 nM, respectively (Table 2) (Ito T et al. 2017; Masuya et al. 2020). These low IC50 values highlight their potential for antimicrobial drug development. Although korormicin was effective against C. trachomatis, it also had significant cytotoxic effects (Dibrov et al. 2017). The newly developed koromicin-like furanone-based NQR inhibitor PEG-2S effectively prevented C. trachomatis-induced alterations in intracellular H+ and Na+ concentrations (Table 2). It also demonstrated exceptional potency and specificity by inhibiting NQR activity in V. cholerae membrane vesicles, with an IC50 of 2 nM. Notably, PEG-2S exhibits minimal cytotoxicity in mammalian cells at therapeutically relevant concentrations (Dibrov et al. 2017), consistent with the low host toxicity seen in other NQR-targeting agents, which is an advantage stemming from the absence of NQR homologs in human cells. This high degree of specificity reinforces the promise of these compounds as safe and selective candidates for antimicrobial drug development (Dibrov et al. 2017).
SDH
Succinate dehydrogenase (SDH) is a membrane-bound protein involved in both the Krebs cycle and electron transport chain (McNeil and Fineran 2013). In bacteria, SDH couples the oxidation of succinate to fumarate in the cytoplasm with quinone reduction in the cell membrane (Yankovskaya et al. 2003). Succinate oxidation by SDH is carried out by four cofactors: FAD and three iron-sulfur clusters (2Fe-2S, 4Fe-4S, and 3Fe-4S) (Cheng et al. 2015). Prokaryotic SDH comprises 3–4 subunits (Figure 4A), while the mitochondrial counterpart is composed of four subunits (SdhA-D). Subunit SdhA is a flavoprotein that harbors a covalently-bound FAD factor (Figure 4A,C). Subunit SdhB contains the three iron-sulfur cofactors that mediate the electron transfer to quinone. In prokaryotes, the hydrophobic membrane anchor of the protein, which is least conserved amongst organisms, either consists of one larger SdhC subunit or two smaller SdhC and SdhD subunits, with the quinone binding site positioned at the interface of the hydrophobic membrane subunit(s) (Figure 4A,B) (Hägerhäll 1997). Eukaryotic SDH contains both SdhC and SdhD subunits and two quinone binding sites, a QP site toward the cytosol, and a QD site near the periplasmic side of the membrane (Oyedotun and Lemire 2001). In addition to these catalytically relevant cofactors, SDH also contains a heme b in the membrane domain that is evolutionarily conserved. Remarkably, heme b stabilizes SDH structure but does not play a role in quinone reduction, as E. coli SDH mutants lacking heme b maintain catalytic activity (Tran QM et al. 2007). In addition to the canonical catalytic subunits, subunit SdhE plays a role in FAD attachment (McNeil and Fineran 2013).
Figure 4.

Structure of SDH accessed from PDB ID: 7D6V (Berman et al. 2000; Zhou X et al. 2021). (A) Tri-dimensional structure of SDH with cofactors/ligand as spheres. Light gray: FAD, yellow: Fe-S clusters, dark gray: Ubiquinone-1. (B) Close view of the ubiquinone-1 (UQ1) site. (C) Close view of FAD binding. (D) Two dimensional structures of SDH inhibitors.
SDH is highly conserved and found in most organisms, including mammals, fungi, and pathogenic bacteria. SDH is present in oxygen-consuming bacteria, including strict aerobes and facultative anaerobes (Hederstedt and Rutberg 1981). SDH plays a crucial role in the metabolism of many clinically relevant, antibiotic-resistant bacterial pathogens, including Acinetobacter baumannii, Neisseria spp., Shigella spp., and P. aeruginosa, all of which have been designated by the World Health Organization as priority pathogens due to their multidrug resistance profiles (Kanehisa and Goto 2000; Kanehisa 2019; André et al. 2021; Kanehisa et al. 2023). In addition, SDH is equally critical in the metabolism of numerous fungal plant pathogens, including Botrytis cinerea and Rhinzoctonia solani (Scalliet et al. 2012). Due to its essential metabolic role, SDH has been recognized as a compelling target for antibacterial and fungicide development, offering broad-spectrum efficacy across diverse applications.
Promysalin is a secondary metabolite produced by P. putida, which exhibits antibiotic activity and specifically targets P. aeruginosa SDH by binding to the ubiquinone site, with an in vitro IC50 of 2.5 μM, and an MIC50 of 1.75 μM (Table 3) (Li W et al. 2011; Keohane et al. 2018). Notably, promysalin preferentially targets the pathogenic enzyme by a 50-fold factor compared to its mitochondrial counterpart, demonstrating the compound’s selectivity for the bacterial enzyme (Keohane et al. 2018). Other studies have highlighted the significance of SDH as a drug target against P. aeruginosa, with siccanin, derived from the fungus Helminthosposium siccans, targeting P. aeruginosa SDH with an IC50 of 0.9 μM, thus exhibiting more potency for the bacterial enzyme compared to porcine heart mitochondria (Table 3) (Mogi et al. 2009). Notably, siccanin, a dual SDH and cytochrome bc1 inhibitor in the malaria-causing agent P. falciparium, is extremely potent against SDH—critical for parasitic survival during the mosquito stage—exhibiting biphasic inhibition with an IC50 of 16 nM and 8.93 μM (Table 3) (Komatsuya et al. 2022). Additionally, in M. tuberculosis, SDH inhibitors have been shown effective against both the enzyme and bacterial growth (Adolph et al. 2024; 2024).
Table 3.
Reported SDH inhibitors.
| Compound | Microorganism targeted | IC50 | MIC50 or EC50 | Toxicity | Inhibition mechanism |
|---|---|---|---|---|---|
|
| |||||
| Boscalid | Botrytis cinerea | 0.8 μM (Bénit et al. 2019) | 2.7 μM (Wang et al. 2016) | Hepatotoxic, thyrotoxicosis (Federal Register 2025) | QP (Sang and Lee 2020) |
| Fluopyram | Botrytis cinerea | 0.2 μM (Bénit et al. 2019) | 75.6–731 nM (Veloukas and Karaoglanidis 2012) | Hepatotoxic, thyrotoxicosis (Federal Register, 2019) | QP (Sang and Lee 2020) |
| Bixafen | Rhinzoctonia solani | 3 μM (Zhang A et al. 2019) | 104 nM (Zhang A et al. 2019) | Hepatotoxic, thyrotoxicosis (solely in the presence of the former) (Federal Register 2018) | QP (Sang and Lee 2020) |
| Promysalin | P. aeruginosa | 2.5 μM (Keohane et al. 2018) | 1.8 μM (Li W et al. 2011) | Ubiquinone-binding site | |
| Siccanin | P. aeruginosa | 0.9 μM (Mogi et al. 2009) | 150 μM (Mogi et al. 2009) | DLD-1 EC50 34 μM HDF EC50 16 μM (Komatsuya et al. 2022) | Ubiquinone-binding site |
| Siccanin | P. falciparum | 16 nM and 8.9 μM (biphasic) (Komatsuya et al. 2022) | 8.4 μM (Komatsuya et al. 2022) | DLD-1 EC50 34 μM HDF EC50 16 μM (Komatsuya et al. 2022) | Ubiquinone-binding site |
SDH has also been extensively exploited as a molecular target for antifungal agents, primarily in agricultural applications. SDH inhibitors act by binding to the QP site of the enzyme, disrupting mitochondrial respiration and impairing fungal viability (Scalliet et al. 2012). These inhibitors encompass diverse drug classes, with prominent groups including carboxamides, benzamines, and pyrazole-carboxamides, such as Boscalid, Fluopyram (Figure 4D), and Bixafen, respectively (Table 3) (Bénit et al. 2019), which are approved by the United States Environmental Protection Agency (EPA) for use as agricultural pesticides. The inhibitory potency of these compounds is species-dependent, with reported IC50 values typically in the low micromolar range (Table 3) (Bénit et al. 2019). Against B. cinerea, Boscalid exhibits an IC50 of 0.8 μM (Bénit et al. 2019), while Fluopyram shows enhanced potency with an IC50 of 0.2 μM (Table 3) (Bénit et al. 2019). Bixafen is considerably more effective, with its IC50 of 0.07 μM (Table 3) (Bénit et al. 2019) highlighting its superior activity against this fungal pathogen (Bénit et al. 2019). However, against the fungal pathogen R. solani, Bixafen is less potent, demonstrating an IC50 of 2.95 μM (Table 3) (Zhang A et al. 2019). Although these molecules were designed to selectively inhibit fungal SDH, off-target activity against the mammalian homolog has been observed. Boscalid, Fluopyram, and Bixafen inhibit human SDH with IC50 values of 4.8 μM, 160 μM, and 0.34 μM, respectively (Bénit et al. 2019). These interactions can impair mitochondrial function in human cells, raising concerns about potential cytotoxic and off-target effects, particularly with high-level exposure. Thus, while these inhibitors are effective and selective antifungals, their use requires careful environmental and toxicological monitoring to mitigate potential risks. Resistance to these molecules has emerged in several important crop pathogens. Amino acid and genetic QP site mutations in the Sdh B, C, and D subunits have been identified as major mechanisms of resistance, reducing inhibitor binding affinity in pathogens such as B. cinerea and Alternaria alternata, underscoring the need for resistance management (Leroux et al. 2010; Scalliet et al. 2012).
Cytochrome bc1
Cytochrome bc1 (complex III) is a critical enzyme essential for energy generation in aerobic prokaryotes and eukaryotes. Cytochrome bc1 is an oligomer spanning the plasma membrane of prokaryotes and the inner mitochondrial membrane of eukaryotes. The enzyme is composed of three distinct electron-transferring proteins: cytochrome b, cytochrome c1, and a Rieske iron-sulfur protein containing an 2Fe-2S cluster. Cytochrome b is a hydrophobic integral membrane protein and houses two noncovalently bound b-type hemes (hemes bL and bH), while cytochrome c1 on the periplasmic side of the membrane contains a single covalently bound c-type heme (heme c1) (Figure 5) (Trumpower 1990). In prokaryotes, the cytochrome bc1 complex typically consists of three or four subunits per monomer, whereas in eukaryotes, each monomer can comprise up to eleven subunits (Berry et al. 2000).
Figure 5.

Structure of cytochrome bc1 accessed from PDB ID: 7TCE (Berman et al. 2000). (A) Tri-dimensional structure with cofactors/ligands shown as spheres. Light gray: Atovaquone, beige: Heme c, pink: Heme, yellow: Fe-S clusters. (B) Close view of atovaquone (AOQ) binding site. (C) Two-dimensional structure of bc1 inhibitors.
Cytochrome bc1 is essential for energy production via the respiratory chain in many pathogenic organisms, including bacteria, fungi, and protozoa. Notably, the enzyme is significant and has been therapeutically targeted in Pneumocystis jirovecii, a fungus which causes life-threatening pneumonia among immunosuppressed patients, P. falciparium, the protozoa responsible for malaria, T. gondii, which causes toxoplasmosis, and Mycobacterium spp., which includes the causative agent of tuberculosis (Kessl et al. 2003; Nixon et al. 2013; Pethe et al. 2013). Cytochrome bc1 is also a critical enzyme in the opportunistic pathogen P. aeruginosa, responsible for respiratory and systemic infections in immunocompromised individuals (Liang P et al. 2020). Within P. aeruginosa, cytochrome bc1 has been demonstrated essential for energy production via its significant contribution to respiration and ion pumping during both logarithmic and stationary growth phases (Hu Y et al. 2024). Furthermore, deletion of the operon encoding for cytochrome bc1 in P. aeruginosa PAO1 severely decreased virulence factor production and pathogenicity (Shen et al. 2021). Despite the fact that some pathogens expressing cytochrome bc1 possess alternative pathways for ubiquinol oxidation, these routes are less efficient for energy production, as they do not couple electron transfer with proton translocation, thus resulting in less ATP production. Furthermore, the widespread presence of this complex across diverse organisms highlights its evolutionary conservation and functional importance (Trumpower 1990).
Several clinically relevant antiparasitic and antifungal agents exert their effects by targeting cytochrome bc1. Fungicides such as Azoxystrobin, Ametoctradin, and Cyazofamid, which are approved by the EPA for use on crops, target the enzyme and disrupt respiration (Figure 5C) (Table 4). The action mechanism of these molecules involves inhibition of the quinone binding sites. For instance, Azoxystrobin is a QO site inhibitor, while Cyazofamid binds to the Qi site. Additionally, it has recently been suggested that Ametoctradin is both a QO and Qi site inhibitor (Table 4). However, resistant strains against these pesticides have emerged, with numerous crop pathogens developing mutations in the cytochrome b gene that alter the binding sites (Dreinert et al. 2018).
Table 4.
Reported complex III inhibitors.
| Compound | Microorganism targeted | IC50 | MIC50 | Toxicity | Inhibition mechanism |
|---|---|---|---|---|---|
|
| |||||
| Azoxystrobin | Pythium spp. | 27 nM (Dreinert et al. 2018) | 0.25–6 μM (Matić et al. 2019) | Acute oral, dermal, inhalation (Rodrigues et al. 2013) | Qo site |
| Ametoctradin | Pythium spp. | 90 nM | Acute oral, dermal, inhalation (European Food Safety Authority 2012) | Qo/Qi site | |
| Cyazofamid | P. insidiosum | >10 mM (Dreinert et al. 2018) | ≤3 μM (Yolanda et al. 2024) | Nephrotoxic (Federal Register 2020) | Qi site |
| Atovaquone | P. falciparum | 3 nM (Biagini et al. 2008) | 1 nM (Biagini et al. 2008) | Hepatotoxic (rare) (National Institute of Diabetes and Digestive and Kidney Diseases 2012d) | Qo site |
| Telacebec | M. tuberculosis | 1 nM (Pethe et al. 2013) | 3 nM (Pethe et al. 2013) | None observed (Kim et al. 2022) | Qo site |
| Lansoprazole | M. tuberculosis | 0.5 μM ((Kovalova et al. 2024) | 2 μM (Rybniker et al. 2015) | Hepatotoxic (rare) (National Institute of Diabetes and Digestive and Kidney Diseases, 2012e) | Qo site |
| Siccanin | P. falciparum | 8 μM (Komatsuya et al. 2022) | 8 μM (Komatsuya et al. 2022) | DLD-1, 34 μM HDF 16 μM (Komatsuya et al. 2022) |
Qi site |
Atovaquone is a naphthoquinone (Figure 5C) approved by the FDA as an antiprotozoal and antifungal agent that is employed against T. gondii and P. jirovecii, and also utilized in combination with proguanil to treat malaria caused by P. falciparum (Atovaquone/Toxoplasmic Encephalitis Study Group 1997; Walker et al. 1998; Baggish and Hill 2002). Atovaquone inhibits the QO site, blocking the transfer of electrons from ubiquinol to the Rieske iron-sulfur protein (Figure 5B). Atovaquone is extremely potent against P. falciparum with MIC50 values of 3 nM against the enzyme and 1 nM against bacterial growth (Table 4) (Fry and Pudney 1992; Biagini et al. 2008; Khositnithikul et al. 2008). However, single nucleotide substitutions in the P. falciparum cytochrome b gene following preventive administration of atovaquone have been demonstrated to result in amino acid changes at the drug’s binding site, enabling the parasite to maintain respiratory activity at drug concentrations that would normally limit this function (Korsinczky et al. 2000). In addition, acridinedione compounds have also been shown to inhibit cytochrome bc1 with higher selectivity compared to atovaquone, also binding the Qo site (Biagini et al. 2008).
Regarding the role of cytochrome bc1 as an antibacterial target, Telacebec (Q203) is a promising candidate targeting the enzyme in M. tuberculosis, binding to the QO site (Table 4) (Pethe et al. 2013) and inhibiting ATP synthesis and bacterial growth (Pethe et al. 2013; Kim J et al. 2022; Capela et al. 2023). Telacebec has been granted Orphan Drug Designation from the FDA and in a Phase 2 clinical trial demonstrated a reduction in M. tuberculosis load in sputum (Janssen et al. 2025). Telacebec exhibits nanomolar-range potency, reducing intracellular ATP levels in M. tuberculosis with an IC50 of 1 nM, while inhibiting bacterial growth in macrophages with an MIC50 of 0.3 nM and in culture medium with an MIC50 of 3 nM (Table 4) (Pethe et al. 2013). Resistance to the drug has been linked to polymorphisms that cause mutations in the target binding site (Sorayah et al. 2019).
Lansoprazole, an FDA approved gastric proton-pump inhibitor which targets H+/K+-ATPase, has also been demonstrated effective against M. tuberculosis, interfering with ATP synthesis by inhibiting cytochrome bc1. Preliminary investigations indicate that the drug inhibits growth of intracellular M. tuberculosis within macrophages with an IC50 of 2.2 μM (Table 4) (Rybniker et al. 2015). Intracellular lansoprazole subsequently undergoes a sulfoxide reaction where it is converted to lansoprazole sulfide prior to binding to the enzymatic QO site (Rybniker et al. 2015; Kovalova et al. 2024). Lansoprazole’s off-target efficacy against cytochrome bc1 offers promising potential for drug repurposing, particularly for drugs with established strong safety profiles.
bd oxidase
bd-type oxidases are absent in human mitochondria, but are widely distributed among bacterial and archaeal species (Borisov et al. 2011; Siletsky and Borisov 2021). This enzyme transfers electrons from reduced quinones to oxygen, pumping two H+ per electron (Borisov et al. 2011; Siletsky and Borisov 2021). bd oxidases comprise a family of enzymes that carry two heme b cofactors and a heme d (Figure 6A) (Iwata et al. 1995; D’mello et al. 1996; Borisov et al. 2021; Siletsky and Borisov 2021), which are grouped based on their genetic organization into bd-I and bd-II type enzymes and are encoded in the cydABX and appCBXA operons, respectively. In E. coli, these operons are expressed differentially depending on environmental conditions. For instance, bd-I is expressed during microaerophilic growth and in oxygen-rich conditions (Brøndsted and Atlung 1996), and bd-type II is expressed during anaerobic growth, phosphate starvation, and at the beginning of the stationary phase (Brøndsted and Atlung 1996; Grauel et al. 2021).
Figure 6.

Structure of bd oxidase accessed from PDB ID: 5DOQ (Berman et al. 2000; Safarian et al. 2016). (A) Tri-dimensional structure with heme groups presented as spheres. Light gray: Heme b/c, dark gray: Heme d. (B) Two-dimensional structures of bd oxidase inhibitors, 1: N-(4-(4-(trifluoromethyl) phenoxy) phenyl) quinazolin-4-amine, 2: 3- methyl-2-[4-[4-(trifluoromethoxy) phenoxy] phenyl]-2,3,4a,5,6,7,8,8a-octahydro-1H-quinolin-4-one.
bd oxidases use different type of quinones, including ubiquinone and menaquinone (Jünemann et al. 1995; Yang et al. 2008; Borisov et al. 2011). In aerobic bacteria such as K. aerogenes (González-Montalvo et al. 2024), M. tuberculosis (Van Der Velden et al. 2025), and E. coli (Van Beilen and Hellingwerf 2016), bd oxidases use ubiquinol. bd oxidases have higher affinity for oxygen than other terminal oxidases (D’mello et al. 1996; Poole and Cook 2000; Forte et al. 2017), which allows them to function in microaerophilic conditions. In addition, these enzymes are highly resistant to cyanide compared to heme-copper oxidases (Sakamoto et al. 1999; Quesada et al. 2007; Forte et al. 2017; Siletsky and Borisov 2021). Organisms like P. aeruginosa actively produce cyanide to eliminate competing bacteria and switch their metabolism to bd oxidase to survive auto poisoning and colonize new environments (Blumer and Haas 2000; Yan et al. 2019). bd-type enzymes have also been linked to ROS protection. In E. coli, bd oxidase has catalase activity, converting H2O2 into O2 (Borisov et al. 2013). Moreover, bd oxidases detoxify other radical species, like peroxynitrite, produced by macrophages phagosomes (Borisov et al. 2015). bd oxidases have also been proposed as electron sinks during anaerobic growth of E. coli, protecting from ROS production upon sudden exposure to oxygen (Korshunov and Imlay 2010). Finally, bd oxidases have also been demonstrated to provide advantages during exposure to adverse conditions like hypoxia or the presence of H2S (Forte et al. 2016), CO (Nastasi et al. 2023), and NO (Mason et al. 2009).
Due to their importance in biological process and their absence in mammalian mitochondria, bd oxidases have been an attractive target for drug development. One of the first compounds found to have inhibitory activity against these enzymes was the Aurachin family of compounds (Meunier et al. 1995), which includes aurachin D and C, which preferentially inhibit bd oxidases and bo3 oxidases, respectively (Figure 6B; Table 5) (Meunier et al. 1995; Kruth and Nett 2023). Due to aurachin D’s significant cytotoxicity (Dejon and Speicher 2013), alternative compounds have been evaluated against the enzyme. The quinazoline ND-011992 was identified to inhibit mycobacterial (Lee BS et al. 2021) and E. coli (Kägi et al. 2023) bd oxidases (Table 5). However, this compound depleted ATP while inhibiting oxygen consumption and growth only in the presence of Bedaquiline (Lee BS et al. 2021), indicating that other terminal oxidases can be used to overcome bd oxidase inhibition. Further studies show that this molecule inhibits mitochondrial and bacterial complex I activities. However, brominated derivatives did not affect NADH oxidase activity of E. coli membranes at micromolar ranges (Kägi et al. 2023).
Table 5.
Reported bd oxidase inhibitors.
| Compound | Microorganism targeted | IC50 | Bacterial inhibition | Toxicity | Inhibition mechanism |
|---|---|---|---|---|---|
|
| |||||
| Aurachin D | E. coli | 18 nM (Radloff et al. 2021) | >128 μg/mL (MIC) (Dejon and Speicher 2013) | HCT-116 1 μg/mL (GI50) U-2 OS 1 μg/mL (GI50) U937 1 μg/mL (GI50) (Dejon and Speicher 2013) |
Blocking of quinone binding site (Miyoshi et al. 1999; Grauel et al. 2021) |
| ND-01192 | E..coli | 0.6–1.3 μM (Kägi et al. 2023) | Mt Complex I IC50 3 μM (Kägi et al. 2023) | ||
| CK-2–63 | M. tuberculosis | 3 nM (Jeffreys et al. 2023) | 37.8% at 5 μM (Jeffreys et al. 2023) | HepG2 IC50 85 μM 181 | Blocking of quinone binding site (Jeffreys et al. 2023) |
More recently, quinolone-based compounds, specifically a 2-aryl-quinolone backbone, was found to have inhibitory activity on mycobacterial bd oxidases and on bacterial growth (Jeffreys et al. 2023). Experimental compounds CK-2–63, SL-2–25, MTD-403 and PG-203 had sub micromolar inhibitory concentrations, with CK-2–63 as the most potent compound (Table 5). While this compound showed inhibitory activity against bovine cytochrome bc1, it showed no toxicity in a HePG2 cell line. This aryl-quinolone synergized with other respiratory chain inhibitors like Bedaquline and ND-011992 to reduce ATP production, oxygen consumption rate, and growth (Jeffreys et al. 2023). The development of drugs against bd oxidases has shown promising results, with some patients already receiving such treatments. Thus, bd oxidases continue to be attractive antibiotic targets since these enzymes are limited to the prokaryotic domains. However, special care is needed to ensure their specificity.
bo3 oxidase
Cytochrome bo3 is a multimeric complex composed of subunits I (cyoB), II (cyoA), III (cyoC), and IV (cyoD) (Chepuri et al. 1990; Minghetti et al. 1992; Nakamura et al. 1997; Richhardt et al. 2013; Choi SK et al. 2017). Subunit I possesses three active redox centers: a high-spin heme o3, a low-spin heme b, and a CuB site (Saiki et al. 1992; 1993; Mogi et al. 1994; Choi SK et al. 2017) and contains all residues required for catalysis and proton translocation (Figure 7A) (Garcia-Horsman 1995; Schäfer and Penefsky 2008). This enzyme belongs to the superfamily of heme-copper oxygen reductases, which is characterized by the presence of subunit I. Heme-copper terminal oxidases can be classified into families A, B, and C (Schäfer and Penefsky 2008; Li J et al. 2021). Cytochrome bo3 oxidase belongs to family A, which is the most extensively distributed and also includes cytochrome c oxidases (Schäfer and Penefsky 2008), such as the mammalian aa3-type cytochrome c oxidases (Chepuri et al. 1990). This complex oxidizes ubiquinol on the periplasmic side and uses four protons from the cytoplasm to reduce dioxygen while pumping four protons to generate a proton motive force across the membrane (Puustinen et al. 1989; Siletsky and Borisov 2021), with a stoichiometry of 2 H+ pumped per e− (Musser and Chan 1998; Siletsky and Borisov 2021). Bo3 oxidase is expressed preferentially under high oxygen concentrations and is under the control of regulators Fnr and ArcAB (Rice and Hempfling 1978; Cotter et al. 1990; Tseng et al. 1996).
Figure 7.

Structure of cytochrome bo3 accessed from PDB ID: 7CUW (Berman et al. 2000; Li J et al. 2021). (A) Tri-dimensional structure with cofactors/ligands shown as spheres, light gray: Ubiquinone-8, dark gray: Heme, slate blue: Cu-heme. (B) Close view of ubiquinone-8 (UQ8) binding. (C) Two-dimensional structures of bo3 inhibitors.
Studies on bo3 oxidase have been mostly focused on elucidating its functions and role in the respiratory chain. This enzyme is widely distributed among bacterial species, and has been identified as an important component of respiration in E. coli (Cheesman et al. 1993), P. aeruginosa (Liang P et al. 2020), Gluconobacter oxydans (Richhardt et al. 2013), K. aerogenes (González-Montalvo et al. 2024), K. pneumoniae (Juty and Hill 1997), Proteus mirabilis (Armbruster et al. 2018), Asaia bogorensis (Kawai et al. 2015), V. alginolyticus (Miyoshi-Akiyama et al. 1993), and Salmonella (Jones-Carson et al. 2016). Although widely distributed, there are only a few studies regarding its metabolic role. In E. coli, bo3 is the favored terminal oxidase in high aeration conditions (Puustinen et al. 1989; Tseng et al. 1996; Weiss et al. 2010; Borisov et al. 2011). This same pattern has been observed in other bacteria, including G. oxydans (Richhardt et al. 2013) and P. aeruginosa (Liang P et al. 2020). On the other hand, in the opportunistic pathogen K. aerogenes, bo3 is not the main terminal oxidase (González-Montalvo et al. 2024). Regarding its role in pathogenicity, it has been shown that bo3 expression is increased in the pathogen P. mirabilis in an urinary tract infection model in mice (Pearson et al. 2011; Armbruster et al. 2018). Moreover, this enzyme is critical during catheter-associated urinary tract infections (Armbruster et al. 2017, 2018).
Regarding drug development against this enzyme, there are major gaps. Previous studies indicate that aurachin C analogs almost completely inhibited bo3 activity. Inhibitor screening showed that aurachin C derivates also showed strong inhibition but were unspecific, as bd-oxidases were also inhibited by these compounds (Table 6) (Radloff et al. 2021). Other studies showed that aurachin D derivatives could be used to develop new bo3 inhibitors, specifically those that contain double bonds in the R1 position, with strong dependence on the length of side chains in position R2 (Radloff et al. 2021).
Table 6.
Reported bo3 oxidase inhibitors.
| Compound | Microorganism targeted | IC50 | MIC | Toxicity | Inhibition mechanism |
|---|---|---|---|---|---|
|
| |||||
| Aurachin C | E. coli | >50 μg/mL (MIC) (Kunze et al. 1987) | 50 nM (IC50) (Mitochondria) (Kunze et al. 1987) | Blocking of quinone binding site (Miyoshi et al. 1999; Grauel et al. 2021) | |
| CORM-3 | E. coli | 4 to >512 μg/mL (MIC) (Southam et al. 2018) | >100 μM for RAW264.7 (Song et al. 2009) | Binding of CO to heme o3 (Jesse et al. 2013; Nastasi et al. 2023) | |
| AC1–10 | E. coli | 10–80 nM (Radloff et al. 2021) | Block quinone binding site (Miyoshi et al. 1999; Grauel et al. 2021) | ||
| AC4–11 | E. coli | 0.1–0.14 μM (Radloff et al. 2021) | Block quinone binding site (Miyoshi et al. 1999; Grauel et al. 2021) | ||
| AC2–11 | E. coli | 30 nM (Radloff et al. 2021) | Block quinone binding site (Miyoshi et al. 1999; Grauel et al. 2021) | ||
AOX
One key difference in the respiratory chain of mammalian mitochondria and many human pathogens is the presence of a cyanide-insensitive alternative oxidase (AOX) (Figure 8). Although AOX was first identified in plants in the 1920s (Schmucker 1925; Moore and Siedow 1991) and later described in Neurospora in the 1950s (Tissieres et al. 1953), its presence in bacteria was not determined until bacterial genomic sequences became available in the early 2000s. The eukaryotic AOX is encoded by the nuclear genome and is a homodimer (32–36 kDa per monomer) associated with the matrix leaf of the inner mitochondrial membrane as a monotopic membrane protein (Elthon and McIntosh 1986; 1987; May et al. 2017). AOX is a terminal diiron quinol oxidase with its active site oriented to the matrix, catalyzing the cyanide and antimycin A-resistant oxidation of ubiquinol and the reduction of oxygen to water (Berthold and Stenmark 2003; Moore et al. 2013; Shiba et al. 2013). This enzyme branches the electron flux of the respiratory chain prior to proton translocation by complexes III and IV (Medentsev et al. 2002; Kern et al. 2007). As AOX is not a proton pump, its activity is not directly linked to the generation of the proton motive force (Berthold et al. 2000). Instead, the energy liberated during ubiquinol oxidation is released as heat (Albury et al. 2009; Vanlerberghe et al. 2009).
Figure 8.

Structure of alternative oxidase accessed form PDB ID: 5ZDR (Berman et al. 2000; Shiba et al. 2019). (A) Tri-dimensional structure with small molecules presented as spheres. (B) Close view of the binding site, FE: Iron, CHW: 3-chloro-4,6-dihydroxy-5-[(2E,6E,8S)-8-hydroxy-3,7-dimethylnona-2,6-dien-1-yl]-2-methylbenzaldehyde.
AOX is ubiquitous in plants (Sharpless and Butow 1970; Dinant et al. 2001; Gérin et al. 2010; Mathy et al. 2010) and many unicellular eukaryotes (Juárez et al. 2004; Sierra-Campos et al. 2009) including pathogenic organisms such as Candida parapsilosis (Guérin and Camougrand 1986; Milani et al. 2001; Ruy et al. 2006), C. albicans (Huh and Kang 2001), P. falciparum (Murphy and Lang-Unnasch 1999), Philasterides dicentrarchi (Mallo et al. 2013), Trypanosoma congolense, T. evansi (Barrett et al. 2003; Roberts CW et al. 2004; Suzuki et al. 2005; Williams et al. 2010), T. brucei (Roberts CW et al. 2004), Cryptococcus neoformans (Akhter et al. 2003), Cryptosporidium parvum, Blastocystis hominis (Moore and Albury 2008; Young et al. 2013; May et al. 2017), and other microsporidia (Williams et al. 2010). Depending on the organism, AOX function can be vastly different, from its thermogenic activity in plants (Albury et al. 2009; Ito K et al. 2011), to the regulation of ubiquinol pools and reduction of ROS production. It is commonly accepted that AOX promotes the metabolic plasticity of cells for rapid adaptation to nutrient sources or to biotic and abiotic stress factors (Albury et al. 2009; Rasmusson et al. 2009; Chai et al. 2010; Hanqing et al. 2010). Moreover, AOX seems to be involved in pathogenicity and virulence, survival of parasites, and regulation of host–pathogen interactions (Black et al. 2021).
Recently, efforts have been made to understand the role of AOX in pathogenic metabolisms, allowing for the discovery of specific AOX inhibitors that could serve as novel antifungals and antiparasitics that do not affect human mitochondrial function. For example, nitroprusside (NO donor) and the AOX inhibitor salicylhydroxamic acid (SHAM) reduce C. albicans viability and increase phagocytic uptake by macrophages (Table 7) (Duvenage et al. 2019). In addition to SHAM, other fungal AOX inhibitors have been identified, including 2,5-dibromo-3-methyl-6-isopropyl-p-benzo quinone, disulfiram, 5-decyl-6-hydroxy-4,7-dioxobenzothiazole, propyl gallate, and various hydroxamic acids (Table 7) (Veiga et al. 2003). Recent findings indicate that P. falciparum AOX can be inhibited by propyl gallate, 8-hydroxyquinoline, and SHAM (Murphy et al. 1997; Murphy and Lang-Unnasch 1999). Moreover, T. brucei AOX, which is essential for the respiratory activity of the bloodstream form of the parasite (Clarkson et al. 1989; Chaudhuri et al. 2006), can be inhibited by ascofuranone (Kido et al. 2010; Menzies et al. 2018).
Table 7.
Reported AOX inhibitors.
| Compound | Microorganism targeted | IC50 |
|---|---|---|
|
| ||
| Ascochlorin | T. brucei brucei | 3.7 nM (Young et al. 2020; Xu et al. 2021) |
| Ascofuranone | T. brucei brucei | 0.03 nM (Shiba et al. 2013) |
| Octyl gallate | T. brucei brucei | 0.23 μM (Young et al. 2020) |
| Salicylhydroxamic acid (SHAM) | T. brucei brucei | 6 μM (Meco-Navas et al. 2018) |
| Salicylic hydroxamic acid | T. brucei brucei | 4 nM (Young et al. 2020) |
| 8-Hydroxyquinoline | P. falciparum | 11 μM (Roberts et al. 2004) |
| 8-Hydroxyquinoline | C. parvum | 0.7 μM (Roberts et al. 2004) |
ATP synthase
ATP synthases convert the transmembrane electrochemical gradient potential into chemical bond energy in the form of adenosine triphosphate (ATP) (Junge and Nelson 2015). Although ATP synthases are ubiquitously distributed in all life domains, they appear to play different roles. ATP synthases are multicomponent membrane enzymes that are highly conserved across species in the phylogenetic tree (von Ballmoos et al. 2009; Walker 2013; Lu P et al. 2014). ATP synthases have two major functional domains, a membrane motor domain (F0) and a cytoplasmic catalytic domain (F1), which are joined together by the central γ and ε-subunits of F1 and the peripheral stalk of F0 (b-subunit). The F0 domain is composed of a, b and c subunits. C-subunits form a ring embedded in the membrane with rotor function. The F1 domain is composed of α, β, γ, δ, and ε subunits. F0 is a motor that rotates, driven by the controlled dissipation of the transmembrane electrochemical gradient (Figure 9A) (Kühlbrandt 2019). Protons flow through a- and c-subunits, which allows the rotation of the γ-subunit, ε-subunits, and c-subunits (Yoshida et al. 2001; Nesci et al. 2016). The rotational energy is transmitted to the catalytic domain in F1 through the γ-subunit, which is then used to synthesize ATP from adenosine diphosphate and inorganic phosphate (Figure 9B,C) (Okuno et al. 2011).
Figure 9.

Structure of ATP synthase accessed from PDB ID: 8J0S (Berman et al. 2000; Zhang Y et al. 2024). (A) Tri-dimensional structure with inhibitors/cofactors visible as spheres. (B–D) Close view of the binding of ADP, ATP, and bedaquiline (BQ1). (E) Two-dimensional structures of ATP synthase inhibitors, upper: Venturicidin A, lower: Bedaquiline.
ATP synthase has been validated as a druggable target against M. tuberculosis. The diarylquinoline bedaquiline (formerly TMC207) (Figure 9D,E), which targets ATP synthase, has potent inhibitory activity against Mycobacterium spp. (Biagini et al. 2008; Mahajan 2013), and it became the first drug approved for tuberculosis treatment with a novel mechanism of action in 40 years (Mahajan 2013). Regimens that included bedaquiline reduced the risk of death of patients with MDR or rifampicin-resistant tuberculosis (Schnippel et al. 2018). Moreover, bedaquiline has strong activity on the growth of a large number of mycobacteria species (Andries et al. 2005). Although the activity of bedaquiline is restricted to mycobacteria, with no activity on Gram-positive of Gram-negative bacteria (Andries et al. 2005), diarylquinolines with novel lateral chains show activity against the ATP synthase and growth of S. aureus (Balemans et al. 2012). Bedaquiline targets the c-ring and the interface of aand c-subunits with high affinity in the lower nanomolar range (Table 8) (Preiss et al. 2015; Guo et al. 2021). Several reports indicate that bedaquiline has little inhibitory activity on human ATP synthase (Haagsma et al. 2009; Balemans et al. 2012). However, a recent study suggests that it may have some toxicity (Luo et al. 2020). There are reported cardiac and liver adverse events, namely QT prolongation and elevated liver enzymes (Table 8) (Kim JH et al. 2023). However, it has been determined that the cardiac effect is due to its metabolites, which inhibit the cardiac delayed rectifier potassium channels (Kakkar and Dahiya 2014). Venturicidin A is an ATP synthase inhibitor that binds to subunit c of fungal ATP synthase, blocking proton translocation and inhibiting ATP synthesis (Figure 9E) (Galanis et al. 1989). Although it has also been shown to deplete ATP in bacteria (Yarlagadda et al. 2020), it has no antibacterial activity on its own. However, it potentiates aminoglycosides against resistant clinical isolates of P. aeruginosa, A. baumannii, methicillin-resistant S. aureus, and vancomycin-resistant enterococci (Yarlagadda et al. 2020), serving as an antibiotic adjuvant. Therefore, ATP synthase inhibitors can prolong the clinical life of certain classes of antibiotics. Finally, in facultative anaerobes, ATP synthase is not essential for growth in aerobic conditions in Gram-negative and Gram-positive bacteria, with the exception of P. aeruginosa. In this bacteria, ATP synthase mutants are avirulent in infection models in vivo (Jensen and Michelsen 1992; Turner et al. 2003; PLOS Pathogens 2025; Skurni et al. 2013; Fuller et al. 2000; Sheehan et al. 2003; Fey et al. 2013; Lee E-J et al. 2013; Koo et al. 2017; Poulsen et al. 2019). Therefore, this is an excellent target for drug development in Pseudomonas.
Table 8.
Reported ATP synthase inhibitors.
| Compound | Microorganism targeted | IC50 | MIC50 | Inhibition mechanism | Toxicity |
|---|---|---|---|---|---|
| Bedaquiline | M. tuberculosis | 20–25 nM (Preiss et al. 2015) | 54 nM (Matteelli et al. 2010) (drug-resistant strain) | Blocks proton flow | Hepatotoxicity |
Quinone synthesis
Ubiquinone (UQ), also known as coenzyme Q, is a critical molecule in cellular energy production and antioxidant defense. It acts as an electron carrier in the mitochondrial and bacterial electron transport chains, enabling ATP generation, and protection from reactive oxygen species. Ubiquinone biosynthesis in humans and microorganisms shares core pathways but differs in some aspects, including isoprenoid chain length and aromatic ring precursor origin. Humans synthesize UQ10, while bacteria produce UQ8, and fungi produce UQ6 (Meganathan 2001). Humans derive 4-hydroxybenzoate (4-HB) from dietary aromatic amino acids, whereas microorganisms synthesize 4-HB via the shikimate pathway (Figure 10). This pathway is also found in plants and apicomplexan parasites (T. gondii, P. falciparum), but is absent in humans (Meganathan 2001), making it a promising antimicrobial target. The shikimate pathway converts phosphoenolpyruvate (PEP) and erythrose-4-phosphate (E4P) into chorismate through seven enzymatic steps. The first step, catalyzed by DAHP synthase (Figure 11B), forms 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) from PEP and E4P (Figures 11B and 12B) (Shende et al. 2024). Inhibiting DAHP synthase could halt the pathway, depriving microbes of aromatic compounds, though redundancy (e.g. three DAHP synthase isoenzymes in E. coli) poses challenges. DAHP oxime (Figure 12E), a product analog, is a potent inhibitor of the aroG encoded E. coli DAHP synthase (Balachandran et al. 2016). Another key target, EPSP synthase (Figure 11E), forms 5-enolpyruvylshikimate-3-phosphate (EPSP) from shikimate-3-phosphate and PEP. Targeted by glyphosate (Pöppe et al. 2019), EPSP synthase is essential for E. coli in minimal medium (Joyce et al. 2006). In bacterial ubiquinone biosynthesis, the enzymes (UbiA through UbiX in E. coli) synthesize and modify the quinone head and isoprenoid tail (Figure 11A,C,D) (Aussel et al. 2014). While some enzymes have human homologs, complicating inhibitor design, others, like UbiC (chorismate pyruvate-lyase), which converts chorismate to 4-HB, lack human equivalents (Figures 11C and 12C) (Mitchell 2011). Humans produce 4-HB from tyrosine or phenylalanine via a distinct pathway. UbiC, a small monomeric enzyme with a unique chorismate-binding site, is a prime target for antibiotic design. Synthetic chorismate analogs could block bacterial 4-HB production without affecting humans, as can 4-HB analogs like vanillate inhibit UbiC (Figure 12D) (Holden et al. 2002). Similarly, UbiD, which decarboxylates 3-polyprenyl-4-hydroxybenzoate to 2-polyprenylphenol, and UbiX, which produces the prenylated Flavin mononucleotide (prFMN) cofactor (Figure 12A) for UbiD, have no human homologs (Aussel et al. 2014). In humans, UQ2 bypasses this decarboxylation, yielding hydroxylated intermediates directly. Inhibitors targeting UbiX (e.g. prFMN synthesis blockers) or UbiD active sites could be suitable inhibitors, for example substrate analogs like vanillate, ferulic and cinnamic acid inhibit P. aeruginosa UbiD (Jacewicz et al. 2013) (Table 9).
Figure 10.

Ubiquinone biosynthesis pathway.
Figure 11.

Tridimensional structure of ubiquinone biosynthesis pathway enzymes. (A) UbiD accessed from PDB ID: 2IDB (Berman et al. 2000). (B) DAHP synthase (ribbons) accessed from PDB ID: 1RZM in complex with erythrose-4-phosphate, phospho-enol-pyruvate, and cadmium (spheres) (Berman et al. 2000; Shumilin et al. 2004). (C) UbiC (ribbons) accessed from PDB ID: 1G1B in complex with Para-hydroxy benzoic acid (spheres) (Berman et al. 2000; Gallagher et al. 2001). (D) UbiX (ribbons) accessed from PDB ID: 6QLK in complex with prenylated flavin-mononucleotide (spheres) (Berman et al. 2000; Marshall et al. 2019). (E) EPSP synthase accessed from PDB ID: 5BUF (Berman et al. 2000; Sutton et al. 2016).
Figure 12.

Close view of ubiquinone synthesis enzyme binding pockets. (A) UbiX in complex with prenylated flavin-mononucleotid1971e (4 LU) (Berman 2000; Marshall et al. 2019). (B) DAHP in complex with erythrose-4-phosphate (E4P), phospho-enolpyruvate (PEP), and cadmium (CD) (Berman 2000; Shumilin et al. 2004). (C) UbiC in complex with Para-hydroxy benzoic acid (PHB) (Berman et al. 2000; Gallagher et al. 2001). (D) UbiC in complex with inhibitor vanillate (VNL) accessed from PDB ID 1XLR (Berman et al. 2000; Smith et al. 2006). (E) Two dimensional structure of DAHP inhibitor, DAHP oxime.
Table 9.
Reported ubiquinone biosynthesis inhibitors.
| Compound | Enzyme target | Microorganism targeted | IC50 | MIC50 | Inhibition mechanism |
|---|---|---|---|---|---|
|
| |||||
| DAHP oxime | DAHP synthase | E.coli | Ki = 1.5 ± 0.4 μM | Product analogue, binds the active site competitive inhibition (Balachandran et al. 2016) | |
| Glyphosate | EPSP synthase | Broad-spectrum herbicide. Bacteria having shikimate pathway |
Not cytotoxic up to 1 mM for Human mononuclear white blood cells (Nagy et al. 2019) | MIC 100 10–80 mg/mL Salmonella spp. | Competitive for PEP (Poppe et al. 2019) |
| Vanillate | chorismate pyruvate-lyase (UbiC) | E. coli | Not cytotoxic up to 100 μM in human monocytes (Zhao D et al. 2019) | Ki 280 ± 50 uM | Likely competitive inhibitor (Holden et al. 2002) |
| Vanillate | UbiD | P. aeruginosa | Not cytotoxic up to 100 μM in human monocytes (Zhao D et al. 2019) | Likely competitive inhibitor (Jacewicz et al. 2013) | |
| Ferulic acid | UbiD | P. aeruginosa | Nontoxic, considered GRAS (Ou and Kwok 2004) | Likely competitive inhibitor (Jacewicz et al. 2013) | |
| Cinnamic acid | UbiD | P. aeruginosa | Nontoxic, considered GRAS (Eilerman 2000) | Likely competitive inhibitor (Jacewicz et al. 2013) | |
Humans and some bacteria (E. coli) use the mevalonate pathway to produce the ubiquinone isoprenoid tail, forming isopentenyl pyrophosphate (IPP) from acetyl-CoA. Others, like M. tuberculosis, use the non-mevalonate (MEP/DOXP) pathway (Meganathan 2001). MEP inhibitors like fosmidomycin target enzymes in bacteria and protozoa (Plasmodium), disrupting isoprenoid and ubiquinone synthesis without affecting human cells (Knak et al. 2022). Additionally, bacteria produce shorter polyisoprenoid chains, and enzymes controlling tail length (e.g. bacterial IspB vs. human UQ1) could be targeted to impair microbial energy metabolism selectively.
Antimicrobial peptides
In addition to traditional drug-like compounds, current research is showing that peptides can be used as antibiotics by disrupting the pathogen’s metabolism. Structurally diverse classes of antimicrobial peptides (AMPs) such as Defensins (Doss et al. 2010), Daptomycin (Alborn et al. 1991; Tally and DeBruin 2000; Nguyen et al. 2010; Tran TT et al. 2015), Lanthipeptides (Breukink and De Kruijff 1999; Dickman et al. 2019; Zhao X and Kuipers 2021), Gramicidin (Hu C et al. 2021), Polymyxins (Slingerland et al. 2022), Cathelicidins (Henzler Wildman et al. 2003; Ridyard and Overhage 2021), Histatin-5 (Puri and Edgerton 2014), Magainins (Kim M et al. 2018; Rashid et al. 2020), and Cecropins (Pillai et al. 2005) interrupt the respiratory chains of pathogens by targeting key membrane components (Table 10). These molecules disrupt membrane integrity, ion gradients, or the proton motive force, which are essential for maintaining cellular respiration and energy production. For instance, the host-produced peptide cathelicidin permeabilizes and depolarizes membranes (Henzler Wildman et al. 2003), which halts ATP synthesis. The antifungal Histatin-5 binds to surface proteins in C. albicans, accumulates in mitochondria, and disrupts the electrochemical gradient (Puri and Edgerton 2014). Another antifungal, HsAFP1, isolated from Hemerocallis pigmenti seeds, binds to fungal cell walls, penetrates the cytoplasm, targets mitochondria, and inhibits the respiratory chain, leading to cell death (Aerts et al. 2011). Magainins also affect fungal membranes by forming transient pores that compromise mitochondrial function (Rashid et al. 2020). Cecropins interact with parasite membrane lipids, compromising integrity and potentially impairing mitochondrial function (Pillai et al. 2005).
Table 10.
Antibacterial, antifungal and antiparasitic peptides targeting respiratory enzymes.
| Type | Target | Compound | Target | Spectrum (microorganisms affected) | Toxicity | Inhibition mechanism |
|---|---|---|---|---|---|---|
|
| ||||||
| AMPs | Antibacterial | Defensins | Unspecific, target the membrane. | Broad | In vitro cytolytic effects on human lymphocytes, endothelial cells, and murine lymphoid cells. | Pore formation in the membrane (Aerts et al. 2011; Doss et al. 2010; Lehrer et al. 1993). |
| Gramicidins | Unspecific, target the membrane. | Broad | Hemolytic | Increases membrane permeability to monovalent cations (Hu et al. 2021). | ||
| Polymyxins | Lipid A | Gram negative | Nephrotoxicity, neurotoxicity, and allergic reactions | Binds to the negatively charged lipid A component of LPS, displacing divalent cations and disorganizing the membrane (Slingerland et al. 2022). | ||
| Lanthipeptides (Nisin) | • Membrane • Lipid II |
Gram positive | None | Creates membrane pores and disrupts cell wall formation (Li et al. 2023; Liang et al. 2023; Zhao and Kuipers 2021). | ||
| Bacteriocins (e.g. Acidocin J1132β and analogues) | Unspecific, target the membrane. | Narrow, non-typhoidal Salmonella | None | |||
| Cathionic peptides (ascaphin-8, XT-7, citropin 1.1, aurein, and dermaseptin) | • ATP synthase • peripheral membrane proteins displacement • phosphatidylglycerol (PG) |
Broad, including antifungal and antiparasitic | None or very low | Inhibit ATP synthase activity (Complex V). Remove cytochrome c and MurG from respiratory chain (Wenzel et al. 2014). | ||
| Daptomycin | Phosphatidylglycerol (PG) | Gram positive | Myopathy and rhabdomyolysis | Cell membrane depolarization (Alborn et al. 1991; Nguyen et al. 2010; Tally and DeBruin 2000; Tran et al. 2015) | ||
| Cathelicidins (LL-37) | Unspecific, target the membrane | Broad | Hemolytic | Pore formation (Choi et al. 2022) | ||
| Antifungal | Histatin-5 and derivatives | A direct mitochondrial target was hypothesized, but the mechanism has not been elucidated. | Broad, including antibacterial | None | Pore formation and mitochondrial disruption (Puri and Edgerton 2014). | |
| Magainins | The mechanism has not been elucidated. | Broad, including antifungal and antiparasitic | None | Permeabilize the inner mitochondrial membrane through pore formation, leading to uncoupling of electron transfer and ATP production (Kim et al. 2018; Rashid et al. 2020). | ||
| Antiparasitic | Cecropins, melittin | Phosphatidylglycerol (PG) | Broad | None | Cell membrane depolarization (Pillai et al. 2005). | |
| Synthetic/Modified Peptides (RP-1, AA-RP-1, (Fxr)3) | A direct mitochondrial target was hypothesized, but the mechanism has not been elucidated. | Unknown | Low | Cell membrane depolarization. | ||
Cationic antimicrobial peptides (CAMPs) like ascaphin-8, XT-7, citropin 1.1, aurein, and dermaseptin have been reported to inhibit E. coli’s ATP synthase (Ahmad Z et al. 2013), likely through membrane depolarization. Another proposed mechanism for small cationic AMPs involves the displacement of peripheral membrane proteins, such as cytochrome c and the lipid II biosynthesis protein MurG, from the membrane (Wenzel et al. 2014). This displacement disrupts the respiratory chain, damaging the electron transfer chain and breaking down the proton gradient. Consequently, ATP synthesis is impaired, leading to altered energy metabolism and reduced energy production for all other macromolecule biosynthesis, including that of the cell wall.
Among the dozens of respiratory chain-affecting AMPs discovered so far, some are of particular interest, especially those that are now in clinical use or preclinical development. Daptomycin, a broad range AMP, binds to bacterial membranes enriched in phosphatidylglycerol, causing pore formation and collapsing the proton motive force essential for respiratory complexes (Rashid et al. 2020). Many AMPs that exhibit membrane-disrupting properties, such as nisin, LL-37, melittin and cecropin, also efficiently bind to teichoic acids, disrupting cell wall biosynthesis and membrane potential of most Gram-positive bacteria (Moreno-Vargas and Prada-Gracia 2024). Gramicidin forms dimeric channels in the lipid bilayer, leading to unregulated cation flux and dissipation of the ion gradient. Polymyxins bind to lipopolysaccharides and inner membrane phospholipids, disrupting membrane integrity and affecting the electron transport chain.
By leveraging these mechanisms, AMPs can effectively impair respiratory function and overall viability of the target cells, making them powerful tools in the fight against resistant pathogens. While most AMPs, such as defensins and cathelicidins, exhibit a broad spectrum of activity against a wide range of pathogens (Li L et al. 2023; Liang H et al. 2023), others, including bacteriocins (microcins) (Telhig et al. 2022; Kim S-Y et al. 2024) and certain lanthipeptides (Lehrer et al. 1993; Choi M et al. 2022), demonstrate a narrow spectrum of activity, targeting specific microorganisms. These variations are closely linked to the distinct molecular interactions and mechanisms of action inherent to each molecule. Notably, molecules that possess multiple mechanisms of action are of particular interest due to their potential to offer both a narrow spectrum of activity and a reduced likelihood of resistance development.
As is evident from the examples presented, most naturally occurring antimicrobial peptides indirectly affect the respiratory chain because the peptides target key components that are essential for maintaining the proton motive force or membrane integrity, and only in a few engineered examples is there evidence for binding to specific proteins (such as cationic AMPs inhibiting ATP synthase). Continued high-resolution structural and biochemical studies are needed to further delineate precise protein targets within pathogen respiratory chains. Although antimicrobial peptides represent a new alternative to target the respiratory activity of pathogens, their clinical implementation faces challenges due to their cytotoxicity, susceptibility to protease degradation, poor pharmacokinetics, and the potential for resistance development (Table 10). Effective delivery systems are needed to target AMPs precisely to infection sites. Despite these challenges, successful AMP therapies like Polymyxins, Daptomycin, LL-37, and Nisin are in use or under development. These therapies demonstrate the clinical potential of AMPs in combating resistant pathogens.
Conclusions
Antimicrobial resistance is a growing problem in bacterial, fungal, and parasitic disease treatment. Annually, millions of deaths are linked to antimicrobial resistance, and the annual economic impact is substantial. Due to these figures, maintaining the status quo regarding microbial disease treatment options is not possible, and would only exacerbate this public health crisis. The respiratory chain complexes and ATP synthase physiology are just as vital to cellular life as other mechanisms that have been therapeutically targeted (cell wall, ribosome, enzymatic pathways, etc.) with success and high chemotherapeutic index. Using aerobic respiratory chain inhibition as a sole disease intervention may be feasible, or, using respiratory chain inhibition synergistically with traditional antimicrobial drugs may increase their efficacy and diminish resistance levels of these traditional drugs. It is important to note that the respiratory chain of pathogens may not all share the same components, and the effect of drugs may vary, which allows for the creation of selective compounds that may have no effect on beneficial bacteria and no impact on the mammalian host.
Funding
This work was supported in part by an NIH grant [NIAID – 1R01AI151152] to OJ and KT. JMS was partially supported by the Pritzker Institute Fellowship Program, Illinois Institute of Technology.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adolph C, Cheung C-Y, McNeil MB, Jowsey WJ, Williams ZC, Hards K, Harold LK, Aboelela A, Bujaroski RS, Buckley BJ, et al. 2024. A dual-targeting succinate dehydrogenase and F1Fo-ATP synthase inhibitor rapidly sterilizes replicating and non-replicating Mycobacterium tuberculosis. Cell Chem Biol. 31(4):683–698.e7. doi: 10.1016/j.chembiol.2023.12.002. [DOI] [PubMed] [Google Scholar]
- Adolph C, Hards K, Williams ZC, Cheung C-Y, Keighley LM, Jowsey WJ, Kyte M, Inaoka DK, Kita K, Mackenzie JS, et al. 2024. Identification of chemical scaffolds that inhibit the Mycobacterium tuberculosis respiratory complex succinate dehydrogenase. ACS Infect Dis. 10(10):3496–3515. doi: 10.1021/acsinfecdis.3c00655. [DOI] [PubMed] [Google Scholar]
- Aerts AM, Bammens L, Govaert G, Carmona-Gutierrez D, Madeo F, Cammue BPA, Thevissen K. 2011. The antifungal plant defensin HsAFP1 from Heuchera sanguinea induces apoptosis in Candida albicans. Front Microbiol. 2:47. doi: 10.3389/fmicb.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Wolberg A, Kahwaji CI. 2023. Biochemistry, electron transport chain. Treasure Island (FL): StatPearls Internet. [PubMed] [Google Scholar]
- Ahmad Z, Okafor F, Azim S, F. Laughlin T 2013. ATP synthase: a molecular therapeutic drug target for antimicrobial and antitumor peptides. Curr Med Chem. 20(15):1956–1973. doi: 10.2174/0929867311320150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter S, McDade HC, Gorlach JM, Heinrich G, Cox GM, Perfect JR. 2003. Role of alternative oxidase gene in pathogenesis of Cryptococcus neoformans. Infect Immun. 71(10):5794–5802. doi: 10.1128/IAI.71.10.5794-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanis AJ. 2005. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 36(6):697–705. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Alborn WE, Allen NE, Preston DA. 1991. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob Agents Chemother. 35(11):2282–2287. doi: 10.1128/AAC.35.11.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albury MS, Elliott C, Moore AL. 2009. Towards a structural elucidation of the alternative oxidase in plants. Physiol Plant. 137(4):316–327. doi: 10.1111/j.1399-3054.2009.01270.x. [DOI] [PubMed] [Google Scholar]
- Amaral L, Kristiansen JE, Viveiros M, Atouguia J. 2001. Activity of phenothiazines against antibiotic-resistant Mycobacterium tuberculosis: a review supporting further studies that may elucidate the potential use of thioridazine as anti-tuberculosis therapy. J Antimicrob Chemother. 47(5):505–511. doi: 10.1093/jac/47.5.505. [DOI] [PubMed] [Google Scholar]
- Andersson DI, Hughes D. 2011. Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol Rev. 35(5):901–911. doi: 10.1111/j.1574-6976.2011.00289.x. [DOI] [PubMed] [Google Scholar]
- André AC, Debande L, Marteyn BS. 2021. The selective advantage of facultative anaerobes relies on their unique ability to cope with changing oxygen levels during infection. Cell Microbiol. 23(8):e13338. doi: 10.1111/cmi.13338. [DOI] [PubMed] [Google Scholar]
- Andries K, Verhasselt P, Guillemont J, Göhlmann HWH, Neefs J-M, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, et al. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 307(5707):223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- Armbruster CE, Forsyth-DeOrnellas V, Johnson AO, Smith SN, Zhao L, Wu W, Mobley HLT. 2017. Genome-wide transposon mutagenesis of Proteus mirabilis: essential genes, fitness factors for catheter-associated urinary tract infection, and the impact of polymicrobial infection on fitness requirements. PLoS Pathog. 13(6):e1006434. doi: 10.1371/journal.ppat.1006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster CE, Mobley HLT, Pearson MM. 2018. Pathogenesis of Proteus mirabilis infection. EcoSal Plus. 8(1):2017. doi: 10.1128/ecosalplus.ESP-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aussel L, Pierrel F, Loiseau L, Lombard M, Fontecave M, Barras F. 2014. Biosynthesis and physiology of coenzyme Q in bacteria. Biochim Biophys Acta. 1837(7):1004–1011. doi: 10.1016/j.bbabio.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Avner BS, Fialho AM, Chakrabarty AM. 2012. Overcoming drug resistance in multi-drug resistant cancers and microorganisms: a conceptual framework. Bioengineered. 3(5):262–270. doi: 10.4161/bioe.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggish AL, Hill DR. 2002. Antiparasitic agent atovaquone. Antimicrob Agents Chemother. 46(5):1163–1173. doi: 10.1128/AAC.46.5.1163-1173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N, Heimhalt M, Liuni P, To F, Wilson DJ, Junop MS, Berti PJ. 2016. Potent inhibition of 3-deoxy-d-arabinoheptulosonate-7-phosphate (DAHP) synthase by DAHP oxime, a phosphate group mimic. Biochemistry. 55(48):6617–6629. doi: 10.1021/acs.biochem.6b00930. [DOI] [PubMed] [Google Scholar]
- Balemans W, Vranckx L, Lounis N, Pop O, Guillemont J, Vergauwen K, Mol S, Gilissen R, Motte M, Lançois D, et al. 2012. Novel antibiotics targeting respiratory ATP synthesis in gram-positive pathogenic bacteria. Antimicrob Agents Chemother. 56(8):4131–4139. doi: 10.1128/AAC.00273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquera B, Hellwig P, Zhou W, Morgan JE, Häse CC, Gosink KK, Nilges M, Bruesehoff PJ, Roth A, Lancaster CRD, et al. 2002. Purification and characterization of the recombinant Na(+)-translocating NADH:quinone oxidoreductase from Vibrio cholerae. Biochemistry. 41(11):3781–3789. doi: 10.1021/bi011873o. [DOI] [PubMed] [Google Scholar]
- Barquera B, Zhou W, Morgan JE, Gennis RB. 2002. Riboflavin is a component of the Na+-pumping NADH-quinone oxidoreductase from Vibrio cholerae. Proc Natl Acad Sci USA. 99(16):10322–10324. doi: 10.1073/pnas.162361299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, Krishna S. 2003. The trypanosomiases. Lancet. 362(9394):1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- Bassetti M, Merelli M, Temperoni C, Astilean A. 2013. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob. 12(1):22. doi: 10.1186/1476-0711-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M, Righi E. 2015. Development of novel antibacterial drugs to combat multiple resistant organisms. Langenbecks Arch Surg. 400(2):153–165. doi: 10.1007/s00423-015-1280-4. [DOI] [PubMed] [Google Scholar]
- Beites T, O’Brien K, Tiwari D, Engelhart CA, Walters S, Andrews J, Yang H-J, Sutphen ML, Weiner DM, Dayao EK, et al. 2019. Plasticity of the Mycobacterium tuberculosis respiratory chain and its impact on tuberculosis drug development. Nat Commun. 10(1):4970. doi: 10.1038/s41467-019-12956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénit P, Kahn A, Chretien D, Bortoli S, Huc L, Schiff M, Gimenez-Roqueplo A-P, Favier J, Gressens P, Rak M, et al. 2019. Evolutionarily conserved susceptibility of the mitochondrial respiratory chain to SDHI pesticides and its consequence on the impact of SDHIs on human cultured cells. PLoS One. 14(11):e0224132. doi: 10.1371/journal.pone.0224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. 2000. The protein data bank. Nucleic Acids Res. 28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry EA, Guergova-Kuras M, Huang L, Crofts AR. 2000. Structure and function of cytochrome bc complexes. Annu Rev Biochem. 69(1):1005–1075. doi: 10.1146/annurev.biochem.69.1.1005. [DOI] [PubMed] [Google Scholar]
- Berthold DA, Andersson ME, Nordlund P. 2000. New insight into the structure and function of the alternative oxidase. Biochim Biophys Acta. 1460(2–3):241–254. doi: 10.1016/s0005-2728(00)00149-3. [DOI] [PubMed] [Google Scholar]
- Berthold DA, Stenmark P. 2003. Membrane-bound diiron carboxylate proteins. Annu Rev Plant Biol. 54(1):497–517. doi: 10.1146/annurev.arplant.54.031902.134915. [DOI] [PubMed] [Google Scholar]
- Biagini GA, Fisher N, Berry N, Stocks PA, Meunier B, Williams DP, Bonar-Law R, Bray PG, Owen A, O’Neill PM, et al. 2008. Acridinediones: selective and potent inhibitors of the malaria parasite mitochondrial bc1 complex. Mol Pharmacol. 73(5):1347–1355. doi: 10.1124/mol.108.045120. [DOI] [PubMed] [Google Scholar]
- Biagini GA, Viriyavejakul P, O’neill PM, Bray PG, Ward SA. 2006. Functional characterization and target validation of alternative complex I of Plasmodium falciparum mitochondria. Antimicrob Agents Chemother. 50(5):1841–1851. doi: 10.1128/AAC.50.5.1841-1851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B, Lee C, Horianopoulos LC, Jung WH, Kronstad JW. 2021. Respiring to infect: emerging links between mitochondria, the electron transport chain, and fungal pathogenesis. PLoS Pathog. 17(7):e1009661. doi: 10.1371/journal.ppat.1009661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaza JN, Bridges HR, Aragão D, Dunn EA, Heikal A, Cook GM, Nakatani Y, Hirst J. 2017. The mechanism of catalysis by type-II NADH:quinone oxidoreductases. Sci Rep. 7(1):40165. doi: 10.1038/srep40165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer C, Haas D. 2000. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch Microbiol. 173(3):170–177. doi: 10.1007/s002039900127. [DOI] [PubMed] [Google Scholar]
- Borisov VB, Forte E, Davletshin A, Mastronicola D, Sarti P, Giuffrè A. 2013. Cytochrome bd oxidase from Escherichia coli displays high catalase activity: an additional defense against oxidative stress. FEBS Lett. 587(14):2214–2218. doi: 10.1016/j.febslet.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Borisov VB, Forte E, Siletsky SA, Sarti P, Giuffrè A. 2015. Cytochrome bd from Escherichia coli catalyzes peroxynitrite decomposition. Biochim Biophys Acta. 1847(2):182–188. doi: 10.1016/j.bbabio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Borisov VB, Gennis RB, Hemp J, Verkhovsky MI. 2011. The cytochrome bd respiratory oxygen reductases. Biochim Biophys Acta. 1807(11):1398–1413. doi: 10.1016/j.bbabio.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisov VB, Siletsky SA, Paiardini A, Hoogewijs D, Forte E, Giuffrè A, Poole RK. 2021. Bacterial oxidases of the cytochrome bd family: redox enzymes of unique structure, function, and utility as drug targets. Antioxid Redox Signal. 34(16):1280–1318. doi: 10.1089/ars.2020.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukink E, De Kruijff B. 1999. The lantibiotic nisin, a special case or not? Biochim Biophys Acta. 1462(1–2):223–234. doi: 10.1016/s0005-2736(99)00208-4. [DOI] [PubMed] [Google Scholar]
- Brøndsted L, Atlung T. 1996. Effect of growth conditions on expression of the acid phosphatase (cyx-appA) operon and the appY gene, which encodes a transcriptional activator of Escherichia coli. J Bacteriol. 178(6):1556–1564. doi: 10.1128/jb.178.6.1556-1564.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela R, Félix R, Clariano M, Nunes D, Perry M, de J, Lopes F. 2023. Target identification in anti-tuberculosis drug discovery. Int J Mol Sci. 24(13):10482. doi: 10.3390/ijms241310482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2024. TB 101 – length of TB disease treatment | TB [Internet]. [accessed 2024 Oct 5]. Available from: https://www.cdc.gov/tb/webcourses/TB101/page16411.html.
- Chai T-T, Colmer TD, Finnegan PM. 2010. Alternative oxidase, a determinant of plant gametophyte fitness and fecundity. Plant Signal Behav. 5(5):604–606. doi: 10.4161/psb.11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri M, Ott RD, Hill GC. 2006. Trypanosome alternative oxidase: from molecule to function. Trends Parasitol. 22(10):484–491. doi: 10.1016/j.pt.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Cheesman MR, Watmough NJ, Pires CA, Turner R, Brittain T, Gennis RB, Greenwood C, Thomson AJ. 1993. Cytochrome bo from Escherichia coli: identification of haem ligands and reaction of the reduced enzyme with carbon monoxide. Biochem J. 289(Pt 3):709–718. doi: 10.1042/bj2890709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng VWT, Piragasam RS, Rothery RA, Maklashina E, Cecchini G, Weiner JH. 2015. Redox state of flavin adenine dinucleotide drives substrate binding and product release in Escherichia coli succinate dehydrogenase. Biochemistry. 54(4):1043–1052. doi: 10.1021/bi501350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepuri V, Lemieux L, Au DC, Gennis RB. 1990. The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J Biol Chem. 265(19):11185–11192. [PubMed] [Google Scholar]
- Choi M, Cho H-S, Ahn B, Prathap S, Nagasundarapandian S, Park C. 2022. Genomewide analysis and biological characterization of cathelicidins with potent antimicrobial activity and low cytotoxicity from three bat species. Antibiot. Basel Switz. 11(8):989. doi: 10.3390/antibiotics11080989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SK, Schurig-Briccio L, Ding Z, Hong S, Sun C, Gennis RB. 2017. Location of the substrate binding site of the cytochrome bo3 ubiquinol oxidase from Escherichia coli. J Am Chem Soc. 139(24):8346–8354. doi: 10.1021/jacs.7b03883. [DOI] [PubMed] [Google Scholar]
- Clarkson ABC, Bienen EJ, Pollakis G, Grady RW. 1989. Respiration of bloodstream forms of the parasite Trypanosoma brucei brucei is dependent on a plant-like alternative oxidase. J Biol Chem. 264(30):17770–17776. doi: 10.1016/S0021-9258(19)84639-2. [DOI] [PubMed] [Google Scholar]
- Cook GM, Greening C, Hards K, Berney M. 2014. Energetics of pathogenic bacteria and opportunities for drug development. Adv Microb Physiol. 65:1–62. doi: 10.1016/bs.ampbs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Cotter PA, Chepuri V, Gennis RB, Gunsalus RP. 1990. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol. 172(11):6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’mello R, Hill S, Poole RK. 1996. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology. 142(4):755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- Dadgostar P 2019. Antimicrobial resistance: implications and costs. Infect Drug Resist. 12:3903–3910. doi: 10.2147/IDR.S234610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Davies D. 2010. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 74(3):417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejon L, Speicher A. 2013. Synthesis of aurachin D and isoprenoid analogues from the myxobacterium Stigmatella aurantiaca. Tetrahedron Lett. 54(49):6700–6702. doi: 10.1016/j.tetlet.2013.09.085. [DOI] [Google Scholar]
- Dhaliwal A, Bajaj T. 2025. Zafirlukast. In: StatPearls. Treasure Island (FL): StatPearls. [PubMed] [Google Scholar]
- Dibrov P, Dibrov E, Maddaford TG, Kenneth M, Nelson J, Resch C, Pierce GN. 2017. Development of a novel rationally designed antibiotic to inhibit a nontraditional bacterial target. Can J Physiol Pharmacol. 95(5):595–603. doi: 10.1139/cjpp-2016-0505. [DOI] [PubMed] [Google Scholar]
- Dickman R, Mitchell SA, Figueiredo AM, Hansen DF, Tabor AB. 2019. Molecular recognition of lipid II by lantibiotics: synthesis and conformational studies of analogues of nisin and mutacin rings A and B. J Org Chem. 84(18):11493–11512. doi: 10.1021/acs.joc.9b01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P 1994. Bacterial sodium ion-coupled energetics. Antonie Van Leeuwenhoek. 65(4):381–395. doi: 10.1007/BF00872221. [DOI] [PubMed] [Google Scholar]
- Dinant M, Baurain D, Coosemans N, Joris B, Matagne RF. 2001. Characterization of two genes encoding the mitochondrial alternative oxidase in Chlamydomonas reinhardtii. Curr Genet. 39(2):101–108. doi: 10.1007/s002940000183. [DOI] [PubMed] [Google Scholar]
- Doss M, White MR, Tecle T, Hartshorn KL. 2010. Human defensins and LL-37 in mucosal immunity. J Leukoc Biol. 87(1):79–92. doi: 10.1189/jlb.0609382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreinert A, Wolf A, Mentzel T, Meunier B, Fehr M. 2018. The cytochrome bc1 complex inhibitor Ametoctradin has an unusual binding mode. Biochim Biophys Acta Bioenerg. 1859(8):567–576. doi: 10.1016/j.bbabio.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Duvenage L, Walker LA, Bojarczuk A, Johnston SA, MacCallum DM, Munro CA, Gourlay CW. 2019. Inhibition of classical and alternative modes of respiration in Candida albicans leads to cell wall remodeling and increased macrophage recognition. mBio. 10(1):e02535–18. doi: 10.1128/mBio.02535-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilerman RG. 2000. Cinnamic acid, cinnamaldehyde, and cinnamyl alcohol. In: Kirk-Othmer encyclopedia of chemical technology. New York: John Wiley & Sons, Ltd. [Google Scholar]
- Elthon TE, McIntosh L. 1986. Characterization and solubilization of the alternative oxidase of Sauromatum guttatum mitochondria. Plant Physiol. 82(1):1–6. doi: 10.1104/pp.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon TE, McIntosh L. 1987. Identification of the alternative terminal oxidase of higher plant mitochondria. Proc Natl Acad Sci U S A. 84(23):8399–8403. doi: 10.1073/pnas.84.23.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority. 2012. Conclusion on the peer review of the pesticide risk assessment of the active substance ametoctradin (BAS 650 F). EFSA J. 10:2921. [Google Scholar]
- Federal Register. 2018. Bixafen; pesticide tolerances [Internet]. [accessed 2025 May 2]. Available from: https://www.federalregister.gov/documents/2018/12/04/2018-26348/bixafen-pesticide-tolerances.
- Federal Register. 2019. Fluopyram; pesticide tolerances [Internet]. [accessed 2025 May 2]. Available from: https://www.federalregister.gov/documents/2019/07/01/2019-13523/fluopyram-pesticide-tolerances.
- Federal Register. 2020. Cyazofamid; Pesticide tolerances [Internet]. [accessed 2025 May 2]. Available from: https://www.federalregister.gov/documents/2020/03/18/2020-04747/cyazofamid-pesticide-tolerances.
- Federal Register. 2025. Boscalid; pesticide tolerances [Internet]. [accessed 2025 May 2]. Available from: https://www.federalregister.gov/documents/2018/10/19/2018-22854/boscalid-pesticide-tolerances.
- Feinberg SM, Fariba KA, Saadabadi A. 2025. Thioridazine. In: StatPearls. Treasure Island (FL): StatPearls. [PubMed] [Google Scholar]
- Feng X, Zhu W, Schurig-Briccio LA, Lindert S, Shoen C, Hitchings R, Li J, Wang Y, Baig N, Zhou T, et al. 2015. Antiinfectives targeting enzymes and the proton motive force. Proc Natl Acad Sci USA. 112(51):E7073–E7082. doi: 10.1073/pnas.1521988112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Li W, Li J, Wang J, Ge J, Xu D, Liu Y, Wu K, Zeng Q, Wu J-W, et al. 2012. Structural insight into the type-II mitochondrial NADH dehydrogenases. Nature. 491(7424):478–482. doi: 10.1038/nature11541. [DOI] [PubMed] [Google Scholar]
- Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio. 4(1):e00537–00512. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte E, Borisov VB, Falabella M, Colaço HG, Tinajero-Trejo M, Poole RK, Vicente JB, Sarti P, Giuffrè A. 2016. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Sci Rep. 6(1):23788. doi: 10.1038/srep23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte E, Borisov VB, Vicente JB, Giuffrè A. 2017. Cytochrome bd and gaseous ligands in bacterial physiology. Adv Microb Physiol. 71:171–234. doi: 10.1016/bs.ampbs.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Franza T, Gaudu P. 2022. Quinones: more than electron shuttles. Res Microbiol. 173(6–7):103953. doi: 10.1016/j.resmic.2022.103953. [DOI] [PubMed] [Google Scholar]
- Fry M, Pudney M. 1992. Site of action of the antimalarial hydroxynaphthoquinone. Biochem Pharmacol. 43(7):1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- Fuller TE, Kennedy MJ, Lowery DE. 2000. Identification of Pasteurella multocida virulence genes in a septicemic mouse model using signature-tagged mutagenesis. Microb Pathog. 29(1):25–38. doi: 10.1006/mpat.2000.0365. [DOI] [PubMed] [Google Scholar]
- Galanis M, Mattoon JR, Nagley P. 1989. Amino acid substitutions in mitochondrial ATP synthase subunit 9 of Saccharomyces cerevisiae leading to venturicidin or ossamycin resistance. FEBS Lett. 249(2):333–336. doi: 10.1016/0014-5793(89)80653-2. [DOI] [PubMed] [Google Scholar]
- Gallagher DT, Mayhew M, Holden MJ, Howard A, Kim K, Vilker VL. 2001. The crystal structure of chorismate lyase shows a new fold and a tightly retained product. Proteins Struct. Funct. Bioinforma. 44(3):304–311. doi: 10.1002/prot.1095. [DOI] [PubMed] [Google Scholar]
- Garcia-Horsman JA. 1995. Proton transfer in cytochrome bo3 ubiquinol oxidase of Escherichia coli: second-site mutations in subunit I that restore proton pumping in the mutant Asp135-Asnt. Biochemistry. 34(13):4428–4433. [DOI] [PubMed] [Google Scholar]
- Gérin S, Mathy G, Blomme A, Franck F, Sluse FE. 2010. Plasticity of the mitoproteome to nitrogen sources (nitrate and ammonium) in Chlamydomonas reinhardtii: the logic of Aox1 gene localization. Biochim Biophys Acta. 1797(6–7):994–1003. doi: 10.1016/j.bbabio.2010.02.034. [DOI] [PubMed] [Google Scholar]
- González-Montalvo MA, Sorescu JM, Baltes G, Juárez O, Tuz K. 2024. The respiratory chain of Klebsiella aerogenes in urine-like conditions: critical roles of NDH-2 and bd-terminal oxidases. Front Microbiol. 15:1479714. doi: 10.3389/fmicb.2024.1479714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandier-Vazeille X, Bathany K, Chaignepain S, Camougrand N, Manon S, Schmitter J-M. 2001. Yeast mitochondrial dehydrogenases are associated in a supramolecular complex. Biochemistry. 40(33):9758–9769. doi: 10.1021/bi010277r. [DOI] [PubMed] [Google Scholar]
- Grauel A, Kägi J, Rasmussen T, Makarchuk I, Oppermann S, Moumbock AFA, Wohlwend D, Müller R, Melin F, Günther S, et al. 2021. Structure of Escherichia coli cytochrome bd-II type oxidase with bound aurachin D. Nat Commun. 12(1):6498. doi: 10.1038/s41467-021-26835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JE, Gawronski JD, DeJesus MA, Ioerger TR, Akerley BJ, Sassetti CM. 2011. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 7(9):e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin M, Camougrand N. 1986. The alternative oxidase of Candida parapsilosis. Eur J Biochem. 159(3):519–524. doi: 10.1111/j.1432-1033.1986.tb09917.x. [DOI] [PubMed] [Google Scholar]
- Guo H, Courbon GM, Bueler SA, Mai J, Liu J, Rubinstein JL. 2021. Structure of mycobacterial ATP synthase bound to the tuberculosis drug bedaquiline. Nature 589:143–147. doi: 10.1038/s41586-020-3004-3. [DOI] [PubMed] [Google Scholar]
- Gyan S, Shiohira Y, Sato I, Takeuchi M, Sato T. 2006. Regulatory loop between redox sensing of the NADH/NAD+ ratio by rex (YdiH) and oxidation of NADH by NADH dehydrogenase Ndh in Bacillus subtilis. J Bacteriol. 188(20):7062–7071. doi: 10.1128/JB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsma AC, Abdillahi-Ibrahim R, Wagner MJ, Krab K, Vergauwen K, Guillemont J, Andries K, Lill H, Koul A, Bald D. 2009. Selectivity of TMC207 towards mycobacterial ATP synthase compared with that towards the eukaryotic homologue. Antimicrob Agents Chemother. 53(3):1290–1292. doi: 10.1128/AAC.01393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägerhäll C 1997. Succinate: quinone oxidoreductases. Variations on a conserved theme. Biochim Biophys Acta. 1320(2):107–141. doi: 10.1016/s0005-2728(97)00019-4. [DOI] [PubMed] [Google Scholar]
- Hanqing F, Kun S, Mingquan L, Hongyu L, Xin L, Yan L, Yifeng W. 2010. The expression, function and regulation of mitochondrial alternative oxidase under biotic stresses. Mol Plant Pathol. 11(3):429–440. doi: 10.1111/j.1364-3703.2010.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häse CC, Barquera B. 2001. Role of sodium bioenergetics in Vibrio cholerae. Biochim Biophys Acta. 1505(1):169–178. doi: 10.1016/s0005-2728(00)00286-3. [DOI] [PubMed] [Google Scholar]
- Häse CC, Mekalanos JJ. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 95(2):730–734. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häse CC, Mekalanos JJ. 1999. Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 96(6):3183–3187. doi: 10.1073/pnas.96.6.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Nakayama Y, Unemoto T. 1996. Existence of Na+-translocating NADH-quinone reductase in Haemophilus influenzae. FEBS Lett. 381(3):174–176. doi: 10.1016/0014-5793(96)00114-7. [DOI] [PubMed] [Google Scholar]
- Hederstedt L, Rutberg L. 1981. Succinate dehydrogenase-a comparative review. Microbiol Rev. 45(4):542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikal A, Hards K, Cheung C-Y, Menorca A, Timmer MSM, Stocker BL, Cook GM. 2016. Activation of type II NADH dehydrogenase by quinolinequinones mediates antitubercular cell death. J Antimicrob Chemother. 71(10):2840–2847. doi: 10.1093/jac/dkw244. [DOI] [PubMed] [Google Scholar]
- Heikal A, Nakatani Y, Dunn E, Weimar MR, Day CL, Baker EN, Lott JS, Sazanov LA, Cook GM. 2014. Structure of the bacterial type II NADH dehydrogenase: a monotopic membrane protein with an essential role in energy generation: structure of bacterial NDH-2. Mol Microbiol. 91(5):950–964. doi: 10.1111/mmi.12507. [DOI] [PubMed] [Google Scholar]
- Henzler Wildman KA, Lee D-K, Ramamoorthy A. 2003. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 42(21):6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- Holden MJ, Mayhew MP, Gallagher DT, Vilker VL. 2002. Chorismate lyase: kinetics and engineering for stability. Biochim Biophys Acta. 1594(1):160–167. doi: 10.1016/s0167-4838(01)00302-8. [DOI] [PubMed] [Google Scholar]
- Hu C, Wen Q, Huang S, Xie S, Fang Y, Jin Y, Campagne R, Alezra V, Miclet E, Zhu J, et al. 2021. Gramicidin-S-inspired cyclopeptidomimetics as potent membrane-active bactericidal agents with therapeutic potential. ChemMedChem. 16(2):368–376. doi: 10.1002/cmdc.202000568. [DOI] [PubMed] [Google Scholar]
- Hu Y, Yuan M, Julian A, Tuz K, Juárez O. 2024. Identification of complex III, NQR, and SDH as primary bioenergetic enzymes during the stationary phase of Pseudomonas aeruginosa cultured in urine-like conditions. Front Microbiol. 15:1347466. doi: 10.3389/fmicb.2024.1347466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W-K, Kang S-O. 2001. Characterization of the gene family encoding alternative oxidase from Candida albicans. Biochem J. 356(Pt 2):595–604. doi: 10.1042/0264-6021:3560595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurdle JG, O’Neill AJ, Chopra I, Lee RE. 2011. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol. 9(1):62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Ogata T, Kakizaki Y, Elliott C, Albury MS, Moore AL. 2011. Identification of a gene for pyruvate-insensitive mitochondrial alternative oxidase expressed in the thermogenic appendices in Arum maculatum. Plant Physiol. 157(4):1721–1732. doi: 10.1104/pp.111.186932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Murai M, Ninokura S, Kitazumi Y, Mezic KG, Cress BF, Koffas MAG, Morgan JE, Barquera B, Miyoshi H. 2017. Identification of the binding sites for ubiquinone and inhibitors in the Na+-pumping NADH-ubiquinone oxidoreductase from Vibrio cholerae by photoaffinity labeling. J Biol Chem. 292(19):7727–7742. doi: 10.1074/jbc.M117.781393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S, Ostermeier C, Ludwig B, Michel H. 1995. Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 376(6542):660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- Jacewicz A, Izumi A, Brunner K, Schnell R, Schneider G. 2013. Structural insights into the UbiD protein family from the crystal structure of PA0254 from Pseudomonas aeruginosa. PLoS One. 8(5):e63161. doi: 10.1371/journal.pone.0063161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen S, Upton C, de Jager VR, van Niekerk C, Dawson R, Hutchings J, Kim J, Choi J, Nam K, Sun E, et al. 2025. Telacebec, a potent agent in the fight against tuberculosis: findings from A randomized, phase 2 clinical trial and beyond. Am J Respir Crit Care Med. 211(8):1504–1512. doi: 10.1164/rccm.202408-1632OC. [DOI] [PubMed] [Google Scholar]
- Jeffreys LN, Ardrey A, Hafiz TA, Dyer L-A, Warman AJ, Mosallam N, Nixon GL, Fisher NE, Hong WD, Leung SC, et al. 2023. Identification of 2-Aryl-quinolone inhibitors of cytochrome bd and chemical validation of combination strategies for respiratory inhibitors against Mycobacterium tuberculosis. ACS Infect Dis. 9(2):221–238. doi: 10.1021/acsinfecdis.2c00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PR and Michelsen O. 1992. Carbon and energy metabolism of ATP mutants of Escherichia coli. J Bacteriol, 174:7635–7641. doi: 10.1128/jb.174.23.7635-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesse HE, Nye TL, McLean S, Green J, Mann BE, Poole RK. 2013. Cytochrome bd-I in Escherichia coli is less sensitive than cytochromes bd-II or bo3 to inhibition by the carbon monoxide-releasing molecule, CORM-3. Biochim Biophys Acta. 1834(9):1693–1703. doi: 10.1016/j.bbapap.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas OB, Irwin A, Berthe FCJ, Le Gall F G, Marquez PV. 2017. Drug-resistant infections: a threat to our economic future (Vol. 2 of 2): final report [Internet]. World Bank. Available from: https://documents.worldbank.org/en/publication/documents-reports/documentdetail/en/323311493396993758. [Google Scholar]
- Jones-Carson J, Husain M, Liu L, Orlicky DJ, Vázquez-Torres A. 2016. Cytochrome bd -Dependent Bioenergetics and Antinitrosative Defenses in Salmonella Pathogenesis. mBio. 7(6):e02052–16. doi: 10.1128/mBio.02052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce AR, Reed JL, White A, Edwards R, Osterman A, Baba T, Mori H, Lesely SA, Palsson BØ, Agarwalla S. 2006. Experimental and computational assessment of conditionally essential genes in Escherichia coli. J Bacteriol. 188(23):8259–8271. doi: 10.1128/JB.00740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez O, Athearn K, Gillespie P, Barquera B. 2009. Acid residues in the transmembrane helices of the Na+-pumping NADH:quinone oxidoreductase from Vibrio cholerae involved in sodium translocation. Biochemistry. 48(40):9516–9524. doi: 10.1021/bi900845y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez O, Barquera B. 2012. Insights into the mechanism of electron transfer and sodium translocation of the Na+-pumping NADH:quinone oxidoreductase. Biochim. Biophys. Acta. 1817(10):1823–1832. doi: 10.1016/j.bbabio.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez O, Guerra G, Martínez F, Pardo JP. 2004. The mitochondrial respiratory chain of Ustilago maydis. Biochim Biophys Acta. 1658(3):244–251. doi: 10.1016/j.bbabio.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Juárez O, Guerra G, Velázquez I, Flores-Herrera O, Rivera-Pérez RE, Pardo JP. 2006. The physiologic role of alternative oxidase in Ustilago maydis. FEBS J. 273(20):4603–4615. doi: 10.1111/j.1742-4658.2006.05463.x. [DOI] [PubMed] [Google Scholar]
- Juárez O, Morgan JE, Barquera B. 2009. The electron transfer pathway of the Na+-pumping NADH: quinone oxidoreductase from Vibrio cholerae. J Biol Chem. 284(13):8963–8972. doi: 10.1074/jbc.M809395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez O, Morgan JE, Nilges MJ, Barquera B. 2010. Energy transducing redox steps of the Na+-pumping NADH:quinone oxidoreductase from Vibrio cholerae. Proc Natl Acad Sci U S A. 107(28):12505–12510. doi: 10.1073/pnas.1002866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez O, Nilges MJ, Gillespie P, Cotton J, Barquera B. 2008. Riboflavin is an active redox cofactor in the Na+-pumping NADH: quinone oxidoreductase (Na+-NQR) from Vibrio cholerae. J Biol Chem. 283(48):33162–33167. doi: 10.1074/jbc.M806913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jünemann S, Butterworth PJ, Wrigglesworth JM. 1995. A suggested mechanism for the catalytic cycle of cytochrome bd terminal oxidase based on kinetic analysis. Biochemistry. 34(45):14861–14867. doi: 10.1021/bi00045a029. [DOI] [PubMed] [Google Scholar]
- Junge W, Nelson N. 2015. ATP synthase. Annu Rev Biochem. 84(1):631–657. doi: 10.1146/annurev-biochem-060614-034124. [DOI] [PubMed] [Google Scholar]
- Juty NS, Hill S. 1997. The KIebsiella pneumoniae cytochrome bd’ terminal oxidase complex and its role in microaerobic nitrogen fixation. Microbiology. 143(Pt 8):2673–2683. [DOI] [PubMed] [Google Scholar]
- Kägi J, Sloan W, Schimpf J, Nasiri HR, Lashley D, Friedrich T. 2023. Exploring ND-011992, a quinazoline-type inhibitor targeting quinone reductases and quinol oxidases. Sci Rep. 13(1):12226. doi: 10.1038/s41598-023-39430-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila VRI, Wikström M. 2021. Architecture of bacterial respiratory chains. Nat Rev Microbiol. 19(5):319–330. doi: 10.1038/s41579-020-00486-4. [DOI] [PubMed] [Google Scholar]
- Kakkar AK, Dahiya N. 2014. Bedaquiline for the treatment of resistant tuberculosis: promises and pitfalls. Tuberculosis. 94(4):357–362. doi: 10.1016/j.tube.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Kamath KS, Pascovici D, Penesyan A, Goel A, Venkatakrishnan V, Paulsen IT, Packer NH, Molloy MP. 2016. Pseudomonas aeruginosa cell membrane protein expression from phenotypically diverse cystic fibrosis isolates demonstrates host-specific adaptations. J Proteome Res. 15(7):2152–2163. doi: 10.1021/acs.jproteome.6b00058. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. 2023. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51(D1):D587–D592. doi: 10.1093/nar/gkac963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. 2000. KEGG: Kyoto encyclopedia of genes and genomes [Internet]. Available from: https://www.genome.jp/kegg/ [DOI] [PMC free article] [PubMed]
- Kanehisa M 2019. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28(11):1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Higashiura N, Hayasaki K, Okamoto N, Takami A, Hirakawa H, Matsushita K, Azuma Y. 2015. Complete genome and gene expression analyses of Asaia bogorensis reveal unique responses to culture with mammalian cells as a potential opportunistic human pathogen. DNA Res. 22(5):357–366. doi: 10.1093/dnares/dsv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane CE, Steele AD, Fetzer C, Khowsathit J, Van Tyne D, Moynié L, Gilmore MS, Karanicolas J, Sieber SA, Wuest WM. 2018. Promysalin elicits species-selective inhibition of Pseudomonas aeruginosa by targeting succinate dehydrogenase. J Am Chem Soc. 140(5):1774–1782. doi: 10.1021/jacs.7b11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A, Hartner FS, Freigassner M, Spielhofer J, Rumpf C, Leitner L, Fröhlich K-U, Glieder A. 2007. Pichia pastoris ‘just in time’ alternative respiration. Microbiology. 153(4):1250–1260. doi: 10.1099/mic.0.2006/001404-0. [DOI] [PubMed] [Google Scholar]
- Kerscher S, Dröse S, Zickermann V, Brandt U. 2008. The three families of respiratory NADH dehydrogenases. Results Probl Cell Differ. 45:185–222. doi: 10.1007/400_2007_028. [DOI] [PubMed] [Google Scholar]
- Kessl JJ, Lange BB, Merbitz-Zahradnik T, Zwicker K, Hill P, Meunier B, Pálsdóttir H, Hunte C, Meshnick S, Trumpower BL. 2003. Molecular basis for atovaquone binding to the cytochrome bc1 complex. J Biol Chem. 278(33):31312–31318. doi: 10.1074/jbc.M304042200. [DOI] [PubMed] [Google Scholar]
- Khositnithikul R, Tan-Ariya P, Mungthin M. 2008. In vitro atovaquone/proguanil susceptibility and characterization of the cytochrome b gene of Plasmodium falciparum from different endemic regions of Thailand. Malar J. 7(1):23. doi: 10.1186/1475-2875-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido Y, Sakamoto K, Nakamura K, Harada M, Suzuki T, Yabu Y, Saimoto H, Yamakura F, Ohmori D, Moore A, et al. 2010. Purification and kinetic characterization of recombinant alternative oxidase from Trypanosoma brucei brucei. Biochim Biophys Acta. 1797(4):443–450. doi: 10.1016/j.bbabio.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Kim J, Choi J, Kang H, Ahn J, Hutchings J, van Niekerk C, Park D, Kim J, Jeon Y, Nam K, et al. 2022. Safety, tolerability, and pharmacokinetics of telacebec (Q203), a new antituberculosis agent, in healthy subjects. Antimicrob. Agents Chemother. 66:e01436–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee H, Oh I-S, Jeong HE, Bea S, Jang SH, Son H, Shin J-Y. 2023. Comparative safety of bedaquiline and delamanid in patients with multidrug resistant tuberculosis: a nationwide retrospective cohort study. J Microbiol Immunol Infect. 56(4):842–852. doi: 10.1016/j.jmii.2023.04.009. [DOI] [PubMed] [Google Scholar]
- Kim M, Kang N, Ko S, Park J, Park E, Shin D, Kim S, Lee S, Lee J, Lee S, et al. 2018. Antibacterial and antibiofilm activity and mode of action of magainin 2 against drug-resistant Acinetobacter baumannii. Int J Mol Sci. 19(10):3041. doi: 10.3390/ijms19103041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-Y, Randall JR, Gu R, Nguyen QD, Davies BW. 2024. Antibacterial action, proteolytic immunity, and in vivo activity of a Vibrio cholerae microcin. Cell Host Microbe. 32(11):1959–1971.e6. doi: 10.1016/j.chom.2024.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishikawa J, Ishikawa M, Masuya T, Murai M, Kitazumi Y, Butler NL, Kato T, Barquera B, Miyoshi H. 2022. Cryo-EM structures of Na+-pumping NADH-ubiquinone oxidoreductase from Vibrio cholerae. Nat Commun. 13(1):4082. doi: 10.1038/s41467-022-31718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knak T, Abdullaziz MA, Höfmann S, Alves Avelar LA, Klein S, Martin M, Fischer M, Tanaka N, Kurz T. 2022. Over 40 years of fosmidomycin drug research: a comprehensive review and future opportunities. Pharmaceuticals. 15(12):1553. doi: 10.3390/ph15121553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Yamamoto K, Kawagishi I, Homma M. 1999. The polar flagellar motor of Vibrio cholerae is driven by an Na+ motive force. J Bacteriol. 181(6):1927–1930. doi: 10.1128/JB.181.6.1927-1930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuya K, Sakura T, Shiomi K, Ōmura S, Hikosaka K, Nozaki T, Kita K, Inaoka DK. 2022. Siccanin is a dual-target inhibitor of Plasmodium falciparum mitochondrial complex II and complex III. Pharmaceuticals. 15(7):903. doi: 10.3390/ph15070903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B-M, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, Wapinski I, Galardini M, Cabal A, Peters JM, et al. 2017. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst. 4(3):291–305.e7. doi: 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov S, Imlay JA. 2010. Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol Microbiol. 75(6):1389–1401. doi: 10.1111/j.1365-2958.2010.07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. 2000. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother. 44(8):2100–2108. doi: 10.1128/AAC.44.8.2100-2108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalova T, Król S, Gamiz-Hernandez AP, Sjöstrand D, Kaila VRI, Brzezinski P, Högbom M. 2024. Inhibition mechanism of potential antituberculosis compound lansoprazole sulfide. Proc Natl Acad Sci U S A. 121(47):e2412780121. doi: 10.1073/pnas.2412780121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruth S, Nett M. 2023. Aurachins, bacterial antibiotics interfering with electron transport processes. Antibiotics. 12(6):1067. doi: 10.3390/antibiotics12061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W 2019. Structure and mechanisms of F-Type ATP synthases. Annu Rev Biochem. 88(1):515–549. doi: 10.1146/annurev-biochem-013118-110903. [DOI] [PubMed] [Google Scholar]
- Kunze B, Höfle G, Reichenbach H. 1987. The aurachins, new quinoline antibiotics from myxobacteria: production, physico-chemical and biological properties. J Antibiot. 40(3):258–265. doi: 10.7164/antibiotics.40.258. [DOI] [PubMed] [Google Scholar]
- Lechartier B, Cole ST. 2015. Mode of action of clofazimine and combination therapy with benzothiazinones against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 59(8):4457–4463. doi: 10.1128/AAC.00395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BS, Hards K, Engelhart CA, Hasenoehrl EJ, Kalia NP, Mackenzie JS, Sviriaeva E, Chong SMS, Manimekalai MSS, Koh VH, et al. 2021. Dual inhibition of the terminal oxidases eradicates antibiotic-tolerant Mycobacterium tuberculosis. EMBO Mol Med. 13(1):e13207. doi: 10.15252/emmm.202013207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E-J, Pontes MH, Groisman EA. 2013. A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium’s own F1Fo ATP synthase. Cell. 154(1):146–156. doi: 10.1016/j.cell.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ding S, Walpole TB, Holding AN, Montgomery MG, Fearnley IM, Walker JE. 2015. Organization of subunits in the membrane domain of the bovine F-ATPase revealed by covalent cross-linking. J Biol Chem. 290(21):13308–13320. doi: 10.1074/jbc.M115.645283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger AL. 1971. Bioenergetics: the molecular basis of biological energy transformations. New York: W.A. Benjamin. [Google Scholar]
- Lehrer RI, Lichtenstein AK, Ganz T. 1993. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 11(1):105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- Lencina AM, Franza T, Sullivan MJ, Ulett GC, Ipe DS, Gaudu P, Gennis RB, Schurig-Briccio LA. 2018. Type 2 NADH dehydrogenase is the only point of entry for electrons into the Streptococcus agalactiae respiratory chain and is a potential drug target. mBio. 9(4):e01034–18. doi: 10.1128/mBio.01034-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux P, Gredt M, Leroch M, Walker A-S. 2010. Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Appl Environ Microbiol. 76(19):6615–6630. doi: 10.1128/AEM.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K 2013. Platforms for antibiotic discovery. Nat Rev Drug Discov. 12(5):371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- Li J, Han L, Vallese F, Ding Z, Choi SK, Hong S, Luo Y, Liu B, Chan CK, Tajkhorshid E, et al. 2021. Cryo-EM structures of Escherichia coli cytochrome bo3 reveal bound phospholipids and ubiquinone-8 in a dynamic substrate binding site. Proc Natl Acad Sci. 118:e2106750118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang J, Zhou L, Shi H, Mai H, Su J, Ma X, Zhong J. 2023. The first lanthipeptide from Lactobacillus iners, Inecin L, exerts high antimicrobial activity against human vaginal pathogens. Appl Environ Microbiol. 89(3):e0212322. doi: 10.1128/aem.02123-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. 2003. DPI induces mitochondrial superoxide-mediated apoptosis. Free Radic Biol Med. 34(4):465–477. doi: 10.1016/s0891-5849(02)01325-4. [DOI] [PubMed] [Google Scholar]
- Li W, Estrada-de los Santos P, Matthijs S, Xie G-L, Busson R, Cornelis P, Rozenski J, De Mot R. 2011. Promysalin, a salicylate-Containing Pseudomonas putida antibiotic, promotes surface colonization and selectively targets other Pseudomonas. Chem Biol. 18(10):1320–1330. doi: 10.1016/j.chembiol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Liang H, Song Z-M, Zhong Z, Zhang D, Yang W, Zhou L, Older EA, Li J, Wang H, Zeng Z, et al. 2023. Genomic and metabolic analyses reveal antagonistic lanthipeptides in archaea. Microbiome. 11(1):74. doi: 10.1186/s40168-023-01521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Fang X, Hu Y, Yuan M, Raba DA, Ding J, Bunn DC, Sanjana K, Yang J, Rosas-Lemus M, et al. 2020. The aerobic respiratory chain of Pseudomonas aeruginosa cultured in artificial urine media: role of NQR and terminal oxidases. PLoS One. 15(4):e0231965. doi: 10.1371/journal.pone.0231965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Rosas-Lemus M, Patel D, Fang X, Tuz K, Juárez O. 2018. Dynamic energy dependency of Chlamydia trachomatis on host cell metabolism during intracellular growth: role of sodium-based energetics in chlamydial ATP generation. J Biol Chem. 293(2):510–522. doi: 10.1074/jbc.M117.797209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Åkerbladh L, Ahmad S, Konda V, Cao S, Vocat A, Maes L, Cole ST, Hughes D, Larhed M, et al. 2022. Synthesis and in vitro biological evaluation of quinolinyl pyrimidines targeting type II NADH-dehydrogenase (NDH-2). ACS Infect Dis. 8(3):482–498. doi: 10.1021/acsinfecdis.1c00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Lill H, Bald D. 2014. ATP synthase in mycobacteria: special features and implications for a function as drug target. Biochim Biophys Acta. 1837(7):1208–1218. doi: 10.1016/j.bbabio.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Luo M, Zhou W, Patel H, Srivastava AP, Symersky J, Bonar MM, Faraldo-Gómez JD, Liao M, Mueller DM. 2020. Bedaquiline inhibits the yeast and human mitochondrial ATP synthases. Commun Biol. 3(1):452. doi: 10.1038/s42003-020-01173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R 2013. Bedaquiline: first FDA-approved tuberculosis drug in 40 years. Int J Appl Basic Med Res. 3(1):1–2. doi: 10.4103/2229-516X.112228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo N, Lamas J, Leiro JM. 2013. Evidence of an alternative oxidase pathway for mitochondrial respiration in the Scuticociliate Philasterides dicentrarchi. Protist. 164(6):824–836. doi: 10.1016/j.protis.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Marreiros BC, Sena FV, Sousa FM, Batista AP, Pereira MM. 2016. Type II NADH:quinone oxidoreductase family: phylogenetic distribution, structural diversity and evolutionary divergences. Environ Microbiol. 18(12):4697–4709. doi: 10.1111/1462-2920.13352. [DOI] [PubMed] [Google Scholar]
- Marreiros BC, Sena FV, Sousa FM, Oliveira ASF, Soares CM, Batista AP, Pereira MM. 2017. Structural and functional insights into the catalytic mechanism of the Type II NADH:quinone oxidoreductase family. Sci Rep. 7(1):42303. doi: 10.1038/srep42303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Payne KAP, Fisher K, White MD, Ní Cheallaigh A, Balaikaite A, Rigby SEJ, Leys D. 2019. The UbiX flavin prenyltransferase reaction mechanism resembles class I terpene cyclase chemistry. Nat Commun. 10(1):2357. doi: 10.1038/s41467-019-10220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Shepherd M, Nicholls P, Dobbin PS, Dodsworth KS, Poole RK, Cooper CE. 2009. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat Chem Biol. 5(2):94–96. doi: 10.1038/nchembio.135. [DOI] [PubMed] [Google Scholar]
- Masuya T, Sano Y, Tanaka H, Butler NL, Ito T, Tosaki T, Morgan JE, Murai M, Barquera B, Miyoshi H. 2020. Inhibitors of a Na+-pumping NADH-ubiquinone oxidoreductase play multiple roles to block enzyme function. J Biol Chem. 295(36):12739–12754. doi: 10.1074/jbc.RA120.014229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathy G, Cardol P, Dinant M, Blomme A, Gérin S, Cloes M, Ghysels B, DePauw E, Leprince P, Remacle C, et al. 2010. Proteomic and functional characterization of a Chlamydomonas reinhardtii mutant lacking the mitochondrial alternative oxidase 1. J Proteome Res. 9(6):2825–2838. doi: 10.1021/pr900866e. [DOI] [PubMed] [Google Scholar]
- Matić S, Gilardi G, Gisi U, Gullino ML, Garibaldi A. 2019. Differentiation of Pythium spp. from vegetable crops with molecular markers and sensitivity to azoxystrobin and mefenoxam. Pest Manag Sci. 75(2):356–365. doi: 10.1002/ps.5119. [DOI] [PubMed] [Google Scholar]
- Matteelli A, Carvalho AC, Dooley KE, Kritski A. 2010. TMC207: the first compound of a new class of potent anti-tuberculosis drugs. Future Microbiol. 5(6):849–858. doi: 10.2217/fmb.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B, Young L, Moore AL. 2017. Structural insights into the alternative oxidases: are all oxidases made equal? Biochem Soc Trans. 45(3):731–740. doi: 10.1042/BST20160178. [DOI] [PubMed] [Google Scholar]
- McNeil MB, Fineran PC. 2013. Prokaryotic assembly factors for the attachment of flavin to complex II. Biochim Biophys Acta. 1827(5):637–647. doi: 10.1016/j.bbabio.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Meco-Navas A, Ebiloma GU, Martín-Domínguez A, Martínez-Benayas I, Cueto-Díaz EJ, Alhejely AS, Balogun EO, Saito M, Matsui M, Arai N, et al. 2018. SAR of 4-Alkoxybenzoic acid inhibitors of the trypanosome alternative oxidase. ACS Med Chem Lett. 9(9):923–928. doi: 10.1021/acsmedchemlett.8b00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medentsev AG, Arinbasarova AY, Golovchenko NP, Akimenko VK. 2002. Involvement of the alternative oxidase in respiration of Yarrowia lipolytica mitochondria is controlled by the activity of the cytochrome pathway. FEMS Yeast Res. 2(4):519–524. doi: 10.1111/j.1567-1364.2002.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Meganathan R 2001. Ubiquinone biosynthesis in microorganisms. FEMS Microbiol Lett. 203(2):131–139. doi: 10.1111/j.1574-6968.2001.tb10831.x. [DOI] [PubMed] [Google Scholar]
- Melo AMP, Bandeiras TM, Teixeira M. 2004. New insights into type II NAD(P)H: quinone oxidoreductases. Microbiol Mol Biol Rev. 68(4):603–616. doi: 10.1128/MMBR.68.4.603-616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies SK, Tulloch LB, Florence GJ, Smith TK. 2018. The trypanosome alternative oxidase: a potential drug target? Parasitology. 145(2):175–183. doi: 10.1017/S0031182016002109. [DOI] [PubMed] [Google Scholar]
- Meunier B, Madgwick SA, Rei E. 1995. New inhibitors of the quinol oxidation sites of bacterial cytochromes bo and bd. Biochemistry. 24;34(3):1076–1083. [DOI] [PubMed] [Google Scholar]
- Milani G, Jarmuszkiewicz W, Sluse-Goffart CM, Schreiber AZ, Vercesi AE, Sluse FE. 2001. Respiratory chain network in mitochondria of Candida parapsilosis: ADP/O appraisal of the multiple electron pathways. FEBS Lett. 508(2):231–235. doi: 10.1016/s0014-5793(01)03060-5. [DOI] [PubMed] [Google Scholar]
- Minghetti KC, Goswitz VC, Gabriel NE, Hill JJ, Barassi CA, Georgiou CD, Chan SI, Gennis RB. 1992. Modified, large-scale purification of the cytochrome o complex (bo-type oxidase) of Escherichia coli yields a two heme/one copper terminal oxidase with high specific activity. Biochemistry. 31(30):6917–6924. doi: 10.1021/bi00145a008. [DOI] [PubMed] [Google Scholar]
- Mitchell P 2011. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. 1966. Biochim Biophys Acta. 1807(12):1507–1538. doi: 10.1016/j.bbabio.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Takegami K, Sakamoto K, Mogi T, Iwamura H. 1999. Characterization of the ubiquinol oxidation sites in cytochromes bo and bd from Escherichia coli using Aurachin C analogues. J Biochem. 125(1):138–142. doi: 10.1093/oxfordjournals.jbchem.a022250. [DOI] [PubMed] [Google Scholar]
- Miyoshi-Akiyama T, Hayashi M, Unemoto T. 1993. Purification and properties of cytochrome bo-type ubiquinol oxidase from a marine bacterium Vibrio alginolyticus. Biochim Biophys Acta. 1141(2–3):283–287. doi: 10.1016/0005-2728(93)90054-j. [DOI] [PubMed] [Google Scholar]
- Mogi T, Kawakami T, Arai H, Igarashi Y, Matsushita K, Mori M, Shiomi K, Omura S, Harada S, Kita K. 2009. Siccanin rediscovered as a species-selective succinate dehydrogenase inhibitor. J Biochem. 146(3):383–387. doi: 10.1093/jb/mvp085. [DOI] [PubMed] [Google Scholar]
- Mogi T, Nakamura H, Anraku Y. 1994. Molecular Structure of a heme-copper redox center of the Escherichia coli ubiquinol oxidase: evidence and Model1. J Biochem. 116(3):471–477. doi: 10.1093/oxfordjournals.jbchem.a124548. [DOI] [PubMed] [Google Scholar]
- Moore AL, Albury MS. 2008. Further insights into the structure of the alternative oxidase: from plants to parasites. Biochem Soc Trans. 36(Pt 5):1022–1026. doi: 10.1042/BST0361022. [DOI] [PubMed] [Google Scholar]
- Moore AL, Shiba T, Young L, Harada S, Kita K, Ito K. 2013. Unraveling the heater: new insights into the structure of the alternative oxidase. Annu Rev Plant Biol. 64(1):637–663. doi: 10.1146/annurev-arplant-042811-105432. [DOI] [PubMed] [Google Scholar]
- Moore AL, Siedow JN. 1991. The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta. 1059(2):121–140. doi: 10.1016/s0005-2728(05)80197-5. [DOI] [PubMed] [Google Scholar]
- Moreno-Vargas LM, Prada-Gracia D. 2024. Exploring the chemical features and biomedical relevance of cell-penetrating peptides. Int J Mol Sci. 26(1):59. doi: 10.3390/ijms26010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AD, Doeller JE, Hearn B, Lang-Unnasch N. 1997. Plasmodium falciparum: cyanide-resistant oxygen consumption. Exp Parasitol. 87(2):112–120. doi: 10.1006/expr.1997.4194. [DOI] [PubMed] [Google Scholar]
- Murphy AD, Lang-Unnasch N. 1999. Alternative oxidase inhibitors potentiate the activity of atovaquone against Plasmodium falciparum. Antimicrob Agents Chemother. 43(3):651–654. doi: 10.1128/AAC.43.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, Han C, Bisignano C, Rao P, Wool E, et al. 2022. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser SM, Chan SI. 1998. Evolution of the cytochrome c oxidase proton pump. J Mol Evol. 46(5):508–520. doi: 10.1007/pl00006332. [DOI] [PubMed] [Google Scholar]
- Naghavi M, Vollset SE, Ikuta KS, Swetschinski LR, Gray AP, Wool EE, Aguilar GR, Mestrovic T, Smith G, Han C, et al. 2024. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet. 404(10459):1199–1226. doi: 10.1016/S0140-6736(24)01867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy K, Tessema RA, Budnik LT, Ádám B. 2019. Comparative cyto- and genotoxicity assessment of glyphosate and glyphosate-based herbicides in human peripheral white blood cells. Environ Res. 179(Pt B):108851. doi: 10.1016/j.envres.2019.108851. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Saiki K, Mogi T, Anraku Y. 1997. Assignment and functional roles of the cyoABCDE gene products required for the Escherichia coli bo-type quinol oxidase. J Biochem. 122(2):415–421. doi: 10.1093/oxfordjournals.jbchem.a021769. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Jiao W, Aragão D, Shimaki Y, Petri J, Parker EJ, Cook GM. 2017. Crystal structure of type II NADH:quinone oxidoreductase from Caldalkalibacillus thermarum with an improved resolution of 2.15 Å. Acta Crystallogr F Struct Biol Commun. 73(Pt 10):541–549. doi: 10.1107/S2053230X17013073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y, Shimaki Y, Dutta D, Muench SP, Ireton K, Cook GM, Jeuken LJC. 2020. Unprecedented properties of phenothiazines unraveled by a NDH-2 bioelectrochemical assay platform. J Am Chem Soc. 142(3):1311–1320. doi: 10.1021/jacs.9b10254. [DOI] [PubMed] [Google Scholar]
- Nastasi MR, Borisov VB, Forte E. 2023. The terminal oxidase cytochrome bd-I confers carbon monoxide resistance to Escherichia coli cells. J Inorg Biochem. 247:112341. doi: 10.1016/j.jinorgbio.2023.112341. [DOI] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. 2012a. Trifluoperazine. In: LiverTox: clinical and research information on drug-induced liver injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. 2012b. Chlorpromazine. In: LiverTox: clinical and research information on drug-induced liver injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. 2012c. Tricalbendazole. In: LiverTox: clinical and research information on drug-induced liver injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. 2012d. Atovaquone. In: LiverTox: clinical and research information on drug-induced liver injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. [accessed 2025 May 2]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK548592/. [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. 2012e. Lansoprazole. In: LiverTox: clinical and research information on drug-induced liver injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. [accessed 2025 May 2]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK548452/. [Google Scholar]
- Nesci S, Trombetti F, Ventrella V, Pagliarani A. 2016. The c-ring of the F1FO-ATP synthase: facts and perspectives. J Membr Biol. 249(1–2):11–21. doi: 10.1007/s00232-015-9860-3. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, He X, Alexander DC, Li C, Gu J-Q, Mascio C, Van Praagh A, Mortin L, Chu M, Silverman JA, et al. 2010. Genetically engineered lipopeptide antibiotics related to A54145 and daptomycin with improved properties. Antimicrob Agents Chemother. 54(4):1404–1413. doi: 10.1128/AAC.01307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. 2002. Bioenergetics. Amsterdam: Elsevier. [Google Scholar]
- Nixon GL, Moss DM, Shone AE, Lalloo DG, Fisher N, O’Neill PM, Ward SA, Biagini GA. 2013. Antimalarial pharmacology and therapeutics of atovaquone. J Antimicrob Chemother. 68(5):977–985. doi: 10.1093/jac/dks504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolfi-Donegan D, Braganza A, Shiva S. 2020. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 37:101674. doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha S, Meng EC, Babbitt PC. 2007. Evolution of function in the “two dinucleotide binding domains” flavoproteins. PLoS Comput Biol. 3(7):e121. doi: 10.1371/journal.pcbi.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno D, Iino R, Noji H. 2011. Rotation and structure of FoF1-ATP synthase. J Biochem. 149(6):655–664. doi: 10.1093/jb/mvr049. [DOI] [PubMed] [Google Scholar]
- O’Neill J 2016. Tackling drug-resistant infections globally: final report and recommendations. Review on Antimicrobial Resistance. Available from: https://amr-review.org/. [Google Scholar]
- Ou S, Kwok K-C. 2004. Ferulic acid: pharmaceutical functions, preparation and applications in foods. J Sci Food Agric. 84(11):1261–1269. doi: 10.1002/jsfa.1873. [DOI] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. 2006. How many drug targets are there? Nat Rev Drug Discov. 5(12):993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Oyedotun KS, Lemire BD. 2001. The quinone-binding sites of the Saccharomyces cerevisiae succinate-ubiquinone oxidoreductase. J Biol Chem. 276(20):16936–16943. doi: 10.1074/jbc.M100184200. [DOI] [PubMed] [Google Scholar]
- Pearson MM, Yep A, Smith SN, Mobley HLT. 2011. Transcriptome of Proteus mirabilis in the murine urinary tract: virulence and nitrogen assimilation gene expression. Infect Immun. 79(7):2619–2631. doi: 10.1128/IAI.05152-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, et al. 2013. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med. 19(9):1157–1160. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- Petri J, Shimaki Y, Jiao W, Bridges HR, Russell ER, Parker EJ, Aragão D, Cook GM, Nakatani Y. 2018. Structure of the NDH-2 – HQNO inhibited complex provides molecular insight into quinone-binding site inhibitors. Biochim Biophys Acta Bioenerg. 1859(7):482–490. doi: 10.1016/j.bbabio.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Ueno S, Zhang H, Lee JM, Kato Y. 2005. Cecropin P1 and novel nematode cecropins: a bacteria-inducible antimicrobial peptide family in the nematode Ascaris suum. Biochem J. 390(Pt 1):207–214. doi: 10.1042/BJ20050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RK, Cook GM. 2000. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv Microb Physiology, 43:165–224. [DOI] [PubMed] [Google Scholar]
- Pöppe J, Bote K, Merle R, Makarova O, Roesler U. 2019. Minimum inhibitory concentration of glyphosate and a glyphosate-containing herbicide in Salmonella enterica isolates originating from different time periods, hosts, and serovars. Eur J Microbiol Immunol (Bp). 9(2):35–41. doi: 10.1556/1886.2019.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen BE, Yang R, Clatworthy AE, White T, Osmulski SJ, Li L, Penaranda C, Lander ES, Shoresh N, Hung DT. 2019. Defining the core essential genome of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 116(20):10072–10080. doi: 10.1073/pnas.1900570116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss L, Langer JD, Yildiz Ö, Eckhardt-Strelau L, Guillemont JEG, Koul A, Meier T. 2015. Structure of the mycobacterial ATP synthase Fo rotor ring in complex with the anti-TB drug bedaquiline. Sci Adv. 1(4):e1500106. doi: 10.1126/sciadv.1500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Edgerton M. 2014. How does it kill?: understanding the candidacidal mechanism of salivary histatin 5. Eukaryot Cell. 13(8):958–964. doi: 10.1128/EC.00095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman M, van Veen HW, Konings WN. 2000. Molecular properties of bacterial multidrug transporters. Microbiol Mol Biol Rev. 64(4):672–693. doi: 10.1128/MMBR.64.4.672-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puustinen A, Finel M, Virkki M, Wikström M. 1989. Cytochrome o (bo) is a proton pump in Paracoccus denitrificans and Escherichia coli. FEBS Lett. 249(2):163–167. doi: 10.1016/0014-5793(89)80616-7. [DOI] [PubMed] [Google Scholar]
- Quesada A, Guijo MI, Merchán F, Blázquez B, Igeño MI, Blasco R. 2007. Essential role of cytochrome bd-related oxidase in cyanide resistance of Pseudomonas pseudoalcaligenes CECT5344. Appl Environ Microbiol. 73(16):5118–5124. doi: 10.1128/AEM.00503-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raba DA, Rosas-Lemus M, Menzer WM, Li C, Fang X, Liang P, Tuz K, Minh DDL, Juárez O. 2018. Characterization of the Pseudomonas aeruginosa NQR complex, a bacterial proton pump with roles in autopoisoning resistance. J Biol Chem. 293(40):15664–15677. doi: 10.1074/jbc.RA118.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff M, Elamri I, Grund TN, Witte LF, Hohmann KF, Nakagaki S, Goojani HG, Nasiri H, Hideto Miyoshi, Bald D, Xie H, Sakamoto J, Schwalbe H, and Safarian S (2021) Short-chain aurachin D derivatives are selective inhibitors of E. coli cytochrome bd-I and bd-II oxidases. Sci Rep, 1. 11, 23852 doi: 10.1038/s41598-021-03288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MMO, Moghal MMR, Billah MM, Hasan M, Yamazaki M. 2020. Effect of membrane potential on pore formation by the antimicrobial peptide magainin 2 in lipid bilayers. Biochim Biophys Acta Biomembr. 1862(10):183381. doi: 10.1016/j.bbamem.2020.183381. [DOI] [PubMed] [Google Scholar]
- Rasmusson AG, Fernie AR, Van Dongen JT. 2009. Alternative oxidase: a defence against metabolic fluctuations? Physiol Plant. 137(4):371–382. doi: 10.1111/j.1399-3054.2009.01252.x. [DOI] [PubMed] [Google Scholar]
- Reyes-Prieto A, Barquera B, Juárez O. 2014. Origin and evolution of the sodium-pumping NADH: ubiquinone oxidoreductase. PLoS One. 9(5):e96696. doi: 10.1371/journal.pone.0096696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CW, Hempfling WP. 1978. Oxygen-linmited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DJ. 2000. Bacterial respiration: a flexible process for a changing environment. Microbiology. 146(Pt 3):551–571. [DOI] [PubMed] [Google Scholar]
- Richhardt J, Luchterhand B, Bringer S, Büchs J, Bott M. 2013. Evidence for a key role of cytochrome bo3 oxidase in respiratory energy metabolism of Gluconobacter oxydans. J Bacteriol. 195(18):4210–4220. doi: 10.1128/JB.00470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridyard KE, Overhage J. 2021. The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics. 10(6):650. doi: 10.3390/antibiotics10060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Roberts F, Henriquez FL, Akiyoshi D, Samuel BU, Richards TA, Milhous W, Kyle D, McIntosh L, Hill GC, et al. 2004. Evidence for mitochondrial-derived alternative oxidase in the apicomplexan parasite Cryptosporidium parvum: a potential anti-microbial agent target. Int J Parasitol. 34(3):297–308. doi: 10.1016/j.ijpara.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Roberts GCK, editor. 2013. Encyclopedia of biophysics. Berlin, Heidelberg: Springer. [Google Scholar]
- Rodrigues ET, Lopes I, Pardal MÂ. 2013. Occurrence, fate and effects of azoxystrobin in aquatic ecosystems: a review. Environ Int. 53:18–28. doi: 10.1016/j.envint.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Ruy F, Vercesi AE, Kowaltowski AJ. 2006. Inhibition of specific electron transport pathways leads to oxidative stress and decreased Candida albicans proliferation. J Bioenerg Biomembr. 38(2):129–135. doi: 10.1007/s10863-006-9012-7. [DOI] [PubMed] [Google Scholar]
- Rybniker J, Vocat A, Sala C, Busso P, Pojer F, Benjak A, Cole ST. 2015. Lansoprazole is an antituberculous prodrug targeting cytochrome bc1. Nat Commun. 6(1):7659. doi: 10.1038/ncomms8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safarian S, Rajendran C, Müller H, Preu J, Langer JD, Ovchinnikov S, Hirose T, Kusumoto T, Sakamoto J, Michel H. 2016. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science. 352(6285):583–586. doi: 10.1126/science.aaf2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki K, Mogi T, Anraku Y. 1992. Heme o biosynthesis in Escherichia coli: the cyoE gene in the cytochrome bo operon encodes a protoheme IX farnesyltransferase. Biochem Biophys Res Commun. 189(3):1491–1497. doi: 10.1016/0006-291x(92)90243-e. [DOI] [PubMed] [Google Scholar]
- Saiki K, Mogi T, Ogura K, Anraku Y. 1993. In vitro heme o synthesis by the cyoE gene product from Escherichia coli. J Biol Chem. 268(35):26041–26044. [PubMed] [Google Scholar]
- Sakamoto J, Koga E, Mizuta T, Sato C, Noguchi S, Sone N. 1999. Gene structure and quinol oxidase activity of a cytochrome bd-type oxidase from Bacillus stearothermophilus. Biochim Biophys Acta. 1411(1):147–158. doi: 10.1016/s0005-2728(99)00012-2. [DOI] [PubMed] [Google Scholar]
- Saleh A, Friesen J, Baumeister S, Gross U, Bohne W. 2007. Growth inhibition of Toxoplasma gondii and Plasmodium falciparum by nanomolar concentrations of 1-hydroxy-2-dodecyl-4(1 H) quinolone, a high-affinity inhibitor of alternative (Type II) NADH dehydrogenases. Antimicrob Agents Chemother. 51(4):1217–1222. doi: 10.1128/AAC.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang H, Lee HB. 2020. Molecular mechanisms of succinate dehydrogenase inhibitor resistance in phytopathogenic fungi. Res Plant Dis. 26:1–7. [Google Scholar]
- Santos G, Torres NV. 2013. New targets for drug discovery against malaria. PLoS One. 8(3):e59968. doi: 10.1371/journal.pone.0059968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti CM, Boyd DH, Rubin EJ. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 48(1):77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- Scalliet G, Bowler J, Luksch T, Kirchhofer-Allan L, Steinhauer D, Ward K, Niklaus M, Verras A, Csukai M, Daina A, et al. 2012. Mutagenesis and functional studies with succinate dehydrogenase inhibitors in the wheat pathogen Mycosphaerella graminicola. PLoS One. 7(4):e35429. doi: 10.1371/journal.pone.0035429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer G, Penefsky HS, editors. 2008. Bioenergetics, results and problems in cell differentiation Berlin, Heidelberg: Springer Berlin Heidelberg. [PubMed] [Google Scholar]
- Schägger H 2002. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim Biophys Acta. 1555(1–3):154–159. doi: 10.1016/s0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- Schmucker T 1925. Beitrage zur Biologie und Physiologie von Arun maculatum. Flora. 118:460–475. [Google Scholar]
- Schnippel K, Ndjeka N, Maartens G, Meintjes G, Master I, Ismail N, Hughes J, Ferreira H, Padanilam X, Romero R, et al. 2018. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med. 6(9):699–706. doi: 10.1016/S2213-2600(18)30235-2. [DOI] [PubMed] [Google Scholar]
- Schurig-Briccio LA, Yano T, Rubin H, Gennis RB. 2014. Characterization of the type 2 NADH:menaquinone oxidoreductases from Staphylococcus aureus and the bactericidal action of phenothiazines. Biochim Biophys Acta. 1837(7):954–963. doi: 10.1016/j.bbabio.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellamuthu S, Singh M, Kumar A, Singh SK. 2017. Type-II NADH dehydrogenase (NDH-2): a promising therapeutic target for antitubercular and antibacterial drug discovery. Expert Opin Ther Targets. 21(6):559–570. doi: 10.1080/14728222.2017.1327577. [DOI] [PubMed] [Google Scholar]
- Shahid M, Sobia F, Singh A, Malik A, Khan HM, Jonas D, Hawkey PM. 2009. Beta-lactams and beta-lactamase-inhibitors in current- or potential-clinical practice: a comprehensive update. Crit Rev Microbiol. 35(2):81–108. doi: 10.1080/10408410902733979. [DOI] [PubMed] [Google Scholar]
- Sharpless T, Butow R. 1970. An inducible alternate terminal oxidase in Euglena gracilis mitochondria. J Biol Chem. 245(1):58–70. doi: 10.1016/S0021-9258(18)63422-2. [DOI] [PubMed] [Google Scholar]
- Sheehan BJ, Bossé JT, Beddek AJ, Rycroft AN, Kroll JS, Langford PR. 2003. Identification of Actinobacillus pleuropneumoniae genes important for survival during infection in its natural host. Infect Immun. 71(7):3960–3970. doi: 10.1128/IAI.71.7.3960-3970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Gao L, Yang M, Zhang J, Wang Y, Feng Y, Wang L, Wang S. 2021. Deletion of the PA4427-PA4431 operon of Pseudomonas aeruginosa PAO1 increased antibiotics resistance and reduced virulence and pathogenicity by affecting quorum sensing and iron uptake. Microorganisms. 9(5):1065. doi: 10.3390/microorganisms9051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende VV, Bauman KD, Moore BS. 2024. The shikimate pathway: gateway to metabolic diversity. Nat Prod Rep. 41(4):604–648. doi: 10.1039/d3np00037k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T, Inaoka DK, Takahashi G, Tsuge C, Kido Y, Young L, Ueda S, Balogun EO, Nara T, Honma T, et al. 2019. Insights into the ubiquinol/dioxygen binding and proton relay pathways of the alternative oxidase. Biochim Biophys Acta Bioenerg. 1860(5):375–382. doi: 10.1016/j.bbabio.2019.03.008. [DOI] [PubMed] [Google Scholar]
- Shiba T, Kido Y, Sakamoto K, Inaoka DK, Tsuge C, Tatsumi R, Takahashi G, Balogun EO, Nara T, Aoki T, et al. 2013. Structure of the trypanosome cyanide-insensitive alternative oxidase. Proc Natl Acad Sci U S A. 110(12):4580–4585. doi: 10.1073/pnas.1218386110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirude PS, Paul B, Roy Choudhury N, Kedari C, Bandodkar B, Ugarkar BG. 2012. Quinolinyl pyrimidines: potent inhibitors of NDH-2 as a novel class of anti-TB agents. ACS Med Chem Lett. 3(9):736–740. doi: 10.1021/ml300134b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumilin IA, Bauerle R, Wu J, Woodard RW, Kretsinger RH. 2004. Crystal structure of the reaction complex of 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase from Thermotoga maritima refines the catalytic mechanism and indicates a new mechanism of allosteric regulation. J Mol Biol. 341(2):455–466. doi: 10.1016/j.jmb.2004.05.077. [DOI] [PubMed] [Google Scholar]
- Sierra-Campos E, Velázquez I, Matuz-Mares D, Villavicencio-Queijeiro A, Pardo JP. 2009. Functional properties of the Ustilago maydis alternative oxidase under oxidative stress conditions. Mitochondrion. 9(2):96–102. doi: 10.1016/j.mito.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Siletsky SA, Borisov VB. 2021. Proton pumping and non-pumping terminal respiratory oxidases: active sites intermediates of these molecular machines and their derivatives. Int J Mol Sci. 22(19):10852. doi: 10.3390/ijms221910852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu T, Debnath P. 2022. Cytochrome bc1-aa3 oxidase supercomplex as emerging and potential drug target against tuberculosis. Curr Mol Pharmacol. 15(2):380–392. doi: 10.2174/1874467214666210928152512. [DOI] [PubMed] [Google Scholar]
- Skurni D, Roux D, Aschard H, Cattoir V, Yoder-Himes D, Lory S, Pier GB. 2013. A comprehensive analysis of in vitro and in vivo genetic fitness of Pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. doi: 10.1371/journal.ppat.1003582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingerland CJ, Kotsogianni I, Wesseling CMJ, Martin NI. 2022. Polymyxin stereochemistry and its role in antibacterial activity and outer membrane disruption. ACS Infect Dis. 8(12):2396–2404. doi: 10.1021/acsinfecdis.2c00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HB, Lee K, Freeman MJ, Stevenson DM, Amador-Noguez D, Sauer J-D. 2023. Listeria monocytogenes requires DHNA-dependent intracellular redox homeostasis facilitated by Ndh2 for survival and virulence. Infect Immun. 91(10):e00022–23. doi: 10.1128/iai.00022-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N, Roitberg AE, Rivera E, Howard A, Holden MJ, Mayhew M, Kaistha S, Gallagher DT. 2006. Structural analysis of ligand binding and catalysis in chorismate lyase. Arch Biochem Biophys. 445(1):72–80. doi: 10.1016/j.abb.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Søballe B, Poole RK. 1999. Microbial ubiquinones: multiple roles in respiration, gene regulation and oxidative stress management. Microbiology. 145(Pt 8):1817–1830. doi: 10.1099/13500872-145-8-1817. [DOI] [PubMed] [Google Scholar]
- Song H, Bergstrasser C, Rafat N, Höger S, Schmidt M, Endres N, Goebeler M, Hillebrands J, Brigelius-Flohé R, Banning A, et al. 2009. The carbon monoxide releasing molecule (CORM-3) inhibits expression of vascular cell adhesion molecule-1 and E-selectin independently of haem oxygenase-1 expression. Br J Pharmacol. 157(5):769–780. doi: 10.1111/j.1476-5381.2009.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorayah R, Manimekalai MSS, Shin SJ, Koh W-J, Grüber G, Pethe K. 2019. Naturally-occurring polymorphisms in QcrB are responsible for resistance to Telacebec in Mycobacterium abscessus. ACS Infect Dis. 5(12):2055–2060. doi: 10.1021/acsinfecdis.9b00322. [DOI] [PubMed] [Google Scholar]
- Southam HM, Smith TW, Lyon RL, Liao C, Trevitt CR, Middlemiss LA, Cox FL, Chapman JA, El-Khamisy SF, Hippler M, et al. 2018. A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3. Redox Biol. 18:114–123. doi: 10.1016/j.redox.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, et al. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 282(5389):754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- Steuber J, Vohl G, Muras V, Toulouse C, Claußen B, Vorburger T, Fritz G. 2015. The structure of Na+-translocating of NADH:ubiquinone oxidoreductase of Vibrio cholerae: implications on coupling between electron transfer and Na + transport. Biol Chem. 396(9–10):1015–1030. doi: 10.1515/hsz-2015-0128. [DOI] [PubMed] [Google Scholar]
- Sutton KA, Breen J, Russo TA, Schultz LW, Umland TC. 2016. Crystal structure of 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase from the ESKAPE pathogen Acinetobacter baumannii. Acta Crystallogr F Struct Biol Commun. 72(Pt 3):179–187. doi: 10.1107/S2053230X16001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Hashimoto T, Yabu Y, Majiwa PAO, Ohshima S, Suzuki M, Lu S, Hato M, Kido Y, Sakamoto K, et al. 2005. Alternative oxidase (AOX) genes of African trypanosomes: phylogeny and evolution of AOX and plastid terminal oxidase families. J Eukaryot Microbiol. 52(4):374–381. doi: 10.1111/j.1550-7408.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- Sykes R 2010. The 2009 Garrod lecture: the evolution of antimicrobial resistance: a Darwinian perspective. J Antimicrob Chemother. 65(9):1842–1852. doi: 10.1093/jac/dkq217. [DOI] [PubMed] [Google Scholar]
- Tally FP, DeBruin MF. 2000. Development of daptomycin for Gram-positive infections. J Antimicrob Chemother. 46(4):523–526. doi: 10.1093/jac/46.4.523. [DOI] [PubMed] [Google Scholar]
- Telhig S, Ben Said L, Torres C, Rebuffat S, Zirah S, Fliss I. 2022. Evaluating the potential and synergetic effects of microcins against multidrug-resistant enterobacteriaceae. Microbiol Spectr. 10(3):e0275221. doi: 10.1128/spectrum.02752-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissieres A, Mitchell HK, Haskins FA. 1953. Studies on the respiratory system of the poky strain of Neurospora. J. Biol. Chem. 205(1):423–433. doi: 10.1016/S0021-9258(19)77267-6. [DOI] [PubMed] [Google Scholar]
- Torres RA, Weinberg W, Stansell J, Leoung G, Kovacs J, Rogers M, Scott J. and Atovaquone/Toxoplasmic Encephalitis Study Group. 1997. Atovaquone for salvage treatment and suppression of toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis. 24(3):422–429. doi: 10.1093/clinids/24.3.422. [DOI] [PubMed] [Google Scholar]
- Tran QM, Rothery RA, Maklashina E, Cecchini G, Weiner JH. 2007. Escherichia coli succinate dehydrogenase variant lacking the heme b. Proc Natl Acad Sci U S A. 104(46):18007–18012. doi: 10.1073/pnas.0707732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Munita JM, Arias CA. 2015. Mechanisms of drug resistance: daptomycin resistance. Ann N Y Acad Sci. 1354(1):32–53. doi: 10.1111/nyas.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpower BL. 1990. Cytochrome bc1 complexes of microorganisms. Microbiol Rev. 54(2):101–129. doi: 10.1128/mr.54.2.101-129.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CP, Albrecht J, Gunsalus RP. 1996. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol. 178(4):1094–1098. doi: 10.1128/jb.178.4.1094-1098.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AK, Barber LZ, Wigley P, Muhammad S, Jones MA, Lovell MA, Hulme S, Barrow PA. 2003. Contribution of proton-translocating proteins to the virulence of Salmonella enterica serovars typhimurium, gallinarum, and dublin in chickens and mice. Infect Immun. 71:3392–3401. Available from: https://journals.asm.org/doi/10.1128/iai.71.6.3392-3401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuz K, Mezic KG, Xu T, Barquera B, Juárez O. 2015. The kinetic reaction mechanism of the Vibrio cholerae sodium-dependent NADH dehydrogenase. J Biol Chem. 290(33):20009–20021. doi: 10.1074/jbc.M115.658773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G, Bongaerts J. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1320(3):217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- Vaillier J, Arselin G, Graves PV, Camougrand N, Velours J. 1999. Isolation of supernumerary yeast ATP synthase subunits e and i. Characterization of subunit i and disruption of its structural gene ATP18. J Biol Chem. 274(1):543–548. doi: 10.1074/jbc.274.1.543. [DOI] [PubMed] [Google Scholar]
- Vamshi Krishna K, Venkata Mohan S. 2019. Purification and characterization of NDH-2 protein and elucidating its role in extracellular electron transport and bioelectrogenic activity. Front Microbiol. 10:880. doi: 10.3389/fmicb.2019.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beilen JWA, Hellingwerf KJ. 2016. All three endogenous quinone species of Escherichia coli are involved in controlling the activity of the aerobic/anaerobic response regulator ArcA. Front. Microbiol. 7:1339. doi: 10.3389/fmicb.2020.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Velden TT, Kayastha K, Waterham CYJ, Brünle S, Jeuken LJC. 2025. Menaquinone-specific turnover by Mycobacterium tuberculosis cytochrome bd is redox regulated by the Q-loop disulfide bond. J. Biol. Chem. 301(2):108094. doi: 10.1016/j.jbc.2024.108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Cvetkovska M, Wang J. 2009. Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase? Physiol Plant. 137(4):392–406. doi: 10.1111/j.1399-3054.2009.01254.x. [DOI] [PubMed] [Google Scholar]
- Veiga A, Arrabaça JD, Loureiro-Dias MC. 2003. Cyanide-resistant respiration, a very frequent metabolic pathway in yeasts. FEMS Yeast Res. 3(3):239–245. doi: 10.1016/S1567-1356(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Veloukas T, Karaoglanidis GS. 2012. Biological activity of the succinate dehydrogenase inhibitor fluopyram against Botrytis cinerea and fungal baseline sensitivity. Pest Manag Sci. 68(6):858–864. doi: 10.1002/ps.3241. [DOI] [PubMed] [Google Scholar]
- Verkhovsky MI, Bogachev AV. 2010. Sodium-translocating NADH:quinone oxidoreductase as a redox-driven ion pump. Biochim Biophys Acta. 1797(6–7):738–746. doi: 10.1016/j.bbabio.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Vitt S, Prinz S, Eisinger M, Ermler U, Buckel W. 2022. Purification and structural characterization of the Na+-translocating ferredoxin: NAD+ reductase (Rnf) complex of Clostridium tetanomorphum. Nat Commun. 13(1):6315. doi: 10.1038/s41467-022-34007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ballmoos C, Wiedenmann A, Dimroth P. 2009. Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem. 78(1):649–672. doi: 10.1146/annurev.biochem.78.081307.104803. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang J, Li L, Hsiang T, Wang M, Shang S, Yu Z. 2016. Metabolic activities of five botryticides against Botrytis cinerea examined using the Biolog FF MicroPlate. Sci Rep, 6:31025. doi: 10.1038/srep31025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Wakefield AE, Dohn MN, Miller RF, Baughman RP, Hossler PA, Bartlett MS, Smith JW, Kazanjian P, Meshnick SR. 1998. Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J Infect Dis. 178(6):1767–1775. doi: 10.1086/314509. [DOI] [PubMed] [Google Scholar]
- Walker JE. 2013. The ATP synthase: the understood, the uncertain and the unknown. Biochem Soc Trans. 41(1):1–16. doi: 10.1042/BST20110773. [DOI] [PubMed] [Google Scholar]
- Weinstein EA, Yano T, Li L-S, Avarbock D, Avarbock A, Helm D, McColm AA, Duncan K, Lonsdale JT, Rubin H. 2005. Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs. Proc Natl Acad Sci U S A. 102(12):4548–4553. doi: 10.1073/pnas.0500469102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Bushby RJ, Evans SD, Jeuken LJC. 2010. A study of cytochrome bo3 in a tethered bilayer lipid membrane. Biochim Biophys Acta. 1797(12):1917–1923. doi: 10.1016/j.bbabio.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel M, Chiriac AI, Otto A, Zweytick D, May C, Schumacher C, Gust R, Albada HB, Penkova M, Krämer U, et al. 2014. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc Natl Acad Sci USA. 111(14):E1409–E1418. doi: 10.1073/pnas.1319900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M 2005. Biophysical and structural aspects of bioenergetics. London: Royal Society of Chemistry. [Google Scholar]
- Williams BAP, Elliot C, Burri L, Kido Y, Kita K, Moore AL, Keeling PJ. 2010. A broad distribution of the alternative oxidase in microsporidian parasites. PLoS Pathog. 6(2):e1000761. doi: 10.1371/journal.ppat.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D, Esser L, Tang W-K, Zhou F, Zhou Y, Yu L, Yu C-A. 2013. Structural analysis of cytochrome bc1 complexes: implications to the mechanism of function. Biochim Biophys Acta. 1827(11–12):1278–1294. doi: 10.1016/j.bbabio.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Copsey AC, Young L, Barsottini MRO, Albury MS, Moore AL. 2021. Comparison of the kinetic parameters of alternative oxidases from Trypanosoma brucei and Arabidopsis thaliana—a tale of two cavities. Front Plant Sci. 12:744218. doi: 10.3389/fpls.2021.744218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T 1991. Bacterial NADH-quinone oxidoreductases. J Bioenerg Biomembr. 23(2):211–225. doi: 10.1007/BF00762218. [DOI] [PubMed] [Google Scholar]
- Yan H, Asfahl KL, Li N, Sun F, Xiao J, Shen D, Dandekar AA, Wang M. 2019. Conditional quorum-sensing induction of a cyanide-insensitive terminal oxidase stabilizes cooperating populations of Pseudomonas aeruginosa. Nat Commun. 10(1):4999. doi: 10.1038/s41467-019-13013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Borisov VB, Konstantinov AA, Gennis RB. 2008. The fully oxidized form of the cytochrome bd quinol oxidase from E. coli does not participate in the catalytic cycle: direct evidence from rapid kinetics studies. FEBS Lett. 582(25–26):3705–3709. doi: 10.1016/j.febslet.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankovskaya V, Horsefield R, Törnroth S, Luna-Chavez C, Miyoshi H, Léger C, Byrne B, Cecchini G, Iwata S. 2003. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 299(5607):700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- Yano T, Kassovska-Bratinova S, Teh JS, Winkler J, Sullivan K, Isaacs A, Schechter NM, Rubin H. 2011. Reduction of clofazimine by mycobacterial type 2 NADH: quinone oxidoreductase. J Biol Chem. 286(12):10276–10287. doi: 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Li L-S, Weinstein E, Teh J-S, Rubin H. 2006. Steady-state kinetics and inhibitory action of antitubercular phenothiazines on Mycobacterium tuberculosis type-II NADH-menaquinone oxidoreductase (NDH-2). J Biol Chem. 281(17):11456–11463. doi: 10.1074/jbc.M508844200. [DOI] [PubMed] [Google Scholar]
- Yano T, Rahimian M, Aneja KK, Schechter NM, Rubin H, Scott CP. 2014. Mycobacterium tuberculosis type II NADH-menaquinone oxidoreductase catalyzes electron transfer through a two-site ping-pong mechanism and has two quinone-binding sites. Biochemistry. 53(7):1179–1190. doi: 10.1021/bi4013897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlagadda V, Medina R, Wright GD. 2020. Venturicidin A, a membrane-active natural product inhibitor of ATP synthase potentiates aminoglycoside antibiotics. Sci Rep. 10(1):8134. doi: 10.1038/s41598-020-64756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Muneyuki E, Hisabori T. 2001. ATP synthase—a marvellous rotary engine of the cell. Nat Rev Mol Cell Biol 2:669–677. doi: 10.1038/35089509 [DOI] [PubMed] [Google Scholar]
- Yolanda H, Jearawuttanakul K, Wannalo W, Kanjanasirirat P, Borwornpinyo S, Rujirawat T, Payattikul P, Kittichotirat W, Wichadakul D, Krajaejun T. 2024. Potential anti-Pythium insidiosum therapeutics identified through screening of agricultural fungicides. Microbiol Spectr. 12(2):e01620–23. doi: 10.1128/spectrum.01620-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Rosell-Hidalgo A, Inaoka DK, Xu F, Albury M, May B, Kita K, Moore AL. 2020. Kinetic and structural characterisation of the ubiquinol-binding site and oxygen reduction by the trypanosomal alternative oxidase. Biochim Biophys Acta Bioenerg. 1861(10):148247. doi: 10.1016/j.bbabio.2020.148247. [DOI] [PubMed] [Google Scholar]
- Young L, Shiba T, Harada S, Kita K, Albury MS, Moore AL. 2013. The alternative oxidases: simple oxidoreductase proteins with complex functions. Biochem Soc Trans. 41(5):1305–1311. doi: 10.1042/BST20130073. [DOI] [PubMed] [Google Scholar]
- Yuan M, González Montalvo MA, Hu Y, Tuz K, Juárez OX. 2025. Repurposing clofazimine as an antibiotic to treat cholera: identification of cellular and structural targets. J Biol Chem. 301(8):110458. doi: 10.1016/j.jbc.2025.110458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Yue Y, Yang Y, Yang J, Tao K, Jin H, Hou T. 2019. Discovery of N-(4-fluoro-2-(phenylamino)phenyl)-pyrazole-4-carboxamides as potential succinate dehydrogenase inhibitors. Pestic Biochem Physiol. 158:175–184. doi: 10.1016/j.pestbp.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lai Y, Zhou S, Ran T, Zhang Y, Zhao Z, Feng Z, Yu L, Xu J, Shi K, et al. 2024. Inhibition of M. tuberculosis and human ATP synthase by BDQ and TBAJ-587. Nature. 631(8020):409–414. doi: 10.1038/s41586-024-07605-8. [DOI] [PubMed] [Google Scholar]
- Zhao D, Jiang Y, Sun J, Li H, Huang M, Sun X, Zhao M. 2019. Elucidation of the anti-inflammatory effect of vanillin in Lps-activated THP-1 cells. J Food Sci. 84(7):1920–1928. doi: 10.1111/1750-3841.14693. [DOI] [PubMed] [Google Scholar]
- Zhao X, Kuipers OP. 2021. Nisin- and ripcin-derived hybrid lanthipeptides display selective antimicrobial activity against Staphylococcus aureus. ACS Synth Biol. 10(7):1703–1714. doi: 10.1021/acssynbio.1c00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J-L, Chen H-H, Xu J, Huang M-Y, Wang J-F, Shen H-J, Shen S-X, Gao C-X, Qian C-D. 2024. Myricetin acts as an inhibitor of type II NADH dehydrogenase from Staphylococcus aureus. Molecules. 29(10):2354. doi: 10.3390/molecules29102354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Gao Y, Wang W, Yang X, Yang X, Liu F, Tang Y, Lam SM, Shui G, Yu L, et al. 2021. Architecture of the mycobacterial succinate dehydrogenase with a membrane-embedded Rieske FeS cluster. Proc Natl Acad Sci. 118:e2022308118. [DOI] [PMC free article] [PubMed] [Google Scholar]


